Ecballium elaterium (L.) A. Rich. (Squirting Cucumber) Plants Cultured Under Different Temperatures: Anatomical and Biochemical Modifications of Their Leaves and the Bioactivity of Leaf Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Setup
2.2. Microscopy
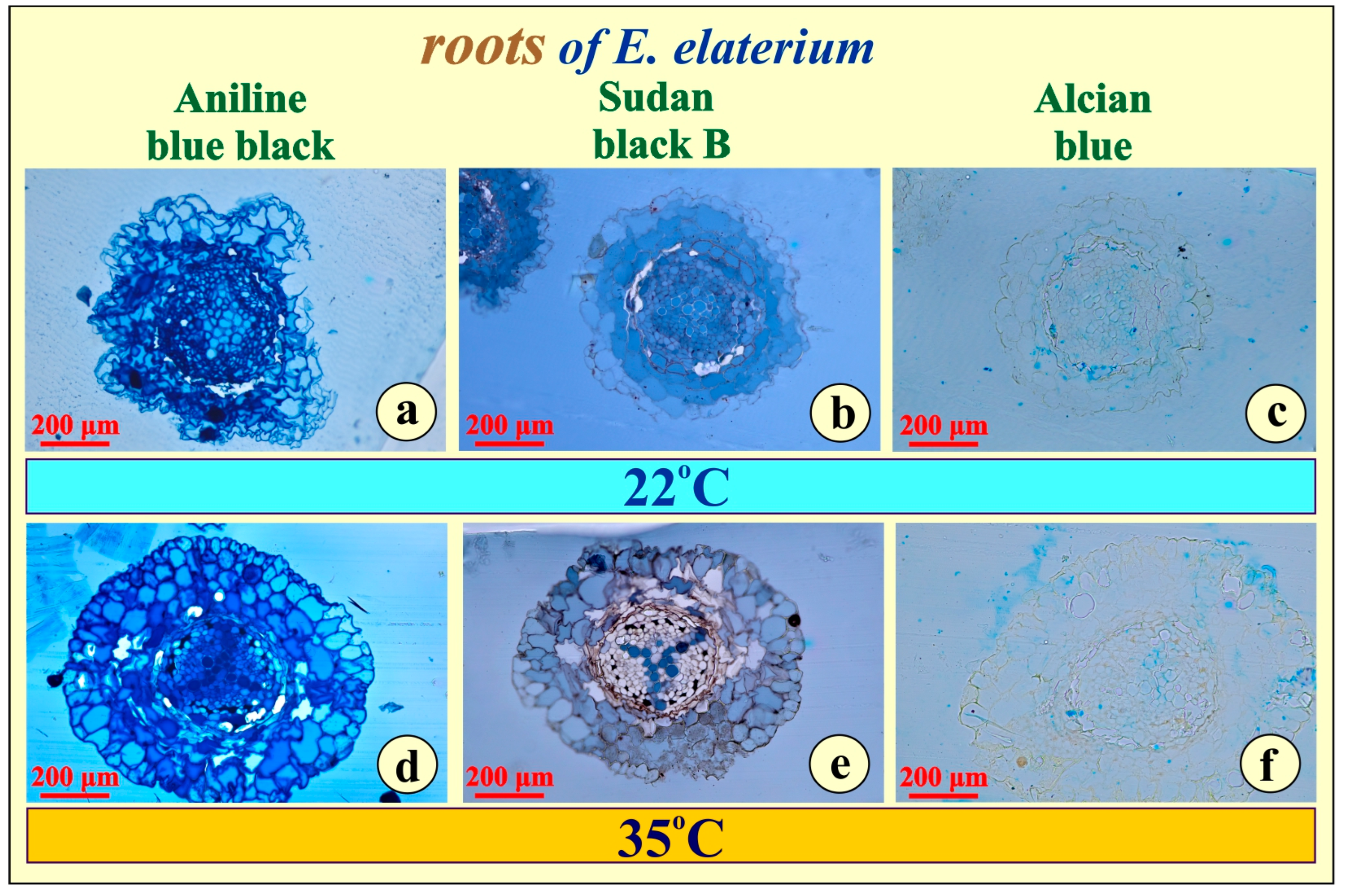
2.3. Histochemistry
- (a)
- A saturated solution of Sudan Black B in 70% ethanol, used to visualize lipid deposits [26].
- (b)
- A saturated solution of Alcian Blue in 3% acetic acid, employed to detect stored polysaccharides [27].
- (c)
- A 1% solution of Aniline Blue Black in 70% acetic acid, used for the histochemical identification of accumulated proteins [28].
2.4. Pigments Protocol
2.5. Protocol for MDA (Malondialdehyde) and H2O2 Determination in Plant Tissues
2.6. Determination of Total Phenolic Content
2.7. Determination of Total Soluble Sugar
2.8. Determination of Proline
2.9. Extraction of Plant Material
2.10. Ultra-High-Performance Liquid Chromatography-High-Resolution Mass Spectrometry (UPLC-HRMS) Analysis
2.11. Cell Lines Bioassays
2.12. ROS Measurements on Cell Cultures
2.13. Cell Death Measurements
2.14. Data Preprocessing and Statistical Analysis
3. Results
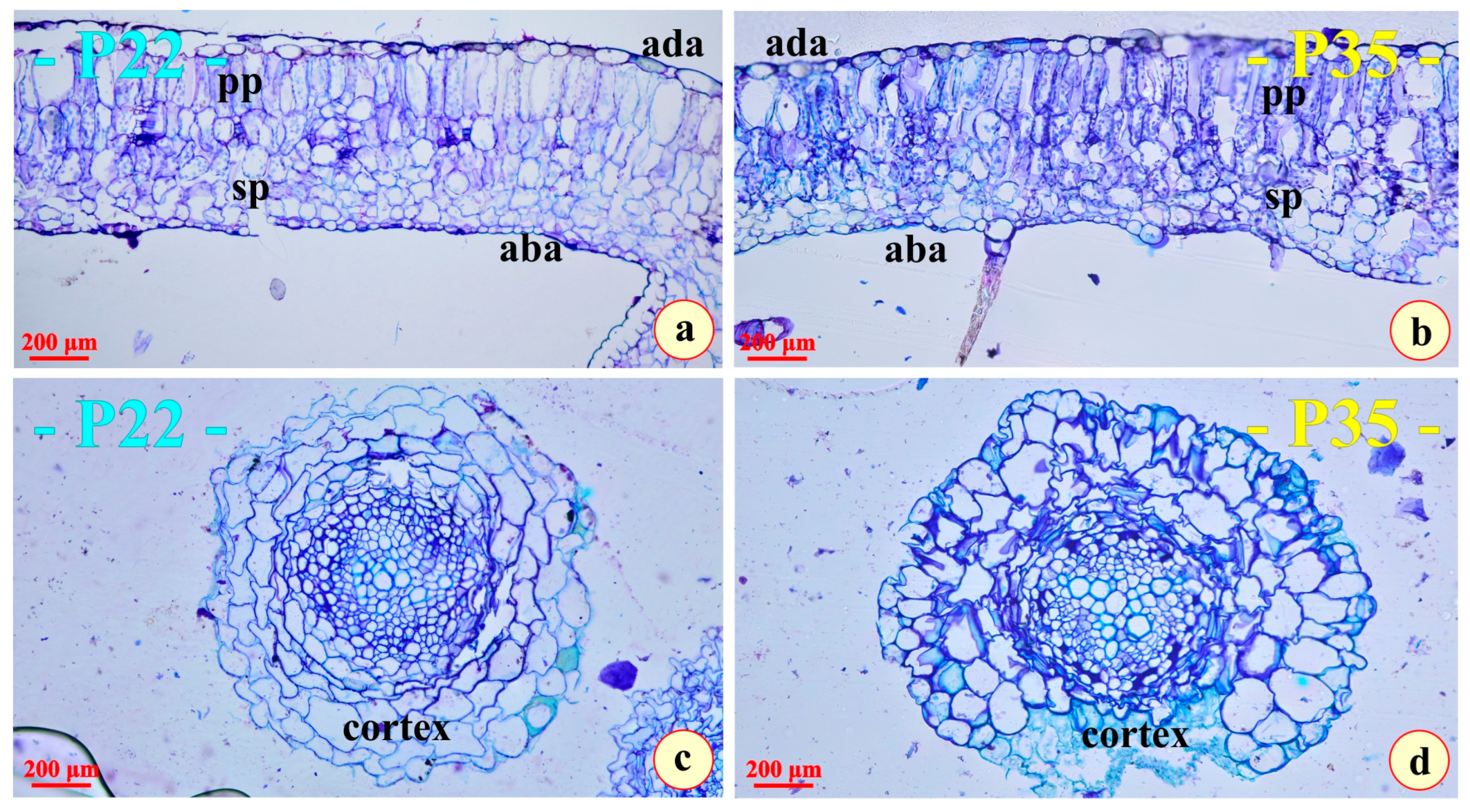
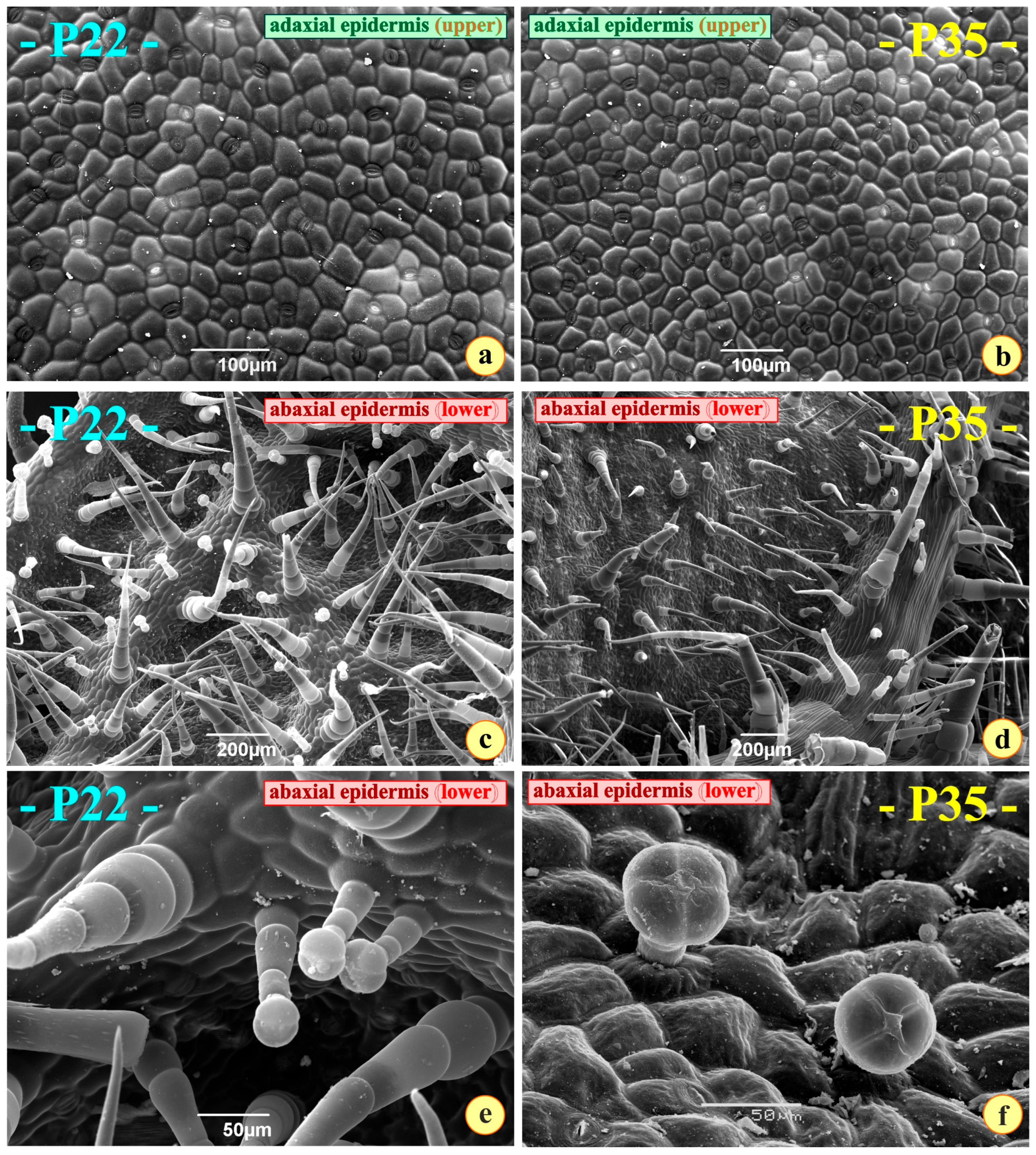
3.1. Morphology, Anatomy, and Histochemistry
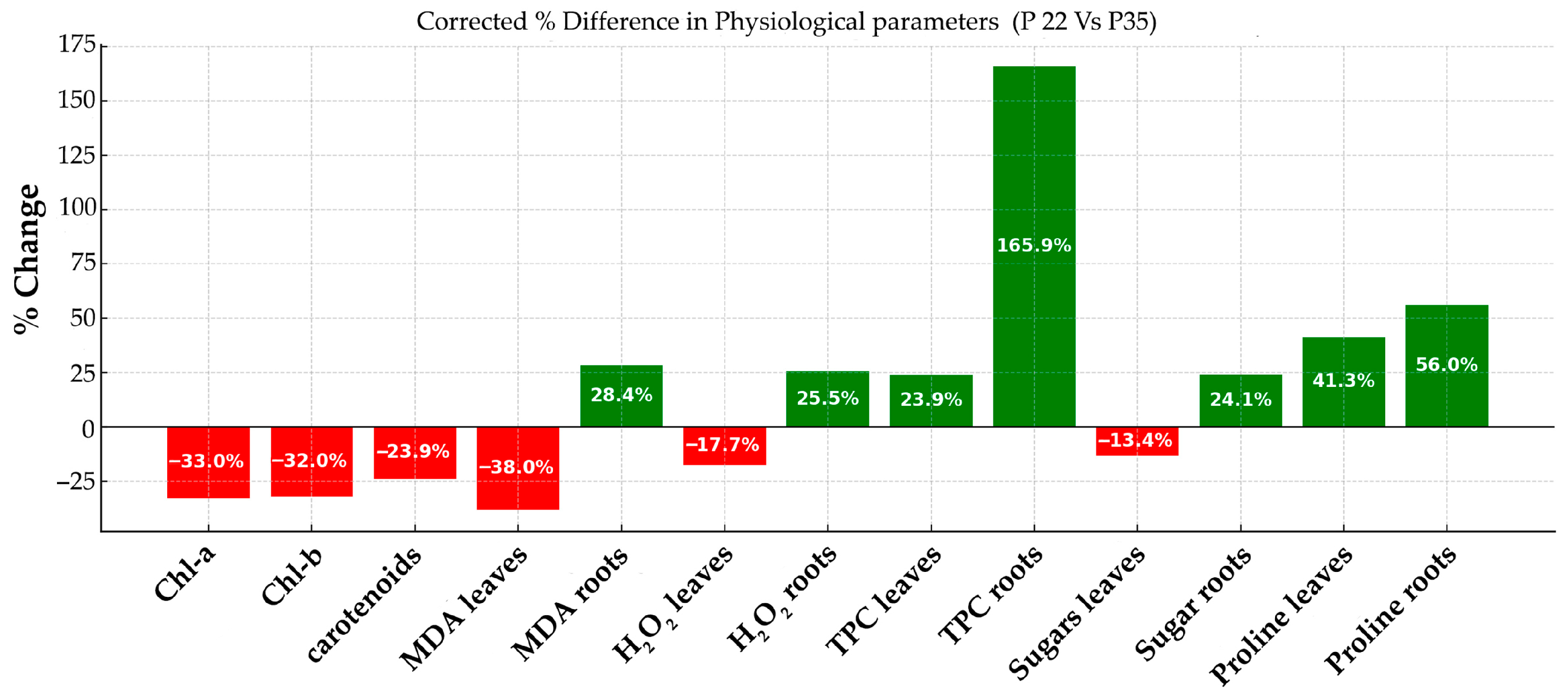
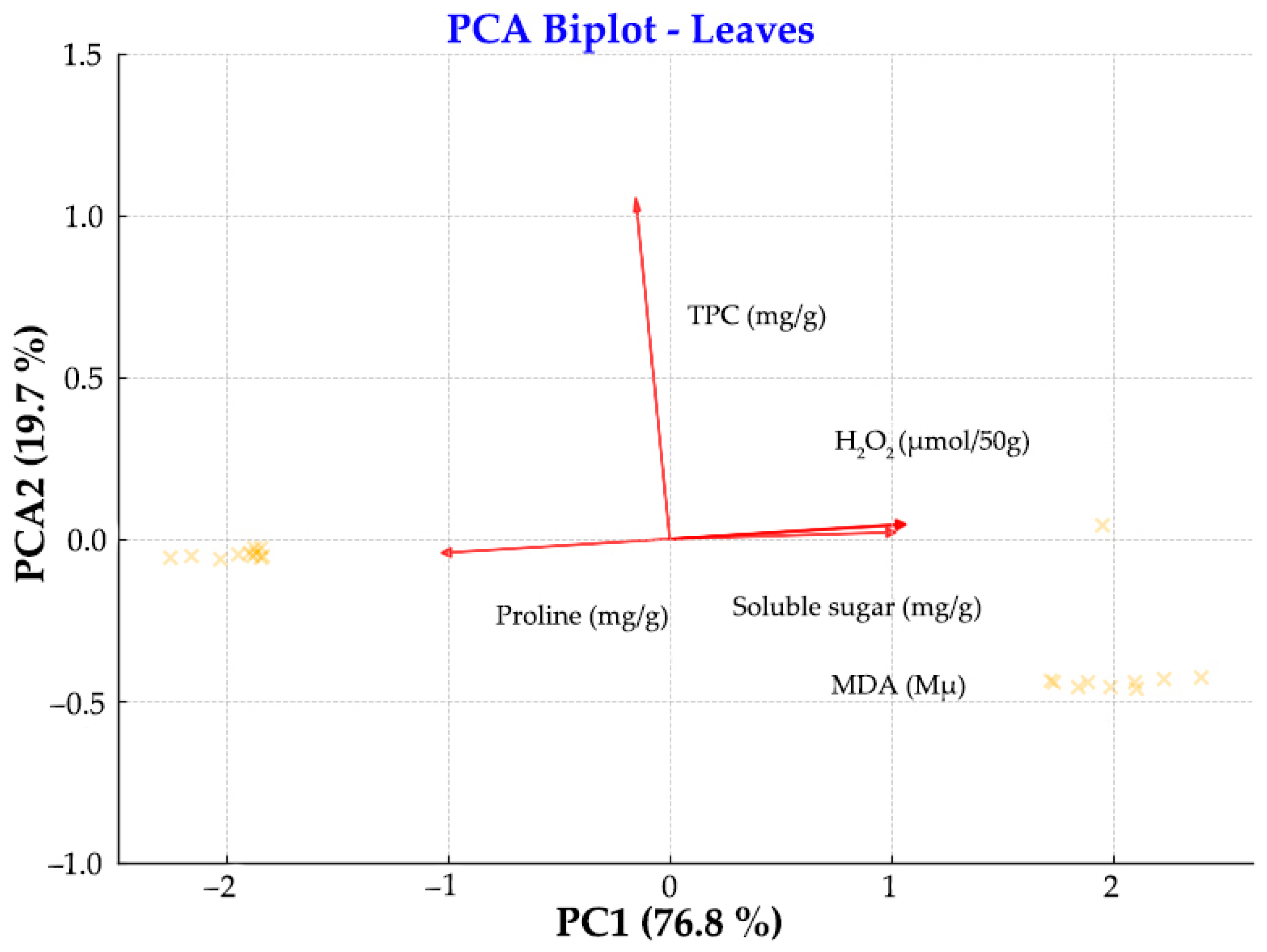
3.2. Physiology
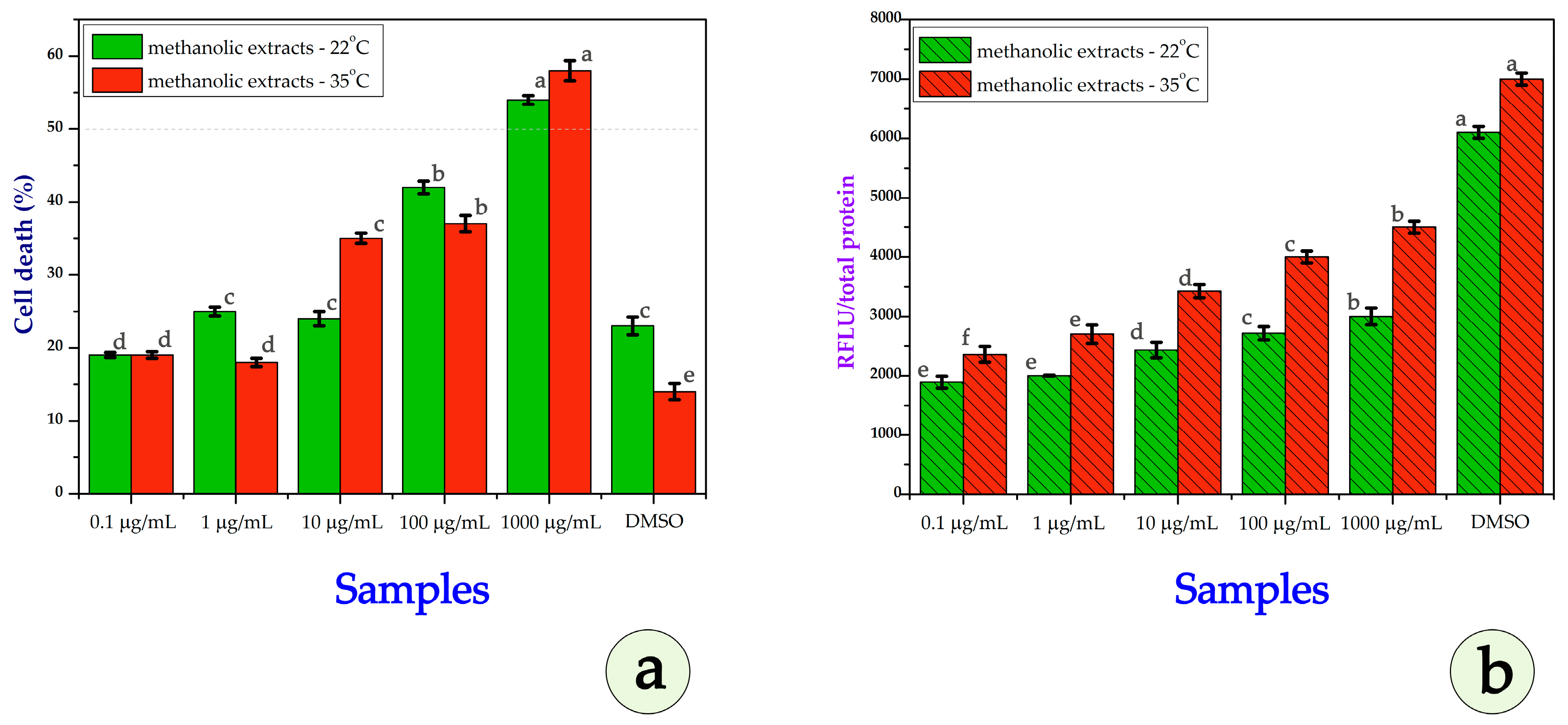
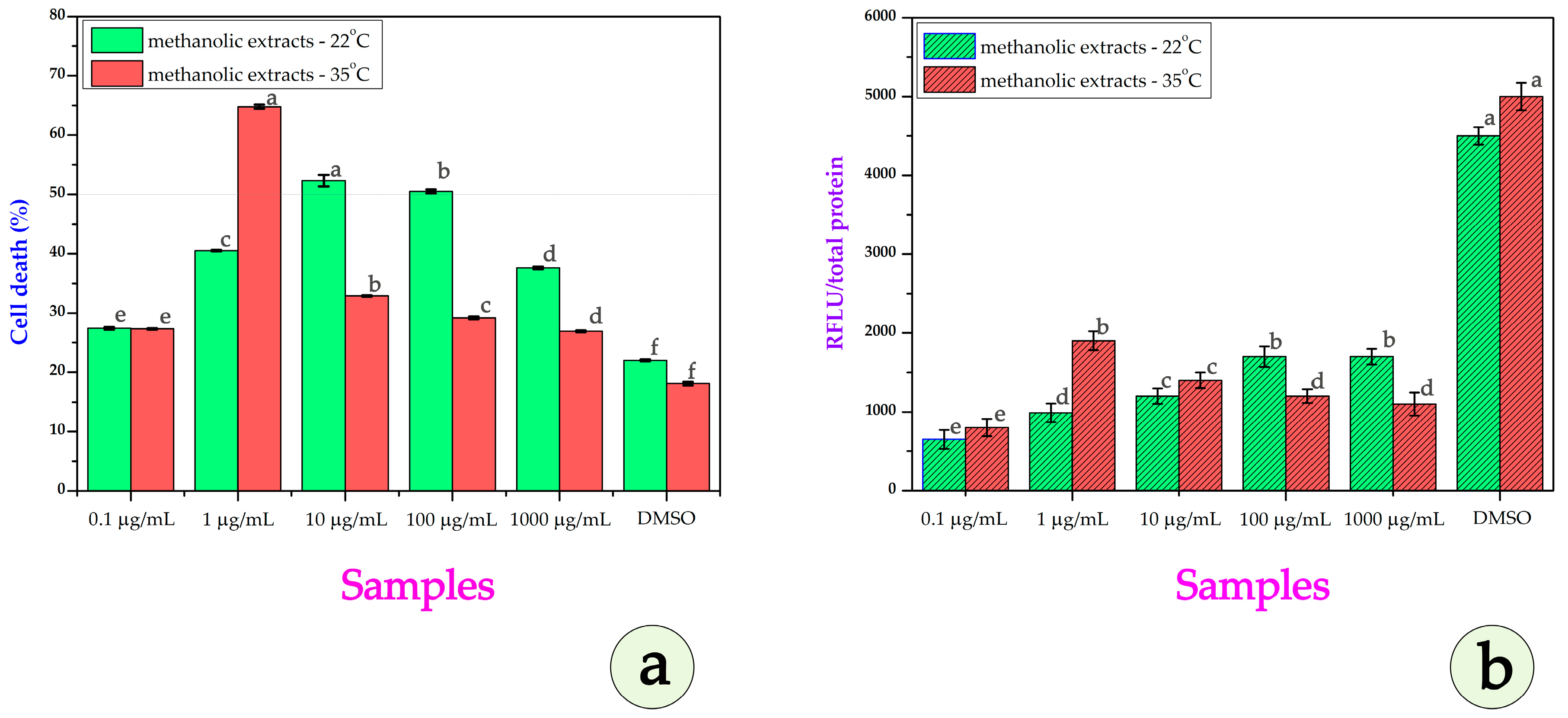
3.3. Cytotoxicity Assays
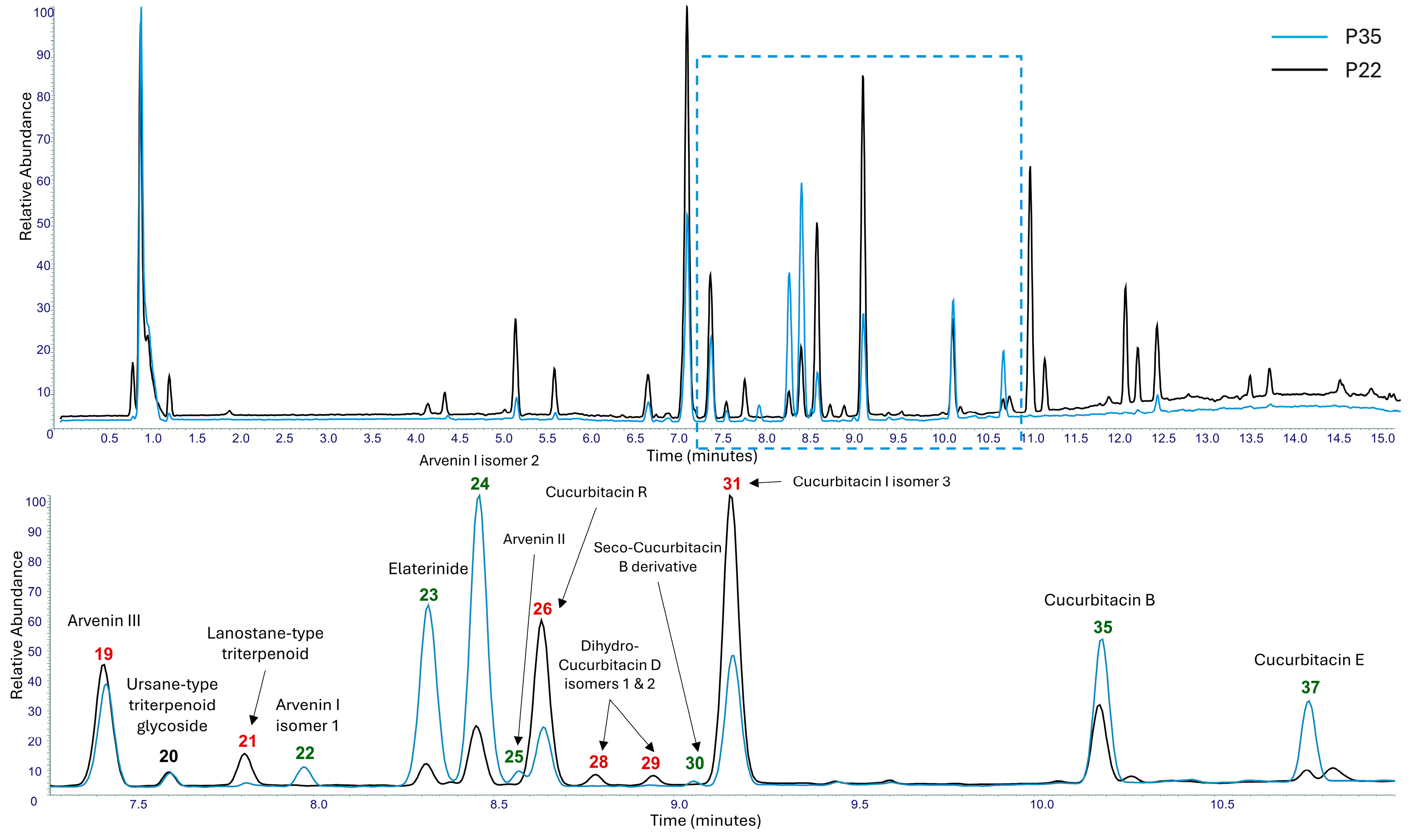
3.4. Extraction and LC-HRMS/MS Analyses of Methanol Extracts
4. Discussion
4.1. Morphology
4.2. On the Plant Function
4.3. Bioassays and LC-HRMS Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dafni, A.; Benítez, G.C.; Blanché, C.; Laca, L.R.; Petanidou, T.; Aytaç, B.; Horvat, M.; Lucchese, F.; Geva Kleinberger, A. The etymological, ecological, historical and ethnobotanical roots of the vernacular names of Ecballium elaterium (L.) Rich. (squirting cucumber). J. Ethnobiol. Tradit. Med. 2013, 119, 515–537. [Google Scholar]
- Muftah, S.A.; Almajberi, S.A.; Mohamed, O.S.; Altayar, M.A.; Nasib, M.A.; Attitalla, I.H.; Al Hejazi, I.O.A.; Elmhdwi, M.F. Anti-bacterial screening and antioxidant and free radical scavenging activity of Ecballium elaterium. Afr. J. Basic Appl. Sci. 2013, 5, 276–283. [Google Scholar]
