Tumor-Associated Glycans and Their Role in Gynecological Cancers: Accelerating Translational Research by Novel High-Throughput Approaches
Abstract
:1. Introduction
1.1. Glycans and Cancer
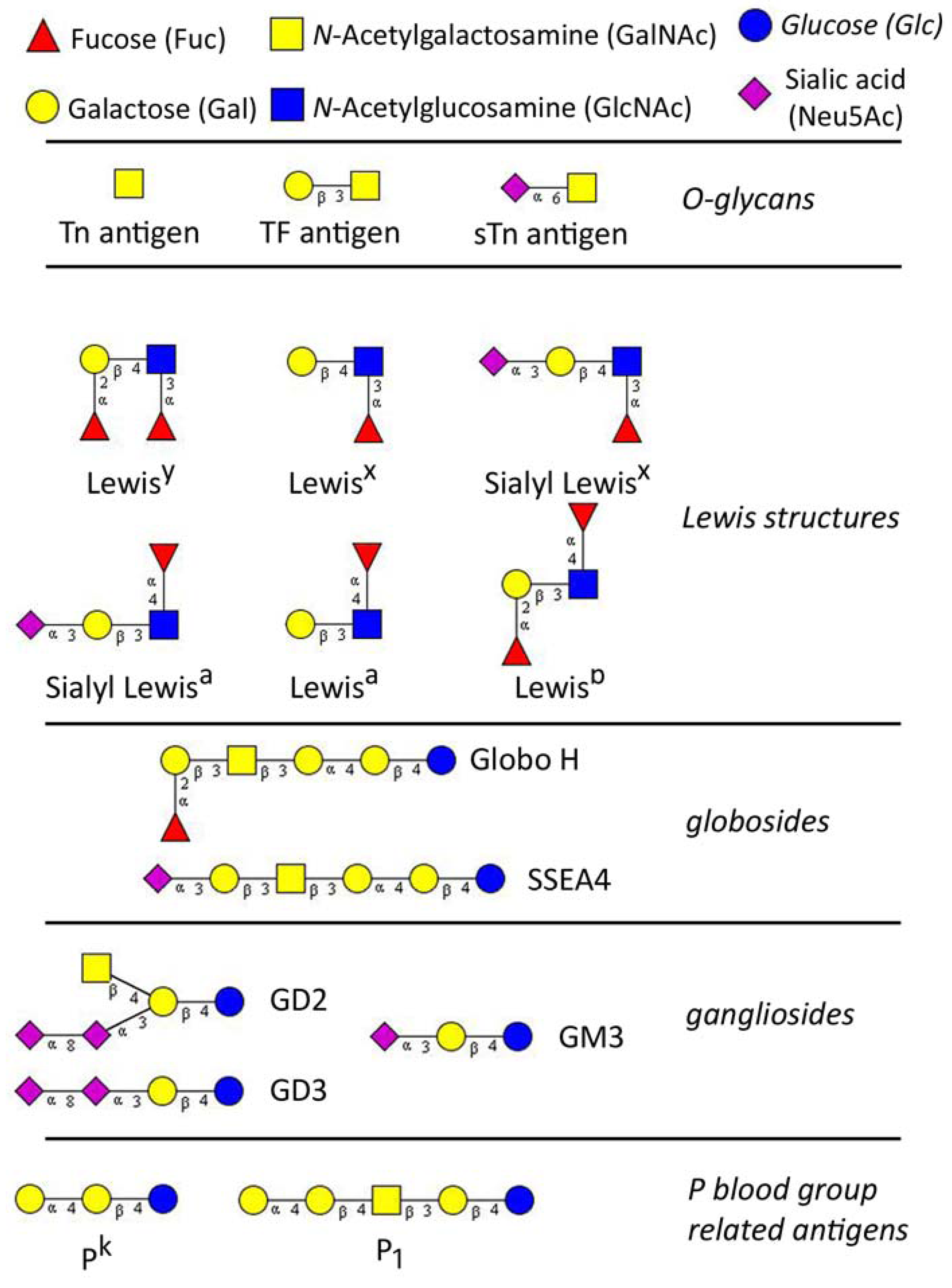
1.2. Naturally Occurring Anti-Glycan Antibodies
1.3. High-Throughput Technologies to Map Glycan-Antibody Interactions
| Array type | Ref. | Glycan-presentation | Assay dynamics | Immobilization (theoretically) | Serum dilution | detection |
|---|---|---|---|---|---|---|
| ELISA (Glycominds) | [36,37,38,39,43,44,45] | n/a | Static | n/a | 1:101 | Anti-human IgA, IgG or IgM separately and each HRPO conjugated |
| ELISA | [26,27,31,33,34,35] | polymeric | Static | Non-covalent and non site-specific | 1:25 (up to 1250 | Anti-human Ig (IgA+IgG+IgM), IgG and IgM HRPO conjugated, IgM and IgG both AP conjugated; |
| ELISA | [32] | polymeric | Static | n/a | Undiluted up to 1:10000 | Anti-human IgD, IgG2 and IgM and anti-mouse IgG HRPO conjugated |
| SPR array | [46] | Monomeric/polymeric | Flow | Covalent and site-specific | 1:50 | Anti-human IgG or IgM |
| Suspension array | [47,48] | polymeric | Flow | Covalent and site-specific | 1:40 | Anti-human IgM or IgG R-phycoerythrin conjugated |
| Glycan array | [24,49,50,51,52] | monomeric | Static | Covalent and site-specific | 1:15 | Anti-human IgA, IgG&IgM biotin conjugated; Streptavidin-Alex555 conjugated |
| Glycan array | [53,54] | monomeric | Static | Covalent and site-specific | 1:20 | Cy3 conjugated anti-human IgG or IgM |
| Glycan array | [19,41,55,56] | monomeric | Static | Covalent and site-specific | 1:20 (up to 1:40) | Anti-human IgA,IgG&IgM or separately all of them biotin conjugated; Streptavidin-europium conjugated |
| Glycan array | [57] | monomeric | Static | Covalent and site-specific | 1:100 | Cy3 conjugated anti-human IgG |
| Glycopeptide array | [58,59,60,61,62] | polymeric | Static | Covalent and (semi-) site specific | 1:50 | Cy3 conjugated anti-human IgG, IgM, IgA together and separately |
| Glycopeptide array | [23,25,63] | polymeric | Static | Covalent and site-specific | 1:25 (up to 1:3000) | Cy3 conjugated anti-human IgG, IgA and IgM separately (in combination and study dependent) |
2. TAC in Gynecological Cancers
2.1. Tn Antigen
2.2. Sialyl-Tn Antigen
2.3. T Antigen
2.4. Lewis Structures
2.5. Glycoshingolipids
2.5.1. Gangliosides
2.5.2. Globosides
3. Conclusions and Discussion
Acknowledgments
Conflict of Interest
References
- Hakomori, S. Tumor-associated carbohydrate antigens. Annu. Rev. Immunol. 1984, 2, 103–126. [Google Scholar] [CrossRef]
- Hakomori, S. Tumor-associated carbohydrate antigens defining tumor malignancy: Basis for development of anti-cancer vaccines. Adv. Exp. Med. Biol 2001, 491, 369–402. [Google Scholar] [CrossRef]
- Konska, G.; Guerry, M.; Caldefie-Chezet, F.; De Latour, M.; Guillot, J. Study of the expression of tn antigen in different types of human breast cancer cells using vva-b4 lectin. Oncol. Rep. 2006, 15, 305–310. [Google Scholar]
- Danussi, C.; Coslovi, A.; Campa, C.; Mucignat, M.T.; Spessotto, P.; Uggeri, F.; Paoletti, S.; Colombatti, A. A newly generated functional antibody identifies tn antigen as a novel determinant in the cancer cell-lymphatic endothelium interaction. Glycobiology 2009, 19, 1056–1067. [Google Scholar] [CrossRef]
- Ghazarian, H.; Idoni, B.; Oppenheimer, S.B. A glycobiology review: Carbohydrates, lectins and implications in cancer therapeutics. Acta Histochem. 2011, 113, 236–247. [Google Scholar] [CrossRef]
- Hakomori, S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv. Cancer Res. 1989, 52, 257–331. [Google Scholar] [CrossRef]
- Hakomori, S. Glycosylation defining cancer malignancy: New wine in an old bottle. Proc. Natl. Acad. Sci. USA 2002, 99, 10231–10233. [Google Scholar] [CrossRef]
- Powlesland, A.S.; Hitchen, P.G.; Parry, S.; Graham, S.A.; Barrio, M.M.; Elola, M.T.; Mordoh, J.; Dell, A.; Drickamer, K.; Taylor, M.E. Targeted glycoproteomic identification of cancer cell glycosylation. Glycobiology 2009, 19, 899–909. [Google Scholar] [CrossRef]
- Fuster, M.M.; Esko, J.D. The sweet and sour of cancer: Glycans as novel therapeutic targets. Nat. Rev. Cancer 2005, 5, 526–542. [Google Scholar] [CrossRef]
- Chandrasekaran, E.V.; Xue, J.; Neelamegham, S.; Matta, K.L. The pattern of glycosyl- and sulfotransferase activities in cancer cell lines: A predictor of individual cancer-associated distinct carbohydrate structures for the structural identification of signature glycans. Carbohydr. Res. 2006, 341, 983–994. [Google Scholar] [CrossRef]
- Hakomori, S. Possible functions of tumor-associated carbohydrate antigens. Curr. Opin. Immunol. 1991, 3, 646–653. [Google Scholar]
- Muramatsu, T. Carbohydrate signals in metastasis and prognosis of human carcinomas. Glycobiology 1993, 3, 291–296. [Google Scholar] [CrossRef]
- Brockhausen, I. Pathways of o-glycan biosynthesis in cancer cells. Biochim. Biophys. Acta 1999, 1473, 67–95. [Google Scholar] [CrossRef]
- Dennis, J.W.; Granovsky, M.; Warren, C.E. Glycoprotein glycosylation and cancer progression. Biochim. Biophys. Acta 1999, 1473, 21–34. [Google Scholar] [CrossRef]
