Abstract
Lignans are plant-derived biphenolic compounds with multiple hydroxyl groups, which, upon ingestion, are metabolized by gut microbiota into enterolignans—enterolactone and enterodiol. These mammalian metabolites exhibit structural similarity to estradiol, enabling lignans to modulate hormonal balance and exert estrogen-like effects. A growing body of evidence highlights their broad spectrum of health-promoting properties, including antioxidant, anti-inflammatory, and hormone-regulating effects. Lignans have shown potential in alleviating menopausal symptoms, preventing estrogen-dependent cancers, and mitigating conditions such as cardiovascular disease, diabetes, and metabolic syndrome. Additionally, their antimicrobial activity against bacteria, fungi, and viruses is being increasingly recognized. This review provides a comprehensive and up-to-date synthesis of current knowledge. It uniquely integrates the latest insights into lignan biosynthesis, gut microbiota-mediated metabolism, and clinically relevant outcomes. Importantly, this review incorporates recent findings from prospective cohort studies and meta-analyses and sheds light on emerging therapeutic applications, including antifungal activity—an area rarely covered in earlier literature. By presenting a holistic perspective, this review advances our understanding of lignans as multifaceted compounds with significant potential in preventive and therapeutic health strategies.
1. Introduction
Lignans are a class of naturally occurring polyphenolic compounds present in a wide range of plant-based foods, particularly seeds (notably flaxseed), whole grains, fruits, and vegetables. Once ingested, they undergo biotransformation by the intestinal microbiota into enterolignans—primarily enterolactone and enterodiol—which closely resemble endogenous estrogens in both structure and biological activity. Due to this similarity, lignans are classified as phytoestrogens and have been the subject of increasing scientific interest for their broad spectrum of health-promoting effects.
Lignans have been shown to exert antioxidant, anti-inflammatory, and hormone-modulating actions. These properties contribute to their potential role in supporting cardiovascular and metabolic health, improving lipid metabolism, enhancing insulin sensitivity, and lowering risk factors associated with conditions such as diabetes and metabolic syndrome. Additionally, lignans may alleviate menopausal symptoms, promote bone health through their interaction with estrogen receptor β and help prevent hormone-dependent cancers such as breast and endometrial cancer by modulating estrogen signaling pathways and have neuroprotective potential.
The present review aims to provide a comprehensive overview of the biological activities of lignans, synthesizing current knowledge while also incorporating recent advances. In particular, we highlight emerging areas of research, including the neuroprotective and antimicrobial potential of lignans, and point out how interindividual differences in gut microbiota composition may influence their metabolism and effectiveness. The review also draws attention to existing knowledge gaps, such as limited clinical data and the unclear role of sex-specific responses, underscoring the need for further, well-designed human studies.
To ensure a balanced and up-to-date perspective, the literature discussed in this review was identified through a comprehensive search of databases including PubMed, Scopus, and Web of Science, using keywords such as lignans, enterolignans, phytoestrogens, health effects, and biological activity. While priority was given to studies published from 2010 onward, earlier references (1998–2006) were retained where they provided foundational mechanistic insights still relevant today.
1.1. Dietary Sources of Lignans
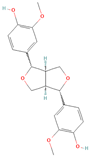
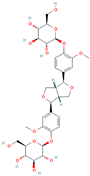
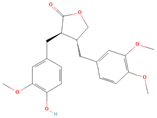
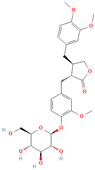
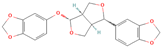
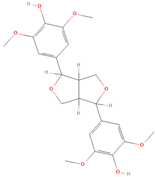
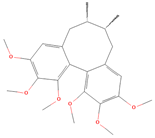
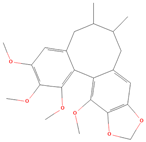
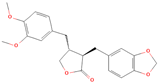
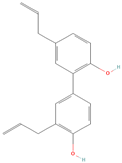
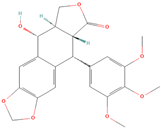
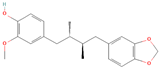
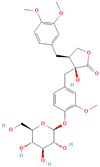
Both plant lignans and their metabolites provide numerous health benefits. As they cannot be synthesized endogenously by humans, lignans must be obtained through dietary sources. Given the broad spectrum of health-promoting effects, it is important to maintain a diet rich in lignans. The primary dietary sources of lignans include flaxseeds and sesame seeds, whole grains (such as rye, barley, and oats), and certain vegetables (e.g., broccoli, kale) and fruits (notably berries) (Table 1).

Table 1.
Lignans and their dietary sources, molecular formulas and structure.
1.2. Biosynthesis and Bioconversion of Lignans
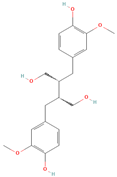
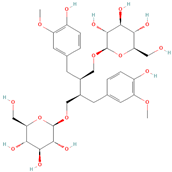
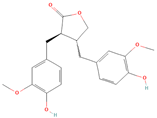
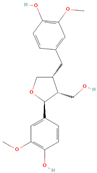
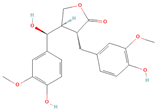
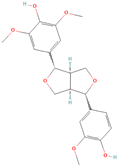
All phytoestrogens, including lignans, are synthesized from phenylpropanoids and simple phenols in plants [39]. Lignans are diphenolic compounds formed by the conjugation of two coniferyl alcohol residues. Stereospecific dimerization of coniferyl alcohol is catalyzed by a dirigent protein resulting in the formation of a dimeric lignan—pinoresinol, a precursor of secoisolariciresinol and matairesinol. Subsequent reactions lead to the formation of secoisolariciresinol diglucoside (SDG) and other derivatives. Sequential enantiospecific reduction in pinoresinol is carried out by reductase, generating lariciresinol followed by secoisolariciresinol (SECO). The glycosylation of SECO is catalyzed by secoisolariciresinol glucosyltransferase, which appears to be primarily localised in seeds [40]. Studies have shown that in flax seeds, SDG occurs in oligomeric form largely with lignan ester linked to 3-hydroxy-3-methylglutaric acid and glucosylated derivatives of hydroxycinnamic acids [41].
In the digestive systems of many animals, lignans ingested with food are converted by intestinal bacteria. SDG and SECO are converted mostly to enterodiol (ED) and enterolactone (EL), which are called mammalian lignans and show structural similarity to estradiol, the most active and dominant form of estrogen in the human body [13]. Their transformations are suspected to be species-specific. In the case of SDG, the gut microbiota first hydrolyzes its sugar residue, releasing SECO. Then, colonic microflora dehydroxylates and demethylates SECO, resulting in the formation of enterodiol. As for enterolactone, it can be formed by oxidation of enterodiol or directly from matairesinol. The main difference between enterolignans and plant lignans is that enterolignans have a hydroxyl group at the meta (3′) position of the aromatic ring, making them chemically stable, whereas plant lignans have oxygenated substituents at the 3′ and 4′ positions [13,40,41]. Following their formation in the gut, mammalian lignans such as enterodiol and enterolactone are absorbed in the colon and enter the hepatic portal system for conjugation in the liver. They are then excreted back into the colon through the bile duct, where they are deconjugated by the enzyme β-glucuronidase and then reabsorbed. Mammalian lignans derived from dietary phytoestrogens are present in blood, bile, faeces, urine, saliva, sperm, and milk [42,43]. Key factors affecting the metabolism of phytoestrogens include the gut microbiome composition and the overall diet, both of which influence bioavailability and conversion to active metabolites [13,44].
2. The Health-Promoting Properties of Lignans
Lignans contribute to animal health through a range of biological effects (Figure 1). They neutralize harmful free radicals, helping to protect cells from oxidative stress and reduce inflammation. Lignans also influence hormonal pathways through their estrogen-like activity, which may affect reproduction and metabolism. Additionally, they can improve cardiovascular function by supporting healthy blood vessels and lipid profiles. Lignans aid in glucose regulation to counteract metabolic disorders and provide antimicrobial and antiviral protection. Their neuroprotective properties may also help preserve brain function, while their anti-cancer potential is linked to their ability to modulate cell growth and reduce the risk of tumor development.
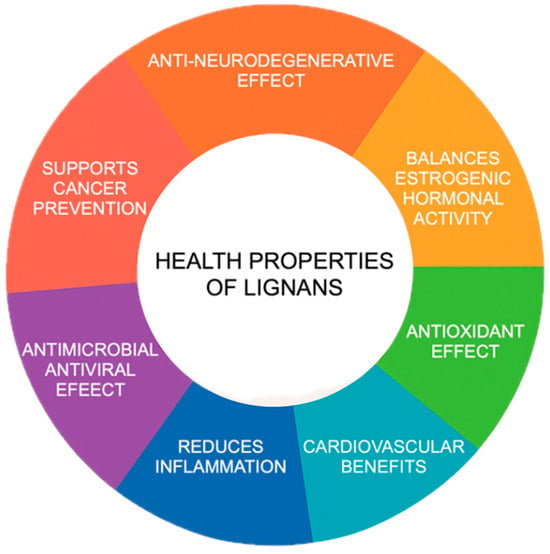
Figure 1.
Health properties of lignans.
2.1. Lignans, Oxidative Stress and Inflammation
Oxidative stress occurs when the body produces too many reactive oxygen species (ROS) compared to its antioxidant capacity. This increased level of oxidative reactions causes inflammation, which can contribute to various diseases such as hypertension [41]. Free radicals originate from various sources, including metabolic reactions, environmental pollution, ionizing radiation, poor diet, or use of stimulants. Excessive free radicals negatively affect the structure and function of the body cells, leading to dangerous diseases. Antioxidant enzymes and vitamins like C, E, and A help the body eliminate ROS more effectively. Lignans also work as antioxidants due to their structure with a high number of hydroxyl groups [40,41].
SDG, ED, and EL have been shown to effectively prevent lipid peroxidation mainly through the quenching of hydroxyl radicals, and this effect is concentration-dependent [45]. The study by Prasad, showed that lignans had higher antioxidant activity compared to vitamin E [46]. SDG and SECO are also effective antioxidants against the DPPH• radical (1,1-diphenyl-2-picrylhydrazyl radical) at concentrations of 25–200 μM, while EL and ED do not exhibit such properties against DPPH. The efficacy of lignans in controlling DNA damage induced by the peroxyl radical AAPH• (2,2′-azo-bis(2-amidinopropane)) has also been confirmed [47]. A study on sauchinone found that it can prevent iron-induced liver damage. Liver iron overload causes oxidative stress leading to hepatocyte damage and inflammation, which can result in liver fibrosis and hepatocellular cancer The action of sauchinone may depend on the activation of activated 5′AMP kinase (AMPK) dependent on LKB1 (human anti-oncogene responsible for encoding the threonine-serine kinase protein) [48].
Table 2 provides a comprehensive overview of the anti-inflammatory and antioxidant properties of lignans, particularly SDG, syringaresinol, schisandrin A, schisandrin B and honokiol. The studies compiled demonstrate that these natural compounds exert protective effects in various in vitro and in vivo models by modulating oxidative stress, inflammatory signaling pathways, and related molecular mechanisms. Syringaresinol was shown to activate Nrf2 signaling [49], inhibit pyroptosis [50], and suppress NF-κB [51] and MAPK pathways [52], resulting in the protection of tissues such as kidney, heart or lung. SchA and SchB primarily protected renal and liver tissues by reducing oxidative stress and fibrosis or ferroptosis [53,54], while honokiol showed neuroprotective effects in a model of kainic acid-induced neurodegeneration [55]. Across the studies, protective outcomes were observed in both animal models (mice or rats) and cultured cells, highlighting the therapeutic potential of these lignans in the prevention or mitigation of inflammation- and oxidative stress-related diseases.

Table 2.
Protective mechanisms of lignans against inflammation and oxidative stress.
2.2. Anti-Neurodegenerative Effect of Lignans
In recent decades, neurodegenerative diseases have become an increasing problem. Neurotoxic factors cause progressive damage to neurons, resulting in motor disorders, memory loss, cognitive impairment, as well as anxiety and depression. The use of lignans seems to be a good solution, as they exhibit neuroprotective properties by regulating apoptosis and modulating different signaling pathways, inhibiting expression of mRNA and inflammatory mediator proteins, and antioxidant effects [99,100,101].
Antioxidative stress, along with accumulation of amyloid beta peptide (Aβ) and tau protein aggregates in the brain and toxicity of metal ions, is one of the causes of Alzheimer’s disease (AD) [102].
Studies in rats and mice have shown that lignans from Schisandra chinensis, such as schisandrin A, may alleviate the neurotoxic effects of inflammation, oxidative stress, Aβ deposition, and tau protein phosphorylation. Moreover, experimental animals showed improved cognitive abilities [103]. Pinoresinol and arctigenin, present in burdock seeds, may also be helpful in the treatment of Alzheimer’s disease. These compounds have reduced memory deficits and learning and memorization problems in experimental animals. The suggested model of action of arctigenin is to reduce hyperphosphorylation of tau protein through the signaling pathway of 3-phosphatidylinositol kinase (PI3K)/Akt protein kinase/glycogen synthase kinase 3 (GSK3β). In the case of pinoresinol, the proposed mode of action is acetylcholinesterase inhibition and facilitation of calcium ion influx into neuronal cells [104,105]. Lignans isolated from Sesame indicum and Acorus tatarinowii mitigated cognitive impairment of Drosophila melanogaster and Caenorhabditis elegans. They showed protective effects against Aβ toxic aggregate [106,107,108].
Another common neurological disorder is Parkinson’s disease (PD). This disease mainly affects the musculoskeletal system, and its pathology is associated with changes in dopaminergic neurons. Studies conducted on a rat model of Parkinson’s disease suggest that sesamin may be useful in PD therapy. After administering 10–20 mg/kg/day to rats for one week, neuroprotective effects were observed. Its potential mechanism of action is through the reduction in oxidative stress, apoptosis and astrogliosis (accumulation of reactive astrocytes at the affected site). Sesamin reduced problems with motor balance in rats, decreased levels of reactive forms of oxygen and malondialdehyde (one of the markers of oxidative stress), increased the activity of superoxide dismutase (SOD), decreased the activity of some enzymes involved in apoptotic processes, and prevented damage to dopaminergic neurons [100,109]. Another compound that may be helpful in PD therapy is honokiol, obtained from the bark and seeds of Magnolia grandiflora. Studies using a mouse model of Parkinson’s disease showed that honokiol has both protective and therapeutic effects on damaged dopaminergic neurons and alleviates motor impairment. One potential mechanism of action of honokiol is modulation of the signaling pathway regulated by nitric oxide [110]. An increase in the number of normal dopaminergic neurons, in mice with induced Parkinson’s disease, was observed following treatment with schisandrine A. This compound also reduced the levels of inflammatory mediators and demonstrated antioxidant activity. In addition, schisandrine A activated proteins associated with autophagy [111]. Giuliano et al. reported that treatment with 7-hydroxymatairesinol was able to slow the progression of dopaminergic neuronal terminal degeneration in the rat PD model. While this treatment did not fully protect dopaminergic cell bodies, it shows potential for alleviating symptoms of Parkinson’s disease [112].
Lignans represent a promising group of compounds with neuroprotective potential, supported by extensive experimental evidence. Specific findings, disease models, lignan types, and molecular targets are summarized in Table 3. This compilation presents an overview of current research on the neuroprotective effects of selected lignans in neurodegenerative diseases, with a focus on Alzheimer’s disease (AD) and Parkinson’s disease. Numerous studies demonstrate that lignans exert multifaceted neuroprotective effects: they reduce oxidative stress, inhibit β-amyloid aggregation, modulate mitochondrial autophagy, improve mitochondrial function, and attenuate neuroinflammatory processes via signaling pathways such as NF-κB, ERK/MAPK, and SIRT3 [66,113,114,115]. In PD models, schisandrin A and 7-hydroxymatairesinol protected dopaminergic neurons and improved motor function [111,112]. In ALS models, honokiol enhanced mitochondrial dynamics and antioxidant capacity, prolonging survival in transgenic mice [116].

Table 3.
Neuroprotective effects of lignans in neurodegenerative disease models.
2.3. Lignans and Osteoporosis
Osteoporosis is a global health problem that affects many older people, especially postmenopausal women, for whom estrogen deficiency is a risk factor for developing the disease [137,138]. Estrogens inhibit bone resorption and block the production of IL-1, IL-6, and TNF-α, which stimulate osteoclasts. Estrogens also stimulate the synthesis of bone matrix components, collagen, and non-collagen proteins. The protective abilities of plant estrogens against bone density loss are most likely due to their affinity for β estrogen receptors, which enables them to inhibit osteoclasts and activate osteoblasts [139,140,141].
A large number of postmenopausal women consume flax seeds as a supplement to their pharmacological drugs. Studies conducted on ovariectomized female rats suggest that lignans, including those from flax, may be useful in protecting against bone fractures or lumbar vertebrae bone loss [142,143,144]. Studies have shown that sezamine can induce osteoblast differentiation by activating the p38 and ERK/MAPK signaling pathway. What is more, it may indirectly influence osteoclast development by enhancing the expression of OPG (osteoprotegerin) and suppressing the expression of RANKL (Receptor Activator for NF-κB Ligand) [141]. It has also been shown that the anti-osteoporotic activity of matairezinol is derived from its ability to counteract osteoclastogenesis Via the p38/ERK-NFATc1 signaling pathway [145].
Table 4 summarizes recent research on specific lignans—such as pinoresinol diglucoside, sesamin, arctigenin, and arctin—highlighting their therapeutic potential in osteoporosis and osteoporotic fracture healing. These compounds were shown to enhance osteogenesis, suppress osteoclast activity, and improve bone structure through modulation of signaling pathways like PI3K/Akt, NF-κB, and Wnt/β-catenin [146,147]. Both in vitro and in vivo studies, suggest their promise as natural treatments for osteoporosis.

Table 4.
Therapeutic Effects of Plant-Derived Lignans in Osteoporosis.
2.4. Lignans and Cardiovascular Diseases
The leading cause of death and disability worldwide are cardiovascular diseases (CVDs), with myocardial infarction and stroke being the predominant causes of disability. Various factors such as hypertension, obesity, inflammation, atherosclerosis, oxidative stress, diabetes, and dyslipidemia promote the development of CVDs. Women are particularly susceptible to CVDs during the postmenopausal period due to the decrease in the concentration of endogenous estrogens, which have a beneficial effect on lipid metabolism, coronary vessels dilation, insulin sensitivity, and blood coagulation. Additionally, estrogens regulate endogenous lipid synthesis by reducing the total cholesterol concentration by approximately 9% and triglyceride (TAG) concentration by approximately 10% [41,158,159]. The development of atherosclerosis is influenced by free radicals and hypercholesterolemia, characterized by high levels of LDL (low density lipoprotein) cholesterol and low levels of HDL (high density lipoprotein) cholesterol.
Lignans exert protective effects both directly, as antioxidants, and indirectly, through the activation of estrogen receptors. SDG, as an antioxidant, has been shown to reduce the development of atherosclerosis caused by high levels of LDL cholesterol. A lignan complex isolated from flax seeds has been shown to reduce the development of atherosclerosis by 34.37%, supporting its potential role in preventing atherosclerosis and lowering the risk of coronary artery disease and stroke. In hypercholesterolemic rabbits, treatment with flaxseed lignans resulted in a 20% reduction in total cholesterol, a 14% decrease in LDL cholesterol, a 34% reduction in the total cholesterol to HDL cholesterol ratio, and a 35% decrease in malondialdehyde (MDA), a marker of lipid peroxidation. Additionally, HDL cholesterol levels rose by 30% in hypercholesterolemic rabbits and by 25% in healthy ones treated with the lignan complex. In contrast, the treatment did not significantly alter total cholesterol, LDL cholesterol, or MDA levels in healthy rabbits. These results indicate that flaxseed lignans may offer cardiovascular benefits primarily in conditions associated with high cholesterol, helping to prevent the progression of atherosclerosis [160].
