A Holistic Approach to Metabolic Health Assessment—Analysis of Bioimpedance, Blood, and Saliva Biochemistry in Population Studies—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Anthropometric Measurements, Blood Pressure Measurement
2.2. BIA Body Composition Analysis
2.3. Collection of Biological Material
2.4. Determination of pH and Buffer Capacity of Saliva
2.5. DPPH Determination in Saliva
2.6. Determination of Antioxidant Activity in Saliva Using the FRAP Method
2.7. Determination of Salivary Amylase Activity
2.8. Determination of Urea Concentration in Saliva
2.9. Statistical Analysis and Other Software
3. Results
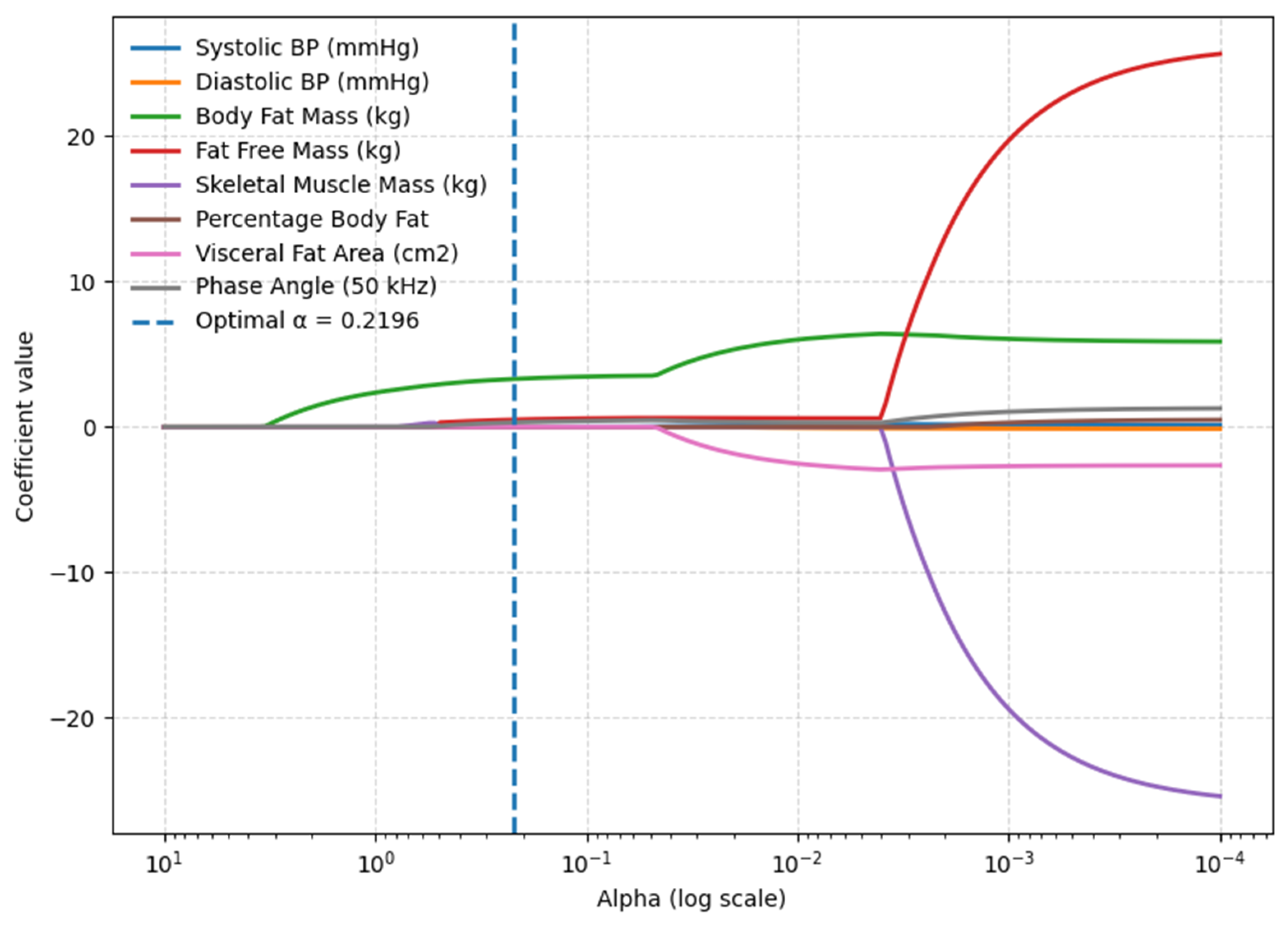
3.1. BMI Model—Body Composition Parameters
3.2. Waist Circumference Model—Body Composition Parameters
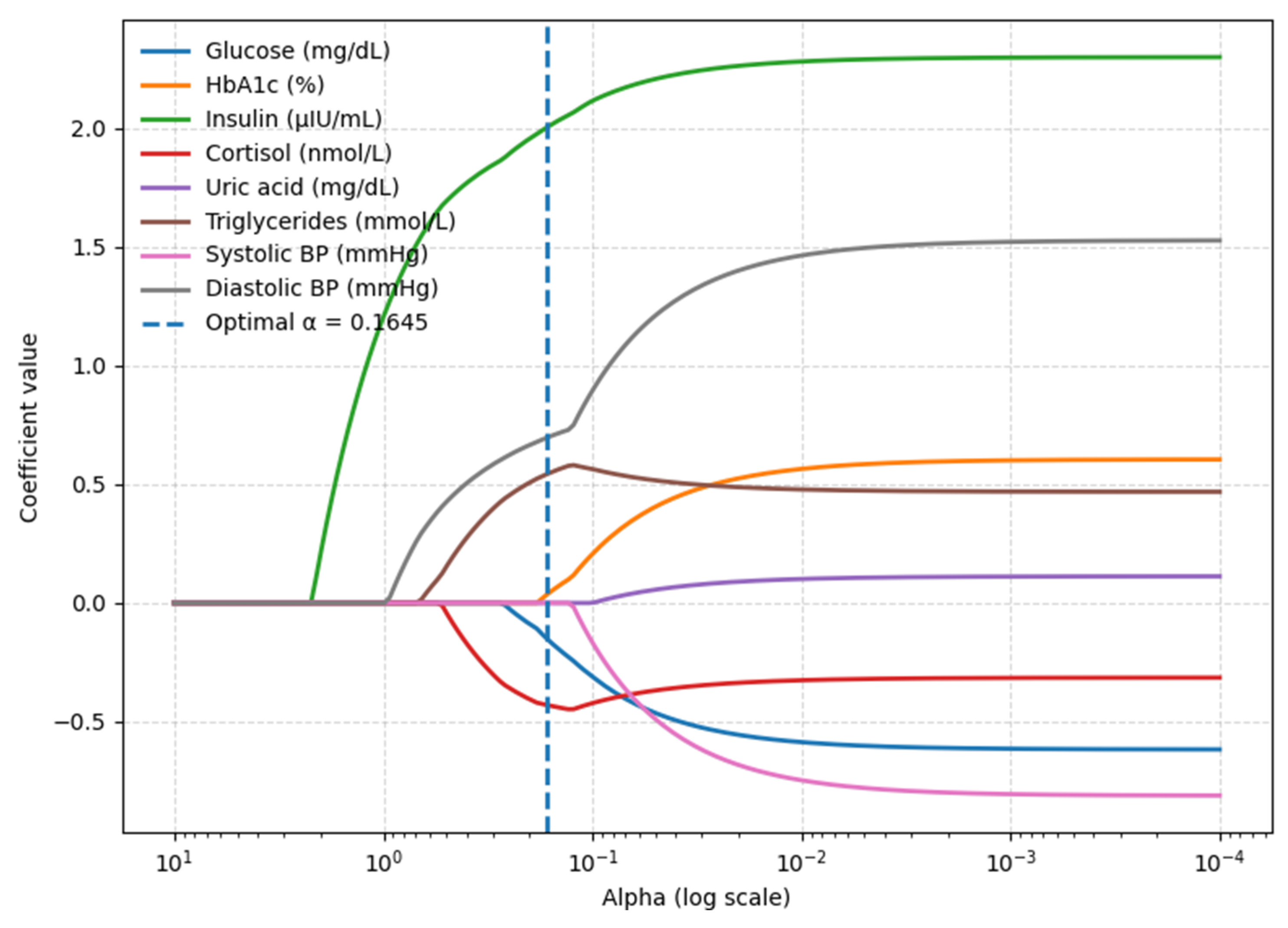
3.3. BMI Model—Blood-Based Parameters
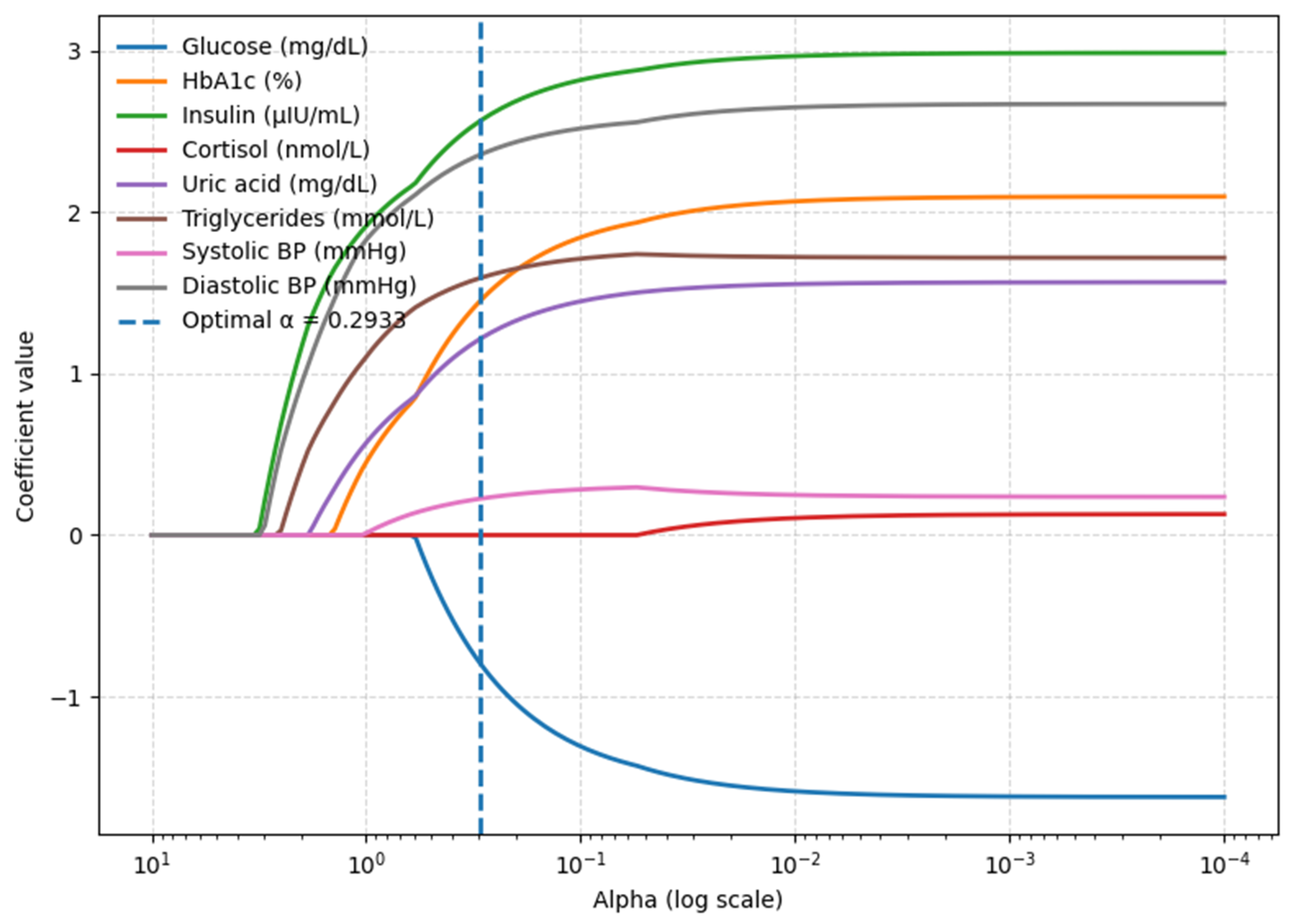
3.4. Waist Circumference Model—Blood-Based Parameters