- Chandrasekaran, E.V.; Xue, J.; Xia, J.; Chawda, R.; Piskorz, C.; Locke, R.D.; Neelamegham, S.; Matta, K.L. Analysis of the specificity of sialyltransferases toward mucin core 2, globo, and related structures. Identification of the sialylation sequence and the effects of sulfate, fucose, methyl, and fluoro substituents of the carbohydrate chain in the biosynthesis of selectin and siglec ligands, and novel sialylation by cloned alpha2,3(o)sialyltransferase. Biochemistry 2005, 44, 15619–15635. [Google Scholar]
- Hebbar, M.; Krzewinski-Recchi, M.A.; Hornez, L.; Verdiere, A.; Harduin-Lepers, A.; Bonneterre, J.; Delannoy, P.; Peyrat, J.P. Prognostic value of tumoral sialyltransferase expression and circulating e-selectin concentrations in node-negative breast cancer patients. Int J. Biol. Markers 2003, 18, 116–122. [Google Scholar]
- Recchi, M.A.; Hebbar, M.; Hornez, L.; Harduin-Lepers, A.; Peyrat, J.P.; Delannoy, P. Multiplex reverse transcription polymerase chain reaction assessment of sialyltransferase expression in human breast cancer. Cancer Res. 1998, 58, 4066–4070. [Google Scholar]
- Ceroni, A.; Maass, K.; Geyer, H.; Geyer, R.; Dell, A.; Haslam, S.M. Glycoworkbench: A tool for the computer-assisted annotation of mass spectra of glycans. J. Proteome Res. 2008, 7, 1650–1659. [Google Scholar] [CrossRef]
- Dotan, I.; Fishman, S.; Dgani, Y.; Schwartz, M.; Karban, A.; Lerner, A.; Weishauss, O.; Spector, L.; Shtevi, A.; Altstock, R.T.; et al. Antibodies against laminaribioside and chitobioside are novel serologic markers in crohn's disease. Gastroenterology 2006, 131, 366–378. [Google Scholar]
- Hirche, T.O.; Stein, J.; Hirche, H.; Hausmann, J.; Wagner, T.O.; Behrens, F.; Schroder, O. Increased levels of anti-glycan antibodies in patients with cystic fibrosis. Eur. J. Med. Res. 2011, 16, 385–390. [Google Scholar] [CrossRef]
- Gyorgy, B.; Tothfalusi, L.; Nagy, G.; Pasztoi, M.; Geher, P.; Lorinc, Z.; Polgar, A.; Rojkovich, B.; Ujfalussy, I.; Poor, G.; et al. Natural autoantibodies reactive with glycosaminoglycans in rheumatoid arthritis. Arthritis Res. Ther. 2008, 10, R110. [Google Scholar] [CrossRef]
- A, V.D.; NS, V.D.V.; Smit, C.H.; Meevissen, M.H.; Hokke, C.H. Parasite glycans and antibody-mediated immune responses in schistosoma infection. Parasitology 2012, 139, 1219–1230. [Google Scholar] [CrossRef]
- Blixt, O.; Bueti, D.; Burford, B.; Allen, D.; Julien, S.; Hollingsworth, M.; Gammerman, A.; Fentiman, I.; Taylor-Papadimitriou, J.; Burchell, J.M. Autoantibodies to aberrantly glycosylated muc1 in early stage breast cancer are associated with a better prognosis. Breast Cancer Res. 2011, 13, R25. [Google Scholar] [CrossRef]
- Jacob, F.; Goldstein, D.R.; Bovin, N.V.; Pochechueva, T.; Spengler, M.; Caduff, R.; Fink, D.; Vuskovic, M.I.; Huflejt, M.E.; Heinzelmann-Schwarz, V. Serum antiglycan antibody detection of nonmucinous ovarian cancers by using a printed glycan array. Int. J. Cancer 2012, 130, 138–146. [Google Scholar]
- Wandall, H.H.; Blixt, O.; Tarp, M.A.; Pedersen, J.W.; Bennett, E.P.; Mandel, U.; Ragupathi, G.; Livingston, P.O.; Hollingsworth, M.A.; Taylor-Papadimitriou, J.; et al. Cancer biomarkers defined by autoantibody signatures to aberrant o-glycopeptide epitopes. Cancer Res. 2010, 70, 1306–1313. [Google Scholar]
- Kurtenkov, O.; Klaamas, K.; Rittenhouse-Olson, K.; Vahter, L.; Sergejev, B.; Miljukhina, L.; Shljapnikova, L. Igg immune response to tumor-associated carbohydrate antigens (tf, tn, alphagal) in patients with breast cancer: Impact of neoadjuvant chemotherapy and relation to the survival. Exp. Oncol. 2005, 27, 136–140. [Google Scholar]
- Smorodin, E.P.; Kurtenkov, O.A.; Sergeyev, B.L.; Pazynina, G.V.; Bovin, N.V. Specificity of human anti-carbohydrate igg antibodies as probed with polyacrylamide-based glycoconjugates. Glycoconj. J. 2004, 20, 83–89. [Google Scholar]
- Springer, G.F. T and tn, general carcinoma autoantigens. Science 1984, 224, 1198–1206. [Google Scholar]
- Brandlein, S.; Pohle, T.; Ruoff, N.; Wozniak, E.; Muller-Hermelink, H.K.; Vollmers, H.P. Natural igm antibodies and immunosurveillance mechanisms against epithelial cancer cells in humans. Cancer Res. 2003, 63, 7995–8005. [Google Scholar]
- Slovin, S.F.; Keding, S.J.; Ragupathi, G. Carbohydrate vaccines as immunotherapy for cancer. Immunol. Cell. Biol. 2005, 83, 418–428. [Google Scholar]
- Klaamas, K.; Kurtenkov, O.; Rittenhouse-Olson, K.; Brjalin, V.; Miljukhina, L.; Shljapnikova, L.; Engstrand, L. Expression of tumor-associated thomsen-friedenreich antigen (T Ag) in helicobacter pylori and modulation of t ag specific immune response in infected individuals. Immunol. Invest. 2002, 31, 191–204. [Google Scholar] [CrossRef]
- Mosedale, D.E.; Chauhan, A.; Schofield, P.M.; Grainger, D.J. A pattern of anti-carbohydrate antibody responses present in patients with advanced atherosclerosis. J. Immunol. Methods 2006, 309, 182–191. [Google Scholar] [CrossRef]
- Obukhova, P.; Rieben, R.; Bovin, N. Normal human serum contains high levels of anti-gal alpha 1-4glcnac antibodies. Xenotransplantation 2007, 14, 627–635. [Google Scholar] [CrossRef]
