Combination of Metabolomic Analysis and Transcriptomic Analysis Reveals Differential Mechanism of Phenylpropanoid Biosynthesis and Flavonoid Biosynthesis in Wild and Cultivated Forms of Angelica sinensis
Abstract
1. Introduction
2. Materials and Methods
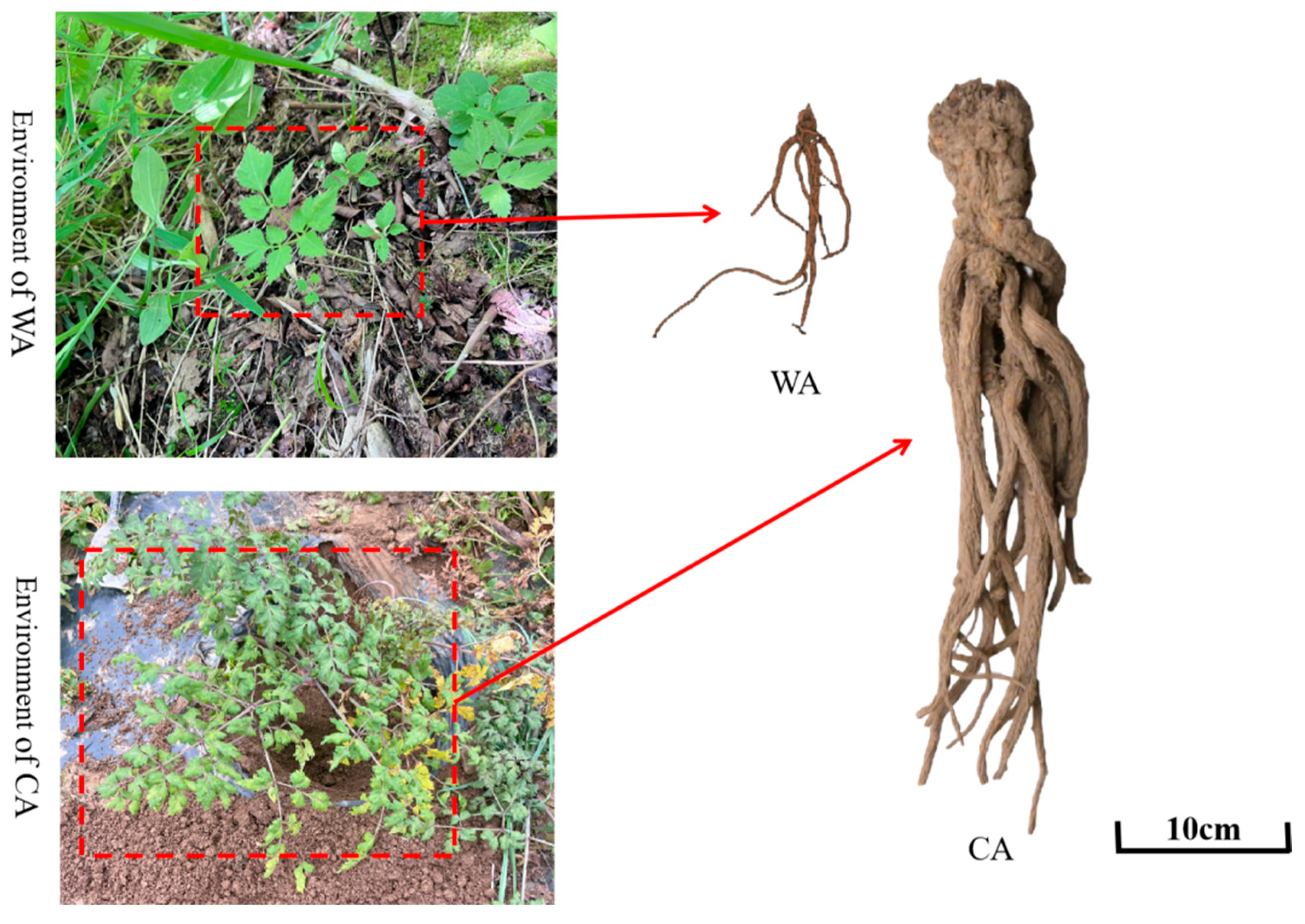
2.1. Plant Material
2.2. Metabolomics Analysis
2.2.1. Extraction of Metabolites
2.2.2. Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS)
2.2.3. Pre-Processing and Annotation of Data
2.2.4. Screening of Differential Metabolites (DMs)
2.3. Transcriptomics Analysis
2.3.1. Extraction, Quantification, and Qualification of RNA
2.3.2. Library Preparation for Transcriptome Sequencing
2.3.3. Gene Expression
2.3.4. Sequencing and Transcriptomics Analysis
2.3.5. Screening of Differentially Expressed Genes (DEGs)
2.4. Combined Use of Metabolomics Analysis and Transcriptomics Analysis
2.5. Validation of Related Genes by Real-Time Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.6. Data Analysis
3. Results
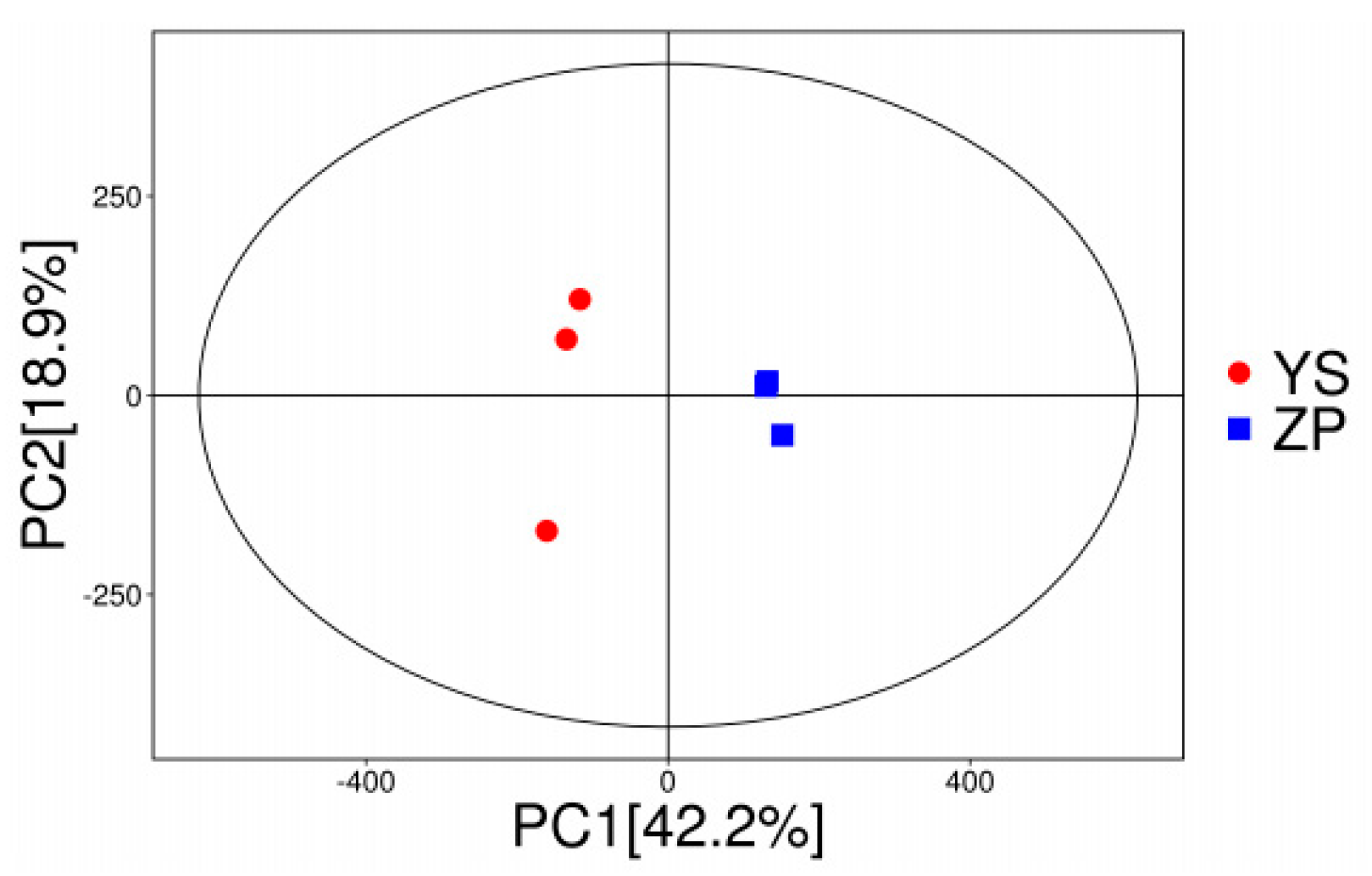
3.1. Metabolomics Analysis of WA and CA
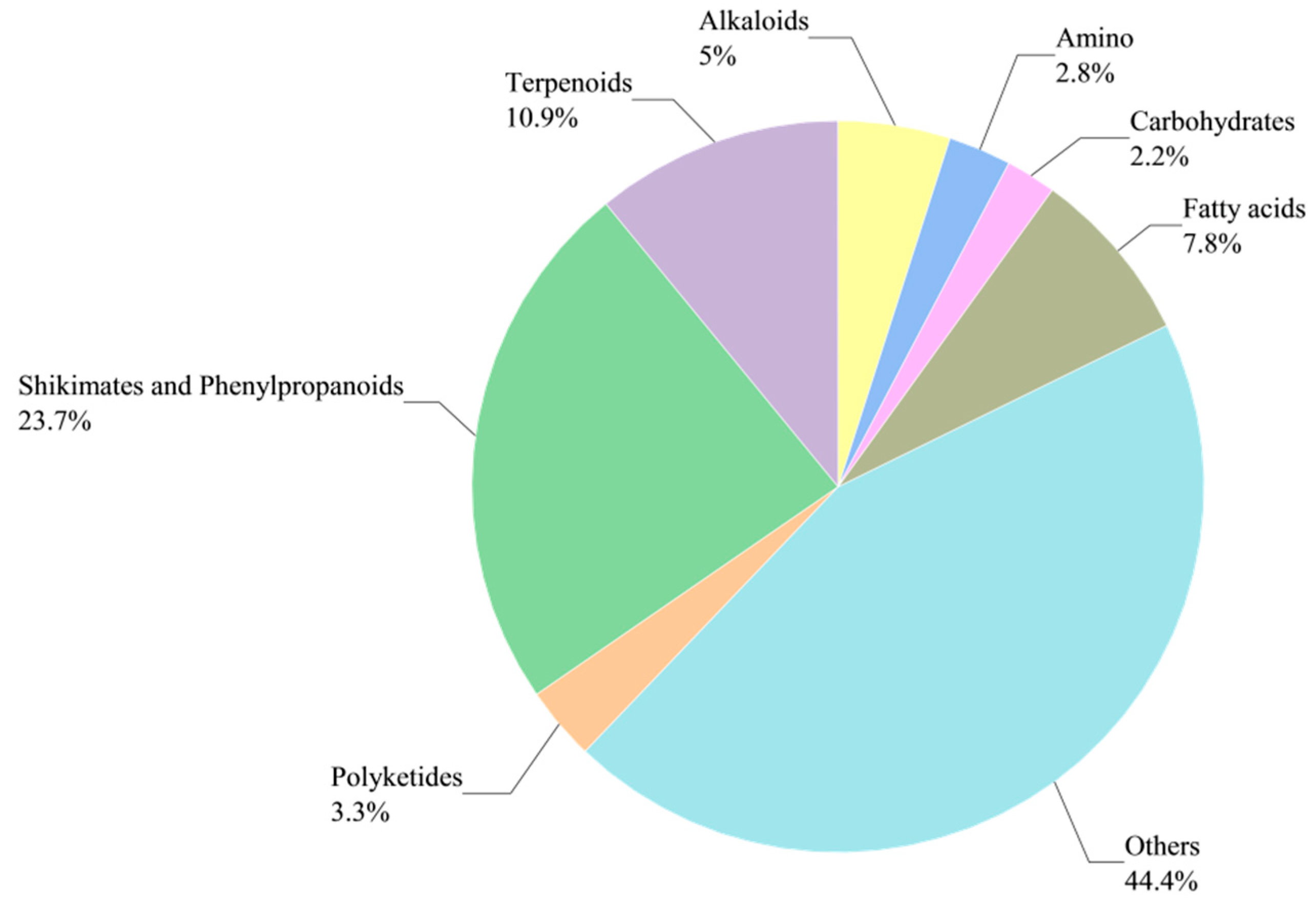
3.1.1. DM Classification
3.1.2. Screening of DMs
3.1.3. Analysis of Enrichment of Pathways of DMs
3.2. Transcriptomics Analysis of WA and CA
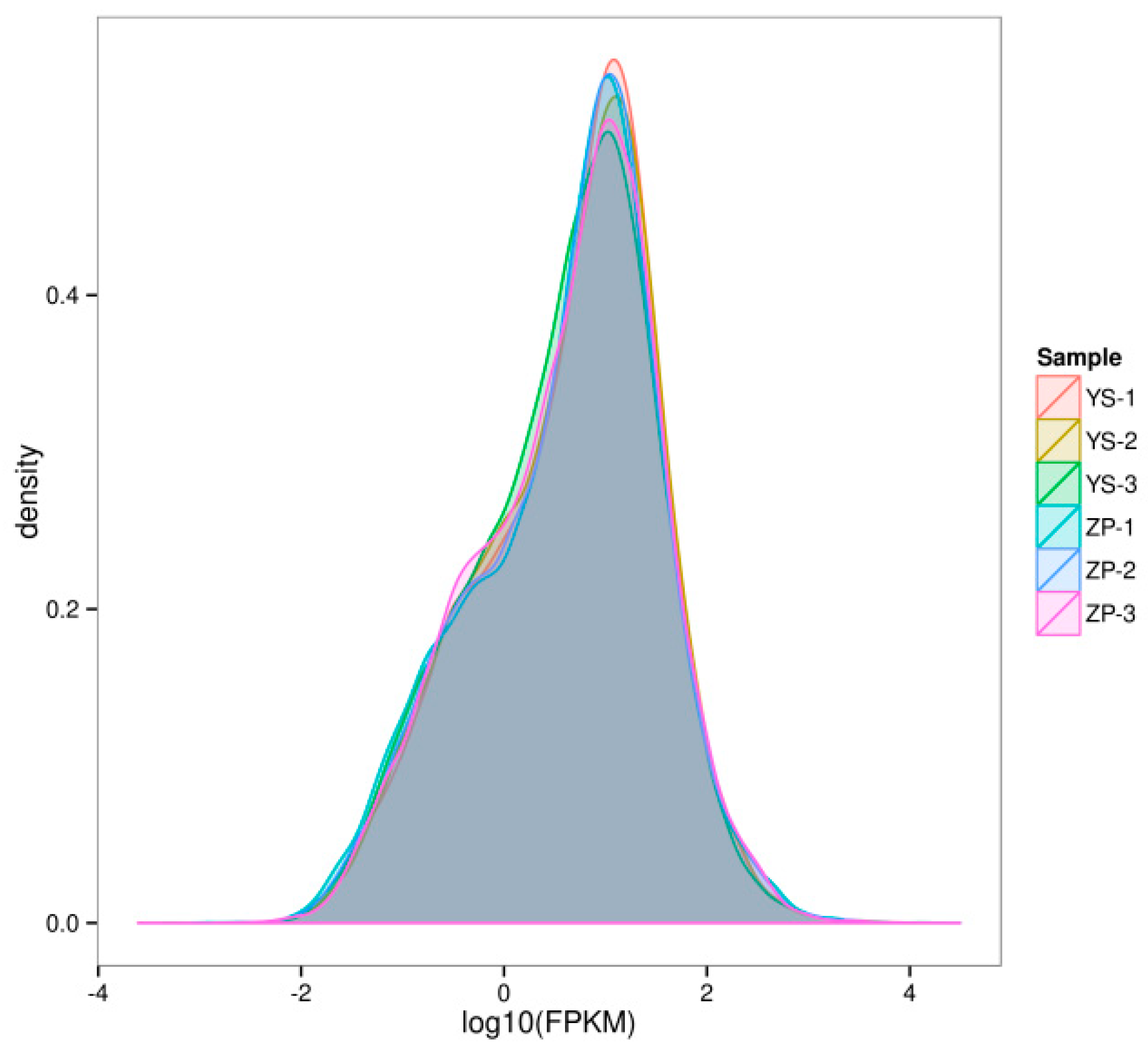
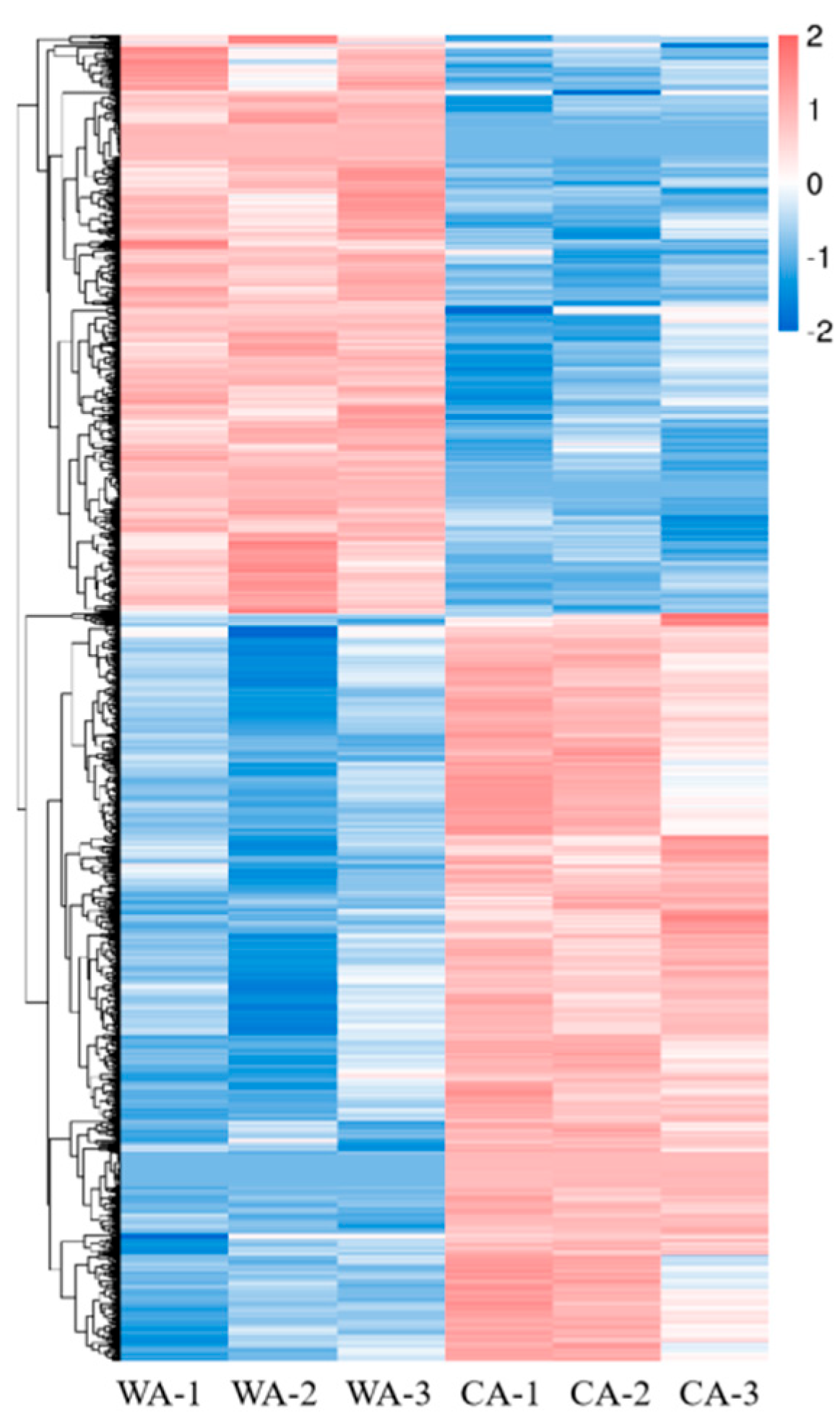
3.2.1. Analysis of Gene Expression Levels
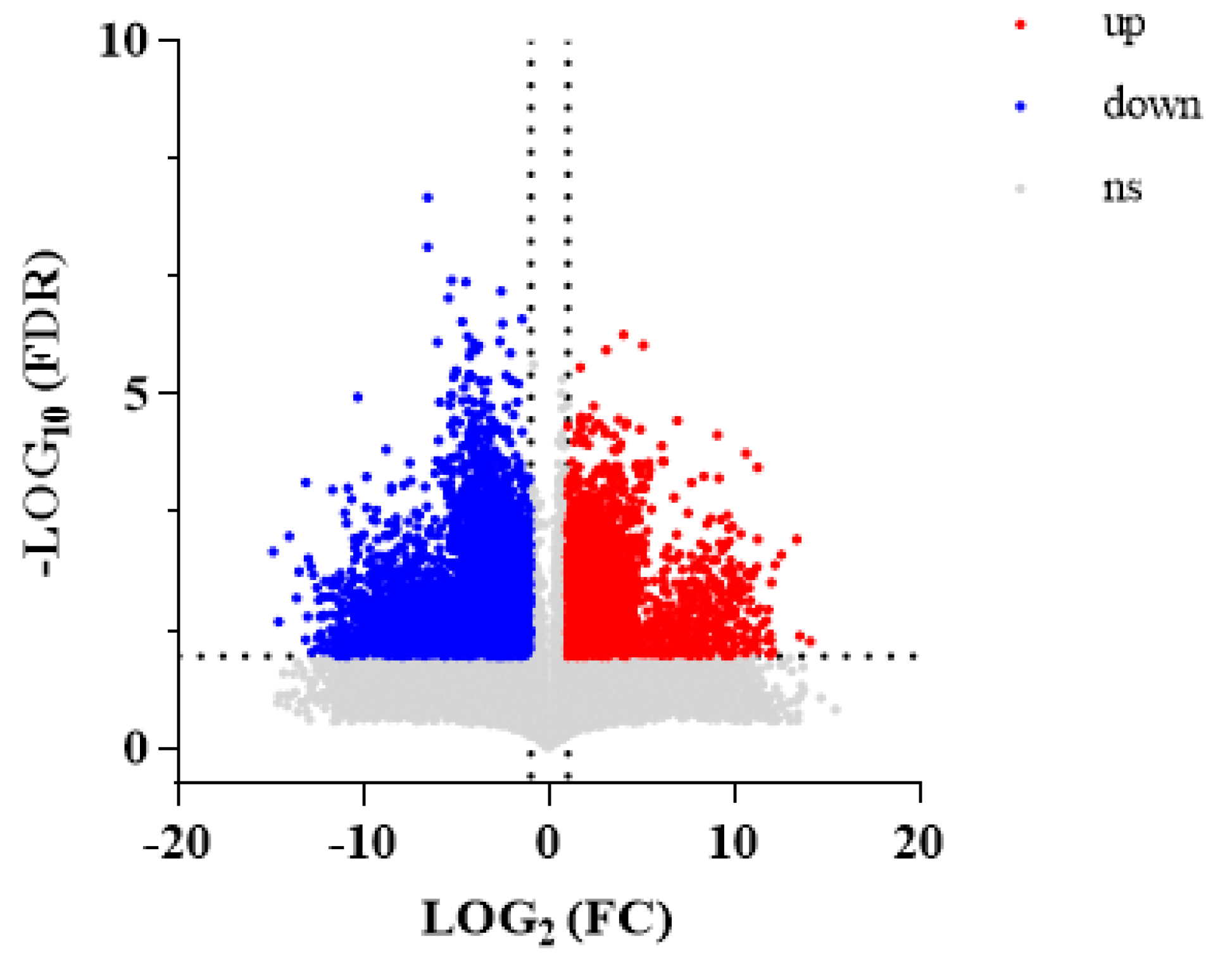
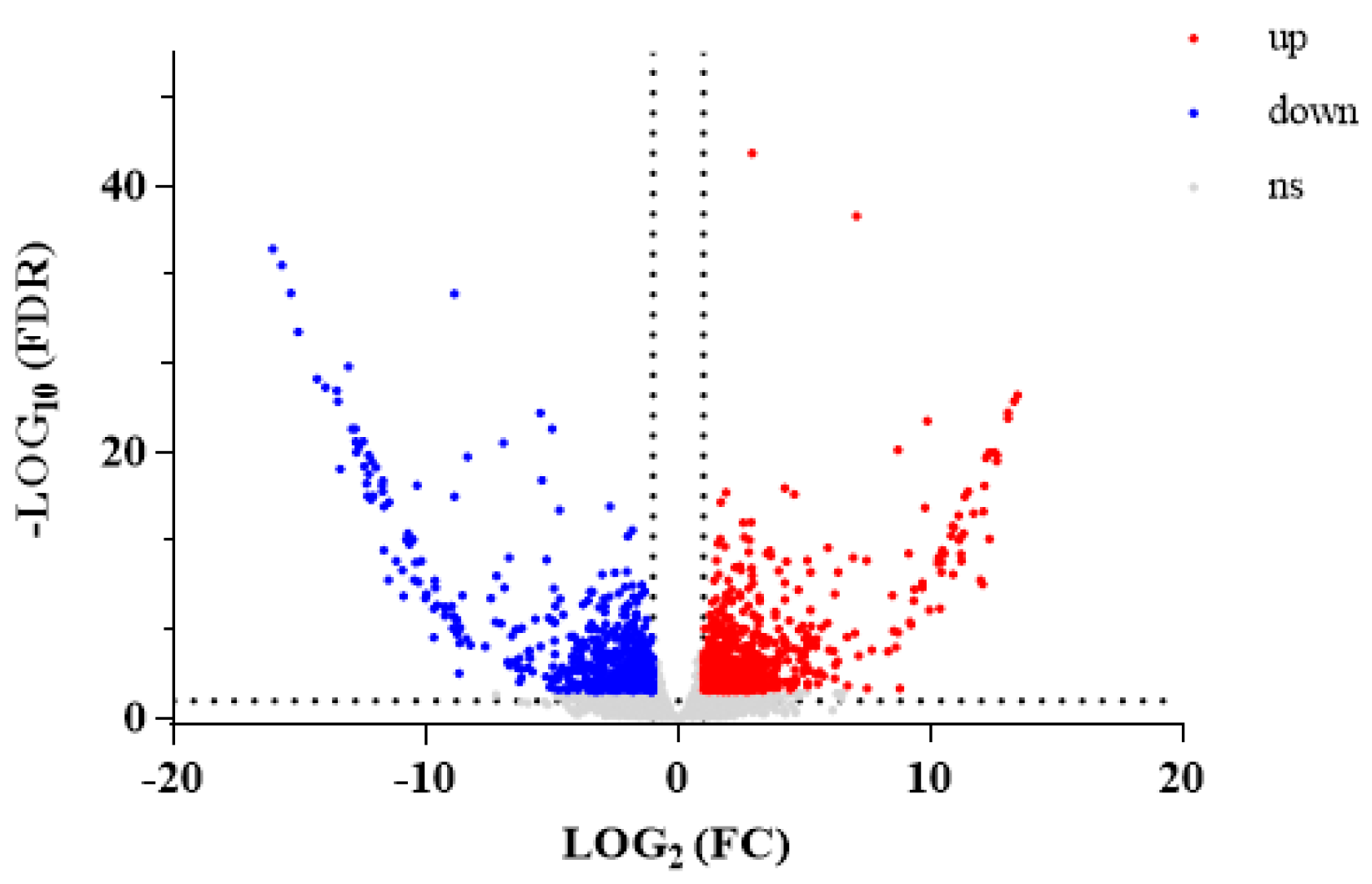
3.2.2. DEGs
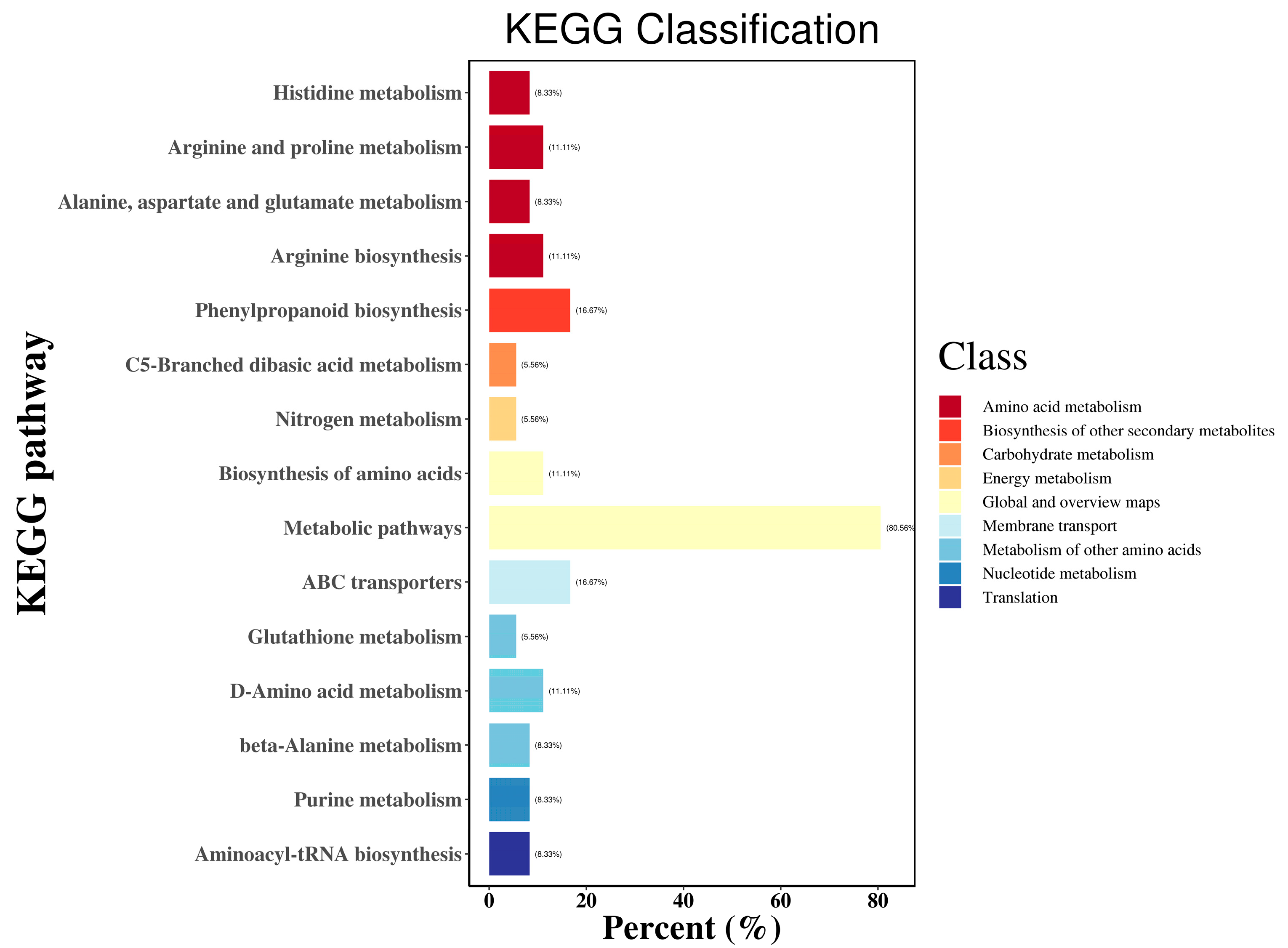
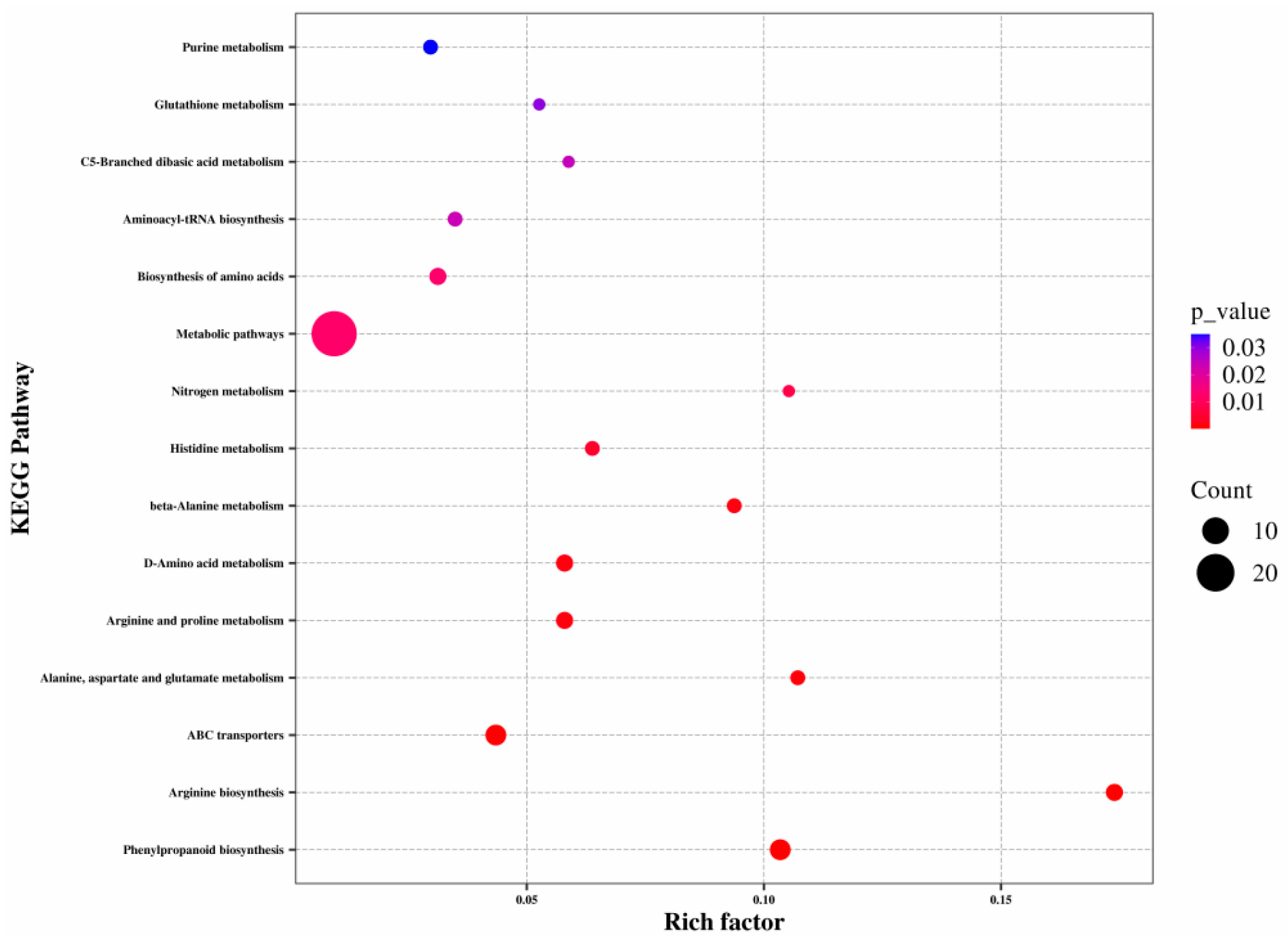
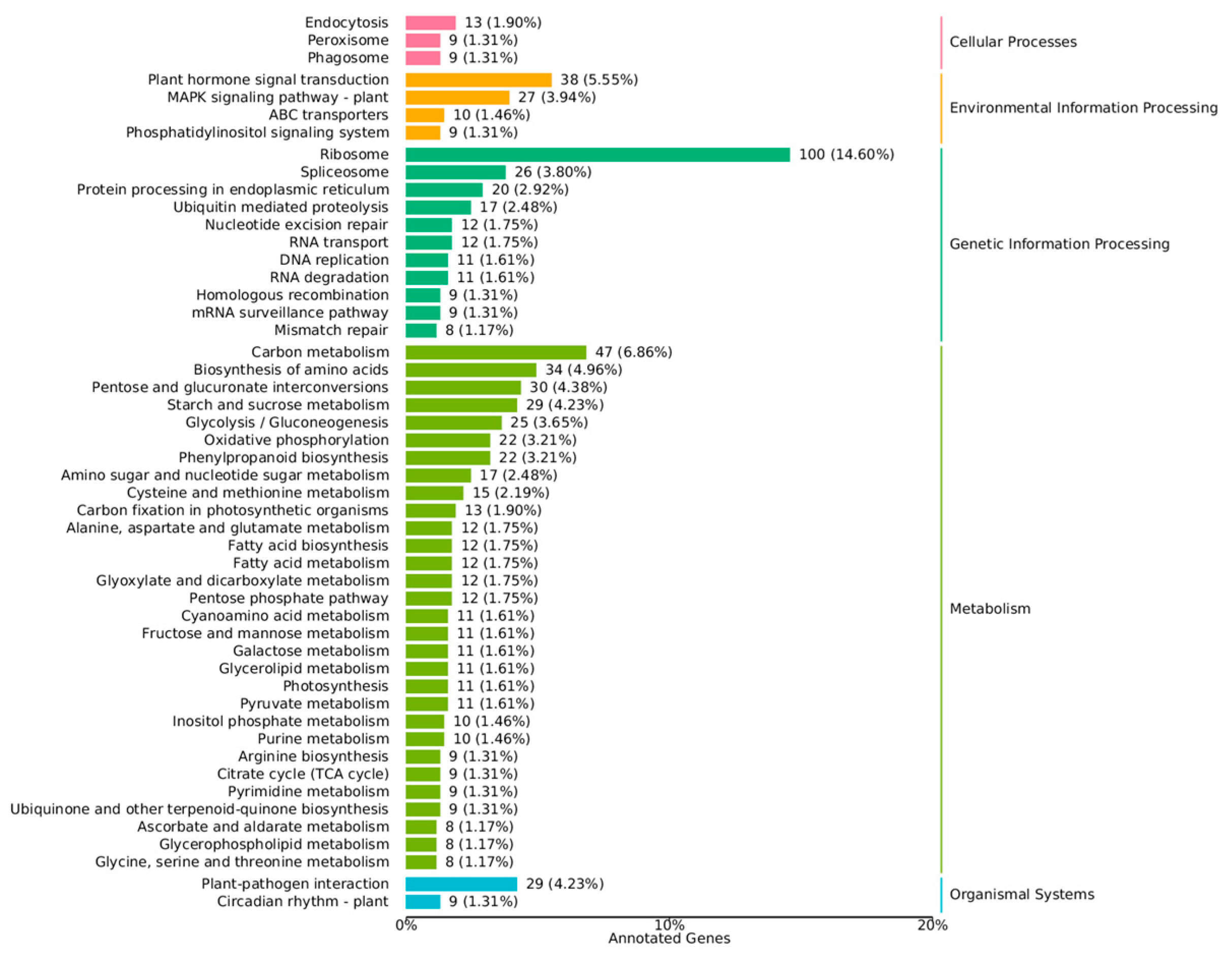
3.2.3. Analyses of Pathway Enrichment of DEGs
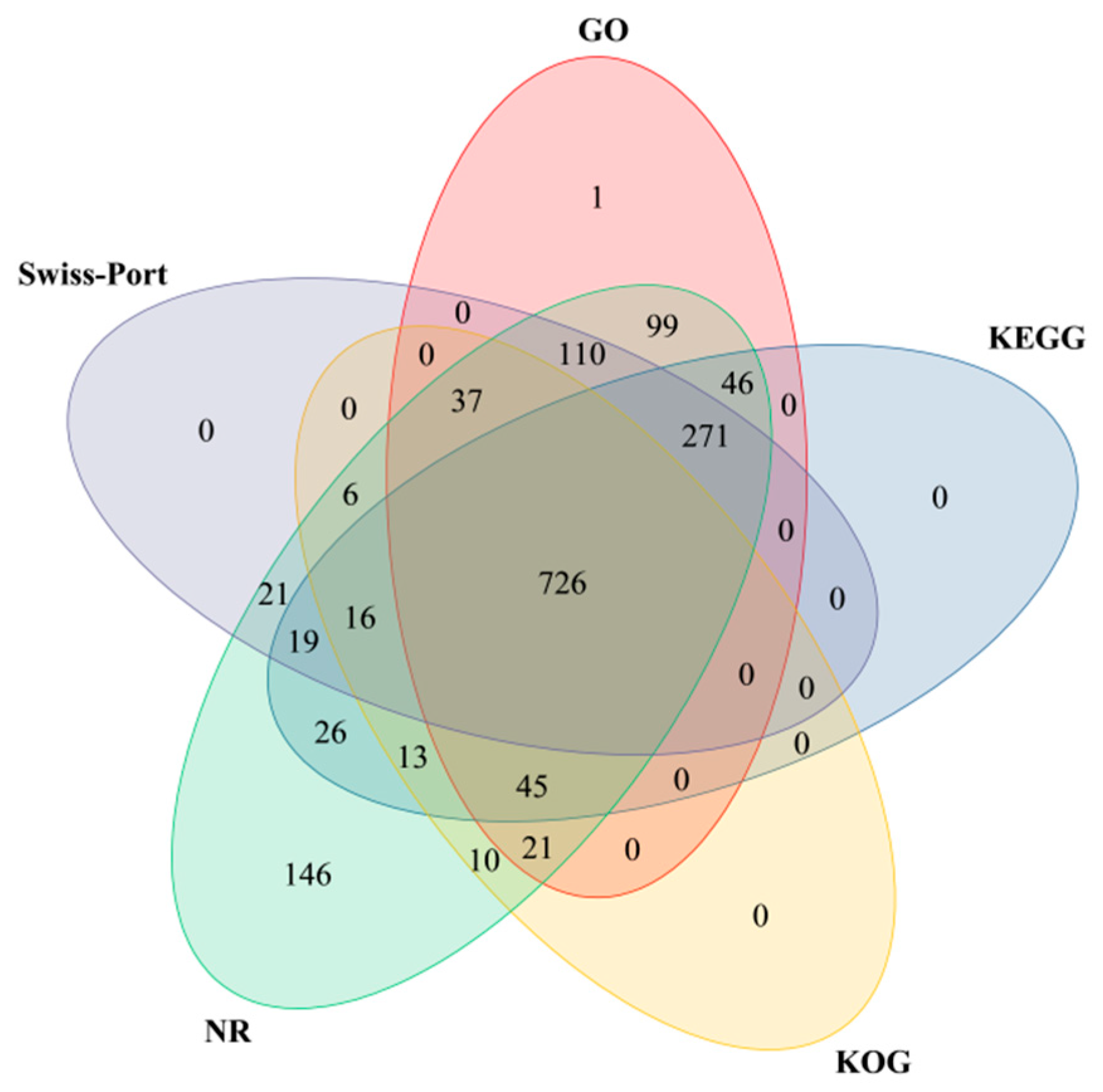
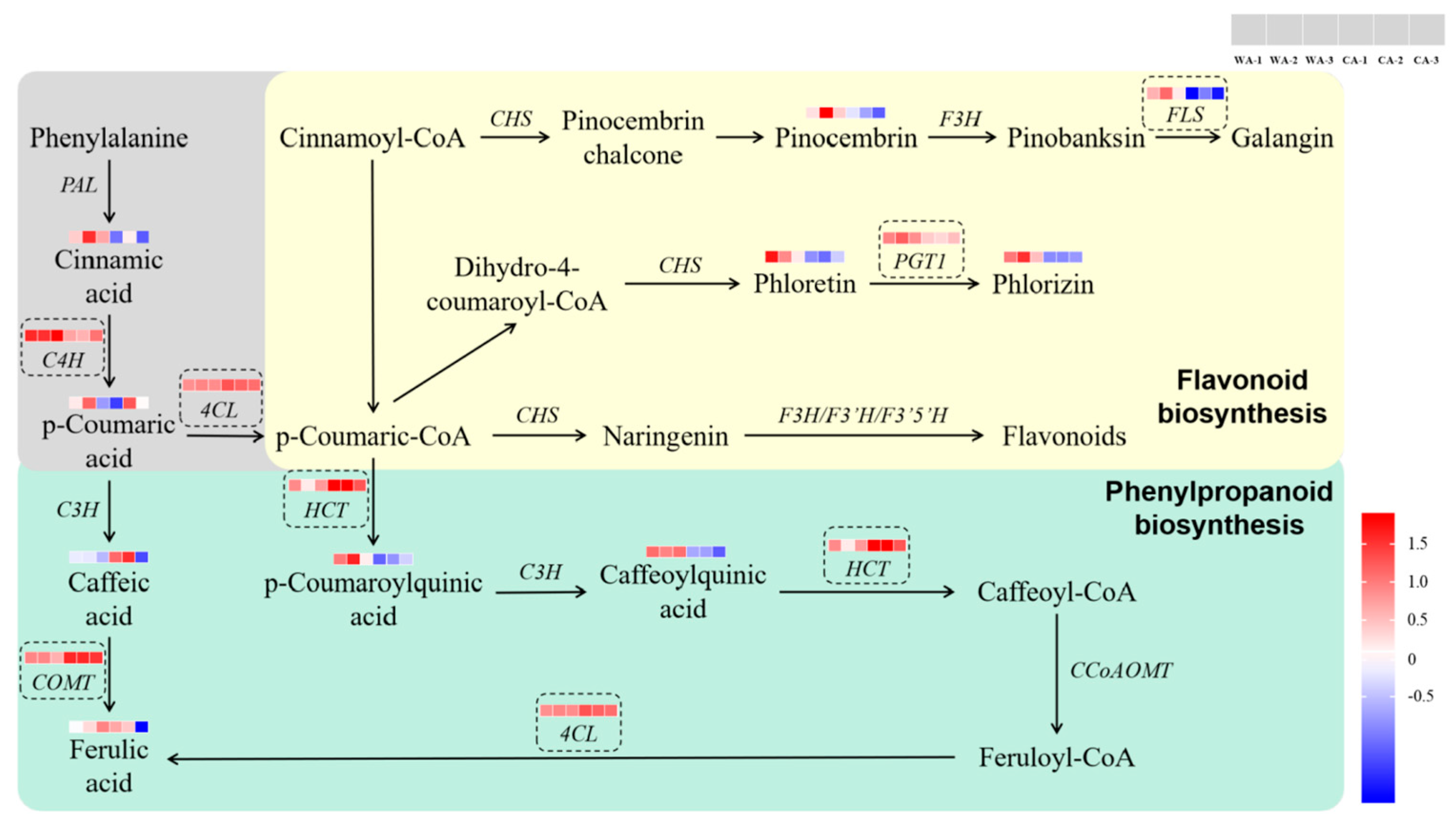
3.3. Combination of Metabolomics Analysis and Transcriptomics Analysis
3.4. Validation of Data Using Real-Time RT-qPCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TCM | Traditional Chinese medicine |
| WA | Wild Angelica sinensis |
| CA | Cultivated Angelica sinensis |
| MA | Metabolomics analysis |
| TA | Transcriptomics analysis |
| RT-qPC | Real-time reverse transcription-quantitative polymerase chain reaction |
| DMs | Differential metabolites |
