Gastric Bypass Associated Hyperammonemia (GaBHA): A Case Study, Scoping Review of the Literature, and Proposed New Pathophysiologic Mechanism
Abstract
1. Introduction
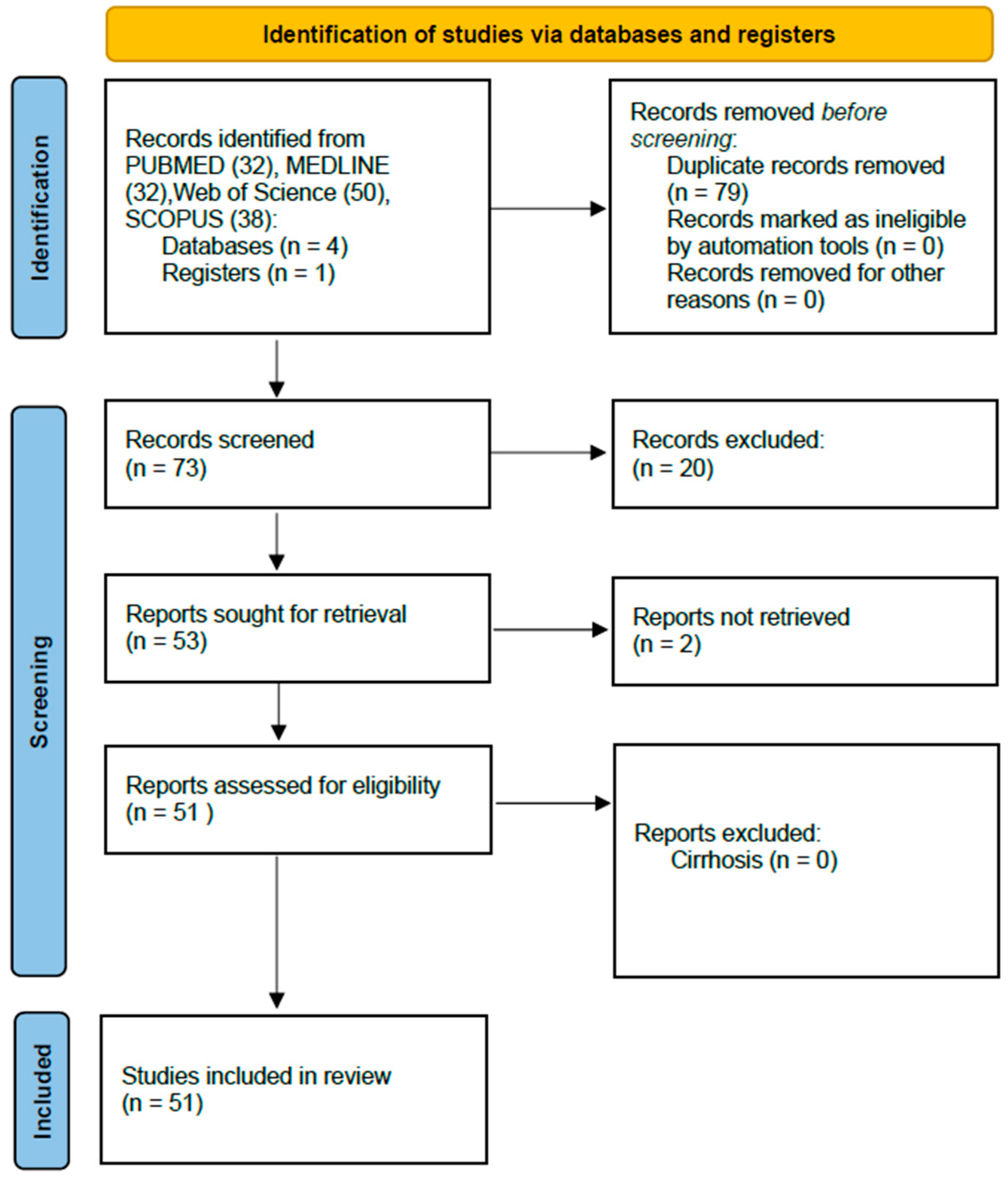
2. Methods
3. Case Presentation
4. Review of Cases in the Literature
5. Discussion
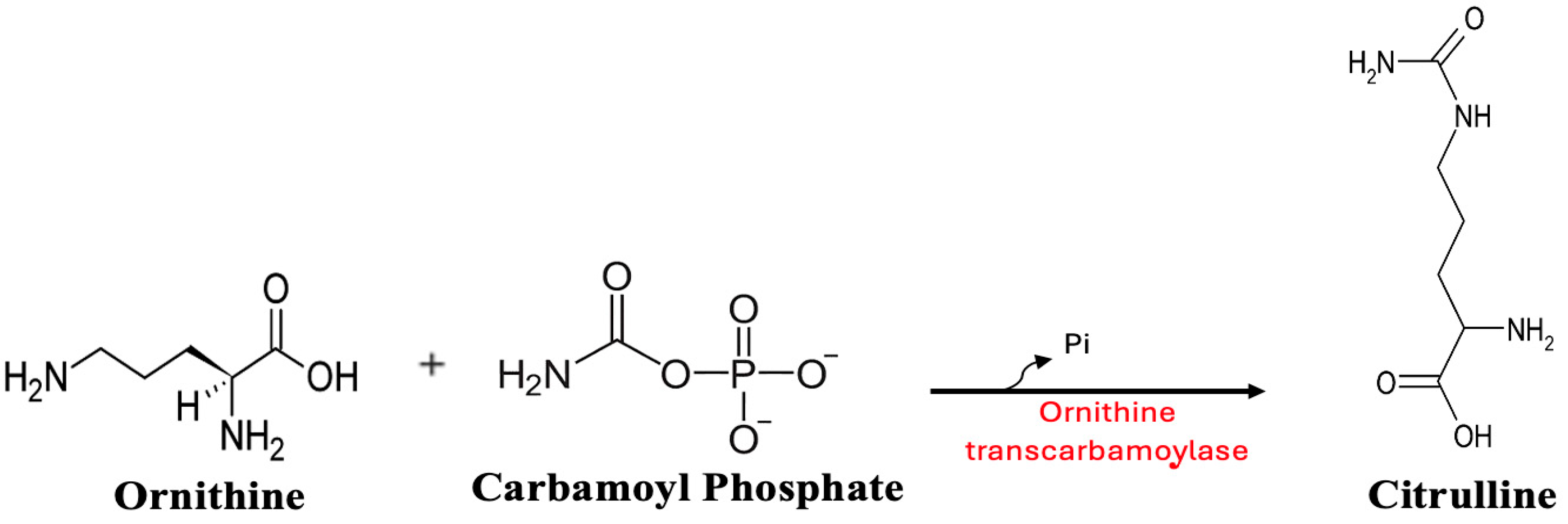
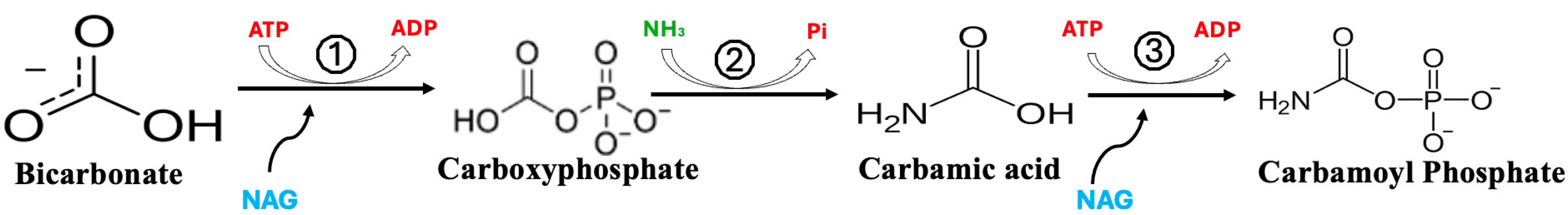
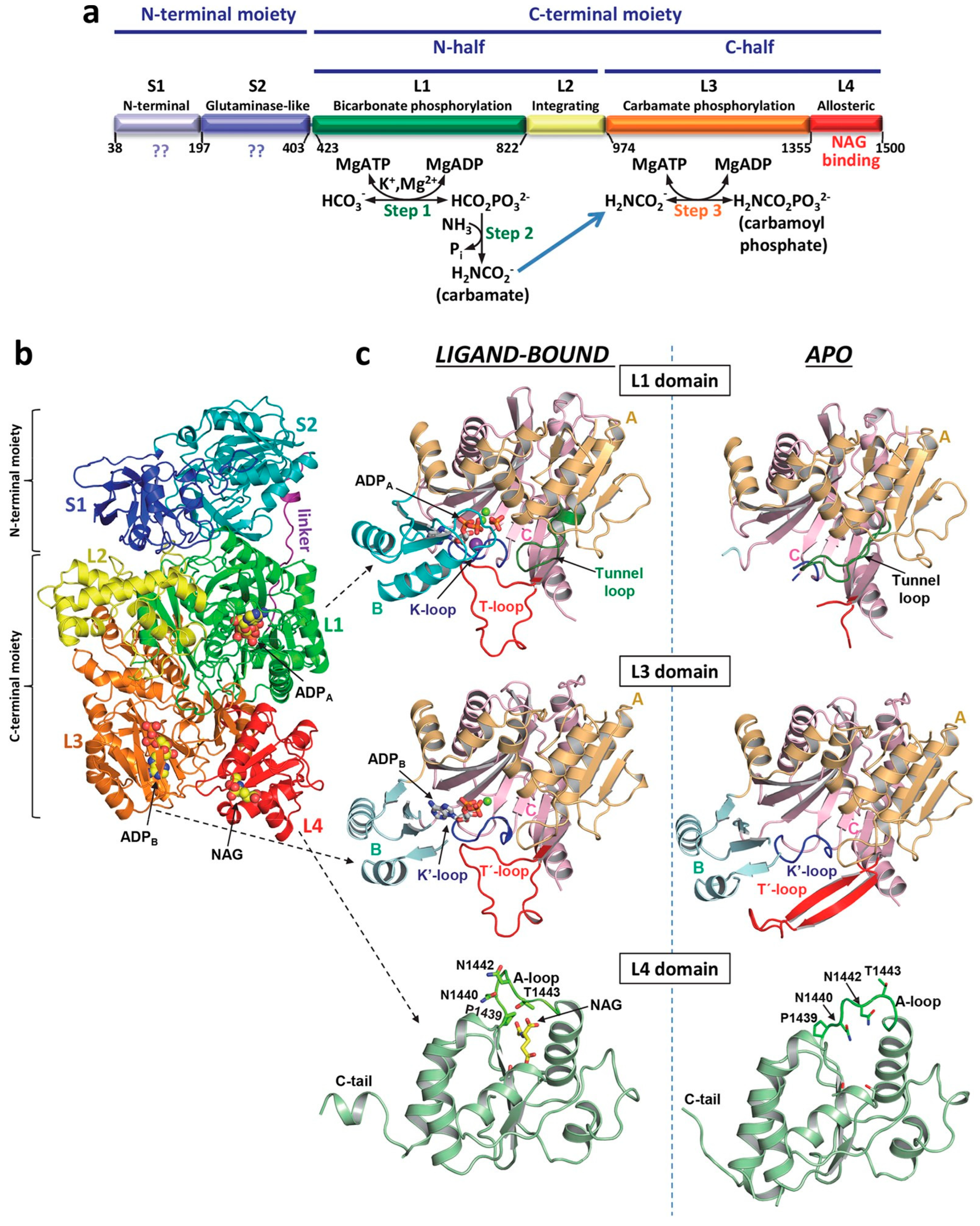
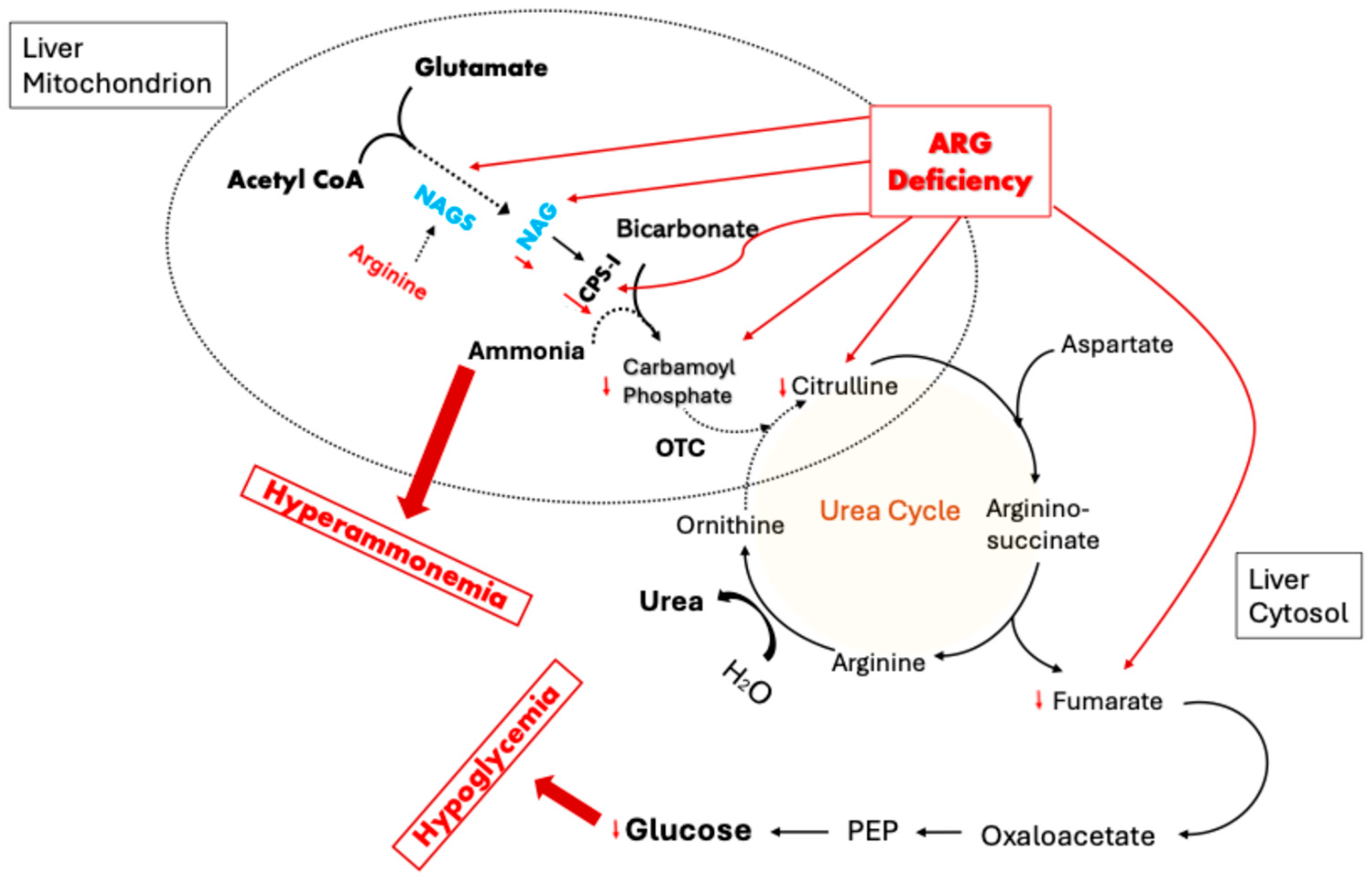
6. Role of OTC Dysfunction in Fasting Hypoglycemia
7. The Conditional Essentiality of Arginine in Stress Metabolism
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchell, B.G.; Collier, S.A.; Gupta, N. Roux-en-Y Gastric Bypass. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Fanous, J.; Bhatt, S.; Cartin-Ceba, R. Gastric bypass-related hyperammonemia: An uncommon yet potentially fatal complication. Chest 2023, 164, A1553–A1554. [Google Scholar] [CrossRef]