- Smorodin, E.P.; Kurtenkov, O.A.; Sergeyev, B.L.; Kodar, K.E.; Chuzmarov, V.I.; Afanasyev, V.P. Postoperative change of anti-thomsen-friedenreich and tn igg level: The follow-up study of gastrointestinal cancer patients. World J. Gastroenterol. 2008, 14, 4352–4358. [Google Scholar] [CrossRef]
- Yeh, P.; Ezzelarab, M.; Bovin, N.; Hara, H.; Long, C.; Tomiyama, K.; Sun, F.; Ayares, D.; Awwad, M.; Cooper, D.K. Investigation of potential carbohydrate antigen targets for human and baboon antibodies. Xenotransplantation 2010, 17, 197–206. [Google Scholar] [CrossRef]
- Ferrante, M.; Henckaerts, L.; Joossens, M.; Pierik, M.; Joossens, S.; Dotan, N.; Norman, G.L.; Altstock, R.T.; Van Steen, K.; Rutgeerts, P.; et al. New serological markers in inflammatory bowel disease are associated with complicated disease behaviour. Gut 2007, 56, 1394–1403. [Google Scholar]
- Rieder, F.; Schleder, S.; Wolf, A.; Dirmeier, A.; Strauch, U.; Obermeier, F.; Lopez, R.; Spector, L.; Fire, E.; Yarden, J.; et al. Association of the novel serologic anti-glycan antibodies anti-laminarin and anti-chitin with complicated crohn's disease behavior. Inflamm. Bowel. Dis. 2010, 16, 263–274. [Google Scholar]
- Rieder, F.; Schleder, S.; Wolf, A.; Dirmeier, A.; Strauch, U.; Obermeier, F.; Lopez, R.; Spector, L.; Fire, E.; Yarden, J.; et al. Serum anti-glycan antibodies predict complicated crohn's disease behavior: A cohort study. Inflamm. Bowel. Dis. 2010, 16, 1367–1375. [Google Scholar]
- Seow, C.H.; Stempak, J.M.; Xu, W.; Lan, H.; Griffiths, A.M.; Greenberg, G.R.; Steinhart, A.H.; Dotan, N.; Silverberg, M.S. Novel anti-glycan antibodies related to inflammatory bowel disease diagnosis and phenotype. Am. J. Gastroenterol. 2009, 104, 1426–1434. [Google Scholar] [CrossRef]
- Blixt, O.; Head, S.; Mondala, T.; Scanlan, C.; Huflejt, M.E.; Alvarez, R.; Bryan, M.C.; Fazio, F.; Calarese, D.; Stevens, J.; et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc. Natl. Acad. Sci. USA 2004, 101, 17033–17038. [Google Scholar]
- Schwarz, M.; Spector, L.; Gargir, A.; Shtevi, A.; Gortler, M.; Altstock, R.T.; Dukler, A.A.; Dotan, N. A new kind of carbohydrate array, its use for profiling antiglycan antibodies, and the discovery of a novel human cellulose-binding antibody. Glycobiology 2003, 13, 749–754. [Google Scholar] [CrossRef]
- Lee, M.R.; Shin, I. Facile preparation of carbohydrate microarrays by site-specific, covalent immobilization of unmodified carbohydrates on hydrazide-coated glass slides. Org. Lett. 2005, 7, 4269–4272. [Google Scholar]
- Papp, M.; Foldi, I.; Altorjay, I.; Palyu, E.; Udvardy, M.; Tumpek, J.; Sipka, S.; Korponay-Szabo, I.R.; Nemes, E.; Veres, G.; et al. Anti-microbial antibodies in celiac disease: Trick or treat? World J. Gastroenterol. 2009, 15, 3891–3900. [Google Scholar]
- Ferrante, M.; Declerck, S.; Coopmans, T.; De Hertogh, G.; Van Assche, G.; Penninckx, F.; Rutgeerts, P.; Geboes, K.; D'Hoore, A.; Vermeire, S. Development of pouchitis following ileal pouch-anal anastomosis (ipaa) for ulcerative colitis: A role for serological markers and microbial pattern recognition receptor genes. J. Crohns. Colitis. 2008, 2, 142–151. [Google Scholar] [CrossRef]
- Sendid, B.; Dotan, N.; Nseir, S.; Savaux, C.; Vandewalle, P.; Standaert, A.; Zerimech, F.; Guery, B.P.; Dukler, A.; Colombel, J.F.; et al. Antibodies against glucan, chitin, and saccharomyces cerevisiae mannan as new biomarkers of candida albicans infection that complement tests based on c. Albicans mannan. Clin. Vaccine Immunol. 2008, 15, 1868–1877. [Google Scholar] [CrossRef]
- de Boer, A.R.; Hokke, C.H.; Deelder, A.M.; Wuhrer, M. Serum antibody screening by surface plasmon resonance using a natural glycan microarray. Glycoconj. J. 2008, 25, 75–84. [Google Scholar] [CrossRef]
- Pochechueva, T.; Chinarev, A.; Spengler, M.; Korchagina, E.; Heinzelmann-Schwarz, V.; Bovin, N.; Rieben, R. Multiplex suspension array for human anti-carbohydrate antibody profiling. Analyst 2010, 136, 560–569. [Google Scholar]
- Pochechueva, T.; Jacob, F.; Goldstein, D.R.; Huflejt, M.E.; Chinarev, A.; Caduff, R.; Fink, D.; Hacker, N.; Bovin, N.V.; Heinzelmann-Schwarz, V. Comparison of printed glycan array, suspension array and elisa in the detection of human anti-glycan antibodies. Glycoconj. J. 2011, 28, 507–517. [Google Scholar] [CrossRef]
- Bovin, N.; Obukhova, P.; Shilova, N.; Rapoport, E.; Popova, I.; Navakouski, M.; Unverzagt, C.; Vuskovic, M.; Huflejt, M. Repertoire of human natural anti-glycan immunoglobulins. Do we have auto-antibodies? Biochim. Biophys. Acta 2012, 1820, 1373–1382. [Google Scholar] [CrossRef]
- Bovin, N.V.; Huflejt, M.E. Unlimited glycochip. Trends Glycosci. Glycotechnol. 2008, 20, 245–258. [Google Scholar] [CrossRef]
- Huflejt, M.E.; Blixt, O.; Vuskovic, M.; Xu, H.; Shaw, L.E.; Reuben, J.M.; Kuerer, H.M. Glycan array identifies specific signatures of anti-glycan autoantibodies in sera of breast cancer patients: Diagnostic, prognostic and therapeutic opportunities. In the 28th Annual San Antonio Breast Cancer Symposium, 8–11 December 2005; p. 2008.