- Costich, D.E. Gender specialization across a climatic gradient: Experimental comparison of monoecious and dioecious Ecballium. Ecology 1995, 76, 1036–1050. [Google Scholar] [CrossRef]
- Fahn, A.; Shimony, C. Nectary structure and ultrastructure of unisexual flowers of Ecballium elaterium (L.) A. Rich. (Cucurbitaceae) and their presumptive pollinators. Ann. Bot. 2001, 87, 27–33. [Google Scholar] [CrossRef]
- Ielciu, I.I.; Frédérich, M.; Tits, M.; Angenot, L.; Păltinean, R.; Cieckiewicz, E.; Crișan, G.; Vlase, L. Bryonia alba L. and Ecballium elaterium (L.) A. Rich.—Two related species of the Cucurbitaceae family with important pharmaceutical potential. Farmacia 2016, 64, 323–332. [Google Scholar]
- Mitrakos, K. A theory for Mediterranean plant life. Acta Oecol. Oecol. Plant. 1980, 1, 245–252. [Google Scholar]
- Christodoulakis, N.S.; Mitrakos, K. Structural analysis of sclerophylly in eleven evergreen phanerophytes in Greece. In Plant Response to Stress: Functional Analysis in Mediterranean Ecosystems; Tenhunen, J.D., Catavino, F.M., Lange, O.L., Oechel, W.C., Eds.; Springer: Berlin/Heidelberg, Germany, 1987; pp. 547–551. [Google Scholar]
- Christodoulakis, N.S.; Fasseas, C. Air pollution effects on the leaf structure of Laurus nobilis, an injury resistant species. Bull. Environ. Contam. Toxicol. 1990, 44, 276–281. [Google Scholar] [CrossRef]
- Christodoulakis, N.S.; Kogia, D.; Mavroeidi, D.; Fasseas, C. Anatomical and cytochemical investigation on the leaf of Teucrium polium L., a pharmaceutical shrub of the Greek phryganic formations. J. Biol. Res. 2010, 14, 199–209. [Google Scholar]
- Christodoulakis, N.S.; Kollia, K.; Fasseas, C. Leaf structure and histochemistry of Ecballium elaterium (L.) A. Rich. (squirting cucumber). Flora 2011, 206, 191–197. [Google Scholar] [CrossRef]
- Mamoucha, S.; Fokialakis, N.; Christodoulakis, N.S. Leaf structure and histochemistry of Ficus carica (Moraceae), the fig tree. Flora 2015, 218, 24–34. [Google Scholar] [CrossRef]
- Stefi, A.L.; Mitsigiorgi, K.; Vassilacopoulou, D.; Christodoulakis, N.S. Response of young Nerium oleander plants to long-term non-ionizing radiation. Planta 2020, 251, 110. [Google Scholar] [CrossRef] [PubMed]
- Stefi, A.L.; Christodoulakis, N.S. Approaching the “secrets” of the resin ducts in the mastic tree (Pistacia lentiscus L. cv. chia). Flora 2021, 285, 151940. [Google Scholar] [CrossRef]