- Fenves, A.; Boland, C.R.; Lepe, R.; Rivera-Torres, P.; Spechler, S.J. Fatal hyperammonemic encephalopathy after gastric bypass surgery. Am. J. Med. 2008, 121, e1–e2. [Google Scholar] [CrossRef]
- Fenves, A.Z.; Shchelochkov, O.A.; Mehta, A. Hyperammonemic syndrome after Roux-en-Y gastric bypass. Obesity (Silver Spring) 2015, 23, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.P.; Marsh, C.B.; Mastronarde, J.G. Wernicke’s encephalopathy in a patient after gastric bypass surgery. Chest 2005, 128, 453S. [Google Scholar] [CrossRef]
- Lloyd, M.R.; Fenves, A.Z. Letter to the Editor Responding to Recurrent Hyperammonemia During Enteral Tube Feeding for Severe Protein Malnutrition After Bariatric Surgery. Obes. Surg. 2020, 30, 1138–1139. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Farzin, R.; Ekta, G.; Timothy, K. Fatal hyperammonemic encephalopathy after gastric bypass: 1027. Am. Coll. Gastroenterol. 2013, 108, S306–S307. Available online: https://journals.lww.com/ajg/Fulltext/2013/10001/Fatal_Hyperammonemic_Encephalopathy_after_Gastric.1027.aspx (accessed on 16 June 2025).
- Katherine, H.; Philip, S.; Kathy, B.-H. Fatal hyperammonemia: A case of urea cycle disorder unmasked after roux-en-y gastric bypass: 917. Am. Coll. Gastroenterol. 2015, 110, S395. [Google Scholar]
- Ahmed, E.; Adnan, A.; Nkem, I. Non-hepatic hyperammonemic encephalopathy after gastric bypass surgery: 787. Off. J. Am. Coll. Gastroenterol. 2015, 110, S344–S345. Available online: https://journals.lww.com/ajg/fulltext/2015/10001/non_hepatic_hyperammonemic_encephalopathy_after.787.aspx (accessed on 16 June 2025).