| DEGs | Differentially expressed genes |
| UHPLC | Ultrahigh-performance liquid chromatography |
| PCA | Principal component analysis |
| VIP | Variable importance projection |
| PCR | polymerase chain reaction |
| FPKM | Fragments per kilobase of transcript per million fragments mapped |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| C4H | 4-hydroxylase |
| HCT | Shikimate o-hydroxycinnamoyltransferase |
| 4CL | 4-coumarate-CoA ligase |
| COMT | Caffeic acid-o-methyltransferase |
| CAD | Cinnamyl-alcohol dehydrogenase |
| FLS | Flavonol synthase |
| CCR | Cinnamoyl-coa reductase |
| K22395 | Cinnamyl-alcohol dehydrogenase |
| MIOX | Inositol oxygenase |
| PLCD | Phosphatidylinositol phospholipase c delta |
| PAL | Phenylalanine ammonia lyase |
| PGT1 | Phlorizin synthase |
| REF1 | Coniferyl-aldehyde dehydrogenase |
| CHS | Chalcone synthase |
| IPK1 | Inositol-pentakisphosphate 2-kinase |
| VTC4 | Inositol-phosphate phosphatase |
| GOLS | 3-alpha-galactosyltransferase |
| ACT | Actin |
| TRAF | factor receptor-associated factor 2 |
| p62 | sequestosome 1 |
Appendix A
References
- Wei, W.; Zeng, R.; Gu, C.; Qu, Y.; Huang, L. Angelica sinensis in China-A review of botanical profile, ethnopharmacology, phytochemistry and chemical analysis. J. Ethnopharmacol. 2016, 190, 116–141. [Google Scholar] [CrossRef]
- Zhang, S.; He, L.; Han, L.; Yang, W.; Liu, L. Study on Wild Angelica Resources. Mod. Chin. Med. 2012, 14, 33–36. [Google Scholar] [CrossRef]
- Yan, H.X.; Zhang, X.; Zhu, S.; Qian, D.; Guo, L.; Huang, L.; Duan, J. Production regionalization study of Chinese angelica based on MaxEnt model. China J. Chin. Mater. Medica 2016, 41, 3139–3147. [Google Scholar] [CrossRef]
- Feng, W.; Liu, P.; Yan, H.; Yu, G.; Guo, Z.; Zhu, L.; Ma, J.; Qian, D.; Duan, J. Transcriptomic data analyses of wild and cultivated Angelica sinensis root by high-throughput sequencing technology. China J. Chin. Mater. Medica 2020, 45, 1879–1886. [Google Scholar] [CrossRef]
- Li, T.; He, X. Analysis of essential oil from the roots of Angelica sinensis by GC—MS. West China J. Pharm. Sci. 2015, 30, 249–250. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, W.; Hu, J.; Duan, Y.; Fan, C.; Feng, T.; Wang, X.; Wu, X. Research Progress on Chemical Constituents and Pharmacological Effects of Angelica sinensis. Acta Chin. Med. Pharmacol. 2022, 50, 111–114. [Google Scholar] [CrossRef]
