Amino Acid Metabolism of the Skin: Control by Specific Enzymes and Contribution to Protective Functions
Abstract
1. Introduction
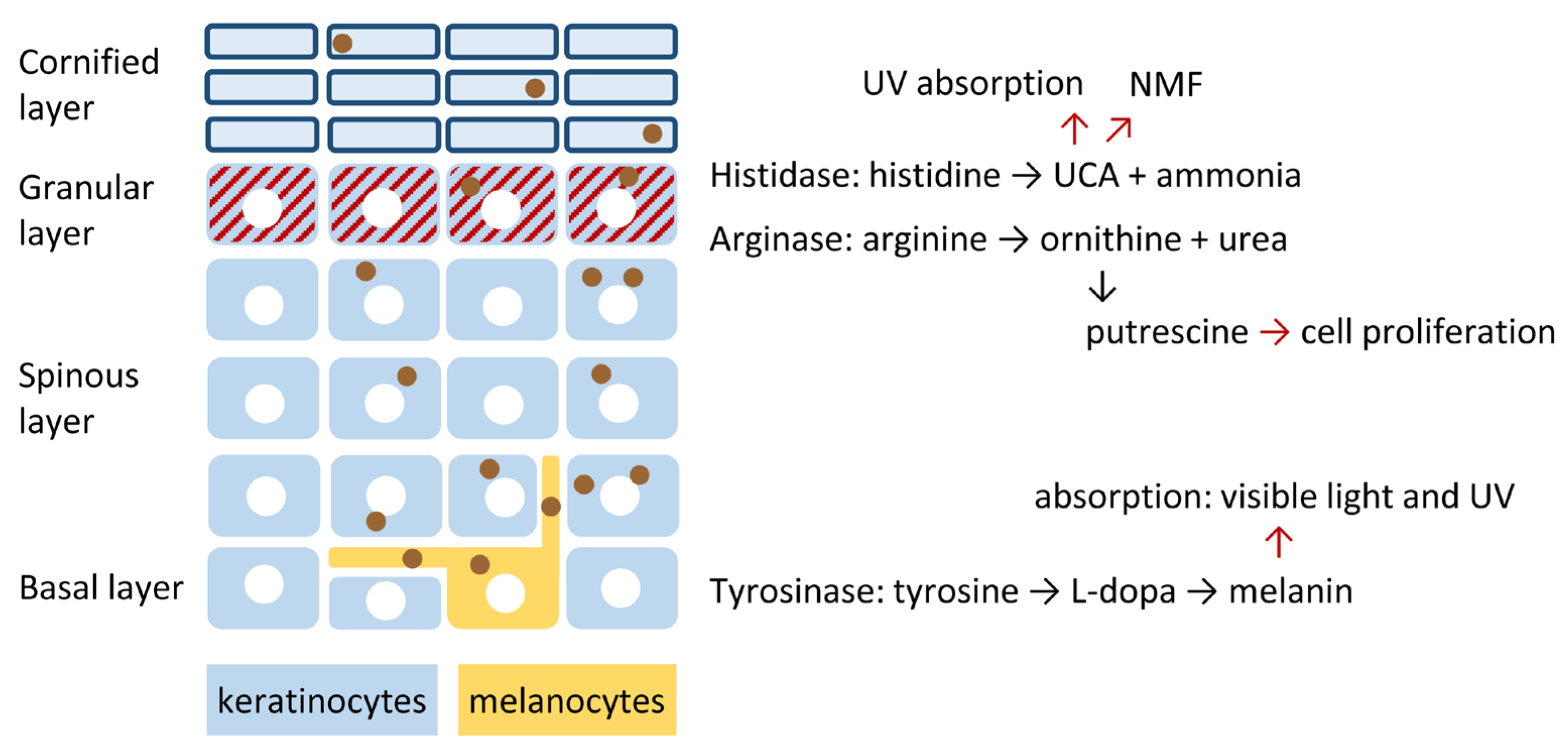
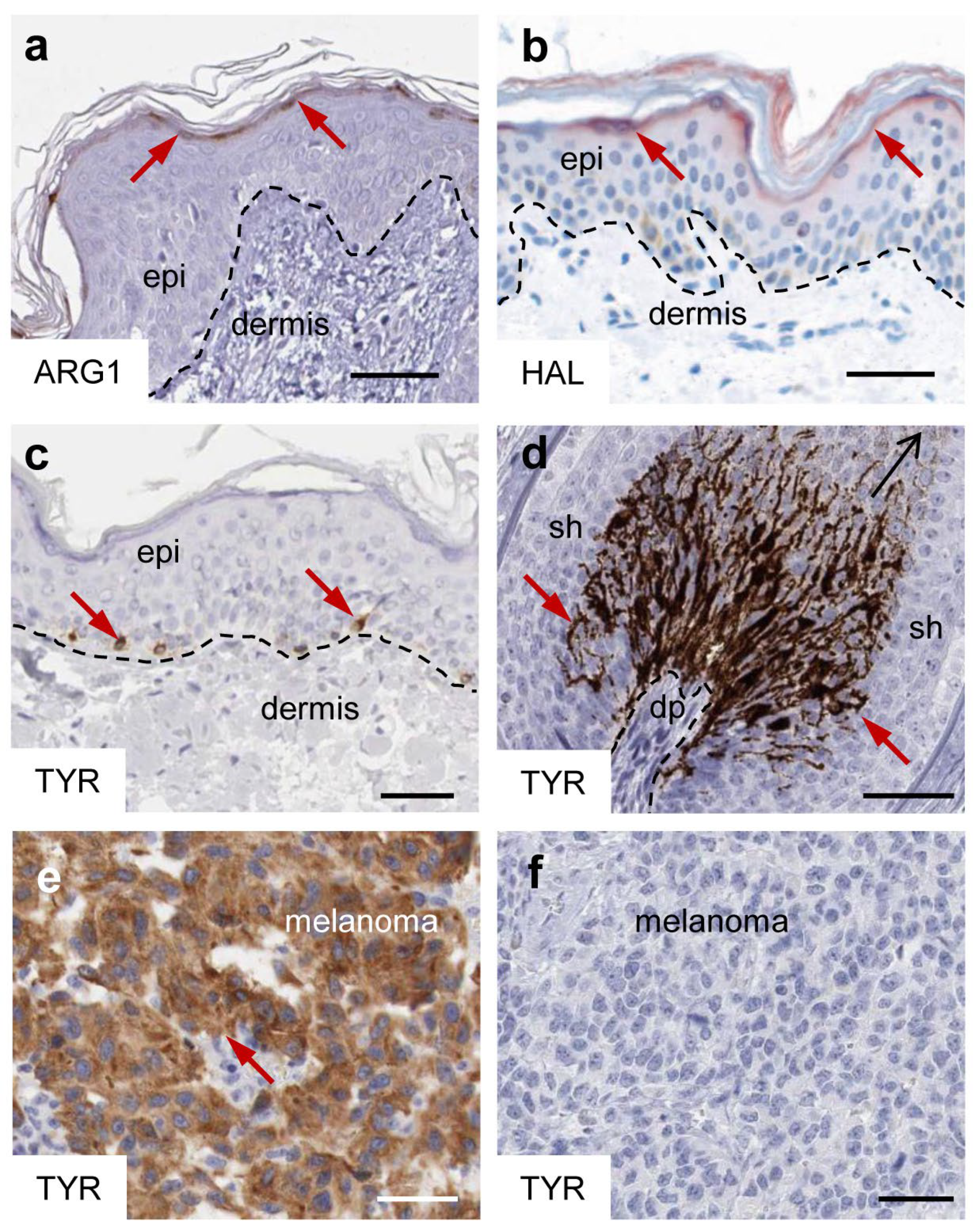
2. Specific Epidermal Expression Patterns of Arginase, Histidase and Tyrosinase Are Hallmarks of the Normal Human Skin
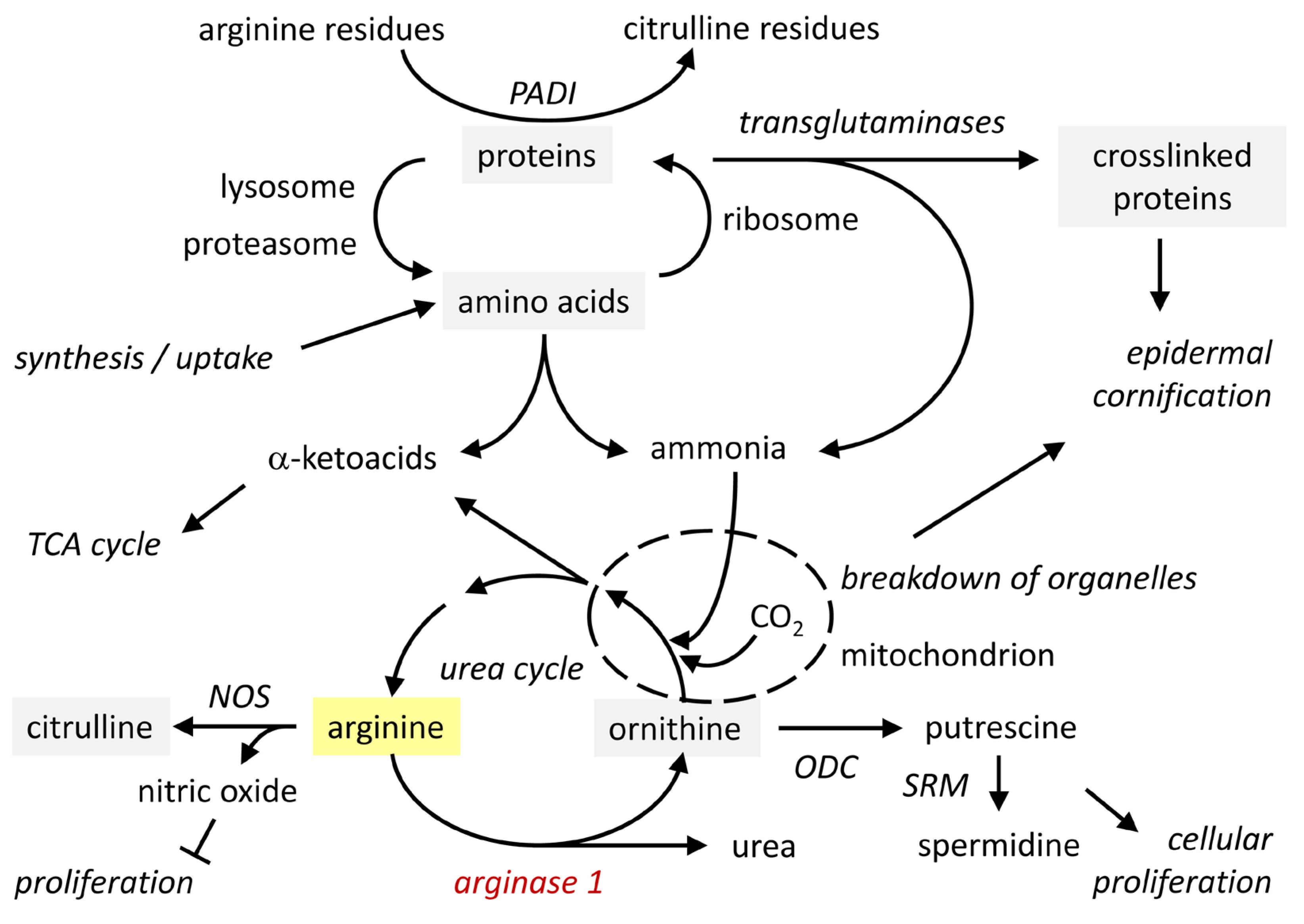
3. Arginase Converts Arginine into Ornithine: Mechanism and Function
3.1. Arginase: Expression, Regulation and Enzymatic Activity in the Skin
3.2. Functions of Arginine and Its Metabolites, Ornithine and Urea
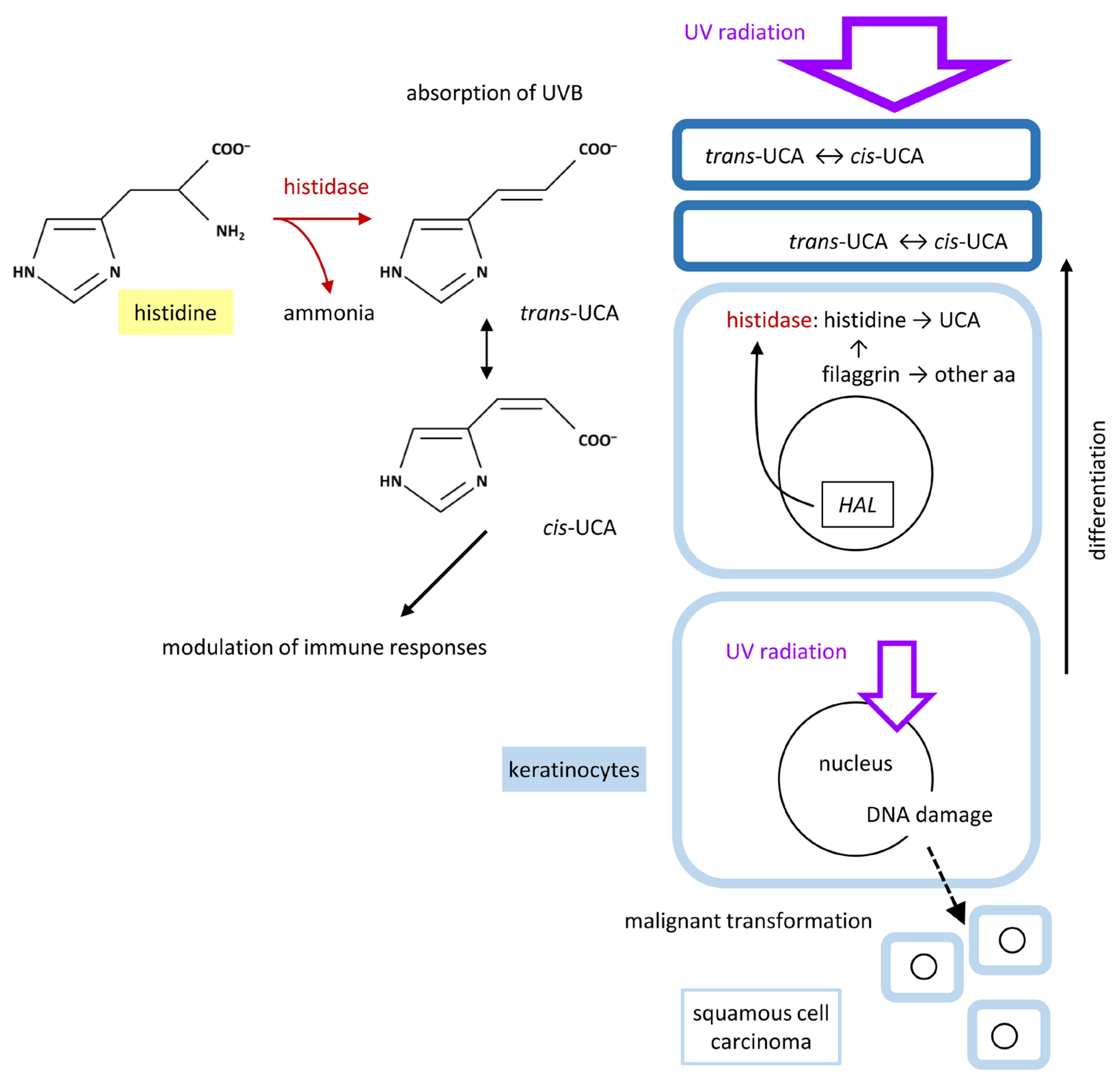
4. Histidase Converts Histidine into Urocanic Acid: Mechanism and Function
4.1. Histidase: Expression, Regulation and Enzymatic Activity in the Skin
4.2. Histidine and UCA
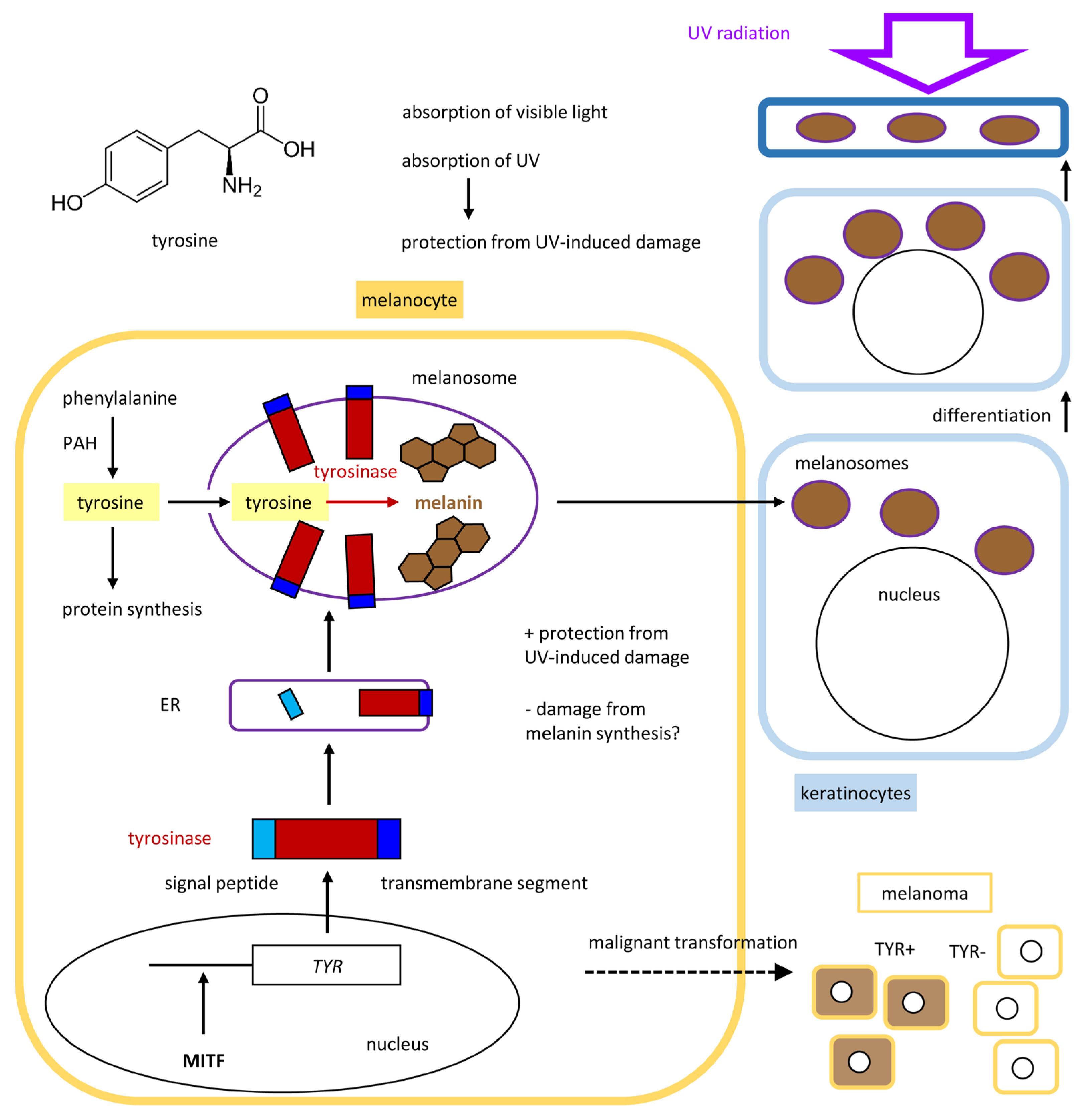
5. Tyrosinase Controls the Polymerization of Tyrosine to Melanin
5.1. Tyrosinase and Related Proteins in the Control of Melanogenesis
5.2. Tyrosine Is the Main Precursor of Melanin
6. Metabolic Reactions of Other Amino Acids in the Skin
6.1. Formation of Free Amino Acids by Hydrolysis of Proteins During Keratinocyte Cornification
6.2. Tryptophan Is Converted to a Ligand of the Aryl Hydrocarbon Receptor
6.3. Glutamine Is Converted to Pyrrolidone Carboxylic Acid, a Component of the NMF
6.4. Serine Regulates Epidermal Cell Proliferation
7. Amino Acid Transporters in the Skin
8. Conclusions
9. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nelson, D.L.; Cox, M.M.; Hoskins, A.A. Lehninger Principles of Biochemistry, 8th ed.; Macmillan Learning: New York, NY, USA, 2021. [Google Scholar]
- Adachi, K.; Lewis, C.; Hershey, F.B. Enzymes of Amino Acid Metabolism in Normal Human Skin. I. Glutamate Dehydrogenase. J. Investig. Dermatol. 1967, 48, 226–229. [Google Scholar] [CrossRef]
- Adachi, K.; Lewis, C.; Hershey, F.B. Enzymes of Amino Acid Metabolism in Normal Human Skin: II. Alanine and Aspartate Transaminases. J. Investig. Dermatol. 1967, 49, 240–245. [Google Scholar] [CrossRef]
- Chandel, N.S. Amino Acid Metabolism. Cold Spring Harb. Perspect. Biol. 2021, 13, a040584. [Google Scholar] [CrossRef]
- Ling, Z.-N.; Jiang, Y.-F.; Ru, J.-N.; Lu, J.-H.; Ding, B.; Wu, J. Amino Acid Metabolism in Health and Disease. Sig Transduct. Target. Ther. 2023, 8, 345. [Google Scholar] [CrossRef]
- Liu, G.Y.; Sabatini, D.M. mTOR at the Nexus of Nutrition, Growth, Ageing and Disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Madison, K.C. Barrier Function of the Skin: “La Raison d’être” of the Epidermis. J. Investig. Dermatol. 2003, 121, 231–241. [Google Scholar] [CrossRef]
- Proksch, E.; Brandner, J.M.; Jensen, J.-M. The Skin: An Indispensable Barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-C.; Fuchs, E. Building and Maintaining the Skin. Cold Spring Harb. Perspect. Biol. 2022, 14, a040840. [Google Scholar] [CrossRef]
- de Szalay, S.; Wertz, P.W. Protective Barriers Provided by the Epidermis. Int. J. Mol. Sci. 2023, 24, 3145. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Murray, P.J.; Pearce, E.J. Metabolic Orchestration of the Wound Healing Response. Cell Metab. 2021, 33, 1726–1743. [Google Scholar] [CrossRef]
- Peña, O.A.; Martin, P. Cellular and Molecular Mechanisms of Skin Wound Healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The Immunological Anatomy of the Skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef]
- Ho, A.W.; Kupper, T.S. T Cells and the Skin: From Protective Immunity to Inflammatory Skin Disorders. Nat. Rev. Immunol. 2019, 19, 490–502. [Google Scholar] [CrossRef]
- Fukuda, K.; Ito, Y.; Amagai, M. Barrier Integrity and Immunity: Exploring the Cutaneous Front Line in Health and Disease. Annu. Rev. Immunol. 2025, 43, 219–252. [Google Scholar] [CrossRef]
- Solano, F. Metabolism and Functions of Amino Acids in the Skin. Adv. Exp. Med. Biol. 2020, 1265, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.D.; Wu, G. Functions and Metabolism of Amino Acids in the Hair and Skin of Dogs and Cats. Adv. Exp. Med. Biol. 2024, 1446, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Karna, E.; Szoka, L.; Huynh, T.Y.L.; Palka, J.A. Proline-Dependent Regulation of Collagen Metabolism. Cell Mol. Life Sci. 2019, 77, 1911–1918. [Google Scholar] [CrossRef]
- Murakami, H.; Shimbo, K.; Inoue, Y.; Takino, Y.; Kobayashi, H. Importance of Amino Acid Composition to Improve Skin Collagen Protein Synthesis Rates in UV-Irradiated Mice. Amino Acids 2012, 42, 2481–2489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Edwards, T.N.; Chaudhri, V.K.; Wu, J.; Cohen, J.A.; Hirai, T.; Rittenhouse, N.; Schmitz, E.G.; Zhou, P.Y.; McNeil, B.D.; et al. Nonpeptidergic Neurons Suppress Mast Cells via Glutamate to Maintain Skin Homeostasis. Cell 2021, 184, 2151–2166. [Google Scholar] [CrossRef]