- Huflejt, M.E.; Vuskovic, M.; Vasiliu, D.; Xu, H.; Obukhova, P.; Shilova, N.; Tuzikov, A.; Galanina, O.; Arun, B.; Lu, K.; et al. Anti-carbohydrate antibodies of normal sera: Findings, surprises and challenges. Mol. Immunol. 2009, 46, 3037–3049. [Google Scholar]
- Wang, C.C.; Huang, Y.L.; Ren, C.T.; Lin, C.W.; Hung, J.T.; Yu, J.C.; Yu, A.L.; Wu, C.Y.; Wong, C.H. Glycan microarray of globo h and related structures for quantitative analysis of breast cancer. Proc. Natl. Acad. Sci.USA 2008, 105, 11661–11666. [Google Scholar]
- Huang, C.Y.; Thayer, D.A.; Chang, A.Y.; Best, M.D.; Hoffmann, J.; Head, S.; Wong, C.H. Carbohydrate microarray for profiling the antibodies interacting with globo h tumor antigen. Proc. Natl. Acad. Sci. USA 2006, 103, 15–20. [Google Scholar]
- Dotan, N.; Altstock, R.T.; Schwarz, M.; Dukler, A. Anti-glycan antibodies as biomarkers for diagnosis and prognosis. Lupus 2006, 15, 442–450. [Google Scholar] [CrossRef]
- Freedman, M.S.; Laks, J.; Dotan, N.; Altstock, R.T.; Dukler, A.; Sindic, C.J. Anti-alpha-glucose-based glycan igm antibodies predict relapse activity in multiple sclerosis after the first neurological event. Mult. Scler. 2009, 15, 422–430. [Google Scholar]
- Padler-Karavani, V.; Hurtado-Ziola, N.; Pu, M.; Yu, H.; Huang, S.; Muthana, S.; Chokhawala, H.A.; Cao, H.; Secrest, P.; Friedmann-Morvinski, D.; et al. Human xeno-autoantibodies against a non-human sialic acid serve as novel serum biomarkers and immunotherapeutics in cancer. Cancer Res. 2011. [Google Scholar]
- Oyelaran, O.; Gildersleeve, J.C. Application of carbohydrate array technology to antigen discovery and vaccine development. Expert Rev. Vaccines 2007, 6, 957–969. [Google Scholar] [CrossRef]
- Oyelaran, O.; Gildersleeve, J.C. Evaluation of human antibody responses to keyhole limpet hemocyanin on a carbohydrate microarray. Proteomics Clin. Appl. 2010, 4, 285–294. [Google Scholar] [CrossRef]
- Oyelaran, O.; Gildersleeve, J.C. Glycan arrays: Recent advances and future challenges. Curr. Opin. Chem. Biol. 2009, 13, 406–413. [Google Scholar] [CrossRef]
- Oyelaran, O.; Li, Q.; Farnsworth, D.; Gildersleeve, J.C. Microarrays with varying carbohydrate density reveal distinct subpopulations of serum antibodies. J. Proteome Res. 2009, 8, 3529–3538. [Google Scholar] [CrossRef]
- Oyelaran, O.; McShane, L.M.; Dodd, L.; Gildersleeve, J.C. Profiling human serum antibodies with a carbohydrate antigen microarray. J. Proteome Res. 2009, 8, 4301–4310. [Google Scholar] [CrossRef]
- Pedersen, J.W.; Blixt, O.; Bennett, E.P.; Tarp, M.A.; Dar, I.; Mandel, U.; Poulsen, S.S.; Pedersen, A.E.; Rasmussen, S.; Jess, P.; et al. Seromic profiling of colorectal cancer patients with novel glycopeptide microarray. Int. J. Cancer 2011, 128, 1860–1871. [Google Scholar] [CrossRef]
- Tarp, M.A.; Sorensen, A.L.; Mandel, U.; Paulsen, H.; Burchell, J.; Taylor-Papadimitriou, J.; Clausen, H. Identification of a novel cancer-specific immunodominant glycopeptide epitope in the muc1 tandem repeat. Glycobiology 2007, 17, 197–209. [Google Scholar]
- Gupta, G.; Surolia, A.; Sampathkumar, S.G. Lectin microarrays for glycomic analysis. OMICS 2010, 14, 419–436. [Google Scholar] [CrossRef]
- Krishhan, V.V.; Khan, I.H.; Luciw, P.A. Multiplexed microbead immunoassays by flow cytometry for molecular profiling: Basic concepts and proteomics applications. Crit. Rev. Biotechnol. 2009, 29, 29–43. [Google Scholar] [CrossRef]
- Pochechueva, T.; Chinarev, A.; Spengler, M.; Korchagina, E.; Heinzelmann-Schwarz, V.; Bovin, N.; Rieben, R. Multiplex suspension array for human anti-carbohydrate antibody profiling. Analyst 2011, 136, 560–569. [Google Scholar] [CrossRef]
- Chinarev, A.A.; Galanina, O.E.; Bovin, N.V. Biotinylated multivalent glycoconjugates for surface coating. Methods Mol. Biol. 2010, 600, 67–78. [Google Scholar] [CrossRef]
- Nolan, J.P.; Sklar, L.A. Suspension array technology: Evolution of the flat-array paradigm. Trends Biotechnol. 2002, 20, 9–12. [Google Scholar] [CrossRef]
- Tyagi, A.; Wang, X.; Deng, L.; Ramstrom, O.; Yan, M. Photogenerated carbohydrate microarrays to study carbohydrate-protein interactions using surface plasmon resonance imaging. Biosens. Bioelectron. 2010, 26, 344–350. [Google Scholar] [CrossRef]
- Moreau, R.; Dausset, J.; Bernard, J.; Moullec, J. Acquired hemolytic anemia with polyagglutinability of erythrocytes by a new factor present in normal blood. Bull. Mem. Soc. Med. Hop. Paris 1957, 73, 569–587. [Google Scholar]
- Cao, Y.; Stosiek, P.; Springer, G.F.; Karsten, U. Thomsen-friedenreich-related carbohydrate antigens in normal adult human tissues: A systematic and comparative study. Histochem Cell. Biol. 1996, 106, 197–207. [Google Scholar] [CrossRef]
- Howard, D.R.; Taylor, C.R. An antitumor antibody in normal human serum: Reaction of anti-t with breast carcinoma cells. Oncology 1980, 37, 142–148. [Google Scholar] [CrossRef]
- Stein, R.; Chen, S.; Grossman, W.; Goldenberg, D.M. Human lung carcinoma monoclonal antibody specific for the thomsen-friedenreich antigen. Cancer Res. 1989, 49, 32–37. [Google Scholar]
- Wang, B.L.; Springer, G.F.; Carlstedt, S.C. Quantitative computerized image analysis of tn and t (thomsen-friedenreich) epitopes in prognostication of human breast carcinoma. J. Histochem. Cytochem. 1997, 45, 1393–1400. [Google Scholar] [CrossRef]
- Yuan, M.; Itzkowitz, S.H.; Boland, C.R.; Kim, Y.D.; Tomita, J.T.; Palekar, A.; Bennington, J.L.; Trump, B.F.; Kim, Y.S. Comparison of t-antigen expression in normal, premalignant, and malignant human colonic tissue using lectin and antibody immunohistochemistry. Cancer Res. 1986, 46, 4841–4847. [Google Scholar]
- Korourian, S.; Siegel, E.; Kieber-Emmons, T.; Monzavi-Karbassi, B. Expression analysis of carbohydrate antigens in ductal carcinoma in situ of the breast by lectin histochemistry. BMC Cancer 2008, 8, 136. [Google Scholar] [CrossRef]
- Inoue, M.; Ogawa, H.; Nakanishi, K.; Tanizawa, O.; Karino, K.; Endo, J. Clinical value of sialyl tn antigen in patients with gynecologic tumors. Obstet. Gynecol. 1990, 75, 1032–1036. [Google Scholar]
- Numa, F.; Tsunaga, N.; Michioka, T.; Nawata, S.; Ogata, H.; Kato, H. Tissue expression of sialyl tn antigen in gynecologic tumors. J. Obstet. Gynaecol. (Tokyo 1995) 1995, 21, 385–389. [Google Scholar]
- van Vliet, S.J.; Saeland, E.; van Kooyk, Y. Sweet preferences of mgl: Carbohydrate specificity and function. Trends Immunol. 2008, 29, 83–90. [Google Scholar] [CrossRef]
