Herbal Weight Loss Supplements Induce Metabolomic In Vitro Changes Indicative of Oxidative Stress
Abstract
1. Introduction
2. Methods
2.1. Toxicological Screening
2.1.1. WLS Preparations
2.1.2. Cell Culture
2.1.3. Bioactive Pre-Treatment
2.1.4. Cell Viability
2.1.5. Statistical Analysis
2.2. NMR Toxicological Analysis
2.2.1. WLS Preparations and Cell Culture
2.2.2. 1H NMR Spectroscopy Data Acquisition and Processing
2.2.3. NMR Data Modelling
3. Results
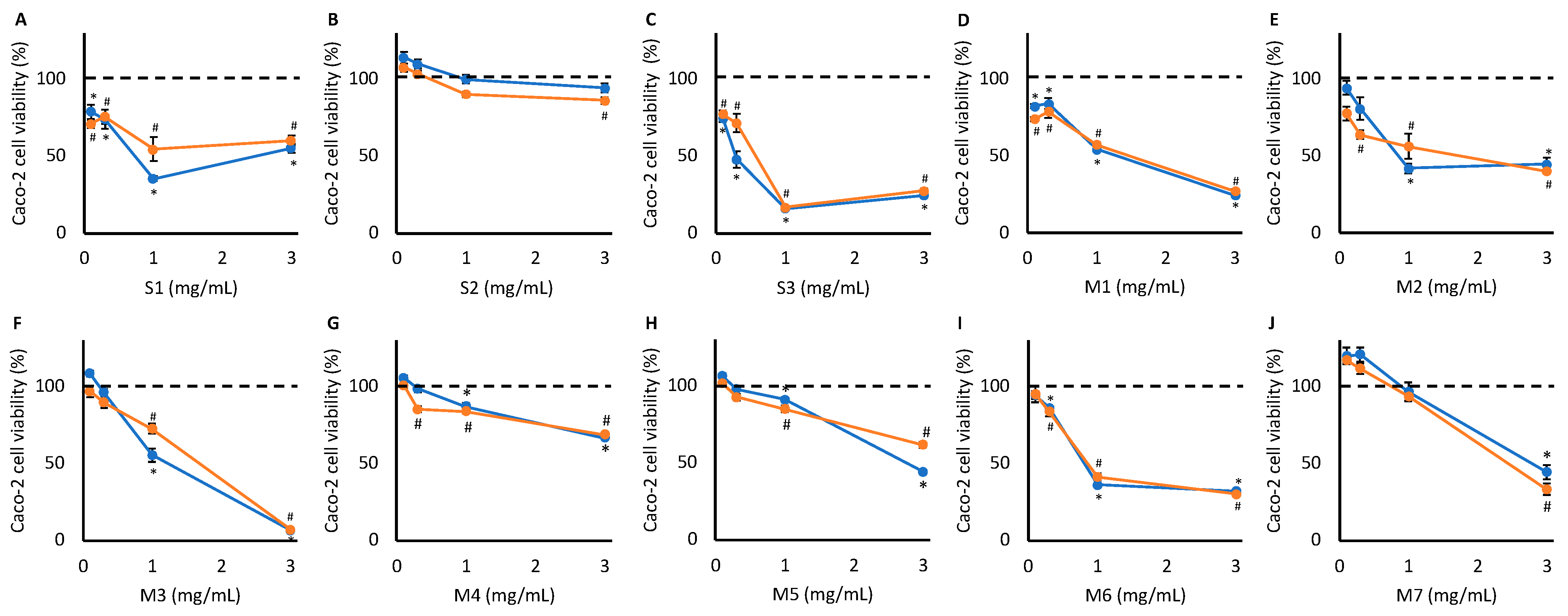
3.1. Toxicological Screening
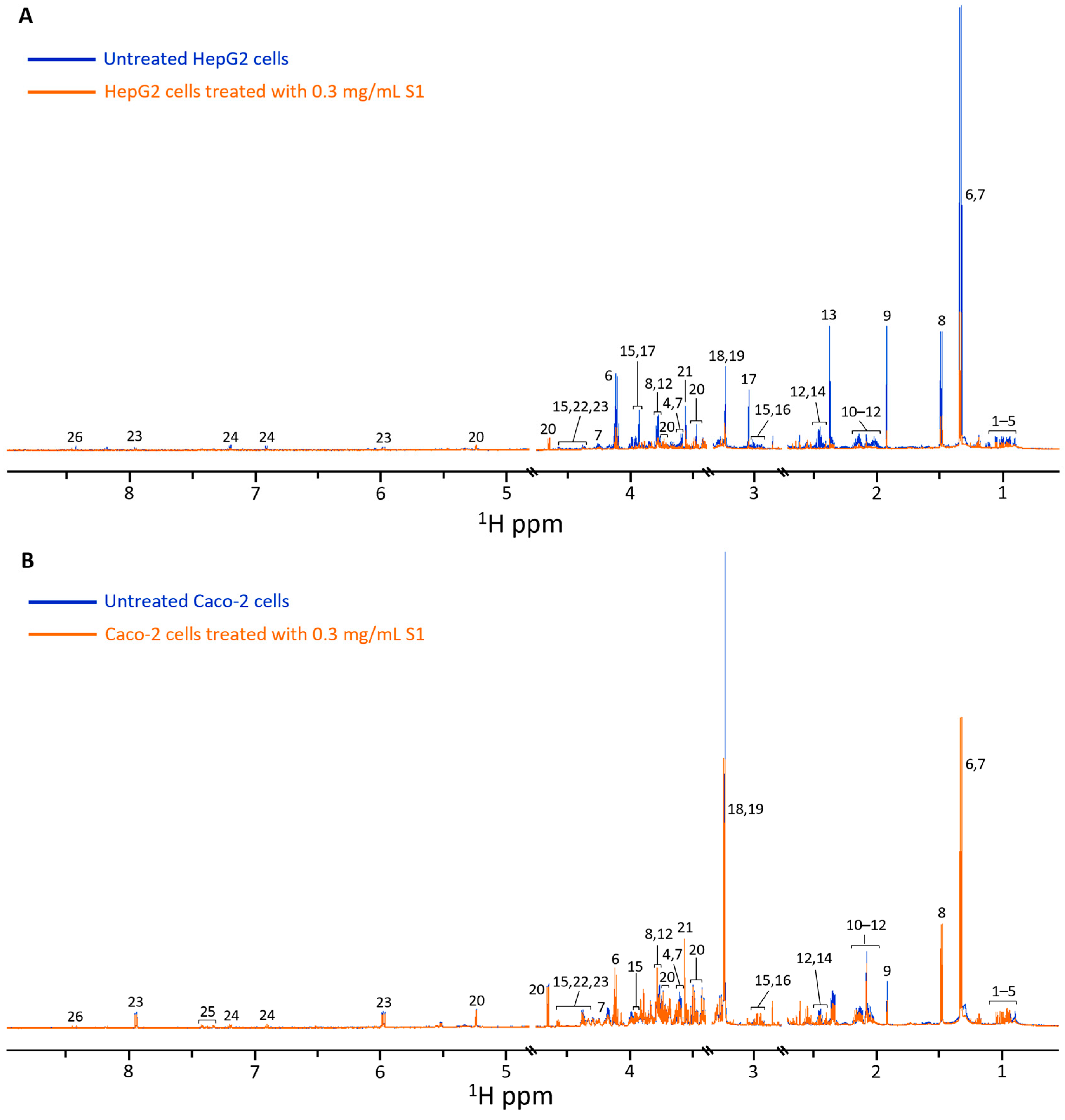
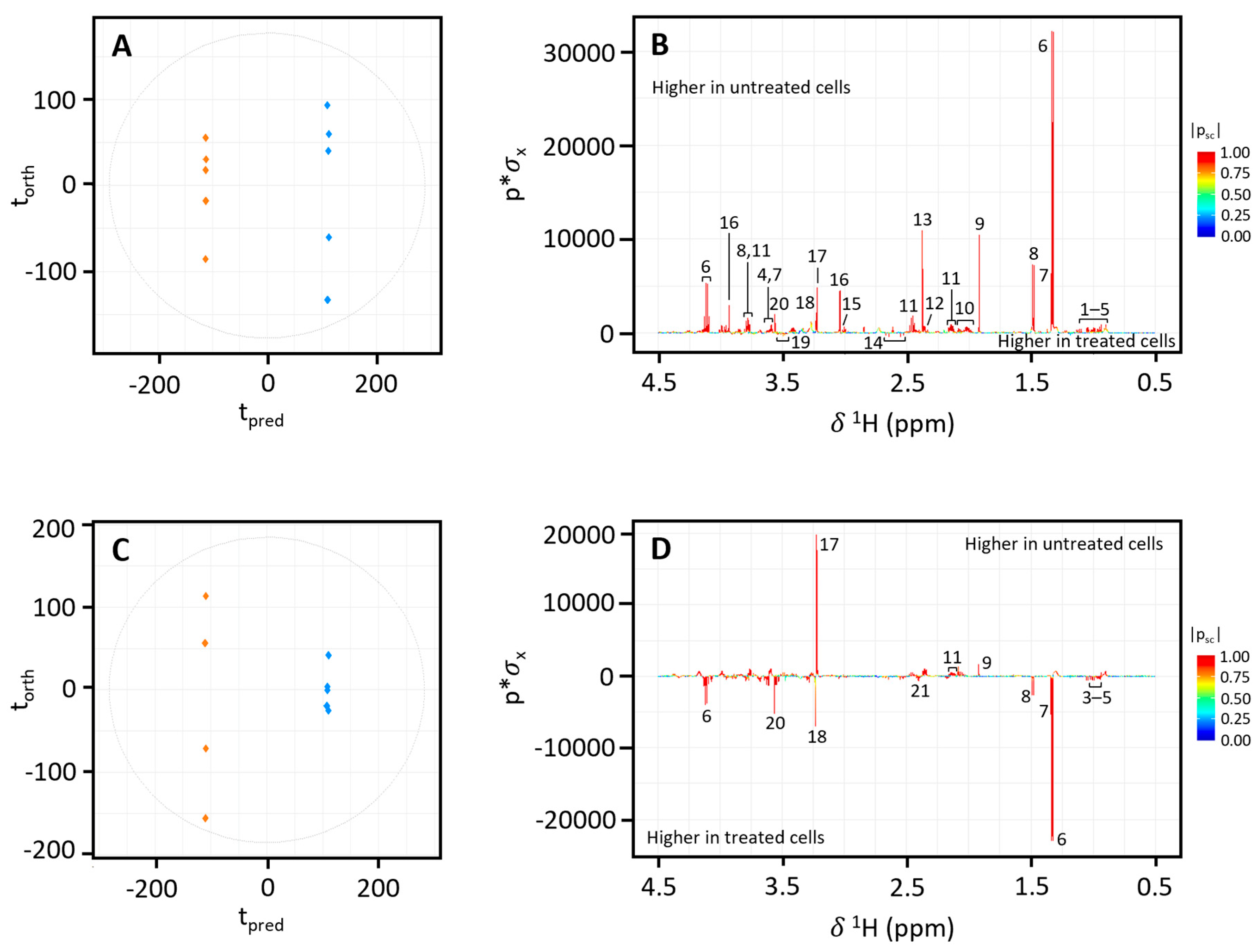
3.2. NMR Analysis of In Vitro Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Australian Bureau of Statistics. National Health Survey: First Results [Internet] Canberra: ABS. 2018. Available online: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/national-health-survey-first-results/latest-release (accessed on 13 May 2023).
- Okunogbe, A.; Nugent, R.; Spencer, G.; Ralston, J.; Wildong, J. Economic impacts of overweight and obesity: Current and future estimates for eight countries. BMJ Global Health 2021, 6, e006351. [Google Scholar] [CrossRef]
