miR-221-3p Exacerbates Obesity-Induced Insulin Resistance by Targeting SOCS1 in Adipocytes
Abstract
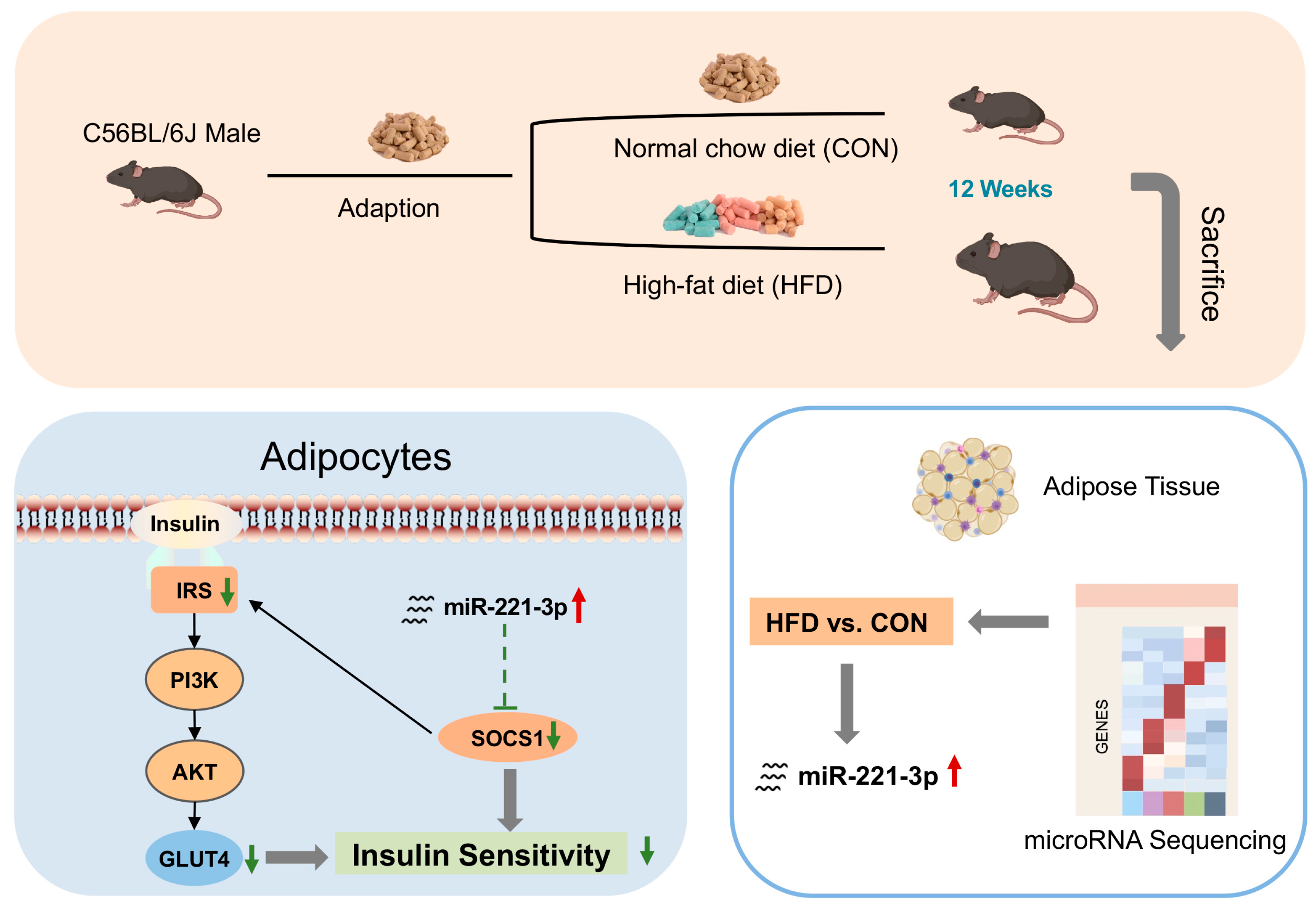
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Glycemic Control
2.3. Histological Analysis
2.4. microRNA Sequencing
2.5. Dual-Luciferase Reporter Assay
2.6. 3T3-L1 Cell Culture and Transfection
2.7. Oil Red O Staining
2.8. Western Blot Analysis
2.9. Quantitative Real-Time Polymerase Chain Reaction Analyses
2.10. Statistical Analysis
3. Results
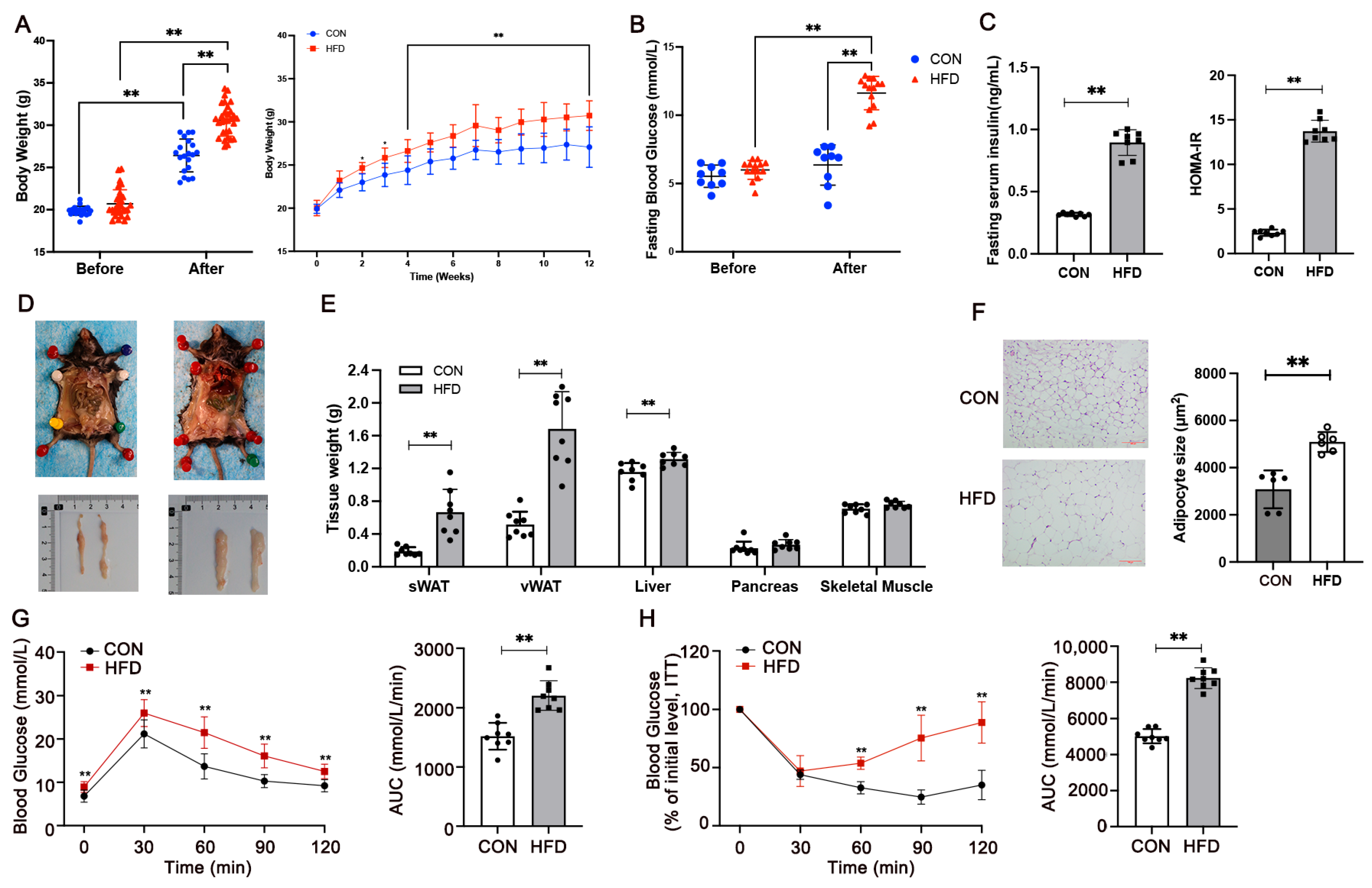
3.1. Mediation of Glucose Metabolism and IR in HFD-Fed Mice
3.2. miR-221-3p Is Upregulated and Is Associated with Insulin Resistance in HFD-Fed Mice
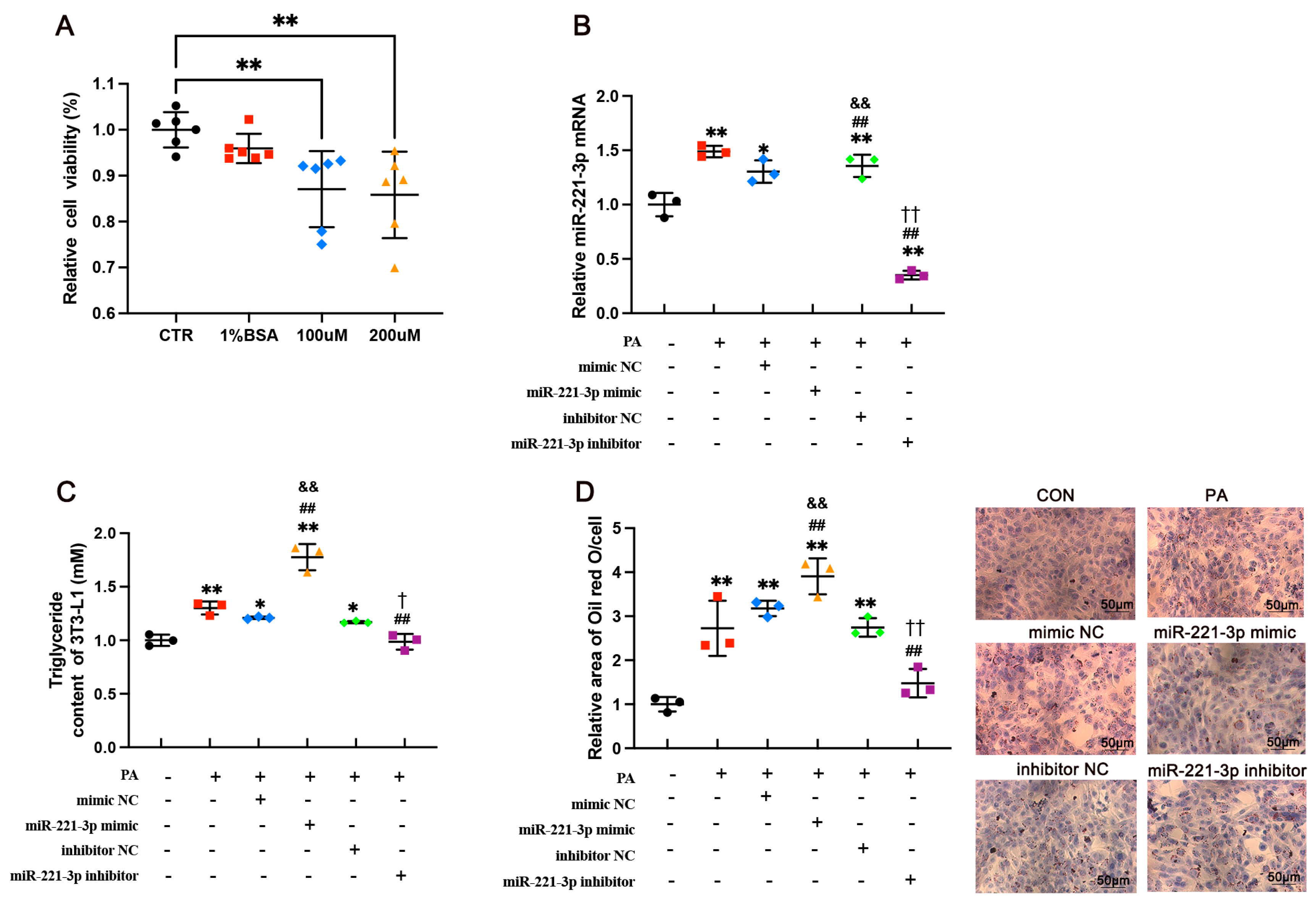
3.3. miR-221-3p Exacerbates Lipid Accumulation in 3T3-L1 Cells
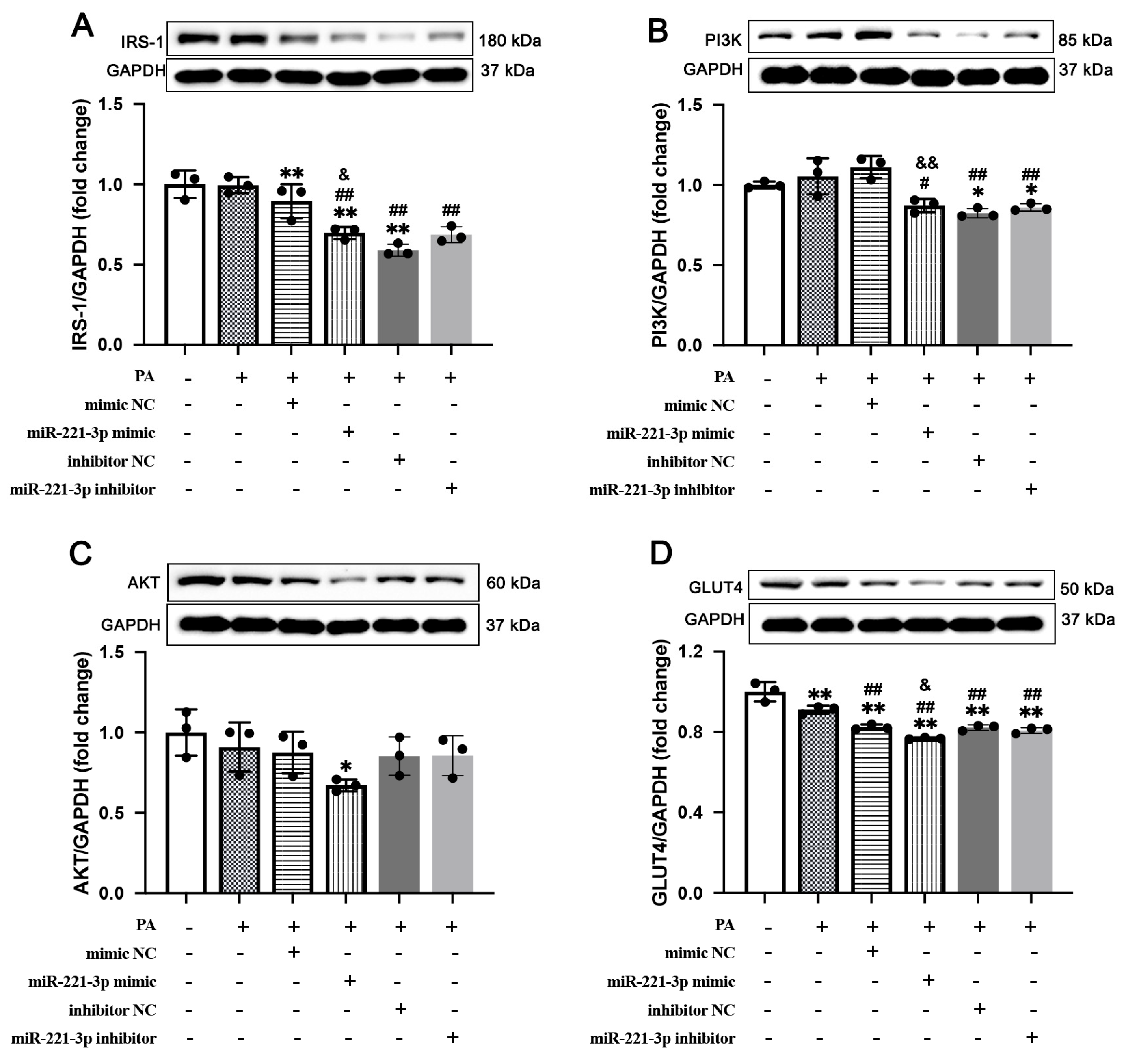
3.4. miR-221-3p Expression Is Associated with GLUT4 Expression and PI3K/AKT Axis Activation in IR Cells
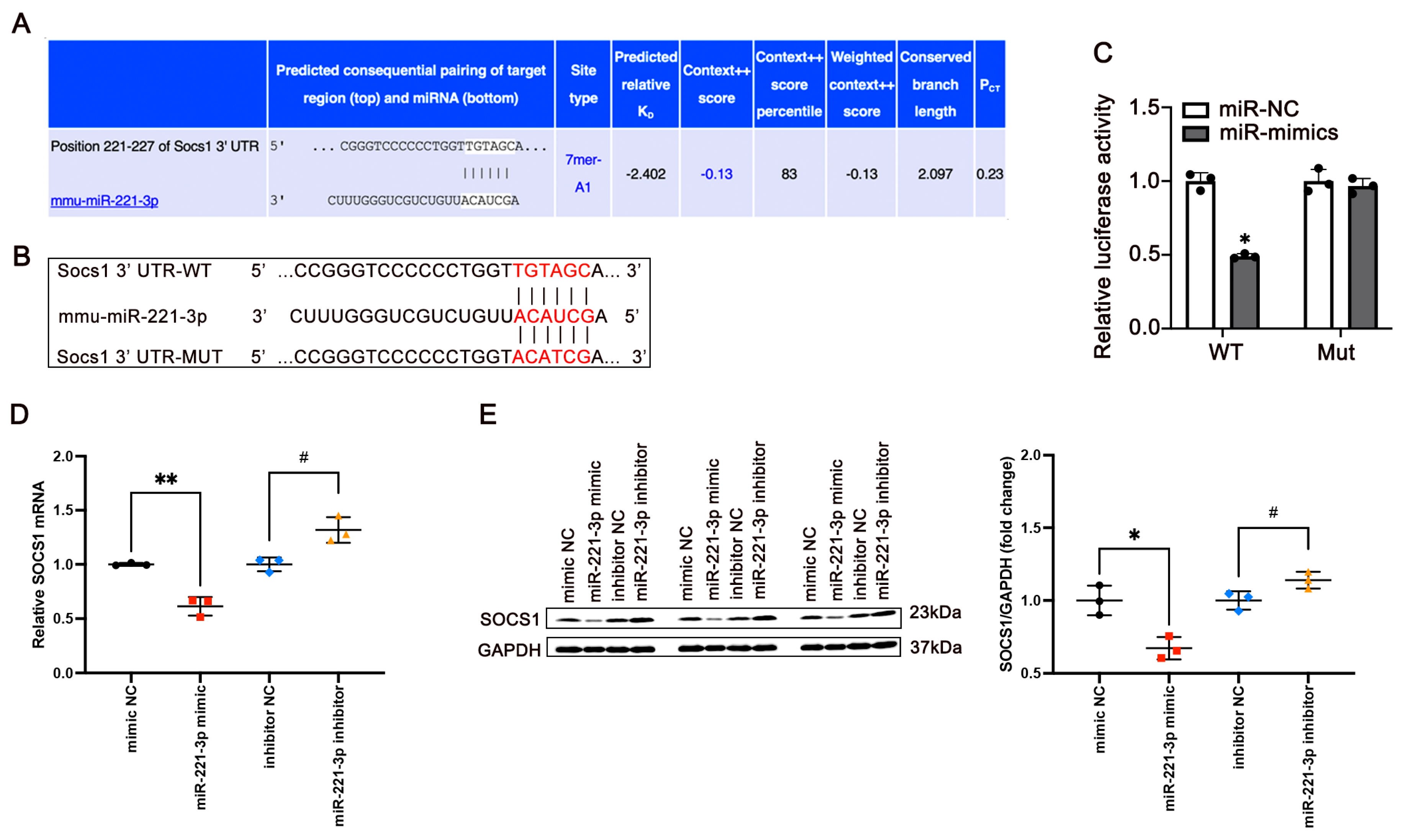
3.5. miR-221-3p Targets Socs1 and Regulates Its Expression
3.6. miR-221-3p Inhibition Protects Against Glucose Metabolism and Insulin Resistance Induced by PA In Vitro by Binding to SOCS1
4. Discussion
5. Limitation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cornejo, P.J.; Vergoni, B.; Ohanna, M.; Angot, B.; Gonzalez, T.; Jager, J.; Tanti, J.F.; Cormont, M. The Stress-Responsive microRNA-34a Alters Insulin Signaling and Actions in Adipocytes through Induction of the Tyrosine Phosphatase PTP1B. Cells 2022, 11, 2581. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Janssen, J. The Causal Role of Ectopic Fat Deposition in the Pathogenesis of Metabolic Syndrome. Int. J. Mol. Sci. 2024, 25, 3238. [Google Scholar] [CrossRef]