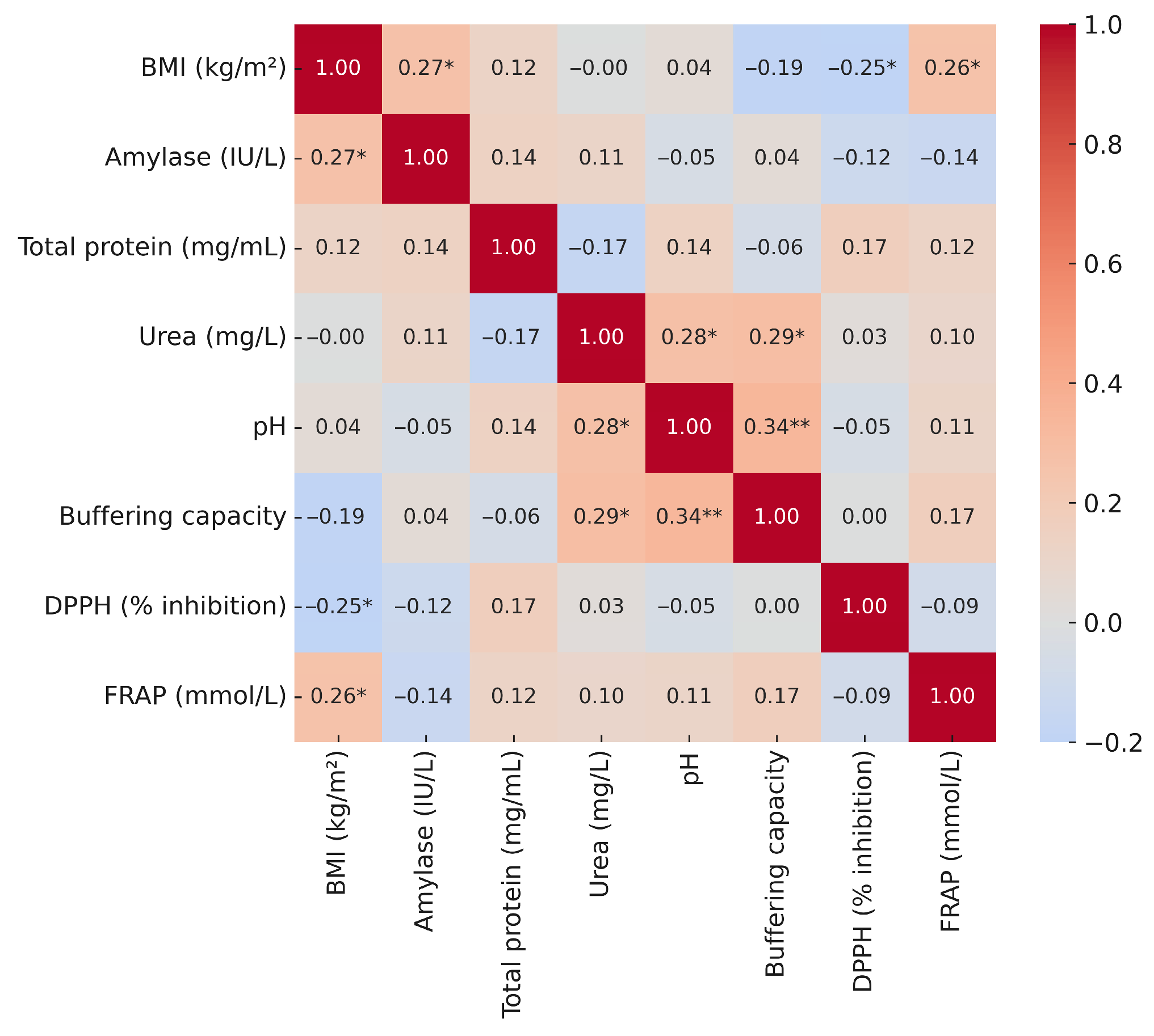
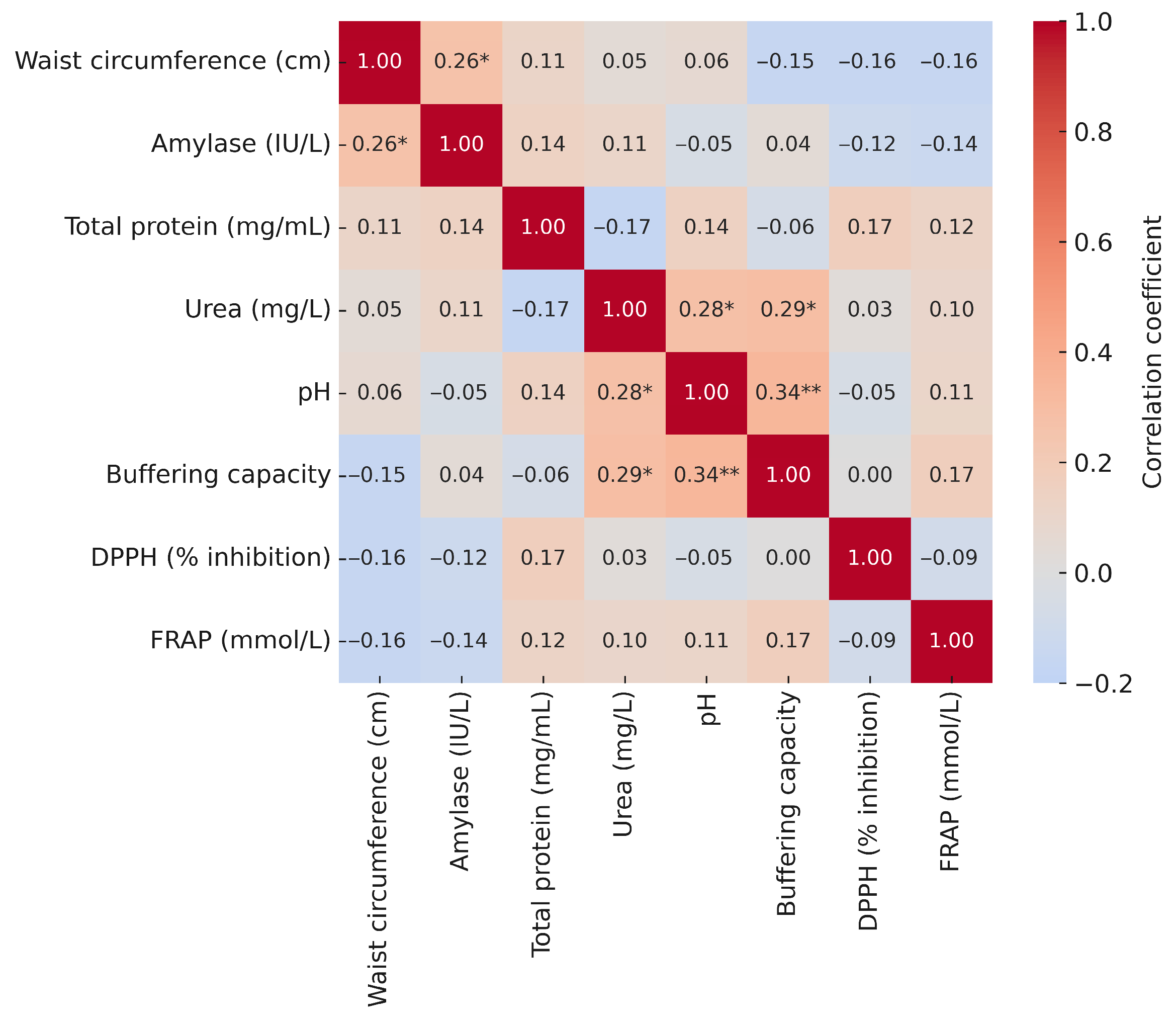
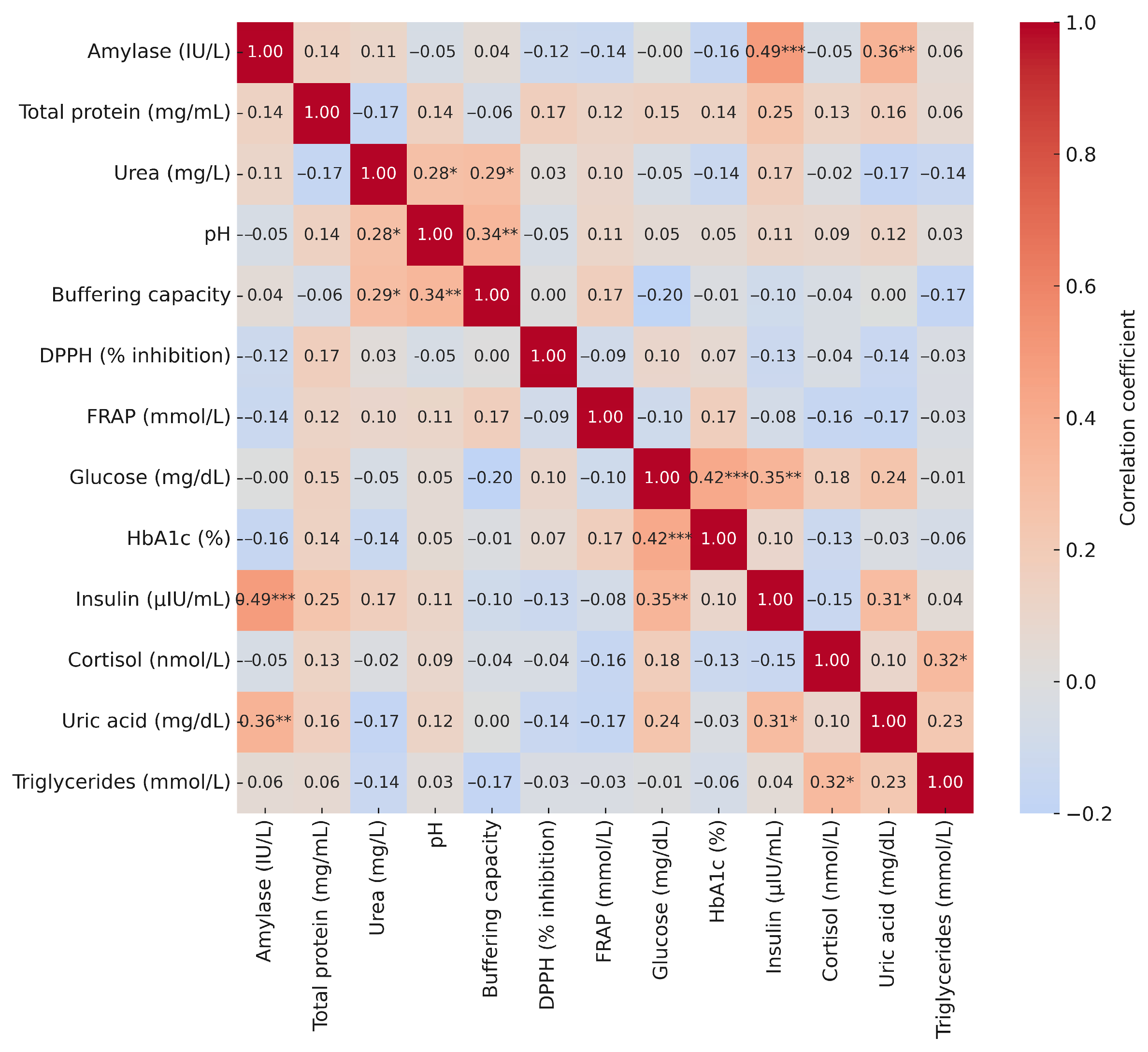
3.5. Correlations of Salivary Biomarkers with Anthropometric and Blood Parameters
4. Discussion
- -
- Carbohydrate metabolism disorders: Fasting glucose concentration ≥ 100 mg/dl or glucose concentration ≥ 140 mg/dl after 120 min in an oral glucose tolerance test (OGTT) or glycated haemoglobin (HbA1c) ≥ 5.7% or use of hypoglycaemic treatment.
