Fruit Quality Characterization and Comprehensive Evaluation of 30 Chionanthus retusus Accessions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Fruit Morphology
2.3. Oil Extraction
2.4. Fatty Acid Determination
2.5. Phytosterols Determination
2.6. Tocopherol Determination
2.7. Statistical Analyses
3. Results
3.1. Fruit Morphological Diversity in Chionanthus retusus
3.2. Oil Content and Fatty Acid Composition of C. retusus Kernels
3.3. Phytosterol and Tocopherol Content and Composition in C. retusus Kernel Oil
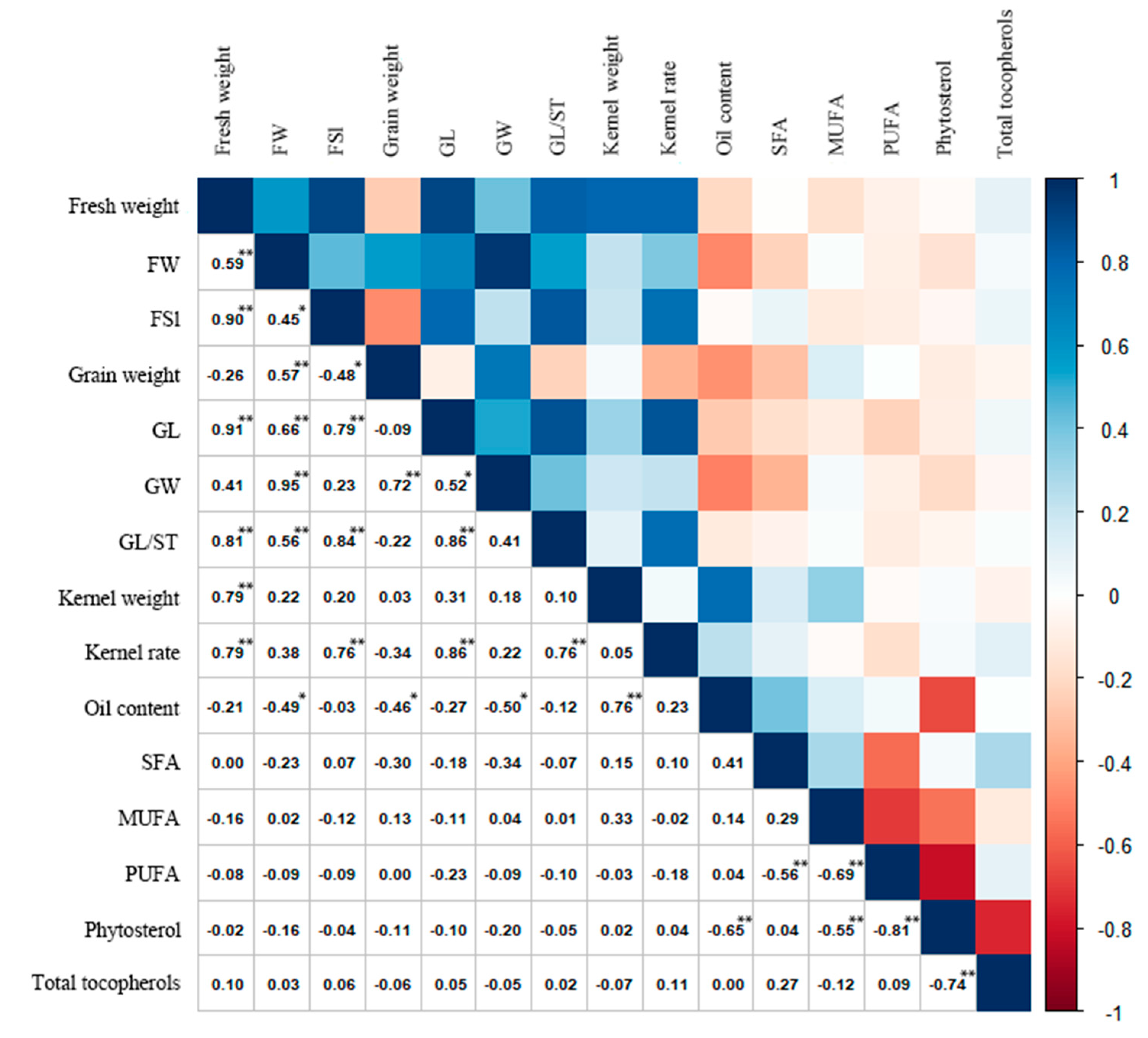
3.4. Correlation Analysis of Fruit Morphological Traits, Fatty Acid Composition, and Oil, Phytosterol, and Tocopherol Content in C. retusus
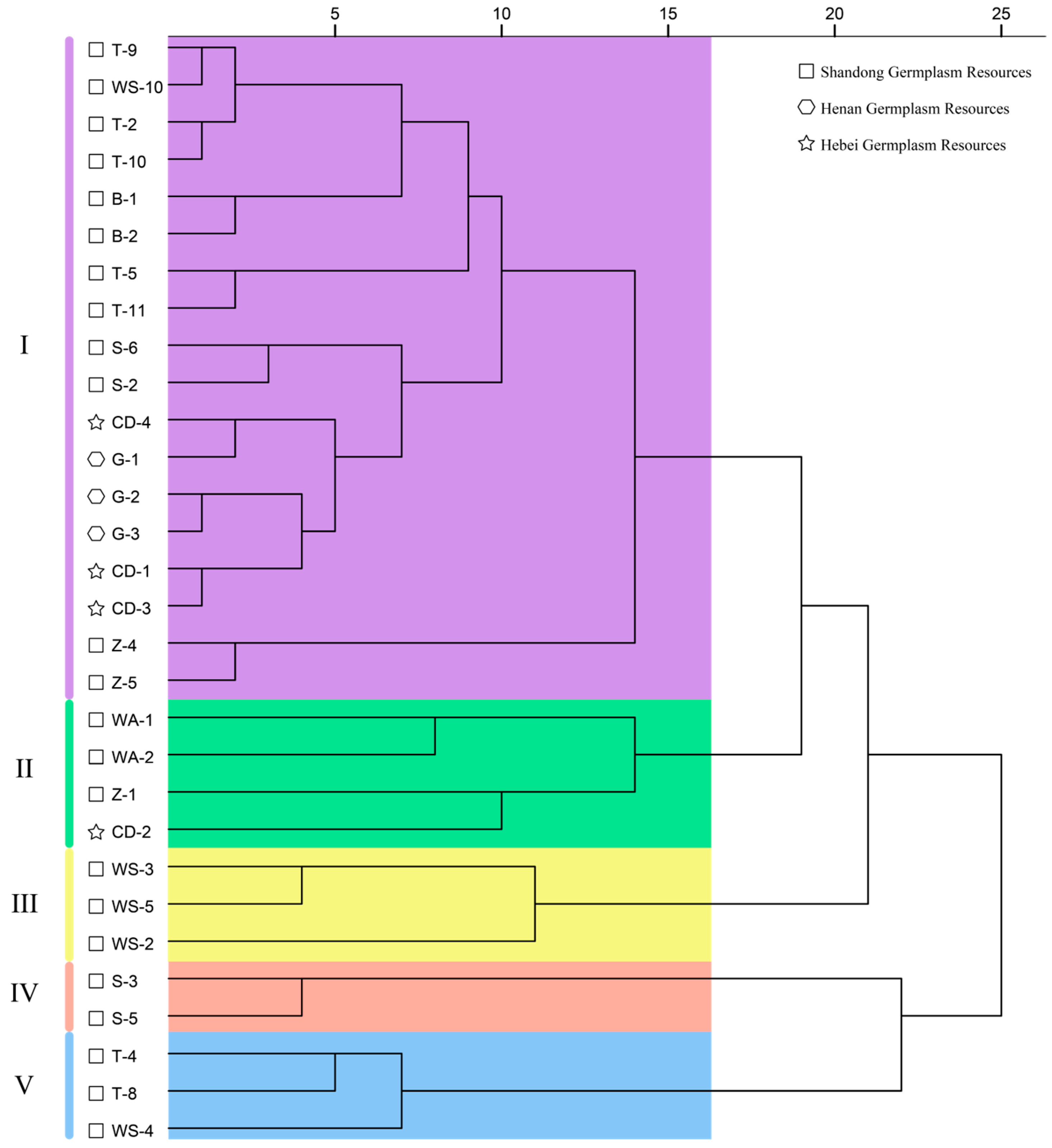
3.5. Cluster Analysis of Fruit Quality Traits in 30 C. retusus Germplasm Accessions
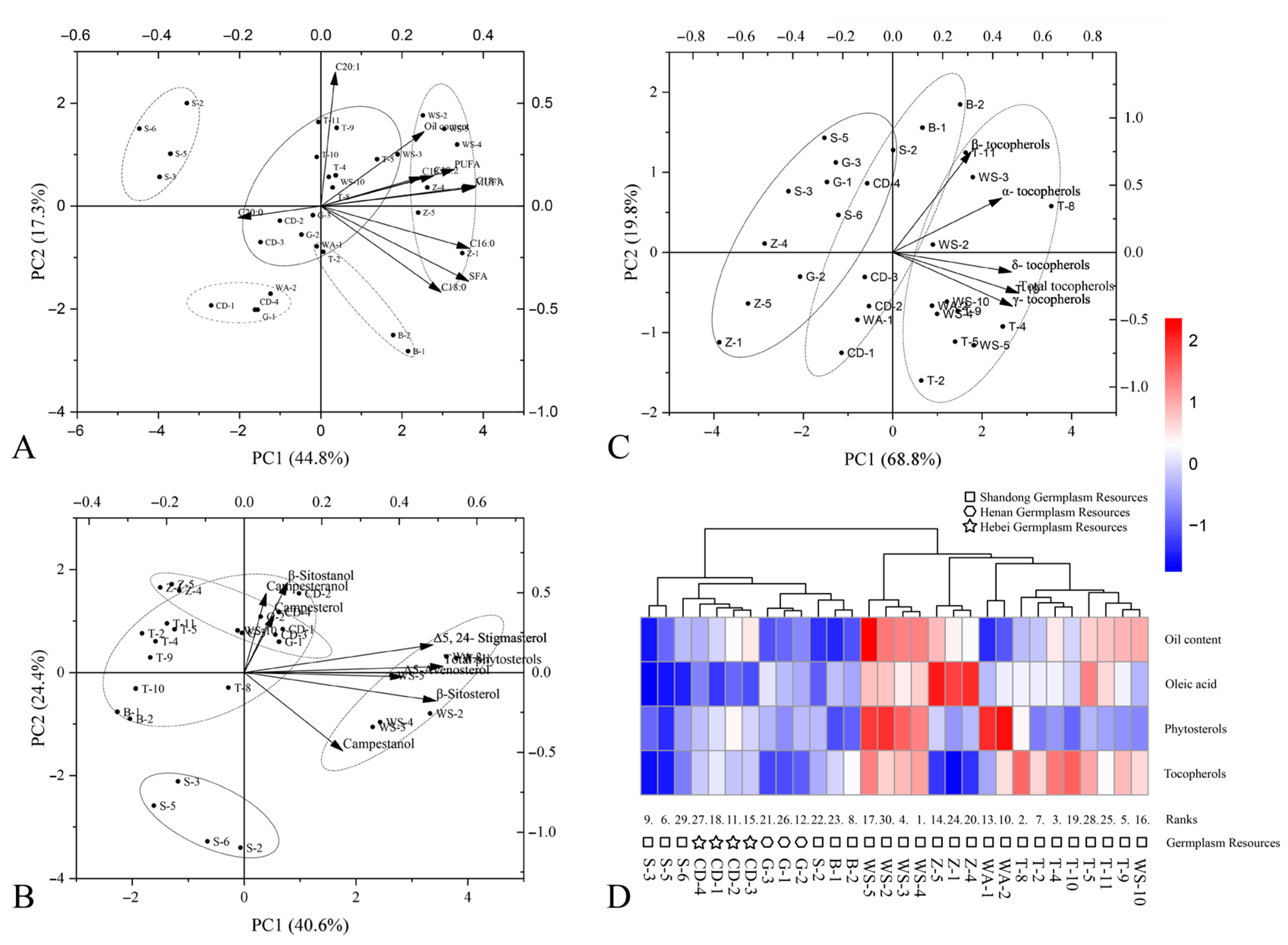
3.6. Principal Component Analysis of Fruit Quality Traits in C. retusus Germplasm Accessions
3.7. Comprehensive Assessment of Fruit Quality in 30 C. retusus Germplasm Accessions
3.8. Analysis of Secondary Metabolite Composition in Kernels from 30 C. retusus Germplasm Accessions
4. Discussion
4.1. Analysis of Fruit Quality in C. retusus Germplasm Accessions
4.2. Comprehensive Evaluation of Fruit Quality Traits in C. retusus Germplasm Accessions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BHA | Butylated hydroxyanisole |
| BHT | Butylated hydroxytoluene |
| EFAs | Essential fatty acids |
| LDL | Low-density lipoprotein |
| HDL | High-density lipoprotein |
| EPA | Eicosapentaenoic acid |
| DHA | Docosahexaenoic acid |
| UV | Ultraviolet |
| FL | Fruit length |
| FW | Fruit width |
| FSI | Fruit shape index |
| GL | Grain length |
| GW | Grain width |
| ST | Shell thickness |
| Kp | Kernel percentage |
| GC | Gas chromatograph |
| CV | Coefficient of variation |
| UFAs | Unsaturated fatty acids |
| SFA | Saturated fatty acids |
| MUFA | Monounsaturated fatty acids |
| PUFA | Polyunsaturated fatty acids |
| PCA | Principal component analysis |
| PC | Principal components |
References
- Hashemi, S.M.B.; Khaneghah, A.M.; Koubaa, M.; Lopez-Cervantes, J.; Yousefabad, S.H.A.; Hosseini, S.F.; Karimi, M.; Motazedian, A.; Asadifard, S. Novel edible oil sources: Microwave heating and chemical properties. Food Res. Int. 2017, 92, 147–153. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, M.; Liu, C.; Liang, R.; Shuai, X.; Chen, J. Characterization of a novel squalene-rich oil: Pachira macrocarpa seed oil. J. Food Sci. 2022, 87, 1696–1707. [Google Scholar] [CrossRef]
- Fan, J.S. Current Situation and Prospect of woody oil production development in China. Non-Wood For. Res. 2008, 77, 116–122. (In Chinese) [Google Scholar] [CrossRef]
- The, S.S.; Lau, H.L.N. Phytonutrient content and oil quality of selected edible oils upon twelve months storage. J. Am. Oil Chem. Soc. 2023, 100, 651–661. [Google Scholar] [CrossRef]
- Cui, T.; Zhang, S.; Chen, N.; Zu, A.; Han, J.; Tao, T. Determination of tert butyl hydroquinone in flaxseed oil by high-performance liquid chromatography. Agric. Prod. Process. 2025, 10, 71–75+82. (In Chinese) [Google Scholar]
- Liang, Q.; Wang, W.; Yuan, F.; Liu, X.; Li, D.; Yang, K. Characterization of yuanbaofeng (Acer truncatum Bunge) samaras: Oil, fatty acid, and phytosterol content. Ind. Crops Prod. 2019, 135, 344–351. [Google Scholar] [CrossRef]
- Chang, M.; Wang, Z.; Zhang, T.; Wang, T.; Liu, R.; Wang, Y.; Jin, Q.; Wang, X. Characterization of fatty acids, triacylglycerols, phytosterols and tocopherols in peony seed oil from five different major areas in China. Food Res. Int. 2020, 137, 109416. [Google Scholar] [CrossRef] [PubMed]
- Shuai, X.; Dai, T.; Chen, M.; Liu, C.; Ruan, R.; Liu, Y.; Chen, J. Characterization of lipid compositions, minor components and antioxidant capacities in macadamia (Macadamia integrifolia) oil from four major areas in China. Food Biosci. 2022, 50, 102009. [Google Scholar] [CrossRef]
- Das, U.N. Long-chain polyunsaturated fatty acids in the growth and development of the brain and memory. Nutrition 2003, 19, 62–65. [Google Scholar] [CrossRef]
- Demaison, L.; Moreau, D. Dietary n-3 polyunsaturated fatty acids and coronary heart disease-related mortality: A possible mechanism of action. Cell. Mol. Life Sci. CMLS 2002, 59, 463–477. [Google Scholar] [CrossRef]
- Li, G.H.; Wang, X.P.; Yang, H.Y.; Zhang, P.F.; Wu, F.Q.; Li, Y.C.; Zhou, Y.J.; Zhang, X.; Ma, H.; Zhang, W.; et al. Alpha Linolenic acid but not linolenic acid protects against hypertension: Critical role of SIRT3 and autophagic flux. Cell Death Dis. 2020, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Hodson, L.; Rosqvist, F.; Parry, S.A. The influence of dietary fatty acids on liver fat content and metabolism. Proc. Nutr. Soc. 2020, 79, 30–41. [Google Scholar] [CrossRef]
- Adlercreutz, H. Phytooestrogens and cancer. Lancet Oncol. 2002, 3, 364–373. [Google Scholar] [CrossRef]
- Baker, E.J.; Valenzuela, C.A.; De Souza, C.O.; Yaqoob, B.P.; Miles, E.A.; Calder, P.C. Comparative anti-inflammatory effects of plant- and marine-derived omega-3 fatty acids explored in an endothelial cell line. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2020, 1865, 158662. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, I.; Blondeau, N.; Heurteaux, C.; Widmann, C.; Romey, G.; Lazdunski, M. Polyunsaturated fatty acids are potent neuroprotectors. Embo J. 2000, 19, 1784–1793. [Google Scholar] [CrossRef]