- Tilg, H.; Ianiro, G.; Gasbarrini, A.; Adolph, T.E. Adipokines: Masterminds of metabolic inflammation. Nat. Rev. Immunol. 2025, 25, 250–265. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef]
- Kwak, S.H.; Park, K.S. Recent progress in genetic and epigenetic research on type 2 diabetes. Exp. Mol. Med. 2016, 48, e220. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Czech, M.P. The GLUT4 glucose transporter. Cell Metab. 2007, 5, 237–252. [Google Scholar] [CrossRef]
- Onogi, Y.; Khalil, A.; Ussar, S. Identification and characterization of adipose surface epitopes. Biochem. J. 2020, 477, 2509–2541. [Google Scholar] [CrossRef]
- Carvalho, E.; Jansson, P.A.; Nagaev, I.; Wenthzel, A.M.; Smith, U. Insulin resistance with low cellular IRS-1 expression is also associated with low GLUT4 expression and impaired insulin-stimulated glucose transport. FASEB J. 2001, 15, 1101–1103. [Google Scholar] [CrossRef]
- He, Y.; Ding, Y.; Liang, B.; Lin, J.; Kim, T.K.; Yu, H.; Hang, H.; Wang, K. A Systematic Study of Dysregulated MicroRNA in Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2017, 18, 456. [Google Scholar] [CrossRef]
- Pang, B.; Qiao, L.; Wang, S.; Guo, X.; Xie, Y.; Han, L. MiR-214-3p plays a protective role in diabetic neuropathic rats by regulating Nav1.3 and TLR4. Cell Biol. Int. 2021, 45, 2294–2303. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Cho, Y.K.; Kim, H.; Choi, C.; Kim, S.; Lee, Y.H. miR-10a regulates cell death and inflammation in adipose tissue of male mice with diet-induced obesity. Mol. Metab. 2024, 90, 102039. [Google Scholar] [CrossRef] [PubMed]
