Expression of miR-210-3p as a Prognostic Marker for Development of Diabetic Neuropathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Diagnosis of Neuropathy
2.3. Laboratory Investigations
- Endogenous Control (Reference Gene): An endogenous control gene with stable expression across samples was used to normalize variability. In this study, RNU6B was selected as the reference.
- Calibrator Sample: A calibrator sample was defined as the reference against which all other samples were compared.
- Ct (Cq) Value: The cycle threshold (Ct) is the PCR cycle number at which the fluorescence signal crosses a defined threshold in the exponential phase of amplification. Lower Ct values indicate higher expression levels of the target microRNA, while higher Ct values indicate lower expression.
- For qPCR we have used TaqMan microRNA assay and Universal master mix, Thermo Fisher Scientific.
- The assay ID for miR-210-3p for ordering in Thermo Fisher scientific site is 000512. The miRbase accession numbers are MI0000286 for miR-210 and MIMAT0000267 for miR-210-3p.
2.4. Statistical Analysis
3. Results
3.1. Participants
3.2. Treatment
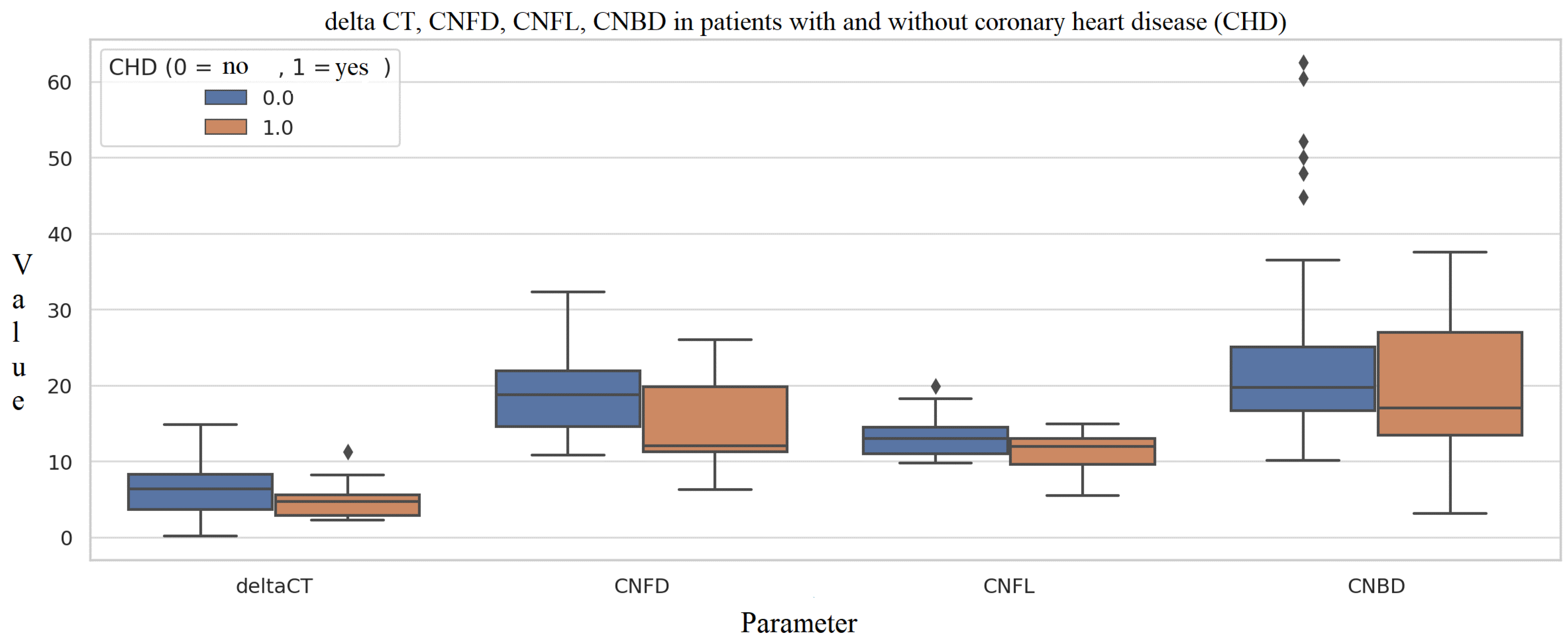
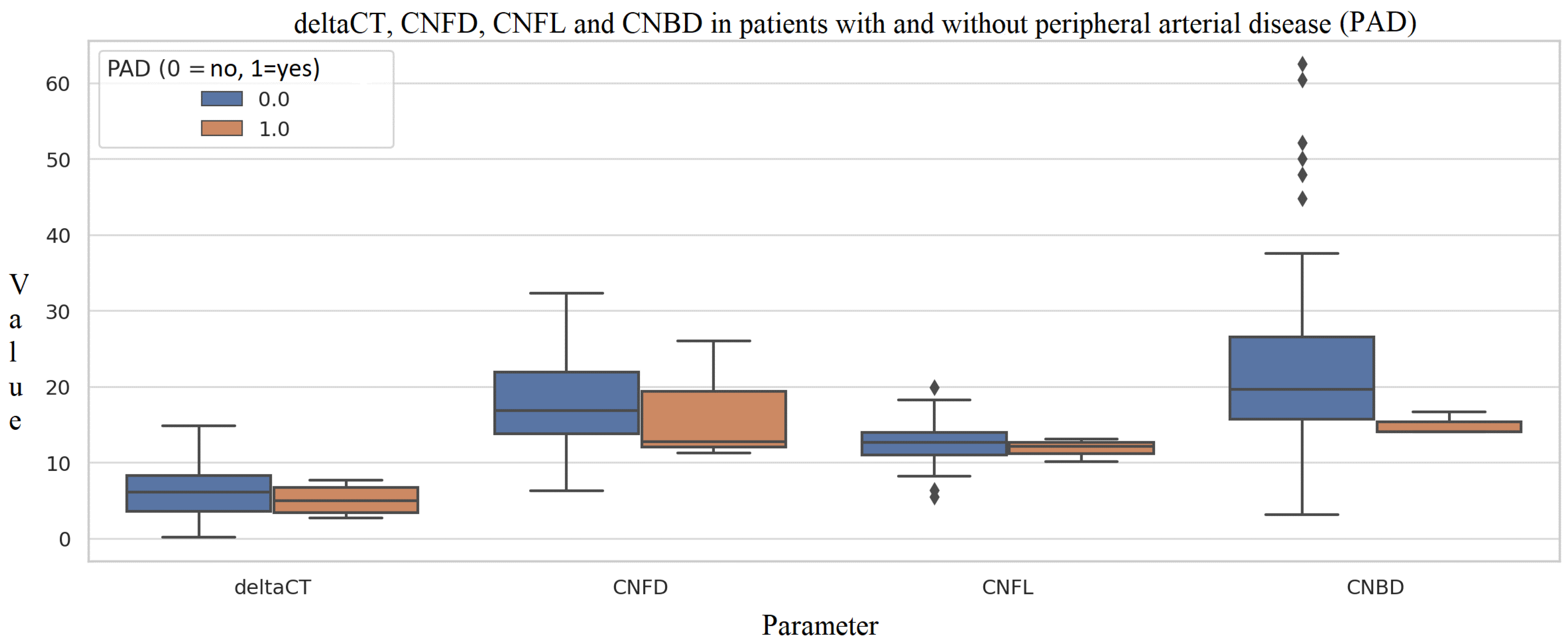
3.3. Diabetes Complications
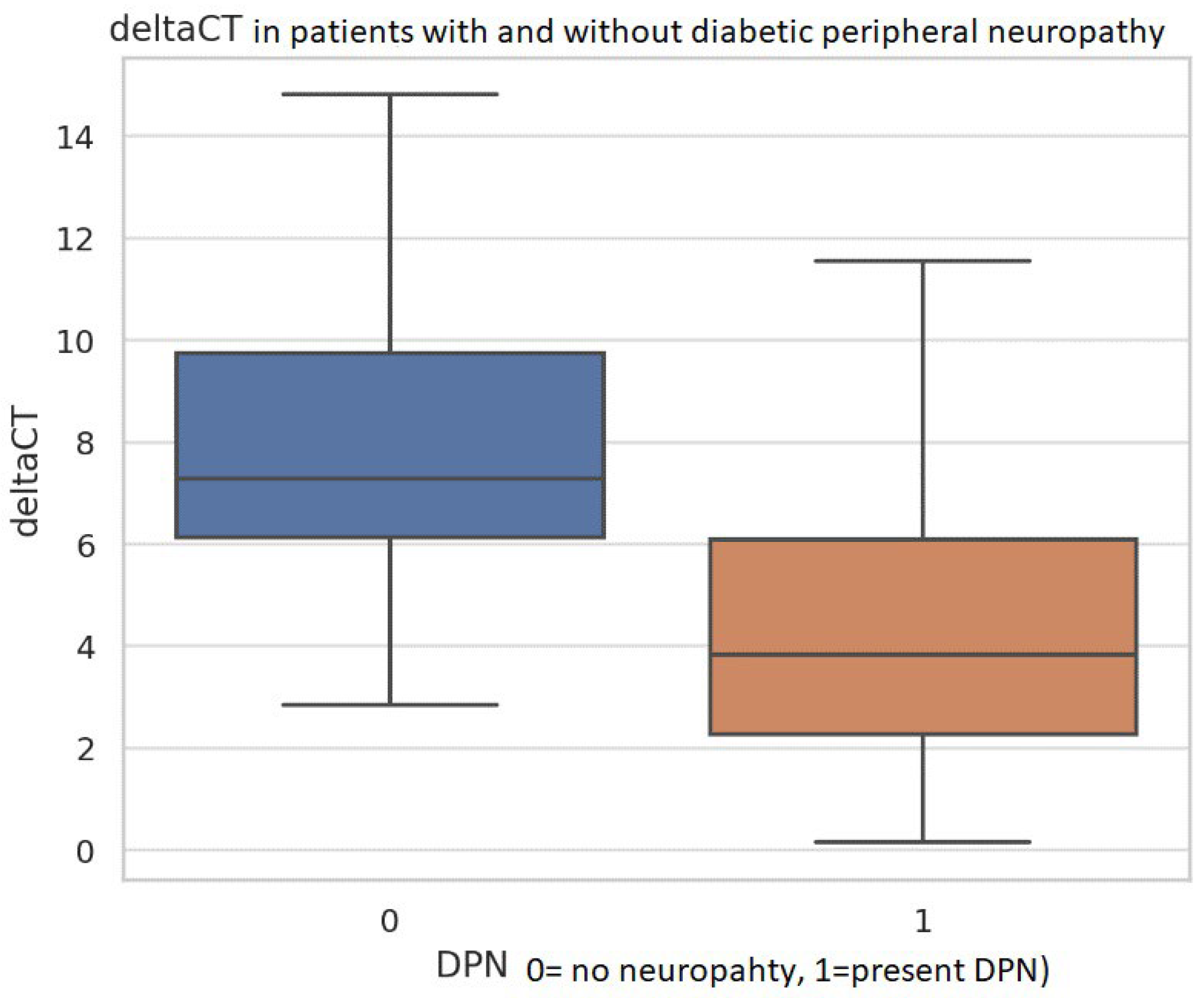
3.4. miR-210-3p Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. Available online: https://idf.org/news/idf-diabetes-atlas-11th-edition/ (accessed on 1 September 2025).