- Stefi, A.L.; Kalouda, G.; Skouroliakou, A.S.; Vassilacopoulou, D.; Christodoulakis, N.S. The counteraction of cultivated Cistus creticus L. (rock rose) plants to the strain imposed by a long-term exposure to non-ionizing radiation and the role of DDC. Biophysica 2022, 2, 248–265. [Google Scholar] [CrossRef]
- Dioscorides. The Greek Herbal of Dioscorides, 1st ed.; Gunther, R.T., Translator; Hafner Publishing Company: New York, NY, USA, 1959. [Google Scholar]
- Theophrastus. Enquiry into Plants; Hort, A.F., Translator; Harvard University Press: Cambridge, MA, USA, 1916; Volume I. [Google Scholar]
- Pliny the Elder. Natural History; Book XX, Chapter 3, Section 85; Rackham, H., Translator; Harvard University Press: Cambridge, MA, USA, 1938. [Google Scholar]
- Yılmaz, K.; Karakuş, F.; Eyol, E.; Tosun, E.; Yılmaz, İ.; Ünüvar, S. Cytotoxic effects of cucurbitacin I and Ecballium elaterium on breast cancer cells. Nat. Prod. Commun. 2018, 13, 1445–1448. [Google Scholar] [CrossRef]
- Oskoui, M.T. Cycloeucalenol from the leaves of Ecballium elaterium. Planta Med. 1986, 52, 159. [Google Scholar] [CrossRef]
- Jafargholizadeh, N.; Zargar, S.J.; Yassa, N.; Tavakoli, S. Purification of cucurbitacins D, E, and I from Ecballium elaterium (L.) A. Rich fruits and study of their cytotoxic effects on the AGS cell line. Asian Pac. J. Cancer Prev. 2016, 17, 4631–4635. [Google Scholar] [CrossRef]
- Anzano, A.; de Falco, B.; Grauso, L.; Lanzotti, V. Squirting cucumber, Ecballium elaterium (L.) A. Ritch: An update of its chemical and pharmacological profile. Molecules 2024, 29, 4377. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, G.; Redmond, L.; Germain, C.; Palazzi, E.; Terzago, S.; Willm, L.; Poulin, B. Predicting the vulnerability of seasonally-flooded wetlands to climate change across the Mediterranean Basin. Sci. Total Environ. 2019, 692, 546–555. [Google Scholar] [CrossRef]
- Noto, L.V.; Cipolla, G.; Pumo, D.; Francipane, A. Climate change in the Mediterranean Basin (Part II): A review of challenges and uncertainties in climate change modeling and impact analyses. Water Resour. Manag. 2023, 37, 2307–2323. [Google Scholar] [CrossRef]
- OBrien, T.P.; McCully, M.E. The Study of Plant Structure Principles and Selected Methods; Termarcarphi Pty. Ltd.: Melbourne, Australia; Scientific Research Publishing: Irvine, CA, USA, 1981; Available online: https://www.scirp.org/reference/referencespapers?referenceid=1453666 (accessed on 22 May 2025).