- Nie, R. Protective Effect of Angelica Sinensis Polysaccharides in Hepatic Injuries by Tetrachloride Intoxication. J. Wuhan Polytech. Univ. 2008, 4, 23–25+105. [Google Scholar]
- Liao, K.F.; Chiu, T.L.; Huang, S.Y.; Hsieh, T.F.; Chang, S.F.; Ruan, J.W.; Chen, S.P.; Pang, C.Y.; Chiu, S.C. Anti-Cancer Effects of Radix Angelica Sinensis (Danggui) and N-Butylidenephthalide on Gastric Cancer: Implications for REDD1 Activation and mTOR Inhibition. Cell. Physiol. Biochem. 2018, 48, 2231–2246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cui, X.; Chen, W.; Chen, Z. Experimental study on mitophagy homeostasis in bone marrow mononuclear cells of aplastic anemia mice intervened by angelica sinensis polysaccharide. Mod. J. Integr. Tradit. Chin. West. Med. 2019, 28, 1939–1942+1946. [Google Scholar] [CrossRef]
- Zhang, G.; Miao, J.; Guo, L.; Jia, J.; Cui, H. Application of multi-omics combination in mechanism studies of traditional Chinese medicine. Chin. Tradit. Herb. Drugs 2021, 52, 3112–3120. [Google Scholar]
- Liu, H.; Zhang, Y.; Wang, J.; Nie, J.; Song, L.; Li, Y. Advances in frontier technologies in metabolomics and their application in modern research of Chinese medicine. Chin. Tradit. Herb. Drugs 2024, 55, 969–977. [Google Scholar]
- Li, M.; Kang, T.; Jin, L.; Wei, J. Research progress on bolting and flowering of Angelica sinensis and regulation pathways. Chin. Tradit. Herb. Drugs 2020, 51, 5894–5899. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016, 44, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.; Basílio, N.; Mateus, N.; de Freitas, V.; Pina, F. Natural and Synthetic Flavylium-Based Dyes: The Chemistry Behind the Color. Chem. Rev. 2022, 122, 1416–1481. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Z. Research Progress in Ferulic Acid. Mod. Chin. Med. 2020, 22, 138–147. [Google Scholar] [CrossRef]
- Liu, J.; Li, W.; Wang, C.; Xie, H. Biosynthesis and regulation of ferulic acid in Angelica sinensis. Chin. Tradit. Herb. Drugs 2008, 39, 1909–1912. [Google Scholar]