- Jasahui, M.D.; Brownback, K. Nonhepatic life threatening hyperammonemia. Chest 2015, 148, 215A. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0012369216361207 (accessed on 16 June 2025). [CrossRef]
- Kromas, M.L.; Mousa, O.Y.; John, S. Hyperammonemia-induced encephalopathy: A rare devastating complication of bariatric surgery. World J. Hepatol. 2015, 7, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.; Ghimire, P.; Bhat, U.; Dogra, M.; France, A.; Landsberg, D. 1695: Reversal of coma and mri findings on correction of bariatric surgery-associated hyperammonemia. Crit. Care Med. 2016, 44, 499. Available online: https://journals.lww.com/00003246-201612001-01653 (accessed on 16 June 2025). [CrossRef]
- Acharya, G.; Mehra, S.; Patel, R.; Frunza-Stefan, S.; Kaur, H. Fatal Nonhepatic Hyperammonemia in ICU Setting: A Rare but Serious Complication following Bariatric Surgery. Case Rep. Crit. Care 2016, 2016, 8531591. [Google Scholar] [CrossRef]
- O’Donnell-Luria, A.H.; Lin, A.P.; Merugumala, S.K.; Rohr, F.; Waisbren, S.E.; Lynch, R.; Tchekmedyian, V.; Goldberg, A.D.; Bellinger, A.; McFaline-Figueroa, J.R.; et al. Brain MRS glutamine as a biomarker to guide therapy of hyperammonemic coma. Mol. Genet. Metab. 2017, 121, 9–15. [Google Scholar] [CrossRef]
- Nagarur, A.; Fenves, A.Z. Late presentation of fatal hyperammonemic encephalopathy after Roux-en-Y gastric bypass. Proc. (Bayl. Univ. Med. Cent.) 2017, 30, 41–43. [Google Scholar] [CrossRef]
- Grogg, J.; Feinman, J.; El Husseini, I.; Hussain, S. 428: Hyperammonemic encephalopathy and new-onset seizures in a roux-en-y and short bowel syndrome patient. Crit. Care Med. 2018, 46, 198. Available online: https://journals.lww.com/00003246-201801001-00394 (accessed on 16 June 2025). [CrossRef]
- Ahmed, Z.; Arora, S.; Seibert, A.; Khanna, R. An Unusual Cause of Hyperammonemic Encephalopathy: 2248. Off. J. Am. Coll. Gastroenterol. 2018, 113, S1274. Available online: https://journals.lww.com/ajg/fulltext/2018/10001/an_unusual_cause_of_hyperammonemic_encephalopathy_.2247.aspx (accessed on 16 June 2025). [CrossRef]
- Borreggine, K.L.; Hosker, D.K.; Rummans, T.A.; Manning, D.M. Psychiatric Manifestations of Hyperammonemic Encephalopathy Following Roux-en-Y Gastric Bypass. Psychosomatics 2018, 59, 90–94. [Google Scholar] [CrossRef]
- Salcedo, J.D.; Goldstein, J.S.; Quinonez, J.M.; Mosetti, M.A. Nonfatal Hyperammonemic Encephalopathy as a Late Complication of Roux-en-Y Gastric Bypass. Case Rep. Gastrointest. Med. 2019, 2019, 9031087. [Google Scholar] [CrossRef] [PubMed]
- Castineira, J.; Goltser, Y.; Vila, M.; Patel, R.; Croix, P.S.; Ashe, D.; Al-Andary, S.; Halleman, C.; Alkurdi, B. Postbariatric Surgery Hyperammonemia: A Rare Cause of Encephalopathy. ACG Case Rep. J. 2019, 6, e00119. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, P.; Ramadas, P.; Landsberg, D. Bariatric Surgery Causing Hyperammonemia. Cureus 2019, 11, e5098. [Google Scholar] [CrossRef]
- Hendrikx, L.; Brandts, H.; van Borren, M.; de Boer, H. Recurrent Hyperammonemia During Enteral Tube Feeding for Severe Protein Malnutrition After Bariatric Surgery. Obes. Surg. 2019, 29, 4127–4130. [Google Scholar] [CrossRef]
- Purpura, R.D.; Zia, Z.; Carlos, W.G. Food Shall Be Thy Remedy: Hyperammonemic Coma Reversed by L-Carnitine Supplementation. Am. Thorac. Soc. 2020, 201, A5152. Available online: https://www.atsjournals.org/doi/10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A5152 (accessed on 16 June 2025).
- Vartanyan, A.; Go, A.; Mwangi, J. Bypassing the urea cycle in the icu. CHEST 2020, 158, A866. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0012369220329901 (accessed on 16 June 2025). [CrossRef]
- Bergagnini, I.; Hogan, D.; Ivanina, E. S2751 When to Rue the Roux-en-y: A Case of Hyperammonemic Encephalopathy Post Bariatric Surgery. Am. J. Gastroenterol. 2020, 115, S1438. Available online: https://journals.lww.com/10.14309/ajg.0000000000000874 (accessed on 16 June 2025). [CrossRef]
- Sun, Y.; Chu, X.; Shan, X.; Shi, Y.; Sun, X. An Effective Way to Treat Hyperammonemic Encephalopathy Complicated Post-Distal Roux-en-Y Gastric Bypass Surgery. Obes. Surg. 2020, 30, 3239–3241. [Google Scholar] [CrossRef]
- Patel, M.; Nash, J.; Khan, S.; Tawfik, M. Post-gastric bypass hyperammonemic encephalopathy: A rare and potentially fatal cause of encephalopathy in the icu. Chest 2021, 160, A601. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0012369221020274 (accessed on 16 June 2025). [CrossRef]
- Rosenberg, C.; Rhodes, M. Hyperammonemia in the setting of Roux-en-Y gastric bypass presenting with osmotic demyelination syndrome. J. Community Hosp. Intern. Med. Perspect. 2021, 11, 708–712. [Google Scholar] [CrossRef]
- Vinegrad, N.; Staretz-Chacham, O.; Barski, L.; Bartal, C. Nonhepatic hyperammonemic encephalopathy complications following bariatric surgery: A case report and review of the literature. J. Med. Case Rep. 2021, 15, 385. [Google Scholar] [CrossRef]