- Christodoulakis, N.S.; Kotsironi, K.; Tsafantakis, N.; Stefi, A.L.; Fokialakis, N. Leaf structure and phytochemical analysis of Aristolochia baetica, a traditionally used pharmaceutical plant. J. Herbs Spices Med. Plants 2019, 25, 88–103. [Google Scholar] [CrossRef]
- Bronner, R. Simultaneous demonstration of lipids and starch in plant tissues. Stain Technol. 1975, 50, 1–4. [Google Scholar] [CrossRef]
- Mowry, R.W. Alcian blue techniques for the histochemical study of acidic carbohydrates. J. Histochem. Cytochem. 1956, 4, 407–411. [Google Scholar]
- Fisher, D.B. Protein staining of ribboned Epon sections for light microscopy. Histochemie 1968, 16, 92–96. [Google Scholar] [CrossRef]
- Gechev, T.; Mehterov, N.; Denev, I.; Hille, J. A simple and powerful approach for isolation of Arabidopsis mutants with increased tolerance to H2O2-induced cell death. In Methods in Enzymology; Walker, J.M., Ed.; Academic Press: San Diego, CA, USA, 2013; Volume 527, pp. 203–220. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. Available online: https://www.researchgate.net/publication/313724134 (accessed on 25 May 2025). [CrossRef]
- Sarropoulou, V.; Sarrou, E.; Angeli, A.; Martens, S.; Maloupa, E.; Grigoriadou, K. Developing an in vitro elicitation strategy for specialized secondary metabolites production in adventitious root cultures of Primula veris subsp. veris. Ind. Crops Prod. 2023, 197, 116618. [Google Scholar] [CrossRef]
- Taulavuori, E.; Hellström, E.K.; Taulavuori, K.; Laine, K. Comparison of two methods used to analyse lipid peroxidation from Vaccinium myrtillus (L.) during snow removal, reacclimation and cold acclimation. J. Exp. Bot. 2001, 52, 2375–2380. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Xu, J.; Duan, X.; Yang, J.; Beeching, J.R.; Zhang, P. Enhanced reactive oxygen species scavenging by overproduction of superoxide dismutase and catalase delays postharvest physiological deterioration of cassava storage roots. Plant Physiol. 2013, 161, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites; Blackwell Scientific Publications: Oxford, UK, 1994; pp. 73–99. Available online: https://www.scirp.org/reference/referencespapers?referenceid=2597356 (accessed on 29 May 2025).