- Calder, P.C. Very long-chain n-3 fatty acids and human health: Fact, fiction and the future. Proc. Nutr. Soc. 2018, 77, 52–72. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Miras-Moreno, B.; Sabaterjara, A.B.; Pedreno, M.A.; Almagro, L. Bioactivity of phytosterols and their production in plant in vitro cultures. J. Agric. Food Chem. 2016, 64, 7049–7058. [Google Scholar] [CrossRef]
- Miettinen, T.A.; Puska, P.; Gylling, H.; Vanhanen, H.; Vartiainen, E. Reduction of serum cholesterol with sitostanol-ester margarine in a mildly hypercholesterolemic population. N. Engl. J. Med. 1995, 333, 1308–1312. [Google Scholar] [CrossRef]
- Moreau, R.A.; Whitaker, B.D.; Hicks, K.B. Phytosterols, phytostanols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid Res. 2002, 41, 457–500. [Google Scholar] [CrossRef]
- Valsta, L.M.; Lemström, A.; Ovaskainen, M.L.; Lampi, A.M.; Toivo, J.; Korhonen, T.; Piironen, V. Estimation of plant sterol and cholesterol intake in Finland: Quality of new values and their effect on intake. Br. J. Nutr. 2004, 92, 671–678. [Google Scholar] [CrossRef]
- Rong, S.; Xu, R.; Li, W.F. Phytosterols and dementia. Plant Foods Hum. Nutr. 2016, 71, 347–354. [Google Scholar] [CrossRef]
- Alemany, L.; Barbera, R.; Alegría, A.; Laparra, J.M. Plant sterols from foods in inflammation and risk of cardiovascular disease: A real threat? Food Chem. Toxicol. 2014, 69, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Othman, R.A.; Moghadasian, M.H. Beyond cholesterol-lowering effects of plant sterols: Clinical and experimental evidence of anti-inflammatory properties. Nutr. Rev. 2011, 69, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Woyengo, T.A.; Ramprasath, V.R.; Jones, P.J. Anticancer effects of phytosterols. Eur. J. Clin. Nutr. 2009, 63, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.C.; Li, S.G.; Wang, Z.L.; Chang, F.G.; Kong, J.J.; Gai, J.Y.; Zhao, T.J. Identification of major quantitative trait loci for seed oil content in soybeans by combining linkage and genome-wide association mapping. Front. Plant Sci. 2017, 8, 1222. [Google Scholar] [CrossRef]
- Tavva, V.S.; Kim, Y.H.; Kagan, I.A.; Dinkins, R.; Kim, K.H.; Collins, G. Increased a-tocopherol content in soybean seed overexpressing the Perilla frutescens y-tocopherol methyltransferase gene. Plant Cell Rep. 2007, 26, 61. [Google Scholar] [CrossRef]
- Raederstorff, D.; Wyss, A.; Calder, P.C.; Weber, P.; Eggersdorfer, M. Vitamin E function and requirements in relation to PUFA. Br. J. Nutr. 2015, 114, 1113–1122. [Google Scholar] [CrossRef]
- Sato, K.; Gosho, M.; Yamamoto, T.; Kobayashi, Y.; Ishii, N.; Ohashi, T.; Nakade, Y.; Ito, K.; Fukuzawa, Y.; Yoneda, M. Vitamin E has a beneficial effect on nonalcoholic fatty liver disease: A meta-anaiysis of randomized controlled trials. Nutrition 2015, 31, 923–930. [Google Scholar] [CrossRef]
- Hanson, C.; Lyden, E.; Furtado, J.; Campos, H.; Litonjua, A.A. Serum tocopherol levels and tocopherols intake are associated with lung function in the normative aging study. Clin. Nutr. 2016, 35, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Munne, B.S. The role of ɑ- tocopherol in plant stress tolerance. J. Plant Physiol. 2005, 162, 743–748. [Google Scholar] [CrossRef]
- Scherder, W.C.; Fehr, W.R.; Welke, G.A.; Tong, W. Tocopherol concent and agronomic performance of soybean lines and reduced palmitate. Crop Sci. 2006, 46, 1286–1290. [Google Scholar] [CrossRef]
- Seker, M.A.; Kemal, M.G.; Ipek, M.; Toplu, C.; Kaleci, N. Screening and comparing tocopherols in the rapeseed (Brassica napus L.) and olive (Olea europaea L.) varieties using high-performance liquid chromatography. Int. J. Food Sci. Nut. 2008, 59, 483–490. [Google Scholar] [CrossRef]
- Wang, J.; Hu, H.; Liang, X.; Tahir ul Qamar, M.; Zhang, Y.; Zhao, J.; Ren, H.; Yan, X.; Ding, B.; Guo, J. High-quality genome assembly and comparative genomic profiling of yellowhorn (Xanthoceras sorbifolia) revealed environmental adaptation footprints and seed oil contents variations. Front. Plant Sci. 2023, 14, 1147946. [Google Scholar] [CrossRef]
- Ding, J.; Wang, L.; Ruan, C. Comparative transcriptome analysis of lipid biosynthesis in seeds and non-seed tissues of sea buckthorn. Genes. Genom. 2017, 39, 1021–1033. [Google Scholar] [CrossRef]
- Song, J.H.; Oak, M.K.; Hong, S.P. Morphological traits in an androdioecious species, Chionanthus retusus (Oleaceae). Flora 2016, 223, 129–137. [Google Scholar] [CrossRef]
- Niu, M.; Zhao, T.; Xu, D.; Liu, C.; Liu, Y.; Sun, M.; Xie, H.; Li, J. Physiological Responses of Chionanthus retusus Seedlings to Drought and Waterlogging Stresses. Forests 2023, 14, 429. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Liu, D.; Yu, M.; Zhao, Z.; Xie, X.; Han, C.; Wu, D.; Bao, Z.; Zhao, Y.J. Discussion on Utilization and Industrialization Development of Woody Oil Plants in Shandong Province. Anhui Agric. Sci. 2020, 48, 146–151. (In Chinese) [Google Scholar] [CrossRef]
- GB 5009.6-2016; National Food Safety Standard—Determination of Fat in Foods. National Health and Family Planning Commission of the PRC: Beijing, China; China Food and Drug Administration: Beijing, China, 2016.
