NMR-Based Metabolomic Profiling Highlights Functional Nutritional Gaps Between Human Milk, Infant Formulas, and Animal Milks
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Collection
2.2. Sample Preparation and NMR Acquisition
2.3. Metabolites Identification
2.4. Statistical Analysis
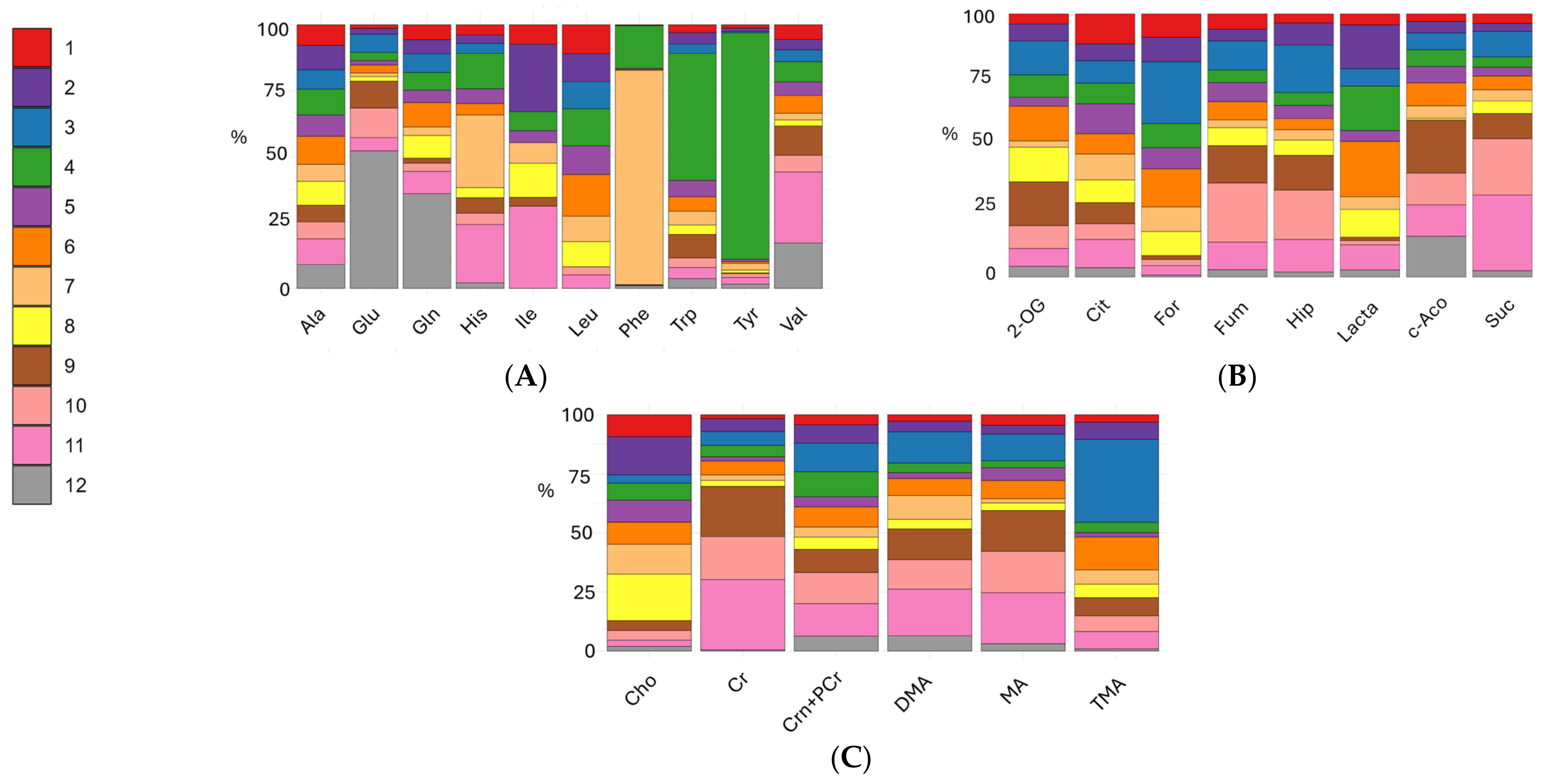
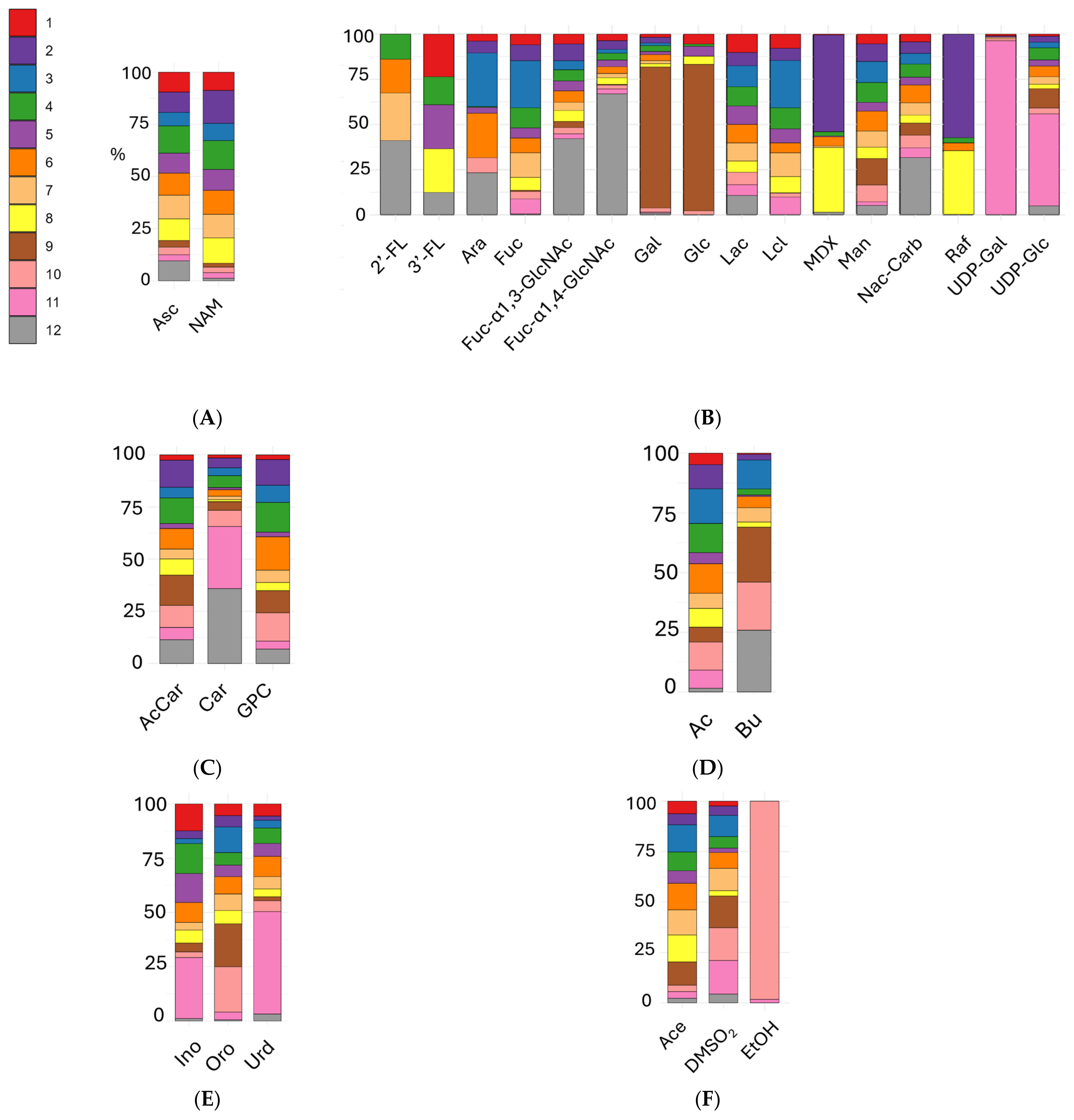
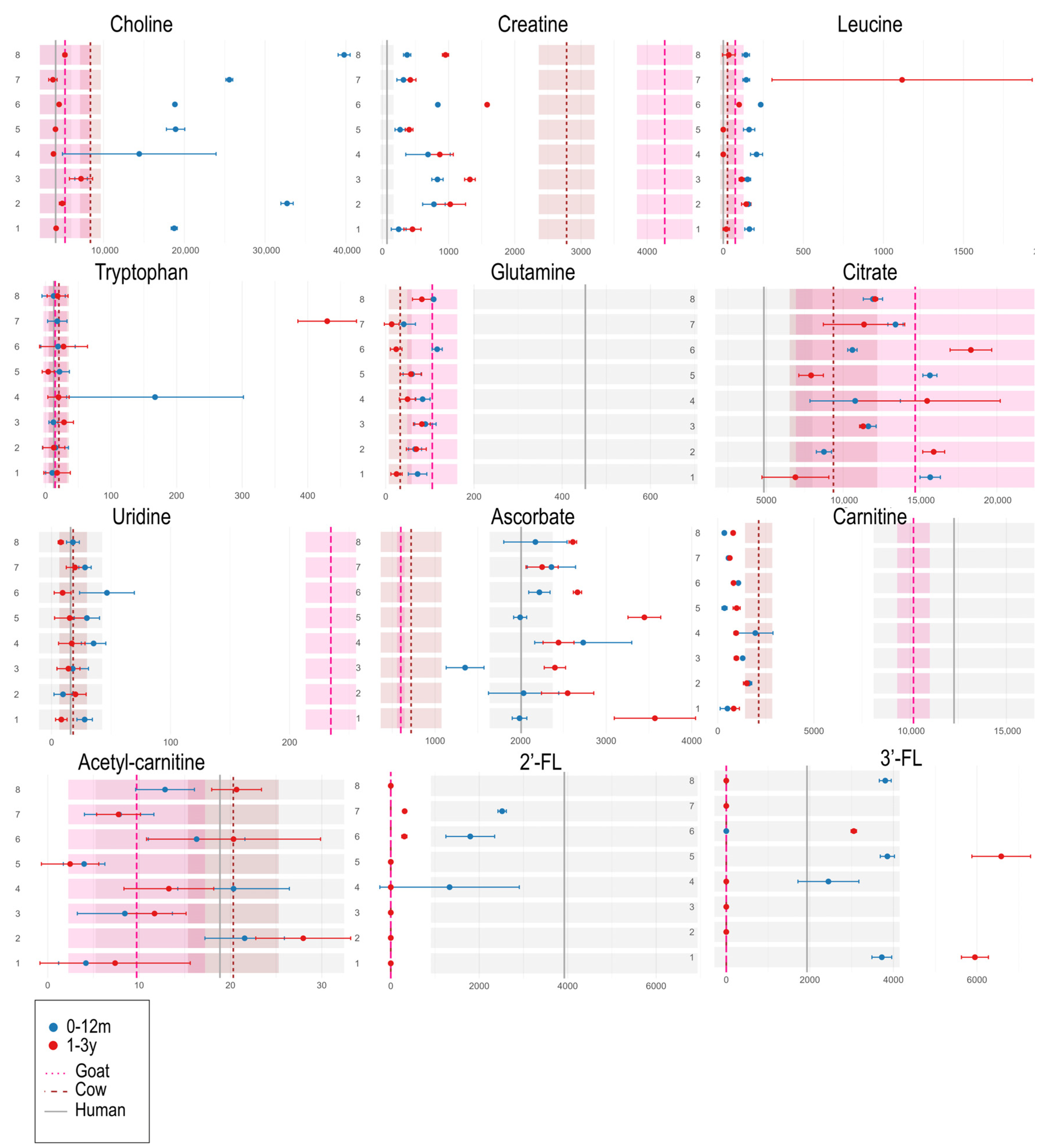
3. Results
3.1. Multivariate Analyses
3.2. Univariate Analyses of Different Milk Types
3.3. Univariate Analyses of Milk Formulas and Human Milk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2′-FL | 2′-Fucosyllactose |
| 3′-FL | 3′-Fucosyllactose |
| AcCar | Acetyl-carnitine |
| Ace | Acetone |
| Ala | Alanine |
| Ara | Arabinose |
| Asc | Ascorbate |
| Bu | Butyrate |
| Car | Carnitine |
| Cho | Choline |
| Cit | Citrate |
| c-Aco | Cis-aconitate |
| Cr | Creatine |
| Crn | Creatinine |
| DMA | Dimethylamine |
| DMSO2 | Dimethylsulfone |
| d | Doublet |
| EtOH | Ethanol |
| FDR | False Discovery Rate |
| FDR p-value | Valore di p corretto per FDR |
| Fuc | Fucose |
| Fuc-α1,3-GlcNAc | Fucosyl-α-1,3-N-acetylglucosamine |
| Fuc-α1,4-GlcNAc | Fucosyl-α-1,4-N-acetylglucosamine |
| FM | Formula Milk |
| For | Formate |
| Fum | Fumarate |
| Gal | Galactose |
| Glc | Glucose |
| Gln | Glutamine |
| Glu | Glutamate |
| GPC | Glycerophosphocholine |
| Hip | Hippurate |
| His | Histidine |
| HM | Human Milk |
| HMO | Human Milk Oligosaccharides |
| ILE | Isoleucine |
| Ino | Inosine |
| Lac | Lactose |
| Lcl | Lactulose |
| LD | Lactose-free |
| Leu | Leucine |
| log2FC | Log2 Fold Change |
| MA | Methylamine |
| Man | Mannose |
| MDX | Maltodextrin |
| m | Multiplet |
| NAc-Carb | N-acetyl carbohydrates |
| NAM | Niacinamide |
| NMR | Nuclear Magnetic Resonance |
| Oro | Orotate |
| PC | Principal Component |
| PCA | Principal Component Analysis |
| PCr | Phosphocreatine |
| Phe | Phenylalanine |
| q | Quartet |
| Raf | Raffinose |
| RCF | Relative Centrifugal Force |
| s | Singlet |
| SCFA | Short Chain Fatty Acids |
| Suc | Sucrose |
| Suc | Succinate |
| t | Triplet |
| TMA | Trimethylamine |
| TSP | Trimethylsilylpropionic acid |
| Trp | Tryptophan |
| Tyr | Tyrosine |
| UDP-Gal | UDP-galactose |
| UDP-Glc | UDP-glucose |
| Urd | Uridine |
| Val | Valine |
References
- Bakshi, S.; Paswan, V.K.; Yadav, S.P.; Bhinchhar, B.K.; Kharkwal, S.; Rose, H.; Kanetkar, P.; Kumar, V.; Al-Zamani, Z.A.S.; Bunkar, D.S. A Comprehensive Review on Infant Formula: Nutritional and Functional Constituents, Recent Trends in Processing and Its Impact on Infants’ Gut Microbiota. Front. Nutr. 2023, 10, 1194679. [Google Scholar] [CrossRef]