- -
- Elevated blood pressure: ≥130/85 mmHg in a doctor’s office or ≥130/80 mmHg at home or use of antihypertensive treatment.
- -
- Atherogenic dyslipidaemia: non-HDL cholesterol concentration ≥ 130 mg/dl or use of lipid-lowering treatment [5]. This definition emphasises the importance of obesity as a central component of MetS and takes into consideration current diagnostic methods, such as HbA1c and non-HDL cholesterol measurements, which better reflect cardiovascular risk than previous indicators. A holistic approach to maintaining and improving metabolic health, taking into account modern diagnostic tools, is of significant importance, and a review of current scientific data indicates the effectiveness of comprehensive interventions, which is in line with contemporary trends in preventive medicine promoting holistic diagnostic methods [37,38]. This study analysed an interdisciplinary approach to assessing metabolic health, taking into account not only standard, clinically validated parameters, but also selected salivary biomarkers whose clinical value is currently being investigated. This study indicates that the interdisciplinary combination of body composition analysis using BIA and blood biochemistry provides a comprehensive picture of metabolic health in the study participants. Although the results regarding salivary biomarkers at this stage of research had limited statistical power and do not allow for precise, generalized clinical conclusions, they are promising. Due to the fact that these are pilot studies, the obtained results regarding salivary biomarkers, i.e., FRAP, DPPH, amylase, and urea, should be treated with caution and thoroughly assessed in further studies on a larger group of individuals. The results of the study indicate that obesity measured by BMI as a screening tool for metabolic syndrome strongly correlates with body fat mass and PA, which strongly and significantly explained the variability of BMI. Similar conclusions were presented in a systematic review by Praget-Bracamontes et al. [39], indicating that obese individuals had a higher PA compared to eutrophic individuals. However, the authors point to the need for further research. The results of current studies in this area are not conclusive, as other authors have shown a negative correlation between BMI and PA. According to Barrea et al. [40], for each unit increase in BMI, PA decreased by 0.54° in individuals with high BMI. The data provided contradicts our results, where we obtained a positive correlation between fat mass, lean body mass, and PA in relation to BMI. The difference in the observations obtained may result from differences in the population and the study design. The authors analysed a specific group of clinical patients in Italy, while our study included a randomly selected sample of adult residents of Poland. A clinical sample may include individuals with more advanced or specific metabolic disorders (e.g., related to the type of diet followed), which may affect body composition and bioelectrical parameters differently than in the general population. In contrast, Fu et al. [41] reported that in overweight and obese individuals, PA increased by 0.006° with increasing BMI. Similarly, these authors agreed that for each one-year increase in age in individuals with a wide range of BMI, PA decreased by 0.11° and 0.014° in overweight and obese individuals, respectively [41]. There are relatively few studies in the current literature evaluating PA in obese individuals/patients, and the results are sometimes contradictory and require further analysis. PA may be useful in assessing muscle quality in obese individuals/patients, but further research is needed on this parameter and its impact on body composition and metabolic functions [42].
5. Conclusions
5.1. Most Accurate Predictive Model
5.2. Most Clinically Relevant Model for Metabolic Syndrome
6. Limitations
7. Directions for Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| BIA | Bioelectrical Impedance Analysis |
| DEXA | Dual-Energy X-Ray Absorptiometry |
| BFM | Body Fat Mass |
| FFM | Fat-Free Mass |
| SMM | Skeletal Muscle Mass |
| PBF | Percentage Body Fat |
| VFA | Visceral Fat Area |
| PA | Phase Angle |
| TC | Total cholesterol |
| TG | Triglycerides |
| LDL | Low-Density Lipoprotein |
| HDL | High-Density Lipoprotein |
| HbA1c | Glycated Hemoglobin |
| non-HDL | Non-High-Density Lipoprotein Cholesterol |
| BUN | Blood Urea Nitrogen |
| FRAP | Ferric Reducing Ability of Plasma |
| DPPH | 2,2-Diphenyl-1-Picrylhydrazyl |
| HCL | Hydrochloric Acid |
| TPTZ | Iron-2,4,6-Tripyridyl-S-Triazine Complex |
| UV-VIS | Ultraviolet–Visible Spectroscopy |
| -SH | Sulfhydryl Group |
| ID | Identification |
| MetS | Metabolic Syndrome |
| IDF | International Diabetes Federation |
| NCEP | National Cholesterol Education Program |
| ATP III | Adult Treatment Panel III |
| CMS | Cardiometabolic syndrome |
| R2 | R-squared |
| SD | Standard Deviation |
| Me | Median |
| HPA | Hypothalamic-Pituitary-Adrenal |
| TNF-α | Tumor Necrosis Factor Alpha |
| IL-6 | Interleukin-6 |
| OLS | Ordinary Least Squares Method |
| LASSO | Least Absolute Shrinkage and Selection Operator |
| VIF | Variance Inflation Factor |
| Systolic BP | Systolic Blood Pressure |
| Diastolic BP | Diastolic Blood Pressure |
References
- Buchanan, L.; Calkins, M.; Kalayjian, T.; Norwitz, N.G.; Teicholz, N.; Unwin, D.; Soto-Mota, A. TOWARD, a metabolic health intervention, demonstrates robust 1-year weight loss and cost-savings through deprescription. Front. Nutr. 2025, 12, 1548609. [Google Scholar] [CrossRef]
- Healy, D.R.; Zarei, I.; Mikkonen, S.; Soininen, S.; Viitasalo, A.; Haapala, E.A.; Auriola, S.; Hanhineva, K.; Kolehmainen, M.; Lakka, T.A. Longitudinal associations of an exposome score with serum metabolites from childhood to adolescence. Commun. Biol. 2024, 7, 890. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, P.; Prejbisz, A.; Kurylowicz, A.; Baska, A.; Burchardt, P.; Chlebus, K.; Dzida, G.; Jankowski, P.; Jaroszewicz, J.; Jaworski, P.; et al. Metabolic Syndrome—A New Definition and Management Guidelines. Arch. Med. Sci. 2022, 18, 1133–1156. [Google Scholar] [CrossRef]