- Bazzini, C.; Bertschi, N.L.; Steck, O.; Luther, F.; Schärli, S.; Rolfes, E.D.; Vallone, A.; Begré, N.; Nuoffer, J.-M.; Radonijc-Hoesli, S.; et al. Human T Helper 9 Cells Rely on Peroxisome Proliferator-Activated Receptor-γ-Mediated Cystine Uptake to Prevent Lipid Peroxidation and Bioenergetic Failure. J. Investig. Dermatol. 2025, 145, 1693–1705. [Google Scholar] [CrossRef]
- Novak, J.S.S.; Polak, L.; Baksh, S.C.; Barrows, D.W.; Schernthanner, M.; Jackson, B.T.; Thompson, E.A.N.; Gola, A.; Abdusselamoglu, M.D.; Bonny, A.R.; et al. The Integrated Stress Response Fine-Tunes Stem Cell Fate Decisions upon Serine Deprivation and Tissue Injury. Cell Metab. 2025, 37, 1715–1731. [Google Scholar] [CrossRef]
- Bu, T.; Zhang, M.; Lee, S.-H.; Cheong, Y.E.; Park, Y.; Kim, K.H.; Kim, D.; Kim, S. GC-TOF/MS-Based Metabolomics for Comparison of Volar and Non-Volar Skin Types. Metabolites 2022, 12, 717. [Google Scholar] [CrossRef]
- Li, Z.; Ju, Y.; Xia, J.; Zhang, Z.; Zhen, H.; Tong, X.; Sun, Y.; Lu, H.; Zong, Y.; Chen, P.; et al. Integrated Human Skin Bacteria Genome Catalog Reveals Extensive Unexplored Habitat-Specific Microbiome Diversity and Function. Adv. Sci. 2023, 10, e2300050. [Google Scholar] [CrossRef]
- Yu, J.; Luo, Y.; Zhu, Z.; Zhou, Y.; Sun, L.; Gao, J.; Sun, J.; Wang, G.; Yao, X.; Li, W. A Tryptophan Metabolite of the Skin Microbiota Attenuates Inflammation in Patients with Atopic Dermatitis through the Aryl Hydrocarbon Receptor. J. Allergy Clin. Immunol. 2019, 143, 2108–2119. [Google Scholar] [CrossRef]
- Guenin-Macé, L.; Morel, J.-D.; Doisne, J.-M.; Schiavo, A.; Boulet, L.; Mayau, V.; Goncalves, P.; Duchatelet, S.; Hovnanian, A.; Bondet, V.; et al. Dysregulation of Tryptophan Catabolism at the Host-Skin Microbiota Interface in Hidradenitis Suppurativa. JCI Insight 2020, 5, e140598. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, F.; Xiang, B.; Chang, X.; Xia, D.; Wu, Y.; Zhang, Y.; Zhang, M. Abnormal Microbial Amino Acid Metabolism and Activated Pathogenesis in Scalp with Dandruff. J. Investig. Dermatol. 2025, 145, 1823–1826. [Google Scholar] [CrossRef]
- Barresi, C.; Stremnitzer, C.; Mlitz, V.; Kezic, S.; Kammeyer, A.; Ghannadan, M.; Posa-Markaryan, K.; Selden, C.; Tschachler, E.; Eckhart, L. Increased Sensitivity of Histidinemic Mice to UVB Radiation Suggests a Crucial Role of Endogenous Urocanic Acid in Photoprotection. J. Investig. Dermatol. 2011, 131, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Bruch-Gerharz, D.; Schnorr, O.; Suschek, C.; Beck, K.-F.; Pfeilschifter, J.; Ruzicka, T.; Kolb-Bachofen, V. Arginase 1 Overexpression in Psoriasis. Am. J. Pathol. 2003, 162, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Abeyakirthi, S.; Mowbray, M.; Bredenkamp, N.; van Overloop, L.; Declercq, L.; Davis, P.J.; Matsui, M.S.; Weller, R.B. Arginase Is Overactive in Psoriatic Skin. Br. J. Dermatol. 2010, 163, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Lou, F.; Sun, Y.; Xu, Z.; Niu, L.; Wang, Z.; Deng, S.; Liu, Z.; Zhou, H.; Bai, J.; Yin, Q.; et al. Excessive Polyamine Generation in Keratinocytes Promotes Self-RNA Sensing by Dendritic Cells in Psoriasis. Immunity 2020, 53, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Kawai, Y.; Moriyama, A.; Asai, K.; Coleman-Campbell, C.M.; Sumi, S.; Morishita, H.; Suchi, M. Molecular Characterization of Histidinemia: Identification of Four Missense Mutations in the Histidase Gene. Hum. Genet. 2005, 116, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Giebel, L.B.; Musarella, M.A.; Spritz, R.A. A Nonsense Mutation in the Tyrosinase Gene of Afghan Patients with Tyrosinase Negative (Type IA) Oculocutaneous Albinism. J. Med. Genet. 1991, 28, 464–467. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Hearing, V.J. Melanocytes and Their Diseases. Cold Spring Harb. Perspect. Med. 2014, 4, a017046. [Google Scholar] [CrossRef] [PubMed]
- Reinke, S.; Königer, P.; Herberth, G.; Audring, H.; Wang, H.; Ma, J.; Guo, Y.; Sterry, W.; Trefzer, U. Differential Expression of MART-1, Tyrosinase, and SM5-1 in Primary and Metastatic Melanoma. Am. J. Dermatopathol. 2005, 27, 401–406. [Google Scholar] [CrossRef]
- Tsao, H.; Chin, L.; Garraway, L.A.; Fisher, D.E. Melanoma: From Mutations to Medicine. Genes. Dev. 2012, 26, 1131–1155. [Google Scholar] [CrossRef]
- Heuser, S.K.; Li, J.; Pudewell, S.; LoBue, A.; Li, Z.; Cortese-Krott, M.M. Biochemistry, Pharmacology, and in Vivo Function of Arginases. Pharmacol. Rev. 2025, 77, 100015. [Google Scholar] [CrossRef]
- Kämpfer, H.; Pfeilschifter, J.; Frank, S. Expression and Activity of Arginase Isoenzymes During Normal and Diabetes-Impaired Skin Repair. J. Investig. Dermatol. 2003, 121, 1544–1551. [Google Scholar] [CrossRef]