- Lobstein, T.; Jackson-Leach, R.; Powis, J.; Brinsden, H.; Gray, M. World Obesity Atlas 2023. World Obesity Federation. 2023. Available online: https://s3-eu-west-1.amazonaws.com/wof-files/World_Obesity_Atlas_2023_Report.pdf (accessed on 30 June 2024).
- Gomez Puente, J.M.; Martinez-Marcos, M. Overweight and obesity: Effectiveness of interventions in adults. Enferm. Clin. 2018, 28, 65–74. [Google Scholar] [CrossRef]
- Therapeutic Goods Administration. Complementary Medicines Overview. 2019. Available online: https://www.tga.gov.au/complementary-medicines-overview (accessed on 4 February 2023).
- Byard, R.W.; Musgrave, I. Herbal medicines and forensic investigations. Forensic Sci. Med. Pathol. 2010, 6, 81–82. [Google Scholar] [CrossRef][Green Version]
- Pajor, E.M.; Eggers, S.M.; Curfs, K.C.J.; Oenema, A.; de Vries, H. Why do Dutch people use dietary supplements? Exploring the role of socio-cognitive and psychosocial determinants. Appetite 2017, 114, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Complementary Medicines Australia. Industry Audit 2022. 2022. Available online: https://cma.mailscampaign.com/annual-reports/index.html (accessed on 21 September 2022).