- Cesare Marincola, F.; Dessì, A.; Corbu, S.; Reali, A.; Fanos, V. Clinical Impact of Human Breast Milk Metabolomics. Clin. Chim. Acta 2015, 451, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human Milk Composition: Nutrients and Bioactive Factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Andreas, N.J.; Kampmann, B.; Mehring Le-Doare, K. Human Breast Milk: A Review on Its Composition and Bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef]
- Sun, W.; Tao, L.; Qian, C.; Xue, P.; Du, S.; Tao, Y. Human Milk Oligosaccharides: Bridging the Gap in Intestinal Microbiota between Mothers and Infants. Front. Cell. Infect. Microbiol. 2025, 14, 1386421. [Google Scholar] [CrossRef]
- Kellman, B.P.; Richelle, A.; Yang, J.-Y.; Chapla, D.; Chiang, A.W.T.; Najera, J.A.; Liang, C.; Fürst, A.; Bao, B.; Koga, N.; et al. Elucidating Human Milk Oligosaccharide Biosynthetic Genes through Network-Based Multi-Omics Integration. Nat. Commun. 2022, 13, 2455. [Google Scholar] [CrossRef]
- Bode, L. Human Milk Oligosaccharides: Every Baby Needs a Sugar Mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef]
- Duman, H.; Bechelany, M.; Karav, S. Human Milk Oligosaccharides: Decoding Their Structural Variability, Health Benefits, and the Evolution of Infant Nutrition. Nutrients 2025, 17, 118. [Google Scholar] [CrossRef]
- Donovan, S.M.; Comstock, S.S. Human Milk Oligosaccharides Influence Neonatal Mucosal and Systemic Immunity. Ann. Nutr. Metab. 2016, 69 (Suppl. S2), 42–51. [Google Scholar] [CrossRef]
- Chen, G.; Chen, L.; Wang, H.; Zhang, J.; Sun, X.; Chen, X.; Fan, J.; Jia, Z.; Huang, Y. 1H Nuclear Magnetic Resonance-Based Metabolomic Profiling and Comparison of Human Milk across Different Lactation Stages in Secretors and Nonsecretors: A Study of Chinese Lactating Women. J. Nutr. 2025, 155, 78–86. [Google Scholar] [CrossRef]
- Zabel, B.E.; Gerdes, S.; Evans, K.C.; Nedveck, D.; Singles, S.K.; Volk, B.; Budinoff, C. Strain-Specific Strategies of 2′-Fucosyllactose, 3-Fucosyllactose, and Difucosyllactose Assimilation by Bifidobacterium Longum Subsp. Infantis Bi-26 and ATCC 15697. Sci. Rep. 2020, 10, 15919. [Google Scholar] [CrossRef]
- Almeida, C.C.; Mendonça Pereira, B.F.; Leandro, K.C.; Costa, M.P.; Spisso, B.F.; Conte-Junior, C.A. Bioactive Compounds in Infant Formula and Their Effects on Infant Nutrition and Health: A Systematic Literature Review. Int. J. Food Sci. 2021, 2021, 8850080. [Google Scholar] [CrossRef] [PubMed]