- Campbell, L.; Saville, C.R.; Murray, P.J.; Cruickshank, S.M.; Hardman, M.J. Local Arginase 1 Activity Is Required for Cutaneous Wound Healing. J. Investig. Dermatol. 2013, 133, 2461–2470. [Google Scholar] [CrossRef]
- Crompton, R.A.; Williams, H.; Campbell, L.; Hui Kheng, L.; Saville, C.; Ansell, D.M.; Reid, A.; Wong, J.; Vardy, L.A.; Hardman, M.J.; et al. An Epidermal-Specific Role for Arginase1 during Cutaneous Wound Repair. J. Investig. Dermatol. 2022, 142, 1206–1216. [Google Scholar] [CrossRef]
- Rothberg, S.; Van Scott, E.J. Evaluation of Arginase Activity in Normal Epidermal Tissue and Pathological Stratum Corneum. J. Investig. Dermatol. 1958, 31, 263–268. [Google Scholar] [CrossRef]
- Michels, V.V.; Beaudet, A.L. Arginase Deficiency in Multiple Tissues in Argininemia. Clin. Genet. 1978, 13, 61–67. [Google Scholar] [CrossRef]
- Surbek, M.; Van de Steene, T.; Sachslehner, A.P.; Golabi, B.; Griss, J.; Eyckerman, S.; Gevaert, K.; Eckhart, L. Cornification of Keratinocytes Is Associated with Differential Changes in the Catalytic Activity and the Immunoreactivity of Transglutaminase-1. Sci. Rep. 2023, 13, 21550. [Google Scholar] [CrossRef]
- Grobben, Y.; Uitdehaag, J.C.M.; Willemsen-Seegers, N.; Tabak, W.W.A.; de Man, J.; Buijsman, R.C.; Zaman, G.J.R. Structural In-Sights into Human Arginase-1 pH Dependence and Its Inhibition by the Small Molecule Inhibitor CB-1158. J. Struct. Biol. X 2019, 4, 100014. [Google Scholar] [CrossRef]
- Fukuda, K.; Ito, Y.; Amagai, M. The Acid Mantle Reimagined: Unveiling the Role of Stepwise pH Zonation in the Stratum Corneum. J. Investig. Dermatol. 2025, 145, 2147–2152. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.; Ishizaki, I.; Kubo, A.; Kawasaki, H.; Nagao, K.; Ohashi, Y.; Amagai, M. The Stratum Corneum Comprises Three Layers with Distinct Metal-Ion Barrier Properties. Sci. Rep. 2013, 3, 1731. [Google Scholar] [CrossRef]
- Fukuda, K.; Ito, Y.; Furuichi, Y.; Matsui, T.; Horikawa, H.; Miyano, T.; Okada, T.; van Logtestijn, M.; Tanaka, R.J.; Miyawaki, A.; et al. Three Stepwise pH Progressions in Stratum Corneum for Homeostatic Maintenance of the Skin. Nat. Commun. 2024, 15, 4062. [Google Scholar] [CrossRef] [PubMed]
- Wellner, K.; Wohlrab, W. Quantitative Evaluation of Urea in Stratum Corneum of Human Skin. Arch. Dermatol. Res. 1993, 285, 239–240. [Google Scholar] [CrossRef]
- Mlitz, V.; Latreille, J.; Gardinier, S.; Jdid, R.; Drouault, Y.; Hufnagl, P.; Eckhart, L.; Guinot, C.; Tschachler, E. Impact of Filaggrin Mutations on Raman Spectra and Biophysical Properties of the Stratum Corneum in Mild to Moderate Atopic Dermatitis. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 983–990. [Google Scholar] [CrossRef]
- Méchin, M.-C.; Simon, M. Deimination in Epidermal Barrier and Hair Formation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2023, 378, 20220245. [Google Scholar] [CrossRef] [PubMed]
- Szondi, D.; Crompton, R.A.; Oon, L.; Subramaniam, N.; Tham, K.-C.; Lee, S.H.; Williams, H.; Pennock, J.; Lim, T.C.; Bonnard, C.; et al. A Role for Arginase in Skin Epithelial Differentiation and Anti-Microbial Peptide Production. Br. J. Dermatol. 2025, 193, 125–135. [Google Scholar] [CrossRef]
- Rahim, A.B.; Lim, H.K.; Tan, C.Y.R.; Jia, L.; Leo, V.I.; Uemura, T.; Hardman-Smart, J.; Common, J.E.A.; Lim, T.C.; Bellanger, S.; et al. The Polyamine Regulator AMD1 Upregulates Spermine Levels to Drive Epidermal Differentiation. J. Invest. Dermatol. 2021, 141, 2178–2188. [Google Scholar] [CrossRef] [PubMed]
- Man, A.W.C.; Zhou, Y.; Xia, N.; Li, H. Endothelial Nitric Oxide Synthase in the Perivascular Adipose Tissue. Biomedicines 2022, 10, 1754. [Google Scholar] [CrossRef] [PubMed]
- Rath, M.; Müller, I.; Kropf, P.; Closs, E.I.; Munder, M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front. Immunol. 2014, 5, 532. [Google Scholar] [CrossRef]
- Liu, T.; Xiao, W.; Chen, M.; Mao, R.; San, X.; Peng, Q.; Zhao, Z.; Wang, Q.; Xie, H.; Deng, Z.; et al. Aberrant Amino Acid Metabolism Promotes Neurovascular Reactivity in Rosacea. JCI Insight 2022, 7, e161870. [Google Scholar] [CrossRef]
- Zannoni, V.G.; La Du, B.N. Determination of Histidine α-Deaminase in Human Stratum Corneum and Its Absence in Histidinaemia. Biochem. J. 1963, 88, 160–162. [Google Scholar] [CrossRef]
- Gibbs, N.K.; Tye, J.; Norval, M. Recent Advances in Urocanic Acid Photochemistry, Photobiology and Photoimmunology. Photochem. Photobiol. Sci. 2008, 7, 655–667. [Google Scholar] [CrossRef]
- Taylor, R.G.; García-Heras, J.; Sadler, S.J.; Lafreniere, R.G.; Willard, H.F.; Ledbetter, D.H.; McInnes, R.R. Localization of Histidase to Human Chromosome Region 12q22→Q24.1 and Mouse Chromosome Region 10C2→D1. Cytogenet. Cell Genet. 1991, 56, 178–181. [Google Scholar] [CrossRef]