- GB 5009.168-2016; National Food Safety Standard—Determination of Fatty Acids in Food. National Health and Family Planning Commission of the PRC: Beijing, China; China Food and Drug Administration: Beijing, China, 2016.
- GB/T 25223-1999; Determination of Sterol Composition and Total Sterol Content in Animal and Plant Oils and Fats. Standardization Administration of China: Beijing, China, 1999.
- GB 5009.82-2016; National Food Safety Standards—Determination of vitamins A, D, and E in food. National Health and Family Planning Commission of the PRC: Beijing, China; China Food and Drug Administration: Beijing, China, 2016.
- El-Esawi, M. Genetic diversity and evolution of Brassica genetic resources: From morphology to novel genomic technologies a review. Plant Genet. Resour. Charact. Util. 2017, 15, 388–399. [Google Scholar] [CrossRef]
- Zubr, J.; Matthäus, B. Effects of growth conditions on fatty acids and tocopherols in Camelina sativa oil. Ind. Crop. Prod. 2002, 15, 155–162. [Google Scholar] [CrossRef]
- Huang, Z. Overview of pharmacological research of Camellia Oil. Mod. Food 2015, 17, 1–4. (In Chinese) [Google Scholar] [CrossRef]
- Yu, L.; Wang, Y.; Wu, G.C.; Jin, J. Quality and composition of virgin olive oils from indigenous and european cultivars grown in China. J. Am. Oil Chem. Soc. 2020, 97, 12315–12327. [Google Scholar] [CrossRef]
- Eguchi, K.; Manabe, I.; Oishi-Tanaka, Y.; Ohsugi, M.; Kono, N.; Ogata, F.; Yagi, N.; Ohto, U.; Kimoto, M.; Miyake, K.; et al. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metab. 2012, 15, 518–533. [Google Scholar] [CrossRef]
- Colla, L.M.; Muccillo-Baisch, A.L.; Costa, J.A.V. Spirulina platensis effects on the levels of total cholesterol, HDL and triacylglycerols in rabbks fed with a hypercholesterolemic diet. Braz. Arch. Biol. Technol. 2008, 51, 405–411. [Google Scholar] [CrossRef]
- Moreau, R.A.; Nyström, L.; Whitaker, B.D.; Winkler-Moser, J.K.; Baer, D.J.; Gebauer, S.K.; Hicks, K.B. Phytosterols and their derivatives: Structural diversity, distribution, metabolism, analysis, and health-promoting uses. Prog. Lipid Res. 2018, 70, 35–61. [Google Scholar] [CrossRef] [PubMed]
- FDA Food Labeling. Health CLaims: Phytosterols and Risk of Coronary Heart Disease. Proposed Rule. 2010. Available online: https://www.federalregister.gov/documents/2010/12/08/2010-30386/food-labeling-health-claim-phytosterols-and-risk-of-coronary-heart-disease (accessed on 21 November 2021).
- Baccouri, B.; Manai, H.; Casas, J.S. Tunisian wild olive (Olea europaea L. subsp. Oleaster) oils: Sterolic and triterpenic dialcohol compounds. Ind. Crop. Prod. 2018, 120, 11–15. [Google Scholar] [CrossRef]
- Venegas-Calerón, M.; Ruíz-Méndez, M.V.; Martínez-Force, E. Characterization of Xan-thoceras sorbifolium Bunge seeds: Lipids, proteins and saponins content. Ind. Crop. Prod. 2017, 109, 192–198. [Google Scholar] [CrossRef]
- Phillips, K.M.; Ruggio, D.M.; Ashraf-Khorassani, M. Phytosterol composition of nuts and seeds commonly consumed in the United States. J. Agric. Food Chem. 2005, 3, 9436–9445. [Google Scholar] [CrossRef]
- Munne-Bosch, S.; Alegre, L. The function of tocopherols and tocotrienols in plants. Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- FDA. Petition for a Qualified Health Claim for a Nutraceutical Formulation and Management of Behavior and Cognitive Difficulties that Can Accompany Dementia. 2013. Available online: https://www.fda.gov/media/119441/download (accessed on 23 November 2021).
- Wang, M.; Hu, L.; Guo, J.; Yu, D.L.; Jiang, L.Z. Determination of main fatty acid composition in fractionated olive oils by FTIR spectroscopy. Trans. Chin. Soc. Agric. Eng. 2011, 6, 365–369. [Google Scholar] [CrossRef]
- Chen, Z.C.; Ni, Z.L.; Mo, R.H.; Zhong, D.L.; Tang, F.B. Comprehensive evaluation on quality of oils from seven kinds of woody oilcrops. China Oils Fats 2018, 43, 80–85. (In Chinese) [Google Scholar] [CrossRef]
- Abrante-Pascua, S.; Goicoechea-Oses, E.; Nieva-Echevarría, B. Insight into the role and fate of a natural tocopherol extract rich in gamma-isoform during sunflower oil degradation under several thermo-oxidative conditions: Formation of 3,4-dehydro-tocopherols. FRI 2025, 221, 117177. [Google Scholar] [CrossRef]
- Zhang, R.; Li, T.; Li, D.; Wang, X.; Wang, T.; Yu, D. Phytosterol ester VE composite antioxidant properties in corn oil. J. Chin. Cereals Oils Assoc. 2018, 33, 58–63. (In Chinese) [Google Scholar]
- Ji, M.; Deng, J.; Yao, B.; Chen, R.; Fan, Z.; Guan, J.; Li, X.; Wu, F.; Nikas, K. Ecogeographical variation of 12 morphological traits within Pinus tabulaeformis: The effects of environmental factors and demographic historie. J. Plant Ecol. 2016, 10, 386–396. [Google Scholar] [CrossRef]
- Tian, W.; Li, Z.; Wang, L.; Sun, S.; Wang, D.; Wang, K.; Wang, G.; Liu, Z.; Lu, X.; Feng, J.; et al. Comprehensive Evaluation of Apple Germplasm Genetic Diversity on the Basis of 26 Phenotypic Traits. Agronomy 2024, 14, 1264. [Google Scholar] [CrossRef]
| Superior Trees No. | Geographic Source | Location | Altitude (m) | Annual Precipitation (mm) | Annual Mean Temperature (°C) | |
|---|---|---|---|---|---|---|
| Latitude (°N) | Longitude (°E) | |||||
| T-2 | Mount Tai, Tai’an City, Shandong Province | 36°13′14″ | 117°7′34″ | 161 | 740 | 11.73 |
| T-4 | Mount Tai, Tai’an City, Shandong Province | 36°13′14″ | 117°7′34″ | 161 | 740 | 11.73 |
| T-5 | Mount Tai, Tai’an City, Shandong Province | 36°13′14″ | 117°7′34″ | 161 | 740 | 11.73 |
| T-8 | Mount Tai, Tai’an City, Shandong Province | 36°13′14″ | 117°7′34″ | 161 | 740 | 11.73 |
| T-9 | Mount Tai, Tai’an City, Shandong Province | 36°13′14″ | 117°7′34″ | 161 | 740 | 11.73 |