- Saeland, E.; van Vliet, S.J.; Backstrom, M.; van den Berg, V.C.; Geijtenbeek, T.B.; Meijer, G.A.; van Kooyk, Y. The c-type lectin mgl expressed by dendritic cells detects glycan changes on muc1 in colon carcinoma. Cancer Immunol. Immunother. 2007, 56, 1225–1236. [Google Scholar] [CrossRef]
- Ju, T.; Aryal, R.P.; Stowell, C.J.; Cummings, R.D. Regulation of protein o-glycosylation by the endoplasmic reticulum-localized molecular chaperone cosmc. J. Cell. Biol. 2008, 182, 531–542. [Google Scholar] [CrossRef]
- Wang, Y.; Ju, T.; Ding, X.; Xia, B.; Wang, W.; Xia, L.; He, M.; Cummings, R.D. Cosmc is an essential chaperone for correct protein o-glycosylation. Proc. Natl. Acad. Sci. USA 2010, 107, 9228–9233. [Google Scholar]
- Springer, G.F.; Desai, P.R.; Tegtmeyer, H.; Spencer, B.D.; Scanlon, E.F. Pancarcinoma t/tn antigen detects human carcinoma long before biopsy does and its vaccine prevents breast carcinoma recurrence. Ann. N. Y. Acad. Sci. 1993, 690, 355–357. [Google Scholar] [CrossRef]
- Buskas, T.; Ingale, S.; Boons, G.J. Glycopeptides as versatile tools for glycobiology. Glycobiology 2006, 16, 113–136. [Google Scholar] [CrossRef]
- Slovin, S.F.; Ragupathi, G.; Musselli, C.; Olkiewicz, K.; Verbel, D.; Kuduk, S.D.; Schwarz, J.B.; Sames, D.; Danishefsky, S.; Livingston, P.O.; et al. Fully synthetic carbohydrate-based vaccines in biochemically relapsed prostate cancer: Clinical trial results with alpha-n-acetylgalactosamine-o-serine/threonine conjugate vaccine. J. Clin. Oncol. 2003, 21, 4292–4298. [Google Scholar] [CrossRef]
- Kaiser, A.; Gaidzik, N.; Westerlind, U.; Kowalczyk, D.; Hobel, A.; Schmitt, E.; Kunz, H. A synthetic vaccine consisting of a tumor-associated sialyl-t(n)-muc1 tandem-repeat glycopeptide and tetanus toxoid: Induction of a strong and highly selective immune response. Angew. Chem. Int. Ed. Engl. 2009, 48, 7551–7555. [Google Scholar]
- Napoletano, C.; Rughetti, A.; Agervig Tarp, M.P.; Coleman, J.; Bennett, E.P.; Picco, G.; Sale, P.; Denda-Nagai, K.; Irimura, T.; Mandel, U.; et al. Tumor-associated tn-muc1 glycoform is internalized through the macrophage galactose-type c-type lectin and delivered to the hla class i and ii compartments in dendritic cells. Cancer Res. 2007, 67, 8358–8367. [Google Scholar]
- Ragupathi, G.; Cereb, N.; Yang, S.Y. The relative distribution of b35 alleles and their ief isotypes in a hla-b35-positive population. Tissue Antigens 1995, 46, 24–31. [Google Scholar] [CrossRef]
- Sabbatini, P.J.; Ragupathi, G.; Hood, C.; Aghajanian, C.A.; Juretzka, M.; Iasonos, A.; Hensley, M.L.; Spassova, M.K.; Ouerfelli, O.; Spriggs, D.R.; et al. Pilot study of a heptavalent vaccine-keyhole limpet hemocyanin conjugate plus qs21 in patients with epithelial ovarian, fallopian tube, or peritoneal cancer. Clin. Cancer Res. 2007, 13, 4170–4177. [Google Scholar] [CrossRef]
- Blixt, O.; Lavrova, O.I.; Mazurov, D.V.; Clo, E.; Kracun, S.K.; Bovin, N.V.; Filatov, A.V. Analysis of tn antigenicity with a panel of new igm and igg1 monoclonal antibodies raised against leukemic cells. Glycobiology 2012, 22, 529–542. [Google Scholar] [CrossRef]
- Kjeldsen, T.; Clausen, H.; Hirohashi, S.; Ogawa, T.; Iijima, H.; Hakomori, S. Preparation and characterization of monoclonal antibodies directed to the tumor-associated o-linked sialosyl-2----6 alpha-n-acetylgalactosaminyl (sialosyl-tn) epitope. Cancer Res. 1988, 48, 2214–2220. [Google Scholar]
- Sewell, R.; Backstrom, M.; Dalziel, M.; Gschmeissner, S.; Karlsson, H.; Noll, T.; Gatgens, J.; Clausen, H.; Hansson, G.C.; Burchell, J.; et al. The st6galnac-i sialyltransferase localizes throughout the golgi and is responsible for the synthesis of the tumor-associated sialyl-tn o-glycan in human breast cancer. J. Biol Chem. 2006, 281, 3586–3594. [Google Scholar]
- Krzewinski-Recchi, M.-A.; Julien, S.; Juliant, S.; Teintenier-Lelievre, M.; Samyn-Petit, B.; Montiel, M.-D.; Mir, A.-M.; Cerutti, M.; Harduin-Lepers, A.; Delannoy, P. Identification and functional expression of a second human beta-galactoside alpha2,6-sialyltransferase, st6gal ii. Eur. J. Biochem. 2003, 270, 950–961. [Google Scholar]
- Ghazizadeh, M.; Oguro, T.; Sasaki, Y.; Aihara, K.; Araki, T.; Springer, G.F. Immunohistochemical and ultrastructural localization of t antigen in ovarian tumors. Am. J. Clin. Pathol. 1990, 93, 315–321. [Google Scholar]
- Kinney, A.Y.; Sahin, A.; Vernon, S.W.; Frankowski, R.F.; Annegers, J.F.; Hortobagyi, G.N.; Buzdar, A.U.; Frye, D.K.; Dhingra, K. The prognostic significance of sialyl-tn antigen in women treated with breast carcinoma treated with adjuvant chemotherapy. Cancer 1997, 80, 2240–2249. [Google Scholar] [CrossRef]
- Leivonen, M.; Nordling, S.; Lundin, J.; von Boguslawski, K.; Haglund, C. Stn and prognosis in breast cancer. Oncology 2001, 61, 299. [Google Scholar] [CrossRef]
- Miles, D.W.; Happerfield, L.C.; Smith, P.; Gillibrand, R.; Bobrow, L.G.; Gregory, W.M.; Rubens, R.D. Expression of sialyl-tn predicts the effect of adjuvant chemotherapy in node-positive breast cancer. Br. J. Cancer 1994, 70, 1272–1275. [Google Scholar] [CrossRef]
- Soares, R.; Marinho, A.; Schmitt, F. Expression of sialyl-tn in breast cancer. Correlation with prognostic parameters. Pathol. Res. Pract. 1996, 192, 1181–1186. [Google Scholar] [CrossRef]
- Kobayashi, H.; Terao, T.; Kawashima, Y. Serum sialyl tn as an independent predictor of poor prognosis in patients with epithelial ovarian cancer. J. Clin. Oncol. 1992, 10, 95–101. [Google Scholar]
- Davidson, B.; Berner, A.; Nesland, J.M.; Risberg, B.; Kristensen, G.B.; Trope, C.G.; Bryne, M. Carbohydrate antigen expression in primary tumors, metastatic lesions, and serous effusions from patients diagnosed with epithelial ovarian carcinoma: Evidence of up-regulated tn and sialyl tn antigen expression in effusion. Hum. Pathol. 2000, 31, 1081–1087. [Google Scholar] [CrossRef]
- Davidson, B.; Gotlieb, W.H.; Ben-Baruch, G.; Kopolovic, J.; Goldberg, I.; Nesland, J.M.; Berner, A.; Bjamer, A.; Bryne, M. Expression of carbohydrate antigens in advanced-stage ovarian carcinomas and their metastases-a clinicopathologic study. Gynecol. Oncol. 2000, 77, 35–43. [Google Scholar] [CrossRef]
- Ikehara, Y.; Kojima, N.; Kurosawa, N.; Kudo, T.; Kono, M.; Nishihara, S.; Issiki, S.; Morozumi, K.; Itzkowitz, S.; Tsuda, T.; et al. Cloning and expression of a human gene encoding an n-acetylgalactosamine-alpha2,6-sialyltransferase (st6galnac i): A candidate for synthesis of cancer-associated sialyl-tn antigens. Glycobiology 1999, 9, 1213–1224. [Google Scholar] [CrossRef]