- Mirska, I.; Kreft, P.; Skalski, D.W.; Rybak, O.; Kowalski, D.; Dyachuk, V. Physical activity as an important element of prevention and treatment of metabolic syndrome. Rehabil. Recreat. 2023, 14, 164–169. [Google Scholar] [CrossRef]
- Swarup, S.; Ahmed, I.; Grigorova, Y.; Zeltser, R. Metabolic Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Pęczkowska, A.; Kaziród, A. Selected Treatment Approaches for Metabolic Syndrome: A Literature Review. Health Probl. Civiliz. 2022, 16, 116–127. [Google Scholar] [CrossRef]
- Cramer, H.; Hohmann, C.; Lauche, R.; Choi, K.-E.; Schneider, N.; Steckhan, N.; Rathjens, F.; Anheyer, D.; Paul, A.; von Scheidt, C.; et al. Effects of Fasting and Lifestyle Modification in Patients with Metabolic Syndrome: A Randomized Controlled Trial. J. Clin. Med. 2022, 11, 4751. [Google Scholar] [CrossRef]
- Alfawaz, H.A.; Wani, K.; Alnaami, A.M.; Al-Saleh, Y.; Aljohani, N.J.; Al-Attas, O.S.; Alokail, M.S.; Kumar, S.; Al-Daghri, N.M. Effects of Different Dietary and Lifestyle Modification Therapies on Metabolic Syndrome in Prediabetic Arab Patients: A 12-Month Longitudinal Study. Nutrients 2018, 10, 383. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, L.; Sun, J.; Zhu, Z.; Xing, Z.; Zhou, S.; Tai, S.; Wang, Y. Association Between Metabolic Syndrome and an Increased Risk of Hospitalization for Heart Failure in Population of HFpEF. Front. Cardiovasc. Med. 2021, 8, 698117. [Google Scholar] [CrossRef] [PubMed]
- Cesaro, A.; De Michele, G.; Fimiani, F.; Acerbo, V.; Scherillo, G.; Signore, G.; Rotolo, F.P.; Scialla, F.; Raucci, G.; Panico, D.; et al. Visceral adipose tissue and residual cardiovascular risk: A pathological link and new therapeutic options. Front. Cardiovasc. Med. 2023, 10, 1187735. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. Optimised Skeletal Muscle Mass as a Key Strategy for Obesity Management. Metabolites 2025, 15, 85. [Google Scholar] [CrossRef]
- Petri, C.; Micheli, M.L.; Izzicupo, P.; Timperanza, N.; Lastrucci, T.; Vanni, D.; Gulisano, M.; Mascherini, G. Bioimpedance Patterns and Bioelectrical Impedance Vector Analysis (BIVA) of Body Builders. Nutrients 2023, 15, 1606. [Google Scholar] [CrossRef]
- Zborowski, M.; Mikulec, A. Comparison between Dual-X-Ray Absorptiometry and Bioelectrical Impedance Analyses in Dietary Practice. J. Educ. Health Sport 2022, 12, 128–136. [Google Scholar] [CrossRef]
- Cimmino, F.; Petrella, L.; Cavaliere, G.; Ambrosio, K.; Trinchese, G.; Monda, V.; D’Angelo, M.; Di Giacomo, C.; Sacconi, A.; Messina, G.; et al. A Bioelectrical Impedance Analysis in Adult Subjects: The Relationship between Phase Angle and Body Cell Mass. J. Funct. Morphol. Kinesiol. 2023, 8, 107. [Google Scholar] [CrossRef]
- Cho, Y.H.; Lee, S.Y. Useful Biomarkers of Metabolic Syndrome. Int. J. Environ. Res. Public Health 2022, 19, 15003. [Google Scholar] [CrossRef] [PubMed]
- Yuguang, L.; Chang, Y.; Chen, N.; Zhao, Y.; Zhang, X.; Song, W.; Lu, J.; Liu, X. Serum klotho as a novel biomarker for metabolic syndrome: Findings from a large national cohort. Front. Endocrinol. 2024, 15, 1295927. [Google Scholar] [CrossRef]
- Szczepańska-Sadowska, E.; Czarzasta, K.; Kowara, M. The Interaction of Vasopressin with Hormones of the Hypothalamo–Pituitary–Adrenal Axis: The Significance for Therapeutic Strategies in Cardiovascular and Metabolic Diseases. Int. J. Mol. Sci. 2024, 25, 7394. [Google Scholar] [CrossRef] [PubMed]
- Makoni, L.; Manduna, I.T.; Mbiriri, A.L. A Review of Whole-Medical Systems and Holistic Care Approach for Type 2 Diabetes and Associated Metabolic Syndrome. J. Integr. Med. 2024, 22, 199–209. [Google Scholar] [CrossRef]
- Birant, S.; İlisulu, S.C.; Özcan, H.; Yanar, K. Examination of the Effect of Treatment of Severe Early Childhood Caries and Fluoride Varnish Applications on Salivary Oxidative Stress Biomarkers and Antioxidants. BMC Oral Health 2024, 24, 1536. [Google Scholar] [CrossRef] [PubMed]
- De Sousa Né, Y.G.; Frazão, D.R.; Bittencourt, L.O.; Fagundes, N.C.F.; Marañón-Vásquez, G.; Crespo-Lopez, M.E.; Maia, L.C.; Lima, R.R. Are Dental Caries Associated with Oxidative Stress in Saliva in Children and Adolescents? A Systematic Review. Metabolites 2022, 12, 858. [Google Scholar] [CrossRef]
- Tothova, L.; Hodosy, J.; Mucska, I.; Celec, P. Salivary Markers of Oxidative Stress in Patients with Obstructive Sleep Apnea Treated with Continuous Positive Airway Pressure. Sleep Breath. 2014, 18, 563–570. [Google Scholar] [CrossRef]
- Etzel, L.; Ye, Q.; Apsley, A.T.; Chiaro, C.; Petri, L.E.; Kozlosky, J.; Propper, C.; Mills-Koonce, R.; Short, S.J.; Garrett-Peters, P.; et al. Maternal telomere length and oxidative stress in pregnancy: Cross-sectional analysis with an exploratory examination of systemic inflammation. BMC Pregnancy Childbirth 2025, 25, 395. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, L.; Smarkusz-Zarzecka, J.; Gornowicz, A.; Lendzion, K.; Zyśk, B.; Pogodziński, D. Analysis of Selected Salivary Adipokines and Cytokines in Patients with Obesity—A Pilot Study. Int. J. Mol. Sci. 2023, 24, 4145. [Google Scholar] [CrossRef] [PubMed]
- Habobe, H.A.; Pieters, R.H.H.; Bikker, F.J. Investigating the salivary biomarker profile in obesity: A systematic review. Curr. Obes. Rep. 2025, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, A.; Kossakowska, A.; Taranta-Janusz, K.; Zięba, S.; Fejfer, K.; Salamonowicz, M.; Kostecka-Sochoń, P.; Wasilewska, A.; Maciejczyk, M. Dysfunction of Salivary Glands, Disturbances in Salivary Antioxidants and Increased Oxidative Damage in Saliva of Overweight and Obese Adolescents. J. Clin. Med. 2020, 9, 548. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Szulimowska, J.; Taranta-Janusz, K.; Werbel, K.; Wasilewska, A.; Zalewska, A. Salivary FRAP as A Marker of Chronic Kidney Disease Progression in Children. Antioxidants 2019, 8, 409. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Żukowski, P.; Zalewska, A. Salivary Biomarkers in Kidney Diseases. In Saliva in Health and Disease: The Present and Future of a Unique Sample for Diagnosis; Tvarijonaviciute, A., Martínez-Subiela, S., López-Jornet, P., Lamy, E., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 193–219. [Google Scholar]