- Steel Am McIntyre, E.; Harnett, J.; Foley, H.; Adams, J.; Sibbritt, D.; Wardle, J.; Frawley, J. Complementary medicine use in the Australian population: Results of a nationally-representative cross-sectional survey. Sci. Rep. 2018, 8, 17325. [Google Scholar]
- Moses, G. The safety of commonly used vitamins and minerals. Aust. Prescr. 2021, 44, 119–123. [Google Scholar] [CrossRef]
- Zheng, E.X.; Rossi, S.; Fontana, R.J.; Vuppalanchi, R.; Hoofnagle, J.H.; Khan, I.; Navarro, V.J. Rick of liver injury associated with green tea extract in SLIMQUICK® weight loss products: Results from the DILIN prospective study. Drug Saf. 2016, 39, 749–754. [Google Scholar] [CrossRef]
- Navarro, V.J.; Khan, I.; Bjornsson, E.; Seeff, L.B.; Serrano, J.; Hoofnagle, J.H. Liver injury from herbal and dietary supplements. Hepatology 2017, 65, 363–373. [Google Scholar] [CrossRef]
- Nash, E.; Sabih, A.-H.; Chetwood, J.; Wood, G.; Pandya, K.; Yip, T.; Majumdar, A.; McCaughan, G.W.; Strasser, S.I.; Liu, K. Drug-induced liver injury in Australia, 2009-2020: The increasing proportion of non-paracetamol cases linked with herbal and dietary supplements. Med. J. Aust. 2021, 215, 261–268. [Google Scholar] [CrossRef]
- Dag, M.S.; Aydinli, M.; Ozturk, Z.A.; Turkbeyler, I.H.; Koruk, I.; Sava, M.C.; Koruk, M.; Kadayifci, A. Drug- and herb-induced liver injury: A case series from a single center. Turk. J. Gastroenterol. 2014, 25, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Byeon, J.-H.; Kil, J.-H.; Ahn, Y.-C.; Son, C.-G. Systemic review of published data on herb induced liver injury. J. Ethnopharmacol. 2019, 233, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Ballotin, V.R.; Bigarella, L.G.; Brandao, A.B.M.; Balbinot, R.A.; Balbinot, S.S.; Soldera, J. Herb-induced liver injury: Systemic review and meta-analysis. World J. Clin. Cases 2021, 9, 5490–5513. [Google Scholar] [CrossRef]
- Mazzanti, G.; Di Sotto, A.; Vitalone, A. Hepatotoxicity of green tea: An update. Arch. Toxicol. 2015, 89, 1175–1191. [Google Scholar] [CrossRef]
- Fong, T.-L.; Klontz, K.C.; Canas-Coto, A.; Casper, S.J.; Durazo, F.A.; Davern, T.J., 2nd; Hayashi, P.; Lee, W.M.; Seeff, L.B. Hepatotoxicity due to hydroxycut: A case series. Am. J. Gastroenterol. 2010, 105, 1561–1566. [Google Scholar] [CrossRef]
- Pillukat, M.H.; Bester, C.; Hensle, A.; Lechtenberg, M.; Petereit, F.; Beckebaum, S.; Mulley, K.-M.; Schmidt, H.H.J. Concentrated green tea extract induces severe acute hepatitis in a 63-year-old woman—A case report with pharmaceutical analysis. J. Ethnopharmacol. 2014, 155, 165–170. [Google Scholar] [CrossRef]
- Teschke, R.; Eickhoff, A.; Schulze, J.; Danan, G. Herb-induced liver injury (HILI) with 12,068 worldwide cases published with causality assessments by Roussel Uclaf Causality Assessment Method (RUCAM): An overview. Transl. Gastroenterol. Hepatol. 2021, 6, 51. [Google Scholar] [CrossRef]
- Gheorghiu, O.R.C.; Ciobanu, A.M.; Gutu, C.M.; Danila, G.-M.; Nitescu, G.V.; Rohnean, S.; Baconi, D.L. Detection of Adulterants in Herbal Weight Loss Supplements. J. Mind Med. Sci. 2025, 12, 23. [Google Scholar] [CrossRef]
- Hachem, R.; Assemat, G.; Martins, N.; Balayssac, S.; Gilard, V.; Martino, R.; Malet-Martino, M. Proton NMR for detection, identification and quantification of adulterants in 160 herbal food supplements marketed for weight loss. J. Pharm. Biomed. Anal. 2016, 124, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, S.; Yang, Q.; Wang, Y.; Yu, X.; Sun, W.; Qiu, S.; Li, X.; Guo, Y.; Xie, Y.; et al. Decoding active compounds and molecular targets of herbal medicine by high-throughput metabolomics technology: A systemic review. Bioorg Chem. 2024, 144, 107090. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Deng, Y.; Li, C.; Hu, Y.; Zhang, Q.; Zhuang, B.; Mosongo, I.; Jiang, J.; Yang, J.; Hu, K. Metabolomics and molecular docking-directed anti-obesity study of the ethanol extract from Gynostemma pentaphyllum (Thunb.) Makino. J. Ethnopharmacol. 2024, 334, 118577. [Google Scholar] [CrossRef] [PubMed]
- Farrington, R. Herbal Medicine Toxicity: The Role of Adulterants, Contaminants and Pharmacokinetic Interactions. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 2019. [Google Scholar]
- Bulutoglu, B.; Rey-Bedon, C.; Mert, S.; Tian, L.; Jang, Y.-Y.; Yarmush, M.L.; Usta, O.B. A comparison of hepato-cellular in vitro platforms to study CYP3A4 induction. PLoS ONE 2020, 15, e0229106. [Google Scholar] [CrossRef]