- Puri, K.; Svenstrup, C.; Vanderpool, C. Functional Infant Formula Additives. NeoReviews 2025, 26, e163–e171. [Google Scholar] [CrossRef] [PubMed]
- Murgia, A.; Scano, P.; Contu, M.; Ibba, I.; Altea, M.; Bussu, M.; Demuru, M.; Porcu, A.; Caboni, P. Characterization of Donkey Milk and Metabolite Profile Comparison with Human Milk and Formula Milk. LWT 2016, 74, 427–433. [Google Scholar] [CrossRef]
- Yang, X.; Meng, L.; Rahman, A.; Wang, J.; Zheng, N. Comparison of Fatty Acid Characteristics in Human Milk, Bovine Milk, and Infant Formula for Better Emulation. Curr. Opin. Food Sci. 2025, 61, 101240. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, B.; Zhu, H.; Hettinga, K.; Pang, X.; Zhang, S.; Li, K.; Jiang, S.; Lyu, J. Comparative Analysis of Skim Milk and Milk Fat Globule Membrane Proteomes between Human and Farm Animal Milk for Infant Formula Production. J. Agric. Food Chem. 2024, 72, 25367–25378. [Google Scholar] [CrossRef]
- Di Francesco, L.; Di Girolamo, F.; Mennini, M.; Masotti, A.; Salvatori, G.; Rigon, G.; Signore, F.; Pietrantoni, E.; Scapaticci, M.; Lante, I.; et al. A MALDI-TOF MS Approach for Mammalian, Human, and Formula Milks’ Profiling. Nutrients 2018, 10, 1238. [Google Scholar] [CrossRef]
- Urrutia-Baca, V.H.; Álvarez-Buylla, J.R.; Gueimonde, M.; Chuck-Hernández, C.; Ruas-Madiedo, P.; González-Iglesias, H. Comparative Study of the Oligosaccharide Profile in Goat, Bovine, Sheep, and Human Milk Whey. Food Chem. 2025, 463, 141123. [Google Scholar] [CrossRef]
- Ceballos, L.S.; Morales, E.R.; de la Torre Adarve, G.; Castro, J.D.; Martínez, L.P.; Sampelayo, M.R.S. Composition of Goat and Cow Milk Produced under Similar Conditions and Analyzed by Identical Methodology. J. Food Compos. Anal. 2009, 22, 322–329. [Google Scholar] [CrossRef]
- Suh, J.H. Critical Review: Metabolomics in Dairy Science—Evaluation of Milk and Milk Product Quality. Food Res. Int. 2022, 154, 110984. [Google Scholar] [CrossRef]
- Hammam, A.R.A.; Salman, S.M.; Elfaruk, M.S.; Alsaleem, K.A. Goat Milk: Compositional, Technological, Nutritional and Therapeutic Aspects: A Review. Asian J. Dairy Food Res. 2022, 41, 367–376. [Google Scholar] [CrossRef]
- Meoni, G.; Tenori, L.; Luchinat, C. Nuclear Magnetic Resonance-Based Metabolomic Comparison of Breast Milk and Organic and Traditional Formula Milk Brands for Infants and Toddlers. OMICS-A J. Integr. Biol. 2020, 24, 424–436. [Google Scholar] [CrossRef]