- Chen, Y.; Zhang, J.; Liu, J.; Hu, H.; Wang, L.; Jin, L. Comparative Study on Morphological Features and Chemical Components of Wild and Cultivated Angelica sinensis Based on Bionic Technologies and Chemometrics. ACS Omega 2024, 9, 40. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, T.; Zhang, M.; Zhang, J.; Xu, L.; Kang, S.; Jin, L. Advances in biosynthesis and regulation of active ingredients of Angelicae Sinensis Radix. Chin. Tradit. Herb. Drugs 2023, 54, 7545–7553. [Google Scholar]
- Shang, J.; Wu, W.; Ma, Y. Phenylpropanoid Metabolism Pathway in Plants, Chinese. J. Biochem. Mol. Biol. 2022, 38, 1467–1476. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Y.; Wen, S.; Xia, Q.; Li, S.; Wang, Z. Gene cloning and sequence analysis of caffeic acid O-methyltransferase in Angelica sinensis. Chin. Tradit. Herb. Drugs 2016, 47, 1180–1186. [Google Scholar]
- Yang, C.; Wang, Y.; Luo, J.; Zhang, Y. Analysis of tissue expression and expression of caffeic acid-O-methyltransferase in Escherichia coli from Angelica sinensis. J. Gansu Univ. Chin. Med. 2019, 36, 1–6. [Google Scholar] [CrossRef]
- Zhang, P.; Hou, Y.; Su, M.; Sun, L.; Xu, D.; Song, T.; Zhou, L.; Zhao, S. Cloning and Expression Analysis of Caffeic Acid–O–Methyltransferase Gene in Angelica sinensis. Chin. Wild Plant Resour. 2021, 40, 20–28. [Google Scholar] [CrossRef]
- Zhu, T.; Zhang, M.; Su, H.; Li, M.; Wang, Y.; JIN, L.; Li, M. Integrated Metabolomic and Transcriptomic Analysis Reveals Differential Mechanism of Flavonoid Biosynthesis in Two Cultivars of Angelica sinensis. Molecules 2022, 27, 306. [Google Scholar] [CrossRef]
- Bai, Q.; Duan, B.; Ma, J.; Fen, Y.; Sun, S.; Long, Q.; Lv, J.; Wan, D. Coexpression of PalbHLH1 and PalMYB90 Genes from Populus alba Enhances Pathogen Resistance in Poplar by Increasing the Flavonoid Content. Front. Plant Sci. 2019, 10, 1772. [Google Scholar] [CrossRef]
- Rao, M.J.; Xu, Y.; Tang, X.; Huang, Y.; Liu, J.; Deng, X.; Xu, Q. CsCYT75B1, a Citrus CYTOCHROME P450 Gene, Is Involved in Accumulation of Antioxidant Flavonoids and Induces Drought Tolerance in Transgenic Arabidopsis. Antioxidants 2020, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Saijo, Y.; Tintor, N.; Lu, X.; Rauf, P.; Pajerowska-Mukhtar, K.; Häweker, H.; Dong, X.; Robatzek, S.; Schulze-Lefert, P. Receptor quality control in the endoplasmic reticulum for plant innate immunity. EMBO J. 2009, 28, 3439–3449. [Google Scholar] [CrossRef]