- Paneru, B.D.; Chini, J.; McCright, S.J.; DeMarco, N.; Miller, J.; Joannas, L.D.; Henao-Mejia, J.; Titchenell, P.M.; Merrick, D.M.; Lim, H.W.; et al. Myeloid-derived miR-6236 potentiates adipocyte insulin signaling and prevents hyperglycemia during obesity. Nat. Commun. 2024, 15, 5394. [Google Scholar] [CrossRef]
- Song, J.; Ouyang, Y.; Che, J.; Li, X.; Zhao, Y.; Yang, K.; Zhao, X.; Chen, Y.; Fan, C.; Yuan, W. Potential Value of miR-221/222 as Diagnostic, Prognostic, and Therapeutic Biomarkers for Diseases. Front. Immunol. 2017, 8, 56. [Google Scholar] [CrossRef]
- Cheng, X.; Zhou, H.; Zhou, Y.; Song, C. M2 Macrophage-Derived Exosomes Inhibit Apoptosis of HUVEC Cell through Regulating miR-221-3p Expression. Biomed. Res. Int. 2022, 2022, 1609244. [Google Scholar] [CrossRef]
- Quero, L.; Tiaden, A.N.; Hanser, E.; Roux, J.; Laski, A.; Hall, J.; Kyburz, D. miR-221-3p Drives the Shift of M2-Macrophages to a Pro-Inflammatory Function by Suppressing JAK3/STAT3 Activation. Front. Immunol. 2019, 10, 3087. [Google Scholar] [CrossRef] [PubMed]
- Gentile, A.M.; Lhamyani, S.; Mengual-Mesa, M.; Garcia-Fuentes, E.; Bermudez-Silva, F.J.; Rojo-Martinez, G.; Clemente-Postigo, M.; Rodriguez-Canete, A.; Olveira, G.; El Bekay, R. MiR-221-3p/222-3p Cluster Expression in Human Adipose Tissue Is Related to Obesity and Type 2 Diabetes. Int. J. Mol. Sci. 2023, 24, 17449. [Google Scholar] [CrossRef]
- Ahonen, M.A.; Asghar, M.Y.; Parviainen, S.J.; Liebisch, G.; Horing, M.; Leidenius, M.; Fischer-Posovszky, P.; Wabitsch, M.; Mikkola, T.S.; Tornquist, K.; et al. Human adipocyte differentiation and composition of disease-relevant lipids are regulated by miR-221-3p. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158841. [Google Scholar] [CrossRef]