- Suchi, M.; Sano, H.; Mizuno, H.; Wada, Y. Molecular Cloning and Structural Characterization of the Human Histidase Gene (HAL). Genomics 1995, 29, 98–104. [Google Scholar] [CrossRef]
- Torres, N.; Martínez, L.; Alemán, G.; Bourges, H.; Tovar, A.R. Histidase Expression Is Regulated by Dietary Protein at the Pretranslational Level in Rat Liver. J. Nutr. 1998, 128, 818–824. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eckhart, L.; Schmidt, M.; Mildner, M.; Mlitz, V.; Abtin, A.; Ballaun, C.; Fischer, H.; Mrass, P.; Tschachler, E. Histidase Expression in Human Epidermal Keratinocytes: Regulation by Differentiation Status and All-Trans Retinoic Acid. J. Dermatol. Sci. 2008, 50, 209–215. [Google Scholar] [CrossRef]
- Eckhart, L.; Lachner, J.; Tschachler, E.; Rice, R.H. TINCR Is Not a Non-coding RNA but Encodes a Protein Component of Cornified Epidermal Keratinocytes. Exp. Dermatol. 2020, 29, 376–379. [Google Scholar] [CrossRef]
- Scott, I.R. Factors Controlling the Expressed Activity of Histidine Ammonia-Lyase in the Epidermis and the Resulting Accumulation of Urocanic Acid. Biochem. J. 1981, 194, 829–838. [Google Scholar] [CrossRef]
- Reaven, E.P.; Cox, A.J. Histidine and Keratinization. J. Invest. Dermatol. 1965, 45, 422–431. [Google Scholar] [CrossRef]
- Gilmour, J.W.; Vestey, J.P.; George, S.; Norval, M. Effect of Phototherapy and Urocanic Acid Isomers on Natural Killer Cell Function. J. Investig. Dermatol. 1993, 101, 169–174. [Google Scholar] [CrossRef]
- Reilly, J.T.; Troester, K.A.; Tyner, T.T.; Vitale, D.A.; Risher, T.R. Inhibition of Histidine Ammonia Lyase by 8-Methoxypsoralen and Psoralen-Oxidized Photoproducts. Photochem. Photobiol. 2010, 86, 1272–1277. [Google Scholar] [CrossRef]
- Lam, W.K.; Cleary, M.A.; Wraith, J.E.; Walter, J.H. Histidinaemia: A Benign Metabolic Disorder. Arch. Dis. Child. 1996, 74, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhang, J.; Yu, S.; Luo, Z.; Hua, F.; Yuan, L.; Zhou, Z.; Liu, Q.; Du, X.; Chen, S.; et al. Protective Effect of Sevoflurane Postconditioning against Cardiac Ischemia/Reperfusion Injury via Ameliorating Mitochondrial Impairment, Oxidative Stress and Rescuing Autophagic Clearance. PLoS ONE 2015, 10, e0134666. [Google Scholar] [CrossRef] [PubMed]
- Levy, H.L.; Baden, H.P.; Shih, V.E. A Simple Indirect Method of Detecting the Enzyme Defect in Histidinemia. J. Pediatr. 1969, 75, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Kacser, H.; Bulfield, G.; Wallace, M.E. Histidinaemic Mutant in the Mouse. Nature 1973, 244, 77–79. [Google Scholar] [CrossRef]
- Taylor, R.G.; Grieco, D.; Clarke, G.A.; McInnes, R.R.; Taylor, B.A. Identification of the Mutation in Murine Histidinemia (His) and Genetic Mapping of the Murine Histidase Locus (Hal) on Chromosome 10. Genomics 1993, 16, 231–240. [Google Scholar] [CrossRef]
- Imamura, I.; Watanabe, T.; Hase, Y.; Sakamoto, Y.; Fukuda, Y.; Yamamoto, H.; Tsuruhara, T.; Wada, H. Histamine Metabolism in Patients with Histidinemia: Determination of Urinary Levels of Histamine, N Tau-Methylhistamine, Imidazole Acetic Acid, and Its Conjugate(s). J. Biochem. 1984, 96, 1925–1929. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, M.; Sakura, N. Hyperhistaminemia in Patients with Histidinemia Due to Increased Decarboxylation of Histidine. Clin. Chim. Acta 1989, 186, 11–17. [Google Scholar] [CrossRef]
- Eckhart, L. Urocanic Acid and Skin Photodamage: New Light on an Old Chromophore. In Skin Stress Response Pathways: Environmental Factors and Molecular Opportunities; Wondrak, G.T., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 79–99. [Google Scholar]
- Hug, D.H.; Dunkerson, D.D.; Hunter, J.K. The Degradation of L-Histidine and Trans- and Cis-Urocanic Acid by Bacteria from Skin and the Role of Bacterial Cis-Urocanic Acid Isomerase. J. Photochem. Photobiol. B 1999, 50, 66–73. [Google Scholar] [CrossRef]
- Patra, V.; Trajanoski, S.; Joshi, A.; Lenief, V.; Goyet, C.; Cornu, A.; Golob-Schwarzl, N.; Somlapura, M.; Mosnier, A.; Zarfl, M.; et al. Urocanase-Positive Skin-Resident Bacteria Metabolize Cis-Urocanic Acid and in Turn Reduce the Immunosuppressive Properties of UVR. J. Investig. Dermatol. 2025, S0022202X25004051. [Google Scholar] [CrossRef]
- Safer, D.; Brenes, M.; Dunipace, S.; Schad, G. Urocanic Acid Is a Major Chemoattractant for the Skin-Penetrating Parasitic Nematode Strongyloides Stercoralis. Proc. Natl. Acad. Sci. USA 2007, 104, 1627–1630. [Google Scholar] [CrossRef]
- de Boer, F.L.; van der Molen, H.F.; Wang, J.-H.; Raun, E.; Pereda, J.; Hwu, E.E.-T.; Jakasa, I.; Dubrac, S.; Rustemeyer, T.; Kezic, S. Skin Barrier- and Immune Response-Related Biomarkers of Solar UVR Exposure Comparing Indoor and Outdoor Workers. JID Innov. 2024, 4, 100280. [Google Scholar] [CrossRef] [PubMed]