What is more, studies demonstrate that natural compounds such as flaxseed, arctigenin, schisandrin B, and sesamine exhibit strong cardioprotective properties. These include antiarrhythmic effects, reduced infarction size, and attenuation of oxidative stress and inflammation, making them promising agents in the prevention and treatment of cardiovascular diseases [108,161,162,163,164]. A summary of the cardioprotective mechanisms of lignans is presented in Table 5. Studies in animal models have shown that a flaxseed-rich diet, as well as supplementation with its key components—alpha-linolenic acid (ALA) and SDG—can significantly reduce the incidence of arrhythmias, minimize infarct size, limit left ventricular dilatation, and lower levels of the pro-inflammatory cytokine TNF-α. These results suggest that flaxseed may support both prevention and treatment of arrhythmias and facilitate cardiac repair after myocardial infarction [164].

Table 5.
Mechanisms of cardioprotective action of lignans.
Myocardial ischemia/reperfusion (MI/R) injury and acute myocardial infarction are associated with oxidative stress, inflammation, and arrhythmias. Arctigenin (ATG) has shown significant protective effects in animal models of these conditions. In rats pre-treated with ATG before MI/R, the frequency and duration of ventricular arrhythmias—including fibrillation and tachycardia—were markedly reduced, along with a decrease in infarct size. These effects were accompanied by increased antioxidant enzyme activity and a reduction in malondialdehyde (MDA) levels, indicating lowered oxidative stress. The antiarrhythmic and cardioprotective effects of ATG are likely mediated by activation of the Nrf2 signaling pathway, as well as modulation of iNOS, COX-2, ERK1/2, and HO pathways. These findings suggest that ATG could be a promising agent in preventing infarction and limiting damage caused by ischemia–reperfusion injury [108,163].
Myocardial infarction causes significant damage to heart tissue through inflammation and apoptosis of cardiac cells. Animal studies have shown that both schisandrin B (Sch B) and sesamine exert cardioprotective effects following infarction. Schisandrin B reduces infarct size and apoptosis by activating the PI3K/Akt signaling pathway, increasing phosphorylated Akt levels, and decreasing the expression of pro-apoptotic markers such as caspase-3 and the Bax/Bcl-2 ratio. Sesamine, on the other hand, mitigates myocardial damage by reducing cardiac cell apoptosis and suppressing inflammation. Its effects are linked to the inhibition of the NF-κB pathway and decreased cytokine expression. Together, these compounds demonstrate strong protective effects on the heart after infarction, supporting their potential role in limiting cardiac injury and improving outcomes following myocardial events [161,162].
Human studies have demonstrated the health benefits of SDG supplementation in cardiovascular diseases. The effects of different SDG doses on total cholesterol, LDL cholesterol, metabolic syndrome, and glucose concentration were studied. Zhang et al. have shown that a dose of 600 mg SDG per day effectively reduces total cholesterol, LDL cholesterol, and fasting glucose in plasma in patients with hypercholesterolemia [204]. In the case of moderate hypercholesterolemia in men, consuming 100 mg SDG per day is enough to effectively lower cholesterol, and such supplementation reduces the risk of liver disease. This is related to a reduced ratio of LDL cholesterol to HDL cholesterol and a decrease in glutamine pyruvate transaminase and γ-glutamyl transpeptidase [205]. Studies on the role of flaxseed supplementation among postmenopausal women show that such dietary supplementation improves the lipid profile, which may have a beneficial effect on the cardiovascular system [206]. Studies on middle-aged Finnish men by Vanharanta et al. have shown a significant association between increased serum enterolactone and a reduced risk of death from ischemic heart disease or cardiovascular disease [207]. One of the key processes in ischemic heart disease is the apoptosis of myocardial cells, mediated by oxidative stress. Studies on rat cardiomyocytes have shown that SDG is able to reduce the adverse effect of H2O2 and act as an anti-apoptotic compound by activating the JAK2/STAT3 signaling pathway [170].
Hypertension is an important risk factor for cardiovascular disease, and diet plays an essential role in its prevention and control. Epidemiological studies have suggested an inverse relationship between polyphenol intake and cardiovascular disease. Clinical studies have confirmed that daily oral supplementation of the lignan complex is significantly associated with plasma concentrations of linseed lignans (free and conjugated forms): secoisolariciresinol, enterodiol (ED), and enterolactone (EL). Participants supplementing with the lignan complex (600 mg SDG/day for 6 months) showed a significant decrease in systolic blood pressure from a mean of 155 ± 13 mm Hg at baseline to 140 ± 11 mm Hg at 24 weeks. These data suggest a relatively safe ability to lower systolic blood pressure, which is an important risk factor for cardiovascular disease [208]. Conversely, a study on the relationship between estimated phytoestrogen intake and hypertension, conducted among 1936 men and women aged at least 18 years, based on dietary forms maintained by the subjects, found a significant association between the intake of pinoresinol and blood pressure reduction among men [209].
It is worth mentioning podophyllotoxin (PPT), a lignan that exhibits strong cardiotoxicity, as demonstrated in in vivo studies by elevated cardiac injury markers and histopathological changes. Its toxic mechanism involves oxidative stress (mediated by CYP2E1), a pronounced inflammatory response driven by arachidonic acid metabolites, and disruptions in cardiomyocyte energy metabolism. Additionally, PPT activates the SIRT1/PPAR/NF-κB and Akt1/SREBP 1c signaling pathways, further exacerbating cardiac damage. Studies suggest that PUFA supplementation may offer partial protection against these effects, opening potential avenues for mitigating PPT-induced toxicity [210,211,212].
2.5. Lignans, Diabetes and Metabolic Syndrome
Metabolic syndrome (MS) increases the risk of developing type 2 diabetes and cardiovascular diseases associated with atherosclerosis, making it a major contributing factor in their pathogenesis. MS is characterized by a cluster of factors, including insulin resistance, hyperinsulinemia, abdominal obesity, impaired glucose tolerance, microalbuminuria, hypertriglyceridemia, decreased HDL cholesterol, hypertension, and pro-inflammatory and pro-thrombotic conditions [213,214]. An example of a lignan that significantly inhibits metabolic syndrome is honokiol, an active ingredient found in the traditional Chinese herb magnolia. This was evidenced by improvements in hepatic steatosis, liver fibrosis, adipose tissue inflammation, and insulin resistance. The effect of honokiol was primarily due to AMPK activation. It directly bound to the AMPKγ1 subunit to potently activate AMPK signaling [215].
Diabetes is a group of metabolic diseases characterized by chronic hyperglycemia, which contributes to diabetic retinopathy, diabetic nephropathy, peripheral and autonomic neuropathy, ischemic heart disease, and hypertension. The main cause of diabetes is either a defect in insulin production by the β-cells of the pancreas (type 1) or impaired insulin signaling/action (type 2). Insulin resistance defined as reduced sensitivity of tissues to insulin, is the main cause of type 2 diabetes, which can later lead to impaired secretory function of pancreatic islet β cells [216,217].
The lignan complex extracted from flax has been shown to improve glycemic control. Studies by Prasad et al. in female rats have demonstrated that diabetes is correlated with increased oxidative stress, total cholesterol, triacylglycerols (TAG), and glycated hemoglobin A1C. No elevation of any of these parameters was observed in non-diabetic rats. Animal models with a predisposition to diabetes have shown that treatment with SDG reduces the incidence of diabetes and delays its development. This effect was associated with a reduction in oxidative stress (decrease in MDA and glycated hemoglobin). It has also been observed that taking 40 mg SDG per kilogram of body weight per day effectively delays the onset of type 2 diabetes [218,219].
Human studies have also demonstrated the beneficial effects of SDG against type 2 diabetes. In a study conducted by Pan et al. on individuals aged 50 to 79 years, it was observed that the participants who included lignans in their diet experienced a significant reduction in their HbA1C (glycated hemoglobin) levels as compared to those in the placebo group. The lower the concentration of glycated hemoglobin HbA1C, the lower the risk of developing complications associated with diabetes [220]. In a follow-up study conducted, one year later, by the same group on patients with type 2 diabetes (26 men and 44 postmenopausal women) who had elevated C-reactive protein (CRP) levels—a marker of inflammation— it was found that the increase in CRP was lower in women supplementing with SDG compared to the placebo group. However, no such relationship was observed in men [221]. The results of studies on the effect of flax lignans during exercise training on metabolic syndrome and the risk of osteoporosis in individuals aged 50 years or older have shown that flax lignans reduce diastolic blood pressure and triacylglycerols, as well as parameters of six metabolic syndrome risk factors (fasting glucose, HDL cholesterol, TAG, abdominal obesity, blood pressure, and inflammatory cytokines). These decreases were observed only in men, and no such changes were observed in women [222].
Examples of molecular mechanisms of action of other selected lignans in managing and preventing diabetes are presented in Table 6. Sesamin is not included in the table because its role as bioactive compounds with antioxidant, anti-inflammatory, anti-hypertensive, anti-infective, anti-obesity, anti-diabetic, anti-thrombotic, and lipid-lowering effects has recently been summarized in detail in several reviews [223,224,225,226].

Table 6.
Mechanisms of action of selected lignans in the treatment and prevention of diabetes mellitus.
2.6. Lignans and Breast Cancer
The most common cancer among women is breast cancer [243]. Breast cancer is characterized by significant heterogeneity. Based on immunohistochemical expression of hormone receptors (ER), progesterone receptor (PR), and human epidermal growth factor receptor (HER2), it is classified into distinct subtypes. Four subtypes of breast cancer are commonly distinguished: luminal A (ER+, PR+, HER2−), luminal B (ER+, PR+/PR−, HER2+/HER2−), HER2-positive (ER−, PR−, HER2+), and triple-negative (ER−, PR−, HER2−). Breast cancer develops through distinct molecular mechanisms depending on its subtype. Luminal breast cancers are driven by estrogen signaling through ERα, which regulates genes involved in cell proliferation, while dysregulation of this pathway—particularly Via PI3K/AKT/mTOR activation due to PIK3CA mutations and PTEN loss—promotes tumor growth. In contrast, HER2-positive breast cancer is characterized by HER2 gene amplification, which triggers hyperactivation of PI3K/AKT and MAPK pathways, leading to aggressive cell proliferation. Triple-negative breast cancer (TNBC), lacking hormone receptors and HER2, is primarily linked to BRCA1/BRCA2 and TP53 mutations, which impair DNA repair and promote genomic instability, making it more aggressive and genetically heterogeneous [244]. Lignans may reduce breast cancer risk through multiple molecular mechanisms. They modulate estrogen receptors, inhibit aromatase, and reduce estrogen-driven cell proliferation. They also have antioxidant and anti-inflammatory effects, and can influence gene expression related to cell cycle control and DNA repair [245]. Lignans not only inhibit the development of breast cancer but also all estrogen-dependent cancers (ovarian, and endometrial cancers) by lowering estrogen levels and other enzymes involved in steroid hormone synthesis. These compounds are antagonistic to estrogens, thereby inhibiting the growth of cancer cells [13,246]. Examples of the mechanism of action of lignans on different breast cancer subtypes are presented in Table 7. A meta-analysis from 2021 found that higher ligan intake was correlated with better survival for breast cancer patients [247], including postmenopausal patients [248]. The risk of breast cancer is also significantly reduced in women consuming higher amounts of lignans [249,250].
Consumption of lignans during mammary gland development can have protective effect later in life. It is believed that the reduction in highly proliferating structures called terminal ending buds (TEBs) in the developing gland, by differentiation into structures called alveolar buds (Abs), leads to a reduced risk of developing mammary gland cancer due to the lower proliferation of AB structures than TEB. During the early development of the mammary glands, increasing levels of endogenous estrogen promote the branching of the milk duct, which ends in TEB structures. AB structures are less proliferative than TEB structures, so they are potentially less susceptible to carcinogens. Animal model studies have confirmed that feeding rats during pregnancy and lactation with flax seeds has a positive effect on the differentiation of the mammary gland [251]. In addition, the metabolites of flax seeds or SDG itself, taken together with the mother’s milk at such an early stage of development of the mammary gland in the offspring, affect the reduction in susceptibility to its carcinogenesis later in life [252]. SDG may affect mammary gland morphogenesis by modulating EGFR (epidermal growth factor receptor) and ER (estrogen receptor) signaling pathways [253]. In addition, when cancer is already present, dietary intake of flaxseed or SDG alone reduces the size and number of mammary gland tumors, as well as the invasiveness and malignancy of the cancer [254].

Table 7.
Mechanism of action of lignans on different subtypes of breast cancer.
Table 7.
Mechanism of action of lignans on different subtypes of breast cancer.
| Lignans | Type of Breast Cancer | Action | Mechanism | Reference |
|---|---|---|---|---|
| Trans-(±)-kusunokinin | triple-negative | attenuation of breast cancer cell migration | inhibition of AKR1B1 enzyme activity resulted in the protection of glucose-induced cellular oxidation | [255] |
| (−)-kusunokinin | luminal A | inhibited of breast cancer cell (MCF-7) migration, proliferation, cell cycle and metastasis | decrease in cell proliferation (c-Src, PI3K, Akt, p-Erk1/2 and c-Myc), cell cycle (E2f-1, cyclin B1 and CDK1) and metastasis (E-cadherin, MMP-2 and MMP-9) proteins | [256] |
| Matairesinol | triple-negative | induction of apoptosis | reduction in the viability of M2a and M2d macrophages and repolarization them to M1 phenotype | [257] |
| Secoisolariciresinol diglucoside (SDG) | luminal A | reducing tumor cell proliferation | reduction in PS2, BCL2, and IGF-1R ERα, ERβ, EGFR, BCL2 mRNA expression and PMAPK protein | [258] |
| inhibition of cell proliferation, induction of apoptosis | decreased mRNA expressions of Bcl2, cyclin D1, pS2, ERα, and ERβ, epidermal growth factor receptor, and insulin-like growth factor receptor; decreased phospho-specific mitogen-activated protein kinase expression | [259] | ||
| triple-negative | reduction in tumor growth | inhibition of NF-κB activity | [260] | |
| SDG derivatives | luminal A | induction of apoptosis; reduction in proliferation | the cleavage of PARP inhibition of ERα | [261] |
| induction of apoptosis | overexpressed pro-apoptotic genes (TP53, CDKN1A, and BAX) and underexpressed anti-apoptotic genes (BCL-2) | [262] | ||
| luminal A, triple-negative | cytotoxic, anti-proliferative and pro-oxidant activity | reduction in intracellular oxidative stress and DNA damage | [263] | |
| Podophyllotoxin | triple-negative | inhibition of cell proliferation, migration and invasion; regulation of cell cycle and induction of apoptosis | inhibition of CDC20, PLK1 expression, and CDK1 and increase the expression of P53 | [264] |
| Lariciresinol | HER2-positive | induction of apoptosis | overexpressed pro-apoptotic genes (TP53, CDKN1A, and BAX) and underexpressed anti-apoptotic genes (BCL-2) | [262] |
| Sauchinone | triple-negative | attenuation of proliferation, migration, and invasion | suppresion of Akt-CREB-MMP13 signaling pathway | [265] |
| HER2-positive | inhibition of progression; | regulation of miR-148a-3p/HER-2 axis; increased miR-148a-3p expression, so downregulated HER-2 expression | [23] | |
| Sesamin | HER2-positive | inhibition of cell proliferation; inducing cell cycle arrest; induction of apoptosis | increasing of P53 and Chk2; activation of the Bax and caspase-3 pathways | [266] |
| triple-negative | suppression of proliferation and migration | decreases the expression of PD-L1 Via the downregulation of AKT, NF-κB, and JAK/Stat signaling | [267] | |
| Schisandrin B | triple-negative | induction of cell cycle arrest and apoptosis, inhibition of migration and colony formation of tumor cells | suppression of signal transducer and activator of transcription-3 (STAT3) phosphorylation and nuclear translocation | [268] |
| suppression the growth, migration, and invasion | inhibits interleukin (IL)-1β production of TNBC cells, hindering its progression | [269] | ||
| Schisandrin A | triple-negative | inhibition of migration and induction of apoptosis | reduction in the activation of EGFR, PIK3R1, and MMP9 and increases the expression of cleaved-caspase 3, | [270] |
| induction of cell cycle arrest and apoptosis | regulation of the Wnt/ER stress signaling pathway | [271] | ||
| Schisandrol A | luminal A | promotion of proliferation | activation of ERK, PI3K, Akt, and Erα | [272] |
| Arctigenin | triple-negative | inhibition of the metastasis | inhibition of the activity of matrix metalloproteases MMP-2, MMP-9 and heparanase | [273] |
| reduction in proliferation and induction of apoptosis | inhibition of binding of STAT3 to genomic DNA | [274] | ||
| luminal A, triple-negative | exhibition of anti-metastatic activity | inhibition of MMP-9 (extracellular matrix metalloproteinase) and uPA (plasminogen ukinase activator) Via Akt, NF-κB and MAPK signaling pathways, regardless of estrogen receptor expression | [275] | |
| Honokiol | luminal A, luminal B, triple-negative, HER2-positive | inhibition of growth associated with a G1-phase cell cycle arrest and induction of caspase-dependent apoptosis | attenuate the PI3K/Akt/mTOR (Phosphoinositide 3-kinases/Akt/mammalian target of rapamycin) signalling by down-regulation of Akt phosphorylation and upregulation of PTEN (Phosphatase and Tensin homolog deleted on chromosome Ten) expression | [276] |
| triple-negative | inhibition of proliferation, suppression of migration and induction of apoptosis | modulating the miR-148a-5p-CYP1B1 Axis | [277] | |
| luminal A | induction of apoptosis influence on the cell cycle | suppression of the expression of Bcl-2 decreases the cyclin D1 expression | [278] |
2.7. Lignans and Menopause
Menopause is the cessation of menstruation, caused by the loss of follicular activity of the ovaries, after which no bleeding has occurred for 12 consecutive months. Symptoms of decreased estrogen secretion include abnormal bleeding from the birth canal, hot flashes with sweating, palpitations, and depressive symptoms. These symptoms can last from six months to several years and occur in 65% of women. In addition, there may be an increased risk of cardiovascular disease and cancer (especially breast cancer). One of the ways to prevent the symptoms of menopause is Hormone Replacement Therapy, which involves the administration of replacement doses of female sex hormones. However, there are many contraindications to its use, including diabetes, osteoporosis, estrogen-dependent cancers, and thromboembolic disease. An alternative to such therapy is the use of phytoestrogens [279,280]. Studies have also confirmed that the use of tablets containing a mixture of isoflavones, lignans, and Cimicifuga racemosa (black cohosh) for a minimum of 3 months effectively lowers the Kupperman Index, which is used to assess menopausal symptoms. The combination of those components shows a reduction in postmenopausal symptoms within 24 h [279,281]. A clinical study published in 2025 confirmed the effectiveness of this combination in alleviating menopausal symptoms compared to a placebo confirming its potential as an effective therapeutic option. Clear results were observed—scores on the Menopause Rating Scale decreased by 48%, with significant improvements across all domains (somatic, psychological, and urogenital). Additionally, a 6.7% reduction in FSH levels and a 12.6% increase in estrogen concentrations were noted. Adverse effects were minimal [282].