- Dona, A.C.; Jimenez, B.; Schafer, H.; Humpfer, E.; Spraul, M.; Lewis, M.R.; Pearce, J.T.M.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Precision High-Throughput Proton NMR Spectroscopy of Human Urine, Serum and Plasma for Large-Scale Metabolic Phenotyping. Anal. Chem. 2014, 86, 9887–9894. [Google Scholar] [CrossRef]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probablistic Quotient Normalization as Robust Method to Account for Dilution of Complex Biological Mixtures. Application in 1H Metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef]
- Bylesjo, M.; Rantalainen, M.; Cloarec, O.; Nicholson, J.K.; Holmes, E.; Trygg, J. OPLS discriminant analysis: Combining the strengths of PLS-DA and SIMCA classification. J. Chemom. 2006, 20, 341–351. [Google Scholar] [CrossRef]
- Jinks, M.; Davies, E.C.; Boughton, B.A.; Lodge, S.; Maker, G.L. 1H NMR spectroscopic characterisation of HepG2 cells as a model metabolic system for toxicology studies. Toxicol. In Vitro 2024, 99, 105881. [Google Scholar] [CrossRef]
- Chen, C.; Gao, J.; Wang, T.-S.; Guo, C.; Yan, Y.-J.; Mao, C.-Y.; Gu, L.-W.; Yang, Y.; Li, Z.-F.; Liu, A. NMR-based Metabolomic Techniques Identify the Toxicity of Emodin in HepG2 cells. Sci. Rep. 2018, 8, 9379. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, L.; Shi, J.; Zhang, G.; Lu, L.; Zhu, L.; Zhnag, J.; Liu, Z. Toxi Markers of Matrine Determined Using (1) H-NMR-Based Metabolomics in Cultured Cells in Vitro and Rats in Vivo. Evid.-Based Complement. Altern. Med. 2015, 2015, 598412. [Google Scholar] [CrossRef] [PubMed]
- Hayton, S.; Maker, G.L.; Mullaney, I.; Trengrove, R.D. Untargeted metabolomics of neuronal cell culture: A model system for the toxicity testing of insecticide chemical exposure. J. Appl. Toxicol. 2017, 37, 1481–1492. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Katerji, M.; Filippova, M.; Duerksen-Hughes, P. Approaches and Methods to Measure Oxidative Stress in Clinical Samples: Research Applications in the Cancer Field. Oxid. Med. Cell. Longev. 2019, 2019, 1279250. [Google Scholar] [CrossRef]
- Kaffe, E.T.; Rigopoulou, E.I.; Koukoulis, G.K.; Dalekos, G.N.; Moulas, A.N. Oxidative stress and antioxidant status in patients with autoimmune liver diseases. Redox Rep. 2015, 20, 33–41. [Google Scholar] [CrossRef]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Mandal, P.K.; Roy, R.G.; Samkaria, A. Oxidative Stress: Glutathione and Its Potential to Protect Methionine-35 of Aβ Peptide from Oxidation. ACS Omega 2022, 7, 27052–27061. [Google Scholar] [CrossRef]
- Kannan, K.; Jain, S.K. Oxidative stress and apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef]
- Ariyoshi, N.; Iga, Y.; Hirata, K.; Sato, Y.; Miura, G.; Ishii, I.; Nagamori, S.; Kitada, M. Enhanced susceptibility of HLA-mediated ticlopidine-induced idiosyncratic hepatotoxicity by CYP2B6 polymorphism in Japenese. Drug Metab. Pharmacokinet. 2010, 25, 298–306. [Google Scholar] [CrossRef]
- Armagan, H.H.; Naziroglu, M. Glutathione depletion induces oxidative injury and apoptosis via TRPM2 channel activation in renal collecting duct cells. Chem. Biol. Interact. 2021, 334, 109306. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; van der Pol, A.; van der Meer, P.; Bischoff, R. LC-MS analysis of key components of the glutathione cycle in tissues and body fluids from mice with myocardial infarction. J. Pharm. Biomed. Anal. 2018, 160, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Geenen, S.; Guallar-Hoyas, C.; Michopoulos, F.; Kenna, J.G.; Kolaja, K.L.; Westerhoff, H.V.; Thomas, P.; Wilson, I.D. HPLC-MS/MS methods for the quantitative analysis of 5-oxoproline (pyroglutamate) in rat plasma and hepatic cell line culture medium. J. Pharm. Biomed. Anal. 2011, 56, 655–663. [Google Scholar] [CrossRef]
- Jin, R.; Banton, S.; Tran, V.T.; Konomi, J.V.; Li, S.; Jones, D.P.; Vos, M.B. Amino acid metabolism is altered in adolescents with nonalcoholic fatty liver disease—An untargeted, high resolution metabolomics study. J. Pediatr. 2016, 172, 14–19.e5. [Google Scholar] [CrossRef] [PubMed]
- Amores-Sanchez, M.I.; Medina, M.A. Glutamine, as a precursor of glutathione, and oxidative stress. Mol. Genet. Metab. 1999, 64, 100–105. [Google Scholar] [CrossRef]
- Yu, J.C.; Jiang, Z.-M.; Li, D.-M. Glutamine: A precursor of glutathione and its effect on the liver. World J. Gastroenterol. 1999, 5, 143–146. [Google Scholar] [CrossRef]
