Organ-Specific Metabolome Reveals Potential Nutritional and Health Benefits of Ampelopsis grossedentata
Abstract
1. Introduction
2. Materials and Methods
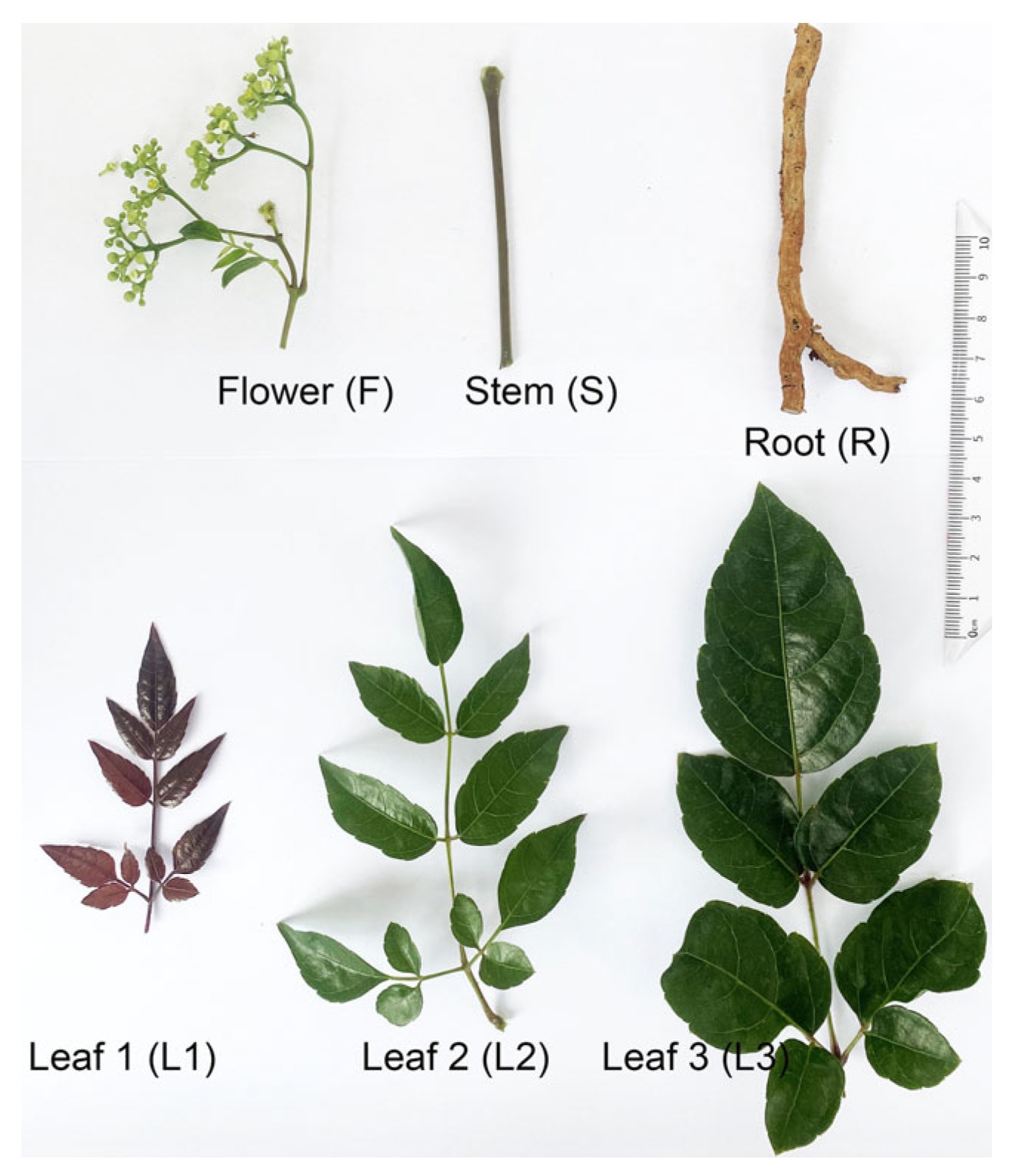
2.1. Sample Collection
2.2. Measurement of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.3. Measurement of Soluble Sugar, Amino Acid, and Soluble Protein Contents
2.4. DPPH Radical Scavenging Assay
2.5. ABTS Radical Cation Scavenging Activity
2.6. Ferric Reducing Antioxidant Power (FRAP)
2.7. Metabolomic Analysis
2.8. Statistical Analysis
3. Results
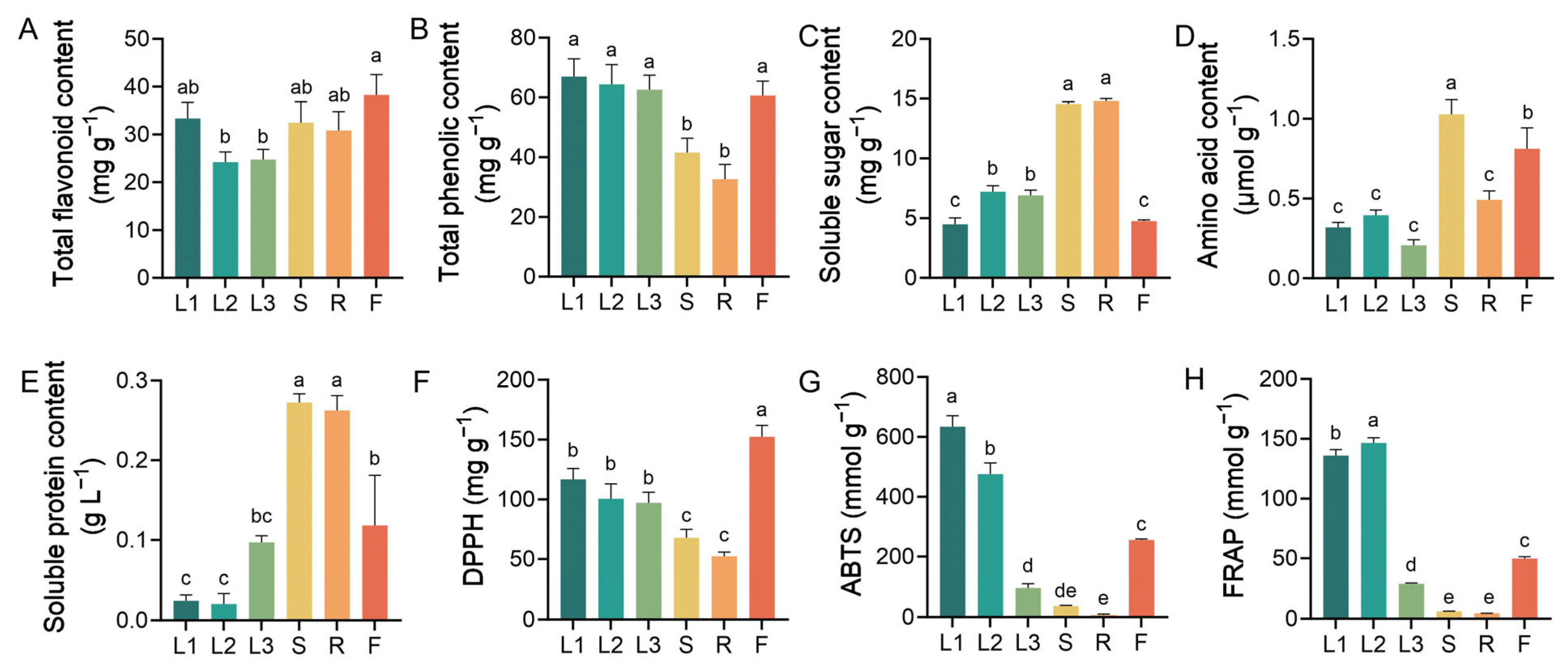
3.1. Biochemical Characteristics of Different A. grossedentata Tissues
3.2. Metabolomic Profiling of Different Tissues of A. grossedentata
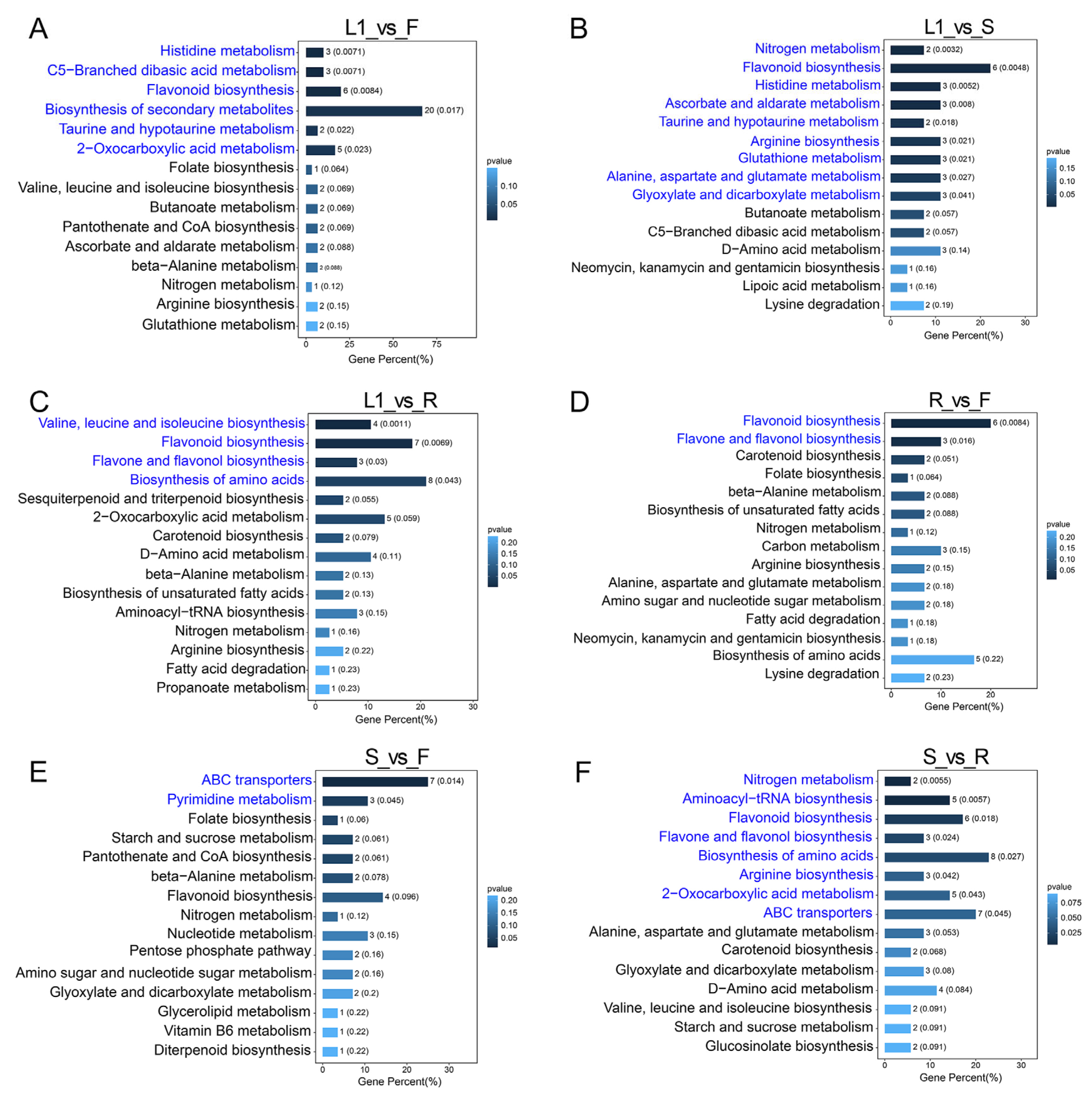
3.3. Identification and KEGG Enrichment Analysis of Differentially Abundant Metabolites
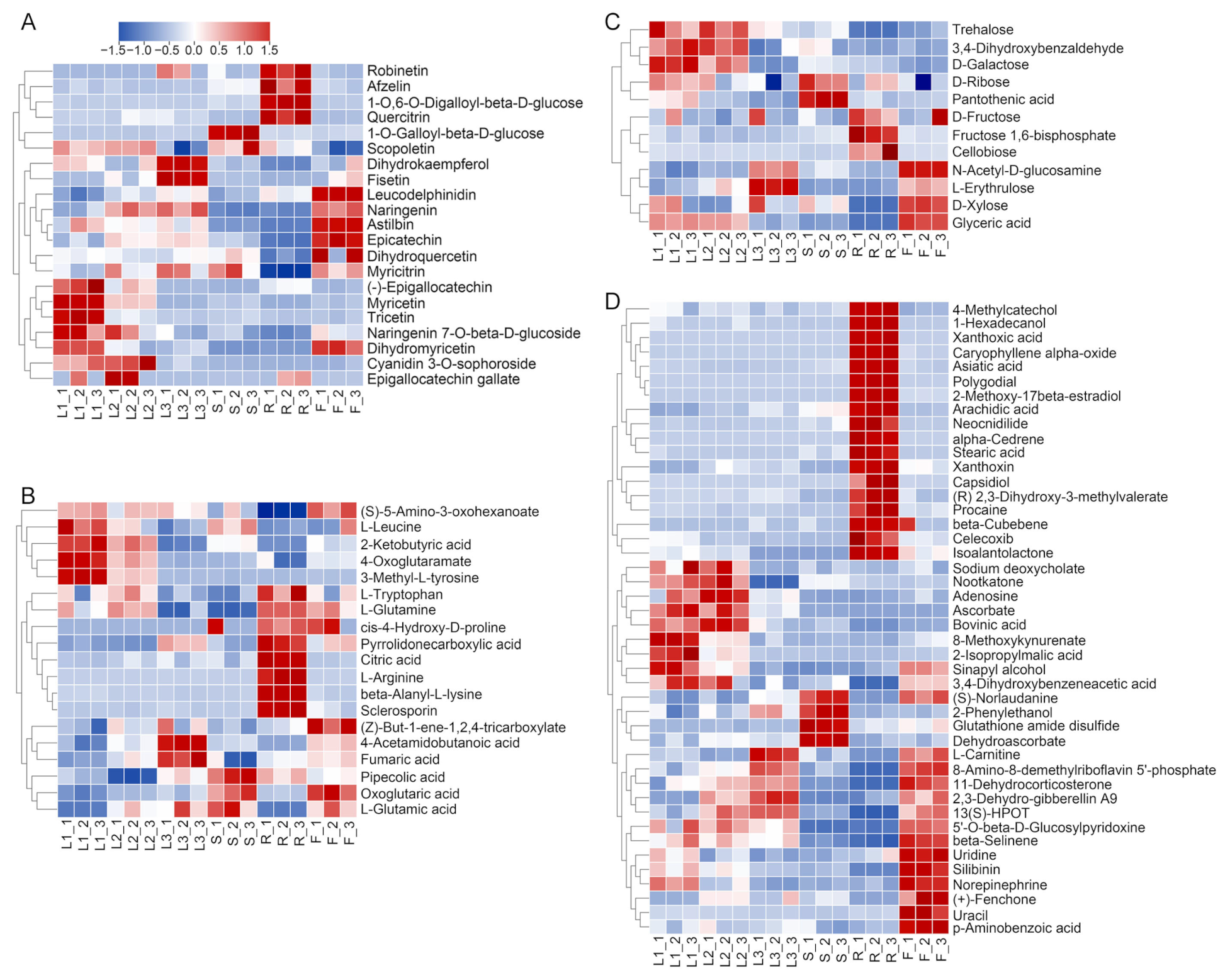
3.4. Clustering Analysis of DAMs
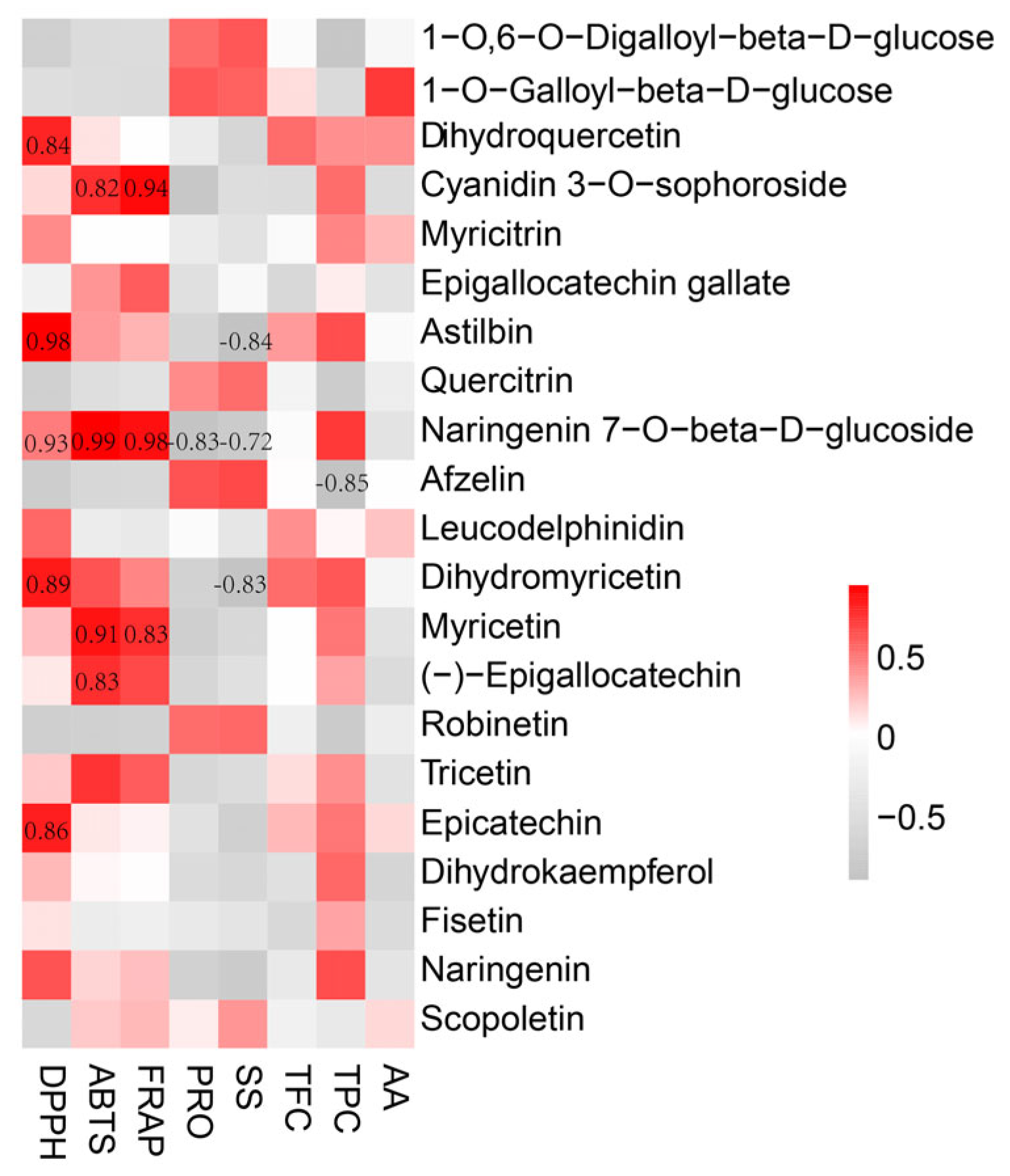
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Tschiggerl, C.; Bucar, F. Guaianolides and volatile compounds in Chamomile tea. Plant Foods Hum. Nutr. 2012, 67, 129–135. [Google Scholar] [CrossRef]
- Wong, L.L.; Liang, Z.; Chen, H.; Zhao, Z. Ingredient authentication of commercial Xihuangcao herbal tea by a microscopic technique combined with UPLC-ESI-QTOF-MS/MS. Anal. Methods 2015, 7, 4257–4268. [Google Scholar] [CrossRef]
- Wenting, W.; Baoping, J.; Sun, L.; Xiong, L.; Xiao, P. Metabolomics reveals that vine tea (Ampelopsis grossedentata) prevents high-fat-diet-induced metabolism disorder by improving glucose homeostasis in rats. PLoS ONE 2017, 12, e182830. [Google Scholar]