A meta-analysis by Touillaud et al. (2009) showed that in postmenopausal women, high levels of lignan intake may be associated with a reduced risk of breast cancer [283]. Cardiovascular disease is less common in premenopausal women than in men, but estrogen deficiency increases its incidence [284]. However, data show that higher long-term lignan intake is significantly associated with a reduced risk of total coronary heart disease (CHD) in both men and women [285]. Estrogen activates its receptors, ERα and ERβ, which stimulate endothelial nitric oxide synthase (eNOS) and promote nitric oxide (NO) production, leading to vascular relaxation. It also improves lipid profiles by increasing HDL and lowering LDL levels. In contrast, estrogen deficiency can result in elevated LDL, triggering chronic inflammation in the aorta and liver through macrophage activation and cytokine release (e.g., TNF-α, IL-1β, PAF-AH), ultimately contributing to hypercholesterolemia and atherosclerosis [286,287].
Most symptoms of menopause are associated with chronic inflammation, as estrogen deficiency often results in a marked increase in pro-inflammatory cytokine levels in the serum, liver, bone, and brain. Activation of the NF-κB pathway and the resulting increase in cytokine production across various organs may contribute to the development of atherosclerosis, osteoporosis, and psychological disorders such as depression. Activation of the NF-κB pathway and the resulting in-crease in cytokine production across various organs may contribute to the development of atherosclerosis, osteoporosis, and psychological disorders [288,289,290].
In menopausal women, estrogen deficiency causes substantial and lasting bone loss, primarily due to the impaired regulation of osteoclast activity. Sesamin appears to be the most promising lignan as a therapeutic agent for postmenopausal osteoporosis. Sesamin promotes bone formation through upregulation of Wnt/β-catenin signaling, while concurrently inhibiting bone resorption by downregulating the NF-κB pathway. Moreover, DANCR has been identified as a central regulator of sesamin-induced modulation of bone formation and resorption [146]. A study in rats demonstrated that daily supplementation with sesame oil can provide osteoprotective effects in osteoporotic rats by increasing aromatase and estradiol levels, as well as by modulating the imbalance between bone formation and resorption [291]. Another lignan with anti-osteoporotic activity is matairesinol, which may exert its effects through anti-osteoclastogenic mechanisms involving the p38/ERK-NFATc1 signaling pathway [145].
In a model of osteoporotic bone fracture, sesamin was shown to significantly enhance callus formation and increase cartilage area during the early healing phase, as well as to reduce fracture gap and increase callus volume during the late phase of femoral fracture healing in OVX mice, indicating the therapeutic potential of this lignan in the treatment of osteoporotic fracture [155]. However, an earlier study conducted also in a rat model showed that administration of methanolic extracts from sesame seeds led to a decrease in bone mass, as indicated by bone densitometry and bone formation markers. This highlights the need for further research to clarify the role of sesamin and sesame seeds in mitigating bone loss observed in postmenopausal women [292].
Secoisolariciresinol (SECO) has been found to have a positive effect on reducing depression, which is one of the symptoms of menopausal syndrome. A dose of 10 mg/kg SECO can counteract depression-like behaviors and probably Via an enhancing effect on norepinephrine and dopamine levels [293]. In mice exposed to stressors for six weeks, daily administration of 50 mg/kg of sesamin increased 5-HT levels and decreased norepinephrine levels in the striatum. Sesamin significantly alleviated memory impairment and depression-like behaviors induced by chronic mild stress by inhibiting neuroinflammation through the suppression of excessive microglial activation and the expression of inflammatory mediators, including iNOS, COX-2, TNF-α, and IL-1β, in the hippocampus and cerebral cortex [294]. Furthermore, lignan-rich extracts from Schisandra chinensis and Kava-Kava have also been suggested to alleviate depressive symptoms [295,296].
Estrogen deficiency can also cause urinary incontinence because estrogen receptors are present in the genitourinary tract and pelvic floor musculoskeletal structures. Increased levels of ED and EL (derived from lignans) in urine have been found to decrease the likelihood of acute and mixed urinary incontinence, as demonstrated by studies conducted among postmenopausal women [297]. A study by Hallund et al. conducted among postmenopausal women found that the lignan complex isolated from flax reduces the concentration of C-reactive protein (CRP), which is not only a marker of inflammation but also a marker for assessing the risk of vascular disease, heart disease, or stroke (its value then increases significantly) [298,299]. In 2024, a study involving 51 women confirmed the beneficial effects of flax lignans on cardiometabolic risk after menopause. Participants consumed 40 g of flaxseed daily for 8 weeks, which led to an improved lipid profile (increased HDL and decreased LDL) and a significant reduction in CRP levels [300].
In summary, lignans may represent a promising compound with broad preventive and therapeutic potential, particularly in addressing health challenges associated with menopause, such as obesity and cardiovascular diseases
2.8. Antimicrobial and Antiviral Properties of Lignans
There are many emerging drug-resistant microbes, making it crucial to find alternative compounds with antimicrobial properties. Besides their already mentioned activities lignans have been shown to possess antiviral, antibacterial, and antifungal properties.
Lignans display broad antimicrobial activity. For example, nortrachelogenin disrupts bacterial membranes and is effective against antibiotic-resistant strains [301]. Hinokinin targets Staphylococcus aureus and MRSA [302]. Sesamin inhibits L-tryptophan indole-lyase, reducing production of indoxyl sulfate, a toxin linked to kidney disease [303]. Flax-derived lignans, including SDG, inhibit growth of S. aureus, S. aureus, E. coli, P. aeruginosa and B. subtilis [304,305]. 7-Hydroxymatairesinol (HMR) and lignans from Sonchus asper also act against various pathogens, including Klebsiella, Proteus, S. cerevisiae, and Staphylococcus spp. [306,307]. One of the key antibacterial mechanisms of lignans is the disorganization or disruption of the bacterial plasma membrane, destabilizing membrane potential, impairing substance transport and in some cases leading to loss of membrane integrity, leakage of intracellular contents, and ultimately cell death. Lignans have been shown to inhibit bacterial biofilm formation by interfering with cell adhesion. Additionally, some lignans act as inhibitors of essential bacterial enzymes, such as L-tryptophan indole-lyase (TIL). TIL plays a significant role in bacterial physiology, influencing biofilm formation, antibiotic resistance, plasmid retention, and virulence. Inhibiting this enzyme with lignans may therefore reduce bacterial pathogenicity and survival. Moreover, lignans can promote oxidative stress in microbial cells and inhibit nucleic acid synthesis, further contributing to their antimicrobial activity [301,303,308,309,310]. Lignans represent a large class of natural compounds with broad antiviral properties or example, lignans isolated from Boesenbergia thorelii roots have shown promising antiviral activity against HIV [311]. Trachelogenin, a dibenzyl lignan, may be a novel inhibitor of hepatitis C because it blocks the penetration of viruses into hepatocytes, preventing the interaction of viruses with the CD81 host protein [312]. Other promising lignans with anti-HCV activity may be flax lignans, which inhibit viral replication [313]. Phillyrin has been shown to inhibit the expression of the influenza A virus nuclear protein (NP) gene and significantly block viral replication. Recent studies also highlight its potential against SARS-CoV-2, the virus behind COVID-19. Bioinformatics analysis revealed 192 shared targets and 25 pathways in co-infection with influenza and SARS-CoV-2, with HIF-1, PI3K-AKT, and RAS signaling pathways likely playing key roles in its antiviral action, offering new prospects for COVID-19 therapy [314]. Studies have shown that lignans from Schisandra chinensis possess antiviral properties. Schizandrin B and deoxyschizandrin effectively inhibit the DNA polymerase activity of HIV-1 reverse transcriptase, disrupting early stages of viral replication. Schizandrin A, on the other hand, suppresses dengue virus replication by activating the STAT1/2 signaling pathway and enhancing the interferon response. Schizandrin C stimulates the cGAS-STING pathway and promotes the production of interferon-β (IFN-β), leading to the inhibition of hepatitis B virus replication. Moreover, its combination with luteolin shows a synergistic antiviral effect in an HBV-infected mouse model [27]. The antiviral activity of lignans has been observed through cytopathic inhibition assays, syncytium formation assays by virus-infected cells, and inhibition of viral reverse transcriptase. The antiviral mechanisms of lignans include the inhibition of key viral enzymes (e.g., integrase, protease), disruption of viral fusion and internalization, as well as suppression of viral antigen expression and genome replication. This makes lignans promising candidates for further research into antiviral drug development. Xu et al. conducted a systematic review that included over 600 lignans evaluated for their antiviral activity [315]. Lignans, acting through various mechanisms, including membrane disruption and enzyme inhibition, exhibit promising antifungal activity against several human pathogenic fungi. Moreover, studies suggest that combining lignans with conventional antifungal drugs or natural antioxidants may produce synergistic effects, potentially reducing the required dosages and minimizing adverse effects [316,317]. According to the conducted studies, linseed extracts containing lignans exhibited moderate (between 70 and 90%) antifungal activity against Aspergillus flavus and Aspergillus niger, plant pathogens that can cause infections or allergic reactions in people with immune disorders. Additionally, the toxins secreted by these fungi are harmful to human health due to their potent hepatotoxic and carcinogenic properties [313]. The extract from Larrea tridentata, rich in two lignans: methylnordihydroguaiaretic acid and nordihydroguaiaretic acid—also demonstrated strong antifungal properties against A.flavus and Aspergillus parasiticus [318]. Antifungal activity of honokiol has been observed in studies on Candida albicans. The simultaneous addition of vitamin C may significantly enhance the antifungal effect of honokiol, whereas vitamin E reduces the effectiveness of honokiol against C. albicans [317]. One of the mechanisms of antifungal action of honokiol is that it acts as a prooxidant in C. albicans, causing mitochondrial dysfunction and increasing apoptosis of fungal cells [319]. Magnolol is another compound exhibiting antifungal activity against C. albicans. Studies have shown that magnolol inhibits yeast biofilm formation by 69.5%. Moreover, cell membrane damage, cell wall and plasma membrane ruptures, cell deformation and intracellular release were observed in magnolol-treated C. albicans cells [320]. Studies on other lignans, benzofuran derivatives, have shown that these compounds have antifungal activity, but this is often associated with toxic effects on mammalian cells [321]. Further pharmacokinetic and in vivo toxicity assessments are necessary to fully evaluate the therapeutic potential of lignans in treating human fungal infections.
In summary, lignans are promising compounds with multifaceted antimicrobial activity. Examples of lignans and microorganisms susceptible to their antimicrobial properties are presented in Table 8.

Table 8.
Examples of lignans and target microbes demonstrating sensitivity to their antimicrobial properties.
3. Summary
In summary, lignans—plant-derived polyphenols metabolized in the gut into estrogen-like enterolignans—exhibit a broad spectrum of biological activities. As presented in this review, their antioxidant, anti-inflammatory, hormone-modulating, and antimicrobial effects contribute to a wide range of health benefits. These include the prevention of hormone-dependent cancers, support for cardiovascular and metabolic functions, protection against neurodegenerative disorders, and the alleviation of menopausal symptoms. Such multifaceted actions position lignans as promising compounds in the prevention and management of chronic diseases, as well as valuable bioactive ingredients in functional foods.
Given their diverse benefits, lignans represent an important and expanding area of interest in both nutritional science and medical research. However, despite substantial evidence from in vitro and animal studies, clinical data remain limited. Further human studies are necessary to determine optimal dosing, improve understanding of their bioavailability, and confirm therapeutic efficacy. Future research should also address how individual factors—such as age, sex, and gut microbiota composition—influence lignan metabolism and activity. Additionally, the exploration of potential synergistic effects with other phytochemicals or pharmaceuticals may enhance their clinical utility. A particularly important direction for future investigation lies in evaluating the role of lignans in neurodegenerative diseases, metabolic syndrome, and hormone-dependent cancers through well-designed, large-scale clinical trials. Finally, the development of lignan-enriched functional foods or supplements with improved bioavailability could further facilitate their practical application in health promotion.
Author Contributions
Conceptualization, M.B. and A.K.; writing—original draft preparation, M.B., J.M., B.A., W.W. and A.K.; writing—review and editing, M.B., J.M., B.A., W.W. and A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Herchi, W.; Arráez-Román, D.; Trabelsi, H.; Bouali, I.; Boukhchina, S.; Kallel, H.; Segura-Carretero, A.; Fernández-Gutierrez, A. Phenolic Compounds in Flaxseed: A Review of Their Properties and Analytical Methods. An Overview of the Last Decade. J. Oleo Sci. 2014, 63, 7–14. [Google Scholar] [CrossRef]
- Frank, J.; Eliasson, C.; Leroy-Nivard, D.; Budek, A.; Lundh, T.; Vessby, B.; Åman, P.; Kamal-Eldin, A. Dietary secoisolariciresinol diglucoside and its oligomers with 3-hydroxy-3-methyl glutaric acid decrease vitamin E levels in rats. Br. J. Nutr. 2004, 92, 169–176. [Google Scholar] [CrossRef]
- Sicilia, T.; Niemeyer, H.B.; Honig, D.M.; Metzler, M. Identification and Stereochemical Characterization of Lignans in Flaxseed and Pumpkin Seeds. J. Agric. Food Chem. 2003, 51, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, G.; Colazo, M.G.; Oba, M.; Dyck, M.K.; Okine, E.K.; Ambrose, D.J. Fecal and Urinary Lignans, Intrafollicular Estradiol, and Endometrial Receptors in Lactating Dairy Cows Fed Diets Supplemented with Hydrogenated Animal Fat, Flaxseed or Sunflower Seed. J. Reprod. Dev. 2008, 54, 439–446. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Milder, I.E.J.; Arts, I.C.W.; Putte, B.V.D.; Venema, D.P.; Hollman, P.C.H. Lignan contents of Dutch plant foods: A database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol. Br. J. Nutr. 2005, 93, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Kezimana, P.; Dmitriev, A.A.; Kudryavtseva, A.V.; Romanova, E.V.; Melnikova, N.V. Secoisolariciresinol Diglucoside of Flaxseed and Its Metabolites: Biosynthesis and Potential for Nutraceuticals. Front. Genet. 2018, 9, 641. [Google Scholar] [CrossRef]
- Thompson, L.U.; Boucher, B.A.; Liu, Z.; Cotterchio, M.; Kreiger, N. Phytoestrogen Content of Foods Consumed in Canada, Including Isoflavones, Lignans, and Coumestan. Nutr. Cancer 2006, 54, 184–201. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally Lignan-Rich Foods: A Dietary Tool for Health Promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef]
- Peterson, J.; Dwyer, J.; Adlercreutz, H.; Scalbert, A.; Jacques, P.; McCullough, M.L. Dietary lignans: Physiology and potential for cardiovascular disease risk reduction. Nutr. Rev. 2010, 68, 571–603. [Google Scholar] [CrossRef]