- Sharma, R.; Romain, D.; Gupta, A. An Unusual Case of Hyperammonemia Following Gastric Bypass Surgery. Am. J. Med. 2021, 134, e451–e452. [Google Scholar] [CrossRef]
- Robinson, J.; Charran, O.; Dietrich, T. 583: Hyperammonemia after roux-en-y gastric bypass. Crit. Care Med. 2022, 50, 283. [Google Scholar] [CrossRef]
- Dace, W.; Srivastava, A.; Milne, D.; Howden, A.; Kemp, H.; Cantley, N. Gastric bypass surgery contributing to hyperammonaemic encephalopathy: An under-reported cause of severe nutritional deficiency and significant patient mortality. Acute Med. 2022, 21, 34–42. [Google Scholar] [CrossRef]
- Kim, G.N.; Ho, S.; Saulino, D.; Liu, X. Severe Protein-Calorie Malnutrition-Associated Hepatic Steatosis in a Woman Who Had Roux-en-Y Gastric Bypass for Morbid Obesity Thirteen Years Ago. Gastroenterol. Res. 2021, 14, 129–137. [Google Scholar] [CrossRef]
- Summar, M.L.; Barr, F.; Dawling, S.; Smith, W.; Lee, B.; Singh, R.H.; Rhead, W.J.; King, L.S.; Christman, B.W. Unmasked adult-onset urea cycle disorders in the critical care setting. Crit. Care Clin. 2005, 21, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.T.; Kantarci, O.H.; Merritt, J.L.; McGrann, P.; Dyck, P.J.B.; Lucchinetti, C.F.; Tippmann-Peikert, M. Ornithine transcarbamylase deficiency presenting as encephalopathy during adulthood following bariatric surgery. Arch. Neurol. 2007, 64, 126–128. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Zucker, S.D. Hyperammonemic encephalopathy caused by carnitine deficiency. J. Gen. Intern. Med. 2008, 23, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Estrella, J.; Yee, G.; Wilcken, B.; Tchan, M.; Talbot, M. Hyperammonemic encephalopathy complicating bariatric surgery: A case study and review of the literature. Surg. Obes. Relat. Dis. 2014, 10, e35–e38. [Google Scholar] [CrossRef]
- Rogal, S.S.; Hu, A.; Bandi, R.; Shaikh, O. Novel therapy for non-cirrhotic hyperammonemia due to a spontaneous splenorenal shunt. World J. Gastroenterol. 2014, 20, 8288–8291. [Google Scholar] [CrossRef]
- Singh, S.; Suresh, S.; McClave, S.A.; Cave, M. Treating Every Needle in the Haystack: Hyperammonemic Encephalopathy and Severe Malnutrition After Bariatric Surgery-A Case Report and Review of the Literature. JPEN J. Parenter. Enter. Nutr. 2015, 39, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, J.; Elfiky, A.; Ravindran, N.; Al Moussawi, H. Hyperammonemia Encephalopathy due to Urea Cycle Disorder Precipitated by Gastrointestinal Bleed in the Setting of Prior Bariatric Surgery. ACG Case Rep. J. 2023, 10, e01164. [Google Scholar] [CrossRef]
- Zeghlache, R.; Samardzic, T.; Norman, K. Nonhepatic Hyperammonemic Encephalopathy: An Unmasked Urea Cycle Disorder in the Setting of Late Gastric Bypass Complication. ACG Case Rep. J. 2024, 11, e01560. [Google Scholar] [CrossRef]
- Kjærgaard, K.; Eriksen, P.L.; Nøhr, T.K.; Pedersen, S.B.; Gravholt, C.H.; Vilstrup, H.; Thomsen, K.L. Hyperammonaemic encephalopathy due to non-functioning urea cycle as a complication to gastric bypass surgery. Metab. Brain Dis. 2024, 40, 46. [Google Scholar] [CrossRef]
- Bonasso, P.C.; Dassinger, M.S. Fatal Hyperammonemic Encephalopathy in a Pediatric Patient After Roux-en-Y Gastric Bypass. Obes. Surg. 2018, 28, 2530–2532. [Google Scholar] [CrossRef]
- Thusay, R.; Sidhu, S.; Masih, D.; Kanmanthareddy, A.; Rangray, R.; Jagan, N. A Rare Case of Hyperammonemic Encephalopathy Arising Without Cirrhosis in an ICU Twenty-Three Years Post Roux-en-y Gastric Bypass. In Proceedings of the American Thoracic Society 2024 International Conference, San Diego, CA, USA, 17–22 May 2024; American Journal of Respiratory and Critical Care: San Diego, CA, USA, 2024. Available online: https://www.researchgate.net/publication/380240351_A_Rare_Case_of_Hyperammonemic_Encephalopathy_Arising_Without_Cirrhosis_in_an_ICU_Twenty-Three_Years_Post_Roux-en-y_Gastric_Bypass (accessed on 16 June 2025).
- Sadlik, G.S.; Angajala, V.; Cruz, A.; Ayyala, D.; Yuan, L. Hyperammonemic Encephalopathy: A Rare Complication of Roux-en-y Gastric Bypass. DDW 2022, 95, AB32. [Google Scholar] [CrossRef]
- Bendrick, T.; Nottoli, M.; Scholtens, A.; Raj, J. Hyperammonemic Encephalopathy as a Late Complication of Gastric Bypass Surgery. J. General. Intern. Med. 2023, 38, S476. [Google Scholar]
- Laoye, A.; Narola, A.; Mikhail, J.; Costanzo, E. Hyperammonemic Encephalopathy after Gastric Bypass Surgery. Am. J. Gastroenterol. 2013, 108, S220. Available online: https://journals.lww.com/ajg/fulltext/2013/10001/hyperammonemic_encephalopathy_after_gastric_bypass.738.aspx (accessed on 16 June 2025). [CrossRef]
- Roth, M.; McRae, L. Urea cycle disorders: A puzzling case of acute encephalopathy. JAAPA 2025, 38, e2–e5. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, T.; Beesen, A. Roux-en-y: Why Isn’t the Patient Waking Up? Correcting Acute Cerebral Edema Related to Hyperammonemia in a Patient Post Gastric Bypass Surgery. Chest 2024, 166, A1809–A1810. [Google Scholar] [CrossRef]