- Zeng, T.; Song, Y.; Qi, S.; Zhang, R.; Xu, L.; Xiao, P. A comprehensive review of vine tea: Origin, research on materia medica, phytochemistry and pharmacology. J. Ethnopharmacol. 2023, 317, 116788. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, Y.; Zhang, M.; Zhang, Y.; Ji, H.; Shen, L. Recent advances in research on vine tea, a potential and functional herbal tea with dihydromyricetin and myricetin as major bioactive compounds. J. Pharm. Anal. 2021, 11, 555–563. [Google Scholar] [CrossRef]
- Fan, L.; Qu, X.; Yi, T.; Peng, Y.; Jiang, M.; Miao, J.; Xiao, P. Metabololmics of the protective effect of Ampelopsis grossedentata and its major active compounds. Evid. Based Complement. Altern. Med. 2020, 28, 3472578. [Google Scholar] [CrossRef]
- Carneiro, R.C.V.; Ye, L.; Baek, N.; Teixeira, G.H.A.; O’Keefe, S.F. Vine tea (Ampelopsis grossedentata): A review of chemical composition, functional properties, and potential food applications. J. Funct. Foods 2021, 76, 10417. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Yang, W.L.; Cui, C. Study on the chemical composition of Ampelopsis grossedentata. Chin. Herb. Med. 2003, 5, 21–22. [Google Scholar]
- He, G.X.; Pei, G.; Zhou, T.D.; Zhou, X.X. Determination of total flavonoids and dihydromyricetin in Ampelopsis grossedentata. Chin. J. Tradit. Chin. Med. 2000, 7, 39–41. [Google Scholar]
- Zhang, J.Y.; Chen, Y.; Luo, H.Q. Recent update on the pharmacological effects and mechanisms of dihydromyricetin. Front. Pharmacol. 2018, 9, 1204. [Google Scholar] [CrossRef]