- Moral, R.; Escrich, E. Influence of Olive Oil and Its Components on Breast Cancer: Molecular Mechanisms. Molecules 2022, 27, 477. [Google Scholar] [CrossRef]
- De Torres, A.; Espínola, F.; Moya, M.; Alcalá, S.; Vidal, A.M.; Castro, E. Assessment of phenolic compounds in virgin olive oil by response surface methodology with particular focus on flavonoids and lignans. LWT 2018, 90, 22–30. [Google Scholar] [CrossRef]
- Smeds, A.I.; Eklund, P.C.; Sjöholm, R.E.; Willför, S.M.; Nishibe, S.; Deyama, T.; Holmbom, B.R. Quantification of a Broad Spectrum of Lignans in Cereals, Oilseeds, and Nuts. J. Agric. Food Chem. 2007, 55, 1337–1346. [Google Scholar] [CrossRef]
- Landete, J.M. Plant and mammalian lignans: A review of source, intake, metabolism, intestinal bacteria and health. Food Res. Int. 2012, 46, 410–424. [Google Scholar] [CrossRef]
- Berenshtein, L.; Okun, Z.; Shpigelman, A. Stability and Bioaccessibility of Lignans in Food Products. ACS Omega 2024, 9, 2022–2031. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Ohno, A.; Yomoda, S.; Inamasu, S. Arctigenin-containing burdock sprout extract prevents obesity in association with modulation of the gut microbiota in mice. Biosci. Microbiota Food Health 2023, 42, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wink, M. Natural lignans from Arctium lappa as antiaging agents in Caenorhabditis elegans. Phytochemistry 2015, 117, 340–350. [Google Scholar] [CrossRef]
- Sun, Y.; Tan, Y.; Lu, Z.; Li, B.; Sun, C.; Li, T.; Zhao, L.; Liu, Z.; Zhang, G.; Yao, J.; et al. Arctigenin Inhibits Liver Cancer Tumorigenesis by Inhibiting Gankyrin Expression via C/EBPα and PPARα. Front. Pharmacol. 2018, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yang, M.; Zuo, Z. Overview of the anti-inflammatory effects, pharmacokinetic properties and clinical efficacies of arctigenin and arctiin from Arctium lappa L. Acta Pharmacol. Sin. 2018, 39, 787–801. [Google Scholar] [CrossRef]
- Chowdhury, R.; Bhuia, M.S.; Wilairatana, P.; Afroz, M.; Hasan, R.; Ferdous, J.; Rakib, A.I.; Sheikh, S.; Mubarak, M.S.; Islam, M.T. An insight into the anticancer potentials of lignan arctiin: A comprehensive review of molecular mechanisms. Heliyon 2024, 10, e32899. [Google Scholar] [CrossRef]
- Smeds, A.I.; Jauhiainen, L.; Tuomola, E.; Peltonen-Sainio, P. Characterization of Variation in the Lignan Content and Composition of Winter Rye, Spring Wheat, and Spring Oat. J. Agric. Food Chem. 2009, 57, 5837–5842. [Google Scholar] [CrossRef]
- Qin, X.; Liu, X.; Guo, C.; Huang, L.; Xu, Q. Medioresinol from Eucommiae cortex improves myocardial infarction-induced heart failure through activation of the PI3K/AKT/mTOR pathway: A network analysis and experimental study. PLoS ONE 2024, 19, e0311143. [Google Scholar] [CrossRef]
- Wang, E.-C.; Shih, M.-H.; Liu, M.-C.; Chen, M.-T.; Lee, G.-H. Studies of Constituents of Saururus chinensis. Heterocycles 1996, 43, 969. [Google Scholar] [CrossRef]
- Hu, X.; Wang, J.; Shang, P.; Wang, S.; Chen, L.; Ye, C.; Yao, G. Sauchinone inhibits breast cancer cell proliferation through regulating microRNA-148a-3p/HER-2 axis. Thorac. Cancer 2023, 14, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Liu, X.; Meng, X.; Cao, L.; Li, H.; Bi, Y.; Wang, M.; Wang, M.; Jiang, Y. Sauchinone alleviates dextran sulfate sodium-induced ulcerative colitis via NAD(P)H dehydrogenase [quinone] 1/NF-kB pathway and gut microbiota. Front. Microbiol. 2023, 13, 1084257. [Google Scholar] [CrossRef]
- Moazzami, A.A.; Haese, S.L.; Kamal-Eldin, A. Lignan contents in sesame seeds and products. Eur. J. Lipid Sci. Technol. 2007, 109, 1022–1027. [Google Scholar] [CrossRef]
- Rosalina, R.; Weerapreeyakul, N. An Insight into Sesamolin: Physicochemical Properties, Pharmacological Activities, and Future Research Prospects. Molecules 2021, 26, 5849. [Google Scholar] [CrossRef]
- Ehambarampillai, D.; Wan, M.L.Y. A comprehensive review of Schisandra chinensis lignans: Pharmacokinetics, pharmacological mechanisms, and future prospects in disease prevention and treatment. Chin. Med. 2025, 20, 47. [Google Scholar] [CrossRef]
- Yu, B.; Sheng, D.; Tan, Q. Determination of Schisandrin A and Schisandrin B in Traditional Chinese Medicine Preparation Huganpian Tablet by RP-HPLC. Chem. Pharm. Bull. 2019, 67, 713–716. [Google Scholar] [CrossRef]
- Nasser, M.I.; Zhu, S.; Chen, C.; Zhao, M.; Huang, H.; Zhu, P. A Comprehensive Review on Schisandrin B and Its Biological Properties. Oxid. Med. Cell. Longev. 2020, 2020, 2172740. [Google Scholar] [CrossRef]
- Sriwiriyajan, S.; Sukpondma, Y.; Srisawat, T.; Madla, S.; Graidist, P. (−)-Kusunokinin and piperloguminine from Piper nigrum: An alternative option to treat breast cancer. Biomed. Pharmacother. 2017, 92, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Poivre, M.; Duez, P. Biological activity and toxicity of the Chinese herb Magnolia officinalis Rehder & E. Wilson (Houpo) and its constituents. J. Zhejiang Univ.-Sci. B 2017, 18, 194–214. [Google Scholar] [CrossRef] [PubMed]
- Siudem, P.; Wasiak, A.; Zielińska, A.; Kowalska, V.; Paradowska, K. Using Lignans from Magnolia officinalis Bark in the Assessment of the Quality of Dietary Supplements—The Application of 1H NMR and HPLC-DAD. Int. J. Mol. Sci. 2025, 26, 1659. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhao, D.; Yin, D.; Duan, K.; Wang, Z. Plant Origin Source, Content Profile and Bioactivity of Podophyllotoxin as an Important Natural Anticancer Agent. Chem. Biodivers. 2025, 22, e202402375. [Google Scholar] [CrossRef] [PubMed]
- Shah, Z.; Gohar, U.F.; Jamshed, I.; Mushtaq, A.; Mukhtar, H.; Zia-UI-Haq, M.; Toma, S.I.; Manea, R.; Moga, M.; Popovici, B. Podophyllotoxin: History, Recent Advances and Future Prospects. Biomolecules 2021, 11, 603. [Google Scholar] [CrossRef]
- Abourashed, E.A.; El-Alfy, A.T. Chemical diversity and pharmacological significance of the secondary metabolites of nutmeg (Myristica fragrans Houtt.). Phytochem. Rev. 2016, 15, 1035–1056. [Google Scholar] [CrossRef]
- Paul, S.; Hwang, J.K.; Kim, H.Y.; Jeon, W.K.; Chung, C.; Han, J.-S. Multiple biological properties of macelignan and its pharmacological implications. Arch. Pharm. Res. 2013, 36, 264–272. [Google Scholar] [CrossRef]
- Yoo, H.H.; Park, J.H.; Kwon, S.W. An Anti-Estrogenic Lignan Glycoside, Tracheloside, from Seeds of Carthamus tinctorius. Biosci. Biotechnol. Biochem. 2006, 70, 2783–2785. [Google Scholar] [CrossRef]
- Wang, H.-F.; Huang, Z.-H.; Zou, W.; Lai, C.-H.; Tan, Q.-G. Tracheloside, the main constituent of the total lignan extract from Trachelospermi Caulis, inhibited rheumatoid arthritis via IL-17/MAPK signaling pathway. Fitoterapia 2025, 180, 106311. [Google Scholar] [CrossRef]
- Kenneth, D.S. Phytoestrogens: The biochemistry, physiology, and implications for human health of soy isoflavones. Am. J. Clin. Nutr. 1998, 68, 1333S–1346S. [Google Scholar] [CrossRef] [PubMed]
- Touré, A.; Xueming, X. Flaxseed Lignans: Source, Biosynthesis, Metabolism, Antioxidant Activity, Bio-Active Components, and Health Benefits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 261–269. [Google Scholar] [CrossRef]
- Adolphe, J.L.; Whiting, S.J.; Juurlink, B.H.J.; Thorpe, L.U.; Alcorn, J. Health effects with consumption of the flax lignan secoisolariciresinol diglucoside. Br. J. Nutr. 2010, 103, 929–938. [Google Scholar] [CrossRef]
- Murkies, A.L.; Wilcox, G.; Davis, S.R. Phytoestrogens. J. Clin. Endocrinol. Metab. 1998, 83, 297–303. [Google Scholar] [CrossRef]
- Rizzolo-Brime, L.; Caro-Garcia, E.M.; Alegre-Miranda, C.A.; Felez-Nobrega, M.; Zamora-Ros, R. Lignan exposure: A worldwide perspective. Eur. J. Nutr. 2022, 61, 1143–1165. [Google Scholar] [CrossRef]
- Raffaelli, B.; Hoikkala, A.; Leppälä, E.; Wähälä, K. Enterolignans. J. Chromatogr. B 2002, 777, 29–43. [Google Scholar] [CrossRef]
- Prasad, K.; Mantha, S.V.; Muir, A.D.; Westcott, N.D. Reduction of hypercholesterolemic atherosclerosis by CDC-flaxseed with very low alpha-linolenic acid. Atherosclerosis 1998, 136, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K. Reduction of Serum Cholesterol and Hypercholesterolemic Atherosclerosis in Rabbits by Secoisolariciresinol Diglucoside Isolated From Flaxseed. Circulation 1999, 99, 1355–1362. [Google Scholar] [CrossRef]
- Hu, C.; Yuan, Y.V.; Kitts, D.D. Antioxidant activities of the flaxseed lignan secoisolariciresinol diglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone in vitro. Food Chem. Toxicol. 2007, 45, 2219–2227. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Lee, S.M.; Shin, S.M.; Hwang, S.J.; Brooks, J.S.; Kang, H.E.; Lee, M.G.; Kim, S.C.; Kim, S.G. Efficacy of sauchinone as a novel AMPK-activating lignan for preventing iron-induced oxidative stress and liver injury. Free Radic. Biol. Med. 2009, 47, 1082–1092. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, T.; Wang, Y.; Li, G.; Wang, Y.; Tian, W.; Feng, L.; Zhang, S.; Xu, Y.; Gao, Y.; et al. Syringaresinol Alleviates Early Diabetic Retinopathy by Downregulating HIF-1α/VEGF via Activating Nrf2 Antioxidant Pathway. Mol. Nutr. Food Res. 2024, 68, 2200771. [Google Scholar] [CrossRef]
- Wei, A.; Liu, J.; Li, D.; Lu, Y.; Yang, L.; Zhuo, Y.; Tian, W.; Cong, H. Syringaresinol attenuates sepsis-induced cardiac dysfunction by inhibiting inflammation and pyroptosis in mice. Eur. J. Pharmacol. 2021, 913, 174644. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, D.; Deng, B.; Yan, L. Syringaresinol attenuates osteoarthritis via regulating the NF-κB pathway. Int. Immunopharmacol. 2023, 118, 109982. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, J.; Tan, L.; Li, Z.; Gao, P.; He, S.; Wang, Q.; Tang, D.; Wang, C.; Wang, F.; et al. (–)-Syringaresinol attenuates ulcerative colitis by improving intestinal epithelial barrier function and inhibiting inflammatory responses. Phytomedicine 2024, 124, 155292. [Google Scholar] [CrossRef]
- Shi, H.; Yan, Y.; Yang, H.; Pu, P.; Tang, H. Schisandrin B Diet Inhibits Oxidative Stress to Reduce Ferroptosis and Lipid Peroxidation to Prevent Pirarubicin-Induced Hepatotoxicity. BioMed Res. Int. 2022, 2022, 5623555. [Google Scholar] [CrossRef]
- Liu, H.-L.; Huang, Z.; Li, Q.-Z.; Cao, Y.-Z.; Wang, H.-Y.; Alolgab, R.N.; Deng, X.-Y.; Zhang, Z.-H. Schisandrin A alleviates renal fibrosis by inhibiting PKCβ and oxidative stress. Phytomedicine 2024, 126, 155372. [Google Scholar] [CrossRef]
- Demir, M.; Cetinavci, D.; Dogan, K.; Elbe, H.; Saruhan, E. Honokiol prevents central kainic acid-induced neurodegeneration by suppressing oxidative stress, inflammation, and TGF-β1 expression. Arch. Physiol. Biochem. 2025, 1–12. [Google Scholar] [CrossRef]
- Aqeel, T.; Gurumallu, S.C.; Bhaskar, A.; Hashimi, S.M.; Lohith, N.C.; Javaraiah, R. Protective role of flaxseed lignan secoisolariciresinol diglucoside against lead-acetate-induced oxidative-stress-mediated nephrotoxicity in rats. Phytomed. Plus 2021, 1, 100038. [Google Scholar] [CrossRef]
- He, X.; Wang, Y.; Wu, M.; Wei, J.; Sun, X.; Wang, A.; Hu, G.; Jia, J. Secoisolariciresinol Diglucoside Improves Ovarian Reserve in Aging Mouse by Inhibiting Oxidative Stress. Front. Mol. Biosci. 2022, 8, 806412. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhu, R.; Wu, T.; Zhao, W. Amelioration of oxidative kidney damage in offspring by maternal trans-fatty acid exposure in mice by secoisolariciresinol diglucoside. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2023, 48, 967–978. [Google Scholar] [CrossRef]
- Zhang, L.; Lan, Y.; Wang, Y.; Yang, Y.; Han, W.; Li, J.; Wang, Y.; Liu, X. Secoisolariciresinol diglucoside ameliorates high fat diet-induced colon inflammation and regulates gut microbiota in mice. Food Funct. 2022, 13, 3009–3022. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Liu, J.; Wang, T.; Liu, Y.; Liu, Y.; Guo, L.; Bai, Z.; Shen, W.; Yan, R.; et al. Dietary secoisolariciresinol diglucoside ameliorates high-fat diet-induced atherosclerosis via regulating immunological inflammation and reshaping gut microbiota in ApoE-/- mice. J. Funct. Foods 2025, 124, 106642. [Google Scholar] [CrossRef]
- Ge, J.; Hao, R.; Rong, X.; Dou, Q.P.; Tan, X.; Li, G.; Li, F.; Li, D. Secoisolariciresinol diglucoside mitigates benzo[a]pyrene-induced liver and kidney toxicity in mice via miR-101a/MKP-1-mediated p38 and ERK pathway. Food Chem. Toxicol. 2022, 159, 112733. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.; Liu, X.; Yang, Y.; Zhang, Y.; Li, B.; Guo, F.; Liang, J.; Hong, X.; Guo, R.; et al. Secoisolariciresinol diglucoside Ameliorates Osteoarthritis via Nuclear factor-erythroid 2-related factor-2/nuclear factor kappa B Pathway: In vitro and in vivo experiments. Biomed. Pharmacother. 2023, 164, 114964. [Google Scholar] [CrossRef]
- Li, A.-L.; Li, G.-H.; Li, Y.-R.; Wu, X.-Y.; Ren, D.-M.; Lou, H.-X.; Wang, X.-N.; Shen, T. Lignan and flavonoid support the prevention of cinnamon against oxidative stress related diseases. Phytomedicine 2019, 53, 143–153. [Google Scholar] [CrossRef]
- Trinh Tat, C.; Duc Thien, D.; Tran, N.T.H.; Anh Duc, N.; Duc Manh, H.; Quang Huy, N.; Ngo, L.H.; Nguyen, N.Q. Effects of Pinoresinol from Vietnamese Gnetum montanum Markgr Inhibits the Inflammation in Macrophages. J. Rep. Pharm. Sci. 2025, 13, e156394. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, Y.; Yao, X.; Yi, J.; Feng, G. Pinoresinol diglucoside alleviates ischemia/reperfusion-induced brain injury by modulating neuroinflammation and oxidative stress. Chem. Biol. Drug Des. 2021, 98, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Wu, S.; Wang, G.; Li, B.; Liu, B.; Lei, X. Pinoresinol diglucoside attenuates neuroinflammation, apoptosis and oxidative stress in a mice model with Alzheimer’s disease. NeuroReport 2021, 32, 259–267. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Wang, R.; Shen, J.; Wang, J.; Li, L. Lariciresinol protects rats from complete Freund’s adjuvant induced arthritis in rats via modulation of transforming growth factor-β and nuclear factor kappa B pathway: An in vivo and in silico study. Chem. Biol. Drug Des. 2023, 102, 168–176. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Y.; Li, Q. Matairesinol exerts anti-inflammatory and antioxidant effects in sepsis-mediated brain injury by repressing the MAPK and NF-κB pathways through up-regulating AMPK. Aging 2021, 13, 23780–23795. [Google Scholar] [CrossRef] [PubMed]
- Salama, S.A.; Mohamadin, A.M.; Abdel-Bakky, M.S. Arctigenin alleviates cadmium-induced nephrotoxicity: Targeting endoplasmic reticulum stress, Nrf2 signaling, and the associated inflammatory response. Life Sci. 2021, 287, 120121. [Google Scholar] [CrossRef] [PubMed]
- Kanawati, G.M.; Al-Khateeb, I.H.; Kandil, Y.I. Arctigenin attenuates CCl4-induced hepatotoxicity through suppressing matrix metalloproteinase-2 and oxidative stress. Egypt. Liver J. 2021, 11, 1. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Zhou, Y.; Chen, T.; Lei, J.-C.; Jiang, X.-J. AMPK/SIRT1 Pathway is Involved in Arctigenin-Mediated Protective Effects Against Myocardial Ischemia-Reperfusion Injury. Front. Pharmacol. 2021, 11, 616813. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Pu, S.; Wang, X.; Guo, L.; Zhang, L.; Wang, Z. Ameliorative Effects of Arctigenin on Pulmonary Fibrosis Induced by Bleomycin via the Antioxidant Activity. Oxid. Med. Cell. Longev. 2022, 2022, 3541731. [Google Scholar] [CrossRef] [PubMed]
- Medras, Z.J.H.; Mostafa, Y.M.; Ahmed, A.A.M.; El-Sayed, N.M. Arctigenin improves neuropathy via ameliorating apoptosis and modulating autophagy in streptozotocin-induced diabetic mice. CNS Neurosci. Ther. 2023, 29, 3068–3080. [Google Scholar] [CrossRef]
- Yuan, Q.; Wu, Y.; Wang, G.; Zhou, X.; Dong, X.; Lou, Z.; Li, S.; Wang, D. Preventive effects of arctigenin from Arctium lappa L. against LPS-induced neuroinflammation and cognitive impairments in mice. Metab. Brain Dis. 2022, 37, 2039–2052. [Google Scholar] [CrossRef]
- Ji, Z.; Guo, R.; Ma, Z.; Li, H. Arctigenin inhibits apoptosis, extracellular matrix degradation, and inflammation in human nucleus pulposus cells by up-regulating miR-483-3p. J. Clin. Lab. Anal. 2022, 36, e24508. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Dou, P.; Zhang, X.; Ran, X.; Liu, L.; Dou, D. The Ameliorative Effects of Arctiin and Arctigenin on the Oxidative Injury of Lung Induced by Silica via TLR-4/NLRP3/TGF- β Signaling Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 5598980. [Google Scholar] [CrossRef] [PubMed]
- Almeaqli, M.T.; Alaidaa, Y.; Alnajjar, F.M.; Al Shararh, A.S.; Alharbi, D.S.; Almslmani, Y.I.; Alotibi, Y.A.; Alrashidi, H.S.; Alshehri, W.A.; Hassan, H.M.; et al. Therapeutic Effects of Arctiin on Alzheimer’s Disease-like Model in Rats by Reducing Oxidative Stress, Inflammasomes and Fibrosis. Curr. Alzheimer Res. 2024, 21, 276–288. [Google Scholar] [CrossRef]