- Schenke, D.; BÖTTCHER, C.; Scheel, D. Crosstalk between abiotic ultraviolet-B stress and biotic (flg22) stress signalling in Arabidopsis prevents flavonol accumulation in favor of pathogen defence compound production. Plant Cell Environ. 2011, 34, 1849–1864. [Google Scholar] [CrossRef]
- Subramanian, S.; Graham, M.Y.; Yu, O.; Graham, T.L. RNA interference of soybean isoflavone synthase genes leads to silencing in tissues distal to the transformation site and to enhanced susceptibility to Phytophthora sojae. Plant Physiol. 2005, 137, 1345–1353. [Google Scholar] [CrossRef]
- Ni, T.; Zhang, S.; Rao, J.; Zhao, J.; Huang, H.; Liu, Y.; Ding, Y.; Liu, Y.; Ma, Y.; Zhang, S.; et al. Phlorizin, an Important Glucoside: Research Progress on Its Biological Activity and Mechanism. Molecules 2024, 29, 741. [Google Scholar] [CrossRef]
- Wang, D.; Chen, J.; Pu, L.; Yu, L.; Xiong, F.; Sun, L.; Yu, Q.; Cao, X.; Chen, Y.; Peng, F.; et al. Galangin: A food-derived flavonoid with therapeutic potential against a wide spectrum of diseases. Phytother. Res. 2023, 37, 5700–5723. [Google Scholar] [CrossRef]
- Lauvergeat, V.; Lacomme, C.; Lacombe, E.; Lasserre, E.; Roby, D.; Grima-Pettenati, J. Two cinnamoyl-CoA reductase (CCR) genes from Arabidopsis thaliana are differentially expressed during development and in response to infection with pathogenic bacteria. Phytochemistry 2001, 57, 1187–1195. [Google Scholar] [CrossRef]
- Sibout, R.; Eudes, A.; Pollet, B.; Goujon, T.; Mila, I.; Granier, F.; Séguin, A.; Lapierre, C.; Jouanin, L. Expression pattern of two paralogs encoding cinnamyl alcohol dehydrogenases in Arabidopsis. Isolation and characterization of the corresponding mutants. Plant Physiol. 2003, 132, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Sibout, R.; Eudes, A.; Mouille, G.; Pollet, B.; Lapierre, C.; Jouanin, L.; Séguin, A. CINNAMYL ALCOHOL DEHYDROGENASE-C and -D are the primary genes involved in lignin biosynthesis in the floral stem of Arabidopsis. Plant Cell 2005, 17, 2059–2076. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, M.R.; Bedgar, D.L.; Moinuddin, S.G.A.; Cardenas, C.L.; Davin, L.B.; Kang, C.; Lewis, N.G. Functional reclassification of the putative cinnamyl alcohol dehydrogenase multigene family in Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 1455–1460. [Google Scholar] [CrossRef]
- Thévenin, J.; Pollet, B.; Letarnec, B.; Saulnier, L.; Gissot, L.; Maia-Grondard, A.; Lapierre, C.; Jouanin, L. The simultaneous repression of CCR and CAD, two enzymes of the lignin biosynthetic pathway, results in sterility and dwarfism in Arabidopsis thaliana. Mol. Plant 2011, 4, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.B.; Bastress, K.L.; Ruegger, M.O.; Denault, J.W.; Chapple, C. The Arabidopsis thaliana REDUCED EPIDERMAL FLUORESCENCE1 gene encodes an aldehyde dehydrogenase involved in ferulic acid and sinapic acid biosynthesis. Plant Cell 2004, 16, 544–554. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Wei, J.; Li, Y.; Yang, X.; Li, S.; Yang, Z.; Duan, G.; Guo, J. Research on herbal research, chemical composition and pharmacological action of different medicinal parts of Angelica sinensis. Chin. Arch. Tradit. Chin. Med. 2024, 42, 127–134. [Google Scholar]
- Zhang, Q.; Feng, Y.; Li, C.; Li, G.; Liu, X.; Li, H.; Wang, H.; Zhou, L.; Zhang, Y. Rapid identification of phthalides and organic acids in Angelica sinensis based on UPLC-Q-TOF/MS. China Pharm. 2022, 33, 579–585+591. [Google Scholar] [CrossRef]
- Feng, W.; Liu, P.; Yan, H.; Yu, G.; Zhang, S.; Jiang, S.; Shang, E.; Qian, D.; Duan, J. Investigation of Enzymes in the Phthalide Biosynthetic Pathway in Angelica sinensis Using Integrative Metabolite Profiles and Transcriptome Analysis. Front Plant Sci. 2022, 13, 928760. [Google Scholar] [CrossRef]
- Shi, Y.; Li, M.; Yang, S.; Hu, Y.; Sun, W.; Li, L. Advances on Chemical Constituents of Volatile Oil from Angelicae Sinensis Radix and Their Pharmacological Effect. Chin. J. Mod. Appl. Pharm. 2024, 41, 1006–1014. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, H.; Qin, J.; Zhang, W.; Gao, H.; Long, X.; He, H.; Zhang, L.; Zhang, C.; Cao, C.; et al. The phthalide compound tokinolide B from Angelica sinensis exerts anti-inflammatory effects through Nur77 binding. Phytomedicine 2024, 133, 155925. [Google Scholar] [CrossRef]