- Ghini, V.; Meoni, G.; Vignoli, A.; Di Cesare, F.; Tenori, L.; Turano, P.; Luchinat, C. Fingerprinting and Profiling in Metabolomics of Biosamples. Prog. Nucl. Magn. Reson. Spectrosc. 2023, 138–139, 105–135. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, A.; Ghini, V.; Meoni, G.; Licari, C.; Takis, P.G.; Tenori, L.; Turano, P.; Luchinat, C. High-Throughput Metabolomics by 1D NMR. Angew. Chem.-Int. Ed. 2019, 58, 968–994. [Google Scholar] [CrossRef] [PubMed]
- Meoni, G.; Sousa, I.; Tenori, L.; Niero, G.; Pozza, M.; De Marchi, M.; Manuelian, C.L. A Metabolic Profiling Approach to Characterize and Discriminate Plant-Based Beverages and Milk. J. Dairy Sci. 2025, 108, 5675–5695. [Google Scholar] [CrossRef] [PubMed]
- An, P.; Lan, D.; Feng, D.; Zhang, Y.; An, H.; Zheng, L.; Wu, Z.; Wang, D.; Zhong, Q. Quantitative Nuclear Magnetic Analysis of Human Milk Oligosaccharides 2′-Fucosyllactose and 3-Fucosyllactose in Complicated Food Matrices. Food Chem. 2025, 473, 142821. [Google Scholar] [CrossRef]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef]
- Tenori, L.; Santucci, C.; Meoni, G.; Morrocchi, V.; Matteucci, G.; Luchinat, C. NMR Metabolomic Fingerprinting Distinguishes Milk from Different Farms. Food Res. Int. 2018, 113, 131–139. [Google Scholar] [CrossRef]
- Niero, G.; Meoni, G.; Tenori, L.; Luchinat, C.; Visentin, G.; Callegaro, S.; Visentin, E.; Cassandro, M.; Marchi, M.D.; Penasa, M. Grazing Affects Metabolic Pattern of Individual Cow Milk. J. Dairy Sci. 2022, 105, 9702–9712. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, H.; Feng, J.; Chen, Z.; Cai, S. 1H NMR-Based Compositional Identification of Different Powdered Infant Formulas. Food Chem. 2017, 230, 164–173. [Google Scholar] [CrossRef]
- Garwolińska, D.; Hewelt-Belka, W.; Kot-Wasik, A.; Sundekilde, U.K. Nuclear Magnetic Resonance Metabolomics Reveals Qualitative and Quantitative Differences in the Composition of Human Breast Milk and Milk Formulas. Nutrients 2020, 12, 921. [Google Scholar] [CrossRef] [PubMed]
- Kortesniemi, M.; Jafari, T.; Zhang, Y.; Yang, B. 1H NMR Metabolomics of Chinese Human Milk at Different Stages of Lactation among Secretors and Non-Secretors. Molecules 2022, 27, 5526. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, E.-M.; Xu, L.-Y. Guide to Metabolomics Analysis: A Bioinformatics Workflow. Metabolites 2022, 12, 357. [Google Scholar] [CrossRef] [PubMed]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.D.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic Profiling, Metabolomic and Metabonomic Procedures for NMR Spectroscopy of Urine, Plasma, Serum and Tissue Extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Meoni, G.; Luchinat, C.; Gotti, E.; Cadena, A.; Tenori, L. Phenotyping Green and Roasted Beans of Nicaraguan Coffea Arabica Varieties Processed with Different Post-Harvest Practices. Appl. Sci. 2021, 11, 11779. [Google Scholar] [CrossRef]
