Predictive Utility and Metabolomic Signatures of TG/HDL-C Ratio for Metabolic Syndrome Without Cardiovascular Disease and/or Diabetes in Qatari Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Study Population
2.2. Definition of Metabolic Syndrome
2.3. Lipid Ratio Calculation
2.4. Biochemical Measurements
2.5. Metabolomic Profiling
2.6. Statistical Analysis
3. Results
3.1. General Characteristics of the Study Population
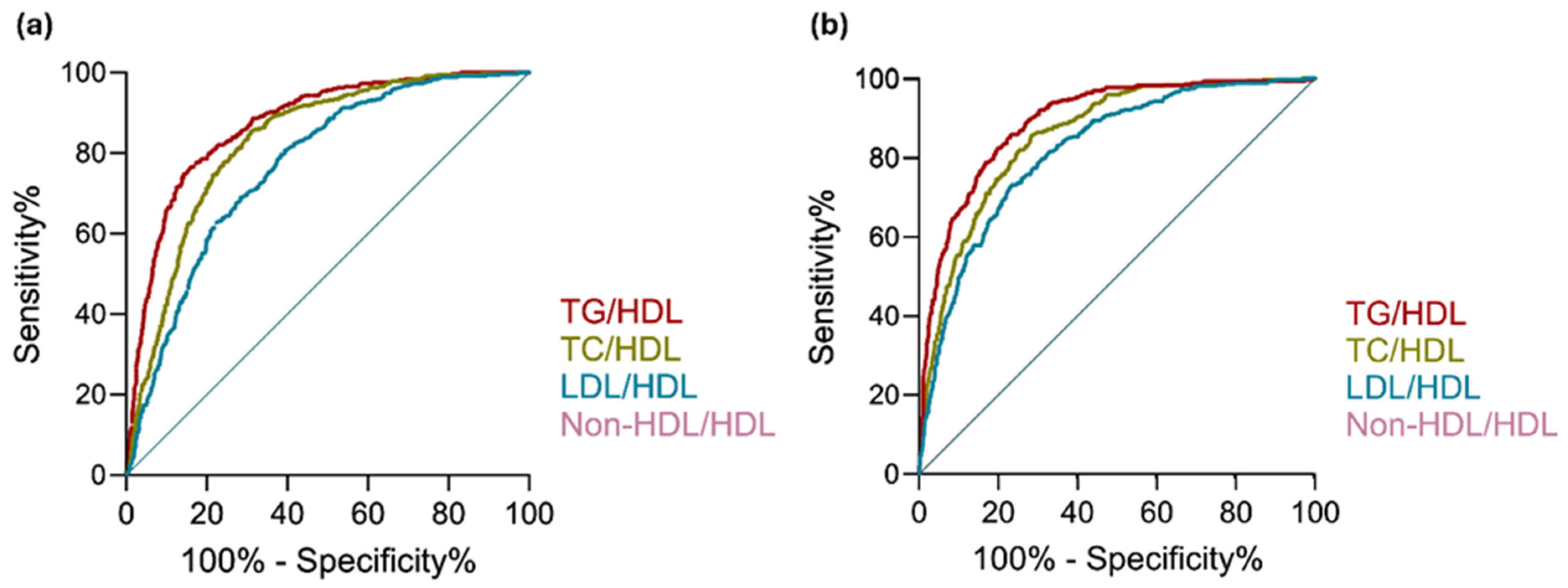
3.2. ROC Curve Analysis
3.3. Multivariate Metabolomics Analysis
3.4. Univariate Metabolite Associations
3.5. Functional Enrichment Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peterseim, C.M.; Jabbour, K.; Kamath Mulki, A. Metabolic Syndrome: An Updated Review on Diagnosis and Treatment for Primary Care Clinicians. J. Prim. Care Community Health 2024, 15, 21501319241309168. [Google Scholar] [CrossRef]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef]
- Lee, M.-K.; Han, K.; Kim, M.K.; Koh, E.S.; Kim, E.S.; Nam, G.E.; Kwon, H.-S. Changes in metabolic syndrome and its components and the risk of type 2 diabetes: A nationwide cohort study. Sci. Rep. 2020, 10, 2313. [Google Scholar] [CrossRef]
- Kurl, S.; Laukkanen, J.A.; Niskanen, L.; Laaksonen, D.; Sivenius, J.; Nyyssönen, K.; Salonen, J.T. Metabolic syndrome and the risk of stroke in middle-aged men. Stroke 2006, 37, 806–811. [Google Scholar] [CrossRef]
- Grundy, S.M.; Hansen, B.; Smith, S.C., Jr.; Cleeman, J.I.; Kahn, R.A.; American Heart, A.; National Heart, L.; Blood, I.; American Diabetes, A. Clinical management of metabolic syndrome: Report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Arterioscler. Thromb. Vasc. Biol. 2004, 24, e19–e24. [Google Scholar] [CrossRef]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Tylutka, A.; Morawin, B.; Walas, Ł.; Michałek, M.; Gwara, A.; Zembron-Lacny, A. Assessment of metabolic syndrome predictors in relation to inflammation and visceral fat tissue in older adults. Sci. Rep. 2023, 13, 89. [Google Scholar] [CrossRef]
- Bhalwar, R. Metabolic syndrome: The Indian public health perspective. Med. J. Armed Forces India 2020, 76, 8–16. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Kraja, A.T.; Rao, D.C.; Weder, A.B.; Mosley, T.H.; Turner, S.T.; Hsiung, C.A.; Quertermous, T.; Cooper, R.; Curb, J.D.; Province, M.A. An evaluation of the metabolic syndrome in a large multi-ethnic study: The Family Blood Pressure Program. Nutr. Metab. 2005, 2, 17. [Google Scholar] [CrossRef][Green Version]
- Krishnadath, I.S.K.; Toelsie, J.R.; Hofman, A.; Jaddoe, V.W.V. Ethnic disparities in the prevalence of metabolic syndrome and its risk factors in the Suriname Health Study: A cross-sectional population study. BMJ Open 2016, 6, e013183. [Google Scholar] [CrossRef]