- Wu, R.R.; Li, X.; Cao, Y.H.; Peng, X.; Liu, G.F.; Liu, Z.K.; Yang, Z.; Liu, Z.Y.; Wu, Y. China medicinal plants of the Ampelopsis grossedentata—A review of their botanical characteristics, use, phytochemistry, active pharmacological components, and toxicology. Molecules 2023, 28, 7145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, T.; Wu, S.; Zhang, Z.; Zhang, Y.; Liu, Q.; Guo, Y.; Guan, H.; Wang, D.; Dong, R.; et al. Vine tea (Ampelopsis grossedentata)—A different kind of tea hidden deep in the mountains of China: A comprehensive review of the nutritional profile, functional effect, and diverse applications as a novel raw material in food practices. Trends Food Sci. Technol. 2025, 159, 104939. [Google Scholar] [CrossRef]
- Huang, H.; Yao, Q.; Xia, E.; Gao, L. Metabolomics and transcriptomics analyses reveal nitrogen influences on the accumulation of flavonoids and amino acids in young shoots of tea plant (Camellia sinensis L.) associated with tea flavor. J. Agric. Food Chem. 2018, 66, 9828–9838. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.D.; Qi, D.D.; Yang, T.; Lv, H.P.; Guo, L.; Zhang, Y.; Zhu, Y.; Peng, Q.H.; Xie, D.C.; Tan, J.F.; et al. Nontargeted analysis using ultraperformance liquid chromatography-quadrupole time-of-flight mass spectrometry uncovers the effects of harvest season on the metabolites and taste quality of tea (Camellia sinensis L.). J. Agric. Food Chem. 2015, 63, 9869–9878. [Google Scholar] [CrossRef]
- Li, X.; Cao, M.; Ma, W.; Jia, C.; Li, J.; Zhang, M.; Liu, C.; Cao, Z.; Faruque, M.O.; Hu, X. Annotation of genes involved in high level of dihydromyricetin production in vine tea (Ampelopsis grossedentata) by transcriptome analysis. BMC Plant Biol. 2020, 20, 131. [Google Scholar] [CrossRef]