- Cai, M.; Shi, Y.; Zheng, T.; Hu, S.; Du, K.; Ren, A.; Jia, X.; Chen, S.; Wang, J.; Lai, S. Mammary epithelial cell derived exosomal MiR-221 mediates M1 macrophage polarization via SOCS1/STATs to promote inflammatory response. Int. Immunopharmacol. 2020, 83, 106493. [Google Scholar] [CrossRef]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008, 2008, pdb.prot4986. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef]
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal. Biochem. 2012, 425, 88–90. [Google Scholar] [CrossRef]
- Boden, G. Fatty acid-induced inflammation and insulin resistance in skeletal muscle and liver. Curr. Diab. Rep. 2006, 6, 177–181. [Google Scholar] [CrossRef]
- Speakman, J.R. Use of high-fat diets to study rodent obesity as a model of human obesity. Int. J. Obes. 2019, 43, 1491–1492. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, F.; Alexiu, A.; Lemonnier, D. Dietary-induced obesity: Effect of dietary fats on adipose tissue cellularity in mice. Br. J. Nutr. 1983, 49, 17–26. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Schwab, U.S. Relationship of dietary fat to glucose metabolism. Atherosclerosis 2000, 150, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Binwal, M.; Babu, V.; Israr, K.M.M.; Kashyap, P.K.; Maurya, A.K.; Padalia, R.C.; Tandon, S.; Bawankule, D.U. Taxoids-rich extract from Taxus wallichiana alleviates high-fat diet-induced insulin resistance in C57BL/6 mice through inhibition of low-grade inflammation. Inflammopharmacology 2023, 31, 451–464. [Google Scholar] [CrossRef]
- Kabeer, S.W.; Pant, R.; Sharma, S.; Tikoo, K. Laccaic acid restores epigenetic alterations responsible for high fat diet induced insulin resistance in C57BL/6J mice. Chem.-Biol. Interact. 2023, 374, 110401. [Google Scholar] [CrossRef]