- Azizi, F.; Hadaegh, F.; Hosseinpanah, F.; Mirmiran, P.; Amouzegar, A.; Abdi, H.; Asghari, G.; Parizadeh, D.; Montazeri, S.A.; Lotfaliany, M.; et al. Metabolic health in the Middle East and north Africa. Lancet Diabetes Endocrinol. 2019, 7, 866–879. [Google Scholar] [CrossRef]
- Alcover, S.; Ramos-Regalado, L.; Giron, G.; Munoz-Garcia, N.; Vilahur, G. HDL-Cholesterol and Triglycerides Dynamics: Essential Players in Metabolic Syndrome. Antioxidants 2025, 14, 434. [Google Scholar] [CrossRef]
- Gasevic, D.; Frohlich, J.; Mancini, G.B.J.; Lear, S.A. Clinical usefulness of lipid ratios to identify men and women with metabolic syndrome: A cross-sectional study. Lipids Health Dis. 2014, 13, 159. [Google Scholar] [CrossRef]
- Cordero, A.; Laclaustra, M.; León, M.; Casasnovas, J.A.; Grima, A.; Luengo, E.; Ordoñez, B.; Bergua, C.; Bes, M.; Pascual, I.; et al. Comparison of serum lipid values in subjects with and without the metabolic syndrome. Am. J. Cardiol. 2008, 102, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Rezapour, M.; Shahesmaeili, A.; Hossinzadeh, A.; Zahedi, R.; Najafipour, H.; Gozashti, M.H. Comparison of Lipid Ratios to Identify Metabolic Syndrome. Arch. Iran. Med. 2018, 21, 572–577. [Google Scholar]
- Wishart, D.S. Emerging applications of metabolomics in drug discovery and precision medicine. Nat. Rev. Drug Discov. 2016, 15, 473–484. [Google Scholar] [CrossRef]
- Lent-Schochet, D.; McLaughlin, M.; Ramakrishnan, N.; Jialal, I. Exploratory metabolomics of metabolic syndrome: A status report. World J. Diabetes 2019, 10, 23–36. [Google Scholar] [CrossRef]
- Surowiec, I.; Noordam, R.; Bennett, K.; Beekman, M.; Slagboom, P.E.; Lundstedt, T.; van Heemst, D. Metabolomic and lipidomic assessment of the metabolic syndrome in Dutch middle-aged individuals reveals novel biological signatures separating health and disease. Metabolomics 2019, 15, 23. [Google Scholar] [CrossRef]
- Hornburg, D.; Wu, S.; Moqri, M.; Zhou, X.; Contrepois, K.; Bararpour, N.; Traber, G.M.; Su, B.; Metwally, A.A.; Avina, M.; et al. Dynamic lipidome alterations associated with human health, disease and ageing. Nat. Metab. 2023, 5, 1578–1594. [Google Scholar] [CrossRef]
- Serna, M.F.; Suarez-Ortegón, M.F.; Jiménez-Charris, E.; Echeverri, I.; Cala, M.P.; Mosquera, M. Lipidomic signatures in Colombian adults with metabolic syndrome. J. Diabetes Metab. Disord. 2024, 23, 1279–1292. [Google Scholar] [CrossRef]
- Yin, X.; Willinger, C.M.; Keefe, J.; Liu, J.; Fernandez-Ortiz, A.; Ibanez, B.; Penalvo, J.; Adourian, A.; Chen, G.; Corella, D.; et al. Lipidomic profiling identifies signatures of metabolic risk. EBioMedicine 2020, 51, 102520. [Google Scholar] [CrossRef]
- Al Thani, A.; Fthenou, E.; Paparrodopoulos, S.; Al Marri, A.; Shi, Z.; Qafoud, F.; Afifi, N. Qatar Biobank Cohort Study: Study Design and First Results. Am. J. Epidemiol. 2019, 188, 1420–1433. [Google Scholar] [CrossRef]