- Vazquez-Martin, C.; Cuevas, E.; Gil-Martin, E.; Fernandez-Briera, A. Correlation analysis between tumor-associated antigen sialyl-tn expression and st6galnac i activity in human colon adenocarcinoma. Oncology 2004, 67, 159–165. [Google Scholar] [CrossRef]
- Ogata, S.; Ho, I.; Chen, A.; Dubois, D.; Maklansky, J.; Singhal, A.; Hakomori, S.; Itzkowitz, S.H. Tumor-associated sialylated antigens are constitutively expressed in normal human colonic mucosa. Cancer Res. 1995, 55, 1869–1874. [Google Scholar]
- Van Elssen, C.H.; Frings, P.W.; Bot, F.J.; Van de Vijver, K.K.; Huls, M.B.; Meek, B.; Hupperets, P.; Germeraad, W.T.; Bos, G.M. Expression of aberrantly glycosylated mucin-1 in ovarian cancer. Histopathology 2010, 57, 597–606. [Google Scholar] [CrossRef]
- Singh, R.; Campbell, B.J.; Yu, L.G.; Fernig, D.G.; Milton, J.D.; Goodlad, R.A.; FitzGerald, A.J.; Rhodes, J.M. Cell surface-expressed thomsen-friedenreich antigen in colon cancer is predominantly carried on high molecular weight splice variants of cd44. Glycobiology 2001, 11, 587–592. [Google Scholar] [CrossRef]
- Akita, K.; Yoshida, S.; Ikehara, Y.; Shirakawa, S.; Toda, M.; Inoue, M.; Kitawaki, J.; Nakanishi, H.; Narimatsu, H.; Nakada, H. Different levels of sialyl-tn antigen expressed on muc16 in patients with endometriosis and ovarian cancer. Int. J. Gynecol. Cancer 2012, 22, 531–538. [Google Scholar]
- Julien, S.; Adriaenssens, E.; Ottenberg, K.; Furlan, A.; Courtand, G.; Vercoutter-Edouart, A.S.; Hanisch, F.G.; Delannoy, P.; Le Bourhis, X. St6galnac i expression in mda-mb-231 breast cancer cells greatly modifies their o-glycosylation pattern and enhances their tumourigenicity. Glycobiology 2006, 16, 54–64. [Google Scholar]
- Julien, S.; Lagadec, C.; Krzewinski-Recchi, M.A.; Courtand, G.; Le Bourhis, X.; Delannoy, P. Stable expression of sialyl-tn antigen in t47-d cells induces a decrease of cell adhesion and an increase of cell migration. Breast Cancer Res. Treat. 2005, 90, 77–84. [Google Scholar] [CrossRef]
- Aruffo, A.; Stamenkovic, I.; Melnick, M.; Underhill, C.B.; Seed, B. Cd44 is the principal cell surface receptor for hyaluronate. Cell 1990, 61, 1303–1313. [Google Scholar] [CrossRef]
- Gunthert, U.; Hofmann, M.; Rudy, W.; Reber, S.; Zoller, M.; Haussmann, I.; Matzku, S.; Wenzel, A.; Ponta, H.; Herrlich, P. A new variant of glycoprotein cd44 confers metastatic potential to rat carcinoma cells. Cell 1991, 65, 13–24. [Google Scholar] [CrossRef]
- Yu, L.G. The oncofetal thomsen-friedenreich carbohydrate antigen in cancer progression. Glycoconj. J. 2007, 24, 411–420. [Google Scholar] [CrossRef]
- Springer, G.F.; Desai, P.R.; Banatwala, I. Blood group mn antigens and precursors in normal and malignant human breast glandular tissue. J. Natl. Cancer Inst. 1975, 54, 335–339. [Google Scholar]
- Springer, G.F.; Desai, P.R.; Murthy, M.S.; Scanlon, E.F. Human carcinoma-associated precursor antigens of the nm blood group system. J. Surg Oncol 1979, 11, 95–106. [Google Scholar] [CrossRef]
- Springer, G.F. Immunoreactive t and tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J. Mol. Med. (Berl) 1997, 75, 594–602. [Google Scholar] [CrossRef]
- Barry, J.D.; Koch, T.J.; Cohen, C.; Brigati, D.J.; Sharkey, F.E. Correlation of immunohistochemical markers with patient prognosis in breast carcinoma: A quantitative study. Am. J. Clin. Pathol. 1984, 82, 582–585. [Google Scholar]
- Wolf, M.F.; Ludwig, A.; Fritz, P.; Schumacher, K. Increased expression of thomsen-friedenreich antigens during tumor progression in breast cancer patients. Tumour Biol. 1988, 9, 190–194. [Google Scholar] [CrossRef]
- Schindlbeck, C.; Jeschke, U.; Schulze, S.; Karsten, U.; Janni, W.; Rack, B.; Krajewski, S.; Sommer, H.; Friese, K. Prognostic impact of thomsen-friedenreich tumor antigen and disseminated tumor cells in the bone marrow of breast cancer patients. Breast Cancer Res. Treat. 2007, 101, 17–25. [Google Scholar] [CrossRef]
- Engelstaedter, V.; Fluegel, B.; Kunze, S.; Mayr, D.; Friese, K.; Jeschke, U.; Bergauer, F. Expression of the carbohydrate tumour marker sialyl lewis a, sialyl lewis x, lewis y and thomsen-friedenreich antigen in normal squamous epithelium of the uterine cervix, cervical dysplasia and cervical cancer. Histol. Histopathol. 2012, 27, 507–514. [Google Scholar]
- Cazet, A.; Julien, S.; Bobowski, M.; Burchell, J.; Delannoy, P. Tumour-associated carbohydrate antigens in breast cancer. Breast Cancer Res. 2010, 12, 204. [Google Scholar] [CrossRef]
- Hanisch, F.G.; Baldus, S.E. The thomsen-friedenreich (tf) antigen: A critical review on the structural, biosynthetic and histochemical aspects of a pancarcinoma-associated antigen. Histol. Histopathol. 1997, 12, 263–281. [Google Scholar]
- Butschak, G.; Karsten, U. Isolation and characterization of thomsen-friedenreich-specific antibodies from human serum. Tumour Biol. 2002, 23, 113–122. [Google Scholar] [CrossRef]
- Bychkov, V.; Dolan, J.R.; Reddy, V.B. Lectin binding to common epithelial tumors of the ovaries. Gynecol. Obstet. Invest. 1991, 31, 166–171. [Google Scholar] [CrossRef]
- Sasano, H.; Saito, Y.; Nagura, H.; Kudo, R.; Rojas, M.; Silverberg, S.G. Lectin histochemistry in mucinous and serous ovarian neoplasms. Int. J. Gynecol. Pathol. 1991, 10, 252–259. [Google Scholar] [CrossRef]
- Glinsky, V.V.; Glinsky, G.V.; Glinskii, O.V.; Huxley, V.H.; Turk, J.R.; Mossine, V.V.; Deutscher, S.L.; Pienta, K.J.; Quinn, T.P. Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Cancer Res. 2003, 63, 3805–3811. [Google Scholar]
- Khaldoyanidi, S.K.; Glinsky, V.V.; Sikora, L.; Glinskii, A.B.; Mossine, V.V.; Quinn, T.P.; Glinsky, G.V.; Sriramarao, P. Mda-mb-435 human breast carcinoma cell homo- and heterotypic adhesion under flow conditions is mediated in part by thomsen-friedenreich antigen-galectin-3 interactions. J. Biol. Chem. 2003, 278, 4127–4134. [Google Scholar]
- Bian, C.F.; Zhang, Y.; Sun, H.; Li, D.F.; Wang, D.C. Structural basis for distinct binding properties of the human galectins to thomsen-friedenreich antigen. PLoS One 2011, 6, e25007. [Google Scholar]
- Almogren, A.; Abdullah, J.; Ghapure, K.; Ferguson, K.; Glinsky, V.V.; Rittenhouse-Olson, K. Anti-thomsen-friedenreich-ag (anti-tf-ag) potential for cancer therapy. Front. Biosci. (Schol Ed.) 2012, 4, 840–863. [Google Scholar]
- MacLean, G.D.; Bowen-Yacyshyn, M.B.; Samuel, J.; Meikle, A.; Stuart, G.; Nation, J.; Poppema, S.; Jerry, M.; Koganty, R.; Wong, T.; et al. Active immunization of human ovarian cancer patients against a common carcinoma (thomsen-friedenreich) determinant using a synthetic carbohydrate antigen. J. Immunother. (1991) 1992, 11, 292–305. [Google Scholar] [CrossRef]