| T-10 | Mount Tai, Tai’an City, Shandong Province | 36°13′14″ | 117°7′34″ | 161 | 740 | 11.73 |
| T-11 | Mount Tai, Tai’an City, Shandong Province | 36°13′14″ | 117°7′34″ | 161 | 740 | 11.73 |
| Z-1 | Boshan District, Zibo City, Shandong Province | 36°21′36″ | 117°59′16″ | 190 | 680 | 13.83 |
| Z-4 | Boshan District, Zibo City, Shandong Province | 36°21′36″ | 117°59′16″ | 190 | 680 | 13.83 |
| Z-5 | Boshan District, Zibo City, Shandong Province | 36°21′36″ | 117°59′16″ | 190 | 680 | 13.83 |
| B-1 | Boshan District, Zibo City, Shandong Province | 36°30′58″ | 117°48′51″ | 190 | 680 | 13.83 |
| B-2 | Boshan District, Zibo City, Shandong Province | 36°30′58″ | 117°48′51″ | 190 | 680 | 13.83 |
| S-2 | Yiyuan Country, Zibo City, Shandong Province | 35°59′42″ | 118°12′59″ | 190 | 680 | 13.83 |
| S-3 | Yiyuan Country, Zibo City, Shandong Province | 35°59′42″ | 118°12′59″ | 190 | 680 | 13.83 |
| S-5 | Yiyuan Country, Zibo City, Shandong Province | 35°59′42″ | 118°12′59″ | 190 | 680 | 13.83 |
| S-6 | Yiyuan Country, Zibo City, Shandong Province | 35°59′42″ | 118°12′59″ | 190 | 680 | 13.83 |
| WS-2 | Qingzhou City, Weifang City, Shandong Province | 36°41′6″ | 118°18′7″ | 101 | 679 | 13.36 |
| WS-3 | Qingzhou City, Weifang City, Shandong Province | 36°41′6″ | 118°18′7″ | 101 | 679 | 13.36 |
| WS-4 | Qingzhou City, Weifang City, Shandong Province | 36°41′6″ | 118°18′7″ | 101 | 679 | 13.36 |
| WS-5 | Qingzhou City, Weifang City, Shandong Province | 36°41′6″ | 118°18′7″ | 101 | 679 | 13.36 |
| WA-1 | Anqiu City, Weifang City, Shandong Province | 36°12′2″ | 119°1′36″ | 101 | 679 | 13.36 |
| WA-2 | Anqiu City, Weifang City, Shandong Province | 36°12′2″ | 119°1′36″ | 101 | 679 | 13.36 |
| CD-1 | Chengde City, Hebei Province | 41°6′53″ | 117°48′18″ | 327 | 493 | 9.09 |
| CD-2 | Chengde City, Hebei Province | 41°6′53″ | 117°48′18″ | 327 | 493 | 9.09 |
| CD-3 | Chengde City, Hebei Province | 41°6′53″ | 117°48′18″ | 327 | 493 | 9.09 |
| CD-4 | Chengde City, Hebei Province | 41°6′53″ | 117°48′18″ | 327 | 493 | 9.09 |
| G-1 | Tongbai City, Nanyang City, Henan Province | 32°25′16″ | 113°17′25″ | 283 | 821 | 13.4 |
| G-2 | Tongbai City, Nanyang City, Henan Province | 32°25′16″ | 113°17′25″ | 283 | 821 | 13.4 |
| G-3 | Tongbai City, Nanyang City, Henan Province | 32°25′16″ | 113°17′25″ | 283 | 821 | 13.4 |
| Germplasm ID | Fruit Traits | Kernel Traits | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh Weight (Per 100 Grains)/g | Fruit Length/mm | Fruit Width/mm | Fruit Shape Index | Kernel Percentage/% | Kernel Weight (Per 100 Grains)/g | Shell Weight (Per 100 Grains)/g | Grain Weight (Per 100 Grains)/g | Grain Length/mm | Grain Transverse Diameter/mm | Shell Thickness/mm | Grain Length/Shell Thickness | |
| T-2 | 79.36 ± 1.56 h | 12.26 ± 0.23 d | 10.38 ± 0.84 ab | 1.18 cd | 62.59 a | 14.99 ± 1.52 c | 8.96 ± 0.91 j | 23.95 ± 0.21 g | 10.56 ± 1.11 e | 7.14 ± 0.43 a | 0.38 ± 0.02 f | 30.03 a |
| T-4 | 90.70 ± 3.81 e | 12.97 ± 1.12 cd | 9.42 ± 0.22 c | 1.39 b | 45.67 i | 14.74 ± 1.30 c | 17.53 ± 1.56 b | 32.27 ± 2,14 c | 11.64 ± 1.31 cd | 7.30 ± 0.55 a | 0.68 ± 0.09 bc | 16.56 jk |
| T-5 | 47.42 ± 0.76 m | 10.90 ± 0.25 ef | 7.81 ± 0.05 e | 1.40 b | 54.07 d | 7.75 ± 0.54 j | 6.58 ± 0.71 l | 14.33 ± 1.11 k | 10.17 ± 1.04 e | 5.76 ± 0.72 bc | 0.39 ± 0.05 f | 24.47 cd |
| T-8 | 121.30 ± 7.51 a | 15.37 ± 0.38 a | 11.38 ± 0.18 a | 1.35 b | 44.84 ij | 16.17 ± 1.83 ab | 19.90 ± 0.25 a | 36.07 ± 0.98 a | 13.57 ± 1.21 ab | 7.49 ± 0.67 a | 0.67 ± 0.10 c | 20.27 fg |
| T-9 | 89.30 ± 2.79 e | 12.43 ± 0.56 d | 10.03 ± 0.47 b | 1.24 bc | 54 d | 14.22 ± 1.25 c | 12.11 ± 0.11 f | 26.33 ± 2.21 de | 10.46 ± 1.03 ef | 6.88 ± 0.52 ab | 0.55 ± 0.04 d | 20.53 fg |
| T-10 | 69.33 ± 1.52 ij | 11.26 ± 0.43 e | 10.30 ± 0.93 ab | 1.09 e | 57.2 c | 11.52 ± 1.11 e | 8.62 ± 0.92 j | 20.15 ± 1.34 i | 9.60 ± 0.87 ef | 6.90 ± 0.29 ab | 0.43 ± 0.03 de | 25.09 c |
| T-11 | 61.02 ± 0.34 k | 11.99 ± 0.25 de | 9.03 ± 0.71 c | 1.33 b | 49.53 g | 9.78 ± 0.99 fg | 9.97 ± 0.93 hi | 19.75 ± 1.23 i | 10.24 ± 1.98 e | 6.22 ± 0.54 ab | 0.51 ± 0.06 d | 21.14 f |
| WS-2 | 49.98 ± 0.63 m | 9.53 ± 0.72 g | 7.81 ± 0.60 e | 1.23 c | 51.09 f | 8.16 ± 0.87 i | 4.93 ± 0.55 m | 15.97 ± 0.94 k | 7.51 ± 3.20 g | 5.12 ± 0.42 c | 0.45 ± 0.07 de | 19.68 fgh |
| WS-3 | 73.85 ± 2.14 i | 11.83 ± 0.89 de | 9.70 ± 0.29 bc | 1.22 c | 58.4 b | 14.53 ± 1.32 c | 10.36 ± 1.31 h | 24.89 ± 2.12 f | 9.70 ± 1.21 ef | 7.15 ± 0.71 a | 0.43 ± 0.02 de | 25.58 bc |
| WS-4 | 112.71 ± 3.58 b | 13.73 ± 0.98 c | 11.08 ± 1.01 a | 1.24 bc | 48.9 g | 17.08 ± 1.98 a | 17.84 ± 2.11 a | 34.92 ± 3.13 b | 11.65 ± 0.85 cd | 7.68 ± 0.66 a | 0.69 ± 0.08 bc | 18.35 i |
| WS-5 | 60.92 ± 2.81 kl | 11.13 ± 1.05 e | 9.00 ± 1.20 cd | 1.24 bc | 57.09 c | 10.19 ± 1.05 f | 7.65 ± 0.45 k | 17.84 ± 1.34 j | 9.95 ± 0.48 ef | 6.31 ± 0.52 ab | 0.45 ± 0.02 de | 22.46 e |
| WS-10 | 78.95 ± 2.14 h | 12.76 ± 0.89 d | 10.70 ± 0.29 ab | 1.19 c | 46.42 i | 11.23 ± 1.32 e | 8.06 ± 1.31 jk | 24.19 ± 2.12 gf | 9.32 ± 1.21 ef | 7.15 ± 0.71 a | 0.43 ± 0.02 de | 21.67 fc |
| WA-1 | 72.75 ± 1.90 i | 15.36 ± 0.45 a | 9.23 ± 0.54 c | 1.67 a | 40.51 k | 10.12 ± 1.00 f | 14.85 ± 1.78 d | 24.97 ± 2.31 f | 14.32 ± 1.21 a | 6.39 ± 0.61 ab | 0.77 ± 0.13 b | 16.42 k |
| WA-2 | 73.21 ± 1.05 i | 13.55 ± 0.21 c | 9.88 ± 1.21 bc | 1.38 b | 45.35 i | 11.09 ± 1.11 e | 13.36 ± 1.19 e | 24.45 ± 2.55 f | 12.09 ± 1.20 c | 7.40 ± 0.70 a | 0.60 ± 0.06 cd | 19.89 fg |
| S-2 | 75.25 ± 2.06 i | 12.34 ± 0.59 d | 9.71 ± 0.76 bc | 1.27 bc | 45.93 i | 9.91 ± 0.78 fg | 13.65 ± 1.67 e | 23.56 ± 2.12 g | 10.34 ± 0.76 e | 6.49 ± 0.49 ab | 0.65 ± 0.04 c | 17.34 j |