- Yu, Z.W.; Zhang, N.; Jiang, C.Y.; Wu, S.X.; Feng, X.Y.; Feng, X.Y. Exploring the genes involved in biosynthesis of dihydroquercetin and dihydromyricetin in Ampelopsis grossedentata. Sci. Rep. 2021, 11, 15596. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, S.; Chen, Y.; Xu, S.; Yu, S.; Zhou, J. Identification of hydroxylation enzymes and the metabolic analysis of dihydromyricetin synthesis in Ampelopsis grossedentata. Genes 2022, 13, 2318. [Google Scholar] [CrossRef]
- Wu, F.; Feng, Z.; Yao, Z.; Zhang, P.; Wang, Y.; Li, M. Integrated transcriptomic and targeted metabolomic analyses elucidate the molecular mechanism underlying dihydromyricetin synthesis in Nekemias grossedentata. Plants 2025, 14, 1561. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Zhang, C.; Sun, B.; Zhou, Y.; Zhou, Y.; Zhao, H. Metabolomics approach, in vitro and in vivo antioxidant activity assay provide insights into impact of multiple variations on the vine tea (Ampelopsis grossedentata). LWT Food Sci. Technol. 2023, 177, 114578. [Google Scholar] [CrossRef]
- Carneiro, R.C.V.; Wang, H.; Duncan, S.E.; O’Keefe, S.F. Flavor compounds in Vine Tea (Ampelopsis grossedentata) infusions. Food Sci. Nutr. 2020, 8, 4505–4511. [Google Scholar] [CrossRef]
- Li, W.; Shao, C.; Yan, H.; Zhang, Y.; Lv, H.; Lin, Z.; Zhu, Y. Distribution and taste characteristics of free amino acid enantiomers in green teas and black teas. J. Food Sci. Technol. 2024, 42, 51–67. [Google Scholar]
- Liu, J.; Wu, Y.; Ma, W.; Zhang, H.; Meng, X.; Zhang, H.; Guo, M.; Ling, X.; Li, L. Anti-inflammatory activity of Panax notoginseng flower saponins quantified using LC/MS/MS. Molecules 2023, 28, 2416. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Fan, J.; Liu, Q.; Luo, H.; Tang, Q.; Li, C.; Zhao, J.; Zhang, X. Phytochemical profiles of edible flowers of medicinal plants of Dendrobium officinale and Dendrobium devonianum. Food Sci. Nutr. 2021, 9, 6575–6586. [Google Scholar] [CrossRef] [PubMed]