- Sedlak, T.W.; Paul, B.D.; Parker, G.M.; Hester, L.D.; Snowman, A.M.; Taniguchi, Y.; Kamiya, A.; Snyder, S.H.; Sawa, A. The glutathione cycle shapes synaptic glutamate activity. Proc. Natl. Acad. Sci. USA 2019, 116, 2701–2706. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Xu, N.; Chen, G.; Liu, H. Antioxidative Categorization of Twenty Amino Acids Based on Experimental Evaluation. Molecules 2017, 22, 2066. [Google Scholar] [CrossRef]
- Maralani, M.N.; Movahedian, A.; Javanmard, S.H.H. Antioxidant and cytoprotective effects of L-Serine on human endothelial cell. Res. Pharm. Sci. 2012, 7, 209–215. [Google Scholar]
- Min, Y.N.; Liu, S.G.; Qu, Z.X.; Meng, G.H.; Gao, Y.P. Effects of dietary threonine levels on growth performance, serum biochemical indexes, antioxidant capacities and gut morphology in broiler chickens. Poult. Sci. 2017, 96, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Cecarini, V.; Gee, J.; Fioretti, E.; Amici, M.; Angeletti, M.; Eleuteri, A.M.; Keller, J.N. Protein oxidation and cellular homestasis: Emphasis on metabolism. Biochim. Biphys. Acta 2007, 1773, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Sivanand, S.; Vander Heiden, M.G. Emerging Roles for Branched-Chain Amino Acid Metabolism in Cancer. Cancer Cell 2020, 37, 147–156. [Google Scholar] [CrossRef]
- Paulusma, C.C.; Lamers, W.H.; Broer, S.; Van de Graff, S.F.K. Amino acid metabolism, transport and signalling in the liver revisited. Biochem. Pharmacol. 2022, 201, 115074. [Google Scholar] [CrossRef] [PubMed]
- Schmalhausen, E.V.; Pleten, A.P.; Muronetz, V.I. Ascorbate-induced oxidation of glyceraldehyde-3-phosphate dehydrogenase. Biochem. Biophys. Res. Commun. 2003, 308, 492–496. [Google Scholar] [CrossRef]
- Gupta, K.J.; Shah, J.K.; Brotman, Y.; Jahnke, K.; Willmitzer, L.; Kaiser, W.M.; Bauwe, H.; Igam berdiev, A.U. Inhibition of aconitase by nitric oxide leads to induction of the alternative oxidase and to a shift of metabolism towards biosynthesis of amino acids. J. Exp. Bot. 2012, 63, 1773–1784. [Google Scholar] [CrossRef]
- Icard, P.; Coquerel, A.; Wu, Z.; Gligorov, J.; Fuks, D.; Fournel, L.; Lincet, H.; Simula, L. Understanding the Central Role of Citrate in the Metabolism of Cancer Cells and Tumours: An Update. Int. J. Mol. Sci. 2021, 22, 6587. [Google Scholar] [CrossRef]
- Van de Wier, B.; Balk, J.M.; Haenen, G.R.M.M.; Giamouridis, D.; Bakker, J.A.; Bast, B.C.; den Hartog, J.M.; Koek, G.H.; Bast, A. Elevated citrate levels in non-alcoholic fatty liver disease: The potential of citrate to promote radical production. FEBS Lett. 2013, 587, 2461–2466. [Google Scholar] [CrossRef]
- Gautier-Luneau, I.; Bertet, P.; Jeunet, A.; Serratrice, G.; Pierre, J.-L. Iron-citrate complexes and free radicals generation: Is citric acid an innocent additive in foods and drinks? Biometals 2007, 20, 793–796. [Google Scholar] [CrossRef]
- Bose, S.; Ramesh, V.; Locasale, J.W. Acetate Metabolism in Physiology, Cancer, and Beyond. Trends Cell Biol. 2019, 29, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, P. Formate as an inhibitor of cytochrome c oxidase. Biochem. Biophys. Res. Commun. 1975, 67, 610–616. [Google Scholar] [CrossRef]
- Tejero, J.D.; Hesterberg, R.S.; Drapela, S.; Ilter, D.; Raizada, D.; Lazure, F.; Kashfi, H.; Liu, M.; Silvane, L.; Avram, D.; et al. Methylmalonic acid induces metabolic abnormalities and exhaustion in CD8+ T cells to suppress anti-tumour immunity. Oncogene 2025, 44, 105–114. [Google Scholar] [CrossRef]
- Kuhnel, J.; Bobik, T.; Procter, J.B.; Burmeister, C.; Hoppner, J.; Wilde, I.; Luersen, K.; Torda, A.E.; Walter, R.D.; Liebau, E. Functional analysis of the methylmalonyl-CoA epimerase from Caenorhabditis elegans. FEBS J. 2005, 272, 1465–1477. [Google Scholar] [CrossRef]
- Weinhold, P.A.; Charles, L.; Feldman, D.A. Regulation of CTP: Phosphocholine cytidylyltransferase in HepG2 cells: Effect of choline depletion on phosphorylation, translocation and phosphatidylcholine levels. Biochim. Biophys. Acta 1994, 1210, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Murtas, G.; Marcone, G.L.; Sacchi, S.; Pollegioni, L. L-serine synthesis via the phosphorylated pathway in humans. Cell. Mol. Life Sci. 2020, 77, 5131–5148. [Google Scholar] [CrossRef] [PubMed]
- Segeritz, C.-P.; Vallier, L. Cell Culture Growing Cells as Model Systems in Vitro. Basic Sci. Methods Clin. Res. 2017, 7, 151–172. [Google Scholar]
- Ohta, Y.; Kazuki, K.; Abe, S.; Oshimura, M.; Kobayashi, K.; Kazuki, Y. Development of Caco-2 cells expressing four CYPs via a mammalian artificial chromosome. BMC Biotechnol. 2020, 20, 44. [Google Scholar] [CrossRef] [PubMed]