- Narita, T.; Funahashi, H.; Satoh, Y.; Watanabe, T.; Sakamoto, J.; Takagi, H. Association of expression of blood group-related carbohydrate antigens with prognosis in breast cancer. Cancer 1993, 71, 3044–3053. [Google Scholar] [CrossRef]
- Ura, Y.; Dion, A.S.; Williams, C.J.; Olsen, B.D.; Redfield, E.S.; Ishida, M.; Herlyn, M.; Major, P.P. Quantitative dot blot analyses of blood-group-related antigens in paired normal and malignant human breast tissues. Int. J. Cancer 1992, 50, 57–63. [Google Scholar] [CrossRef]
- Idikio, H.A.; Manickavel, V. Lewis blood group antigens (a and b) in human breast tissues. Loss of lewis-b in breast cancer cells and correlation with tumor grade. Cancer 1991, 68, 1303–1308. [Google Scholar] [CrossRef]
- Idikio, H.A.; Manickavel, V. A, B, H, and lewis-a and lewis-b blood group antigens in human breast cancer: Correlation with steroid hormone receptor and disease status. J. Cancer Res. Clin. Oncol. 1993, 119, 486–492. [Google Scholar] [CrossRef]
- Abd Hamid, U.M.; Royle, L.; Saldova, R.; Radcliffe, C.M.; Harvey, D.J.; Storr, S.J.; Pardo, M.; Antrobus, R.; Chapman, C.J.; Zitzmann, N.; et al. A strategy to reveal potential glycan markers from serum glycoproteins associated with breast cancer progression. Glycobiology 2008, 18, 1105–1118. [Google Scholar] [CrossRef]
- Matsuura, N.; Narita, T.; Mitsuoka, C.; Kimura, N.; Kannagi, R.; Imai, T.; Funahashi, H.; Takagi, H. Increased level of circulating adhesion molecules in the sera of breast cancer patients with distant metastases. Jpn. J. Clin. Oncol. 1997, 27, 135–139. [Google Scholar] [CrossRef]
- Ordonez, N.G.; Freedman, R.S.; Herlyn, M. Lewis and related tumor-associated determinants on ovarian carcinoma. Gynecol. Oncol. 1987, 26, 1–10. [Google Scholar] [CrossRef]
- Takehara, K.; Kubushiro, K.; Kiguchi, K.; Ishiwata, I.; Tsukazaki, K.; Nozawa, S.; Iwamori, M. Expression of glycolipids bearing lewis phenotypes in tissues and cultured cells of human gynecological cancers. Jpn J. Cancer Res. 2002, 93, 1129–1137. [Google Scholar] [CrossRef]
- Dabelsteen, E. Cell surface carbohydrates as prognostic markers in human carcinomas. J. Pathol 1996, 179, 358–369. [Google Scholar] [CrossRef]
- Muller, S.; Hanisch, F.-G. Recombinant muc1 probe authentically reflects cell-specific o-glycosylation profiles of endogenous breast cancer mucin. High density and prevalent core 2-based glycosylation. J. Biol. Chem. 2002, 277, 26103–26112. [Google Scholar] [CrossRef]
- Nakagoe, T.; Fukushima, K.; Itoyanagi, N.; Ikuta, Y.; Oka, T.; Nagayasu, T.; Ayabe, H.; Hara, S.; Ishikawa, H.; Minami, H. Expression of abh/lewis-related antigens as prognostic factors in patients with breast cancer. J. Cancer Res. Clin. Oncol. 2002, 128, 257–264. [Google Scholar] [CrossRef]
- Kurebayashi, J.; Nomura, T.; Hirono, M.; Okubo, S.; Udagawa, K.; Shiiki, S.; Ikeda, M.; Nakashima, K.; Tanaka, K.; Sonoo, H. Combined measurement of serum sialyl lewis x with serum ca15-3 in breast cancer patients. Jpn. J. Clin. Oncol. 2006, 36, 150–153. [Google Scholar] [CrossRef]
- Sozzani, P.; Arisio, R.; Porpiglia, M.; Benedetto, C. Is sialyl lewis x antigen expression a prognostic factor in patients with breast cancer? Int J. Surg. Pathol. 2008, 16, 365–374. [Google Scholar] [CrossRef]
- Saldova, R.; Royle, L.; Radcliffe, C.M.; Abd Hamid, U.M.; Evans, R.; Arnold, J.N.; Banks, R.E.; Hutson, R.; Harvey, D.J.; Antrobus, R.; et al. Ovarian cancer is associated with changes in glycosylation in both acute-phase proteins and igg. Glycobiology 2007, 17, 1344–1356. [Google Scholar] [CrossRef]
- Berg, E.L.; Robinson, M.K.; Mansson, O.; Butcher, E.C.; Magnani, J.L. A carbohydrate domain common to both sialyl le(a) and sialyl le(x) is recognized by the endothelial cell leukocyte adhesion molecule elam-1. J. Biol. Chem. 1991, 266, 14869–14872. [Google Scholar]
- Narita, T.; Kawasaki-Kimura, N.; Matsuura, N.; Funahashi, H.; Kannagi, R. Adhesion of human breast cancer cells to vascular endothelium mediated by sialyl lewis &supx; /e-selectin. Breast Cancer 1996, 3, 19–23. [Google Scholar] [CrossRef]
- Ohyama, C.; Tsuboi, S.; Fukuda, M. Dual roles of sialyl lewis x oligosaccharides in tumor metastasis and rejection by natural killer cells. EMBO J. 1999, 18, 1516–1525. [Google Scholar] [CrossRef]
- Phillips, M.L.; Nudelman, E.; Gaeta, F.C.; Perez, M.; Singhal, A.K.; Hakomori, S.; Paulson, J.C. Elam-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-lex. Science 1990, 250, 1130–1132. [Google Scholar]
- Takada, A.; Ohmori, K.; Takahashi, N.; Tsuyuoka, K.; Yago, A.; Zenita, K.; Hasegawa, A.; Kannagi, R. Adhesion of human cancer cells to vascular endothelium mediated by a carbohydrate antigen, sialyl lewis a. Biochem. Biophys. Res. Commun. 1991, 179, 354–361. [Google Scholar]
- Takada, A.; Ohmori, K.; Yoneda, T.; Tsuyuoka, K.; Hasegawa, A.; Kiso, M.; Kannagi, R. Contribution of carbohydrate antigens sialyl lewis a and sialyl lewis x to adhesion of human cancer cells to vascular endothelium. Cancer Res. 1993, 53, 354–361. [Google Scholar]
- Matsuura, N.; Narita, T.; Mitsuoka, C.; Kimura, N.; Kannagi, R.; Imai, T.; Funahashi, H.; Takagi, H. Increased concentration of soluble e-selectin in the sera of breast cancer patients. Anticancer Res. 1997, 17, 1367–1372. [Google Scholar]
- Renkonen, R.; Mattila, P.; Majuri, M.L.; Rabina, J.; Toppila, S.; Renkonen, J.; Hirvas, L.; Niittymaki, J.; Turunen, J.P.; Renkonen, O.; et al. In vitro experimental studies of sialyl lewis x and sialyl lewis a on endothelial and carcinoma cells: Crucial glycans on selectin ligands. Glycoconj. J. 1997, 14, 593–600. [Google Scholar] [CrossRef]
- Julien, S.; Ivetic, A.; Grigoriadis, A.; QiZe, D.; Burford, B.; Sproviero, D.; Picco, G.; Gillett, C.; Papp, S.L.; Schaffer, L.; et al. Selectin ligand sialyl-lewis x antigen drives metastasis of hormone-dependent breast cancers. Cancer Res. 2011, 71, 7683–7693. [Google Scholar] [CrossRef]
- Somers, W.S.; Tang, J.; Shaw, G.D.; Camphausen, R.T. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of p- and e-selectin bound to sle(x) and psgl-1. Cell 2000, 103, 467–479. [Google Scholar] [CrossRef]
- Sperandio, M.; Gleissner, C.A.; Ley, K. Glycosylation in immune cell trafficking. Immunol Rev. 2009, 230, 97–113. [Google Scholar] [CrossRef]