- Stefi, A.L.; Chalkiadaki, M.; Dimitriou, K.; Mitsigiorgi, K.; Gkikas, D.; Papageorgiou, D.; Ntroumpogianni, G.C.; Vassilacopoulou, D.; Halabalaki, M.; Christodoulakis, N.S. Oregano young plants cultured at low temperature reveal an enhanced healing effect of their extracts: Anatomical, physiological and cytotoxicity approach. Metabolites 2025, 15, 103. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Buysse, J.; Merckx, R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 4, 1627–1629. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Ain-Lhout, F.; Zunzunegui, M.; Barradas, M.D.; Tirado, R.; Clavijo, A.; Novo, F.G. Comparison of proline accumulation in two Mediterranean shrubs subjected to natural and experimental water deficit. Plant Soil 2001, 230, 175–183. [Google Scholar] [CrossRef]
- Pouris, J.; Meletiou-Christou, M.S.; Chimona, C.; Rhizopoulou, S. Seasonal functional partitioning of carbohydrates and proline among plant parts of the sand daffodil. Agronomy 2020, 10, 539. [Google Scholar] [CrossRef]
- Kovalevich, J.; Langford, D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 1078, pp. 9–21. [Google Scholar]
- Comşa, Ş.; Cimpean, A.M.; Raica, M. The story of MCF-7 breast cancer cell line: 40 years of experience in research. Anticancer Res. 2015, 35, 3147–3154. [Google Scholar] [PubMed]
- Dini, L. Apoptosis induction in DU-145 human prostate carcinoma cells. Tissue Cell 2005, 37, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Stefi, A.L.; Margaritis, L.H.; Skouroliakou, A.S.; Vassilacopoulou, D. Mobile phone electromagnetic radiation affects amyloid precursor protein and α-synuclein metabolism in SH-SY5Y cells. Pathophysiology 2019, 26, 203–212. [Google Scholar] [CrossRef]
- Crowley, L.C.; Marfell, B.J.; Christensen, M.E.; Waterhouse, N.J. Measuring cell death by trypan blue uptake and light microscopy. Cold Spring Harb. Protoc. 2016, 2016, 643–646. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. arXiv 2012, arXiv:1201.0490. [Google Scholar]
- Metcalfe, C.R.; Chalk, L. Anatomy of Dicotyledons, 2nd ed.; Clarendon Press: Oxford, UK, 1979; pp. 456–473. [Google Scholar]
- Bansal, S.; Wang, B. A critical factor in reactive oxygen species (ROS) studies: The need to understand the chemistry of the solvent used: The case of DMSO. Chem. Sci. 2024, 15, 17843–17851. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Kong, W.; Tang, T.; Cheng, H. Identification of CmACL genes in melon and analysis of their potential functions in fruit sugar and acid accumulation. Front. Plant Sci. 2023, 14, 1239482. [Google Scholar] [CrossRef] [PubMed]
- Ul Haq, F.; Ali, A.; Khan, M.N.; Shah, S.M.Z.; Kandel, R.C.; Aziz, N.; Adhikari, A.; Choudhary, M.I.; ur-Rahman, A.; El-Seedi, H.R.; et al. Metabolite profiling and quantitation of cucurbitacins in Cucurbitaceae plants by liquid chromatography coupled to tandem mass spectrometry. Sci. Rep. 2019, 9, 15992. [Google Scholar] [CrossRef] [PubMed]
- Bourebaba, L.; Gilbert-Lopez, B.; Oukil, N.; Bedjou, F. Phytochemical composition of Ecballium elaterium extracts with antioxidant and anti-inflammatory activities: Comparison among leaves, flowers and fruits extracts. Arab. J. Chem. 2018, 13, 3286–3300. [Google Scholar] [CrossRef]
- Chen, J.C.; Chiu, M.H.; Nie, R.L.; Cordell, G.A.; Qiu, S.X. Cucurbitacins and cucurbitane glycosides: Structures and biological activities. Nat. Prod. Rep. 2005, 22, 386–399. [Google Scholar] [CrossRef]
- Sayed, Z.; Badr, W. Cucurbitacin glucosides and biological activities of the ethyl acetate fraction from ethanolic extract of Egyptian Ecballium elaterium. J. Appl. Sci. Res. 2012, 8, 1252–1258. [Google Scholar]
- Chen, C.; Qiang, S.; Lou, L.; Zhao, W. Cucurbitane-type triterpenoids from the stems of Cucumis melo. J. Nat. Prod. 2009, 72, 824–829. [Google Scholar] [CrossRef]
- Takemoto, M.; Delghandi, S.; Abo, M.; Yurimoto, K.; Odagi, M.; Singh, V.P.; Wang, J.; Nakagawa, R.; Sato, S.; Takemoto, Y.; et al. Covalent plant natural product that potentiates antitumor immunity. J. Am. Chem. Soc. 2025, 147, 2902–2912. [Google Scholar] [CrossRef]
- Cohen, I.; Zandalinas, S.I.; Huck, C.; Fritschi, F.B.; Mittler, R. Meta-analysis of drought and heat stress combination impact on crop yield and yield components. Physiol. Plant. 2020, 171, 66–76. [Google Scholar] [CrossRef]
- Kong, X.; Liu, G.; Liu, J.; Ding, Z. The root transition zone: A hot spot for signal crosstalk. Trends Plant Sci. 2018, 23, 403–409. [Google Scholar] [CrossRef]
- Saab, I.M.; Sharp, R.E.; Pritchard, J.; Voetberg, G.S. Increased endogenous abscisic acid maintains primary root growth and inhibits shoot growth of maize seedlings at low water potentials. Plant Physiol. 1990, 93, 1329–1336. [Google Scholar] [CrossRef]
- Ober, E.S.; Sharp, R.E. Electrophysiological responses of maize roots to low water potentials: Relationship to growth and ABA accumulation. J. Exp. Bot. 2003, 54, 813–824. [Google Scholar] [CrossRef]
- Parkhurst, D.F. The adaptive significance of stomatal occurrence on one or both surfaces of leaves. J. Ecol. 1978, 66, 367–383. [Google Scholar] [CrossRef]
- Parkhurst, D.F.; Mott, K.A. Intercellular diffusion limits to CO2 uptake in leaves: Studies in air and helox. Plant Physiol. 1990, 94, 1024–1032. [Google Scholar] [CrossRef]
- Drake, P.L.; de Boer, H.J.; Schymanski, S.J.; Veneklaas, E.J. Two sides to every leaf: Water and CO2 transport in hypostomatous and amphistomatous leaves. New Phytol. 2019, 222, 1179–1187. [Google Scholar] [CrossRef]
- Asargew, M.F.; Masutomi, Y.; Kobayashi, K.; Aono, M. Water stress changes the relationship between photosynthesis and stomatal conductance. Sci. Total Environ. 2023, 907, 167886. [Google Scholar] [CrossRef] [PubMed]
- Pirasteh-Anosheh, H.; Saed-Moucheshi, A.; Pakniyat, H.; Pessarakli, M. Stomatal responses to drought stress. In Water Stress and Crop Plants; Wiley-Blackwell: Hoboken, NJ, USA, 2016; pp. 24–40. [Google Scholar]
- Flexas, J.; Ribas-Carbó, M.; Bota, J.; Galmés, J.; Henkle, M.; Martínez-Cañellas, S.; Medrano, H. Decreased Rubisco activity during water stress is not induced by decreased relative water content but related to conditions of low stomatal conductance and chloroplast CO2 concentration. New Phytol. 2006, 172, 73–82. [Google Scholar] [CrossRef]
- Siebert, D.J. Localization of salvinorin A and related compounds in glandular trichomes of the psychoactive sage, Salvia divinorum. Ann. Bot. 2004, 93, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Demir, M.; Taylan, M.; Kaya, H.; Ekinci, A.; Arslan, D.; Aslan, E.; Keles, A.; Yılmaz, S.; Sezgi, C. Histopathological and biochemical effects of Ecballium elaterium on sepsis-induced lung injury. J. Investig. Surg. 2016, 29, 302–308. [Google Scholar] [CrossRef]
- Hussain, H.; Green, I.R.; Saleem, M.; Khattak, K.F.; Irshad, M.; Ali, M. Cucurbitacins as Anticancer Agents: A Patent Review. Recent Pat. Anti-Cancer Drug Discov. 2019, 14, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, T.; Rehman, S.U.; Smith, D.; Sultan, T.; Seleiman, M.F.; Alsadon, A.A.; Amna; Ali, S.; Chaudhary, H.J.; Solieman, T.H.I.; et al. Mitigation of heat stress in Solanum lycopersicum L. by ACC-deaminase and exopolysaccharide producing Bacillus cereus: Effects on biochemical profiling. Sustainability 2020, 12, 2159. [Google Scholar] [CrossRef]
- Shen, H.; Zhao, B.; Xu, J.; Liang, W.; Huang, W.; Li, H. Effects of heat stress on changes in physiology and anatomy in two cultivars of Rhododendron. S. Afr. J. Bot. 2017, 112, 338–345. [Google Scholar] [CrossRef]
- Yang, D.; Qiu, H.; Pei, Y.; Hu, S.; Ma, S.; Liu, Z.; Zhang, Y.; Cao, M. Spatial and temporal evolution of the infiltration characteristics of a loess landslide. ISPRS Int. J. Geo-Inf. 2020, 9, 26. [Google Scholar] [CrossRef]