- Neuhäuser, M. Test. In International Encyclopedia of Statistical Science; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1656–1658. [Google Scholar]
- Kim, S.Y.; Yi, D.Y. Components of Human Breast Milk: From Macronutrient to Microbiome and microRNA. Clin. Exp. Pediatr. 2020, 63, 301–309. [Google Scholar] [CrossRef]
- Urashima, T.; Ajisaka, K.; Ujihara, T.; Nakazaki, E. Recent Advances in the Science of Human Milk Oligosaccharides. BBA Adv. 2025, 7, 100136. [Google Scholar] [CrossRef]
- Walsh, C.; Lane, J.A.; van Sinderen, D.; Hickey, R.M. Human Milk Oligosaccharides: Shaping the Infant Gut Microbiota and Supporting Health. J. Funct. Foods 2020, 72, 104074. [Google Scholar] [CrossRef]
- Al-Beltagi, M. Human Milk Oligosaccharide Secretion Dynamics during Breastfeeding and Its Antimicrobial Role: A Systematic Review. World J. Clin. Pediatr. 2025, 14, 104797. [Google Scholar] [CrossRef]
- Yang, Y.; Thorhallsson, A.T.; Rovira, C.; Holck, J.; Meyer, A.S.; Yang, H.; Zeuner, B. Improved Enzymatic Production of the Fucosylated Human Milk Oligosaccharide LNFP II with GH29B α-1,3/4-l-Fucosidases. J. Agric. Food Chem. 2024, 72, 11013–11028. [Google Scholar] [CrossRef]
- Willemsen, Y.; Beijers, R.; Gu, F.; Vasquez, A.A.; Schols, H.A.; de Weerth, C. Fucosylated Human Milk Oligosaccharides during the First 12 Postnatal Weeks Are Associated with Better Executive Functions in Toddlers. Nutrients 2023, 15, 1463. [Google Scholar] [CrossRef] [PubMed]
- O’Connell Motherway, M.; O’Brien, F.; O’Driscoll, T.; Casey, P.G.; Shanahan, F.; van Sinderen, D. Carbohydrate Syntrophy Enhances the Establishment of Bifidobacterium Breve UCC2003 in the Neonatal Gut. Sci. Rep. 2018, 8, 10627. [Google Scholar] [CrossRef] [PubMed]
- Grabinger, T.; Glaus Garzon, J.F.; Hausmann, M.; Geirnaert, A.; Lacroix, C.; Hennet, T. Alleviation of Intestinal Inflammation by Oral Supplementation With 2-Fucosyllactose in Mice. Front. Microbiol. 2019, 10, 1385. [Google Scholar] [CrossRef] [PubMed]
- van Sadelhoff, J.H.J.; Wiertsema, S.P.; Garssen, J.; Hogenkamp, A. Free Amino Acids in Human Milk: A Potential Role for Glutamine and Glutamate in the Protection Against Neonatal Allergies and Infections. Front. Immunol. 2020, 11, 1007. [Google Scholar] [CrossRef]
- Baldeón, M.E.; Zertuche, F.; Flores, N.; Fornasini, M. Free Amino Acid Content in Human Milk Is Associated with Infant Gender and Weight Gain during the First Four Months of Lactation. Nutrients 2019, 11, 2239. [Google Scholar] [CrossRef]