- Klimiuk, A.; Zalewska, A.; Sawicki, R.; Knapp, M.; Maciejczyk, M. Salivary Oxidative Stress Increases with the Progression of Chronic Heart Failure. J. Clin. Med. 2020, 9, 769. [Google Scholar] [CrossRef]
- Morawska, K.; Maciejczyk, M.; Popławski, Ł.; Popławska-Kita, A.; Kretowski, A.; Zalewska, A. Enhanced Salivary and General Oxidative Stress in Hashimoto’s Thyroiditis Women in Euthyreosis. J. Clin. Med. 2020, 9, 2102. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Bielas, M.; Zalewska, A.; Gerreth, K. Salivary Biomarkers of Oxidative Stress and Inflammation in Stroke Patients: From Basic Research to Clinical Practice. Oxidative Med. Cell. Longev. 2021, 2021, 5545330. [Google Scholar] [CrossRef]
- Nunes, L.A.S.; Mussavira, S.; Bindhu, O.S. Clinical and Diagnostic Utility of Saliva as a Non-Invasive Diagnostic Fluid: A Systematic Review. Biochem. Med. 2015, 25, 177–192. [Google Scholar] [CrossRef]
- Van Nieuw Amerongen, A.; Bolscher, J.G.; Veerman, E.C. Salivary proteins: Protective and diagnostic value in cariology? Caries Res. 2004, 38, 247–253. [Google Scholar] [CrossRef]
- Zych, I.; Krzepiłko, A. Measurement of total antioxidant capacity of selected antioxidants and infusions using DPPH radical reduction. Chem. Educ. Ecol. Metrol. 2010, 15, 51–54. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of ‘antioxidant Power’: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Ramírez-Gallegos, I.; Busquets-Cortes, C.; Paublini, H.; López-González, Á.A.; Martínez-Almoyna-Rifá, E.; Tárraga López, P.J.; Ramírez-Manent, J.I. Association Between Bioimpedance-Determined Metabolic Age and MASLD Risk Scores in Spanish Workers. Metabolites 2025, 15, 343. [Google Scholar] [CrossRef]
- Freitas, F.P.C.; Rodrigues, C.E.M. Effect of Liraglutide on Cardiometabolic Profile and on Bioelectrical Impedance Analysis in Patients with Obesity and Metabolic Syndrome. Dent. Sci. Rep. 2023, 13, 13090. [Google Scholar] [CrossRef] [PubMed]
- Praget-Bracamontes, S.; González-Arellanes, R.; Aguilar-Salinas, C.A.; Martagón, A.J. Phase Angle as a Potential Screening Tool in Adults with Metabolic Diseases in Clinical Practice: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 1608. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Laudisio, D.; Di Somma, C.; Salzano, C.; Pugliese, G.; de Alteriis, G.; Colao, A.; Savastano, S. Phase Angle: A Possible Biomarker to Quantify Inflammation in Subjects with Obesity and 25(OH)D Deficiency. Nutrients 2019, 11, 1747. [Google Scholar] [CrossRef]
- Fu, L.; Ren, Z.; Liu, X.; Wu, N.; Zhao, K.; Luo, G.; Yang, H.; Zhang, Y.; Yan, T.; Liu, Y.; et al. Reference Data of Phase Angle Using Bioelectrical Impedance Analysis in Overweight and Obese Chinese. Front. Endocrinol. 2022, 13, 924199. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, O.; Marra, M.; Sacco, A.M.; Pasanisi, F.; Scalfi, L. Bioelectrical Impedance (BIA)-Derived Phase Angle in Adults with Obesity: A Systematic Review. Clin. Nutr. 2021, 40, 5238–5248. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-H.; Tsao, Y.-C.; Tzeng, I.-S.; Chuang, H.-H.; Li, W.-C.; Tung, T.-H.; Chen, J.-Y. Body Mass Index and Waist Circumference Are Better Predictors of Insulin Resistance than Total Body Fat Percentage in Middle-Aged and Elderly Taiwanese. Medicine 2017, 96, e8126. [Google Scholar] [CrossRef] [PubMed]
- Gołacki, J.; Witczak, K.; Górecki, K.; Szafraniec-Porada, A.; Wronecki, J.; Porada, D.; Matyjaszek-Matuszek, B. Bioimpedance Body Composition Analysis in Estimating Insulin Resistance in Women with Overweight and Obesity (LUCAS 1.1): A Retrospective Analysis. Available online: https://journals.viamedica.pl/clinical_diabetology/article/view/102456 (accessed on 11 July 2025).
- Mirarefin, M.; Sharifi, F.; Fakhrzadeh, H.; Amini, M.R.; Ghaderpanahi, M.; Zerafati Shoa, N.; Badamchizadeh, Z.; Tajalizadekhoob, Y.; Nazari, N.; Larijani, B. Waist Circumference and Insulin Resistance in Elderly Men: An Analysis of Kahrizak Elderly Study. J. Diabetes Metab. Disord. 2014, 13, 28. [Google Scholar] [CrossRef]
- Wu, L.; Liu, H.; Cui, Z.; Hou, F.; Gong, X.; Zhang, Y.; Lu, C.; Liu, H.; Yu, P. Fluctuations in Waist Circumference Increase Diabetes Risk: A 4-Year Cohort Study in 61,587 Older Adults. Nutr. Metab. 2021, 18, 99. [Google Scholar] [CrossRef]
- Robledo-Millán, C.R.; Diaz-Domínguez, M.R.; Castañeda-Ramírez, A.E.; Quiñones-Lara, E.; Valencia-Marín, S.; Suárez-García, R.X.; López-Desiderio, N.G.; Ramos-Cortés, C.A.; Gaytán Gómez, A.M.; Bello-López, J.M.; et al. A Novel Metabolic Risk Classification System Incorporating Body Fat, Waist Circumference, and Muscle Strength. J. Funct. Morphol. Kinesiol. 2025, 10, 72. [Google Scholar] [CrossRef]
- Ramírez-Manent, J.I.; Jover, A.M.; Martinez, C.S.; Tomás-Gil, P.; Martí-Lliteras, P.; López-González, Á.A. Waist Circumference Is an Essential Factor in Predicting Insulin Resistance and Early Detection of Metabolic Syndrome in Adults. Nutrients 2023, 15, 257. [Google Scholar] [CrossRef]
- Wei, J.; Liu, X.; Xue, H.; Wang, Y.; Shi, Z. Comparisons of Visceral Adiposity Index, Body Shape Index, Body Mass Index and Waist Circumference and Their Associations with Diabetes Mellitus in Adults. Nutrients 2019, 11, 1580. [Google Scholar] [CrossRef]
- Gagnon, É.; Mitchell, P.L.; Arsenault, B.J. Body Fat Distribution, Fasting Insulin Levels and Insulin Secretion: A Bidirectional Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2023, 108, 1308–1317. [Google Scholar] [CrossRef]