- Kuo, H.F.; Chang, T.Y.; Chiang, S.F.; Wang, W.D.; Charng, Y.Y.; Chiou, T.J. Arabidopsis inositol pentakisphosphate 2-kinase, AtIPK1, is required for growth and modulates phosphate homeostasis at the transcriptional level. Plant J. 2014, 80, 503–515. [Google Scholar] [CrossRef]
- Aggarwal, S.; Kumar, A.; Bhati, K.K.; Kaur, G.; Shukla, V.; Tiwari, S.; Pandey, A.K. RNAi-Mediated Downregulation of Inositol Pentakisphosphate Kinase (IPK1) in Wheat Grains Decreases Phytic Acid Levels and Increases Fe and Zn Accumulation. Front Plant Sci. 2018, 9, 259. [Google Scholar] [CrossRef]
- Belgaroui, N.; Lacombe, B.; Rouached, H.; Hanin, M. Phytase overexpression in Arabidopsis improves plant growth under osmotic stress and in combination with phosphate deficiency. Sci. Rep. 2018, 8, 1137. [Google Scholar] [CrossRef]
- Ibrahim, S.; Saleem, B.; Rehman, N.; Zafar, S.A.; Naeem, M.K.; Khan, M.R. CRISPR/Cas9 mediated disruption of Inositol Pentakisphosphate 2-Kinase 1 (TaIPK1) reduces phytic acid and improves iron and zinc accumulation in wheat grains. J. Adv. Res. 2022, 37, 33–41. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Kang, L.; Zhan, Z.; Nan, T. Comparison of Wild and Cultivated Bupleurum chinense Based on Traditional Quality Evaluation. Chin. J. Exp. Tradit. Med. Formulae 2024, 30, 145–155. [Google Scholar] [CrossRef]
- Torabinejad, J.; Donahue, J.L.; Gunesekera, B.N.; Allen-Daniels, M.J.; Gillaspy, G.E. VTC4 is a bifunctional enzyme that affects myoinositol and ascorbate biosynthesis in plants. Plant Physiol. 2009, 150, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Taji, T.; Ohsumi, C.; Iuchi, S.; Seki, M.; Kasuga, M.; Kobayashi, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J. 2002, 29, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Panikulangara, T.J.; Eggers-Schumacher, G.; Wunderlich, M.; Stransky, H.; Schöffl, F. Galactinol synthase1. A novel heat shock factor target gene responsible for heat-induced synthesis of raffinose family oligosaccharides in Arabidopsis. Plant Physiol. 2004, 136, 3148–3158. [Google Scholar] [CrossRef] [PubMed]