- Coleman, K.J.; Wellman, R.; Fitzpatrick, S.L.; Conroy, M.B.; Hlavin, C.; Lewis, K.H.; Coley, R.Y.; McTigue, K.M.; Tobin, J.N.; McBride, C.L.; et al. Comparative Safety and Effectiveness of Roux-en-Y Gastric Bypass and Sleeve Gastrectomy for Weight Loss and Type 2 Diabetes Across Race and Ethnicity in the PCORnet Bariatric Study Cohort. JAMA Surg. 2022, 157, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Kamel, A.Y.; Emtiazjoo, A.M.; Adkins, L.; Shahmohammadi, A.; Alnuaimat, H.; Pelaez, A.; Machuca, T.; Pipkin, M.; Lee, H.-W.; Weiner, I.D.; et al. Hyperammonemia After Lung Transplantation: Systematic Review and a Mini Case Series. Transpl. Int. 2022, 35, 10433. [Google Scholar] [CrossRef]
- Tavasoli, F.; Liu, M.; Machuca, T.; Bonato, R.; Grant, D.R.; Cypel, M.; Keshavjee, S.; Grasemann, H. Increased Arginase Expression and Decreased Nitric Oxide in Pig Donor Lungs after Normothermic Ex Vivo Lung Perfusion. Biomolecules 2020, 10, 300. [Google Scholar] [CrossRef]
- Tymoczko, J.L.B.; Jeremy, M.; Stryer, L. BIochemistry: A Short Course; Freeman Macmillan: New York, NY, USA, 2015. [Google Scholar]
- de Cima, S.; Polo, L.M.; Díez-Fernández, C.; Martínez, A.I.; Cervera, J.; Fita, I.; Rubio, V. Structure of human carbamoyl phosphate synthetase: Deciphering the on/off switch of human ureagenesis. Sci. Rep. 2015, 5, 16950. [Google Scholar] [CrossRef]
- Yao, S.; Nguyen, T.-V.; Rolfe, A.; Agrawal, A.A.; Ke, J.; Peng, S.; Colombo, F.; Yu, S.; Bouchard, P.; Wu, J.; et al. Small Molecule Inhibition of CPS1 Activity through an Allosteric Pocket. Cell Chem. Biol. 2020, 27, 259–268.e5. [Google Scholar] [CrossRef]
- Kenneson, A.; Singh, R.H. Presentation and management of N-acetylglutamate synthase deficiency: A review of the literature. Orphanet J. Rare Dis. 2020, 15, 279. [Google Scholar] [CrossRef]
- Limón, I.D.; Angulo-Cruz, I.; Sánchez-Abdon, L.; Patricio-Martínez, A. Disturbance of the Glutamate-Glutamine Cycle, Secondary to Hepatic Damage, Compromises Memory Function. Front. Neurosci. 2021, 15, 578922. [Google Scholar] [CrossRef] [PubMed]
- Holecek, M. Roles of malate and aspartate in gluconeogenesis in various physiological and pathological states. Metabolism 2023, 145, 155614. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Davis, T.A.; Kim, S.W.; Li, P.; Rhoads, J.M.; Satterfield, M.C.; Smith, S.B.; Spencer, T.E.; Yin, Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 2009, 37, 153–168. [Google Scholar] [CrossRef]
- van de Poll, M.C.; Siroen, M.P.; van Leeuwen, P.A.; Soeters, P.B.; Melis, G.C.; Boelens, P.G.; Deutz, N.E.; Dejong, C.H. Interorgan amino acid exchange in humans: Consequences for arginine and citrulline metabolism. Am. J. Clin. Nutr. 2007, 85, 167–172. [Google Scholar] [CrossRef]
- Lopez, M.J.; Mohiuddin, S.S. Biochemistry, Essential Amino Acids; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Patel, J.J.; Miller, K.R.; Rosenthal, C.; Rosenthal, M.D. When Is It Appropriate to Use Arginine in Critical Illness? Nutr. Clin. Pract. 2016, 31, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, L.; Ren, Y.; Huang, Y.; Liu, W.; Lv, Z.; Qian, L.; Yu, Y.; Xiong, Y. Arginase: Shedding light on the mechanisms and opportunities in cardiovascular diseases. Cell Death Discov. 2022, 8, 413. [Google Scholar] [CrossRef]
- Uygun, V.; Karasu, G.; Daloglu, H.; Hazar, V.; Yesilipek, A. Idiopathic hyperammonemia after hematopoietic stem cell transplantation: A case report. Pediatr. Transplant. 2015, 19, E104–E105. [Google Scholar] [CrossRef] [PubMed]
- Kiberenge, R.K.; Lam, H. Fatal hyperammonemia after repeat renal transplantation. J. Clin. Anesth. 2015, 27, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Ghabril, M.; Nguyen, J.; Kramer, D.; Genco, T.; Mai, M.; Rosser, B.G. Presentation of an acquired urea cycle disorder post liver transplantation. Liver Transplant. 2007, 13, 1714–1716. [Google Scholar] [CrossRef]
- Seethapathy, H.; Fenves, A.Z. Pathophysiology and Management of Hyperammonemia in Organ Transplant Patients. Am. J. Kidney Dis. 2019, 74, 390–398. [Google Scholar] [CrossRef] [PubMed]

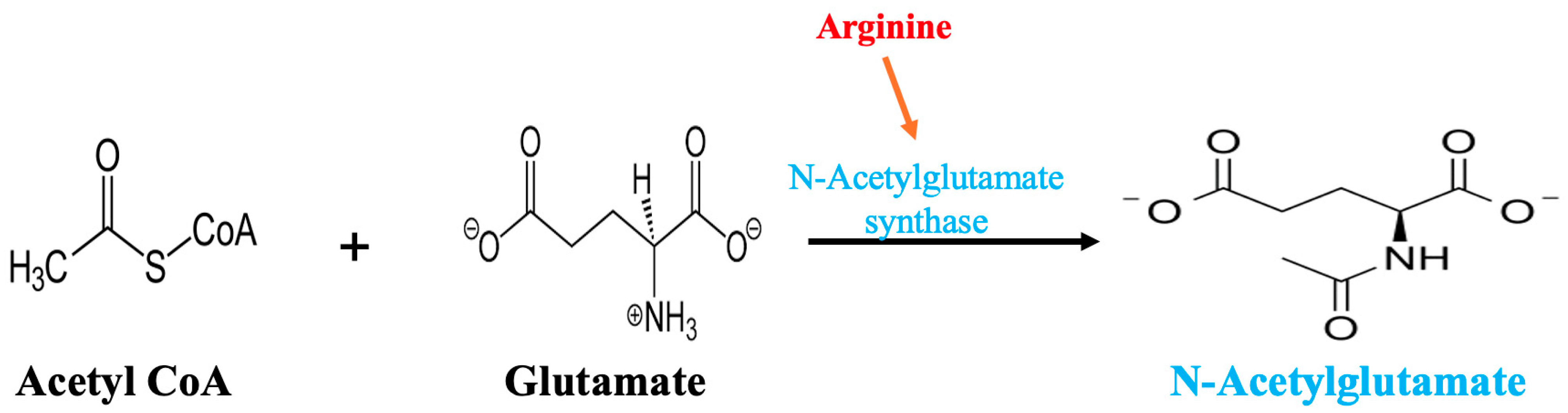
| Parameter | Result | Reference Range |
|---|---|---|
| Phosphoserine | 0 | <18 |
| Phosphoethanolamine | <2 | <12 |
| Taurine | 26 (L) | 42–156 |
| Asparagine | 62 | 37–92 |
| Serine | 180 | 63–187 |
| Hydroxyproline | 37 (H) | 4–29 |
| Glycine | 257 | 126–490 |
| Glutamine | 1078 (H) | 371–957 |
| Aspartic acid | 5 | <7 |
| Ethanolamine | 24 | <67 |
| Histidine | 77 | 39–123 |
| Threonine | 162 | 85–231 |
| Citrulline | 21 | 17–46 |
| Sarcosine | 5 (H) | <5 |
| Beta-Alanine | 8 | <29 |
| Alanine | 327 | 200–579 |
| Glutamic acid | 78 | 12–113 |
| 1-Methylhistidine | 2 | <28 |
| 3-Methylhistidine | 3 | 2–9 |