| S-3 | 100.76 ± 5.14 c | 11.68 ± 2.14 de | 11.19 ± 0.11 a | 1.04 e | 52.5 e | 14.68 ± 1.65 c | 17.28 ± 1.82 b | 31.96 ± 3.02 c | 9.42 ± 0.81 ef | 7.71 ± 0.95 a | 0.67 ± 0.07 bc | 17.21 j |
| S-5 | 89.52 ± 3.84 e | 13.44 ± 0.78 c | 10.29 ± 1.00 ab | 1.31 b | 52.03 e | 16.67 ± 1.80 a | 15.07 ± 1.87 d | 31.74 ± 3.08 c | 11.74 ± 1.53 cd | 7.41 ± 0.83 a | 0.57 ± 0.02 cd | 21.08 f |
| S-6 | 63.32 ± 1.81 k | 11.63 ± 0.37 de | 8.96 ± 0.51 cd | 1.30 b | 42.05 k | 9.53 ± 0.99 fg | 8.79 ± 0.88 j | 18.32 ± 1.51 j | 9.85 ± 0.49 ef | 6.12 ± 1.01 b | 0.51 ± 0.04 d | 20.44 fg |
| B-1 | 49.24 ± 0.94 m | 11.62 ± 0.11 de | 8.74 ± 0.71 cd | 1.33 b | 47.58 h | 10.43 ± 1.21 ef | 11.49 ± 1.20 fg | 21.92 ± 1.93 h | 10.17 ± 1.10 e | 6.83 ± 1.14 ab | 0.42 ± 0.05 e | 24.82 cd |
| B-2 | 70.55 ± 1.58 ij | 12.21 ± 0.17 d | 10.01 ± 1.11 b | 1.22 bc | 58.49 b | 15.95 ± 1.65 ab | 11.32 ± 1.10 fg | 27.27 ± 2.22 d | 9.88 ± 0.89 ef | 6.72 ± 0.72 ab | 0.42 ± 0.01 e | 26.79 b |
| Z-1 | 58.14 ± 0.47 l | 14.13 ± 0.26 bc | 8.86 ± 0.39 cd | 1.60 a | 45.19 i | 8.95 ± 0.65 gh | 10.86 ± 1.03 gh | 19.81 ± 1.32 i | 12.67 ± 1.00 bc | 6.92 ± 0.53 ab | 0.45 ± 0.03 de | 26.12 b |
| Z-4 | 83.89 ± 2.08 ef | 12.35 ± 0.62 d | 9.93 ± 0.64 bc | 1.24 bc | 39.9 lk | 10.71 ± 1.09 ef | 16.13 ± 1.47 c | 26.84 ± 2.11 d | 10.85 ± 0.82 e | 6.95 ± 0.71 ab | 0.89 ± 0.09 a | 12.64 l |
| Z-5 | 98.98 ± 0.56 cd | 12.48 ± 0.23 d | 11.60 ± 1.12 a | 1.07 e | 50.69 f | 12.53 ± 1.20 d | 12.19 ± 1.54 f | 24.72 ± 3.10 f | 9.72 ± 0.91 ef | 7.41 ± 0.45 a | 0.69 ± 0.08 bc | 17.65 ij |
| CD-1 | 78.28 ± 0.56 h | 12.21 ± 0.23 d | 10.13 ± 1.41 ab | 1.21 bc | 56.15 c | 12.78 ± 1.24 d | 10.81 ± 1.52 gh | 22.76 ± 2.84 gh | 10.92 ± 0.83 e | 6.96 ± 0.65 ab | 0.49 ± 0.04 de | 22.29 e |
| CD-2 | 72.55 ± 0.56 i | 13.76 ± 0.58 c | 9.53 ± 0.95 c | 1.44 b | 47.48 i | 11.47 ± 1.14 e | 12.64 ± 1.44 f | 24.16 ± 3.00 g | 13.27 ± 0.56 abc | 6.41 ± 0.42 ab | 0.43 ± 0.06 de | 30.86 a |
| CD-3 | 80.79 ± 0.56 h | 11.42 ± 0.31 de | 11.05 ± 0.67 a | 1.03 ef | 55.61 d | 13.29 ± 1.07 d | 12.45 ± 1.23 f | 23.90 ± 3.34 g | 12.64 ± 0.72 bc | 6.08 ± 0.81 abc | 0.57 ± 0.08 cd | 22.18 e |
| CD-4 | 66.41 ± 0.56 jk | 11.88 ± 0.37 de | 9,03 ± 0.95 cd | 1.32 b | 50.07 g | 10.33 ± 1.11 ef | 9.08 ± 1.31 j | 20.63 ± 3.61 i | 9.16 ± 0.80 ef | 6.21 ± 0.25 ab | 0.61 ± 0.05 bc | 15.02 k |
| G-1 | 84.53 ± 0.56 ef | 12.62 ± 0.43 d | 10.87 ± 1.32 ab | 1.16 cd | 39.84 k | 10.64 ± 1.28 ef | 11.74 ± 1.44 fg | 26.71 ± 3.74 d | 10.48 ± 0.63 e | 5.96 ± 0.41 abc | 0.53 ± 0.07 d | 19.77 fgh |
| G-2 | 86.24 ± 0.56 ef | 13.18 ± 0.33 cd | 11.71 ± 0.82 a | 1.13 cd | 53.11 d | 14.62 ± 1.34 c | 13.80 ± 1.56 e | 27.53 ± 2.10 d | 11.94 ± 0.79 cd | 6.31 ± 0.97 ab | 0.72 ± 0.03 b | 16.58 k |
| G-3 | 81.44 ± 0.56 efg | 11.65 ± 0.27 de | 11.07 ± 0.88 a | 1.05 e | 52.22 e | 12.45 ± 1.20 d | 12.72 ± 1.54 f | 23.84 ± 3.11 g | 11.73 ± 0.47 cd | 5.81 ± 0.45 bc | 0.51 ± 0.08 de | 23.00 e |
| Maximum | 121.30 | 15.37 | 11.71 | 1.60 | 62.59 | 17.08 | 19.90 | 36.07 | 14.43 | 7.68 | 0.89 | 30.03 |
| Minimum | 47.42 | 9.53 | 7.81 | 1.03 | 39.84 | 7.75 | 4.93 | 14.33 | 7.51 | 5.12 | 0.38 | 12.64 |
| Average | 77.02 ± 17.69 | 12.46 ± 1.23 | 9.98 ± 1.03 | 1.26 ± 0.15 | 50.15 ± 5.83 | 12.22 ± 2.57 | 12.02 ± 3.47 | 24.33 ± 5.56 | 10.85 ± 1.47 | 6.71 ± 0.63 | 0.55 ± 0.13 | 21.20 ± 4.20 |
| CV(%) | 27.15 | 11.18 | 10.63 | 11.48 | 11.62 | 26.52 | 32.42 | 26.75 | 14.09 | 9.53 | 25.18 | 19.83 |
| Germplasm ID | Oil Content/% | Palmitic Acid (C16:0) | Stearic Acid (C18:0) | Oleic Acid (C18:1) | Linoleic Acid (C18:2) | Linolenic Acid (C18:3) | Arachidic Acid (C20:0) | Gondoic Acid (C20:1) | Saturated Fatty Acids (SFA) | Monounsaturated Fatty Acids (MUFA) | Polyunsaturated Fatty Acids (PUFA) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T-2 | 34.9 ± 0.65 ef | 1.28 ± 0.03 f | 1.16 ± 0.01 e | 54.63 ± 0.09 cd | 22.41 ± 0.02 gh | 9.17 ± 0.05 cde | — | 0.28 ± 0.04 i | 2.44 ± 0.07 cde | 54.91 ± 0.13 ef | 31.58 ± 0.07 hi |
| T-4 | 38.2 ± 0.10 cd | 1.23 ± 0.12 f | 1.10 ± 0.01 e | 54.70 ± 0.17 cd | 22.44 ± 0.03 fg | 9.33 ± 0.09 cde | — | 0.35 ± 0.04 efg | 2.33 ± 0.31 de | 55.05 ± 0.21 de | 31.77 ± 0.12 hi |
| T-5 | 39.2 ± 0.38 bcd | 1.25 ± 0.05 f | 1.34 ± 0.06 cd | 57.67 ± 0.25 b | 21.41 ± 0.03 gh | 11.47 ± 0.12 b | — | 0.38 ± 0.03 cd | 2.59 ± 0.14 cd | 55.05 ± 0.28 de | 32.88 ± 0.15 ghi |
| T-8 | 34.5 ± 0.53 ef | 1.21 ± 0.12 f | 1.31 ± 0.09 cd | 54.73 ± 0.47 cd | 23.40 ± 0.05 efg | 8.60 ± 0.05 de | — | 0.38 ± 0.03 cd | 2.52 ± 0.24 cd | 55.11 ± 0.50 de | 32.00 ± 0.10 hi |
| T-9 | 40.3 ± 0.87 bc | 1.22 ± 0.02 f | 0.98 ± 0.15 fg | 54.67 ± 0.45 cd | 24.42 ± 0.04 ef | 9.30 ± 0.17 cd | — | 0.38 ± 0.06 cd | 2.20 ± 0.21 def | 55.05 ± 0.51 de | 33.72 ± 0.21 fgh |
| T-10 | 35.5 ± 0.60 ef | 1.21 ± 0.01 f | 1.04 ± 0.15 ef | 54.03 ± 0.08 cd | 23.48 ± 0.04 efg | 9.27 ± 0.16 cde | — | 0.38 ± 0.03 cd | 2.25 ± 0.19 def | 54.41 ± 0.11 efg | 32.75 ± 0.20 ghi |
| T-11 | 39.6 ± 0.64 bcd | 1.06 ± 0.32 gh | 0.74 ± 0.08 gh | 55.77 ± 0.06 c | 23.40 ± 0.02 efg | 9.30 ± 0.55 cd | — | 0.35 ± 0.03 efg | 1.80 ± 0.42 g | 56.12 ± 0.09 de | 32.70 ± 0.52 ghi |
| Z-1 | 37.8 ± 1.15 cd | 1.98 ± 0.06 ab | 1.52 ± 0.24 bc | 58.97 ± 0.28 ab | 25.47 ± 0.03 cde | 9.43 ± 0.11 cd | — | 0.32 ± 0.05 h | 3.50 ± 0.33 a | 59.29 ± 0.33 ab | 34.90 ± 0.14 fg |
| Z-4 | 37.2 ± 0.96 cde | 1.28 ± 0.01 f | 1.53 ± 0.11 bc | 59.43 ± 0.04 ab | 26.48 ± 0.05 cd | 8.70 ± 0.06 de | — | 0.36 ± 0.09 e | 2.81 ± 0.16 bc | 59.79 ± 0.13 ab | 35.18 ± 0.11 f |
| Z-5 | 40.0 ± 0.96 bc | 1.15 ± 0.01 fg | 1.64 ± 0.09 b | 59.67 ± 0.15 ab | 22.44 ± 0.04 fg | 9.40 ± 0.07 cd | — | 0.33 ± 0.03 gh | 2.79 ± 0.14 bc | 60.00 ± 0.18 a | 31.84 ± 0.11 de |