- Li, Q.M.; Zha, X.Q.; Zhang, W.N.; Liu, J.; Pan, L.H.; Luo, J.P. Laminaria japonica polysaccharide prevents high-fat-diet-induced insulin resistance in mice via regulating gut microbiota. Food Funct. 2021, 12, 5260–5273. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tan, J.; Zhang, H.; Hou, D.X.; He, J. Tissue-specific mechanisms of fat metabolism that focus on insulin actions. J. Adv. Res. 2023, 53, 187–198. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, Z.; Lin, Q.; Luo, Q.; Cen, Y.; Li, J.; Fang, X.; Gong, C. MiRNAs and Cancer: Key Link in Diagnosis and Therapy. Genes 2021, 12, 1289. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ma, D.; Zhao, W.; Wang, D.; Liu, T.; Liu, Y.; Yang, Y.; Liu, Y.; Mu, J.; Li, B.; et al. Obesity-induced overexpression of miR-802 impairs insulin transcription and secretion. Nat. Commun. 2020, 11, 1822. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Yu, Y.; Feng, L.; Li, J.; Zhang, M.; Lan, X.; Yan, X.; Liu, Y.; Guan, F.; Zhang, M.; et al. Adipogenic miR-27a in adipose tissue upregulates macrophage activation via inhibiting PPARgamma of insulin resistance induced by high-fat diet-associated obesity. Exp. Cell Res. 2017, 355, 105–112. [Google Scholar] [CrossRef]
- Li, Y.; Yan, C.; Fan, J.; Hou, Z.; Han, Y. MiR-221-3p targets Hif-1alpha to inhibit angiogenesis in heart failure. Lab. Investig. 2021, 101, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Zhang, D.; Katayama, A.; Kurooka, N.; Sugawara, R.; Albuayjan, H.H.H.; Nakatsuka, A.; Eguchi, J.; Wada, J. Adipocyte-Specific Inhibition of Mir221/222 Ameliorates Diet-Induced Obesity Through Targeting Ddit4. Front. Endocrinol. 2021, 12, 750261. [Google Scholar] [CrossRef]