- Fu, Y.; Li, X.; Zeng, Y.; Zhang, A.; Qiu, S. Arctiin attenuated NASH by inhibiting glycolysis and inflammation via FGFR2/CSF1R signaling. Eur. J. Pharmacol. 2025, 996, 177424. [Google Scholar] [CrossRef]
- Li, J.; Du, X.; Mu, Z.; Han, X. Arctiin Alleviates Atopic Dermatitis Against Inflammation and Pyroptosis Through Suppressing TLR4/MyD88/NF-κB and NLRP3/Caspase-1/GSDMD Signaling Pathways. J. Inflamm. Res. 2024, 17, 8009–8026. [Google Scholar] [CrossRef]
- Yang, J.; Chen, D.; He, Q.; Chen, B.; Pan, Z.; Zhang, G.; Li, M.; Li, S.; Xiao, J.; Wang, H.; et al. Arctiin alleviates knee osteoarthritis by suppressing chondrocyte oxidative stress induced by accumulated iron via AKT/NRF2/HO-1 signaling pathway. Sci. Rep. 2024, 14, 31935. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Xiao, F.; Wang, Z.; Liu, J. Arctiin attenuates lipid accumulation, inflammation and oxidative stress in nonalcoholic fatty liver disease through inhibiting MAPK pathway. Qual. Assur. Saf. Crops Foods 2022, 14, 105–114. [Google Scholar] [CrossRef]
- Yuan, L.; Sun, C. The protective effects of Arctiin in asthma by attenuating airway inflammation and inhibiting p38/NF-κB signaling. Aging 2024, 16, 5038–5049. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, L.; Liang, Y.; Li, J.; Pan, X. Arctiin suppresses H9N2 avian influenza virus-mediated inflammation via activation of Nrf2/HO-1 signaling. BMC Complement. Med. Ther. 2021, 21, 289. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Bai, J.; Wang, X.; Yuan, Q.; Mi, Y.; Zhang, C. Bioactive Lignan Honokiol Alleviates Ovarian Oxidative Stress in Aging Laying Chickens by Regulating SIRT3/AMPK Pathway. Antioxidants 2024, 13, 377. [Google Scholar] [CrossRef]
- Kuk, M.U.; Lee, Y.H.; Kim, D.; Lee, K.S.; Park, J.H.; Yoon, J.H.; Lee, Y.J.; So, B.; Kim, M.; Kwon, H.W.; et al. Sauchinone Ameliorates Senescence Through Reducing Mitochondrial ROS Production. Antioxidants 2025, 14, 259. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Yang, H.; Heng, X.; Xu, T.; Zhang, K.; Zhao, Y.; Liu, Y.; Han, D.; Wu, Y.; Zhang, W.; et al. Sauchinone preserves cardiac function in doxorubicin-induced cardiomyopathy by inhibiting the NLRP3 inflammasome. Phytomedicine 2025, 140, 156624. [Google Scholar] [CrossRef]
- Alshahrani, S.; Ali Thubab, H.M.; Ali Zaeri, A.M.; Anwer, T.; Ahmed, R.A.; Jali, A.M.; Qadri, M.; Nomier, Y.; Moni, S.S.; Alam, M.F. The Protective Effects of Sesamin against Cyclophosphamide-Induced Nephrotoxicity through Modulation of Oxidative Stress, Inflammatory-Cytokines and Apoptosis in Rats. Int. J. Mol. Sci. 2022, 23, 11615. [Google Scholar] [CrossRef]
- Du, H.; Tong, S.; Kuang, G.; Gong, X.; Jiang, N.; Yang, X.; Liu, H.; Li, N.; Xie, Y.; Xiang, Y.; et al. Sesamin Protects against APAP-Induced Acute Liver Injury by Inhibiting Oxidative Stress and Inflammatory Response via Deactivation of HMGB1/TLR4/NFκB Signal in Mice. J. Immunol. Res. 2023, 2023, 1116841. [Google Scholar] [CrossRef]
- Chang, C.; Cheng, H.; Chou, W.; Huang, Y.; Hsieh, P.; Chu, P.; Lee, S. Sesamin suppresses angiotensin-II-enhanced oxidative stress and hypertrophic markers in H9c2 cells. Environ. Toxicol. 2023, 38, 2165–2172. [Google Scholar] [CrossRef]
- Kitipaspallop, W.; Phuwapraisirisan, P.; Kim, W.-K.; Chanchao, C.; Pimtong, W. Sesamin lacks zebrafish embryotoxicity but exhibits evidence of anti-angiogenesis, anti-oxidant and anti-inflammatory activities. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 269, 109637. [Google Scholar] [CrossRef]
- Zheng, W.; Song, Z.; Li, S.; Hu, M.; Shaukat, H.; Qin, H. Protective Effects of Sesamol against Liver Oxidative Stress and Inflammation in High-Fat Diet-Induced Hepatic Steatosis. Nutrients 2021, 13, 4484. [Google Scholar] [CrossRef]
- Ruankham, W.; Suwanjang, W.; Wongchitrat, P.; Prachayasittikul, V.; Prachayasittikul, S.; Phopin, K. Sesamin and sesamol attenuate H2O2-induced oxidative stress on human neuronal cells via the SIRT1-SIRT3-FOXO3a signaling pathway. Nutr. Neurosci. 2021, 24, 90–101. [Google Scholar] [CrossRef]
- Ramazani, E.; Ebrahimpour, F.; Emami, S.A.; Shakeri, A.; Javadi, B.; Sahebkar, A.; Tayarani-Najaran, Z. Neuroprotective Effects of Sesamum indicum, Sesamin and SesamolinAgainst 6-OHDA-induced Apoptosis in PC12 Cells. Recent Adv. Food Nutr. Agric. 2023, 14, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Karrar, E.; Liu, R.; Chang, M.; Wang, X. Comparative effects of sesame lignans (sesamin, sesamolin, and sesamol) on oxidative stress and lipid metabolism in steatosis HepG2 cells. J. Food Biochem. 2022, 46, e14180. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Kim, H.S.; Park, S.H.; Kim, D.; Hong, Y.D.; Kim, J.H.; Cho, J.Y. Syringaresinol derived from Panax ginseng berry attenuates oxidative stress-induced skin aging via autophagy. J. Ginseng Res. 2022, 46, 536–542. [Google Scholar] [CrossRef]
- Wisidsri, N.; Chansriniyom, C.; Limpanasitikul, W. Syringaresinol prevents human epidermal keratinocytes from oxidative stress, DNA damage, and cellular senescence induced by UVB irradiation. J. Pharm. Pharmacogn. Res. 2025, 13, 991–1002. [Google Scholar] [CrossRef]
- Zhuo, Y.; Yang, L.; Li, D.; Zhang, L.; Zhang, Q.; Zhang, S.; Li, C.; Cui, L.; Hao, J.; Li, J.; et al. Syringaresinol Resisted Sepsis-Induced Acute Lung Injury by Suppressing Pyroptosis Via the Oestrogen Receptor-β Signalling Pathway. Inflammation 2022, 45, 824–837. [Google Scholar] [CrossRef]
- Li, G.; Liu, C.; Yang, L.; Feng, L.; Zhang, S.; An, J.; Li, J.; Gao, Y.; Pan, Z.; Xu, Y.; et al. Syringaresinol protects against diabetic nephropathy by inhibiting pyroptosis via NRF2-mediated antioxidant pathway. Cell Biol. Toxicol. 2023, 39, 621–639. [Google Scholar] [CrossRef]
- Garlapati, P.K.; Raghavan, A.K.; Shivanna, N. Phytochemicals Having Neuroprotective Properties from Dietary Sources and Medicinal Herbs. Pharmacogn. J. 2014, 7, 1–17. [Google Scholar] [CrossRef]
- Shimoyoshi, S.; Takemoto, D.; Ono, Y.; Kitagawa, Y.; Shibata, H.; Tomono, S.; Unno, K.; Wakabayashi, K. Sesame Lignans Suppress Age-Related Cognitive Decline in Senescence-Accelerated Mice. Nutrients 2019, 11, 1582. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. An overview of neuroprotective and cognitive enhancement properties of lignans from Schisandra chinensis. Biomed. Pharmacother. 2018, 97, 958–968. [Google Scholar] [CrossRef]
- Kantham, S.; Chan, S.; McColl, G.; Miles, J.A.; Veliyath, S.K.; Deora, G.S.; Dighe, S.N.; Khabbazi, S.; Parat, M.-O.; Ross, B.P. Effect of the Biphenyl Neolignan Honokiol on Aβ42-Induced Toxicity in Caenorhabditis elegans, Aβ42 Fibrillation, Cholinesterase Activity, DPPH Radicals, and Iron(II) Chelation. ACS Chem. Neurosci. 2017, 8, 1901–1912. [Google Scholar] [CrossRef]
- Wei, M.; Liu, Z.; Liu, Y.; Li, S.; Hu, M.; Yue, K.; Liu, T.; He, Y.; Pi, Z.; Liu, Z.; et al. Urinary and plasmatic metabolomics strategy to explore the holistic mechanism of lignans in S. chinensis in treating Alzheimer’s disease using UPLC-Q-TOF-MS. Food Funct. 2019, 10, 5656–5668. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Dou, D.-Q.; Jiang, H.; Zhang, B.-B.; Qin, W.-Y.; Kang, K.; Zhang, N.; Jia, D. Arctigenin Attenuates Learning and Memory Deficits through PI3k/Akt/GSK-3β Pathway Reducing Tau Hyperphosphorylation in Aβ-Induced AD Mice. Planta Med. 2016, 83, 51–56. [Google Scholar] [CrossRef]
- Yu, J.; Kwon, H.; Cho, E.; Jeon, J.; Kang, R.H.; Youn, K.; Jun, M.; Lee, Y.C.; Ryu, J.H.; Kim, D.H. The effects of pinoresinol on cholinergic dysfunction-induced memory impairments and synaptic plasticity in mice. Food Chem. Toxicol. 2019, 125, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Keowkase, R.; Shoomarom, N.; Bunargin, W.; Sitthithaworn, W.; Weerapreeyakul, N. Sesamin and sesamolin reduce amyloid-β toxicity in a transgenic Caenorhabditis elegans. Biomed. Pharmacother. 2018, 107, 656–664. [Google Scholar] [CrossRef]
- Luo, X.-H.; Zhang, Y.-Y.; Chen, X.-Y.; Sun, M.-L.; Li, S.; Wang, H.-B. Lignans from the roots of Acorus tatarinowii Schott ameliorate β amyloid-induced toxicity in transgenic Caenorhabditis elegans. Fitoterapia 2016, 108, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Y. Arctigenin exerts protective effects against myocardial infarction via regulation of iNOS, COX-2, ERK1/2 and HO-1 in rats. Mol. Med. Rep. 2018, 17, 4839–4845. [Google Scholar] [CrossRef]
- Baluchnejadmojarad, T.; Mansouri, M.; Ghalami, J.; Mokhtari, Z.; Roghani, M. Sesamin imparts neuroprotection against intrastriatal 6-hydroxydopamine toxicity by inhibition of astroglial activation, apoptosis, and oxidative stress. Biomed. Pharmacother. 2017, 88, 754–761. [Google Scholar] [CrossRef]
- Chen, H.-H.; Chang, P.-C.; Chen, C.; Chan, M.-H. Protective and therapeutic activity of honokiol in reversing motor deficits and neuronal degeneration in the mouse model of Parkinson’s disease. Pharmacol. Rep. 2018, 70, 668–676. [Google Scholar] [CrossRef]
- Zhi, Y.; Jin, Y.; Pan, L.; Zhang, A.; Liu, F. Schisandrin A ameliorates MPTP-induced Parkinson’s disease in a mouse model via regulation of brain autophagy. Arch. Pharm. Res. 2019, 42, 1012–1020. [Google Scholar] [CrossRef]
- Giuliano, C.; Siani, F.; Mus, L.; Ghezzi, C.; Cerri, S.; Pacchetti, B.; Bigogno, C.; Blandini, F. Neuroprotective effects of lignan 7-hydroxymatairesinol (HMR/lignan) in a rodent model of Parkinson’s disease. Nutrition 2020, 69, 110494. [Google Scholar] [CrossRef]
- Li, H.; Sun, J.; Wu, Y.; Yang, Y.; Zhang, W.; Tian, Y. Honokiol relieves hippocampal neuronal damage in Alzheimer’s disease by activating the SIRT3-mediated mitochondrial autophagy. CNS Neurosci. Ther. 2024, 30, e14878. [Google Scholar] [CrossRef]
- Li, H.; Jia, J.; Wang, W.; Hou, T.; Tian, Y.; Wu, Q.; Xu, L.; Wei, Y.; Wang, X. Honokiol Alleviates Cognitive Deficits of Alzheimer’s Disease (PS1V97L) Transgenic Mice by Activating Mitochondrial SIRT3. J. Alzheimer’s Dis. 2018, 64, 291–302. [Google Scholar] [CrossRef]
- Jia, S.; Guan, H.; Zhang, S.; Li, Q. Schisandrin A Alleviates Inflammation and Oxidative Stress in Aβ25−35-Induced Alzheimer’s Disease in Vitro Model. Actas Esp. Psiquiatr. 2024, 52, 724–732. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, J.; Lan, J.; Zhang, Y.; Wang, H.; Chen, Q.; Kang, Y.; Sun, Y.; Feng, X.; Wu, L.; et al. Honokiol alleviated neurodegeneration by reducing oxidative stress and improving mitochondrial function in mutant SOD1 cellular and mouse models of amyotrophic lateral sclerosis. Acta Pharm. Sin. B 2023, 13, 577–597. [Google Scholar] [CrossRef]
- Jia, M.; Ning, F.; Wen, J.; Wang, X.; Chen, J.; Hu, J.; Chen, X.; Liu, Z. Secoisolariciresinol diglucoside attenuates neuroinflammation and cognitive impairment in female Alzheimer’s disease mice via modulating gut microbiota metabolism and GPER/CREB/BDNF pathway. J. Neuroinflamm. 2024, 21, 201. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Y.; Ni, C.; Song, G. Honokiol Attenuates Oligomeric Amyloid β1-42-Induced Alzheimer’s Disease in Mice Through Attenuating Mitochondrial Apoptosis and Inhibiting the Nuclear Factor Kappa-B Signaling Pathway. Cell. Physiol. Biochem. 2017, 43, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Sasia, C.; Borgonetti, V.; Mancini, C.; Lori, G.; Arbiser, J.L.; Taddei, M.L.; Galeotti, N. The Neolignan Honokiol and Its Synthetic Derivative Honokiol Hexafluoro Reduce Neuroinflammation and Cellular Senescence in Microglia Cells. Cells 2024, 13, 1652. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.T.; Vu, C.M.; Ly, T.T.B.; Nguyen, N.T.; Nguyen, P.T.M.; Chu, H.H. Effect of Honokiol on culture time and survival of Alzheimer’s disease iPSC-derived neurons. Bioimpacts 2023, 14, 27652. [Google Scholar] [CrossRef]
- Singh, L.; Singh, S. Neuroprotective potential of Honokiol in ICV-STZ induced neuroinflammation, Aβ(1–42) and NF-kB expression in experimental model of rats. Neurosci. Lett. 2023, 799, 137090. [Google Scholar] [CrossRef]
- Gu, L.; Cai, N.; Li, M.; Bi, D.; Yao, L.; Fang, W.; Wu, Y.; Hu, Z.; Liu, Q.; Lin, Z.; et al. Inhibitory Effects of Macelignan on Tau Phosphorylation and Aβ Aggregation in the Cell Model of Alzheimer’s Disease. Front. Nutr. 2022, 9, 892558. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Cho, E.; Kwon, H.; Jeon, J.; Seong Sin, J.; Kwon Park, J.; Kim, J.-S.; Woong Choi, J.; Jin Park, S.; Jun, M.; et al. Akt and calcium-permeable AMPA receptor are involved in the effect of pinoresinol on amyloid β-induced synaptic plasticity and memory deficits. Biochem. Pharmacol. 2021, 184, 114366. [Google Scholar] [CrossRef]
- Han, R.; Yu, Y.; Zhao, K.; Wei, J.; Hui, Y.; Gao, J.-M. Lignans from Eucommia ulmoides Oliver leaves exhibit neuroprotective effects via activation of the PI3K/Akt/GSK-3β/Nrf2 signaling pathways in H2O2-treated PC-12 cells. Phytomedicine 2022, 101, 154124. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Dan, D.; Xu, J.; Qiu, C.; He, K.; Zhang, C.-E.; Li, S.; Yang, X.; Xu, P.; Zhu, F. Arctigenin attenuated spatial memory impairment in pR5 mice by regulating mitochondrial energy metabolism. J. Pharm. Pharmacol. 2024, 76, 154–161. [Google Scholar] [CrossRef]
- Wei, L.; Xue, Z.; Lan, B.; Yuan, S.; Li, Y.; Guo, C.; Zhang, R.; Ding, R.; Shen, H. Arctigenin Exerts Neuroprotective Effect by Ameliorating Cortical Activities in Experimental Autoimmune Encephalomyelitis In Vivo. Front. Immunol. 2021, 12, 691590. [Google Scholar] [CrossRef]
- Arabi, A.; Karimi, S.A.; Salehi, I.; Haddadi, R.; Komaki, A. Effects of sesamin on Aβ1-42-induced oxidative stress and LTP impairment in a rat model of Alzheimer’s disease. Metab. Brain Dis. 2023, 38, 1503–1511. [Google Scholar] [CrossRef]
- Udomruk, S.; Wudtiwai, B.; Hla Shwe, T.; Phitak, T.; Pothacharoen, P.; Phimphilai, M.; Kongtawelert, P. Sesamin suppresses advanced glycation end products induced microglial reactivity using BV2 microglial cell line as a model. Brain Res. Bull. 2021, 172, 190–202. [Google Scholar] [CrossRef]
- Piao, Z.; Song, L.; Yao, L.; Zhang, L.; Lu, Y. Schisandrin Restores the Amyloid β-Induced Impairments on Mitochondrial Function, Energy Metabolism, Biogenesis, and Dynamics in Rat Primary Hippocampal Neurons. Pharmacology 2021, 106, 254–264. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Q.; Guan, H.; Zhou, Y.; Liu, L. Schisandrin Inhibits NLRP1 Inflammasome-Mediated Neuronal Pyroptosis in Mouse Models of Alzheimer’s Disease. Neuropsychiatr. Dis. Treat. 2021, 17, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Y.; Zhao, T.; Mou, T.; Jing, W.; Chen, J.; Hao, W.; Gu, S.; Cui, M.; Sun, Y.; et al. Schisandrin alleviates the cognitive impairment in rats with Alzheimer’s disease by altering the gut microbiota composition to modulate the levels of endogenous metabolites in the plasma, brain, and feces. Front. Pharmacol. 2022, 13, 888726. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Song, M.; Wu, Y.; Li, Z.; Zhang, S.; Fan, X. Schisandrin B ameliorates Alzheimer’s disease by suppressing neuronal ferroptosis and ensuing microglia M1 polarization. Phytomedicine 2025, 142, 156780. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Guo, N.; Luo, G.; Wang, L.; Wang, J.; Gao, J.; Qin, W.; Yao, L.; Li, G. Schisandrin B loaded carrier with synergistic antioxidation alleviates Alzheimer’s disease in Caenorhabditis elegans by prolonging lifespan and inhibiting Aβ aggregation. J. Ind. Eng. Chem. 2025, in press. [CrossRef]
- Meng, X.; Zhao, W.; Yang, R.; Xu, S.; Wang, S.; Li, M.; Jiang, Y.; Hao, Z.; Guan, W.; Kuang, H.; et al. Lignans from Schisandra chinensis (Turcz.) Baill ameliorates cognitive impairment in Alzheimer’s disease and alleviates ferroptosis by activating the Nrf2/FPN1 signaling pathway and regulating iron levels. J. Ethnopharmacol. 2025, 341, 119335. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Li, M.-M.; Wu, J.-T.; Sun, Y.; Pan, J.; Guan, W.; Naseem, A.; Algradi, A.M.; Kuang, H.-X.; Jiang, Y.-K.; et al. Lignans of Schisandra chinensis (Turcz.) Baill inhibits Parkinson’s disease progression through mediated neuroinflammation-TRPV1 expression in microglia. Phytomedicine 2024, 135, 156146. [Google Scholar] [CrossRef]
- Kim, N.-Y.; Ko, M.S.; Lee, C.H.; Lee, T.J.; Hwang, K.-W.; Park, S.-Y. Inhibitory Effects of Forsythia velutina and its Chemical Constituents on LPS-induced Nitric Oxide Production in BV2 Microglial Cells. Nat. Prod. Sci. 2022, 28, 153–160. [Google Scholar] [CrossRef]