| B-1 | 28.9 ± 0.58 h | 1.76 ± 0.01 cd | 1.88 ± 0.01 a | 53.60 ± 0.08 cd | 28.82 ± 0.04 b | 9.87 ± 0.35 cd | — | 0.27 ± 0.02 j | 3.64 ± 0.20 a | 53.87 ± 0.10 fgh | 38.69 ± 0.41 d |
| B-2 | 30.5 ± 0.36 g | 1.69 ± 0.22 cde | 1.83 ± 0.05 ab | 53.10 ± 0.11 cd | 28.49 ± 0.06 b | 9.40 ± 0.06 cd | — | 0.28 ± 0.03 i | 3.52 ± 0.27 ab | 53.38 ± 0.14 fgh | 37.89 ± 0.12 de |
| S-3 | 28.3 ± 0.35 h | 0.88 ± 0.04 h | 0.45 ± 0.01 hij | 49.53 ± 0.06 ih | 21.41 ± 0.03 gh | 8.27 ± 0.29 de | 0.13 ± 0.03 de | 0.34 ± 0.05 fgh | 1.33 ± 0.05 hi | 49.87 ± 0.11 jkl | 29.68 ± 0.32 ijk |
| S-5 | 31.4 ± 0.95 g | 0.44 ± 0.08 i | 0.73 ± 0.08 gh | 50.17 ± 0.12 gi | 20.71 ± 0.03 ghi | 9.37 ± 1.15 cd | 0.13 ± 0.03 de | 0.35 ± 0.03 efg | 1.17 ± 0.16 ij | 50.52 ± 0.15 jkl | 30.08 ± 1.18 hij |
| S-6 | 34.5 ± 1.60 ef | 0.41 ± 0.02 i | 0.72 ± 0.04 gh | 50.30 ± 0.06 gi | 20.39 ± 0.03 ghi | 5.00 ± 0.80 f | 0.13 ± 0.03 de | 0.40 ± 0.04 ab | 1.13 ± 0.06 ij | 50.70 ± 0.10 jkl | 25.39 ± 0.83 g |
| S-2 | 29.2 ± 0.38 h | 0.42 ± 0.06 i | 0.58 ± 0.01 hi | 51.53 ± 0.37 fg | 24.41 ± 0.04 ef | 8.47 ± 0.81 de | 0.12 ± 0.03 ef | 0.39 ± 0.01 bc | 1.00 ± 0.07 jk | 51.92 ± 0.38 ij | 32.88 ± 0.85 ef |
| WS-2 | 42.8 ± 1.01 b | 2.02 ± 0.31 ab | 0.80 ± 0.08 gh | 56.20 ± 0.04 bc | 29.38 ± 0.04 ab | 11.03 ± 1.01 b | 0.13 ± 0.03 de | 0.38 ± 0.04 cd | 2.82 ± 0.39 cd | 56.58 ± 0.08 ab | 40.41 ± 1.05 bc |
| WS-3 | 42.4 ± 1.30 b | 1.63 ± 0.02 cde | 1.10 ± 0.09 e | 55.40 ± 0.08 bc | 25.34 ± 0.02 cde | 12.80 ± 0.51 a | 0.15 ± 0.03 c | 0.35 ± 0.03 efg | 2.73 ± 0.11 cde | 55.75 ± 0.11 bc | 38.14 ± 0.53 d |
| WS-4 | 42.3 ± 0.49 b | 2.09 ± 0.02 ab | 1.09 ± 0.06 ef | 56.03 ± 0.06 bc | 30.34 ± 0.03 a | 12.40 ± 0.46 a | 0.13 ± 0.02 de | 0.37 ± 0.04 ef | 3.18 ± 0.08 bc | 56.40 ± 0.10 ab | 42.74 ± 0.49 a |
| WS-5 | 47.5 ± 0.67 a | 1.80 ± 0.32 cd | 1.15 ± 0.11 e | 56.37 ± 0.12 bc | 30.35 ± 0.03 a | 11.43 ± 1.11 b | 0.13 ± 0.03 de | 0.36 ± 0.06 ef | 2.95 ± 0.43 bc | 55.73 ± 0.18 ab | 41.78 ± 1.14 ab |
| WS-10 | 40.6 ± 0.62 bc | 1.06 ± 0.24 fg | 1.15 ± 0.07 e | 53.42 ± 0.10 cd | 18.36 ± 0.02 ijk | 13.06 ± 1.43 a | 0.13 ± 0.02 de | 0.36 ± 0.03 ef | 2.95 ± 0.49 bc | 55.73 ± 0.48 ab | 31.42 ± 127 bc |
| WA-1 | 29.0 ± 0.45 h | 1.21 ± 0.02 f | 1.06 ± 0.01 ef | 53.53 ± 0.03 cd | 30.51 ± 0.05 a | 9.80 ± 0.10 d | 0.18 ± 0.04 b | 0.27 ± 0.04 j | 2.27 ± 0.03 def | 53.80 ± 0.07 fgh | 40.31 ± 0.15 bc |
| WA-2 | 30.8 ± 1.00 g | 1.48 ± 0.03 e | 0.93 ± 0.09 fg | 54.63 ± 0.25 cd | 25.47 ± 0.04 cde | 5.03 ± 0.15 f | 0.21 ± 0.04 a | 0.26 ± 0.03 k | 2.41 ± 0.12 de | 54.89 ± 0.28 ef | 30.50 ± 0.19 hij |
| CD-1 | 35.6 ± 0.73 ef | 1.27 ± 0.07 f | 1.04 ± 0.05 ef | 50.78 ± 0.57 gi | 15.33 ± 0.04 k | 9.05 ± 0.91 de | — | 0.25 ± 0.01 k | 2.24 ± 0.27 de | 51.03 ± 0.48 ijk | 24.38 ± 0.56 lm |
| CD-2 | 37.2 ± 1.54 cd | 1.19 ± 0.05 f | 0.94 ± 0.07 fg | 51.77 ± 1.74 fg | 24.43 ± 0.02 ef | 10.08 ± 2.21 cd | — | 0.29 ± 0.01 i | 2.33 ± 0.94 de | 52.06 ± 0.56 ij | 34.51 ± 0.34 fg |
| CD-3 | 38.2 ± 2.65 ef | 1.14 ± 0.07 f | 1.21 ± 0.09 de | 51.22 ± 0.25 fg | 19.37 ± 0.04 ijk | 10.24 ± 0.15 cd | — | 0.31 ± 0.03 k | 2.48 ± 0.52 de | 51.53 ± 0.28 ijk | 29.61 ± 0.19 ijk |
| CD-4 | 34.2 ± 1.94 ef | 1.35 ± 0.03 f | 1.15 ± 0.10 e | 52.33 ± 0.73 fg | 15.34 ± 0.02 k | 9.67 ± 1.15 de | — | 0.27 ± 0.02 j | 2.79 ± 0.91 cde | 52.60 ± 0.28 hij | 25.01 ± 0.19 l |
| G-1 | 31.3 ± 1.75 g | 1.31 ± 0.03 f | 1.24 ± 0.12 e | 53.54 ± 1.23 cd | 15.38 ± 0.06 k | 8.42 ± 1.48 de | — | 0.30 ± 0.03 i | 2.92 ± 0.63 bc | 53.84 ± 0.28 fgh | 23.80 ± 0.19 lm |
| G-2 | 32.5 ± 1.83 g | 1.21 ± 0.07 f | 1.11 ± 0.11 ef | 53.21 ± 1.63 cd | 23.40 ± 0.04 efg | 9.82 ± 0.94 d | — | 0.32 ± 0.03 h | 2.67 ± 1.27 cd | 53.53 ± 0.28 fgh | 33.22 ± 0.19 fgh |
| G-3 | 30.4 ± 1.41 g | 1.38 ± 0.03 f | 1.06 ± 0.05 ef | 54.38 ± 0.85 cd | 24.43 ± 0.05 ef | 10.06 ± 0.61 d | — | 0.33 ± 0.03 h | 2.26 ± 0.53 de | 54.71 ± 0.28 ef | 34.49 ± 0.19 fg |
| Maximum | 47.50 | 2.09 | 1.88 | 59.67 | 30.51 | 13.06 | 0.21 | 0.40 | 3.64 | 60.00 | 41.78 |
| Minimum | 28.30 | 0.41 | 0.45 | 49.53 | 15.33 | 5.00 | — | 0.26 | 1.00 | 49.87 | 23.80 |
| Average | 35.83 ± 4.87 | 1.29 ± 0.41 | 1.12 ± 0.33 | 54.18 ± 2.61 | 23.57 ± 4.19 | 9.57 ± 1.72 | 0.14 ± 0.03 | 0.33 ± 0.04 | 2.41 ± 0.64 | 54.51 ± 2.61 | 33.23 ± 4.83 |
| CV/% | 15.81 | 11.40 | 17.65 | 5.84 | 11.90 | 10.07 | 20.00 | 22.22 | 12.02 | 6.17 | 10.22 |
| Germplasm ID | Phytosterols mg/100 g | |||||||
|---|---|---|---|---|---|---|---|---|
| Campesterol | Campestanol | Campesteranol | β-Sitosterol | β-Sitostanol | Δ5-Avenasterol | Δ5,24-Stigmasterol | Total Sterols | |
| T-2 | 11.41 ± 0.25 h | 15.25 ± 0.85 hi | 18.63 ± 0.31 ab | 166.19 ± 1.05 ef | 37.48 ± 0.94 de | — | — | 248.96 ± 0.93 de |
| T-4 | 12.95 ± 0.57 fg | 12.09 ± 1.79 k | 16.74 ± 0.89 cd | 178.88 ± 1.98 de | 36.12 ± 0.74 e | — | — | 256.78 ± 0.56 cde |
| T-5 | 11.49 ± 0.05 h | 12.76 ± 0.79 k | 19.50 ± 0.92 a | 187.63 ± 2.16 bcd | 35.18 ± 0.50 ef | — | — | 266.56 ± 0.78 cd |
| T-8 | 14.13 ± 1.04 e | 28.10 ± 1.17 e | 13.44 ± 0.76 f | 195.13 ± 1.53 abc | 49.65 ± 0.64 a | — | — | 290.45 ± 1.93 c |
| T-9 | 10.99 ± 0.05 hi | 17.53 ± 0.52 g | 15.92 ± 0.67 cd | 167.70 ± 2.91 def | 42.28 ± 0.20 b | — | — | 254.42 ± 1.25 cde |
| T-10 | 11.98 ± 0.50 g | 23.57 ± 0.70 f | 14.96 ± 1.13 de | 155.93 ± 6.67 fg | 36.26 ± 0.16 e | — | — | 242.70 ± 1.06 de |
| T-11 | 16.95 ± 0.13 d | 17.21 ± 0.28 g | 18.73 ± 0.58 ab | 174.53 ± 1.39 de | 32.57 ± 0.23 h | — | — | 259.99 ± 0.63 cde |
| Z-1 | 22.03 ± 0.33 a | 17.58 ± 0.26 g | 19.33 ± 0.43 a | 163.85 ± 1.83 ef | 33.56 ± 0.89 g | — | — | 256.35 ± 0.31 cde |
| Z-4 | 22.95 ± 0.81 a | 18.44 ± 0.98 g | 17.80 ± 0.47 bc | 170.28 ± 2.69 def | 38.60 ± 1.08 d | — | — | 268.07 ± 0.50 cd |