- Faselis, C.; Katsimardou, A.; Imprialos, K.; Deligkaris, P.; Kallistratos, M.; Dimitriadis, K. Microvascular Complications of Type 2 Diabetes Mellitus. Curr. Vasc. Pharmacol. 2020, 18, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Dillon, B.R.; Ang, L.; Pop-Busui, R. Spectrum of Diabetic Neuropathy: New Insights in Diagnosis and Treatment. Annu. Rev. Med. 2024, 75, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Stoian, A.; Muntean, C.; Babă, D.-F.; Manea, A.; Dénes, L.; Simon-Szabó, Z.; Kosovski, I.B.; Nemes-Nagy, E.; Gliga, F.I.; Stoian, M. Update on Biomarkers of Chronic Inflammatory Processes Underlying Diabetic Neuropathy. Int. J. Mol. Sci. 2024, 25, 10395. [Google Scholar] [CrossRef] [PubMed]
- Margaritis, K.; Margioula-Siarkou, G.; Giza, S.; Kotanidou, E.P.; Tsinopoulou, V.R.; Christoforidis, A.; Galli-Tsinopoulou, A. Micro-RNA Implications in Type-1 Diabetes Mellitus: A Review of Literature. Int. J. Mol. Sci. 2021, 22, 12165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crosby, M.E.; Kulshreshtha, R.; Ivan, M.; Glazer, P.M. MicroRNA regulation of DNA repair gene expression in hypoxic stress. Cancer Res. 2009, 69, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Aderinto, N.; Olatunji, G.; Kokori, E.; Sanker, V.; Yusuf, I.A.; Adefusi, T.O.; Egbunu, E.; Aboje, J.E.; Apampa, O.O.; Ogieuhi, I.J.; et al. miR-210 in ischaemic stroke: Biomarker potential, challenges and future perspectives. Eur. J. Med. Res. 2024, 29, 432. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pasculli, B.; Barbano, R.; Rendina, M.; Fontana, A.; Copetti, M.; Mazza, T.; Valori, V.M.; Morritti, M.; Maiello, E.; Graziano, P.; et al. Hsa-miR-210-3p expression in breast cancer and its putative association with worse outcome in patients treated with Docetaxel. Sci. Rep. 2019, 9, 14913. [Google Scholar] [CrossRef]
- Cao, X.; Lu, B.; Gu, Y.; Li, X.; Guo, D.; Xia, F. miR-210-3p Impairs Pancreatic β-Cell Function by Targeting Dtx1 in Gestational Diabetes Mellitus. J. Environ. Pathol. Toxicol. Oncol. 2022, 41, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Patra, D.; Roy, S.; Arora, L.; Kabeer, S.W.; Singh, S.; Dey, U.; Banerjee, D.; Sinha, A.; Dasgupta, S.; Tikoo, K.; et al. miR-210-3p Promotes Obesity-Induced Adipose Tissue Inflammation and Insulin Resistance by Targeting SOCS1-Mediated NF-κB Pathway. Diabetes 2023, 72, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Patra, D.; Ramprasad, P.; Sharma, S.; Dey, U.; Kumar, V.; Singh, S.; Dasgupta, S.; Kumar, A.; Tikoo, K.; Pal, D. Adipose tissue macrophage-derived microRNA-210-3p disrupts systemic insulin sensitivity by silencing GLUT4 in obesity. J. Biol. Chem. 2024, 300, 107328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tavakoli, M.; Ferdousi, M.; Petropoulos, I.N.; Morris, J.; Pritchard, N.; Zhivov, A.; Ziegler, D.; Pacaud, D.; Romanchuk, K.; Perkins, B.A.; et al. Normative values for corneal nerve morphology assessed using corneal confocal microscopy: A multinational normative data set. Diabetes Care 2015, 38, 838–843. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pichu, S.; Vimalraj, S.; Viswanathan, V. Impact of microRNA-210 on wound healing among the patients with diabetic foot ulcer. PLoS ONE 2021, 16, e0254921. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, C.; Ding, M.; Liang, X.; Zhuo, L. Application and progress of corneal confocal microscopy in the evaluation of diabetes-related peripheral neuropathy. Endokrynol. Pol. 2024, 75, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Ponirakis, G.; Al-Janahi, I.; Elgassim, E.; Homssi, M.; Petropoulos, I.N.; Gad, H.; Khan, A.; Zaghloul, H.B.; Ali, H.; Siddique, M.A.; et al. Sustained corneal nerve loss predicts the development of diabetic neuropathy in type 2 diabetes. Front. Neurosci. 