- Gandhi, G.D.; Aamer, W.; Krishnamoorthy, N.; Syed, N.; Aliyev, E.; Al-Maraghi, A.; Kohailan, M.; Alenbawi, J.; Elanbari, M.; Mifsud, B.; et al. Assessing the genetic burden of familial hypercholesterolemia in a large middle eastern biobank. J. Transl. Med. 2022, 20, 502. [Google Scholar] [CrossRef]
- Ismail Umlai, U.K.; Toor, S.M.; Al-Sarraj, Y.A.; Mohammed, S.; Al Hail, M.S.H.; Ullah, E.; Kunji, K.; El-Menyar, A.; Gomaa, M.; Jayyousi, A.; et al. A multi-ancestry genome-wide association study and evaluation of polygenic scores of LDL-C levels. J. Lipid Res. 2025, 66, 100752. [Google Scholar] [CrossRef]
- Mousa, H.; Islam, N.; Ganji, V.; Zughaier, S.M. Serum 25-Hydroxyvitamin D Is Inversely Associated with Monocyte Percentage to HDL Cholesterol Ratio among Young Healthy Adults in Qatar. Nutrients 2020, 13, 127. [Google Scholar] [CrossRef]
- Ullah, E.; Mall, R.; Rawi, R.; Moustaid-Moussa, N.; Butt, A.A.; Bensmail, H. Harnessing Qatar Biobank to understand type 2 diabetes and obesity in adult Qataris from the First Qatar Biobank Project. J. Transl. Med. 2018, 16, 99. [Google Scholar] [CrossRef]
- Al-Khelaifi, F.; Diboun, I.; Donati, F.; Botrè, F.; Alsayrafi, M.; Georgakopoulos, C.; Suhre, K.; Yousri, N.A.; Elrayess, M.A. A pilot study comparing the metabolic profiles of elite-level athletes from different sporting disciplines. Sports Med. Open 2018, 4, 2. [Google Scholar] [CrossRef]
- Bridgewater Br, E.A.M. High Resolution Mass Spectrometry Improves Data Quantity and Quality as Compared to Unit Mass Resolution Mass Spectrometry in High-Throughput Profiling Metabolomics. J. Postgenom. Drug Biomark. Dev. 2014, 4, 2. [Google Scholar] [CrossRef]
- Nie, G.; Hou, S.; Zhang, M.; Peng, W. High TG/HDL ratio suggests a higher risk of metabolic syndrome among an elderly Chinese population: A cross-sectional study. BMJ Open 2021, 11, e041519. [Google Scholar] [CrossRef]
- Saeedi, F.; Baqeri, E.; Bidokhti, A.; Moodi, M.; Sharifi, F.; Riahi, S.M. Clinical utility of lipid ratios as potential predictors of metabolic syndrome among the elderly population: Birjand Longitudinal Aging Study (BLAS). BMC Geriatr. 2023, 23, 403. [Google Scholar] [CrossRef]
- Yuan, J.; He, X.; Lu, Y.; Pu, X.; Liu, L.; Zhang, X.; Liao, J.; Li, G.; Luo, Y.; Zhang, T. Triglycerides/high-density lipoprotein-cholesterol ratio outperforms traditional lipid indicators in predicting metabolic dysfunction-associated steatotic liver disease among U.S. adults. Front. Endocrinol. 2025, 16, 1591241. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Rodriguez Polanco, S.; Bousvarou, M.D.; Papakonstantinou, E.J.; Peña Genao, E.; Guzman, E.; Kostara, C.E. The Triglyceride/High-Density Lipoprotein Cholesterol (TG/HDL-C) Ratio as a Risk Marker for Metabolic Syndrome and Cardiovascular Disease. Diagnostics 2023, 13, 929. [Google Scholar] [CrossRef]