| Z-5 | 21.93 ± 0.28 a | 16.88 ± 0.86 gh | 19.30 ± 0.26 a | 162.70 ± 2.00 ef | 33.88 ± 0.40 g | — | — | 274.69 ± 0.64 cd |
| B-1 | 12.43 ± 0.15 g | 13.31 ± 0.80 k | 10.07 ± 0.41 h | 165.56 ± 2.76 ef | 33.74 ± 0.23 g | — | — | 235.11 ± 0.71 def |
| B-2 | 12.18 ± 0.21 g | 14.21 ± 0.17 hij | 10.46 ± 0.55 gh | 172.60 ± 0.66 def | 31.66 ± 0.02 hi | — | — | 241.11 ± 0.88 de |
| S-3 | 17.59 ± 0.30 c | 31.74 ± 1.56 cd | 13.94 ± 1.07 ef | 176.40 ± 0.46 de | — | 2.99 ± 0.44 de | — | 242.66 ± 1.62 de |
| S-5 | 12.17 ± 0.14 g | 32.37 ± 0.39 c | 14.44 ± 0.28 de | 174.47 ± 3.32 de | — | 2.26 ± 0.46 de | — | 225.71 ± 1.21 ef |
| S-6 | 11.83 ± 0.19 h | 32.84 ± 1.02 c | 11.75 ± 0.46 g | 198.70 ± 4.17 bc | — | 3.17 ± 0.09 de | — | 258.29 ± 0.84 cde |
| S-2 | 14.43 ± 0.35 e | 36.60 ± 1.05 b | 10.95 ± 1.01 gh | 199.40 ± 1.91 bc | — | 5.97 ± 0.28 c | — | 267.35 ± 1.05 cd |
| WS-2 | 11.90 ± 0.23 h | 36.28 ± 0.97 b | 15.75 ± 1.72 cd | 232.30 ± 4.69 a | 41.26 ± 0.65 bc | 7.92 ± 0.14 abc | 5.96 ± 0.46 c | 351.37 ± 0.54 a |
| WS-3 | 11.95 ± 0.09 gh | 42.90 ± 5.30 a | 18.42 ± 1.08 ab | 224.67 ± 34.56 a | 32.59 ± 0.10 h | 2.43 ± 1.6 de | 4.46 ± 3.50 d | 337.42 ± 1.75 ab |
| WS-4 | 12.43 ± 0.07 g | 31.89 ± 1.47 de | 15.46 ± 0.37 cde | 221.30 ± 2.61 a | 33.31 ± 0.29 g | 9.32 ± 0.37 a | 4.05 ± 0.58 e | 327.76 ± 1.83 ab |
| WS-5 | 13.04 ± 0.18 f | 13.86 ± 1.20 jk | 15.24 ± 2.27 cde | 239.23 ± 3.43 a | 32.38 ± 0.11 h | 9.87 ± 0.31 a | 4.33 ± 0.32 d | 347.95 ± 1.43 a |
| WS-10 | 19.13 ± 0.60 b | 14.82 ± 0.32 hij | 12.21 ± 1.17 fg | 154.78 ± 0.11 fg | 40.94 ± 2.11 bc | 9.13 ± 0.60 a | 4.82 ± 0.32 d | 252.21 ± 11.17 cde |
| WA-1 | 20.39 ± 0.22 b | 28.19 ± 1.33 e | 15.75 ± 0.98 cd | 237.03 ± 2.25 a | 34.57 ± 0.10 f | 9.15 ± 0.21 a | 7.91 ± 0.64 ab | 352.99 ± 1.67 a |
| WA-2 | 20.22 ± 0.58 b | 32.19 ± 1.13 c | 15.74 ± 0.17 cd | 240.67 ± 1.07 a | 39.52 ± 0.29 c | 3.75 ± 0.41 d | 8.50 ± 0.39 a | 360.59 ± 1.54 a |
| CD-1 | 15.07 ± 0.32 de | 18.03 ± 0.24 g | 16.86 ± 1.06 cd | 183.58 ± 0.54 cde | 38.54 ± 1.90 d | 7.11 ± 0.45 b | 5.07 ± 0.32 cd | 278.03 ± 0.24 cd |
| CD-2 | 20.65 ± 0.64 b | 19.01 ± 0.16 fg | 18.64 ± 9.05 ab | 197.52 ± 0.60 bc | 37.82 ± 4.05 de | 4.82 ± 0.32 cd | 4.65 ± 0.64 d | 291.01 ± 0.16 c |
| CD-3 | 13.59 ± 0.70 f | 17.26 ± 0.30 g | 17.12 ± 6.41 bcd | 186.50 ± 0.33 cde | 38.47 ± 3.88 d | 6.93 ± 0.55 bc | 4.59 ± 0.70 d | 275.26 ± 0.30 cd |
| CD-4 | 16.94 ± 1.54 cde | 16.77 ± 0.45 gh | 16.23 ± 5.55 cd | 181.64 ± 0.41 cde | 41.58 ± 3.27 bc | 6.48 ± 0.21 bc | 5.94 ± 1.54 c | 268.77 ± 0.45 cd |
| G-1 | 14.60 ± 1.07 e | 15.84 ± 0.31 hi | 15.43 ± 4.84 cde | 190.32 ± 0.74 bcd | 38.19 ± 5.32 d | 8.43 ± 0.33 ab | 5.60 ± 1.07 c | 253.84 ± 0.31 cde |
| G-2 | 16.22 ± 0.44 cde | 16.72 ± 0.57 gh | 18.67 ± 7.39 ab | 176.83 ± 0.68 de | 32.44 ± 4.44 h | 7.56 ± 0.52 b | 4.22 ± 0.44 d | 267.72 ± 0.57 cd |
| G-3 | 15.34 ± 0.65 de | 18.61 ± 0.72 bcd | 16.89 ± 0.23 cd | 168.42 ± 2.73 def | 36.22 ± 2.04 e | 6.54 ± 0.15 bc | 3.82 ± 0.34 ef | 265.84 ± 9.32 cd |
| Maximum | 22.95 | 42.90 | 19.50 | 240.67 | 49.65 | 9.87 | 8.50 | 360.59 |
| Minimum | 10.99 | 12.09 | 10.07 | 154.78 | — | — | — | 225.71 |
| Average | 15.30 ± 3.59 | 22.10 ± 8.40 | 15.81 ± 2.63 | 188.16 ± 24.80 | 36.88 ± 4.04 | 6.32 ± 2.46 | 5.09 ± 1.49 | 279.58 ± 35.79 |
| CV/% | 27.22 | 22.55 | 18.77 | 14.92 | 12.54 | 14.45 | 16.19 | 15.68 |
| Germplasm ID | Tocopherols µg/g | ||||
|---|---|---|---|---|---|
| α-Tocopherol | β-Tocopherol | γ-Tocopherol | δ-Tocopherol | Total Tocopherols | |
| T-2 | 32.82 ± 0.45 def | 5.75 ± 0.11 efg | 555.60 ± 10.45 bcd | 11.65 ± 0.63 bcd | 605.82 ± 2.37 bcd |
| T-4 | 37.53 ± 0.22 c | 7.33 ± 0.32 d | 586.55 ± 14.76 a | 14.12 ± 0.87 a | 645.53 ± 5.84 a |
| T-5 | 33.86 ± 0.83 | 6.85 ± 0.17 de | 572.71 ± 4.56 ab | 11.94 ± 0.42 bc | 625.36 ± 4.28 abc |
| T-8 | 44.21 ± 1.56 a | 9.16 ± 0.23 ab | 583.85 ± 8.39 a | 14.88 ± 0.61 a | 652.10 ± 8.56 a |
| T-9 | 36.38 ± 1.05 cde | 7.00 ± 0.61 de | 560.84 ± 5.28 abc | 12.24 ± 0.70 ab | 616.46 ± 6.10 abc |
| T-10 | 40.19 ± 0.74 b | 7.83 ± 0.30 cd | 593.94 ± 13.92 a | 12.26 ± 0.61 ab | 654.22 ± 1.94 a |
| T-11 | 39.64 ± 0.92 bc | 9.11 ± 0.45 ab | 524.26 ± 15.21 cd | 12.61 ± 0.94 ab | 585.62 ± 5.45 bcd |
| Z-1 | 19.13 ± 0.60 h | 4.82 ± 0.32 fg | 452.21 ± 11.17 fg | 4.78 ± 0.11 i | 480.94 ± 2.11 efg |
| Z-4 | 21.20 ± 0.67 h | 6.93 ± 0.55 de | 465.27 ± 6.82 efg | 4.96 ± 0.20 i | 498.36 ± 4.06 def |
| Z-5 | 15.12 ± 0.59 i | 6.48 ± 0.21 a | 472.14 ± 9.76 efg | 5.25 ± 0.16 i | 501.99 ± 3.17 def |
| B-1 | 44.15 ± 0.62 a | 8.43 ± 0.33 bc | 508.31 ± 7.24 cde | 8.03 ± 0.44 g | 568.92 ± 4.85 cde |
| B-2 | 42.64 ± 0.71 ab | 9.56 ± 0.52 a | 523.63 ± 8.18 cd | 10.02 ± 0.53 def | 585.85 ± 1.81 bcd |
| S-3 | 32.37 ± 0.61 ef | 6.54 ± 0.15 def | 439.49 ± 11.34 fgh | 6.54 ± 0.47 h | 484.94 ± 1.79 efg |
| S-5 | 34.83 ± 0.38 de | 7.56 ± 0.41 cd | 438.73 ± 6.23 fgh | 8.51 ± 0.58 g | 489.63 ± 5.72 efg |
| S-6 | 30.13 ± 0.45 g | 7.37 ± 0.50 cd | 484.36 ± 8.42 def | 7.67 ± 0.36 gh | 529.53 ± 6.44 def |
| S-2 | 33.58 ± 0.79 de | 8.84 ± 0.39 bc | 498.91 ± 6.71 def | 9.25 ± 0.30 efg | 550.58 ± 5.13 cde |
| WS-2 | 35.07 ± 0.32 de | 8.03 ± 0.24 bcd | 556.86 ± 7.06 abc | 8.58 ± 0.54 g | 608.54 ± 1.90 bcd |
| WS-3 | 40.65 ± 0.64 b | 9.01 ± 0.16 ab | 558.64 ± 9.05 abc | 9.52 ± 0.60 efg | 617.82 ± 4.05 abc |
| WS-4 | 33.59 ± 0.70 de | 7.26 ± 0.30 cde | 579.12 ± 6.41 a | 8.50 ± 0.33 g | 628.47 ± 3.88 abc |
| WS-5 | 36.94 ± 1.54 cde | 6.77 ± 0.45 de | 586.23 ± 5.55 a | 11.64 ± 0.41 bcd | 641.58 ± 3.27 a |
| WS-10 | 32.03 ± 0.33 de | 7.58 ± 0.26 cd | 569.33 ± 0.43 a | 11.85 ± 1.83 bcd | 603.56 ± 0.89 bc |
| WA-1 | 30.60 ± 1.07 g | 5.84 ± 0.31 efg | 501.43 ± 4.84 cd | 10.32 ± 0.74 de | 548.19 ± 5.32 cde |