- Rinschen, M.M.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 2019, 20, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, Y.; Yang, X.; Zhu, G.; Lu, X.; Jia, F.; Diao, B.; Yu, S.; Ali, A.; Zhang, H.; et al. Investigation of flavonoid components and their associated antioxidant capacity in different pigmented rice varieties. Food Res. Int. 2022, 161, 111726. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Li, D.; Huang, D.; Huo, J.; Wu, H.; Sui, X.; Zhang, Y. Non-extractable polyphenols from blue honeysuckle fruit pomace with strong antioxidant capacity: Extraction, characterization, and their antioxidant capacity. Food Res. Int. 2023, 174, 113495. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, L.; Guan, L.; Zhang, L. Metabolome and transcriptome reveal the biosynthesis of flavonoids and amino acids in Isatis indigotica fruit during development. Physiol. Plant. 2024, 176, e14617. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing Mass Spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- Navarro-Reig, M.; Jaumot, J.; García-Reiriz, A.; Tauler, R. Evaluation of changes induced in rice metabolome by Cd and Cu exposure using LC-MS with XCMS and MCR-ALS data analysis strategies. Anal. Bioanal. Chem. 2015, 407, 8835–8847. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Human Serum Metabolome Consortium. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 1999, 27, 29–34. [Google Scholar] [CrossRef]