- Yuan, L.; Tang, L.; Zhu, S.; Hou, J.; Chen, G.; Liu, F.; Liu, S.; Wang, C. Influence of heat stress on leaf morphology and nitrogen–carbohydrate metabolisms in two wucai (Brassica campestris L.) genotypes. Acta Soc. Bot. Pol. 2017, 86, 3587. [Google Scholar] [CrossRef]
- Matysik, J.; Alia; Bhalu, B.; Mohanty, P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 2002, 82, 525–532. [Google Scholar]
- Meena, M.; Divyanshu, K.; Kumar, S.; Swapnil, P.; Zehra, A.; Shukla, V.; Yadav, M.; Upadhyay, R.S. Regulation of L-proline biosynthesis, signal transduction, transport, accumulation and its vital role in plants during variable environmental conditions. Heliyon 2019, 5, e02952. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M. Proline alleviates abiotic stress-induced oxidative stress in plants. J. Plant Growth Regul. 2023, 42, 4629–4651. [Google Scholar] [CrossRef]
- Khan, P.; Abdelbacki, A.M.M.; Albaqami, M.; Jan, R.; Kim, K.-M. Proline promotes drought tolerance in maize. Biology 2025, 14, 41. [Google Scholar] [CrossRef]
- Singh, P.K.; Tewari, R.K. Cadmium toxicity induced changes in plant water relations and oxidative metabolism of Brassica juncea L. plants. J. Environ. Biol. 2003, 24, 107–112. [Google Scholar]
- Afzal, S.; Chaudhary, N.; Singh, N.K. Role of soluble sugars in metabolism and sensing under abiotic stress. In Plant Growth Regulators; Aftab, T., Hakeem, K.R., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Farooq, M.; Hussain, M.; Ul-Allah, S.; Siddique, K.H. Physiological and agronomic approaches for improving water-use efficiency in crop plants. Agric. Water Manag. 2019, 219, 95–108. [Google Scholar] [CrossRef]
- Kaushal, N.; Gupta, K.; Bhandhari, K.; Kumar, S.; Thakur, P.; Nayyar, H. Proline induces heat tolerance in chickpea (Cicer arietinum L.) plants by protecting vital enzymes of carbon and antioxidative metabolism. Physiol. Mol. Biol. Plants 2011, 17, 203–213. [Google Scholar] [CrossRef]
- Jahan, M.S.; Wang, Y.; Shu, S.; Zhong, M.; Chen, Z.; Wu, J.; Sun, J.; Guo, S. Exogenous salicylic acid increases the heat tolerance in tomato (Solanum lycopersicum L.) by enhancing photosynthesis efficiency and improving antioxidant defense system through scavenging of reactive oxygen species. Sci. Hortic. 2019, 247, 421–429. [Google Scholar] [CrossRef]
- Wassie, M.; Zhang, W.; Zhang, Q.; Ji, K.; Cao, L.; Chen, L. Exogenous salicylic acid ameliorates heat stress-induced damages and improves growth and photosynthetic efficiency in alfalfa (Medicago sativa L.). Ecotoxicol. Environ. Saf. 2020, 191, 110206. [Google Scholar] [CrossRef]
- Zahra, N.; Hafeez, M.B.; Ghaffar, A.; Kausar, A.; Zeidi, M.A.; Siddique, K.H.; Farooq, M. Plant photosynthesis under heat stress: Effects and management. Environ. Exp. Bot. 2022, 206, 105178. [Google Scholar] [CrossRef]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant carotenoids: Recent advances and future perspectives. Mol. Hortic. 2022, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ding, Y.; Zhu, C. Sensitivity and responses of chloroplasts to heat stress in plants. Front. Plant Sci. 2020, 11, 375. [Google Scholar] [CrossRef]
- Bolouri-Moghaddam, M.R.; Le Roy, K.; Xiang, L.; Rolland, F.; Van den Ende, W. Sugar signalling and antioxidant network connections in plant cells. FEBS J. 2010, 277, 2022–2037. [Google Scholar] [CrossRef] [PubMed]
- Hussin, S.; Geissler, N.; Koyro, H.W. Effect of NaCl salinity on Atriplex nummularia with special emphasis on carbon and nitrogen metabolism. Acta Physiol. Plant. 2013, 35, 1025–1038. [Google Scholar] [CrossRef]
- Tejera, N.A.; Soussi, M.; Lluch, C. Physiological and nutritional indicators of tolerance to salinity in chickpea plants growing under symbiotic conditions. Environ. Exp. Bot. 2006, 58, 17–24. [Google Scholar] [CrossRef]
- Negro, C.; Tommasi, L.; Miceli, A. Phenolic compounds and antioxidant activity from red grape marc extracts. Bioresour. Technol. 2003, 87, 41–44. [Google Scholar] [CrossRef]
- Elavarthi, S.; Martin, B. Spectrophotometric assays for antioxidant enzymes in plants. In Plant Stress Tolerance, Methods in Molecular Biology; Sunkar, R., Ed.; Humana Press: Totowa, NJ, USA, 2010; Volume 639, pp. 273–281. [Google Scholar]
- Kumar, K.; Debnath, P.; Singh, S.; Kumar, N. An overview of plant phenolics and their involvement in abiotic stress tolerance. Stresses 2023, 3, 570–585. [Google Scholar] [CrossRef]
- Raychaudhuri, R.; Lin, D.W.; Montgomery, R.B. Prostate cancer: A review. JAMA 2025, 333, 1433–1446. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Tang, J.; Liu, X.; Sun, Y.; Gong, B.; Gao, Q. Identification of the optimal candidates to benefit from surgery and chemotherapy among elderly female breast cancer patients with bone metastases. Sci. Rep. 2025, 15, 4678. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Biyik-Sit, R.; Uzun, Y.; Chen, C.H.; Thadi, A.; Sussman, J.H.; Pang, M.; Wu, C.Y.; Grossmann, L.D.; Gao, P.; et al. Longitudinal single-cell multiomic atlas of high-risk neuroblastoma reveals chemotherapy-induced tumor microenvironment rewiring. Nat. Genet. 2025, 57, 1142–1154. [Google Scholar] [CrossRef]
- Johnsen, J.I.; Dyberg, C.; Wickström, M. Neuroblastoma—A neural crest derived embryonal malignancy. Front. Mol. Neurosci. 2019, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Attard, E. Use of extracts from squirting cucumber (Ecballium elaterium) seeds in health. In Nuts and Seeds in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: London, UK, 2011; pp. 1079–1086. [Google Scholar]
- Verheijen, M.; Lienhard, M.; Schrooders, Y.; Clayton, O.; Nudischer, R.; Boerno, S.; Timmermann, B.; Selevsek, N.; Schlapbach, R.; Gmuender, H.; et al. DMSO induces drastic changes in human cellular processes and epigenetic landscape in vitro. Sci. Rep. 2019, 9, 4641. [Google Scholar] [CrossRef]
- Kaczor-Kamińska, M.; Kaszuba, K.; Bilska-Wilkosz, A.; Iciek, M.; Wróbel, M.; Kamiński, K. Dimethyl Sulfoxide (DMSO) as a potential source of interference in research related to Sulfur Metabolism—A preliminary study. Antioxidants 2024, 13, 582. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Maliar, T.; Maliarová, M.; Blažková, M.; Kunštek, M.; Uváčková, Ľ.; Viskupičová, J.; Purdešová, A.; Beňovič, P. Simultaneously determined antioxidant and Pro-Oxidant activity of randomly selected plant secondary metabolites and plant extracts. Molecules 2023, 28, 6890. [Google Scholar] [CrossRef]
- Amrati, F.E.-Z.; Chebaibi, M.; Galvão de Azevedo, R.; Conte, R.; Slighoua, M.; Mssillou, I.; Kiokias, S.; de Freitas Gomes, A.; Soares Pontes, G.; Bousta, D. Phenolic Composition, Wound Healing, Antinociceptive, and Anticancer Effects of Caralluma europaea Extracts. Molecules 2023, 28, 1780. [Google Scholar] [CrossRef]
- Spissu, Y.; Gil, K.A.; Dore, A.; Sanna, G.; Palmieri, G.; Sanna, A.; Cossu, M.; Belhadj, F.; Gharbi, B.; Pinna, M.B.; et al. Anti- and Pro-Oxidant activity of polyphenols extracts of syrah and chardonnay grapevine pomaces on melanoma cancer cells. Antioxidants 2022, 12, 80. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, B.; Sharma, U.; Parashar, G.; Parashar, N.C.; Rani, I.; Ramniwas, S.; Kaur, S.; Haque, S.; Tuli, H.S. Apoptotic and antimetastatic effect of cucurbitacins in cancer: Recent trends and advancement. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 1867–1878. [Google Scholar] [CrossRef]
- Molavi, O.; Torkzaban, F.; Jafari, S.; Asnaashari, S.; Asgharian, P. Chemical compositions and anti-proliferative activity of the aerial parts and rhizomes of squirting cucumber, Cucurbitaceae. Jundishapur J. Nat. Pharm. Prod. 2019, 15, e82990. [Google Scholar] [CrossRef]