- Li, N.; Zhang, L.; Guo, Q.; Shi, H.; Gan, Y.; Wang, W.; Yang, X.; Zhou, Y. Aerobic exercise improves inflammation and insulin resistance in skeletal muscle by regulating miR-221-3p via JAK/STAT signaling pathway. Front. Physiol. 2025, 16, 1534911. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, M.; Zhou, Q.; Cheng, Y.; Che, L.; Xu, J.; Xiao, J.; Shen, Z.; Bei, Y. Dynamic Regulation of Circulating microRNAs During Acute Exercise and Long-Term Exercise Training in Basketball Athletes. Front. Physiol. 2018, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Pairet, S.; Alvarez-Larran, A.; Pons, A.; Ferrer, G.; Longaron, R.; Fernandez-Rodriguez, C.; Camacho, L.; Monzo, M.; Besses, C.; et al. miR-203 and miR-221 regulate SOCS1 and SOCS3 in essential thrombocythemia. Blood Cancer J. 2016, 6, e406. [Google Scholar] [CrossRef]
- Liau, N.P.D.; Laktyushin, A.; Lucet, I.S.; Murphy, J.M.; Yao, S.; Whitlock, E.; Callaghan, K.; Nicola, N.A.; Kershaw, N.J.; Babon, J.J. The molecular basis of JAK/STAT inhibition by SOCS1. Nat. Commun. 2018, 9, 1558. [Google Scholar] [CrossRef]
- He, J.; Xie, J.; Zhou, G.; Jia, C.; Han, D.; Li, D.; Wei, J.; Li, Y.; Huang, R.; Li, C.; et al. Active Fraction of Polyrhachis Vicina Roger (AFPR) Ameliorate Depression Induced Inflammation Response by FTO/miR-221-3p/SOCS1 Axis. J. Inflamm. Res. 2023, 16, 6329–6348. [Google Scholar] [CrossRef]
- Galic, S.; Sachithanandan, N.; Kay, T.W.; Steinberg, G.R. Suppressor of cytokine signalling (SOCS) proteins as guardians of inflammatory responses critical for regulating insulin sensitivity. Biochem. J. 2014, 461, 177–188. [Google Scholar] [CrossRef]
- Gurzov, E.N.; Stanley, W.J.; Pappas, E.G.; Thomas, H.E.; Gough, D.J. The JAK/STAT pathway in obesity and diabetes. FEBS J. 2016, 283, 3002–3015. [Google Scholar] [CrossRef] [PubMed]