- Szendroedi, J.; Yoshimura, T.; Phielix, E.; Koliaki, C.; Marcucci, M.; Zhang, D.; Jelenik, T.; Muller, J.; Herder, C.; Nowotny, P.; et al. Role of diacylglycerol activation of PKCtheta in lipid-induced muscle insulin resistance in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 9597–9602. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Roles of Diacylglycerols and Ceramides in Hepatic Insulin Resistance. Trends Pharmacol. Sci. 2017, 38, 649–665. [Google Scholar] [CrossRef]
- Jornayvaz, F.R.; Shulman, G.I. Diacylglycerol activation of protein kinase Cepsilon and hepatic insulin resistance. Cell Metab. 2012, 15, 574–584. [Google Scholar] [CrossRef]
- Tonks, K.T.; Coster, A.C.; Christopher, M.J.; Chaudhuri, R.; Xu, A.; Gagnon-Bartsch, J.; Chisholm, D.J.; James, D.E.; Meikle, P.J.; Greenfield, J.R.; et al. Skeletal muscle and plasma lipidomic signatures of insulin resistance and overweight/obesity in humans. Obesity 2016, 24, 908–916. [Google Scholar] [CrossRef]
- Calzada, E.; Onguka, O.; Claypool, S.M. Phosphatidylethanolamine Metabolism in Health and Disease. Int. Rev. Cell Mol. Biol. 2016, 321, 29–88. [Google Scholar] [CrossRef]
- Dawaliby, R.; Trubbia, C.; Delporte, C.; Noyon, C.; Ruysschaert, J.M.; Van Antwerpen, P.; Govaerts, C. Phosphatidylethanolamine Is a Key Regulator of Membrane Fluidity in Eukaryotic Cells. J. Biol. Chem. 2016, 291, 3658–3667. [Google Scholar] [CrossRef]
- Naja, K.; Anwardeen, N.; Albagha, O.; Elrayess, M.A. Lipid Subclasses Differentiate Insulin Resistance by Triglyceride-Glucose Index. Metabolites 2025, 15, 342. [Google Scholar] [CrossRef]
- Al-Sulaiti, H.; Anwardeen, N.; Bashraheel, S.S.; Naja, K.; Elrayess, M.A. Alterations in Choline Metabolism in Non-Obese Individuals with Insulin Resistance and Type 2 Diabetes Mellitus. Metabolites 2024, 14, 457. [Google Scholar] [CrossRef]
- Grapentine, S.; Singh, R.K.; Bakovic, M. Skeletal Muscle Consequences of Phosphatidylethanolamine Synthesis Deficiency. Function 2023, 4, zqad020. [Google Scholar] [CrossRef]
- Pani, A.; Giossi, R.; Menichelli, D.; Fittipaldo, V.A.; Agnelli, F.; Inglese, E.; Romandini, A.; Roncato, R.; Pintaudi, B.; Del Sole, F.; et al. Inositol and Non-Alcoholic Fatty Liver Disease: A Systematic Review on Deficiencies and Supplementation. Nutrients 2020, 12, 3379. [Google Scholar] [CrossRef]
- Shirouchi, B.; Nagao, K.; Inoue, N.; Furuya, K.; Koga, S.; Matsumoto, H.; Yanagita, T. Dietary phosphatidylinositol prevents the development of nonalcoholic fatty liver disease in Zucker (fa/fa) rats. J. Agric. Food Chem. 2008, 56, 2375–2379. [Google Scholar] [CrossRef]
- Shimizu, K.; Ida, T.; Tsutsui, H.; Asai, T.; Otsubo, K.; Oku, N. Anti-obesity effect of phosphatidylinositol on diet-induced obesity in mice. J. Agric. Food Chem. 2010, 58, 11218–11225. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, Y.; Li, M.; Han, Y.; Liu, L. Unraveling the causal pathway between phosphatidylinositol, metabolites, and metabolic syndrome: A Mendelian randomization study. Diabetol. Metab. Syndr. 2025, 17, 162. [Google Scholar] [CrossRef]