- Barazzoni, R.; Gortan Cappellari, G.; Ragni, M.; Nisoli, E. Insulin Resistance in Obesity: An Overview of Fundamental Alterations. Eat. Weight Disord. -Stud. Anorex. Bulim. Obes. 2018, 23, 149–157. [Google Scholar] [CrossRef]
- Wondmkun, Y.T. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3611–3616. [Google Scholar] [CrossRef] [PubMed]
- Gołacki, J.; Matuszek, M.; Matyjaszek-Matuszek, B. Link between Insulin Resistance and Obesity—From Diagnosis to Treatment. Diagnostics 2022, 12, 1681. [Google Scholar] [CrossRef] [PubMed]
- Shariq, O.A.; McKenzie, T.J. Obesity-Related Hypertension: A Review of Pathophysiology, Management, and the Role of Metabolic Surgery. Gland Surg. 2020, 9, 80–93. [Google Scholar] [CrossRef]
- Linderman, G.C.; Lu, J.; Lu, Y.; Sun, X.; Xu, W.; Nasir, K.; Schulz, W.L.; Jiang, L.; Krumholz, H.M. Association of Body Mass Index with Blood Pressure Among 1.7 Million Chinese Adults. JAMA Netw. Open 2018, 1, e181271. [Google Scholar] [CrossRef]
- Hossain, F.B.; Adhikary, G.; Chowdhury, A.B.; Shawon, S.R. Association between Body Mass Index (BMI) and Hypertension in South Asian Population: Evidence from Nationally-Representative Surveys. Clin. Hypertens. 2019, 25, 28. [Google Scholar] [CrossRef]
- Song, L.; He, H.; Lun, J. Body Mass Index Is Associated with Blood Pressure and Vital Capacity in Medical Students. Lipids Health Dis. 2023, 22, 174. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-Y.; Su, Z.; Shen, H.; Yang, H.; Sun, W.; Kong, X. Abdominal Fat Accumulation Increases the Risk of High Blood Pressure: Evidence of 47,037 Participants from Chinese and US National Population Surveys. Nutr. J. 2024, 23, 153. [Google Scholar] [CrossRef]
- Chielle, E.O.; Casarin, J.N. Evaluation of Salivary Oxidative Parameters in Overweight and Obese Young Adults. Arch. Endocrinol. Metab. 2017, 61, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiari, A.; Hajian-Tilaki, K.; Omidvar, S.; Nasiri Amiri, F. Association of Lipid Peroxidation and Antioxidant Status with Metabolic Syndrome in Iranian Healthy Elderly Women. Biomed. Rep. 2017, 7, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Manjunathan, K.; Menon, M.; Bavle, R.M.; Makarla, S.; Venugopal, R.; Santo, A.J. Evaluation of Antioxidant Activity in Saliva among Young Adults Having Diverging Food Habits and Its Relation to Oral Health: A Pilot Study. J. Oral Maxillofac. Pathol. 2024, 28, 226–231. [Google Scholar] [CrossRef]
- Oluwadaisi, A.; Ogunlade, O.; Owotade, F.; Oyetola, E.O.; Aborisade, A.; Abiola, A.M. Caries experience, periodontal disease and salivary antioxidants as biomarkers of cardiometabolic syndrome among hospital attendees in a Nigerian suburban setting. BMC Oral Health 2025, 1118, 25. [Google Scholar] [CrossRef]
- Suzuki, D.; Yamada, S.; Sakurai, A.; Karasawa, I.; Kondo, E.; Sakai, H.; Tanaka, H.; Shimane, T.; Kurita, H. Correlations between the Properties of Saliva and Metabolic Syndrome: A Prospective Observational Study. Medicine 2020, 99, e23688. [Google Scholar] [CrossRef]
- Alqaderi, H.; Hegazi, F.; Al-Mulla, F.; Chiu, C.-J.; Kantarci, A.; Al-Ozairi, E.; Abu-Farha, M.; Bin-Hasan, S.; Alsumait, A.; Abubaker, J.; et al. Salivary Biomarkers as Predictors of Obesity and Intermediate Hyperglycemia in Adolescents. Front. Public Health 2022, 10, 800373. [Google Scholar] [CrossRef]
- Surdu, A.; Foia, L.G.; Luchian, I.; Trifan, D.; Tatarciuc, M.S.; Scutariu, M.M.; Ciupilan, C.; Budala, D.G. Saliva as a Diagnostic Tool for Systemic Diseases—A Narrative Review. Medicina 2025, 61, 243. [Google Scholar] [CrossRef]
- Soukup, M.; Biesiada, I.; Henderson, A.T.; Idowu, B.; Rodeback, D.; Ridpath, L.; Bridges, E.G.; Nazar, A.M.; Bridges, K.G. Salivary Uric Acid as a Noninvasive Biomarker of Metabolic Syndrome. Diabetol. Metab. Syndr. 2012, 4, 14. [Google Scholar] [CrossRef]
- Goodson, J.M.; Kantarci, A.; Hartman, M.-L.; Denis, G.V.; Stephens, D.; Hasturk, H.; Yaskell, T.; Vargas, J.; Wang, X.; Cugini, M.; et al. Metabolic Disease Risk in Children by Salivary Biomarker Analysis. PLoS ONE 2014, 9, e98799. [Google Scholar] [CrossRef] [PubMed]
- Ittichaicharoen, J.; Phrommintikul, A.; Chattipakorn, N.; Chattipakorn, S.C. Reduced Salivary Amylase Activity in Metabolic Syndrome Patients with Obesity Could Be Improved by Treatment with a Dipeptidyl Peptidase IV Inhibitor. Clin. Oral Investig. 2018, 22, 3113–3120. [Google Scholar] [CrossRef]
- Bonnefond, A.; Yengo, L.; Yengo, L.; Dechaume, A.; Canouil, M.; Castelain, M.; Roger, E.; Allegaert, F.; Caiazzo, R.; Raverdy, V.; et al. Relationship between Salivary/Pancreatic Amylase and Body Mass Index: A Systems Biology Approach. BMC Med. 2017, 15, 37. [Google Scholar] [CrossRef] [PubMed]
- Aldossari, N.M.; Aldossari, N.M.; El Gabry, E.E.; El Gabry, E.E.; Gawish, G.E.H. Association between Salivary Amylase Enzyme Activity and Obesity in Saudi Arabia. Medicine 2019, 98, e15878. [Google Scholar] [CrossRef]
- Al Akl, N.S.; Khalifa, O.; Habibullah, M.; Arredouani, A. Salivary α-amylase activity is associated with cardiometabolic and inflammatory biomarkers in overweight/obese, non-diabetic Qatari women. Front. Endocrinol. 2024, 15, 1348853. [Google Scholar] [CrossRef]
- Erta, G.; Gersone, G.; Jurka, A.; Tretjakovs, P. Decoding metabolic connections: The role of salivary amylase activity in modulating visceral fat and triglyceride glucose index. Lipids Health Dis. 2025, 24, 98. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-L.; Evans, R.D.R.; Unwin, R.J.; Norman, J.T.; Rich, P.R. Assessment of Measurement of Salivary Urea by ATR-FTIR Spectroscopy to Screen for CKD. Kidney360 2022, 3, 357–363. [Google Scholar] [CrossRef]
- Pandya, D.; Nagrajappa, A.K.; Ravi, K.S. Assessment and Correlation of Urea and Creatinine Levels in Saliva and Serum of Patients with Chronic Kidney Disease, Diabetes and Hypertension- A Research Study. J. Clin. Diagn. Res. JCDR 2016, 10, ZC58–ZC62. [Google Scholar] [CrossRef] [PubMed]