| WLS Code | Active Ingredients(s) | ARTG Status |
|---|---|---|
| S1 | Camellia sinensis (L.) Kuntz (Theaceae) (green tea catechins (GTCs), caffeine) | Listed |
| S2 | G. gummi-gutta (hydroxycitric acid (HCA)) | Listed |
| S3 | Coffea canephora (chlorogenic acid (CGA), caffeine) | Listed |
| M1 | C. sinensis (GTCs, caffeine); Coffea canephora Pierre ex A.Froehner (Rubiaceae) (CGA, caffeine); caffeine anhydrous; Capsicum annuum (L.) (Solanaceae) (capsaicin); Citrus aurantium L. (Rutaceae) (synephrine); “Theacrine”; “Dynamine”; Higenamine HCL; Piper nigrum L. (Piperaceae) (piperine); chromium picolinate | Not listed |
| M2 | Taurine; Paullinia cupana Kunth (Sapindaceae) (guarana); tyrosine; Theobroma cacao L. (Malvaceae) (theobromine, caffeine); thiamine (vitamin B1); C. sinensis (GTCs, caffeine); C. aurantium (synephrine); Nandina domestica Thunb. (Berberidaceae); Siberian Rhodiola rosea L. (Crassulaceae); Tetradium Lour. (Rutaceae) extract; naringen | Not listed |
| M3 | Medium chain triglyceride (MCT) powder, decaffeinated C. canephora (CGA), L-carnitine, P. cupana (guarana), C. annuum (capsaicin) | Not listed |
| M4 | Caralluma fimbriata Wall. (Apocynaceae) herb extract; G. gummi-gutta (HCA); C. aurantium (synephrine), chromium chloride hexahydrate; chromium picolinate | Listed |
| M5 | C. sinensis (GTCs, caffeine); Fucus vesiculosus; chromium; thiamine (vitamin B1); riboflavin (vitamin B2); nicotinamide (vitamin B3); pyridoxine (vitamin B6); cyanocobalamin (vitamin B12); Plantago afra L. (Plantaginaceae); Garcinia quaesita Pierre (Clusiaceae); P. cupana (guarana); C. aurantium (synephrine); Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. (Araliaceae) (Siberian ginseng); Coleus barbatus (Andrews) Benth. ex G.Don (Lamiaceae) (forskolin); Ilex paraguariensis A.St.-Hil. (Aquifoliaceae); C. canephora (CGA, caffeine) | Listed |
| M6 | Moringa oleifera Lam. (Moringaceae), Bergera koenigii L. (Rutaceae) (curry tree extract), Curcuma longa L. (Zingiberaceae) (curcumin), potassium iodide, chromic chloride hexahydrate, C. sinensis (GTCs, caffeine), P. cupana (guarana), caffeine, riboflavin (Vitamin B2), nicotinamide (Vitamin B3), calcium pantothenate, C. annuum (capsaicin) | Listed |
| M7 | C. canephora (CGA, caffeine), T. cacao (theobromine, caffeine), caffeine, C. barbatus (forskolin), C. annuum (capsaicin) | Listed |
| Weight Loss Supplement | HepG2 Cells | Caco-2 Cells | ||
|---|---|---|---|---|
| Concentration (mg/mL) | Reduction in Cell Viability (%) | Concentration (mg/mL) | Reduction in Cell Viability (%) | |
| S1 | 0.3 | 49.8 | 0.3 | 24.3 |
| S3 | 1.0 | 28.8 | 0.3 | 28.6 |
| M1 | 1.0 | 31.6 | 1.0 | 42.7 |
| M2 | 1.0 | 30.9 | 0.3 | 36.3 |
| M3 | 3.0 | 36.5 | 1.0 | 27.1 |
| M4 | - | - | 3.0 | 31.3 |
| M5 | - | - | 3.0 | 38.0 |
| M6 | 1.0 | 49.4 | - | - |
| Metabolites | δ 1H ppm and Multiplicity | δ 13C ppm | Fold Change Relative to Untreated Cells | |||||
|---|---|---|---|---|---|---|---|---|
| S1 | S3 | M1 | M2 | M3 | M6 | |||
| Acetate | 1.92 (s) | 26.10 | 0.15 ** | 0.67 * | 0.24 ** | 0.47 ** | 0.69 | 0.17 ** |
| Alanine | 1.49 (d), 3.78 (q) | 17.20, 54.60 | 0.33 ** | - | - | 0.63 * | - | 0.20 ** |
| Citrate | 2.54 (d), 2.69 (d) | 48.70, 48.70 | 2.28 ** | 1.40 ** | 2.31 ** | 0.99 | - | 1.66 ** |
| Creatine | 3.04 (s), 3.93 (s) | 40.10, 56.24 | 0.22 ** | 0.87 * | 0.66 ** | 0.64 * | 0.48 ** | 0.12 ** |
| Formate | 8.43 (s) | 172.10 | 0.43 ** | - | 0.75 ** | 0.87 | 0.69 * | 0.43 ** |
| Glucose | 3.25 (dd), 3.41 (t), 3.42 (t), 3.47 (m), 3.50 (t), 3.54 (dd), 3.72 (t), 3.73 (dd), 3.77 (dd), 3.83 (m), 3.84 (m), 3.90 (dd), 4.65 (d), 5.24 (d) | 77.02, 73.50, 78.65, 63.53, 74.15, 63.32, 63.39, 96.00 | 1.22 * | 1.02 | 1.05 | 0.90 | - | 1.56 ** |
| Glutamate | 2.05 (m), 2.14 (m), 2.34 (m), 2.37 (m), 3.76 (q) | 29.70, 36.24, 57.57 | 1.24 * | 1.05 | 1.19 ** | 0.98 | 0.92 | 1.57 |
| Glutamine | 2.14 (m), 2.46 (m), 3.78 (t) | 29.50, 32.93, 57.10 | 0.15 ** | 0.88 | 0.86 * | 0.76 | 0.71 | 0.14 ** |
| Glutathione | 2.16 (m), 2.19 (m), 2.54 (m), 2.57 (m), 2.93 (dd), 2.98 (dd), 3.76 (dd), 3.77 (t), 3.80 (dd), 4.57 (dd), 8.26 (m), 8.51 (s) | 29.06, 34.05, 28.32, 46.35, 57.17, 59.21, 58.55 | 0.93 | 0.54 ** | 1.46 * | 1.22 | 0.59 * | 0.84 |
| Glycerophosphocholine | 3.23 (s), 3.69 (m), 3.92 (m), 4.32 (dd) | 55.90, 68.10, 73.90, 64.40 | 0.65 ** | 1.56 ** | 2.00 ** | 1.19 | 2.37 * | 0.38 ** |