- Arjmandi, B.H.; Smith, B.J. Soy isoflavones’ osteoprotective role in postmenopausal women: Mechanism of action. J. Nutr. Biochem. 2002, 13, 130–137. [Google Scholar] [CrossRef]
- Sozen, T.; Ozisik, L.; Calik Basaran, N. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Gruber, C.J.; Tschugguel, W.; Schneeberger, C.; Huber, J.C. Production and Actions of Estrogens. N. Engl. J. Med. 2002, 346, 340–352. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Harrath, A.H. Phytoestrogens and their effects. Eur. J. Pharmacol. 2014, 741, 230–236. [Google Scholar] [CrossRef]
- Wanachewin, O.; Boonmaleerat, K.; Pothacharoen, P.; Reutrakul, V.; Kongtawelert, P. Sesamin stimulates osteoblast differentiation through p38 and ERK1/2 MAPK signaling pathways. BMC Complement. Altern. Med. 2012, 12, 71. [Google Scholar] [CrossRef]
- Sacco, S.M.; Jiang, J.M.Y.; Reza-López, S.; Ma, D.W.L.; Thompson, L.U.; Ward, W.E. Flaxseed combined with low-dose estrogen therapy preserves bone tissue in ovariectomized rats. Menopause 2009, 16, 545–554. [Google Scholar] [CrossRef]
- Yin, J.; Tezuka, Y.; Subehan; Shi, L.; Nobukawa, M.; Nobukawa, T.; Kadota, S. In vivo anti-osteoporotic activity of isotaxiresinol, a lignan from wood of Taxus yunnanensis. Phytomedicine 2006, 13, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Pan, Y.-L.; Hu, S.-J.; Kong, X.-H.; Juan, W.; Mei, Q.-B. Effects of total lignans from Eucommia ulmoides barks prevent bone loss in vivo and in vitro. J. Ethnopharmacol. 2014, 155, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-W.; Park, K.-I.; Yeon, J.-T.; Ryu, B.J.; Kim, K.-J.; Kim, S.H. Anti-osteoclastogenic activity of matairesinol via suppression of p38/ERK-NFATc1 signaling axis. BMC Complement. Altern. Med. 2014, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Feng, L.; Wang, H.; Li, Y.; Lo, J.H.T.; Zhang, X.; Lu, X.; Wang, Y.; Lin, S.; Tortorella, M.D.; et al. DANCR Mediates the Rescuing Effects of Sesamin on Postmenopausal Osteoporosis Treatment via Orchestrating Osteogenesis and Osteoclastogenesis. Nutrients 2021, 13, 4455. [Google Scholar] [CrossRef]
- Li, M.; Pan, Z.; He, Q.; Xiao, J.; Chen, B.; Wang, F.; Kang, P.; Luo, H.; Li, J.; Zeng, J.; et al. Arctiin attenuates iron overload-induced osteoporosis by regulating the PI3K/Akt pathway. Int. J. Mol. Med. 2023, 52, 108. [Google Scholar] [CrossRef]
- Chen, G.; Chen, Y.; Hong, J.; Gao, J.; Xu, Z. Secoisolariciresinol diglucoside regulates estrogen receptor expression to ameliorate OVX-induced osteoporosis. J. Orthop. Surg. Res. 2023, 18, 792. [Google Scholar] [CrossRef]
- Thomas, E.; Panjagari, N.R.; Ganguly, S.; Deepika, S.; Kapila, S.; Singh, A.K. Development and Validation of Flaxseed Lignan-Enriched Set-Type Fermented Milk to Manage Postmenopausal Osteoporosis. Fermentation 2024, 10, 72. [Google Scholar] [CrossRef]
- Yang, X.; Wu, D.; Zhuang, C.; Ma, C. Anti-osteoporosis effects of mammalian lignans and their precursors from flaxseed and safflower seed using zebrafish model. J. Food Sci. 2023, 88, 5278–5290. [Google Scholar] [CrossRef]
- Tu, X.; Wu, S.; Li, M.; Chen, Z.; Liu, C.; Ruan, Y.; Zeng, J.; Shi, W.; Liu, J.; Zhang, F. Characterization of metabolic features and potential anti-osteoporosis mechanism of pinoresinol diglucoside using metabolite profiling and network pharmacology. Rapid Commun. Mass Spectrom. 2024, 38, e9872. [Google Scholar] [CrossRef]
- Zuo, Y.; Chen, C.; Liu, F.; Hu, H.; Dong, S.; Shen, Q.; Zeng, J.; Huang, L.; Liao, X.; Cao, Z.; et al. Pinoresinol diglucoside mitigates dexamethasone-induced osteoporosis and chondrodysplasia in zebrafish. Toxicol. Appl. Pharmacol. 2024, 484, 116884. [Google Scholar] [CrossRef]
- Jin, Z.; Li, H.; Bi, F.; Cao, H. The effects of Pinoresinol diglucoside on the differentiation and bone resorption of osteoclast RAW264.7. Food Sci. Technol. 2022, 42, e89221. [Google Scholar] [CrossRef]
- Li, H.; Liao, X.; Lan, M.; He, J.; Gao, J.; Fan, Z.; Huang, J.; Wu, X.; Chen, J.; Sun, G. Arctigenin Modulates Adipogenic-Osteogenic Balance in the Bone Marrow Microenvironment of Ovariectomized Rats via the MEK1/PPARγ/Wnt/β-Catenin Pathway. Chem. Biol. Drug Des. 2024, 104, e14625. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Feng, L.; Wang, M.; Li, Y.; Bai, S.; Lu, X.; Wang, H.; Zhang, X.; Wang, Y.; Lin, S.; et al. Sesamin Promotes Osteoporotic Fracture Healing by Activating Chondrogenesis and Angiogenesis Pathways. Nutrients 2022, 14, 2106. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.-H.; Mok, D.K.-W.; Yao, X.-S.; Wong, M.-S. Lignans from Sambucus williamsii Protect Bone Via Microbiome. Curr. Osteoporos. Rep. 2024, 22, 497–501. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, B.; Li, B.; Wang, C.; Li, G.; Cao, W.; Zeng, F.; Chen, Y. Greater Consumption of Total and Individual Lignans and Dietary Fibers Were Significantly Associated with Lowered Risk of Hip Fracture—A 1:1 Matched Case–Control Study among Chinese Elderly Men and Women. Nutrients 2022, 14, 1100. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Johnstone, B.M.; Cook-Newell, M.E. Meta-Analysis of the Effects of Soy Protein Intake on Serum Lipids. N. Engl. J. Med. 1995, 333, 276–282. [Google Scholar] [CrossRef]
- van der Schouw, Y.T.; de Kleijn, M.J.; Peeters, P.H.; Grobbee, D.E. Phyto-oestrogens and cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. 2000, 10, 154–167. [Google Scholar]
- Prasad, K. Hypocholesterolemic and antiatherosclerotic effect of flax lignan complex isolated from flaxseed. Atherosclerosis 2005, 179, 269–275. [Google Scholar] [CrossRef]
- Fan, D.; Yang, Z.; Yuan, Y.; Wu, Q.-Q.; Xu, M.; Jin, Y.-G.; Tang, Q.-Z. Sesamin prevents apoptosis and inflammation after experimental myocardial infarction by JNK and NF-κB pathways. Food Funct. 2017, 8, 2875–2885. [Google Scholar] [CrossRef]
- Zhao, X.; Xiang, Y.; Cai, C.; Zhou, A.; Zhu, N.; Zeng, C. Schisandrin B protects against myocardial ischemia/reperfusion injury via the PI3K/Akt pathway in rats. Mol. Med. Rep. 2017, 17, 556–561. [Google Scholar] [CrossRef]
- Yang, J.; Yin, H.; Cao, Y.; Jiang, Z.; Li, Y.; Song, M.; Wang, Y.; Wang, Z.; Yang, R.; Jiang, Y.; et al. Arctigenin Attenuates Ischemia/Reperfusion Induced Ventricular Arrhythmias by Decreasing Oxidative Stress in Rats. Cell. Physiol. Biochem. 2018, 49, 728–742. [Google Scholar] [CrossRef]
- Parikh, M.; Raj, P.; Austria, J.A.; Yu, L.; Garg, B.; Netticadan, T.; Pierce, G.N. Dietary flaxseed protects against ventricular arrhythmias and left ventricular dilation after a myocardial infarction. J. Nutr. Biochem. 2019, 71, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, A.H.; Negm, A.M.; Mahmoud, E.S.; Salama, R.M.; Schaalan, M.F.; El-Sheikh, A.A.K.; Ramadan, B.K. The cardioprotective effects of secoisolariciresinol diglucoside (flaxseed lignan) against cafeteria diet-induced cardiac fibrosis and vascular injury in rats: An insight into apelin/AMPK/FOXO3a signaling pathways. Front. Pharmacol. 2023, 14, 1199294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cheng, M.; Wang, Z.; Liu, Y.; Ren, Y.; Rong, S.; Wang, X. Secoisolariciresinol Diglucoside Exerts Anti-Inflammatory and Antiapoptotic Effects through Inhibiting the Akt/IκB/NF-κB Pathway on Human Umbilical Vein Endothelial Cells. Mediat. Inflamm. 2020, 2020, 3621261. [Google Scholar] [CrossRef]
- Penumathsa, S.V.; Koneru, S.; Zhan, L.; John, S.; Menon, V.P.; Prasad, K.; Maulik, N. Secoisolariciresinol diglucoside induces neovascularization-mediated cardioprotection against ischemia–reperfusion injury in hypercholesterolemic myocardium. J. Mol. Cell. Cardiol. 2008, 44, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Penumathsa, S.V.; Koneru, S.; Thirunavukkarasu, M.; Zhan, L.; Prasad, K.; Maulik, N. Secoisolariciresinol Diglucoside: Relevance to Angiogenesis and Cardioprotection against Ischemia-Reperfusion Injury. J. Pharmacol. Exp. Ther. 2007, 320, 951–959. [Google Scholar] [CrossRef]
- Jalal, I.A.; Elkhoely, A.; Mohamed, S.K.; Ahmed, A.A.E. Linagliptin and secoisolariciresinol diglucoside attenuate hyperlipidemia and cardiac hypertrophy induced by a high-methionine diet in rats via suppression of hyperhomocysteinemia-induced endoplasmic reticulum stress. Front. Pharmacol. 2023, 14, 1275730. [Google Scholar] [CrossRef]
- Huang, G.; Huang, X.; Liu, M.; Hua, Y.; Deng, B.; Jin, W.; Yan, W.; Tan, Z.; Wu, Y.; Liu, B.; et al. Secoisolariciresinol diglucoside prevents the oxidative stress-induced apoptosis of myocardial cells through activation of the JAK2/STAT3 signaling pathway. Int. J. Mol. Med. 2018, 41, 3570–3576. [Google Scholar] [CrossRef]
- Zhang, T.; Li, L.; Mo, X.; Xie, S.; Liu, S.; Zhao, N.; Zhang, H.; Chen, S.; Zeng, X.; Wang, S.; et al. Matairesinol blunts adverse cardiac remodeling and heart failure induced by pressure overload by regulating Prdx1 and PI3K/AKT/FOXO1 signaling. Phytomedicine 2024, 135, 156054. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, R.; Zhang, J.; Liang, T.; Guo, J.; Sun, T.; Fu, X.; Wang, L.; Zhang, L. Pinoresinol diglucoside (PDG) attenuates cardiac hypertrophy via AKT/mTOR/NF-κB signaling in pressure overload-induced rats. J. Ethnopharmacol. 2021, 272, 113920. [Google Scholar] [CrossRef]
- Wei, Y.; Xiao, L.; Yingying, L.; Haichen, W. Pinoresinol diglucoside ameliorates H/R-induced injury of cardiomyocytes by regulating miR-142-3p and HIF1AN. J. Biochem. Mol. Toxicol. 2022, 36, e23175. [Google Scholar] [CrossRef]
- Xie, Y.; Sui, W.; Qin, S.; Yao, Q.; Fan, D.; Li, T.; Wang, F.; Fu, X.; Zhang, L. Pinoresinol diglucoside alleviates pressure overload-induced cardiac injury via the AMPK/SIRT3/RIG-1 pathway. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, X.; Liang, L.; Liu, H.; Li, L.; Shan, Q. Intramyocardial delivery of injectable hydrogel with arctigenin alleviated myocardial ischemia–reperfusion injury in rats. Biotechnol. Appl. Biochem. 2024, 71, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.-H.; Sun, S.-N.; Zhou, Z.; Li, Y.; Huang, Y.-S.; Li, H.; Wang, J.-J.; Xiao, W.; Xian, S.-X.; Yang, Z.-Q.; et al. Arctigenin alleviates myocardial infarction injury through inhibition of the NFAT5-related inflammatory phenotype of cardiac macrophages/monocytes in mice. Lab. Investig. 2020, 100, 527–541. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, G.; Yang, M.; Chen, H.; Zhao, Y.; Yang, S.; Sun, C. Arctigenin reduces blood pressure by modulation of nitric oxide synthase and NADPH oxidase expression in spontaneously hypertensive rats. Biochem. Biophys. Res. Commun. 2015, 468, 837–842. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Huang, X.; Ma, S.; Xing, Y.; Geng, X.; He, X. Sauchinone inhibits angiotensin II-induced proliferation and migration of vascular smooth muscle cells. Clin. Exp. Pharmacol. Physiol. 2020, 47, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Jeong, C.W.; Bae, H.B.; Kwak, S.H.; Son, J.-K.; Seo, C.-S.; Lee, H.-J.; Lee, J.; Yoo, K.Y. Protective Effect of Sauchinone Against Regional Myocardial Ischemia/Reperfusion Injury: Inhibition of p38 MAPK and JNK Death Signaling Pathways. J. Korean Med. Sci. 2012, 27, 572. [Google Scholar] [CrossRef]
- Zhao, M.; Tu, P.; Ren, Y.; Tao, S.; Zheng, S. Sesamin Preconditioning Attenuates Myocardial Ischemia Reperfusion Injury in Rats Through Activation of Akt/eNOS Signaling Pathway. Zhong Yao Cai J. Chin. Med. Mater. 2016, 39, 1633–1637. [Google Scholar]
- Wei, P.; Liu, Y.; Cheng, M. Mechanism of sesamin on myocardial apoptosis in rats with coronary heart disease. Northwest Pharm. J. 2023, 38, 59–64. [Google Scholar]
- Feng, L.; Sun, R.; Zhang, H.; Zhang, J.; Peng, Z.; Li, J.; Gao, Y.; Xu, Y.; Cui, J.; Liu, J.; et al. Exploring the protective mechanisms of syringaresinol against myocardial infarction by experimental validation and network pharmacology. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2025, 1871, 167728. [Google Scholar] [CrossRef]
- Gao, L.; Li, T.; Li, S.; Song, Z.; Chang, Y.; Yuan, L. Schisandrin A protects against isoproterenol-induced chronic heart failure via miR-155. Mol. Med. Rep. 2021, 25, 24. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Ning, Z. In vivo and in vitro investigations of schisandrin B against angiotensin II induced ferroptosis and atrial fibrosis by regulation of the SIRT1 pathway. Sci. Rep. 2025, 15, 6200. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Pang, S.; Yang, N.; Meng, H.; Liu, J.; Zhou, N.; Zhang, M.; Xu, Z.; Gao, W.; Chen, B.; et al. Beneficial Effects of Schisandrin B on the Cardiac Function in Mice Model of Myocardial Infarction. PLoS ONE 2013, 8, e79418. [Google Scholar] [CrossRef]
- Fan, X.; Elkin, K.; Shi, Y.; Zhang, Z.; Cheng, Y.; Gu, J.; Liang, J.; Wang, C.; Ji, X. Schisandrin B improves cerebral ischemia and reduces reperfusion injury in rats through TLR4/NF-κB signaling pathway inhibition. Neurol. Res. 2020, 42, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, Z.; Meng, F. Schisandrin B Ameliorates Myocardial Ischemia/Reperfusion Injury Through Attenuation of Endoplasmic Reticulum Stress-Induced Apoptosis. Inflammation 2017, 40, 1903–1911. [Google Scholar] [CrossRef]
- Ai, F.; Guo, Q.; Yu, B.; Li, W.; Guo, X.; Chen, Z. Schisandrin B attenuates pressure overload-induced cardiac remodeling in mice by inhibiting the MAPK signaling pathway. Exp. Ther. Med. 2019, 18, 4645–4652. [Google Scholar] [CrossRef]
- Han, J.; Shi, X.; Zheng, Z.; Zhang, B.; Shi, F.; Jiang, L.; Xu, J. Schisandrin B protects against angiotensin II-induced endotheliocyte deficits by targeting Keap1 and activating Nrf2 pathway. Drug Des. Devel. Ther. 2018, 12, 3985–3997. [Google Scholar] [CrossRef]
- You, S.; Qian, J.; Wu, G.; Qian, Y.; Wang, Z.; Chen, T.; Wang, J.; Huang, W.; Liang, G. Schizandrin B attenuates angiotensin II induced endothelial to mesenchymal transition in vascular endothelium by suppressing NF-κB activation. Phytomedicine 2019, 62, 152955. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, M.; Xing, Z.; Su, J.; Gu, Y.; Ning, Z. Schisandrin B regulates the SIRT1/PI3K/Akt signaling pathway to ameliorate Ang II-infused cardiac fibrosis. Iran. J. Basic Med. Sci. 2025, 28, 946–954. [Google Scholar] [CrossRef]
- Xu, S.; Hu, C.; Han, J.; Luo, W.; Huang, L.; Jiang, Y.; Samorodov, A.V.; Wang, Y.; Huang, J. Schisandrin B alleviates angiotensin II-induced cardiac inflammatory remodeling by inhibiting the recruitment of MyD88 to TLRs in mouse cardiomyocytes. Int. Immunopharmacol. 2024, 139, 112660. [Google Scholar] [CrossRef]
- Duan, H.; Li, H.; Liu, T.; Chen, Y.; Luo, M.; Shi, Y.; Zhou, J.; Rashed, M.M.A.; Zhai, K.; Li, L.; et al. Exploring the Molecular Mechanism of Schisandrin C for the Treatment of Atherosclerosis via the PI3K/AKT/mTOR Autophagy Pathway. ACS Omega 2024, 9, 32920–32930. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Yu, J.; Liu, Y.; Song, H.; Zhang, X.; Zhou, L.; Wang, S.; Niu, X.; Li, W. Schisandrin inhibits VSMCs proliferation and migration by arresting cell cycle and targeting JAK2 to regulating the JAK2/STAT3 pathway. Tissue Cell 2024, 89, 102440. [Google Scholar] [CrossRef]
- Yang, M.; Jiang, X.-C.; Wang, L.; Cui, D.-A.; Zhang, J.-Y.; Wang, X.-R.; Feng, H.-P.; Zhang, K.; Zhang, K.; Li, J.-X.; et al. Schisandrin Protects against Norepinephrine-Induced Myocardial Hypertrophic Injury by Inhibiting the JAK2/STAT3 Signaling Pathway. Evid. Based Complement. Alternat. Med. 2021, 2021, 8129512. [Google Scholar] [CrossRef]
- Liu, J.; Tang, M.; Li, T.; Su, Z.; Zhu, Z.; Dou, C.; Liu, Y.; Pei, H.; Yang, J.; Ye, H.; et al. Honokiol Ameliorates Post-Myocardial Infarction Heart Failure Through Ucp3-Mediated Reactive Oxygen Species Inhibition. Front. Pharmacol. 2022, 13, 811682. [Google Scholar] [CrossRef]
- Tan, Z.; Liu, H.; Song, X.; Ling, Y.; He, S.; Yan, Y.; Yan, J.; Wang, S.; Wang, X.; Chen, A. Honokiol post-treatment ameliorates myocardial ischemia/reperfusion injury by enhancing autophagic flux and reducing intracellular ROS production. Chem. Biol. Interact. 2019, 307, 82–90. [Google Scholar] [CrossRef]
- Chi, Z.; Le, T.P.H.; Lee, S.K.; Guo, E.; Kim, D.; Lee, S.; Seo, S.; Lee, S.Y.; Kim, J.H.; Lee, S.Y. Honokiol ameliorates angiotensin II-induced hypertension and endothelial dysfunction by inhibiting HDAC6-mediated cystathionine γ-lyase degradation. J. Cell. Mol. Med. 2020, 24, 10663–10676. [Google Scholar] [CrossRef] [PubMed]