- Pan, W.; Yu, J.; Shi, R.; Yan, L.; Yang, T.; Li, Y.; Zhang, Z.; Yu, G.; Bai, Y.; Schuchman, E.H.; et al. Elevation of ceramide and activation of secretory acid sphingomyelinase in patients with acute coronary syndromes. Coron. Artery Dis. 2014, 25, 230–235. [Google Scholar] [CrossRef]
- Jiang, X.C.; Paultre, F.; Pearson, T.A.; Reed, R.G.; Francis, C.K.; Lin, M.; Berglund, L.; Tall, A.R. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2614–2618. [Google Scholar] [CrossRef]
- Hanamatsu, H.; Ohnishi, S.; Sakai, S.; Yuyama, K.; Mitsutake, S.; Takeda, H.; Hashino, S.; Igarashi, Y. Altered levels of serum sphingomyelin and ceramide containing distinct acyl chains in young obese adults. Nutr. Diabetes 2014, 4, e141. [Google Scholar] [CrossRef]
- Samad, F.; Hester, K.D.; Yang, G.; Hannun, Y.A.; Bielawski, J. Altered Adipose and Plasma Sphingolipid Metabolism in Obesity: A Potential Mechanism for Cardiovascular and Metabolic Risk. Diabetes 2006, 55, 2579–2587. [Google Scholar] [CrossRef]
- Bergman, B.C.; Brozinick, J.T.; Strauss, A.; Bacon, S.; Kerege, A.; Bui, H.H.; Sanders, P.; Siddall, P.; Kuo, M.S.; Perreault, L. Serum sphingolipids: Relationships to insulin sensitivity and changes with exercise in humans. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E398–E408. [Google Scholar] [CrossRef]
- Bozelli, J.C., Jr.; Azher, S.; Epand, R.M. Plasmalogens and Chronic Inflammatory Diseases. Front. Physiol. 2021, 12, 730829. [Google Scholar] [CrossRef]
- Dorninger, F.; Werner, E.R.; Berger, J.; Watschinger, K. Regulation of plasmalogen metabolism and traffic in mammals: The fog begins to lift. Front. Cell Dev. Biol. 2022, 10, 946393. [Google Scholar] [CrossRef]
- Lee, Y.S.; Olefsky, J. Chronic tissue inflammation and metabolic disease. Genes. Dev. 2021, 35, 307–328. [Google Scholar] [CrossRef]
- Sutherland, J.P.; McKinley, B.; Eckel, R.H. The metabolic syndrome and inflammation. Metab. Syndr. Relat. Disord. 2004, 2, 82–104. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Beyene, H.B.; Huynh, K.; Wang, T.; Paul, S.; Cinel, M.; Mellett, N.A.; Olshansky, G.; Meikle, T.G.; Watts, G.F.; Hung, J.; et al. Development and validation of a plasmalogen score as an independent modifiable marker of metabolic health: Population based observational studies and a placebo-controlled cross-over study. EBioMedicine 2024, 105, 105187. [Google Scholar] [CrossRef]
- Razquin, C.; Toledo, E.; Clish, C.B.; Ruiz-Canela, M.; Dennis, C.; Corella, D.; Papandreou, C.; Ros, E.; Estruch, R.; Guasch-Ferré, M.; et al. Plasma Lipidomic Profiling and Risk of Type 2 Diabetes in the PREDIMED Trial. Diabetes Care 2018, 41, 2617–2624. [Google Scholar] [CrossRef]