- Xu, W.; Dong, Q.; Zhao, G.; Han, B. Analysis of metabolites of bactrain camel milk in Alxa of China before and after fermentation with fermenting agent TR1 based on untargeted LC-MS/MS based metabolomics. Heliyon 2023, 9, e18522. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Saeed, F.; Hussain, G.; Imran, A.; Mehmood, Z.; Gondal, T.A.; El-Ghorab, A.; Ahmad, I.; Pezzani, R.; Arshad, M.U.; et al. Myricetin: A comprehensive review on its biological potentials. Food Sci. Nutr. 2021, 9, 5854–5868. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Pan, J.; Ren, D.; Cheng, Y.; Fan, P.; Lou, H. Naringenin-7-O-glucoside protects against doxorubicin-induced toxicity in H9c2 cardiomyocytes by induction of endogenous antioxidant enzymes. Food Chem. Toxicol. 2008, 46, 3140–3146. [Google Scholar] [CrossRef]
- Lu, R.; Wen, Y.; Zhang, J.; Capanoglu, E.; Wang, H. Advances on Resources, Biosynthesis Pathway, Bioavailability, Bioactivity, and Pharmacology of Tricetin. In Handbook of Dietary Flavonoids; Xiao, J., Ed.; Springer: Cham, Switzerland, 2024. [Google Scholar]
- Teng, H.; Fang, T.; Lin, Q.; Song, H.; Liu, B.; Chen, L. Red raspberry and its anthocyanins: Bioactivity beyond antioxidant capacity. Trends Food Sci. Technol. 2017, 66, 153–165. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, M.; Ma, W.; Li, J.; Zhao, S.; Xiong, S.; Hu, X.; Li, X. Evaluation of antioxidant properties of the different tissues of vine tea (Ampelopsis grossedentata) in stripped canola oil and sunflower oil. J. Food. Sci. 2020, 85, 1082–1089. [Google Scholar] [CrossRef]
- Jiang, X.; Chi, J.; Feng, Q.; Wu, H.; Wang, Z.; Dai, L. Isolation and identification of antioxidant constituents from the flowers of Salvia miltiorrhiza. Nat. Prod. Res. 2024, 38, 2653–2657. [Google Scholar] [CrossRef]