- Tabachnick, J. Urocanic Acid, the Major Acid-Soluble, Ultraviolet-Absorbing Compound in Guinea Pig Epidermis. Arch. Biochem. Biophys. 1957, 70, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Mildner, M.; Jin, J.; Eckhart, L.; Kezic, S.; Gruber, F.; Barresi, C.; Stremnitzer, C.; Buchberger, M.; Mlitz, V.; Ballaun, C.; et al. Knockdown of Filaggrin Impairs Diffusion Barrier Function and Increases UV Sensitivity in a Human Skin Model. J. Investig. Dermatol. 2010, 130, 2286–2294. [Google Scholar] [CrossRef]
- Denecker, G.; Hoste, E.; Gilbert, B.; Hochepied, T.; Ovaere, P.; Lippens, S.; Van den Broecke, C.; Van Damme, P.; D’Herde, K.; Hachem, J.-P.; et al. Caspase-14 Protects against Epidermal UVB Photodamage and Water Loss. Nat. Cell Biol. 2007, 9, 666–674. [Google Scholar] [CrossRef]
- Sarin, K.Y.; Lin, Y.; Daneshjou, R.; Ziyatdinov, A.; Thorleifsson, G.; Rubin, A.; Pardo, L.M.; Wu, W.; Khavari, P.A.; Uitterlinden, A.; et al. Genome-Wide Meta-Analysis Identifies Eight New Susceptibility Loci for Cutaneous Squamous Cell Carcinoma. Nat. Commun. 2020, 11, 820. [Google Scholar] [CrossRef] [PubMed]
- Revez, J.A.; Lin, T.; Qiao, Z.; Xue, A.; Holtz, Y.; Zhu, Z.; Zeng, J.; Wang, H.; Sidorenko, J.; Kemper, K.E.; et al. Genome-Wide Association Study Identifies 143 Loci Associated with 25 Hydroxyvitamin D Concentration. Nat. Commun. 2020, 11, 1647. [Google Scholar] [CrossRef]
- Backman, J.D.; Li, A.H.; Marcketta, A.; Sun, D.; Mbatchou, J.; Kessler, M.D.; Benner, C.; Liu, D.; Locke, A.E.; Balasubramanian, S.; et al. Exome Sequencing and Analysis of 454,787 UK Biobank Participants. Nature 2021, 599, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Kezic, S.; Kemperman, P.M.J.H.; Koster, E.S.; de Jongh, C.M.; Thio, H.B.; Campbell, L.E.; Irvine, A.D.; McLean, I.W.H.; Puppels, G.J.; Caspers, P.J. Loss-of-Function Mutations in the Filaggrin Gene Lead to Reduced Level of Natural Moisturizing Factor in the Stratum Corneum. J. Investig. Dermatol. 2008, 128, 2117–2119. [Google Scholar] [CrossRef] [PubMed]
- Thyssen, J.P.; Thuesen, B.; Huth, C.; Standl, M.; Carson, C.G.; Heinrich, J.; Krämer, U.; Kratzsch, J.; Berg, N.D.; Menné, T.; et al. Skin Barrier Abnormality Caused by Filaggrin (FLG) Mutations Is Associated with Increased Serum 25-Hydroxyvitamin D Concentrations. J. Allergy Clin. Immunol. 2012, 130, 1204–1207. [Google Scholar] [CrossRef]
- Schwarz, T.; Schwarz, A. Molecular Mechanisms of Ultraviolet Radiation-Induced Immunosuppression. Eur. J. Cell Biol. 2011, 90, 560–564. [Google Scholar] [CrossRef]
- Yu, Z.; Wolf, P. How It Works: The Immunology Underlying Phototherapy. Dermatol. Clin. 2020, 38, 37–53. [Google Scholar] [CrossRef]
- Noonan, F.P.; De Fabo, E.C. Immunosuppression by Ultraviolet B Radiation: Initiation by Urocanic Acid. Immunol. Today 1992, 13, 250–254. [Google Scholar] [CrossRef]
- Norval, M.; Gibbs, N.K.; Gilmour, J. The Role of Urocanic Acid in Uv-Induced Immunosuppression: Recent Advances (1992–1994). Photochem. Photobiol. 1995, 62, 209–217. [Google Scholar] [CrossRef]
- el-Ghorr, A.A.; Norval, M. A Monoclonal Antibody to Cis-Urocanic Acid Prevents the Ultraviolet-Induced Changes in Langerhans Cells and Delayed Hypersensitivity Responses in Mice, Although Not Preventing Dendritic Cell Accumulation in Lymph Nodes Draining the Site of Irradiation and Contact Hypersensitivity Responses. J. Investig. Dermatol. 1995, 105, 264–268. [Google Scholar] [CrossRef]
- Leitch, C.S.; Natafji, E.; Yu, C.; Abdul-Ghaffar, S.; Madarasingha, N.; Venables, Z.C.; Chu, R.; Fitch, P.M.; Muinonen-Martin, A.J.; Campbell, L.E.; et al. Filaggrin-Null Mutations Are Associated with Increased Maturation Markers on Langerhans Cells. J. Allergy Clin. Immunol. 2016, 138, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Bruhs, A.; Eckhart, L.; Tschachler, E.; Schwarz, T.; Schwarz, A. Urocanic Acid: An Endogenous Regulator of Langerhans Cells. J. Investig. Dermatol. 2016, 136, 1735–1737. [Google Scholar] [CrossRef] [PubMed]
- Bentley, N.J.; Eisen, T.; Goding, C.R. Melanocyte-Specific Expression of the Human Tyrosinase Promoter: Activation by the Microphthalmia Gene Product and Role of the Initiator. Mol. Cell Biol. 1994, 14, 7996–8006. [Google Scholar] [CrossRef]
- Yasumoto, K.; Yokoyama, K.; Takahashi, K.; Tomita, Y.; Shibahara, S. Functional Analysis of Microphthalmia-Associated Transcription Factor in Pigment Cell-Specific Transcription of the Human Tyrosinase Family Genes. J. Biol. Chem. 1997, 272, 503–509. [Google Scholar] [CrossRef]
- Bertolotto, C.; Abbe, P.; Hemesath, T.J.; Bille, K.; Fisher, D.E.; Ortonne, J.-P.; Ballotti, R. Microphthalmia Gene Product as a Signal Transducer in cAMP-Induced Differentiation of Melanocytes. J. Cell Biol. 1998, 142, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Marks, M.S. Melanosomes—Dark Organelles Enlighten Endosomal Membrane Transport. Nat. Rev. Mol. Cell Biol. 2007, 8, 786–797. [Google Scholar] [CrossRef]