- Guzzardi, M.A.; La Rosa, F.; Granziera, F.; Panetta, D.; Pardo-Tendero, M.; Barone, M.; Turroni, S.; Faita, F.; Kusmic, C.; Brigidi, P.; et al. Gut-Derived Metabolites Mediating Cognitive Development in 5-Year-Old Children: Early-Life Transplant in Mice Has Lasting Effects throughout Adulthood. Brain Behav. Immun. 2023, 114, 94–110. [Google Scholar] [CrossRef]
- Lee, S.; Goodson, M.; Vang, W.; Kalanetra, K.; Barile, D.; Raybould, H. 2′-Fucosyllactose Supplementation Improves Gut-Brain Signaling and Diet-Induced Obese Phenotype and Changes the Gut Microbiota in High Fat-Fed Mice. Nutrients 2020, 12, 1003. [Google Scholar] [CrossRef]
- Fan, Y.; McMath, A.L.; Donovan, S.M. Review on the Impact of Milk Oligosaccharides on the Brain and Neurocognitive Development in Early Life. Nutrients 2023, 15, 3743. [Google Scholar] [CrossRef]
- O’Callaghan, T.F.; O’Donovan, M.; Murphy, J.P.; Sugrue, K.; Tobin, J.T.; McNamara, A.E.; Yin, X.; Sundaramoorthy, G.; Brennan, L. The Bovine Colostrum and Milk Metabolome at the Onset of Lactation as Determined by 1H-NMR. Int. Dairy J. 2021, 113, 104881. [Google Scholar] [CrossRef]
- Mendelson, S.D. (Ed.) Syndrome. In Metabolic Syndrome and Psychiatric Illness; Academic Press: San Diego, CA, USA, 2008; pp. 141–186. ISBN 978-0-12-374240-7. [Google Scholar]
- Gentili, A.; Caretti, F.; D’Ascenzo, G.; Marchese, S.; Perret, D.; Di Corcia, D.; Rocca, L.M. Simultaneous Determination of Water-Soluble Vitamins in Selected Food Matrices by Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 2029–2043. [Google Scholar] [CrossRef]
- Bobbo, T.; Meoni, G.; Niero, G.; Tenori, L.; Luchinat, C.; Cassandro, M.; Penasa, M. Nuclear Magnetic Resonance Spectroscopy to Investigate the Association between Milk Metabolites and Udder Quarter Health Status in Dairy Cows. J. Dairy. Sci. 2021, 105, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Gómez, P.; Pariyani, R.; Bateman, L.M.; Lynch, D.; Timlin, M.; Dineen, M.; McCarthy, N.A.; Brodkorb, A.; Maguire, A.R.; O’Donovan, M.; et al. Effect of Proportion of Pasture in the Cow Diet and Seasonality on the Milk Metabolome as Determined by 1H-NMR. J. Dairy Sci. 2025, 108, 4659–4673. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Pan, J.; Pang, S.; Hu, S.; Liu, M.; Zhang, X.; Song, L.; Ren, X.; Wang, Z. Comparative Analysis of the Nutritional Composition, Digestibility, Metabolomics Profiles and Growth Influence of Cow, Goat and Sheep Milk Powder Diets in Rat Models. Front. Nutr. 2024, 11, 1428938. [Google Scholar] [CrossRef] [PubMed]