| Argininosuccinic acid | 0 | <2 |
| Carnosine | 0 | <1 |
| Anserine | 0 | <1 |
| Homocitrulline | 0 | <2 |
| Arginine | 31 (L) | 32–120 |
| Alpha-aminoadipic acid | 1 | <3 |
| Gamma-amino-n-butyric acid | 0 | <2 |
| Beta-aminoisobutyric acid | 7 (H) | <5 |
| Alpha-amino-n-butyric acid | 31 | 9–37 |
| Hydroxylysine | 1 | <2 |
| Proline | 283 | 97–368 |
| Ornithine | 60 | 38–130 |
| Cystathionine | <1 | <5 |
| Cystine | 23 | 3–95 |
| Lysine | 149 | 103–255 |
| Methionine | 13 | 4–44 |
| Valine | 93 (L) | 136–309 |
| Tyrosine | 42 | 31–90 |
| Isoleucine | 29 (L) | 36–107 |
| Leucine | 37 (L) | 68–183 |
| Phenylalanine | 52 | 35–80 |
| Tryptophan | 18 (L) | 29–77 |
| Allo-isoleucine | 1 | <5 |
| Review Article | Age (y) | Sex | Years Since RYGB Surgery | Outcome | Peak Ammonia (μmol/L) | Urine Orotic Acid (mmol/mol) | Gln (μmol/L) | Cit (μmol/L) | Albumin (g/dL) | Zinc Level (mcg/dL) | Arginine Level (μmol/L) | Report of Hypoglycemia | OTC Testing | Treatment Received |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fenves [3,4] | 50 | F | 1.4 | Deceased | 138 | 2 | Yes | |||||||
| 48 | F | 0.3 | Deceased | 50.7 | 2 | Yes | ||||||||
| 26 | F | 1.4 | Deceased | 286.7 | 1.3 | Yes | ||||||||
| 58 | F | 28 | Deceased | 44.8 | 1.5 | Yes | ||||||||
| 41 | F | 6 | Deceased | 40.1 | 2.1 | Yes | ||||||||
| 54 | F | Deceased | 205 | 1824 | 0.8 | 19 | No | |||||||
| 52 | F | 1 | Survived | 171 | 1004 | 33 | 1.1 | 58 | Yes | 15% activity of OTC in liver bx | ||||
| 49 | F | 11 | Survived | 96 | 1.5 | 23 | Yes | |||||||
| 46 | F | Deceased | 450 | 3.6 | 1.7 | 35 | No | |||||||
| 38 | F | 4.4 | Survived | 157 | 1.8 | 905 | 1.4 | 25 | Yes | |||||
| 44 | F | 10 | Deceased | 258 | 1700 | 1.7 | 86 | Yes | ||||||
| 41 | F | 0.5 | Survived | 99 | 2.7 | 858 | 13 | 1.8 | 41 | Yes | ||||
| 69 | F | 1.3 | Survived | 201 | 0.9 | 811 | 40 | 2.5 | 41 | Normal activity of OTC in liver bx | ||||
| 37 | F | 3 | Deceased | 180 | 1.8 | 31 | No | |||||||
| Rashti [8] | 43 | F | 10 | Deceased | 191 | Normal | Lactulose, Rifaximin | |||||||
| Hahn [9] | 41 | F | 11 | Deceased | 446 | 1998 | Negative gene testing for OTC and CPS-1 mutation | Dialysis, ammonul, cyclinex-2 (aminoacid modified food), citrulline | ||||||
| Elhassan [10] | 47 | F | 9 | Survived | 116 | Normal | Negative screening for urea cycle disorders | Lactulose, rifaximin, pyridoxine, thiamine, zinc | ||||||
| Postigo Jasahu [11] | 52 | F | Survived | Normal | Normal | Normal | Lactulose, rifaximin, Golytely | |||||||
| Kromas [12] | 56 | M | 2 | Survived | 91.5 | 1.4 | Normal | Lactulose, sodium benzoate, protein restriction | ||||||
| Khanal [13] | 45 | F | Survived | Normal | CRRT | |||||||||
| Acharya [14] | 42 | F | 2 | Deceased | 491 | 2.3 | 1363 | 43 | 1.8 | 23 | 130 | No | Lactulose, rifaximin, D5, VitK, | |
| O’Donnell-Luria [15] | 47 | F | 10 | Survived | 138 | 2.8 | 1391 | 17 | 1.9 | Normal | 20 | Negative testing for OTC, GLUD1, and PCCA | Lactulose, rifaximin, added sodium phenylacetate and sodium benzoate, arginine, N-carbamoyl glutamate. IVF without protein and then TPN with slow protein advancement. Vitamin and minerals | |
| Nagarur and Fenves [16] | 42 | F | 11 | Deceased | 498 | Normal | Normal | 12 | 1.4 | 34 | Lactulose, rifaximin, HDx1, Zinc, thiamine, Dextrose | |||
| Grogg [17] | 45 | F | 15 | Survived | 82.6 | 2.4 | Normal | 9 | Normal | Normal | 24 | Lactulose, rifaximin, low-protein parenteral diet | ||
| Ahmed [18] | 50 | F | 10 | Survived | . | Normal | Normal | Normal | Normal | Normal | normal | Lactulose | ||
| Borreggine [19] | 44 | F | 11 | Survived | 186 | 1.9 | Normal | Normal | 2.2 | 29 | Negative Sequencing analysis and deletion/duplication analysis of OTC deficiency gene | OVF, thiamine, selenium, zinc, then lactulose, rifaximin, and levocarnitine | ||
| Salcedo [20] | 48 | F | 20 | Survived | 173 | 2.3 | 54 | yes | Lactulose | |||||
| Castineira [21] | 42 | F | 1 | Survived | 190 | 1.63 | 1.7 | Lactulose, sodium benzoate, sodium phenylacetate, rifaximin, zinc, arginine (po), IV lipids, and dextrose | ||||||
| Krishnan [22] | 45 | F | 4 | Survived | 432 | 396 (mmol/L) | 9 | Normal | 49 | 34 | CRRT, supplemental nutrition fortifying her parenteral feeds with the essential amino acid combinations found deficient | |||
| Hendrikx/ Lloyd and Fenves [6,23] | 28 | F | 7 | Survived | 251 | 1 | 45 | Adjustment to enteral nutrition to slowly increase protein as IV glucose was tapered | ||||||
| Purpura [24] | 57 | F | Survived | 251 | 14 | CRRT, protein restriction, vitamin and L-carnitine supplementation | ||||||||
| Vartanyan [25] | 45 | F | Survived | 192 | Normal | Lactulose, rifaximin, CRRT, arginine, sodium benzoate, zinc and copper supplementation | ||||||||
| Bergagnini [26] | 45 | F | 16 | Survived | 227 | Normal | CVVHD, lactulose, rifaximin, low protein | |||||||