- Bento-Lopes, L.; Cabaço, L.C.; Charneca, J.; Neto, M.V.; Seabra, M.C.; Barral, D.C. Melanin’s Journey from Melanocytes to Keratinocytes: Uncovering the Molecular Mechanisms of Melanin Transfer and Processing. Int. J. Mol. Sci. 2023, 24, 11289. [Google Scholar] [CrossRef]
- Ito, S. A Chemist’s View of Melanogenesis. Pigment. Cell Res. 2003, 16, 230–236. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. Chemistry of Mixed Melanogenesis—Pivotal Roles of Dopaquinone. Photochem. Photobiol. 2008, 84, 582–592. [Google Scholar] [CrossRef]
- Snyman, M.; Walsdorf, R.E.; Wix, S.N.; Gill, J.G. The Metabolism of Melanin Synthesis—From Melanocytes to Melanoma. Pigment. Cell Melanoma Res. 2024, 37, 438–452. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Wazir, U.; Kothari, S.; Gibbons, N.C.J.; Moore, J.; Wood, J.M. Human Phenylalanine Hydroxylase Is Activated by H2O2: A Novel Mechanism for Increasing the l-Tyrosine Supply for Melanogenesis in Melanocytes. Biochem. Biophys. Res. Commun. 2004, 322, 88–92. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Nagao, A.; Watanabe, M.; Nakao, K.; Ito, S. Pheomelanogenesis Is Promoted at a Weakly Acidic pH. Pigment. Cell Melanoma Res. 2017, 30, 372–377. [Google Scholar] [CrossRef]
- Ito, S.; Palumbo, A.; Prota, G. Tyrosinase-Catalyzed Conjugation of Dopa with Glutathione. Experientia 1985, 41, 960–961. [Google Scholar] [CrossRef]
- Walker, G.J.; Khosrotehrani, K. Assessment of the Influence of UVR in Cutaneous Melanoma. Photodermatol. Photoimmunol. Photomed. 2025, 41, e70024. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Luo, X.; Morgan, A.; Wang, J.; Hoang, M.P.; Lo, J.; Guerrero, C.R.; Lennerz, J.K.; Mihm, M.C.; Wargo, J.A.; et al. An Ultraviolet-Radiation-Independent Pathway to Melanoma Carcinogenesis in the Red Hair/Fair Skin Background. Nature 2012, 491, 449–453. [Google Scholar] [CrossRef]
- Morgan, A.M.; Lo, J.; Fisher, D.E. How Does Pheomelanin Synthesis Contribute to Melanomagenesis?: Two Distinct Mechanisms Could Explain the Carcinogenicity of Pheomelanin Synthesis. Bioessays 2013, 35, 672–676. [Google Scholar] [CrossRef]
- Eckhart, L.; Gruber, F.; Sukseree, S. Autophagy-Mediated Cellular Remodeling during Terminal Differentiation of Keratinocytes in the Epidermis and Skin Appendages. Cells 2024, 13, 1675. [Google Scholar] [CrossRef]
- Stamatas, G.N. Protein Degradation in the Stratum Corneum. Int. J. Cosmet. Sci. 2024, 46, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Adelmann, C.H.; Traunbauer, A.K.; Chen, B.; Condon, K.J.; Chan, S.H.; Kunchok, T.; Lewis, C.A.; Sabatini, D.M. MFSD12 Mediates the Import of Cysteine into Melanosomes and Lysosomes. Nature 2020, 588, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Kezic, S.; Kammeyer, A.; Calkoen, F.; Fluhr, J.W.; Bos, J.D. Natural Moisturizing Factor Components in the Stratum Corneum as Biomarkers of Filaggrin Genotype: Evaluation of Minimally Invasive Methods. Br. J. Dermatol. 2009, 161, 1098–1104. [Google Scholar] [CrossRef]
- Schmuth, M.; Eckmann, S.; Moosbrugger-Martinz, V.; Ortner-Tobider, D.; Blunder, S.; Trafoier, T.; Gruber, R.; Elias, P.M. Skin Barrier in Atopic Dermatitis. J. Investig. Dermatol. 2024, 144, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Thyssen, J.P.; Jakasa, I.; Riethmüller, C.; Schön, M.P.; Braun, A.; Haftek, M.; Fallon, P.G.; Wróblewski, J.; Jakubowski, H.; Eckhart, L.; et al. Filaggrin Expression and Processing Deficiencies Impair Corneocyte Surface Texture and Stiffness in Mice. J. Investig. Dermatol. 2020, 140, 615–623. [Google Scholar] [CrossRef]
- Palmer, C.N.A.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.D.; et al. Common Loss-of-Function Variants of the Epidermal Barrier Protein Filaggrin Are a Major Predisposing Factor for Atopic Dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Rannug, A.; Rannug, U.; Rosenkranz, H.S.; Winqvist, L.; Westerholm, R.; Agurell, E.; Grafström, A.K. Certain Photooxidized Derivatives of Tryptophan Bind with Very High Affinity to the Ah Receptor and Are Likely to Be Endogenous Signal Substances. J. Biol. Chem. 1987, 262, 15422–15427. [Google Scholar] [CrossRef] [PubMed]
- Wincent, E.; Amini, N.; Luecke, S.; Glatt, H.; Bergman, J.; Crescenzi, C.; Rannug, A.; Rannug, U. The Suggested Physiologic Aryl Hydrocarbon Receptor Activator and Cytochrome P4501 Substrate 6-Formylindolo [3,2-b]Carbazole Is Present in Humans. J. Biol. Chem. 2009, 284, 2690–2696. [Google Scholar] [CrossRef]
- Dawe, H.R.; Di Meglio, P. The Aryl Hydrocarbon Receptor (AHR): Peacekeeper of the Skin. Int. J. Mol. Sci. 2025, 26, 1618. [Google Scholar] [CrossRef]