- Zheng, Q.; Liu, Y.; Liu, W.; Ma, F.; Zhou, Y.; Chen, M.; Chang, J.; Wang, Y.; Yang, G.; He, G. Cucurbitacin B inhibits growth and induces apoptosis through the JAK2/STAT3 and MAPK pathways in SH-SY5Y human neuroblastoma cells. Mol. Med. Rep. 2014, 10, 89–94. [Google Scholar] [CrossRef]
- Patel, A.; Rasheed, A.; Reilly, I.; Pareek, Z.; Hansen, M.; Haque, Z.; Simon-Fajardo, D.; Davies, C.; Tummala, A.; Reinhardt, K.; et al. Modulation of cytoskeleton, protein trafficking, and signaling pathways by metabolites from Cucurbitaceae, Ericaceae, and Rosaceae plant families. Pharmaceuticals 2022, 15, 1380. [Google Scholar] [CrossRef] [PubMed]
- Chau, M.N.; Banerjee, P.P. Development of a STAT3 reporter prostate cancer cell line for high throughput screening of STAT3 activators and inhibitors. Biochem. Biophys. Res. Commun. 2008, 377, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Srivastava, S.K. Inhibition of HER2-integrin signaling by cucurbitacin B leads to in vitro and in vivo breast tumor growth suppression. Oncotarget 2014, 5, 1812–1828. [Google Scholar] [CrossRef] [PubMed]
- Duangmano, S.; Sae-Lim, P.; Suksamrarn, A.; Patmasiriwat, P.; Domann, F.E. Cucurbitacin B causes increased radiation sensitivity of human breast cancer cells via G2/M cell cycle arrest. J. Oncol. 2012, 2012, 601682, Erratum in J. Oncol. 2015, 2015, 486850. [Google Scholar] [CrossRef]
- Guo, J.; Wu, G.; Bao, J.; Hao, W.; Lu, J.; Chen, X. Cucurbitacin B induced ATM-mediated DNA damage causes G2/M cell cycle arrest in a ROS-dependent manner. PLoS ONE 2014, 9, e88140. [Google Scholar] [CrossRef]
- Ren, G.; Sha, T.; Guo, J.; Li, W.; Lu, J.; Chen, X. Cucurbitacin B induces DNA damage and autophagy mediated by reactive oxygen species (ROS) in MCF-7 breast cancer cells. J. Nat. Med. 2015, 69, 522–530. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, X.; Qu, Y.; Ding, Y. Curcumin alleviates oxygen-glucose-deprivation/reperfusion-induced oxidative damage by regulating miR-1287-5p/LONP2 axis in SH-SY5Y cells. Anal. Cell. Pathol. 2021, 2021, 5548706. [Google Scholar] [CrossRef]
- Duncan, K.L.K.; Duncan, M.D.; Alley, M.C.; Sausville, E.A. Cucurbitacin E-induced disruption of the actin and vimentin cytoskeleton in prostate carcinoma cells. Biochem. Pharmacol. 1996, 52, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Balding, K.K.; Zimmerman, S.J.; Chang, C.-Y.; Lu, S.-M.; Huang, H.-C. Reduction of cancer stem cells and invasiveness of human melanoma and breast cancer by cucurbitacin B from Lagenaria siceraria. Drugs Drug Candidates 2023, 2, 19. [Google Scholar] [CrossRef]
- Delgado-Tiburcio, E.E.; Cadena-Iñiguez, J.; Santiago-Osorio, E.; Ruiz-Posadas, L.M.; Castillo-Juárez, I.; Aguiñiga-Sánchez, I.; Soto-Hernández, M. Pharmacokinetics and biological activity of cucurbitacins. Pharmaceuticals 2022, 15, 1325. [Google Scholar] [CrossRef]
- Minh, C.V.; Nhiem, N.X.; Yen, H.T.; Kiem, P.V.; Tai, B.H.; Le Tuan Anh, H.; Hien, T.T.T.; Park, S.; Kim, N.; Kim, S.H. Chemical constituents of Trichosanthes kirilowii and their cytotoxic activities. Arch. Pharm. Res. 2015, 38, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Tannin-Spitz, T.; Grossman, S.; Dovrat, S.; Gottlieb, H.E.; Bergman, M. Growth inhibitory activity of cucurbitacin glucosides isolated from Citrullus colocynthis on human breast cancer cells. Biochem. Pharmacol. 2007, 73, 56–67. [Google Scholar] [CrossRef]




| Treatment (Both Experiments) | Total Dry Mass (g) | Transition Zone Diameter (mm) |
|---|---|---|
| Ecballium elaterium—P22 | 2.8 ± 0.4 * | 6.96 ± 0.22 ** |
| Ecballium elaterium—P35 | 1.7 ± 0.3 * | 8.26 ± 0.56 ** |
| Treatment (Both Experiments) | Number of Stomata/mm2—Adaxial | Number of Stomata/mm2—Abaxial | Number of Glandular Trichomes/mm2 —Abaxial |
|---|---|---|---|
| Ecballium elaterium—P22 | 221 ± 28 * | N/A | 29 ± 10 ** |
| Ecballium elaterium—P35 | 119 ± 9 * | 61 ± 19 | 2 ± 1.2 ** |
| ID | Annotated Compound | Rt (min) | Experimental m/z [M-H]− | Experimental m/z [M+H]+ | Elemental Composition (EC) | RDBeq. Values | Δm (ppm) | HRMS/MS Fragments | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Trehalose | 0.92 | 377.0850 [M+Cl]− | C12H22O11 | 1.50 | −1.73 | 341.1086 (100), 89.0243 (72), 179.0563 (72) 119.0352 (42), 161.0455 (33), 59.0140 (24), 101.0246 (24) 71.0139 (22), 113.0247 (20), 221.0670 (11) | - | |
| 2 | Unknown | 0.95 | 323.1447 | C12H22N2O8 | 2.50 | −0.64 | 239.1026 (100), 195.1127 (55), 287.1238 (47), 108.0443 (41), 150.0549 (40), 81.0335 (39), 115.0865 (37), 100.0756 (29), 225.1235 (24), 161.0920 (19), 173.0920 (15) | - | |
| 3 | Unknown | 1.23 | 323.1446 | C12H22N2O8 | 2.50 | −0.93 | 239.1026 (100), 195.1128 (55), 81.0334 (43), 287.1239 (40), 108.0443 (39), 150.0549 (36), 115.0865 (31), 100.0756 (30), 225.1233 (23), 161.0920 (16), 173.0921 (16), 122.0600 (15) | - | |
| 4 | Citric acid | 1.24 | 191.0195 | C6H8O7 | 3.50 | −0.94 | 111.0087 (100), 87.0088 (42), 85.0295 (28), 191.0198 (11) | [51] | |
| 5 | Gentisic acid O-glycoside | 1.91 | 315.0721 | C13H16O9 | 6.50 | −0.04 | 152.0115 (100), 315.0721 (74), 153.0194 (50), 108.0217, (31), 109.0295 (26) | - | |
| 6 | Unknown | 4.19 | 367.1605 | C15H28O10 | 2.50 | −1.22 | 235.1186 (100), 101.0244 (80), 59.0138 (46), 71.0138 (44), 89.0244 (42), 161.0455 (34),131.0350 (31), 113.0244 (26), 73.0295 (18), 85.0295 (16) | - | |
| 7 | Megastigmane 9-O glucopyranoside | 4.39 | 451.2180 [M+FA-H]− | C19H34O9 | 3.50 | −1.17 | 167.1078 (100), 89.0246 (77), 71.0139 (41), 101.0245 (39), 59.0139 (36), 113.0247 (31), 149.0970 (25) | [52] | |
| 8 | Kaempferol 3-O diglycoside | 4.56 | 593.1515 | C27H30O15 | 13.50 | 0.09 | 473.1093 (100), 503.1187 (36), 593.1496 (20) | [52] | |
| 9 | Quercetin 3-O diglycoside | 5.19 | 741.1876 | C32H38O20 | 14.50 | −1.09 | 300.0273 (100), 741.1880 (41), 301.0352 (13) | [52] | |
| 10 | Luteolin 8-C glycoside | 5.27 | 447.0933 | C21H20O11 | 12.50 | −0.53 | 327.0512 (100), 357.0611 (69), 285.0407 (10) | [53] | |
| 11 | Rutin | 5.64 | 609.1456 | C27H30O16 | 13.50 | −0.99 | 300.0273 (100), 609.1457 (63), 301.0352 (57) | [53] | |
| 12 | Kaempferol 3-O diglycoside | 5.99 | 593.1513 | C27H30O15 | 13.50 | 0.19 | 285.0403 (100), 284.0327 (61), 593.1509 (45) | [53] | |
| 13 | Narcissin | 6.08 | 623.1616 | C28H32O16 | 13.50 | −0.12 | 315.0509 (100), 314.0431 (41), 623.1614 (20) | - | |
| 14 | Khekadaengoside K | 6.68 | 561.2701 | C30H42O10 | 10.50 | −0.68 | 561.2705 (100), 398.2093 (40), 399.2171 (35), 543.2598 (30) | [52] | |
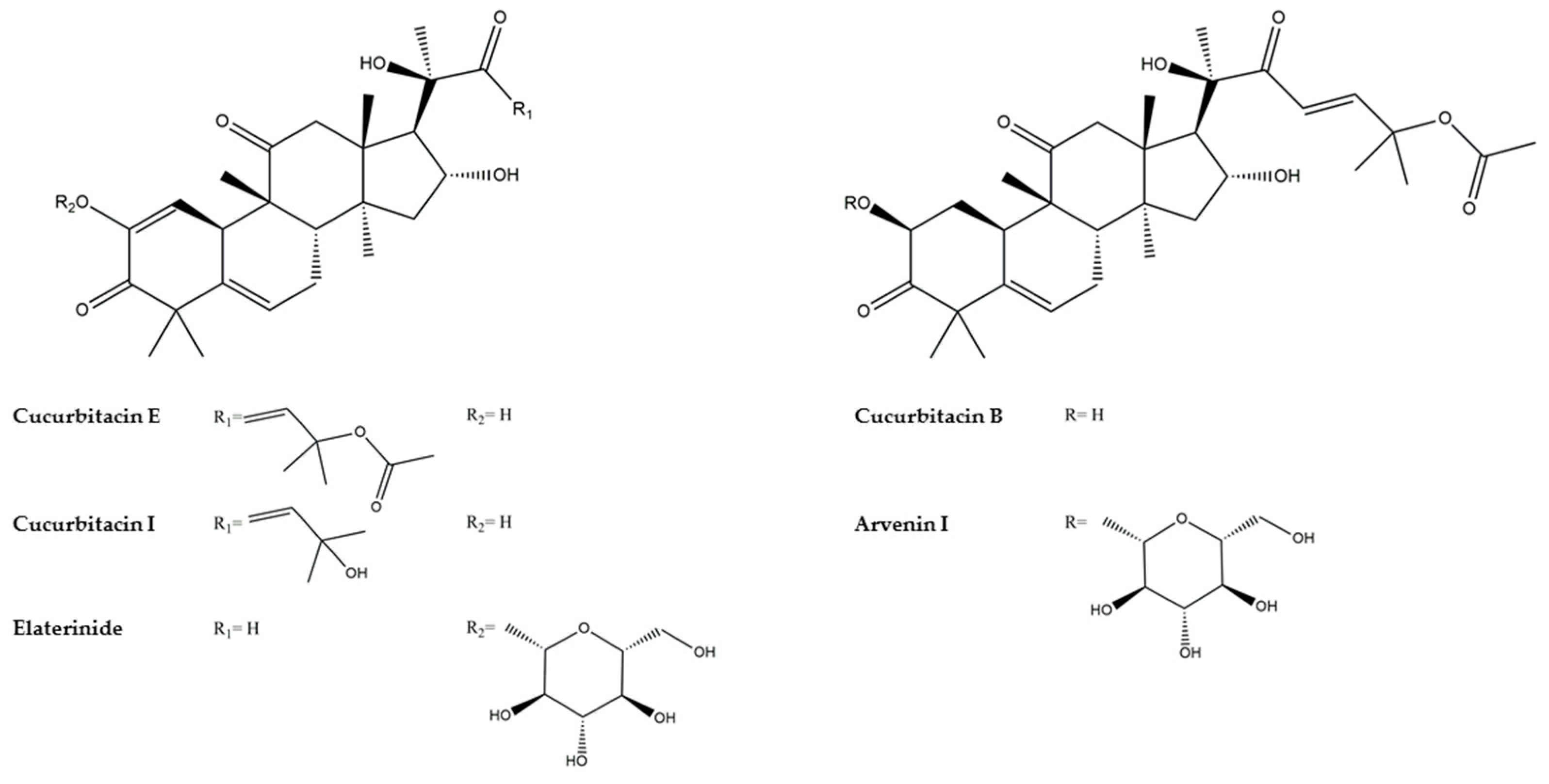
| 15 | Cucurbitacin J 2-O glucopyranoside | 6.70 | 693.3488 | C36H54O13 | 10.50 | −0.59 | 605.2968 (100), 425.2329 (63), 675.3386 (28), 443.2439 (16), 101.0608 (14), 341.1765 (13), 383.2227 (13), 587.2856 (12), 513.2847 (12), 495.2741 (12), 657.3276 (11) | [54] | |
| 16 | Cucurbitacin I isomer 1 | 6.70 | 515.3000 | C30H42O7 | 9.50 | −0.66 | 515.2998 (100), 91.0540 (18), 205.1222 (17), 111.0804 (16), 123.0802 (14), 95.0491 (14), 128.0618 (12), 115.0542 (12), 215.1428 (10), 139.0753 (10), 187.1117 (10) | [55] | |
| 17 | Khekadaengoside D | 7.15 | 721.3434 [M+FA-H]− | C36H52O12 | 11.50 | −1.47 | 675.3384 (100), 657.3282 (26) 495.2751 (22), 513.2860 (14) 341.1757 (12) 477.2645 (12) 411.2178 (11) | [54] | |
| 18 | Cucurbitacin S | 7.17 | 499.3045 | C30H42O6 | 9.50 | −0.60 | 499.3045 (100), 113.0960 (62), 91.0541 (55), 123.0804 (44), 149.0960 (41), 95.0491 (40), 95.0854 (40), 128.0622 (31), 115.0542 (40), 67.0542 (27), 79.0542 (23), 111.0804 (22) | [56] | |
| 19 | Arvenin III | 7.41 | 723.3588 [M+FA-H]− | C36H54O12 | 10.50 | −1.08 | 659.3441 (100), 575.2862 (99), 413.2334 (88) 87.0087 (87), 101.0244 (71), 89.0243 (64), 505.2449 (58), 113.0244 (55), 119.0350 (51), 497.2910 (51), 343.1914 (46), 641.3337 (45), 191.1081 (37), 677.3530 (35), 161.0453 (34), 165.0921 (31), 437.2694 (30), 455.2806 (29), 479.2805 (27), 485.2531 (25), 599.3241 (23), 617.3338 (22), 325.1815 (21), 547.2554 (20) | [57] | |