- Elbarbry, F.; Moshirian, N. The Modulation of Arachidonic Acid Metabolism and Blood Pressure-Lowering Effect of Honokiol in Spontaneously Hypertensive Rats. Molecules 2022, 27, 3396. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Kong, Q.; Li, Z.; Zhang, Y.; Chen, B.; Lv, L.; Zhang, Y. Honokiol Provides Cardioprotection from Myocardial Ischemia/Reperfusion Injury (MI/RI) by Inhibiting Mitochondrial Apoptosis via the PI3K/AKT Signaling Pathway. Cardiovasc. Ther. 2022, 2022, 1001692. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, H.; Chu, Y.; Zhang, Y.; Xu, C.; Xie, H.; Ruan, Q.; Lin, J.; Huang, C.; Chai, D. Honokiol ameliorates angiotensin II-induced cardiac hypertrophy by promoting dissociation of the Nur77–LKB1 complex and activating the AMPK pathway. J. Cell. Mol. Med. 2024, 28, e18028. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, P.; Wu, A.-H. Honokiol inhibits carotid artery atherosclerotic plaque formation by suppressing inflammation and oxidative stress. Aging 2020, 12, 8016–8028. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Z.; Hu, C.; Li, Z.; Hu, J. Honokiol suppresses TNF-α-induced migration and matrix metalloproteinase expression by blocking NF-κB activation via the ERK signaling pathway in rat aortic smooth muscle cells. Acta Histochem. 2014, 116, 588–595. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Liu, Y.; Tian, H.; Flickinger, B.; Empie, M.W.; Sun, S.Z. Dietary flaxseed lignan extract lowers plasma cholesterol and glucose concentrations in hypercholesterolaemic subjects. Br. J. Nutr. 2008, 99, 1301–1309. [Google Scholar] [CrossRef]
- Fukumitsu, S.; Aida, K.; Shimizu, H.; Toyoda, K. Flaxseed lignan lowers blood cholesterol and decreases liver disease risk factors in moderately hypercholesterolemic men. Nutr. Res. 2010, 30, 441–446. [Google Scholar] [CrossRef]
- Lucas, E.A.; Wild, R.D.; Hammond, L.J.; Khalil, D.A.; Juma, S.; Daggy, B.P.; Stoecker, B.J.; Arjmandi, B.H. Flaxseed Improves Lipid Profile without Altering Biomarkers of Bone Metabolism in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2002, 87, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Vanharanta, M.; Voutilainen, S.; Rissanen, T.H.; Adlercreutz, H.; Salonen, J.T. Risk of Cardiovascular Disease–Related and All-Cause Death According to Serum Concentrations of Enterolactone: Kuopio Ischaemic Heart Disease Risk Factor Study. Arch. Intern. Med. 2003, 163, 1099. [Google Scholar] [CrossRef] [PubMed]
- Di, Y.; Jones, J.; Mansell, K.; Whiting, S.; Fowler, S.; Thorpe, L.; Billinsky, J.; Viveky, N.; Cheng, P.C.; Almousa, A.; et al. Influence of Flaxseed Lignan Supplementation to Older Adults on Biochemical and Functional Outcome Measures of Inflammation. J. Am. Coll. Nutr. 2017, 36, 646–653. [Google Scholar] [CrossRef]
- Godos, J.; Bergante, S.; Satriano, A.; Pluchinotta, F.; Marranzano, M. Dietary Phytoestrogen Intake is Inversely Associated with Hypertension in a Cohort of Adults Living in the Mediterranean Area. Molecules 2018, 23, 368. [Google Scholar] [CrossRef]
- Jiang, T.; Sun, L.; Wang, Y.; Zhang, F.; Guo, J.; Sun, L.; Jiang, Y.; Xue, J.; Duan, J.; Liu, C. Podophyllotoxin via SIRT1/PPAR/NF-κB axis induced cardiac injury in rats based on the toxicological evidence chain (TEC) concept. Phytomedicine 2024, 130, 155655. [Google Scholar] [CrossRef]
- Liu, C.; Kong, J.; Lai, Y.; Zhang, Y.; Li, Y.; Du, J.; Sun, L.; Tian, Y. Investigating podophyllotoxin-induced cardiotoxicity in rats by Toxicological Evidence Chain (TEC): Focus on the Akt1/Srebp-1c/PUFAs axis. Chem. Biol. Interact. 2025, 419, 111634. [Google Scholar] [CrossRef]
- Ma, K.; Sun, L.; Jia, C.; Kui, H.; Xie, J.; Zang, S.; Huang, S.; Que, J.; Liu, C.; Huang, J. Potential mechanisms underlying podophyllotoxin-induced cardiotoxicity in male rats: Toxicological evidence chain (TEC) concept. Front. Pharmacol. 2024, 15, 1378758. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Samson, S.L.; Garber, A.J. Metabolic Syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23. [Google Scholar] [CrossRef]
- Tian, R.; Yang, J.; Wang, X.; Liu, S.; Dong, R.; Wang, Z.; Yang, Z.; Zhang, Y.; Cai, Z.; Yang, H.; et al. Honokiol acts as an AMPK complex agonist therapeutic in non-alcoholic fatty liver disease and metabolic syndrome. Chin. Med. 2023, 18, 30. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2014, 37 (Suppl. S1), S81–S90. [Google Scholar] [CrossRef]
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of Diabetes and Diabetes-Related Complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef]
- Prasad, K. Secoisolariciresinol diglucoside from flaxseed delays the development of type 2 diabetes in Zucker rat. J. Lab. Clin. Med. 2001, 138, 32–39. [Google Scholar] [CrossRef]
- Prasad, K.; Dhar, A. Flaxseed and Diabetes. Curr. Pharm. Des. 2015, 22, 141–144. [Google Scholar] [CrossRef]
- Pan, A.; Sun, J.; Chen, Y.; Ye, X.; Li, H.; Yu, Z.; Wang, Y.; Gu, W.; Zhang, X.; Chen, X.; et al. Effects of a Flaxseed-Derived Lignan Supplement in Type 2 Diabetic Patients: A Randomized, Double-Blind, Cross-Over Trial. PLoS ONE 2007, 2, e1148. [Google Scholar] [CrossRef]
- Pan, A.; Demark-Wahnefried, W.; Ye, X.; Yu, Z.; Li, H.; Qi, Q.; Sun, J.; Chen, Y.; Chen, X.; Liu, Y.; et al. Effects of a flaxseed-derived lignan supplement on C-reactive protein, IL-6 and retinol-binding protein 4 in type 2 diabetic patients. Br. J. Nutr. 2008, 101, 1145–1149. [Google Scholar] [CrossRef]
- Cornish, S.M.; Chilibeck, P.D.; Paus-Jennsen, L.; Biem, H.J.; Khozani, T.; Senanayake, V.; Vatanparast, H.; Little, J.P.; Whiting, S.J.; Pahwa, P. A randomized controlled trial of the effects of flaxseed lignan complex on metabolic syndrome composite score and bone mineral in older adults. Appl. Physiol. Nutr. Metab. 2009, 34, 89–98. [Google Scholar] [CrossRef]
- Huang, S.-M.; Chuang, C.-H.; Rejano, C.J.F.; Tayo, L.L.; Hsieh, C.-Y.; Huang, S.K.-H.; Tsai, P.-W. Sesamin: A Promising Therapeutic Agent for Ameliorating Symptoms of Diabetes. Molecules 2023, 28, 7255. [Google Scholar] [CrossRef] [PubMed]
- Parsa, E.; Javadi, B.; Sahebkar, A. The protective effects of sesamin against metabolic syndrome: A mechanistic review. Phytomed. Plus 2024, 4, 100625. [Google Scholar] [CrossRef]
- Zuo, J.; Ren, J.; Yin, B.; Wang, Z.; Cui, Q.; Liu, J.; Huang, D.; Pei, H.; Wen, R.; Zhang, Y.; et al. Effects of Sesamin in Animal Models of Obesity-Associated Diseases: A Systematic Review and Meta-Analysis. Nutr. Rev. 2025, 83, e838–e851. [Google Scholar] [CrossRef]
- Hadipour, E.; Emami, S.A.; Tayarani-Najaran, N.; Tayarani-Najaran, Z. Effects of sesame (Sesamum indicum L.) and bioactive compounds (sesamin and sesamolin) on inflammation and atherosclerosis: A review. Food Sci. Nutr. 2023, 11, 3729–3757. [Google Scholar] [CrossRef]
- Prasad, K. Suppression of phosphoenolpyruvate carboxykinase gene expression by secoisolariciresinol diglucoside (SDG), a new antidiabetic agent. Int. J. Angiol. 2011, 11, 107–109. [Google Scholar] [CrossRef]
- Moree, S.S.; Kavishankar, G.B.; Rajesha, J. Antidiabetic effect of secoisolariciresinol diglucoside in streptozotocin-induced diabetic rats. Phytomedicine 2013, 20, 237–245. [Google Scholar] [CrossRef]
- Yang, L.; Wang, C. Lignan matairesinol illustrates anti-diabetic effect via inhibition of DPP-4 and hepato-protective effect via inhibition of apoptosis in diabetic rats. Acta Pol. Pharm.—Drug Res. 2022, 79, 393–400. [Google Scholar] [CrossRef]
- Alam, M.B.; Ra, J.; Lim, J.; Song, B.; Javed, A.; Lee, S. Lariciresinol Displays Anti-Diabetic Activity through Inhibition of α-Glucosidase and Activation and Enhancement of Insulin Signaling. Mol. Nutr. Food Res. 2022, 66, 2100751. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, L.; Xiang, R.; Bu, X.; Qin, G.; Dai, J.; Zhao, Z.; Fang, X.; Yang, S.; Han, J.; et al. Arctigenin mitigates insulin resistance by modulating the IRS2/GLUT4 pathway via TLR4 in type 2 diabetes mellitus mice. Int. Immunopharmacol. 2023, 114, 109529. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, J.; Yan, J.; He, J.C.; Li, Y.; Zhong, Y. Additive renal protective effects between arctigenin and puerarin in diabetic kidney disease. Biomed. Pharmacother. 2024, 171, 116107. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yang, L.; Feng, L.; Yang, J.; Li, Y.; An, J.; Li, D.; Xu, Y.; Gao, Y.; Li, J.; et al. Syringaresinol Protects against Type 1 Diabetic Cardiomyopathy by Alleviating Inflammation Responses, Cardiac Fibrosis, and Oxidative Stress. Mol. Nutr. Food Res. 2020, 64, 2000231. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Wang, X. Syringaresinol-di-O-β-D-glucoside, a phenolic compound from Polygonatum sibiricum, exhibits an antidiabetic and antioxidative effect on a streptozotocin-induced mouse model of diabetes. Mol. Med. Rep. 2018, 18, 5511–5519. [Google Scholar] [CrossRef]
- Shang, J.; Yan, W.; Cui, X.; Ma, W.; Wang, Z.; Liu, N.; Yi, X.; Guo, T.; Wei, X.; Sun, Y.; et al. Schisandrin B, a potential GLP-1R agonist, exerts anti-diabetic effects by stimulating insulin secretion. Mol. Cell. Endocrinol. 2023, 577, 112029. [Google Scholar] [CrossRef]
- Luo, W.; Lin, K.; Hua, J.; Han, J.; Zhang, Q.; Chen, L.; Khan, Z.A.; Wu, G.; Wang, Y.; Liang, G. Schisandrin B Attenuates Diabetic Cardiomyopathy by Targeting MyD88 and Inhibiting MyD88-Dependent Inflammation. Adv. Sci. 2022, 9, 2202590. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, M.; Zhang, W.; Liu, W.; Li, H.; Ren, S.; Jiang, S.; Song, M.; Wang, Z.; Li, W. Schisandrin ameliorates diabetic nephropathy via regulating of PI3K/Akt/NF-κB-mediated inflammation and TGF-β1-induced fibrosis in HFD/STZ-induced C57BL/6J mice. J. Funct. Foods 2023, 100, 105376. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, R.; Liang, J.; Chen, Y. Antidiabetic and Anti-oxidative Effects of Honokiol on Diabetic Rats Induced by High-fat Diet and Streptozotocin. Chin. Herb. Med. 2014, 6, 42–46. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Jung, U.J. Honokiol Improves Insulin Resistance, Hepatic Steatosis, and Inflammation in Type 2 Diabetic db/db Mice. Int. J. Mol. Sci. 2019, 20, 2303. [Google Scholar] [CrossRef]
- He, A.; Yu, H.; Hu, Y.; Chen, H.; Li, X.; Shen, J.; Zhuang, R.; Chen, Y.; Sasmita, B.R.; Luo, M.; et al. Honokiol improves endothelial function in type 2 diabetic rats via alleviating oxidative stress and insulin resistance. Biochem. Biophys. Res. Commun. 2022, 600, 109–116. [Google Scholar] [CrossRef]
- Hu, X.; Sun, J.; Fu, X.; Liu, Y.; Wang, Y.; Huo, B.; Guo, Y.; Gao, X.; Li, W. Hypoglycemic effect and mechanism of honokiol on type 2 diabetic mice. Drug Des. Devel. Ther. 2015, 2015, 96327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhai, M.; Li, B.; Liu, Z.; Li, K.; Jiang, L.; Zhang, M.; Yi, W.; Yang, J.; Yi, D.; et al. Honokiol Ameliorates Myocardial Ischemia/Reperfusion Injury in Type 1 Diabetic Rats by Reducing Oxidative Stress and Apoptosis through Activating the SIRT1-Nrf2 Signaling Pathway. Oxid. Med. Cell. Longev. 2018, 2018, 3159801. [Google Scholar] [CrossRef]
- Petrelli, N.J.; Winer, E.P.; Brahmer, J.; Dubey, S.; Smith, S.; Thomas, C.; Vahdat, L.T.; Obel, J.; Vogelzang, N.; Markman, M.; et al. Clinical Cancer Advances 2009: Major Research Advances in Cancer Treatment, Prevention, and Screening—A Report From the American Society of Clinical Oncology. J. Clin. Oncol. 2009, 27, 6052–6069. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.; Canberk, S.; Schmitt, F.; Vale, N. Molecular Subtypes and Mechanisms of Breast Cancer: Precision Medicine Approaches for Targeted Therapies. Cancers 2025, 17, 1102. [Google Scholar] [CrossRef]
- Jang, W.Y.; Kim, M.-Y.; Cho, J.Y. Antioxidant, Anti-Inflammatory, Anti-Menopausal, and Anti-Cancer Effects of Lignans and Their Metabolites. Int. J. Mol. Sci. 2022, 23, 15482. [Google Scholar] [CrossRef]
- Mense, S.M.; Hei, T.K.; Ganju, R.K.; Bhat, H.K. Phytoestrogens and Breast Cancer Prevention: Possible Mechanisms of Action. Environ. Health Perspect. 2008, 116, 426–433. [Google Scholar] [CrossRef]
- Micek, A.; Godos, J.; Brzostek, T.; Gniadek, A.; Favari, C.; Mena, P.; Libra, M.; Del Rio, D.; Galvano, F.; Grosso, G. Dietary phytoestrogens and biomarkers of their intake in relation to cancer survival and recurrence: A comprehensive systematic review with meta-analysis. Nutr. Rev. 2021, 79, 42–65. [Google Scholar] [CrossRef]
- McCann, S.E.; Thompson, L.U.; Nie, J.; Dorn, J.; Trevisan, M.; Shields, P.G.; Ambrosone, C.B.; Edge, S.B.; Li, H.-F.; Kasprzak, C.; et al. Dietary lignan intakes in relation to survival among women with breast cancer: The Western New York Exposures and Breast Cancer (WEB) Study. Breast Cancer Res. Treat. 2010, 122, 229–235. [Google Scholar] [CrossRef]
- Linseisen, J.; Piller, R.; Hermann, S.; Chang-Claude, J. Dietary phytoestrogen intake and premenopausal breast cancer risk in a German case-control study. Int. J. Cancer 2004, 110, 284–290. [Google Scholar] [CrossRef]
- Cotterchio, M.; Boucher, B.A.; Kreiger, N.; Mills, C.A.; Thompson, L.U. Dietary phytoestrogen intake—Lignans and isoflavones—And breast cancer risk (Canada). Cancer Causes Control 2008, 19, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Tou, J.C.L.; Thompson, L.U. Exposure to flaxseed or its lignan component during different developmental stages influences rat mammary gland structures. Carcinogenesis 1999, 20, 1831–1835. [Google Scholar] [CrossRef]
- Chen, J.; Tan, K.P.; Ward, W.E.; Thompson, L.U. Exposure to Flaxseed or Its Purified Lignan during Suckling Inhibits Chemically Induced Rat Mammary Tumorigenesis. Exp. Biol. Med. 2003, 228, 951–958. [Google Scholar] [CrossRef]
- Tan, K.P.; Chen, J.; Ward, W.E.; Thompson, L.U. Mammary Gland Morphogenesis is Enhanced by Exposure to Flaxseed or Its Major Lignan During Suckling in Rats. Exp. Biol. Med. 2004, 229, 147–157. [Google Scholar] [CrossRef]
- Rickard, S.E.; Yuan, Y.V.; Chen, J.; Thompson, L.U. Dose Effects of Flaxseed and Its Lignan on N-Methyl-N-Nitrosourea-Induced Mammary Tumorigenesis in Rats. Nutr. Cancer 1999, 35, 50–57. [Google Scholar] [CrossRef]
- Tanawattanasuntorn, T.; Rattanaburee, T.; Thongpanchang, T.; Graidist, P. Trans-(±)-Kusunokinin Binding to AKR1B1 Inhibits Oxidative Stress and Proteins Involved in Migration in Aggressive Breast Cancer. Antioxidants 2022, 11, 2347. [Google Scholar] [CrossRef]
- Tedasen, A.; Dokduang, S.; Sukpondma, Y.; Lailerd, N.; Madla, S.; Sriwiriyajan, S.; Rattanaburee, T.; Tipmanee, V.; Graidist, P. (−)-Kusunokinin inhibits breast cancer in N-nitrosomethylurea-induced mammary tumor rats. Eur. J. Pharmacol. 2020, 882, 173311. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Patil, P.; Raina, P.; Kaul-Ghanekar, R. Matairesinol repolarizes M2 macrophages to M1 phenotype to induce apoptosis in triple-negative breast cancer cells. Immunopharmacol. Immunotoxicol. 2025, 47, 8–22. [Google Scholar] [CrossRef]
- Saggar, J.K.; Chen, J.; Corey, P.; Thompson, L.U. The Effect of Secoisolariciresinol Diglucoside and Flaxseed Oil, Alone and in Combination, on MCF-7 Tumor Growth and Signaling Pathways. Nutr. Cancer 2010, 62, 533–542. [Google Scholar] [CrossRef]
- Chen, J.; Saggar, J.K.; Corey, P.; Thompson, L.U. Flaxseed and Pure Secoisolariciresinol Diglucoside, but Not Flaxseed Hull, Reduce Human Breast Tumor Growth (MCF-7) in Athymic Mice. J. Nutr. 2009, 139, 2061–2066. [Google Scholar] [CrossRef] [PubMed]
- Bowers, L.W.; Lineberger, C.G.; Ford, N.A.; Rossi, E.L.; Punjala, A.; Camp, K.K.; Kimler, B.K.; Fabian, C.J.; Hursting, S.D. The flaxseed lignan secoisolariciresinol diglucoside decreases local inflammation, suppresses NFκB signaling, and inhibits mammary tumor growth. Breast Cancer Res. Treat. 2019, 173, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Scherbakov, A.M.; Stasevich, O.V.; Salnikova, D.I.; Andreeva, O.E.; Mikhaevich, E.I. Antiestrogenic and antiproliferative potency of secoisolariciresinol diglucoside derivatives on MCF-7 breast cancer cells. Nat. Prod. Res. 2021, 35, 6099–6105. [Google Scholar] [CrossRef]
- Soltani, M.; Fotovat, R.; Sharifi, M.; Ahmadian Chashmi, N.; Behmanesh, M. In Vitro Comparative Study on Antineoplastic Effects of Pinoresinol and Lariciresinol on Healthy Cells and Breast Cancer-Derived Human Cells. Iran. J. Med. Sci. 2024, 49, 30–39. [Google Scholar] [CrossRef] [PubMed]
- López-Biedma, A.; Sánchez-Quesada, C.; Beltrán, G.; Delgado-Rodríguez, M.; Gaforio, J.J. Phytoestrogen (+)-pinoresinol exerts antitumor activity in breast cancer cells with different oestrogen receptor statuses. BMC Complement. Altern. Med. 2016, 16, 350. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, C.; Li, J.; Liu, R.; Zhuang, J.; Feng, F.; Yao, Y.; Sun, C. Target Analysis and Mechanism of Podophyllotoxin in the Treatment of Triple-Negative Breast Cancer. Front. Pharmacol. 2020, 11, 1211. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Sung, N.J.; Shin, S.; Ryu, D.-S.; Youn, H.-S.; Park, S.-A. Sauchinone inhibits the proliferation, migration and invasion of breast cancer cells by suppressing Akt-CREB-MMP13 signaling pathway. Biosci. Rep. 2021, 41, BSR20211067. [Google Scholar] [CrossRef] [PubMed]