2024, 18, 1393105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaplan, H.; Yüzbaşıoğlu, S.; Vural, G.; Gümüşyayla, Ş. Investigation of small fiber neuropathy in patients with diabetes mellitus by corneal confocal microscopy. Neurophysiol. Clin. 2024, 54, 102955. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Z.M.; Burgess, J.; Ooi, C.; Ferdousi, M.; Azmi, S.; Kalteniece, A.; Anson, M.; Cuthbertson, D.J.; Petropoulos, I.N.; Malik, R.A.; et al. Corneal Confocal Microscopy Predicts Cardiovascular and Cerebrovascular Events and Demonstrates Greater Peripheral Neuropathy in Patients with Type 1 Diabetes and Foot Ulcers. Diagnostics 2023, 13, 2793. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, Z.; Collado, A.; Sun, C.; Tratsiakovich, Y.; Mahdi, A.; Winter, H.; Chernogubova, E.; Seime, T.; Narayanan, S.; Jiao, T.; et al. Downregulation of Erythrocyte miR-210 Induces Endothelial Dysfunction in Type 2 Diabetes. Diabetes 2022, 71, 285–297. [Google Scholar] [CrossRef]
- Song, R.; Dasgupta, C.; Mulder, C.; Zhang, L. MicroRNA-210 Controls Mitochondrial Metabolism and Protects Heart Function in Myocardial Infarction. Circulation 2022, 145, 1140–1153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Collado, A.; Jiao, T.; Kontidou, E.; Carvalho, L.R.R.A.; Chernogubova, E.; Yang, J.; Zaccagnini, G.; Zhao, A.; Tengbom, J.; Zheng, X.; et al. miR-210 as a therapeutic target in diabetes-associated endothelial dysfunction. Br. J. Pharmacol. 2025, 182, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Duisenbek, A.; Avilés Pérez, M.D.; Pérez, M.; Aguilar Benitez, J.M.; Pereira Pérez, V.R.; Gorts Ortega, J.; Ussipbek, B.; Yessenbekova, A.; López-Armas, G.C.; Ablaikhanova, N.; et al. Unveiling the Predictive Model for Macrovascular Complications in Type 2 Diabetes Mellitus: microRNAs Expression, Lipid Profile, and Oxidative Stress Markers. Int. J. Mol. Sci. 2024, 25, 11763. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, X.; Tian, F.; Sun, Z.; Zeng, G.; Tang, P. Elevation of Circulating miR-210 Participates in the Occurrence and Development of Type 2 Diabetes Mellitus and Its Complications. J. Diabetes Res. 2022, 2022, 9611509. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yin, C.; Lin, X.; Sun, Y.; Ji, X. Dysregulation of miR-210 is involved in the development of diabetic retinopathy and serves a regulatory role in retinal vascular endothelial cell proliferation. Eur. J. Med Res. 2020, 25, 20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Yordanova, S.G.; Nikolova, D.; Kamenov, Z.; Karamfilova, V.; Lachezar, T.; Assyov, Y.; Gatev, T.; Kaneva, R.; Belcheva, O.; Kachakova, D.; et al. Expression of miR-210-3p as a Prognostic Marker for Development of Diabetic Neuropathy. Metabolites 2026, 16, 13. https://doi.org/10.3390/metabo16010013
Yordanova SG, Nikolova D, Kamenov Z, Karamfilova V, Lachezar T, Assyov Y, Gatev T, Kaneva R, Belcheva O, Kachakova D, et al. Expression of miR-210-3p as a Prognostic Marker for Development of Diabetic Neuropathy. Metabolites. 2026; 16(1):13. https://doi.org/10.3390/metabo16010013
Chicago/Turabian StyleYordanova, Savelia G., Diana Nikolova, Zdravko Kamenov, Vera Karamfilova, Traykov Lachezar, Yavor Assyov, Tsvetan Gatev, Radka Kaneva, Olga Belcheva, Darina Kachakova, and et al. 2026. "Expression of miR-210-3p as a Prognostic Marker for Development of Diabetic Neuropathy" Metabolites 16, no. 1: 13. https://doi.org/10.3390/metabo16010013
APA StyleYordanova, S. G., Nikolova, D., Kamenov, Z., Karamfilova, V., Lachezar, T., Assyov, Y., Gatev, T., Kaneva, R., Belcheva, O., Kachakova, D., Petkova, V., Zhelev, Y., & Gateva, A. T. (2026). Expression of miR-210-3p as a Prognostic Marker for Development of Diabetic Neuropathy. Metabolites, 16(1), 13. https://doi.org/10.3390/metabo16010013