- Ding, C.; Zhao, Y.; Chen, X.; Zheng, Y.; Liu, W.; Liu, X. Taxifolin, a novel food, attenuates acute alcohol-induced liver injury in mice through regulating the NF-κB-mediated inflammation and PI3K/Akt signalling pathways. Pharm. Biol. 2021, 59, 868–879. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, D.; Yang, T.; Su, J.; Liu, C.; Su, X.; Li, A.; Sun, P.; Li, J.; Yan, L.; et al. Research progress of dihydroquercetin in the treatment of skin diseases. Molecules 2023, 28, 6989. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, S.; Chauhan, S.; Nair, A.; Sharma, P. Astilbin: A promising unexplored compound with multidimensional medicinal and health benefits. Pharmacol. Res. 2020, 158, 104894. [Google Scholar] [CrossRef]
- Zhu, M.Z.; Li, N.; Zhao, M.; Yu, W.L.; Wu, J.L. Metabolomic profiling delineate taste qualities of tea leaf pubescence. Food Res. Int. 2017, 94, 36–44. [Google Scholar] [CrossRef]
- Stompor-Goracy, M.; Machaczka, M. Recent advances in biological activity, new formulations and prodrugs of ferulic acid. Int. J. Mol. Sci. 2021, 22, 12889. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi-Asl, N.; Garrido, J.; Khazraei, H.; Borges, F.; Firuzi, O. Antioxidant properties of hydroxycinnamic acids: A review of structure-activity relationships. Curr. Med. Chem. 2013, 20, 4436–4450. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yang, Z. Understanding different regulatory mechanisms of proteinaceous and non-proteinaceous amino acid formation in tea (Camellia sinensis) provides new insights into the safe and effective alteration of tea flavor and function. Crit. Rev. Food Sci. Nutr. 2020, 60, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Mehmood, A.; Battino, M.; Xiao, J.; Chen, X. Enrichment of gamma-aminobutyric acid in foods: From conventional methods to innovative technologies. Food Res. Int. 2022, 162, 111801. [Google Scholar] [CrossRef]