| Sun [27] | 24 | F | 3 | Survived | 169 | 1.7 | Initially, PN and albumin but stopped those and started duphalac enema, lactulose, probiotics, ornithine aspartate THEN gastrostomy and enteral nutrition | |||||||
| Patel [28] | 43 | F | Deceased | 169 | Lactulose, rifaximin, zinc, MTV, L-carnitine | |||||||||
| Rosenberg [29] | 58 | F | 20 | Deceased | 290 | 21.1 | Genetic testing for urea cycle disorder negative | Lactulose, rifaximin, CRRT | ||||||
| Vinegard [30] | 44 | F | 14 | Survived | 268.5 | Normal | 4 | 1.5 | sodium benzoate, N-carbamylglutamate, l-arginine, carnitine, and low-protein TPN, Zinc, thiamine, Vit B, C, E; Hemofiltration | |||||
| Sharma [31] | 38 | F | 16 | Survived | 314 | 1.9 | 35.3 | lactulose, albumin, thiamine, low protein, IV sodium benzoate, nutrient supplementation; HD | ||||||
| Robinson [32] | 52 | M | 7 | Deceased | 176 | 1.9 | 21 | lactulose, rifaximin, zinc, MTV with minerals | ||||||
| Dace [33] | 49 | F | 14 | Survived | 234 | Normal | 2235 | 8 | 1.5 | 4.7 | (BIMDG guidelines) sodium benzoate, L-arginine, rifaximin, lactulose, oral amino acid, vitamins supplementation, insulin, pancreatic enzymes | |||
| Kim [34] | F | 13 | Deceased | 135 | <1.5 | 29.7 | Lactulose, enteral tube-feeding, antibiotics | |||||||
| Summar [35] | 34 | F | 0.7 | Deceased | 442 | Normal | Low | NAGs deficiency found on liver biopsy testing | Dialysis, phenylbutyrate, citrulline | |||||
| Hu [36] | 29 | F | 0.1 | Survived | 92 | 3.2 | 2018 | 17 | 2.1 | 30 | 102 | OTC deficiency on biopsy of liver, <1% enzyme activity. Genetic testing was negative for OTC mutation | Lactulose, parenteral nutrition, carnitine supplementation, | |
| Limketkai [37] | 35 | F | 6 | Survived | 342 | 19 | 1.6 | 39 | No | Thiamin, lactulose, neomycin, hemodialysis, oral levocarnitine | ||||
| Estrella [38] | 52 | F | 0.5 | Survived | 155 | Normal | 448 | 19 | 1.8 | 28 | No | Negative CPS1 and NAGS gene sequencing | Oral metronidazole, cholestyramine, erthyromycin, sodium benzoate, arginine, parenteral nutrition, reversal of RYGB | |
| Rogal [39] | 58 | M | 7 | Survived | 306 | High | 3.8 | No | Closure of splenorenal shunt | |||||
| Singh [40] | 39 | F | 4 | Survived | 477 | 1.4 | 644 | 15 | 2.8 | 16 | Thiamine, lactulose, multivitamin, zinc, L-carnitine, rifaximin, IV arginine, dialysis | |||
| Loeffler [41] | 41 | F | 16 | Survived | 200 | 808.7 | 7.3 | 38 | Yes | Glucose, thiamine, lactulose, rifaximin, zinc | ||||
| Zeghlache [42] | 49 | F | 7 | Survived | 104 | normal | 13 | 1.9 | 38 | 42 | Yes | Lactulose, rifaximin | ||
| Kjaergaard [43] | 46 | F | 11 | Survived | 115 | normal | 1.7 | low | Yes | Parenteral nutrition, vitamin K, lactulose | ||||
| Bonasso [44] | 17 | F | 0.05 | Deceased | 903 | Yes | Hemodialysis | |||||||
| Thusay [45] | 54 | F | 23 | Deceased | 168 | CRRT | ||||||||
| Sadlik [46] | 44 | F | Survived | 305 | low | low | CRRT, lactulose, rifaximin, carnitine, vitamin and nutrient supplementation, parenteral nutrition | |||||||
| Fanous [2] | 65 | F | Deceased | 264 | high | 2254 | low | low | Negative urea cycle disorder genetic panel | Lactulose, rifaximin, dialysis, levocarnitine, sodium phenylbutyrate, zinc | ||||
| Bendrick [47] | 56 | M | 19 | Survived | 123 | Negative urea cycle disorder genetic panel | Low-protein diet, lactulose, TPN | |||||||
| Laoye [48] | 42 | F | 6 | Survived | 192 | 18 | 2.4 | 44 | Intravenous L-carnitine, hemodialysis, lactulose, rifaximin | |||||
| Roth [49] | 65 | F | Survived | 216 | 1.6 | 2254 | 14 | 2.7 | 27 | Negative urea cycle disorder genetic panel | Lactulose, rifaximin, L-carnitine, zinc, multivitamin, IV sodium phenylacetate-sodium benzoate, arginine infusion | |||
| Lloyd [50] | 35 | F | Survived | 100 | 1.79 | Lactulose, rifaximin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fenves, A.Z.; Hatipoglu, D.; Robinson, J.C.; Rothkopf, M.M. Gastric Bypass Associated Hyperammonemia (GaBHA): A Case Study, Scoping Review of the Literature, and Proposed New Pathophysiologic Mechanism. Metabolites 2025, 15, 573. https://doi.org/10.3390/metabo15090573
Fenves AZ, Hatipoglu D, Robinson JC, Rothkopf MM. Gastric Bypass Associated Hyperammonemia (GaBHA): A Case Study, Scoping Review of the Literature, and Proposed New Pathophysiologic Mechanism. Metabolites. 2025; 15(9):573. https://doi.org/10.3390/metabo15090573
Chicago/Turabian StyleFenves, Andrew Z., Dilara Hatipoglu, John C. Robinson, and Michael M. Rothkopf. 2025. "Gastric Bypass Associated Hyperammonemia (GaBHA): A Case Study, Scoping Review of the Literature, and Proposed New Pathophysiologic Mechanism" Metabolites 15, no. 9: 573. https://doi.org/10.3390/metabo15090573
APA StyleFenves, A. Z., Hatipoglu, D., Robinson, J. C., & Rothkopf, M. M. (2025). Gastric Bypass Associated Hyperammonemia (GaBHA): A Case Study, Scoping Review of the Literature, and Proposed New Pathophysiologic Mechanism. Metabolites, 15(9), 573. https://doi.org/10.3390/metabo15090573