| 20 | Unknown Ursane-type triterpenoid glycoside | 7.60 | 707.3646 [M+FA-H]− | C36H54O11 | 10.50 | −0.36 | 87.0089 (100), 101.0247 (95), 499.3076 (84), 661.3618 (77), 571.3281 (77), 191.1081 (71), 161.0458 (68), 113.0247 (47), 309.2073 (33), 89.0244 (33), 448.8047 (30), 119.0352 (29), 135.6041 (27) | - | |
| 21 | Unknown Lanostane-type triterpenoid | 7.81 | 547.2910 | C30H44O9 | 9.50 | −0.55 | 135.0815 (100), 331.1915 (18), 153.0921 (9), 485.2908 (9) | - | |
| 22 | Arvenin I Isomer 1 | 7.97 | 765.3698 [M+FA-H]− | C38H56O13 | 11.50 | −0.63 | 701.3542 (100), 497.2903 (31), 719.3652 (30), 101.0244 (27), 659.3442 (24), 87.0087 (22), 573.2700 (21), 161.0455 (16), 113.0244 (16), 89.0244 (15) | [54] | |
| 23 | Elaterinide | 8.31 | 763.3542 [M+FA-H]− | C38H54O13 | -1.59 | 12.50 | 657.3273 (100), 495.2751 (43), 717.3499 (34), 615.3170 (23), 561.2706 (17), 675.3434 (14), 477.2666 (11), 699.3367 (11) | [52] | |
| 24 | Arvenin I Isomer 2 | 8.45 | 765.3696 [M+FA-H]− | C39H58O15 | 11.50 | −0.87 | 701.3543 (100), 497.2909 (32), 719.3641 (27), 659.3430 (26), 101.0244 (24), 87.0088 (22), 113.0245 (17) | ||
| 25 | Arvenin II | 8.58 | 767.3851 [M+FA-H]− | C38H58O13 | 10.50 | −1.06 | 643.3481 (100), 481.2959 (85), 609.2919 (82), 661.3586 (80), 703.3665 (67), 101.0244 (48), 87.0087 (39), 161.0455 (37), 553.3176 (33), 113.0243 (29), 89.0246 (22), 541.3177 (20), | [55] | |
| 26 | Cucurbitacin R | 8.64 | 561.3063 [M+FA-H]− | C30H46O7 | 8.50 | −0.59 | 165.0921 (100), 385.2382 (93), 369.2068 (77), 59.0138 (66), 179.1078 (48), 499.3072 (44), 137.0973 (42), 457.2961 (42), 367.2273 (41), 325.1810 (41), 341.2112 (29), 173.1184 (16), 439.2820 (16) | [54] | |
| 27 | Deoxocucurbitoside B | 8.75 | 835.4116 [M+FA-H]− | C42H62O14 | 12.50 | −0.72 | 789.4066 (100), 481.2956 (32), 505.2957 (22), 480.2877 (19), 101.0242 (12) | [52] | |
| 28 | Dihydro-Cucurbitacin D isomer 1 | 8.78 | 561.3063 [M+FA-H]− | C30H44O7 | 9.50 | 0.88 | 165.0921 (100), 497.2917 (44), 439.2494 (40), 479.2809 (36), 437.2708 (31), 515.3033 (30), 385.2021 (25), 455.2806 (24), 385.2386 (22), 369.2079 (20), 497.2515 (18), 343.1927 (17), 87.0454 (10) | [54] | |
| 29 | Dihydro-Cucurbitacin D isomer 2 | 8.94 | 561.3063 [M+FA-H]− | C30H44O7 | 9.50 | −0.54 | 165.0920 (100), 479.28109 (36), 437.2702 (32), 497.2921 (29), 455.2823 (28), 439.2477 (22), 385.2008 (18) | [56] | |
| 30 | Seco-Cucurbitacin B derivative | 9.05 | 589.3016 | C32H46O10 | 10.50 | −0.42 | 135.0815 (100), 331.1913 (17), 485.2906 (8), 467.2805 (6) | [54] | |
| 31 | Cucurbitacin I isomer 3 | 9.16 | 559.2906 [M+FA-H]− | C30H44O7 | 9.50 | −0.66 | 163.0766 (100), 497.2905 (71), 383.2230 (62), 59.0138 (42), 203.1081 (33), 367.1915 (32), 179.1081 (30), 455.2811 (27), 437.2665 (22), 339.1970 (21), 173.1189 (21) | - | |
| 32 | Unknown (Sphingolipid) | 9.38 | 288.2896 | C17H37NO2 | −0.50 | −0.96 | 288.2898 (100), 106.0862 (40), 88.0757 (21), 57.0699 (14) | - | |
| 33 | Unknown (Sphingolipid) | 9.58 | 288.2891 | C17H37NO2 | −0.50 | −2.13 | 288.2893 (100), 88.0756 (12), 106.0861 (9) | - | |
| 34 | Unknown (Sphingolipid) | 9.71 | 304.2844 | C17H37NO3 | −0.50 | −0.85 | 304.2843 (100), 256.2633 (57), 88.0756 (21) 56.0494 (14), 74.0600 (12), 122.0810 (10) | - | |
| 35 | Cucurbitacin B | 10.18 | 603.3168 [M+FA-H]− | C32H46O8 | 10.50 | −1.06 | 497.2909 (100), 411.2176 (90), 301.1445 (34), 273.1486 (19) | [52] | |
| 36 | Unknown (Sphingolipid) | 10.42 | 316.3206 | C19H41NO2 | −0.50 | −1.23 | 316.3206 (100), 106.0861 (31), 88.0756 (19), 57.0699 (12) | - | |
| 37 | Cucurbitacin E | 10.76 | 601.3015 [M+FA-H]− | C32H44O8 | 11.50 | −0.41 | 409.2027 (100), 495.2733 (96), 163.0764 (93), 299.1280 (53), 463.7701 (51), 426.2051 (47), 299.0011 (41), 165.9267 (41), 175.3833 (40), 195.3096 (39), 87.3690 (39), 322.1431 (39) | [52] | |
| 38 | Gingerglycolipid A-Glycerolipid | 11.07 | 721.3643 [M+FA-H]− | C33H56O14 | 6.50 | −1.52 | 277.2171 (100), 397.1364 (37), 101.0247 (24), 415.1458 (19), 89.0245 (18) | - | |
| 39 | LysoPC (18:3)-Glycerophospholipid | 11.24 | 562.3146 [M+FA-H]− | C26H48NO7P | 4.50 | −0.83 | 277.2173 (100), 502.2940 (12), 224.0692 (11) | - | |
| 40 | MGMG (18:3)-Glycolipid | 12.15 | 559.3120 [M+FA-H]− | C27H46O9 | 5.50 | −0.64 | 277.2172 (100), 253.0928 (11), 101.0245 (3), 278.2206 (3) | - | |
| 41 | DGMG(16:0)-Glycolipid | 12.30 | 653.3747 [M+FA-H]− | C31H58O14 | 3.50 | −1.05 | 255.2328 (100), 89.0244 (31), 101.0244 (30), 397.1349 (23), 71.0138 (16), 415.1457 (15) | - | |
| 42 | LysoPC (16:0)-Glycerophospholipid | 12.51 | 540.3303 | C25H52NO9P | 1.50 | 0.80 | 255.2328 (100), 480.4096 (17), 224.0693 (10) | - | |
| 43 | PC(16:0/9:0(COOH))-Glycerophospholipid | 13.80 | 666.4337 | C33H64NO10P | 2.50 | −0.52 | 184.0734 (100), 86.0964 (21), 98.9842 (16), 124.9998 (10), 71.0730 (5) | - | |
| 44 | SQMG(18:3)-Glycerolipid | 14.60 | 577.2684 | C27H46O11S | 5.50 | −0.75 | 577.2684 (100), 225.0074 (13), 64.1417 (5) | - | |
| 45 | Unknown (Triterpenoid) | 14.73 | 485.2894 | C29H40O6 | 9.50 | −0.82 | 133.1012 (100), 91.0542 (36) | - | |
| 46 | Unknown (Sphingolipid) | 15.31 | 526.5188 | C33H67NO3 | 0.50 | −0.62 | 270.2790 (100), 283.2630 (97), 526.5189 (94), 88.0756 (18), 102.0913 (12) | - | |
| 47 | Monopalmitin | 15.87 | 331.2841 | C19H38O4 | 0.50 | −0.51 | 57.0699 (100), 71.0855 (70), 95.0855 (54), 85.1012 (40), 109.1012 (26), 81.0698 (26), 313.2741 (22), 83.0855 (20), 67.0542 (18), 69.0699 (16), 55.0542 (12), 97.1011 (11) | - | |
| 48 | Pheophorbide a | 16.07 | 593.2751 | C35H36N4O5 | 19.50 | −1.18 | 593.2753 (100), 533.2546 (23), 460.2255 (9), 461.2329 (6), 505.2217 (6) | - | |
| 49 | Monostearin | 17.19 | 359.3155 | C21H42O4 | 0.50 | −0.18 | 57.0698 (100), 116.0527 (81), 71.0854 (70), 59.9902 (69), 95.0855 (52), 85.1012 (38), 88.0215 (37), 67.0543 (29), 81.0698 (28), 341.3047 (25), 109.1014 (25), 55.0541 (24), 69.0699 (23), 83.0855 (22), 57.0335 (13), 97.1009 (12), 158.1506 (11), 229.3898 (11), 111.1167 (10) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefi, A.L.; Chalkiadaki, M.; Bashari, E.; Mitsigiorgi, K.; Szczeblewski, P.; Papageorgiou, D.; Gkikas, D.; Vassilacopoulou, D.; Christodoulakis, N.S.; Halabalaki, M. Ecballium elaterium (L.) A. Rich. (Squirting Cucumber) Plants Cultured Under Different Temperatures: Anatomical and Biochemical Modifications of Their Leaves and the Bioactivity of Leaf Extracts. Metabolites 2025, 15, 585. https://doi.org/10.3390/metabo15090585
Stefi AL, Chalkiadaki M, Bashari E, Mitsigiorgi K, Szczeblewski P, Papageorgiou D, Gkikas D, Vassilacopoulou D, Christodoulakis NS, Halabalaki M. Ecballium elaterium (L.) A. Rich. (Squirting Cucumber) Plants Cultured Under Different Temperatures: Anatomical and Biochemical Modifications of Their Leaves and the Bioactivity of Leaf Extracts. Metabolites. 2025; 15(9):585. https://doi.org/10.3390/metabo15090585
Chicago/Turabian StyleStefi, Aikaterina L., Maria Chalkiadaki, Emily Bashari, Konstantina Mitsigiorgi, Paweł Szczeblewski, Danae Papageorgiou, Dimitrios Gkikas, Dido Vassilacopoulou, Nikolaos S. Christodoulakis, and Maria Halabalaki. 2025. "Ecballium elaterium (L.) A. Rich. (Squirting Cucumber) Plants Cultured Under Different Temperatures: Anatomical and Biochemical Modifications of Their Leaves and the Bioactivity of Leaf Extracts" Metabolites 15, no. 9: 585. https://doi.org/10.3390/metabo15090585
APA StyleStefi, A. L., Chalkiadaki, M., Bashari, E., Mitsigiorgi, K., Szczeblewski, P., Papageorgiou, D., Gkikas, D., Vassilacopoulou, D., Christodoulakis, N. S., & Halabalaki, M. (2025). Ecballium elaterium (L.) A. Rich. (Squirting Cucumber) Plants Cultured Under Different Temperatures: Anatomical and Biochemical Modifications of Their Leaves and the Bioactivity of Leaf Extracts. Metabolites, 15(9), 585. https://doi.org/10.3390/metabo15090585