| Glycine | 3.56 (s) | 44.50 | 0.50 ** | 1.25 ** | 1.52 ** | 0.88 | - | 0.31 ** |
| Isobutyrate | 1.13 (d), 2.38 (m) | 22.01, 39.58 | 0.51 ** | - | 0.56 ** | 0.75 | 0.66 * | 0.47 ** |
| Isoleucine | 0.96 (t), 1.02 (d), 1.26 (m), 1.47 (m), 1.98 (m), 3.67 (d) | 13.91, 17.37, 27.00 | 0.71 ** | 1.25 ** | 1.19 ** | - | 1.17 | 0.68 * |
| Lactate | 1.33 (d), 4.11 (q) | 20.20, 68.60 | 0.31 ** | 0.74 ** | 0.58 ** | 0.59 ** | 0.62 * | 0.17 ** |
| Leucine | 0.96 (d), 0.97 (d), 1.68 (m), 1.71 (m), 1.73 (m), 3.74 (dd) | 23.40, 24.30, 42.60, 56.90 | 0.69 ** | 1.34 ** | 1.28 ** | - | 1.26 | 0.70 * |
| Lysine | 1.44 (m), 1.48 (m), 1.72 (m), 1.88 (m), 1.92 (m), 3.02 (t), 3.77 (t) | 24.04, 29.15, 42.12, 57.45 | 0.52 ** | 0.94 | 0.72 * | 0.84 | - | 0.57 ** |
| Methylamine | 2.61 (s) | 28.30 | 0.48 | 0.81 | 0.63 * | 0.59 | 0.62 | 0.39 ** |
| Methylmalonate | 1.22 (d), 3.17 (q) | 17.91 | 0.45 ** | 0.70 ** | 0.58 ** | 0.56 ** | 0.56 ** | 0.38 ** |
| NAD+ | 4.22 (m), 4.24 (m), 4.26 (m), 4.37 (m), 4.38 (m), 4.43 (m), 4.49 (t), 4.51 (dd), 4.54 (m), 4.77 (t), 6.04 (d), 6.10 (d), 8.18 (s), 8.20 (m), 8.43 (s), 8.83 (m), 9.15 (d), 9.35 (s)) | 68.13, 73.47, 80.47, 102.73, 131.34 | 0.67 ** | - | 0.85 ** | 0.94 | 0.82 * | 0.62 ** |
| Phenylalanine | 3.13 (dd), 3.29 (m), 3.98 (m), 7.34 (m), 7.38 (m), 7.43 (m) | 38.90, 38.90, 58.90, 132.20, 129.80, 131.90 | - | 1.13 * | 1.15 ** | 1.51 * | 1.21 | - |
| Phosphocholine | 3.22 (s), 3.60 (m), 4.15 (m) | 56.52, 68.90, 60.61 | 0.24 ** | 0.86 | 0.56 ** | 0.67 | 0.63 * | 0.19 ** |
| Proline | 1.99 (m), 2.03 (m), 2.07 (m), 2.37 (m), 3.34 (m), 3.42 (m), 4.13 (dd) | 24.84, 30.13, 47.42, 62.29, 175.27 | 0.48 ** | 1.46 * | 1.27 ** | 0.74 * | 1.09 | 0.48 ** |
| Pyroglutamate | 2.03 (m), 2.39 (m), 2.42 (m), 2.50 (m), 4.17 (dd) | 32.28, 27.98, 60.97 | 0.58 ** | - | - | 0.81 * | - | 0.59 ** |
| Pyruvate | 2.38 (s) | 29.50 | 0.08 ** | 0.50 * | 0.19 ** | 0.46 * | 0.40 * | 0.05 ** |
| Serine | 3.85 (dd), 3.96 (d), 3.99 (d), 4.01 (d) | 57.40, 61.31, 173.37 | 0.35 ** | - | 0.74 ** | 0.64 * | 0.77 | 0.24 ** |
| Threonine | 1.34 (d), 3.59 (d), 4.26 (m) | 22.30, 63.46 | 0.37 ** | - | 0.89 | 0.78 | 0.79 | 0.31 ** |
| Trimethylamine | 2.85 (s) | 47.55 | 0.46 ** | 0.70 ** | 0.60 ** | 0.57 * | 0.58 * | 0.36 ** |
| Tyrosine | 3.06 (dd), 3.20 (dd), 3.97 (dd), 6.91 (m), 7.20 (m) | 37.90, 59.40, 118.80, 133.40 | 0.62 ** | - | - | 0.83 | - | 0.56 ** |
| UDP-glucose | 3.47 (t), 3.55 (m), 3.77 (t), 3.79 (dd), 3.86 (dd), 3.89 (m), 4.20 (m), 4.24 (m), 4.29 (m), 4.36 (m), 4.38 (m), 5.60 (dd), 5.98 (d), 5.99 (d), 7.96 (d) | 60.08, 64.69, 68.94, 71.33, 73.52, 82.96, 88.19, 95.31, 102.36, 141.18, 151.26, 164.21 | 0.76 ** | 1.65 ** | 1.26 ** | 1.09 | 1.43 | 0.61 ** |
| Valine | 0.99 (d), 1.05 (d), 2.28 (m), 3.61 (d) | 19.41, 20.75, 31.89 | 0.57 ** | 1.20 ** | 1.07 | - | - | 0.54 ** |
| 2-oxoisocaproate | 0.94 (d), 2.08 (m), 2.60 (d) | 22.40, 22.40, 24.20, 47.90, 161.57, 194.07 | 0.25 ** | 0.80 ** | 0.53 ** | 0.57 * | 0.52 ** | 0.17 ** |
| 3-methyl-2-oxovalerate | 0.90 (t), 1.10 (d), 1.45 (m), 1.69 (m), 2.93 (m) | 11.20, 14.40, 24.70, 43.40, 162.25, 195.00 | 0.47 ** | 0.79 ** | 0.57 ** | 0.78 | 0.65 ** | 0.45 ** |
| Metabolites | δ 1H ppm and Multiplicity | δ 13C ppm | Fold Change Relative to Untreated Cells | ||||||
|---|---|---|---|---|---|---|---|---|---|
| S1 | S3 | M1 | M2 | M3 | M4 | M5 | |||
| Acetate | 1.92 (s) | 26.10 | 0.65 ** | 1.08 | 1.14 | 0.71 ** | 1.36 ** | 0.83 * | 1.21 |
| Alanine | 1.49 (d), 3.78 (q) | 17.21, 54.60 | 1.28 ** | - | 0.76 ** | 0.80 ** | 1.27 ** | 1.58 ** | 1.10 |
| Asparagine | 2.88 (dd), 2.98 (dd), 3.99 (t) | 35.70, 52.40, 174.10, 175.30 | 0.99 | - | 0.82 | 0.90 | - | - | 0.82 |
| Aspartate | 2.68 (dd), 2.80 (dd), 3.90 (t) | 39.30, 55.09 | 1.24 ** | - | 0.65 ** | 0.78 ** | - | - | 0.69 ** |
| Citrate | 2.54 (d), 2.65 (d) | 48.70, 48.70 | 1.50 ** | - | 1.43 | 1.08 | - | 0.83 * | - |
| Formate | 8.43 (s) | 172.10 | - | - | 1.17 ** | 1.09 | - | 1.14 ** | 1.16 ** |
| Fumarate | 6.52 (s) | 132.45, 166.73 | 1.38 | - | - | 0.75 * | - | - | - |
| Glucose | 3.25 (dd), 3.41 (t), 3.42 (t), 3.47 (m), 3.50 (t), 3.54 (dd), 3.72 (t), 3.73 (dd), 3.77 (dd), 3.83 (m), 3.84 (m), 3.90 (dd), 4.65 (d), 5.24 (d) | 77.02, 73.50, 78.65, 63.53, 74.15, 63.32, 63.39, 96.00 | 0.90 ** | 0.93 | 0.73 ** | 1.09 | 0.97 | 1.01 | 0.69 ** |
| Glutamate | 2.05 (m), 2.14 (m), 2.34 (m), 2.37 (m), 3.76 (q) | 29.70, 36.24, 57.57 | 0.73 ** | 0.90 ** | 0.58 ** | 1.02 | 0.88 ** | 0.52 ** | 0.60 ** |