- Rosca, C.I.; Lighezan, D.F.; Nisulescu, D.-D.; Sharma, A.; Neagu, M.N.; Nistor, D.; Georgescu, D.; Kundnani, N.R. Metabolic Syndrome: A Strange Companion of Atrial Fibrillation; A Blessing in Disguise from the Neuropsychiatric Point of View. Biomedicines 2023, 11, 2012. [Google Scholar] [CrossRef]
- Koutsonida, M.; Markozannes, G.; Bouras, E.; Aretouli, E.; Tsilidis, K.K. Metabolic syndrome and cognition: A systematic review across cognitive domains and a bibliometric analysis. Front. Psychol. 2022, 13, 981379. [Google Scholar] [CrossRef]

| MetS-Negative (n = 1811) | MetS-Positive (n = 368) | p-Value | ||
|---|---|---|---|---|
| General characteristics | Gender | |||
| Male | 870 (48%) | 225 (61%) | <0.001 | |
| Female | 941 (52%) | 143 (39%) | ||
| Age | 33 (27–43) | 42 (34–50) | <0.001 | |
| SBP (mmHg) | 109 (101–118) | 120.5 (111–133) | <0.001 | |
| DBP (mmHg) | 71 (65–77) | 79 (71–87) | <0.001 | |
| BMI (Kg/m2) | 27 (23.85–30.48) | 31.83 (28.66–35.23) | <0.001 | |
| Weight (Kg) | 74.3 (63.9–85.05) | 88.8 (75.9–100.43) | <0.001 | |
| Lipid profile | TG/HDL-C | 0.72 (0.47–1.08) | 1.8 (1.32–2.62) | <0.001 |
| TC/HDL-C | 3.46 (2.84–4.14) | 4.83 (4.23–5.74) | <0.001 | |
| LDL-C/HDL-C | 2.1 (1.58–2.7) | 3.04 (2.44–3.7) | <0.001 | |
| NonHDL-C/HDL-C | 2.46 (1.84–3.14) | 3.83 (3.23–4.74) | <0.001 | |
| NonHDL-C (mmol/L) | 3.4 (2.84–4) | 4.14 (3.55–4.73) | <0.001 | |
| TG (mmol/L) | 1 (0.73–1.34) | 2 (1.4–2.58) | <0.001 | |
| HDL-C (mmol/L) | 1.39 (1.18–1.63) | 1.06 (0.94–1.19) | <0.001 | |
| LDL-C Calc (mmol/L) | 3 (2.38–3.46) | 3.17 (2.66–3.89) | <0.001 | |
| TC (mmol/L) | 4.8 (4.3–5.4) | 5.2 (4.6–5.8) | <0.001 | |
| Blood Sugar | FBG (mmol/L) | 4.9 (4.6–5.2) | 5.6 (5–5.9) | <0.001 |
| Insulin (uU/mL) | 8.1 (6–13) | 17 (11.95–30) | <0.001 | |
| C-Peptide (ng/mL) | 2 (1.49–2.77) | 3.33 (2.56–4.93) | <0.001 | |
| HbA1C (%) | 5.3 (5.1–5.5) | 5.6 (5.3–5.8) | <0.001 | |
| Unadjusted Model | Adjusted Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC | AUC 95% CI | OR | OR 95% CI | p | AUC | AUC 95% CI | OR | OR 95% CI | p | |
| TG/HDL | 0.873 | 0.85–0.89 | 4.134 | 3.53–4.88 | <0.0001 | 0.896 | 0.88–0.91 | 4.356 | 3.63–5.28 | <0.0001 |
| TC/HDL | 0.829 | 0.81–0.85 | 2.141 | 1.95–2.36 | <0.0001 | 0.857 | 0.84–0.88 | 2.100 | 1.88–2.35 | <0.0001 |
| LDL/HDL | 0.769 | 0.75–0.79 | 2.049 | 1.84–2.30 | <0.0001 | 0.819 | 0.79–0.84 | 1.846 | 1.63–2.10 | <0.0001 |
| Non-HDL/HDL | 0.829 | 0.81–0.85 | 2.141 | 1.95–2.36 | <0.0001 | 0.857 | 0.84–0.88 | 2.100 | 1.88–2.35 | <0.0001 |
| Unadjusted Model | Adjusted Model | |||||
|---|---|---|---|---|---|---|
| Comparison | AUC | p-Value | Adjusted p-Value | AUC | p-Value | Adjusted p-Value |
| TG/HDL | 0.872 | - | - | 0.896 | - | - |
| vs. TC/HDL | 0.829 | 4.22 × 10−7 | 1.27 × 10−6 | 0.857 | 0.166 | 0.497 |
| vs. LDL/HDL | 0.769 | 2.2 × 10−16 | 6.6 × 10−16 | 0.819 | 8.8 × 10−5 | 2.64 × 10−4 |
| vs. Non-HDL/HDL | 0.829 | 4.22 × 10−7 | 1.27 × 10−6 | 0.857 | 0.166 | 0.497 |