- Slupsky, C.M.; He, X.; Hernell, O.; Andersson, Y.; Rudolph, C.; Lönnerdal, B.; West, C.E. Postprandial Metabolic Response of Breast-Fed Infants and Infants Fed Lactose-Free vs Regular Infant Formula: A Randomized Controlled Trial. Sci. Rep. 2017, 7, 3640. [Google Scholar] [CrossRef]
- Falaina, V.; Fotakis, C.; Boutsikou, T.; Tsiaka, T.; Moros, G.; Ouzounis, S.; Andreou, V.; Iliodromiti, Z.; Xanthos, T.; Vandenplas, Y.; et al. Urine Metabolomic Profile of Breast- versus Formula-Fed Neonates Using a Synbiotic-Enriched Formula. Int. J. Mol. Sci. 2022, 23, 10476. [Google Scholar] [CrossRef]
- Shoff, S.; He, X.; Lee, H.; Zhang, Z.; Mishchuk, D.O.; Ozturk, G.; Barile, D.; Hartvigsen, M.L.; Kvistgaard, A.S.; Slupsky, C.M. The Role of Alpha-Lactalbumin in Modulating Tryptophan Metabolism and Serotonin Synthesis. npj Sci. Food 2025, 9, 120. [Google Scholar] [CrossRef]
- Sabater, C.; Prodanov, M.; Olano, A.; Corzo, N.; Montilla, A. Quantification of Prebiotics in Commercial Infant Formulas. Food Chem. 2016, 194, 6–11. [Google Scholar] [CrossRef]
- Lund, M.N.; Ray, C.A. Control of Maillard Reactions in Foods: Strategies and Chemical Mechanisms. J. Agric. Food Chem. 2017, 65, 4537–4552. [Google Scholar] [CrossRef]
- Wang, X.; Yang, S.; Zheng, C.; Huang, C.; Yao, H.; Guo, Z.; Wu, Y.; Wang, Z.; Wu, Z.; Ge, R.; et al. Multi-Omics Profiles of Small Intestine Organoids in Reaction to Breast Milk and Different Infant Formula Preparations. Nutrients 2024, 16, 2951. [Google Scholar] [CrossRef]
- Guo, M. (Ed.) Chapter 10—Human Milk Biochemistry and Infant Formula Manufacturing Technology. In Functional Foods, 2nd ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2025; pp. 327–362. ISBN 978-0-443-19100-8. [Google Scholar]
- Connolly, C.; Yin, X.; Brennan, L. Impact of Lactation Stage on the Metabolite Composition of Bovine Milk. Molecules 2023, 28, 6608. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Wang, B. Metabolomics in Ruminant Food: Bridging Nutritional Quality and Safety Evaluation. Anim. Nutr. 2025, 2, e7. [Google Scholar] [CrossRef]
- Pintus, R.; Dessì, A.; Mussap, M.; Fanos, V. Metabolomics Can Provide New Insights into Perinatal Nutrition. Acta Paediatr. 2023, 112, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gallego, C.; Morales, J.M.; Monleón, D.; Du Toit, E.; Kumar, H.; Linderborg, K.M.; Zhang, Y.; Yang, B.; Isolauri, E.; Salminen, S.; et al. Human Breast Milk NMR Metabolomic Profile across Specific Geographical Locations and Its Association with the Milk Microbiota. Nutrients 2018, 10, 1355. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, K.O.; Meng, F.; Lanfranchi, E.; Young, J.F.; Stanton, C.; Ryan, C.A.; Kelly, A.L.; Sundekilde, U.K. Dynamic Changes in the Human Milk Metabolome Over 25 Weeks of Lactation. Front. Nutr. 2022, 9, 917659. [Google Scholar] [CrossRef] [PubMed]