- Park, S.L.; Justiniano, R.; Williams, J.D.; Cabello, C.M.; Qiao, S.; Wondrak, G.T. The Tryptophan-Derived Endogenous Aryl Hydrocarbon Receptor Ligand 6-Formylindolo [3,2-b]Carbazole Is a Nanomolar UVA Photosensitizer in Epidermal Keratinocytes. J. Investig. Dermatol. 2015, 135, 1649–1658. [Google Scholar] [CrossRef]
- Justiniano, R.; de Faria Lopes, L.; Perer, J.; Hua, A.; Park, S.L.; Jandova, J.; Baptista, M.S.; Wondrak, G.T. The Endogenous Tryptophan-Derived Photoproduct 6-Formylindolo [3,2-b]Carbazole (FICZ) Is a Nanomolar Photosensitizer That Can Be Harnessed for the Photodynamic Elimination of Skin Cancer Cells in Vitro and in Vivo. Photochem. Photobiol. 2021, 97, 180–191. [Google Scholar] [CrossRef]
- Xue, C.; Li, G.; Zheng, Q.; Gu, X.; Shi, Q.; Su, Y.; Chu, Q.; Yuan, X.; Bao, Z.; Lu, J.; et al. Tryptophan Metabolism in Health and Disease. Cell Metab. 2023, 35, 1304–1326. [Google Scholar] [CrossRef]
- Walczak, K.; Langner, E.; Makuch-Kocka, A.; Szelest, M.; Szalast, K.; Marciniak, S.; Plech, T. Effect of Tryptophan-Derived AhR Ligands, Kynurenine, Kynurenic Acid and FICZ, on Proliferation, Cell Cycle Regulation and Cell Death of Melanoma Cells-In Vitro Studies. Int. J. Mol. Sci. 2020, 21, 7946. [Google Scholar] [CrossRef]
- Steinert, P.M.; Marekov, L.N. Direct Evidence That Involucrin Is a Major Early Isopeptide Cross-Linked Component of the Keratinocyte Cornified Cell Envelope. J. Biol. Chem. 1997, 272, 2021–2030. [Google Scholar] [CrossRef]
- Barrett, J.G.; Scott, I.R. Pyrrolidone Carboxylic Acid Synthesis in Guinea Pig Epidermis. J. Investig. Dermatol. 1983, 81, 122–124. [Google Scholar] [CrossRef]
- Cheng, J.B.; Sedgewick, A.J.; Finnegan, A.I.; Harirchian, P.; Lee, J.; Kwon, S.; Fassett, M.S.; Golovato, J.; Gray, M.; Ghadially, R.; et al. Transcriptional Programming of Normal and Inflamed Human Epidermis at Single-Cell Resolution. Cell Rep. 2018, 25, 871–883. [Google Scholar] [CrossRef]
- Cappello, A.; Mancini, M.; Madonna, S.; Rinaldo, S.; Paone, A.; Scarponi, C.; Belardo, A.; Zolla, L.; Zuccotti, A.; Panatta, E.; et al. Extracellular Serine Empowers Epidermal Proliferation and Psoriasis-like Symptoms. Sci. Adv. 2022, 8, eabm7902. [Google Scholar] [CrossRef] [PubMed]
- Labuschagne, C.F.; van den Broek, N.J.F.; Mackay, G.M.; Vousden, K.H.; Maddocks, O.D.K. Serine, but Not Glycine, Supports One-Carbon Metabolism and Proliferation of Cancer Cells. Cell Rep. 2014, 7, 1248–1258. [Google Scholar] [CrossRef]
- Amelio, I.; Cutruzzolá, F.; Antonov, A.; Agostini, M.; Melino, G. Serine and Glycine Metabolism in Cancer. Trends Biochem. Sci. 2014, 39, 191–198. [Google Scholar] [CrossRef]
- Li, A.M.; Ye, J. Reprogramming of Serine, Glycine and One-Carbon Metabolism in Cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165841. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhong, B.; Qin, X.; Xu, H.; Li, Z.; Li, L.; Wang, X.; Zhang, W.; Lou, Z.; Fan, Y.; et al. An Epidermal Serine Sensing System for Skin Healthcare. Nat. Commun. 2025, 16, 2681. [Google Scholar] [CrossRef]
- Yoshihisa, Y.; Rehman, M.U.; Nakagawa, M.; Matsukuma, S.; Makino, T.; Mori, H.; Shimizu, T. Inflammatory Cytokine-mediated Induction of Serine Racemase in Atopic Dermatitis. J. Cell Mol. Med. 2018, 22, 3133–3138. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Zhong, D.; Li, Q.; Yu, Y.; Zhang, S.; Liang, J.; Zhang, X. LC-MS Metabolomics Reveal Skin Metabolic Signature of Psoriasis Vulgaris. Exp. Dermatol. 2023, 32, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Kim, H.; Hong, S.-C.; Yoo, S.-Y.; Shin, D.; Lee, C.-L.; Na, S.-J.; Kim, Y.H.; Jo, K.; Yun, G.; et al. Moisturizing Effect of Serine-Loaded Solid Lipid Nanoparticles and Polysaccharide-Rich Extract of Root Phragmites Communis Incorporated in Hydrogel Bases. Arch. Pharm. Res. 2017, 40, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, P.; Gyimesi, G.; Kanai, Y.; Hediger, M.A. Amino Acid Transporters Revisited: New Views in Health and Disease. Trends Biochem. Sci. 2018, 43, 752–789. [Google Scholar] [CrossRef]
- Hushmandi, K.; Einollahi, B.; Saadat, S.H.; Lee, E.H.C.; Farani, M.R.; Okina, E.; Huh, Y.S.; Nabavi, N.; Salimimoghadam, S.; Kumar, A.P. Amino Acid Transporters within the Solute Carrier Superfamily: Underappreciated Proteins and Novel Opportunities for Cancer Therapy. Mol. Metab. 2024, 84, 101952. [Google Scholar] [CrossRef]
- Wiedmer, T.; Teoh, S.T.; Christodoulaki, E.; Wolf, G.; Tian, C.; Sedlyarov, V.; Jarret, A.; Leippe, P.; Frommelt, F.; Ingles-Prieto, A.; et al. Metabolic Mapping of the Human Solute Carrier Superfamily. Mol. Syst. Biol. 2025, 21, 560–598. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, C.; Chen, X.; Li, J. The Role of Solute Carriers in the Metabolic Reprogramming of Skin Diseases. Clinic Rev. Allerg. Immunol. 2025, 68, 80. [Google Scholar] [CrossRef]