| Metabolite | Superpathway | Subpathway | Estimate | Std. Error | p-Value | FDR |
|---|---|---|---|---|---|---|
| oleoyl-linoleoyl-glycerol (18:1/18:2) (2) | Lipid | Diacylglycerol | 0.739 | 0.033 | 1.51 × 10−94 | 7.57 × 10−92 |
| oleoyl-linoleoyl-glycerol (18:1/18:2) (1) | Lipid | Diacylglycerol | 0.766 | 0.034 | 1.70 × 10−94 | 7.57 × 10−92 |
| 1-(1-enyl-palmitoyl)-2-oleoyl-GPC (P-16:0/18:1) * | Lipid | Plasmalogen | −0.39 | 0.018 | 1.96 × 10−87 | 5.82 × 10−85 |
| 1-stearoyl-2-linoleoyl-GPE (18:0/18:2) * | Lipid | Phosphatidylethanolamine | 0.547 | 0.028 | 1.47 × 10−73 | 3.26 × 10−71 |
| 1-(1-enyl-palmitoyl)-2-linoleoyl-GPC (P-16:0/18:2) * | Lipid | Plasmalogen | −0.331 | 0.017 | 8.27 × 10−73 | 1.47 × 10−70 |
| 1-stearoyl-GPE (18:0) | Lipid | Lysophospholipid | 0.353 | 0.019 | 1.99 × 10−70 | 2.94 × 10−68 |
| 1-palmitoyl-2-linoleoyl-GPE (16:0/18:2) | Lipid | Phosphatidylethanolamine | 0.549 | 0.03 | 2.76 × 10−68 | 3.50 × 10−66 |
| 1-palmitoyl-2-oleoyl-GPE (16:0/18:1) | Lipid | Phosphatidylethanolamine | 0.576 | 0.032 | 4.92 × 10−67 | 5.46 × 10−65 |
| 1-stearoyl-2-oleoyl-GPE (18:0/18:1) | Lipid | Phosphatidylethanolamine | 0.544 | 0.03 | 3.48 × 10−66 | 3.44 × 10−64 |
| 1-linoleoylglycerol (18:2) | Lipid | Monoacylglycerol | 0.547 | 0.03 | 3.13 × 10−65 | 2.79 × 10−63 |
| p-Value | FDR | |
|---|---|---|
| Sphingomyelins | 2.81 × 10−11 | 2.56 × 10−9 |
| Plasmalogen | 5.51 × 10−8 | 2.51 × 10−6 |
| Phosphatidylethanolamine | 4.17 × 10−6 | 1.26 × 10−4 |
| Monoacylglycerol | 2.26 × 10−5 | 5.15 × 10−4 |
| Phosphatidylinositol | 2.41 × 10−4 | 4.39 × 10−3 |
| Lysoplasmalogen | 5.55 × 10−4 | 8.41 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kano, N.; Anwardeen, N.; Naja, K.; Elashi, A.A.; Malki, A.; Elrayess, M.A. Predictive Utility and Metabolomic Signatures of TG/HDL-C Ratio for Metabolic Syndrome Without Cardiovascular Disease and/or Diabetes in Qatari Adults. Metabolites 2025, 15, 574. https://doi.org/10.3390/metabo15090574
Kano N, Anwardeen N, Naja K, Elashi AA, Malki A, Elrayess MA. Predictive Utility and Metabolomic Signatures of TG/HDL-C Ratio for Metabolic Syndrome Without Cardiovascular Disease and/or Diabetes in Qatari Adults. Metabolites. 2025; 15(9):574. https://doi.org/10.3390/metabo15090574
Chicago/Turabian StyleKano, Noora, Najeha Anwardeen, Khaled Naja, Asma A. Elashi, Ahmed Malki, and Mohamed A. Elrayess. 2025. "Predictive Utility and Metabolomic Signatures of TG/HDL-C Ratio for Metabolic Syndrome Without Cardiovascular Disease and/or Diabetes in Qatari Adults" Metabolites 15, no. 9: 574. https://doi.org/10.3390/metabo15090574
APA StyleKano, N., Anwardeen, N., Naja, K., Elashi, A. A., Malki, A., & Elrayess, M. A. (2025). Predictive Utility and Metabolomic Signatures of TG/HDL-C Ratio for Metabolic Syndrome Without Cardiovascular Disease and/or Diabetes in Qatari Adults. Metabolites, 15(9), 574. https://doi.org/10.3390/metabo15090574