- Yago, T.; Fu, J.; McDaniel, J.M.; Miner, J.J.; McEver, R.P.; Xia, L. Core 1-derived o-glycans are essential e-selectin ligands on neutrophils. Proc. Natl. Acad. Sci. USA 2010, 107, 9204–9209. [Google Scholar]
- Hellstrom, I.; Garrigues, H.J.; Garrigues, U.; Hellstrom, K.E. Highly tumor-reactive, internalizing, mouse monoclonal antibodies to le(y)-related cell surface antigens. Cancer Res. 1990, 50, 2183–2190. [Google Scholar]
- Madjd, Z.; Parsons, T.; Watson, N.F.; Spendlove, I.; Ellis, I.; Durrant, L.G. High expression of lewis y/b antigens is associated with decreased survival in lymph node negative breast carcinomas. Breast Cancer Res. 2005, 7, R780–787. [Google Scholar] [CrossRef]
- Federici, M.F.; Kudryashov, V.; Saigo, P.E.; Finstad, C.L.; Lloyd, K.O. Selection of carbohydrate antigens in human epithelial ovarian cancers as targets for immunotherapy: Serous and mucinous tumors exhibit distinctive patterns of expression. Int. J. Cancer 1999, 81, 193–198. [Google Scholar] [CrossRef]
- Iwamori, M.; Iwamori, Y.; Kubushiro, K.; Ishiwata, I.; Kiguchi, K. Characteristic expression of lewis-antigenic glycolipids in human ovarian carcinoma-derived cells with anticancer drug-resistance. J. Biochem. 2007, 141, 309–317. [Google Scholar]
- Wang, C.; Yan, L.; Wang, Y.; Lin, B.; Liu, S.; Li, Q.; Gao, L.; Zhang, S.; Iwamori, M. Overexpression of lewis(y) antigen protects ovarian cancer rmg-1 cells from carboplatin-induced apoptosis by the upregulation of topo-i and topo-ii beta. Anat. Rec. (Hoboken) 2011, 294, 961–969. [Google Scholar] [CrossRef]
- Gao, L.; Yan, L.; Lin, B.; Gao, J.; Liang, X.; Wang, Y.; Liu, J.; Zhang, S.; Iwamori, M. Enhancive effects of lewis y antigen on cd44-mediated adhesion and spreading of human ovarian cancer cell line rmg-i. J. Exp. Clin. Cancer Res. 2011, 30, 15. [Google Scholar] [CrossRef]
- Fukushi, Y.; Kannagi, R.; Hakomori, S.; Shepard, T.; Kulander, B.G.; Singer, J.W. Location and distribution of difucoganglioside (vi3neuacv3iii3fuc2nlc6) in normal and tumor tissues defined by its monoclonal antibody fh6. Cancer Res. 1985, 45, 3711–3717. [Google Scholar]
- Bos, P.D.; Zhang, X.H.F.; Nadal, C.; Shu, W.; Gomis, R.R.; Nguyen, D.X.; Minn, A.J.; van de Vijver, M.J.; Gerald, W.L.; Foekens, J.A.; et al. Genes that mediate breast cancer metastasis to the brain. Nature 2009, 459, 1005–1009. [Google Scholar]
- Cazet, A.; Groux-Degroote, S.; Teylaert, B.; Kwon, K.-M.; Lehoux, S.; Slomianny, C.; Kim, C.-H.; Le Bourhis, X.; Delannoy, P. Gd3 synthase overexpression enhances proliferation and migration of mda-mb-231 breast cancer cells. Biol. Chem. 2009, 390, 601–609. [Google Scholar]
- Battula, V.L.; Shi, Y.; Evans, K.W.; Wang, R.Y.; Spaeth, E.L.; Jacamo, R.O.; Guerra, R.; Sahin, A.A.; Marini, F.C.; Hortobagyi, G.; et al. Ganglioside gd2 identifies breast cancer stem cells and promotes tumorigenesis. J. Clin. Invest. 2012, 122, 2066–2078. [Google Scholar]
- Marquina, G.; Waki, H.; Fernandez, L.E.; Kon, K.; Carr, A.; Valiente, O.; Perez, R.; Ando, S. Gangliosides expressed in human breast cancer. Cancer Res. 1996, 56, 5165–5171. [Google Scholar]
- Oliva, J.P.; Valdes, Z.; Casaco, A.; Pimentel, G.; Gonzalez, J.; Alvarez, I.; Osorio, M.; Velazco, M.; Figueroa, M.; Ortiz, R.; et al. Clinical evidences of gm3 (neugc) ganglioside expression in human breast cancer using the 14f7 monoclonal antibody labelled with (99m)tc. Breast Cancer Res. Treat. 2006, 96, 115–121. [Google Scholar] [CrossRef]
- Santin, A.D.; Ravindranath, M.H.; Bellone, S.; Muthugounder, S.; Palmieri, M.; O'Brien, T.J.; Roman, J.; Cannon, M.J.; Pecorelli, S. Increased levels of gangliosides in the plasma and ascitic fluid of patients with advanced ovarian cancer. BJOG 2004, 111, 613–618. [Google Scholar] [CrossRef]
- Webb, T.J.; Li, X.; Giuntoli, R.L.; Lopez, P.H.H.; Heuser, C.; Schnaar, R.L.; Tsuji, M.; Kurts, C.; Oelke, M.; Schneck, J.P. Molecular identification of gd3 as a suppressor of the innate immune response in ovarian cancer. Cancer Res. 2012, 72, 3744. [Google Scholar]
- Bremer, E.G.; Levery, S.B.; Sonnino, S.; Ghidoni, R.; Canevari, S.; Kannagi, R.; Hakomori, S. Characterization of a glycosphingolipid antigen defined by the monoclonal antibody mbr1 expressed in normal and neoplastic epithelial cells of human mammary gland. J. Biol. Chem. 1984, 259, 14773–14777. [Google Scholar]
- Perrone, F.; Menard, S.; Canevari, S.; Calabrese, M.; Boracchi, P.; Bufalino, R.; Testori, S.; Baldini, M.; Colnaghi, M.I. Prognostic significance of the cambr1 antigen on breast carcinoma: Relevance of the type of recognised glycoconjugate. Eur. J. Cancer 1993, 29A, 2113–2117. [Google Scholar]
- Mariani-Costantini, R.; Colnaghi, M.I.; Leoni, F.; Menard, S.; Cerasoli, S.; Rilke, F. Immunohistochemical reactivity of a monoclonal antibody prepared against human breast carcinoma. Virchows Arch. A Pathol Anat Histopathol 1984, 402, 389–404. [Google Scholar] [CrossRef]
- Sharon, N. Lectins: Carbohydrate-specific reagents and biological recognition molecules. J. Biol. Chem. 2007, 282, 2753–2764. [Google Scholar] [CrossRef]
- Kyselova, Z.; Mechref, Y.; Kang, P.; Goetz, J.A.; Dobrolecki, L.E.; Sledge, G.W.; Schnaper, L.; Hickey, R.J.; Malkas, L.H.; Novotny, M.V. Breast cancer diagnosis and prognosis through quantitative measurements of serum glycan profiles. Clin. Chem. 2008, 54, 1166–1175. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pochechueva, T.; Jacob, F.; Fedier, A.; Heinzelmann-Schwarz, V. Tumor-Associated Glycans and Their Role in Gynecological Cancers: Accelerating Translational Research by Novel High-Throughput Approaches. Metabolites 2012, 2, 913-939. https://doi.org/10.3390/metabo2040913
Pochechueva T, Jacob F, Fedier A, Heinzelmann-Schwarz V. Tumor-Associated Glycans and Their Role in Gynecological Cancers: Accelerating Translational Research by Novel High-Throughput Approaches. Metabolites. 2012; 2(4):913-939. https://doi.org/10.3390/metabo2040913
Chicago/Turabian StylePochechueva, Tatiana, Francis Jacob, Andre Fedier, and Viola Heinzelmann-Schwarz. 2012. "Tumor-Associated Glycans and Their Role in Gynecological Cancers: Accelerating Translational Research by Novel High-Throughput Approaches" Metabolites 2, no. 4: 913-939. https://doi.org/10.3390/metabo2040913
APA StylePochechueva, T., Jacob, F., Fedier, A., & Heinzelmann-Schwarz, V. (2012). Tumor-Associated Glycans and Their Role in Gynecological Cancers: Accelerating Translational Research by Novel High-Throughput Approaches. Metabolites, 2(4), 913-939. https://doi.org/10.3390/metabo2040913