- Siao, A.-C.; Hou, C.-W.; Kao, Y.-H.; Jeng, K.-C. Effect of Sesamin on Apoptosis and Cell Cycle Arrest in Human Breast Cancer MCF-7 Cells. Asian Pac. J. Cancer Prev. 2015, 16, 3779–3783. [Google Scholar] [CrossRef]
- Kongtawelert, P.; Wudtiwai, B.; Shwe, T.H.; Pothacharoen, P.; Phitak, T. Inhibition of programmed death ligand 1 (PD-L1) expression in breast cancer cells by sesamin. Int. Immunopharmacol. 2020, 86, 106759. [Google Scholar] [CrossRef]
- Dai, X.; Yin, C.; Guo, G.; Zhang, Y.; Zhao, C.; Qian, J.; Wang, O.; Zhang, X.; Liang, G. Schisandrin B exhibits potent anticancer activity in triple negative breast cancer by inhibiting STAT3. Toxicol. Appl. Pharmacol. 2018, 358, 110–119. [Google Scholar] [CrossRef]
- Chang, C.-M.; Liang, T.-R.; Lam, H.Y.P. The Use of Schisandrin B to Combat Triple-Negative Breast Cancers by Inhibiting NLRP3-Induced Interleukin-1β Production. Biomolecules 2024, 14, 74. [Google Scholar] [CrossRef]
- Chen, L.; Ren, L.-Q.; Liu, Z.; Liu, X.; Tu, H.; Huang, X.-Y. Bio-informatics and in Vitro Experiments Reveal the Mechanism of Schisandrin A Against MDA-MB-231 cells. Bioengineered 2021, 12, 7678–7693. [Google Scholar] [CrossRef]
- Xu, X.; Rajamanicham, V.; Xu, S.; Liu, Z.; Yan, T.; Liang, G.; Guo, G.; Zhou, H.; Wang, Y. Schisandrin A inhibits triple negative breast cancer cells by regulating Wnt/ER stress signaling pathway. Biomed. Pharmacother. 2019, 115, 108922. [Google Scholar] [CrossRef]
- Lee, D.; Kim, Y.-M.; Chin, Y.-W.; Kang, K.S. Schisandrol A Exhibits Estrogenic Activity via Estrogen Receptor α-Dependent Signaling Pathway in Estrogen Receptor-Positive Breast Cancer Cells. Pharmaceutics 2021, 13, 1082. [Google Scholar] [CrossRef]
- Lou, C.; Zhu, Z.; Zhao, Y.; Zhu, R.; Zhao, H. Arctigenin, a lignan from Arctium lappa L., inhibits metastasis of human breast cancer cells through the downregulation of MMP-2/−9 and heparanase in MDA-MB-231 cells. Oncol. Rep. 2017, 37, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Cao, W.; Shen, W.; Zhang, L.; Gu, X.; Guo, Y.; Tsai, H.; Liu, X.; Li, J.; Zhang, J.; et al. Arctigenin inhibits STAT3 and exhibits anticancer potential in human triple-negative breast cancer therapy. Oncotarget 2017, 8, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, T.; Chun, S.-Y.; Lee, K.-S.; Kim, S.; Nam, K.-S. The anti-metastatic effects of the phytoestrogen arctigenin on human breast cancer cell lines regardless of the status of ER expression. Int. J. Oncol. 2017, 50, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zang, C.; Emde, A.; Planas-Silva, M.D.; Rosche, M.; Kühnl, A.; Schulz, C.-O.; Elstner, E.; Possinger, K.; Eucker, J. Anti-tumor effect of honokiol alone and in combination with other anti-cancer agents in breast cancer. Eur. J. Pharmacol. 2008, 591, 43–51. [Google Scholar] [CrossRef]
- Han, X.; Cheng, Y.; Jiang, Z.; Alu, A.; Ma, X. Honokiol Exhibits Anti-Tumor Effects in Breast Cancer by Modulating the miR-148a-5p-CYP1B1 Axis. Am. J. Chin. Med. 2024, 52, 1843–1861. [Google Scholar] [CrossRef]
- Mikhaevich, E.I.; Sorokin, D.V.; Scherbakov, A.M. Honokiol inhibits the growth of hormone-resistant breast cancer cells: Its promising effect in combination with metformin. Res. Pharm. Sci. 2023, 18, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Lin, C.; Liu, C. Efficacy of phytoestrogens for menopausal symptoms: A meta-analysis and systematic review. Climacteric 2015, 18, 260–269. [Google Scholar] [CrossRef]
- Takahashi, T.A.; Johnson, K.M. Menopause. Med. Clin. N. Am. 2015, 99, 521–534. [Google Scholar] [CrossRef]
- Sammartino, A.; Tommaselli, G.A.; Gargano, V.; Di Carlo, C.; Attianese, W.; Nappi, C. Short-term effects of a combination of isoflavones, lignans and Cimicifuga racemosa on climacteric-related symptoms in postmenopausal women: A double-blind, randomized, placebo-controlled trial. Gynecol. Endocrinol. 2006, 22, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Pokushalov, E.; Ponomarenko, A.; Garcia, C.; Kasimova, L.; Pak, I.; Shrainer, E.; Romanova, A.; Kudlay, D.; Johnson, M.; Miller, R. Assessing the combined effects of Black Cohosh, Soy Isoflavones, and SDG Lignans on menopausal symptoms: A randomized, double-blind, placebo-controlled clinical trial. Eur. J. Nutr. 2025, 64, 138. [Google Scholar] [CrossRef] [PubMed]
- Velentzis, L.S.; Cantwell, M.M.; Cardwell, C.; Keshtgar, M.R.; Leathem, A.J.; Woodside, J.V. Lignans and breast cancer risk in pre- and post-menopausal women: Meta-analyses of observational studies. Br. J. Cancer 2009, 100, 1492–1498. [Google Scholar] [CrossRef]
- Mosca, L.; Barrett-Connor, E.; Kass Wenger, N. Sex/Gender Differences in Cardiovascular Disease Prevention: What a Difference a Decade Makes. Circulation 2011, 124, 2145–2154. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Y.; Sampson, L.; Wang, M.; Manson, J.E.; Rimm, E.; Sun, Q. Lignan Intake and Risk of Coronary Heart Disease. J. Am. Coll. Cardiol. 2021, 78, 666–678. [Google Scholar] [CrossRef]
- Chambliss, K.L.; Yuhanna, I.S.; Mineo, C.; Liu, P.; German, Z.; Sherman, T.S.; Mendelsohn, M.E.; Anderson, R.G.W.; Shaul, P.W. Estrogen Receptor α and Endothelial Nitric Oxide Synthase Are Organized Into a Functional Signaling Module in Caveolae. Circ. Res. 2000, 87, e44–e52. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, S.; Naderi Nabi, F.; Sugiyama, M.G.; Lee, W.L. Estrogen Inhibits LDL (Low-Density Lipoprotein) Transcytosis by Human Coronary Artery Endothelial Cells via GPER (G-Protein–Coupled Estrogen Receptor) and SR-BI (Scavenger Receptor Class B Type 1). Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2283–2294. [Google Scholar] [CrossRef]
- Miklowitz, D.J.; Portnoff, L.C.; Armstrong, C.C.; Keenan-Miller, D.; Breen, E.C.; Muscatell, K.A.; Eisenberger, N.I.; Irwin, M.R. Inflammatory cytokines and nuclear factor-kappa B activation in adolescents with bipolar and major depressive disorders. Psychiatry Res. 2016, 241, 315–322. [Google Scholar] [CrossRef]
- Monaco, C. Nuclear factor κB: A potential therapeutic target in atherosclerosis and thrombosis. Cardiovasc. Res. 2004, 61, 671–682. [Google Scholar] [CrossRef]
- Abu-Amer, Y. NF-κB signaling and bone resorption. Osteoporos. Int. 2013, 24, 2377–2386. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Ko, P.-Y.; Kwan, T.-H.; Liu, M.-Y.; Jou, I.-M.; Lin, C.-W.; Wu, P.-T. Daily supplement of sesame oil prevents postmenopausal osteoporosis via maintaining serum estrogen and aromatase levels in rats. Sci. Rep. 2024, 14, 321. [Google Scholar] [CrossRef]
- Tachibana, R.; Matsushita, H.; Minami, A.; Morita, N.; Shimizu, S.; Kanazawa, H.; Suzuki, T.; Watanabe, K.; Wakatsuki, A. Dietary sesame diminishes bone mass and bone formation indices in ovariectomized rats. Clin. Exp. Obstet. Gynecol. 2020, 47, 546. [Google Scholar] [CrossRef]
- Wang, C.; Lin, Y.; Lin, Y.; Chung, W. Modified Primers for the Identification of Nonpathogenic Fusarium oxysporum Isolates That Have Biological Control Potential against Fusarium Wilt of Cucumber in Taiwan. PLoS ONE 2013, 8, e65093. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.; Jia, M.; Fu, S.; Pan, J.; Chu, C.; Liu, X.; Liu, X.; Liu, Z. (+)-Sesamin attenuates chronic unpredictable mild stress-induced depressive-like behaviors and memory deficits via suppression of neuroinflammation. J. Nutr. Biochem. 2019, 64, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, H.; Yan, P.; Guo, L.; Li, J.; Han, J.; Qiu, J.; Yang, K. Efficacy and safety of phytoestrogens in the treatment of perimenopausal and postmenopausal depressive disorders: A systematic review and meta-analysis. Int. J. Clin. Pract. 2021, 75, e14360. [Google Scholar] [CrossRef]
- Yan, T.; He, B.; Wan, S.; Xu, M.; Yang, H.; Xiao, F.; Bi, K.; Jia, Y. Antidepressant-like effects and cognitive enhancement of Schisandra chinensis in chronic unpredictable mild stress mice and its related mechanism. Sci. Rep. 2017, 7, 6903. [Google Scholar] [CrossRef] [PubMed]
- Kreydin, E.I.; Kim, M.M.; Barrisford, G.W.; Rodriguez, D.; Sanchez, A.; Santiago-Lastra, Y.; Ko, D.S.C. Urinary Lignans Are Associated With Decreased Incontinence in Postmenopausal Women. Urology 2015, 86, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Boncler, M.; Luzak, B.; Watała, C. Role of C-reactive protein in atherogenesis. Postep. Hig Med Dosw 2006, 60, 538–546. [Google Scholar]
- Hallund, J.; Tetens, I.; Bügel, S.; Tholstrup, T.; Bruun, J.M. The effect of a lignan complex isolated from flaxseed on inflammation markers in healthy postmenopausal women. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 497–502. [Google Scholar] [CrossRef]
- Lenártová, P.; Gažarová, M.; Kopčeková, J.; Mrázová, J. Effect of Crushed Flaxseed Consumption on Cardiovascular Risk Indicators in Menopausal Women. Life 2024, 14, 849. [Google Scholar] [CrossRef]
- Lee, H.; Ji, Y.R.; Ryoo, Z.Y.; Choi, M.-S.; Woo, E.-R.; Lee, D.G. Antibacterial Mechanism of (−)-Nortrachelogenin in Escherichia coli O157. Curr. Microbiol. 2016, 72, 48–54. [Google Scholar] [CrossRef]
- De Souza Pereira, J.J.; Pereira, A.D.P.C.; Jandú, J.J.B.; Da Paz, J.A.; Crovella, S.; Dos Santos Correia, M.T.; De Azevêdo Silva, J. Commiphora leptophloeos Phytochemical and Antimicrobial Characterization. Front. Microbiol. 2017, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, D.; Yamashita, S.; Takahashi, S.; Waki, T.; Kikuchi, K.; Abe, T.; Katayama, T.; Nakayama, T. (+)-Sesamin, a sesame lignan, is a potent inhibitor of gut bacterial tryptophan indole-lyase that is a key enzyme in chronic kidney disease pathogenesis. Biochem. Biophys. Res. Commun. 2022, 590, 158–162. [Google Scholar] [CrossRef]
- Czemplik, M.; Żuk, M.; Kulma, A.; Kuc, S.; Szopa, J. GM flax as a source of effective antimicrobial compounds. Sci. Microb. Pathog. Commun. Curr. Res. Technol. Adv. 2011, 76, 39–47. [Google Scholar]
- Kyselka, J.; Rabiej, D.; Dragoun, M.; Kreps, F.; Burčová, Z.; Němečková, I.; Smolová, J.; Bjelková, M.; Szydłowska-Czerniak, A.; Schmidt, Š.; et al. Antioxidant and antimicrobial activity of linseed lignans and phenolic acids. Eur. Food Res. Technol. 2017, 243, 1633–1644. [Google Scholar] [CrossRef]
- Al-Ani, W.M.K.; Aziz, F.M. Antimicrobial Activity of HMR lignans. Iraqi J. Pharm. Sci. 2013, 22, 30–34. [Google Scholar] [CrossRef]
- Nie, H.; Guan, X.-L.; Li, J.; Zhang, Y.-J.; He, R.-J.; Huang, Y.; Liu, B.-M.; Zhou, D.-X.; Deng, S.-P.; Chen, H.-C.; et al. Antimicrobial lignans derived from the roots of Streblus asper. Phytochem. Lett. 2016, 18, 226–231. [Google Scholar] [CrossRef]
- Davidova, S.; Galabov, A.S.; Satchanska, G. Antibacterial, Antifungal, Antiviral Activity, and Mechanisms of Action of Plant Polyphenols. Microorganisms 2024, 12, 2502. [Google Scholar] [CrossRef]
- García Hernández, L.C.; Higuera-Piedrahita, R.I.; Rivero-Perez, N.; Morales-Ubaldo, A.L.; Valladares-Carranza, B.; De La Cruz-Cruz, H.A.; Cuéllar-Ordaz, J.A.; González-Ruiz, C.; Nicolás-Vázquez, M.I.; Zaragoza-Bastida, A. Antibacterial Activity and Molecular Docking of Lignans Isolated from Artemisia cina Against Multidrug-Resistant Bacteria. Pharmaceuticals 2025, 18, 781. [Google Scholar] [CrossRef] [PubMed]
- Elbakush, A.M.; Fulano, A.M.; Gomelsky, M. Lignan-containing maple products inhibit Listeria monocytogenes biofilms on fresh produce. Front. Microbiol. 2023, 14, 1258394. [Google Scholar] [CrossRef] [PubMed]
- Thongphichai, W.; Tuchinda, P.; Pohmakotr, M.; Reutrakul, V.; Akkarawongsapat, R.; Napaswad, C.; Limthongkul, J.; Jenjittikul, T.; Saithong, S. Anti-HIV-1 activities of constituents from the rhizomes of Boesenbergia thorelii. Fitoterapia 2019, 139, 104388. [Google Scholar] [CrossRef]
- Qian, X.-J.; Jin, Y.-S.; Chen, H.-S.; Xu, Q.-Q.; Ren, H.; Zhu, S.-Y.; Tang, H.-L.; Wang, Y.; Zhao, P.; Qi, Z.-T.; et al. Trachelogenin, a novel inhibitor of hepatitis C virus entry through CD81. J. Gen. Virol. 2016, 97, 1134–1144. [Google Scholar] [CrossRef]
- Barbary, O.M.; El-Sohaimy, S.A.; El-Saadani, M.A.; Zeitoun, A. Antioxidant, Antimicrobial and Anti-HCV Activities of Lignan Extracted from Flaxseed. Res. J. Agric. Biol. Sci. 2010, 6, 247–256. [Google Scholar]
- Zhou, C.; Lu, M.; Cheng, J.; Rohani, E.R.; Hamezah, H.S.; Han, R.; Tong, X. Review on the Pharmacological Properties of Phillyrin. Molecules 2022, 27, 3670. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-Y.; Wang, D.-Y.; Li, Y.-P.; Deyrup, S.T.; Zhang, H.-J. Plant-derived lignans as potential antiviral agents: A systematic review. Phytochem. Rev. 2022, 21, 239–289. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Guo, N.; Zhang, J.; Ding, Y.; Tang, X.; Liang, J.; Li, L.; Deng, X.; Yu, L. The synergy of honokiol and fluconazole against clinical isolates of azole-resistant Candida albicans: Synergy of HNK and FLC on Candida. Lett. Appl. Microbiol. 2010, 51, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Ye, X.; Ding, D.; Liao, K. Opposite effects of vitamin C and vitamin E on the antifungal activity of honokiol. J. Microbiol. Biotechnol. 2019, 29, 538–547. [Google Scholar] [CrossRef]
- Vargas-Arispuro, I.; Reyes-Báez, R.; Rivera-Castañeda, G.; Martínez-Téllez, M.A.; Rivero-Espejel, I. Antifungal lignans from the creosotebush (Larrea tridentata). Ind. Crops Prod. 2005, 22, 101–107. [Google Scholar] [CrossRef]
- Sun, L.; Liao, K.; Hang, C.; Wang, D. Honokiol induces reactive oxygen species-mediated apoptosis in Candida albicans through mitochondrial dysfunction. PLoS ONE 2017, 12, e0172228. [Google Scholar] [CrossRef]
- Behbehani, J.; Shreaz, S.; Irshad, M.; Karched, M. The natural compound magnolol affects growth, biofilm formation, and ultrastructure of oral Candida isolates. Microb. Pathog. 2017, 113, 209–217. [Google Scholar] [CrossRef]
- Xiao, S.-J.; Guo, D.-L.; Xia, B.; Allen, S.; Gu, Y.-C.; Chen, F.; Ding, L.-S.; Zhou, Y. Polycyclic Spiro Lignans and Biphenyl Tetrahydrofuranone Lignans from Gymnotheca involucrata. Planta Med. 2016, 82, 723–728. [Google Scholar] [CrossRef]
- Sriphana, U.; Thongsri, Y.; Ardwichai, P.; Poopasit, K.; Prariyachatigul, C.; Simasathiansophon, S.; Yenjai, C. New lignan esters from Alyxia schlechteri and antifungal activity against Pythium insidiosum. Fitoterapia 2013, 91, 39–43. [Google Scholar] [CrossRef]
- Zafar, S.; -Ur-Rehman, F.; Shah, Z.A.; Rauf, A.; Khan, A.; Humayun Khan, M.; Ur Rahman, K.; Khan, S.; Ullah, A.; Shaheen, F. Potent leishmanicidal and antibacterial metabolites from Olea ferruginea. J. Asian Nat. Prod. Res. 2019, 21, 679–687. [Google Scholar] [CrossRef]
- Guo, Y.; Hou, E.; Wen, T.; Yan, X.; Han, M.; Bai, L.-P.; Fu, X.; Liu, J.; Qin, S. Development of Membrane-Active Honokiol/Magnolol Amphiphiles as Potent Antibacterial Agents against Methicillin-Resistant Staphylococcus aureus (MRSA). J. Med. Chem. 2021, 64, 12903–12916. [Google Scholar] [CrossRef]
- Ekalu, A.; Ayo, R.G.-O.; Habila, J.D.; Hamisu, I. In vitro antimicrobial activity of lignan from the stem bark of Strombosia grandifolia Hook.f. ex Benth. Bull. Natl. Res. Cent. 2019, 43, 115. [Google Scholar] [CrossRef]
- Asano, J.; Chiba, K.; Tada, M.; Yoshii, T. Antiviral activity of lignans and their glycosides from Justicia procumbens. Phytochemistry 1996, 42, 713–717. [Google Scholar] [CrossRef] [PubMed]
- MacRae, W.; Hudson, J.; Towers, G. The Antiviral Action of Lignans. Planta Med. 1989, 55, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.D.C.; Pitorro, T.E.A.; Santiago, M.B.; Franco, R.R.; Silva, T.D.C.; Prado, D.G.; Cunha, L.C.S.; Espindola, F.S.; Tavares, D.C.; Nicolella, H.D.; et al. In vitro evaluation of the antibacterial and cytotoxic activities of the Euclea natalensis crude extract and fractions against oral infection agents. Arch. Oral Biol. 2022, 143, 105546. [Google Scholar] [CrossRef]
- Huh, J.; Song, J.H.; Kim, S.R.; Cho, H.M.; Ko, H.-J.; Yang, H.; Sung, S.H. Lignan Dimers from Forsythia viridissima Roots and Their Antiviral Effects. J. Nat. Prod. 2019, 82, 232–238. [Google Scholar] [CrossRef]
- Xu, L.; Tan, J.-B.; Zheng, Y.-T.; Sang, Z.-H.; Qin, S.-Y.; Huang, Y.-T.; Li, M.-F.; Zou, Z.-X. New lignans from Phyllanthodendron dunnianum. Nat. Prod. Res. 2024, 1–9. [Google Scholar] [CrossRef]
- Liu, C.-H.; Jassey, A.; Hsu, H.-Y.; Lin, L.-T. Antiviral Activities of Silymarin and Derivatives. Molecules 2019, 24, 1552. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Woo, E.-R.; Lee, D.G. Anti-Candida property of a lignan glycoside derived from Styrax japonica S. et Z. via membrane-active mechanisms. Mol. Cells 2010, 29, 581–584. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).