- Calice-Silva, V.; Sacomboio, E.N.M.; Sacomboio, E.N.M.; Raimann, J.G.; Evans, R.; Sebastião, C.S.; Tchivango, A.T.; Kotanko, P.; Levin, N.W.; Pecoits-Filho, R. Diagnostic Performance of Salivary Urea Nitrogen Dipstick to Detect and Monitor Acute Kidney Disease in Patients with Malaria. Malar. J. 2018, 17, 477. [Google Scholar] [CrossRef]
- Evans, R.; Hemmila, U.; Mzinganjira, H.; Mtekateka, M.; Banda, E.; Sibale, N.; Kawale, Z.; Phiri, C.; Dreyer, G.; Calice-Silva, V.; et al. Diagnostic Performance of a Point-of-Care Saliva Urea Nitrogen Dipstick to Screen for Kidney Disease in Low-Resource Settings Where Serum Creatinine Is Unavailable. BMJ Glob. Health 2020, 5, e002312. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Qiao, H.; Zhang, Y.; Xu, Y.; Chen, R.; Liu, K.; Zhang, S.; Zhuang, G. Metabolic Syndrome and Cancer Risk: A Bidirectional Two-Sample Mendelian Randomization Study. Exp. Gerontol. 2025, 207, 112802. [Google Scholar] [CrossRef]
- Kołota, A.; Górczyńska, P. Assessment of nutritional status and nutritional habits in a selected group of middle-aged people as related to metabolic syndrome risk. Hygeia Public Health 2020, 55, 63–70. [Google Scholar]
- Iwaniak, A.; Mogut, D.; Darewicz, M.; Hrynkiewicz, M. Use of biologically active Peptides from food proteins in the prevention of metabolic syndrome. Probl. Issues Prog. Agric. Sci. 2016, 47, 213–236. [Google Scholar]
- Karvane, H.; Esfandiari, H.; Qutaiba, O.; Allela, B.; Mahdi, M.S.; Al-Nuaimi, A.M.A.; Alhussein, R.; Jawad, M.J.; Ghayourvahdat, A.; Keshavarzian, A. Metabolic Syndrome in Association with Novel Dietary Index, Metabolic Parameters, Nesfatin-1 and Omentin-1. BMC Endocr. Disord. 2024, 24, 257. [Google Scholar] [CrossRef]
- Reeder, N.K.; Reneker, J.C. Food Insecurity Is Associated with Metabolic Syndrome among US Adults: NHANES 2005–2016. Nutr. Res. 2024, 126, 159–166. [Google Scholar] [CrossRef]
- Chomiuk, T.; Niezgoda, N.; Mamcarz, A.; Śliż, D. Physical Activity in Metabolic Syndrome. Front. Physiol. 2024, 15, 1365761. [Google Scholar] [CrossRef] [PubMed]
| Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Average | SD | Scope | Me | Average | SD | Scope | Me |
| Age (years) | 57.7 | 8.6 | 41.0–70.0 | 56.5 | 52.3 | 8.7 | 40.0–71.0 | 52.0 |
| BMI (kg/m2) | 27.9 | 4.2 | 20.9–41.5 | 27.5 | 27.6 | 3.1 | 21.8–34.1 | 27.8 |
| Waist circumference (cm) | 92.3 | 9.3 | 74.0–109.0 | 93.0 | 94.5 | 8.0 | 78.0–110.0 | 96.8 |
| Predictor | Estimate | SE | z | p-Value | 95% CI Lower | 95% CI Upper |
|---|---|---|---|---|---|---|
| Intercept | 9.131 | 1.936 | 4.716 | 0.000 | 5.336 | 12.926 |
| Body Fat Mass (kg) | 0.411 | 0.035 | 11.715 | 0.000 | 0.342 | 0.479 |
| Fat Free Mass (kg) | 0.061 | 0.021 | 2.938 | 0.003 | 0.020 | 0.101 |
| Phase Angle (50 kHz) | 0.895 | 0.406 | 2.203 | 0.028 | 0.099 | 1.692 |
| Predictor | Estimate | SE | z | p-Value | 95% CI Lower | 95% CI Upper |
|---|---|---|---|---|---|---|
| Intercept | 43.405 | 9.413 | 4.611 | 0.000 | 24.956 | 61.853 |
| Systolic BP (mmHg) | 0.202 | 0.094 | 2.140 | 0.032 | 0.017 | 0.386 |
| Diastolic BP (mmHg) | −0.076 | 0.158 | −0.480 | 0.631 | −0.387 | 0.235 |
| Body Fat Mass (kg) | 0.585 | 0.113 | 5.167 | 0.000 | 0.363 | 0.806 |
| Phase Angle (50 kHz) | 2.831 | 1.414 | 2.002 | 0.045 | 0.059 | 5.602 |
| Predictor | Estimate | SE | z | p-Value | 95% CI Lower | 95% CI Upper |
|---|---|---|---|---|---|---|
| Intercept | 19.338 | 7.236 | 2.673 | 0.008 | 5.156 | 33.520 |
| Glucose (mg/dL) | −0.025 | 0.042 | −0.599 | 0.549 | −0.106 | 0.057 |
| HbA1c (%) | −0.736 | 1.246 | −0.590 | 0.555 | −3.178 | 1.707 |
| Insulin (µIU/mL) | 0.478 | 0.066 | 7.283 | 0.000 | 0.349 | 0.606 |
| Cortisol (nmol/L) | −0.004 | 0.004 | −1.159 | 0.246 | −0.011 | 0.003 |
| Triglycerides (mmol/L) | 0.933 | 0.683 | 1.366 | 0.172 | −0.406 | 2.271 |
| Diastolic BP (mmHg) | 0.121 | 0.031 | 3.937 | 0.000 | 0.061 | 0.181 |
| Predictor | Estimate | SE | z | p-Value | 95% CI Lower | 95% CI Upper |
|---|---|---|---|---|---|---|
| Intercept | 12.579 | 24.377 | 0.516 | 0.606 | −35.198 | 60.357 |
| Glucose (mg/dL) | −0.215 | 0.081 | −2.658 | 0.008 | −0.373 | −0.056 |
| HbA1c (%) | 8.839 | 3.782 | 2.337 | 0.019 | 1.426 | 16.252 |
| Insulin (µIU/mL) | 0.533 | 0.171 | 3.119 | 0.002 | 0.198 | 0.868 |
| Uric acid (mg/dL) | 1.889 | 0.888 | 2.128 | 0.033 | 0.149 | 3.629 |
| Triglycerides (mmol/L) | 1.422 | 1.513 | 0.940 | 0.347 | −1.544 | 4.387 |
| Systolic BP (mmHg) | 0.005 | 0.099 | 0.049 | 0.961 | −0.190 | 0.200 |
| Diastolic BP (mmHg) | 0.387 | 0.166 | 2.335 | 0.020 | 0.062 | 0.711 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stawiarska, A.; Francik, R.; Mikulec, A.; Zborowski, M.; Cisoń-Apanasewicz, U.; Gajdosz, R.; Zaczyk, I.; Potok, H.; Radom, A.; Ogonowska, D.; et al. A Holistic Approach to Metabolic Health Assessment—Analysis of Bioimpedance, Blood, and Saliva Biochemistry in Population Studies—A Pilot Study. Metabolites 2025, 15, 591. https://doi.org/10.3390/metabo15090591
Stawiarska A, Francik R, Mikulec A, Zborowski M, Cisoń-Apanasewicz U, Gajdosz R, Zaczyk I, Potok H, Radom A, Ogonowska D, et al. A Holistic Approach to Metabolic Health Assessment—Analysis of Bioimpedance, Blood, and Saliva Biochemistry in Population Studies—A Pilot Study. Metabolites. 2025; 15(9):591. https://doi.org/10.3390/metabo15090591
Chicago/Turabian StyleStawiarska, Aleksandra, Renata Francik, Anna Mikulec, Marek Zborowski, Urszula Cisoń-Apanasewicz, Ryszard Gajdosz, Iwona Zaczyk, Halina Potok, Agnieszka Radom, Dorota Ogonowska, and et al. 2025. "A Holistic Approach to Metabolic Health Assessment—Analysis of Bioimpedance, Blood, and Saliva Biochemistry in Population Studies—A Pilot Study" Metabolites 15, no. 9: 591. https://doi.org/10.3390/metabo15090591
APA StyleStawiarska, A., Francik, R., Mikulec, A., Zborowski, M., Cisoń-Apanasewicz, U., Gajdosz, R., Zaczyk, I., Potok, H., Radom, A., Ogonowska, D., & Rafa, E. (2025). A Holistic Approach to Metabolic Health Assessment—Analysis of Bioimpedance, Blood, and Saliva Biochemistry in Population Studies—A Pilot Study. Metabolites, 15(9), 591. https://doi.org/10.3390/metabo15090591