- Abdou, A.M.; Higashiguchi, S.; Horie, K.; Kim, M.; Hatta, H.; Yokogoshi, H. Relaxation and immunity enhancement effects of gamma-aminobutyric acid (GABA) administration in humans. Biofactors 2006, 26, 201–208. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Talebi, M.; Matkowski, A. The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech.f. Phytochemistry 2019, 162, 90–98. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Valtat, A.; Abiven, S.; Studer, M.S.; Ong, R.; Schmid, B. The role of soluble sugars during drought in tropical tree seedlings with contrasting tolerances. J. Plant Ecol. 2020, 13, 389–397. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Ye, R.; Yang, J.; Deng, S.; Rong, D.; Luo, Y.; Huang, H. Organ-Specific Metabolome Reveals Potential Nutritional and Health Benefits of Ampelopsis grossedentata. Metabolites 2025, 15, 604. https://doi.org/10.3390/metabo15090604
Li Y, Ye R, Yang J, Deng S, Rong D, Luo Y, Huang H. Organ-Specific Metabolome Reveals Potential Nutritional and Health Benefits of Ampelopsis grossedentata. Metabolites. 2025; 15(9):604. https://doi.org/10.3390/metabo15090604
Chicago/Turabian StyleLi, Yanna, Ran Ye, Ju Yang, Siting Deng, Dongqing Rong, Yinling Luo, and Hui Huang. 2025. "Organ-Specific Metabolome Reveals Potential Nutritional and Health Benefits of Ampelopsis grossedentata" Metabolites 15, no. 9: 604. https://doi.org/10.3390/metabo15090604
APA StyleLi, Y., Ye, R., Yang, J., Deng, S., Rong, D., Luo, Y., & Huang, H. (2025). Organ-Specific Metabolome Reveals Potential Nutritional and Health Benefits of Ampelopsis grossedentata. Metabolites, 15(9), 604. https://doi.org/10.3390/metabo15090604