- Korovljev, D.; Todorovic, N.; Stajer, V.; Ostojic, S.M. Dietary Intake of Creatine in Children Aged 0–24 Months. Ann. Nutr. Metab. 2021, 77, 185–188. [Google Scholar] [CrossRef]
- Singh, P.; Sanchez-Fernandez, L.L.; Ramiro-Cortijo, D.; Ochoa-Allemant, P.; Perides, G.; Liu, Y.; Medina-Morales, E.; Yakah, W.; Freedman, S.D.; Martin, C.R. Maltodextrin-Induced Intestinal Injury in a Neonatal Mouse Model. Dis. Model. Mech. 2020, 13, dmm044776. [Google Scholar] [CrossRef]



| Milk Type/Brand (Year) | n° Batches | ||
|---|---|---|---|
| 0–12 Months | 1–3 Years | ||
| Formula Milk—Brand 1 (2019) | 5 | 5 | / |
| Formula Milk—Brand 2 (2019) | 5 | 5 | / |
| Formula Milk—Brand 3 (2019) | 4 | 4 | / |
| Formula Milk—Brand 4 (2019) | 5 | 5 | / |
| Formula Milk—Brand 4 (2025) | 4 | 3 | / |
| Formula Milk—Brand 5 (2019) | 5 | 5 | / |
| Formula Milk—Brand 6 (2025) | 3 | 3 | / |
| Formula Milk—Brand 7 (2025) | 4 | 3 | / |
| Formula Milk—Brand 8 (2025) | 4 | 3 | / |
| Cow milk (conventional, 2025) | / | / | 4 brands, 1 batch each |
| Cow milk (lactose-free, 2025) | / | / | 3 batches, same brand |
| Goat milk (2025) | / | / | 4 brands, 1 batch each |
| Human milk (2019) | / | / | 4 individual donors |
| TOTAL | 39 | 36 | 15 |
| 90 samples overall | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meoni, G.; Tenori, L.; Niero, G.; De Marchi, M.; Luchinat, C. NMR-Based Metabolomic Profiling Highlights Functional Nutritional Gaps Between Human Milk, Infant Formulas, and Animal Milks. Metabolites 2025, 15, 620. https://doi.org/10.3390/metabo15090620
Meoni G, Tenori L, Niero G, De Marchi M, Luchinat C. NMR-Based Metabolomic Profiling Highlights Functional Nutritional Gaps Between Human Milk, Infant Formulas, and Animal Milks. Metabolites. 2025; 15(9):620. https://doi.org/10.3390/metabo15090620
Chicago/Turabian StyleMeoni, Gaia, Leonardo Tenori, Giovanni Niero, Massimo De Marchi, and Claudio Luchinat. 2025. "NMR-Based Metabolomic Profiling Highlights Functional Nutritional Gaps Between Human Milk, Infant Formulas, and Animal Milks" Metabolites 15, no. 9: 620. https://doi.org/10.3390/metabo15090620
APA StyleMeoni, G., Tenori, L., Niero, G., De Marchi, M., & Luchinat, C. (2025). NMR-Based Metabolomic Profiling Highlights Functional Nutritional Gaps Between Human Milk, Infant Formulas, and Animal Milks. Metabolites, 15(9), 620. https://doi.org/10.3390/metabo15090620