- Rui, L.; Yuan, M.; Frantz, D.; Shoelson, S.; White, M.F. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J. Biol. Chem. 2002, 277, 42394–42398. [Google Scholar] [CrossRef]
- Jamieson, E.; Chong, M.M.; Steinberg, G.R.; Jovanovska, V.; Fam, B.C.; Bullen, D.V.; Chen, Y.; Kemp, B.E.; Proietto, J.; Kay, T.W.; et al. Socs1 deficiency enhances hepatic insulin signaling. J. Biol. Chem. 2005, 280, 31516–31521. [Google Scholar] [CrossRef]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef]
- Huang, F.; Chen, J.; Wang, J.; Zhu, P.; Lin, W. Palmitic Acid Induces MicroRNA-221 Expression to Decrease Glucose Uptake in HepG2 Cells via the PI3K/AKT/GLUT4 Pathway. Biomed Res. Int. 2019, 2019, 8171989. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Jayaraman, S.; Natarajan, S.R.; Veeraraghavan, V.P.; Krishnamoorthy, R.; Gatasheh, M.K.; Palanisamy, C.P.; Elrobh, M. Piperine modulates IR/Akt/GLUT4 pathways to mitigate insulin resistance: Evidence from animal and computational studies. Int. J. Biol. Macromol. 2023, 253 Pt 5, 127242. [Google Scholar] [CrossRef]
- Li, D.; Song, H.; Shuo, L.; Wang, L.; Xie, P.; Li, W.; Liu, J.; Tong, Y.; Zhang, C.Y.; Jiang, X.; et al. Gonadal white adipose tissue-derived exosomal MiR-222 promotes obesity-associated insulin resistance. Aging 2020, 12, 22719–22743. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primer Sequences (5′-3′) | |
|---|---|---|
| Socs1 | F | CCAACGGAACTGCTTCTTCG |
| R | AAAGGCAGTCGAAGGTCTCG | |
| β-actin | F | GTGCTATGTTGCTCTAGACTTCG |
| R | ATGCCACAGGATTCCATACC | |
| miR-221-3p | RT | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGAAACCCA |
| F | ACACTCCAGCTGGG AGCTACATTGTCTGC | |