| WA-2 | 36.22 ± 0.44 cde | 6.72 ± 0.57 de | 548.67 ± 7.39 bcd | 10.83 ± 0.68 cde | 602.44 ± 4.44 bcd |
| CD-1 | 31.41 ± 0.25 g | 5.25 ± 0.85 efg | 518.63 ± 0.31 cde | 6.19 ± 1.05 h | 577.48 ± 0.94 cd |
| CD-2 | 32.95 ± 0.57 de | 6.09 ± 1.79 efg | 516.74 ± 0.89 cde | 8.88 ± 1.98 g | 562.12 ± 0.74 cde |
| CD-3 | 31.49 ± 0.05 g | 6.76 ± 0.79 ef | 519.50 ± 0.92 cde | 7.63 ± 2.16 gh | 563.18 ± 0.50 cde |
| CD-4 | 34.13 ± 1.04 de | 8.10 ± 1.17 bcd | 513.44 ± 0.76 cde | 5.13 ± 1.53 i | 564.65 ± 0.64 cde |
| G-1 | 30.99 ± 0.05 g | 7.53 ± 0.52 cd | 465.92 ± 0.67 efg | 7.70 ± 2.91 gh | 510.28 ± 0.20 def |
| G-2 | 31.98 ± 0.50 g | 5.57 ± 0.70 efg | 474.96 ± 1.13 efg | 5.93 ± 6.67 h | 516.26 ± 0.16 de |
| G-3 | 36.95 ± 0.13 cde | 7.21 ± 0.28 cd | 458.73 ± 0.58 fg | 7.53 ± 1.39 gh | 508.57 ± 0.23 ef |
| Maximum | 44.21 | 9.11 | 593.94 | 14.88 | 654.22 |
| Minimum | 15.12 | 4.82 | 438.73 | 4.78 | 480.94 |
| Average | 33.76 ± 6.43 | 7.24 ± 1.18 | 520.97 ± 47.47 | 9.16 ± 2.75 | 571.13 ± 53.70 |
| CV/% | 22,17 | 17.07 | 9.47 | 31,77 | 10.14 |
| Traits | Principal Components (PC) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Fresh weight | 0.178 | 0.016 | 0.170 | −0.109 | 0.059 |
| FW | 0.150 | 0.125 | −0.234 | 0.060 | −0.070 |
| FSI | −0.001 | 0.157 | −0.382 | 0.071 | 0.039 |
| Grain weight | 0.192 | 0.030 | 0.103 | −0.005 | 0.005 |
| GL | 0.120 | 0.103 | −0.286 | 0.139 | 0.028 |
| GW | 0.145 | 0.064 | 0.136 | 0.105 | −0.117 |
| VD | −0.060 | 0.036 | 0.004 | 0.660 | −0.145 |
| Kernel weight | 0.155 | 0.014 | 0.231 | 0.228 | 0.040 |
| Kernel rate | 0.190 | 0.033 | −0.039 | −0.123 | 0.022 |
| Oil content | −0.080 | 0.199 | 0.218 | −0.066 | 0.133 |
| SFA | −0.044 | 0.221 | 0.072 | 0.059 | −0.486 |
| MUFA | −0.036 | 0.244 | 0.082 | −0.277 | −0.412 |
| PUFA | −0.027 | 0.267 | 0.031 | 0.010 | 0.023 |
| Phytosterol mg | −0.030 | 0.229 | −0.064 | −0.193 | 0.476 |
| Total tocopherols | −0.038 | 0.174 | 0.168 | 0.275 | 0.439 |
| Eigenvalue | 4.922 | 2.775 | 2.050 | 1.333 | 1.121 |
| Contribution rate (%) | 32.810 | 18.502 | 13.666 | 8.884 | 7.471 |
| Cumulative contribution rate (%) | 32.810 | 51.312 | 64.978 | 73.862 | 81.333 |
| NO. | Principal Component Score | F Scores | Ranks | ||||
|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | |||
| WS-4 | 1.51596 | 2.09272 | 1.36305 | −0.34555 | 0.57238 | 1.33147 | 1 |
| T-8 | 2.24169 | 1.14722 | −0.01586 | 0.49376 | 0.69923 | 1.280788 | 2 |
| T-4 | 1.12543 | 0.531 | 0.358 | −0.15575 | 0.59919 | 0.672984 | 3 |
| WS-3 | −0.3522 | 1.14704 | 1.176 | 0.6128 | 0.71118 | 0.448708 | 4 |
| T-9 | 0.24627 | 0.18168 | 0.87142 | 0.16868 | 0.35447 | 0.338082 | 5 |
| S-5 | 1.49493 | −1.33189 | −0.3518 | 0.65656 | 0.12274 | 0.323965 | 6 |
| T-2 | −0.05712 | −0.16646 | 0.80731 | 1.97169 | −0.52507 | 0.241865 | 7 |
| B-2 | 0.05508 | 0.05648 | 0.65429 | 1.67989 | −1.22328 | 0.216122 | 8 |
| S-3 | 1.38769 | −2.00214 | 0.90201 | −0.62671 | 0.2812 | 0.213296 | 9 |
| WA-2 | 0.37248 | 0.65631 | −1.02351 | −0.23319 | 1.00006 | 0.19398 | 10 |
| CD-2 | 0.08004 | 0.44214 | −1.41426 | 1.87668 | 0.34656 | 0.132052 | 11 |
| G-2 | 0.68922 | −0.39802 | −0.06349 | −0.68584 | −0.39746 | 0.065407 | 12 |
| WA-1 | 0.66734 | 1.26569 | −3.06885 | −0.7231 | 1.05342 | 0.059275 | 13 |
| Z-5 | 0.27237 | 0.28316 | 1.27042 | −1.70987 | −1.61776 | 0.052386 | 14 |
| CD-3 | −0.00897 | −0.67567 | 0.39362 | 0.35875 | 0.56125 | −0.00044 | 15 |
| WS-10 | −0.37951 | 0.19109 | 0.91748 | 0.1539 | −0.68416 | −0.00151 | 16 |
| WS-5 | −1.50018 | 1.51688 | 0.73461 | −0.1742 | 1.20883 | −0.04468 | 17 |
| CD-1 | 0.04012 | −0.91603 | 0.07991 | 0.62953 | 0.64957 | −0.05034 | 18 |
| T-10 | −0.73141 | −0.45018 | 1.03498 | 1.07417 | 0.16342 | −0.09123 | 19 |
| Z-4 | 0.37528 | 0.46615 | 0.12362 | −2.49828 | −1.4951 | −0.13201 | 20 |
| G-3 | −0.04337 | −0.76749 | −0.04268 | −0.18696 | −0.5495 | −0.27015 | 21 |
| S-2 | 0.10542 | −1.22841 | −0.78898 | −0.74678 | 1.14105 | −0.34623 | 22 |
| B-1 | −0.65094 | 0.02443 | −0.43194 | 1.02443 | −1.68655 | −0.37265 | 23 |
| Z-1 | −0.40033 | 1.20091 | −1.91016 | 0.28417 | −2.76518 | −0.43223 | 24 |
| T-11 | −0.84981 | −0.13058 | −0.2986 | −0.2989 | 0.23186 | −0.43405 | 25 |
| G-1 | 0.05024 | −1.12709 | −0.25769 | −0.63757 | −1.0383 | −0.44444 | 26 |
| CD-4 | −0.67125 | −0.93193 | −0.29619 | −0.93952 | 0.05132 | −0.63046 | 27 |
| T-5 | −1.84504 | 0.04488 | −0.58909 | 0.50691 | 0.16995 | −0.7621 | 28 |
| S-6 | −0.76152 | −1.78822 | −0.80252 | −0.18202 | 0.98794 | −0.77797 | 29 |
| WS-2 | −2.4679 | 0.66633 | 0.66887 | −1.34767 | 1.07676 | −0.7799 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, M.; Wang, J.; Huang, B.; Tian, H.; Sun, M.; Li, J.; Ren, J.; Liu, C. Fruit Quality Characterization and Comprehensive Evaluation of 30 Chionanthus retusus Accessions. Metabolites 2025, 15, 588. https://doi.org/10.3390/metabo15090588
Niu M, Wang J, Huang B, Tian H, Sun M, Li J, Ren J, Liu C. Fruit Quality Characterization and Comprehensive Evaluation of 30 Chionanthus retusus Accessions. Metabolites. 2025; 15(9):588. https://doi.org/10.3390/metabo15090588
Chicago/Turabian StyleNiu, Muge, Jinnan Wang, Baoqiang Huang, Hui Tian, Maotong Sun, Jihong Li, Jing Ren, and Cuishuang Liu. 2025. "Fruit Quality Characterization and Comprehensive Evaluation of 30 Chionanthus retusus Accessions" Metabolites 15, no. 9: 588. https://doi.org/10.3390/metabo15090588
APA StyleNiu, M., Wang, J., Huang, B., Tian, H., Sun, M., Li, J., Ren, J., & Liu, C. (2025). Fruit Quality Characterization and Comprehensive Evaluation of 30 Chionanthus retusus Accessions. Metabolites, 15(9), 588. https://doi.org/10.3390/metabo15090588