- Cibrian, D.; De La Fuente, H.; Sánchez-Madrid, F. Metabolic Pathways That Control Skin Homeostasis and Inflammation. Trends Mol. Med. 2020, 26, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Cibrian, D.; Castillo-González, R.; Fernández-Gallego, N.; de la Fuente, H.; Jorge, I.; Saiz, M.L.; Punzón, C.; Ramírez-Huesca, M.; Vicente-Manzanares, M.; Fresno, M.; et al. Targeting L-Type Amino Acid Transporter 1 in Innate and Adaptive T Cells Efficiently Controls Skin Inflammation. J. Allergy Clin. Immunol. 2020, 145, 199–214. [Google Scholar] [CrossRef]
- Tina, E.; Prosén, S.; Lennholm, S.; Gasparyan, G.; Lindberg, M.; Göthlin Eremo, A. Expression Profile of the Amino Acid Transporters SLC7A5, SLC7A7, SLC7A8 and the Enzyme TDO2 in Basal Cell Carcinoma. Br. J. Dermatol. 2019, 180, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Kaira, K.; Kato, M.; Yasuda, M.; Takahashi, A.; Tominaga, H.; Oriuchi, N.; Nagamori, S.; Kanai, Y.; Oyama, T.; et al. Prognostic Significance of L-Type Amino Acid Transporter 1 (LAT1) Expression in Cutaneous Melanoma. Melanoma Res. 2015, 25, 399–405. [Google Scholar] [CrossRef]
- Bauwens, E.; Parée, T.; Meurant, S.; Bouriez, I.; Hannart, C.; Wéra, A.-C.; Khelfi, A.; Fattaccioli, A.; Burteau, S.; Demazy, C.; et al. Senescence Induced by UVB in Keratinocytes Impairs Amino Acids Balance. J. Investig. Dermatol. 2023, 143, 554–565. [Google Scholar] [CrossRef]
- Luo, L.; Zeng, H.; Hu, Y.; Jiang, L.; Fu, C.; Huang, J.; Chen, J.; Zeng, Q. The Amino Acid Transporter SLC16A10 Promotes Melanogenesis by Facilitating the Transportation of Phenylalanine. Exp. Dermatol. 2024, 33, e15165. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, J.; Yin, H.; Wang, X.; Li, Q.; Li, H.; Wu, J.; Lu, Q. Solute Carrier Family 15 Member 4, an Emerging Therapeutic Target for Systemic Lupus Erythematosus. Int. Rev. Immunol. 2025, 44, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, D.; Palumbo, S.; Antonini, D.; D’Auria, L.; Cerbone, V.; Porcelli, T.; Cavallo, F.; Calautti, E.; Riganti, C.; Missero, C. Ankyloblepharon-Ectodermal Defects-Cleft Lip/Palate Syndrome-Linked P63 Mutations Disrupt Keratinocyte Proliferation and Survival Through Oxidative Stress and Impaired Slc7a11 Expression. Int. J. Mol. Sci. 2025, 26, 5231. [Google Scholar] [CrossRef]
- Zhou, D.; Ota, K.; Nardin, C.; Feldman, M.; Widman, A.; Wind, O.; Simon, A.; Reilly, M.; Levin, L.R.; Buck, J.; et al. Mammalian Pigmentation Is Regulated by a Distinct cAMP-Dependent Mechanism That Controls Melanosome pH. Sci. Signal 2018, 11, eaau7987. [Google Scholar] [CrossRef]
- Liu, Y.; Chi, W.; Tao, L.; Wang, G.; Deepak, R.N.V.K.; Sheng, L.; Chen, T.; Feng, Y.; Cao, X.; Cheng, L.; et al. Ablation of Proton/Glucose Exporter SLC45A2 Enhances Melanosomal Glycolysis to Inhibit Melanin Biosynthesis and Promote Melanoma Metastasis. J. Investig. Dermatol. 2022, 142, 2744–2755. [Google Scholar] [CrossRef]
- Newton, J.M.; Cohen-Barak, O.; Hagiwara, N.; Gardner, J.M.; Davisson, M.T.; King, R.A.; Brilliant, M.H. Mutations in the Human Orthologue of the Mouse Underwhite Gene (Uw) Underlie a New Form of Oculocutaneous Albinism, OCA4. Am. J. Hum. Genet. 2001, 69, 981–988. [Google Scholar] [CrossRef]
- Bertolotti, A.; Lasseaux, E.; Plaisant, C.; Trimouille, A.; Morice-Picard, F.; Rooryck, C.; Lacombe, D.; Couppie, P.; Arveiler, B. Identification of a Homozygous Mutation of SLC24A5 (OCA6) in Two Patients with Oculocutaneous Albinism from French Guiana. Pigment. Cell Melanoma Res. 2016, 29, 104–106. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dörner, C.; Steinbinder, J.; Sachslehner, A.P.; Sukseree, S.; Eckhart, L. Amino Acid Metabolism of the Skin: Control by Specific Enzymes and Contribution to Protective Functions. Metabolites 2025, 15, 601. https://doi.org/10.3390/metabo15090601
Dörner C, Steinbinder J, Sachslehner AP, Sukseree S, Eckhart L. Amino Acid Metabolism of the Skin: Control by Specific Enzymes and Contribution to Protective Functions. Metabolites. 2025; 15(9):601. https://doi.org/10.3390/metabo15090601
Chicago/Turabian StyleDörner, Corina, Julia Steinbinder, Attila Placido Sachslehner, Supawadee Sukseree, and Leopold Eckhart. 2025. "Amino Acid Metabolism of the Skin: Control by Specific Enzymes and Contribution to Protective Functions" Metabolites 15, no. 9: 601. https://doi.org/10.3390/metabo15090601
APA StyleDörner, C., Steinbinder, J., Sachslehner, A. P., Sukseree, S., & Eckhart, L. (2025). Amino Acid Metabolism of the Skin: Control by Specific Enzymes and Contribution to Protective Functions. Metabolites, 15(9), 601. https://doi.org/10.3390/metabo15090601