| miR-183-5p | RT | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAGTGAATT |
| F | ACACTCCAGCTGGGTATGGCACTGGTAG | |
| miR-219a-5p | RT | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAG AGAATTG |
| F | CACTCCAGCTGGG TGATTGTCCAAACG | |
| miR-741-3p | RT | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAG TCTACATA |
| F | ACACTCCAGCTGGG TGAGAGATGCCATTC | |
| miR-96-5p | RT | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAGCAAAAAT |
| F | ACACTCCAGCTGGG TTTGGCACTAGCAC | |
| All R | TGGTGTCGTGGAGTCG | |
| U6 | F | CTCGCTTCGGCAGCACA |
| R | AACGCTTCACGAATTTGCGT | |
| miRNA | Log FC | HS-NS |
|---|---|---|
| mmu-miR-183-5p | 1.13 | up |
| mmu-miR-219a-5p | −1.50 | down |
| mmu-miR-221-3p | 3.40 | up |
| mmu-miR-741-3p | 2.84 | up |
| mmu-miR-96-5p | 2.34 | up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Zhang, L.; Guo, Q.; Yang, X.; Liu, C.; Zhou, Y. miR-221-3p Exacerbates Obesity-Induced Insulin Resistance by Targeting SOCS1 in Adipocytes. Metabolites 2025, 15, 572. https://doi.org/10.3390/metabo15090572
Li N, Zhang L, Guo Q, Yang X, Liu C, Zhou Y. miR-221-3p Exacerbates Obesity-Induced Insulin Resistance by Targeting SOCS1 in Adipocytes. Metabolites. 2025; 15(9):572. https://doi.org/10.3390/metabo15090572
Chicago/Turabian StyleLi, Nan, Liang Zhang, Qiaofeng Guo, Xiaoying Yang, Changjiang Liu, and Yue Zhou. 2025. "miR-221-3p Exacerbates Obesity-Induced Insulin Resistance by Targeting SOCS1 in Adipocytes" Metabolites 15, no. 9: 572. https://doi.org/10.3390/metabo15090572
APA StyleLi, N., Zhang, L., Guo, Q., Yang, X., Liu, C., & Zhou, Y. (2025). miR-221-3p Exacerbates Obesity-Induced Insulin Resistance by Targeting SOCS1 in Adipocytes. Metabolites, 15(9), 572. https://doi.org/10.3390/metabo15090572