| Glutamine | 2.14 (m), 2.46 (m), 3.78 (t) | 29.51, 32.94, 57.10 | 0.67 ** | 0.76 ** | 0.40 ** | 0.86 ** | 0.95 | 0.52 ** | 0.46 ** |
| Glutathione | 2.16 (m), 2.19 (m), 2.54 (m), 2.57 (m), 2.93 (dd), 2.98 (dd), 3.76 (dd), 3.77 (m), 3.80 (dd), 4.57 (dd), 8.27 (m), 8.51 (s) | 29.06, 34.05, 28.32, 46.35, 57.17, 59.21, 58.55 | 1.04 | 0.55 ** | 0.66 ** | 0.91 * | 0.55 ** | - | 0.64 ** |
| Glycerophosphocholine | 3.23 (s), 3.69 (m), 3.92 (m), 4.32 (dd) | 55.90, 68.10, 73.90, 64.40 | 1.29 ** | 1.04 | 1.21 | 1.27 * | 1.25 ** | 0.45 ** | 1.07 |
| Glycine | 3.56 (s) | 44.50 | 2.13 ** | 0.78 ** | 0.86 | 1.18 | 0.81 | 0.92 | 0.62 ** |
| Isoleucine | 0.96 (t), 1.02 (d), 1.26 (m), 1.47 (m), 1.98 (m), 3.67 (d) | 13.91, 17.37, 27.00 | 1.58 ** | - | 0.83 ** | 1.25 ** | 1.20 ** | 1.61 ** | 0.85 * |
| Lactate | 1.33 (d), 4.11 (q) | 20.20, 68.60 | 2.26 ** | 1.14 | 1.85 ** | 0.95 | 1.54 | 3.21 ** | 2.04 ** |
| Leucine | 0.96 (d), 0.97 (d), 1.68 (m), 1.71 (m), 1.73 (m), 3.74 (dd) | 23.40, 24.30, 42.60, 56.90 | 1.40 ** | - | 0.68 ** | 1.20 ** | 1.14 ** | 1.57 ** | 0.71 ** |
| Lysine | 1.44 (m), 1.48 (m), 1.72 (m), 1.88 (m), 1.92 (m), 3.02 (t), 3.77 (t) | 24.04, 29.15, 42.12, 57.45 | - | - | - | 0.81 | - | - | - |
| Methylamine | 2.61 (s) | 28.30 | - | - | - | - | - | - | 0.74 |
| Methylmalonate | 1.22 (d), 3.17 (q) | 17.91 | 1.28 * | - | 1.33 * | - | - | 1.80 ** | 1.34 * |
| Myoinositol | 3.28 (t), 3.54 (dd), 3.63 (t), 4.07 (t) | 75.24 | 2.39 ** | 0.89 | 0.70 ** | 1.30 ** | - | - | - |
| NAD+ | 4.22 (m), 4.24 (m), 4.26 (m), 4.37 (m), 4.38 (m), 4.43 (m), 4.49 (t), 4.51 (dd), 4.54 (m), 4.77 (t), 6.04 (d), 6.10 (d), 8.18 (s), 8.20 (m), 8.43 (s), 8.83 (m), 9.15 (d), 9.35 (s) | 68.13, 73.47, 80.47, 102.73, 131.34 | - | - | 1.12 | 1.15 | - | 1.11 | - |
| Pantothenate | 0.88 (s), 0.92 (s), 2.42 (t), 3.39 (d), 3.44 (q), 3.44 (q), 3.51 (d), 3.98 (s), 8.01 (m) | 21.83, 23.15, 39.08, 71.21, 38.80, 71.34, 78.65 | 0.41 ** | 1.02 | - | - | 0.72 ** | 0.87 | 0.79 |
| Phenylalanine | 3.13 (dd), 3.29 (m), 3.98 (m), 7.34 (m), 7.38 (m), 7.43 (m) | 38.90, 38.90, 58.90, 132.20, 129.80, 131.90 | 1.07 | - | 1.50 ** | - | - | 1.58 ** | 0.96 |
| Phosphocholine | 3.22 (s), 3.60 (m), 4.15 (m) | 56.52, 68.90, 60.61 | 0.62 ** | 0.95 | 0.84 | 0.79 ** | 0.89 | 1.18 ** | 0.95 |
| Pyroglutamate | 2.03 (m), 2.39 (m), 2.42 (m), 2.50 (m), 4.17 (dd) | 32.28, 27.98, 60.97 | 1.24 ** | 0.98 | 0.86 * | 1.15 ** | 1.21 ** | 1.04 | 1.01 |
| Pyruvate | 2.38 (s) | 29.50 | 1.10 | - | 1.48 | 0.83 | 1.46 | 1.59 ** | - |
| Serine | 3.85 (dd), 3.96 (d), 3.99 (d) | 57.40, 61.31, 173.37 | - | - | - | 0.84 * | - | - | - |
| Succinate | 2.41 (s) | 37.30 | 0.72 ** | 0.82 * | 0.53 ** | - | 1.08 | - | - |
| Threonine | 1.34 (d), 3.59 (d), 4.26 (m) | 22.30, 63.46 | 0.67 ** | 0.95 | 0.83 | 0.79 ** | 0.93 | - | 0.95 |
| Trimethylamine | 2.85 (s) | - | - | - | - | - | - | 0.71 | |
| Tyrosine | 3.06 (dd), 3.20 (dd), 3.97 (dd), 6.91 (m), 7.20 (m) | 37.90, 59.40, 118.80, 133.40 | 1.20 ** | - | 0.90 | - | - | 1.19 ** | 0.80 ** |
| UDP-glucose | 3.47 (t), 3.55 (m), 3.77 (t), 3.79 (dd), 3.86 (dd), 3.89 (m), 4.20 (m), 4.24 (m), 4.29 (m), 4.36 (m), 4.38 (m), 5.60 (dd), 5.98 (d), 5.99 (d), 7.96 (d) | 60.08, 64.69, 68.94, 71.33, 73.52, 82.96, 88.19, 95.31, 102.36, 141.18, 151.26, 164.21 | 0.84 ** | 0.97 | 0.91 | 1.05 | 0.96 | 0.53 ** | 1.05 |
| Valine | 0.99 (d), 1.05 (d), 2.28 (m), 3.61 (d) | 19.41, 20.75, 31.89 | 1.82 ** | - | 0.91 | 1.34 ** | 1.21 * | 1.52 ** | 0.81 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davies, E.C.; Maker, G.L.; Musgrave, I.F.; Lodge, S. Herbal Weight Loss Supplements Induce Metabolomic In Vitro Changes Indicative of Oxidative Stress. Metabolites 2025, 15, 587. https://doi.org/10.3390/metabo15090587
Davies EC, Maker GL, Musgrave IF, Lodge S. Herbal Weight Loss Supplements Induce Metabolomic In Vitro Changes Indicative of Oxidative Stress. Metabolites. 2025; 15(9):587. https://doi.org/10.3390/metabo15090587
Chicago/Turabian StyleDavies, Emily C., Garth L. Maker, Ian F. Musgrave, and Samantha Lodge. 2025. "Herbal Weight Loss Supplements Induce Metabolomic In Vitro Changes Indicative of Oxidative Stress" Metabolites 15, no. 9: 587. https://doi.org/10.3390/metabo15090587
APA StyleDavies, E. C., Maker, G. L., Musgrave, I. F., & Lodge, S. (2025). Herbal Weight Loss Supplements Induce Metabolomic In Vitro Changes Indicative of Oxidative Stress. Metabolites, 15(9), 587. https://doi.org/10.3390/metabo15090587