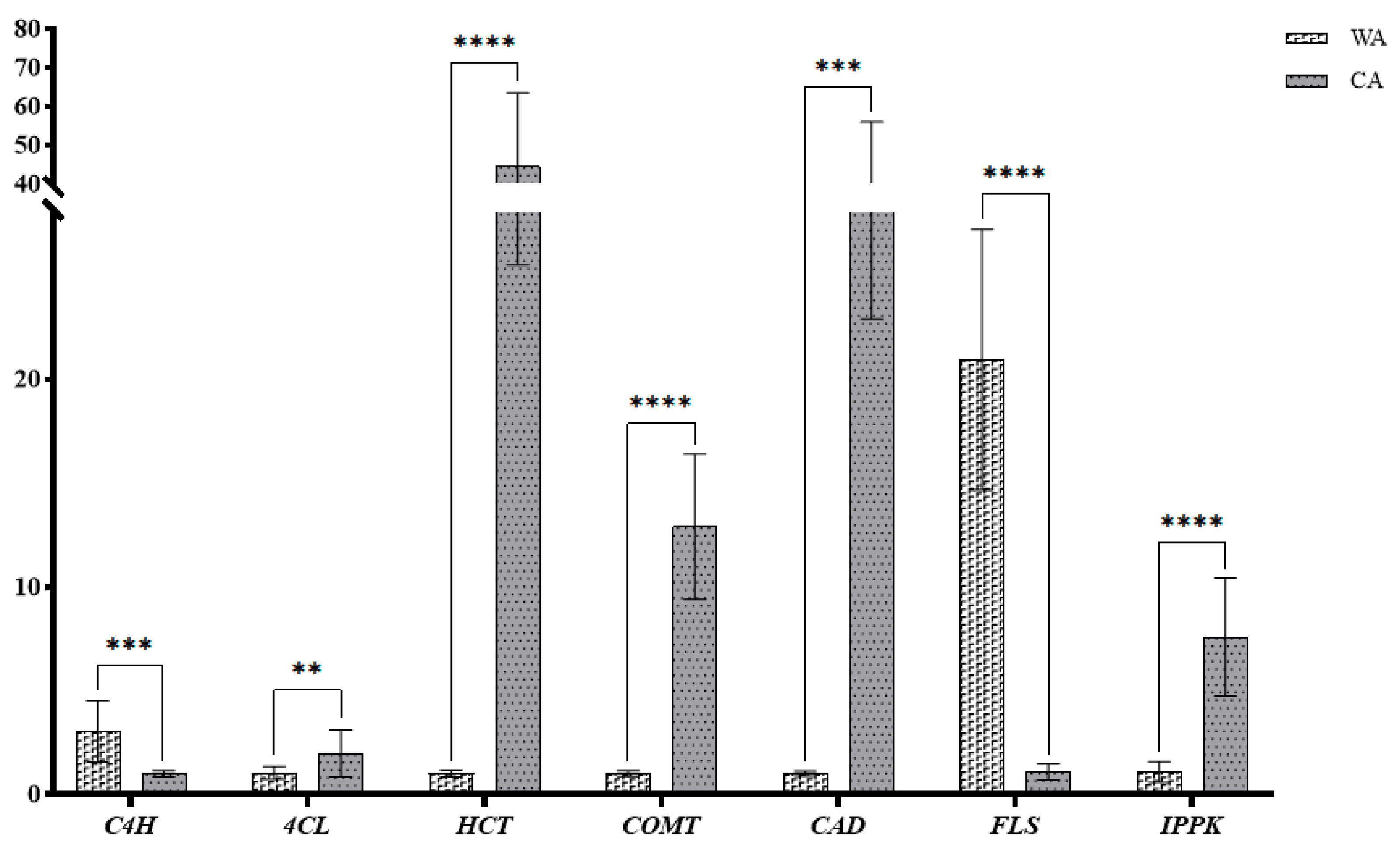
| Graph 5. | Primer Sequences (5′—3′) |
|---|---|
| ACT | Forward: TGGTATTGTGCTGGATTCTGGT |
| Reverse: TGAGATCACCACCAGCAAGG | |
| C4H | Forward: GCTCACTGGGAAAACCCTGA |
| Reverse: ATGCAAGGATGATTCCGGGG | |
| 4CL | Forward: GTTGTCAGATCCCCCGACAG |
| Reverse: TCCCTGAAGCTGACTTTGGC | |
| HCT | Forward: TCAGTTTGGGATGAGGTGCC |
| Reverse: TTTGTGTGAAACGCTGGCTG | |
| COMT | Forward: AGCTTCTCATGTCGAACCCC |
| Reverse: TCCAGCAGTAGAGCCACTCA | |
| CAD | Forward: ACAGGAGCAACGCTAGACAG |
| Reverse: AAGGACCGGCAGTCTTGA | |
| FLS | Forward: AGTGTGGCGTGAGTTCTTCC |
| Reverse: ACGGCTGCCATAACCTTCAT | |
| IPPK | Forward: CTAAGCGTCCTTCATGGCGA |
| Reverse: TCCGCACTTGGGCTTTATCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, J.; Chen, Y.; Liu, J.; Li, K.; Jin, L. Combination of Metabolomic Analysis and Transcriptomic Analysis Reveals Differential Mechanism of Phenylpropanoid Biosynthesis and Flavonoid Biosynthesis in Wild and Cultivated Forms of Angelica sinensis. Metabolites 2025, 15, 633. https://doi.org/10.3390/metabo15090633
Wang Y, Zhang J, Chen Y, Liu J, Li K, Jin L. Combination of Metabolomic Analysis and Transcriptomic Analysis Reveals Differential Mechanism of Phenylpropanoid Biosynthesis and Flavonoid Biosynthesis in Wild and Cultivated Forms of Angelica sinensis. Metabolites. 2025; 15(9):633. https://doi.org/10.3390/metabo15090633
Chicago/Turabian StyleWang, Yuanyuan, Jialing Zhang, Yiyang Chen, Juanjuan Liu, Ke Li, and Ling Jin. 2025. "Combination of Metabolomic Analysis and Transcriptomic Analysis Reveals Differential Mechanism of Phenylpropanoid Biosynthesis and Flavonoid Biosynthesis in Wild and Cultivated Forms of Angelica sinensis" Metabolites 15, no. 9: 633. https://doi.org/10.3390/metabo15090633
APA StyleWang, Y., Zhang, J., Chen, Y., Liu, J., Li, K., & Jin, L. (2025). Combination of Metabolomic Analysis and Transcriptomic Analysis Reveals Differential Mechanism of Phenylpropanoid Biosynthesis and Flavonoid Biosynthesis in Wild and Cultivated Forms of Angelica sinensis. Metabolites, 15(9), 633. https://doi.org/10.3390/metabo15090633







