Review of Biological Activities of Some Rare Oils from Amazonian Plants
Abstract
1. Introduction
2. Methods
3. Lipid Composition of Some Rare Amazonian Oils
3.1. Lipids of Açai (Euterpe oleracea Mart.) Fruit Oil
3.2. Lipids of Andiroba (Carapa guianensis Aubl.) Seed Oil
3.3. Lipids of Bacuri (Platonia insignis Mart.) Seed Butter
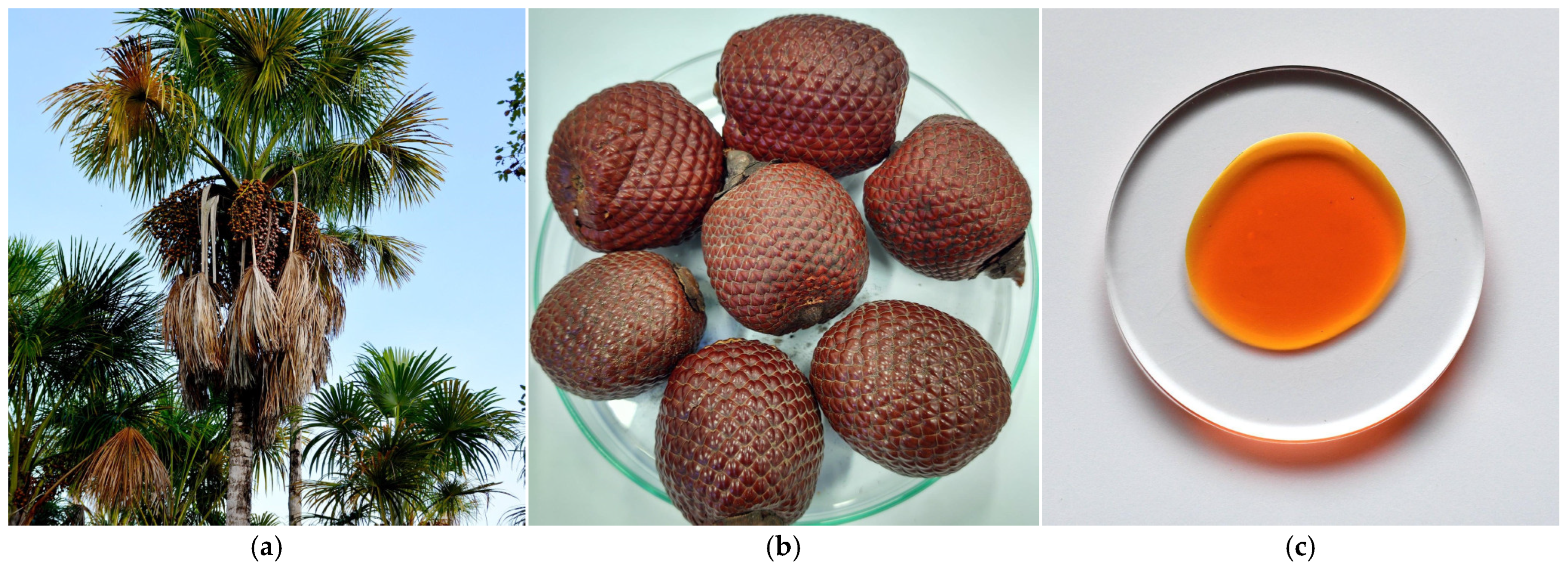
3.4. Lipids of Buriti (Mauritia flexuosa L.f.) Fruit Oil
3.5. Lipids of Cupuaçu (Theobroma grandiflorum Willd.) Seed Butter
3.6. Lipids of Pracaxi (Pentaclethra macroloba (Willd.) Kuntze) Seed Oil
4. Biological Activities of Some Rare Amazonian Oils and Butters
4.1. Some Biological Activities of Açai (Euterpe oleracea Mart.) Fruit Oil
4.1.1. Antioxidant Activity
4.1.2. Anti-Inflammatory Activity
4.1.3. Antitumoral Activity
4.1.4. Antimicrobial Activity
4.1.5. Antihypercholesterolemic and Antihypertriglyceridemic Activity
4.1.6. Other Biological Activities
4.2. Some Biological Activities of Andiroba (Carapa guianensis Aubl.) Seed Oil
4.2.1. Antioxidant Activity
4.2.2. Anti-Inflammatory and Tissue-Healing Activity
4.2.3. Antitumoral Activity
4.2.4. Antimicrobial Activity
4.2.5. Antiparasitic Activity
4.2.6. Other Main Biological Activities
4.3. Some Biological Activities of Bacuri (Platonia insignis Mart.) Seed Butter
4.3.1. Antioxidant Activity
4.3.2. Anti-Inflammatory Activity
4.3.3. Wound-Healing Activity
4.3.4. Cardio-Protective Activity
4.3.5. Vasorelaxant Activity
4.3.6. Immunomodulatory Activity
4.3.7. Antiparasitic Activity
4.3.8. Central Nervous System Stimulator
4.3.9. Gastroprotective
4.4. Some Biological Activities of Buriti (Mauritia flexuosa L.f.) Fruit Oil
4.4.1. Antioxidant Activity
4.4.2. Anti-Inflammatory Activity
4.4.3. Healing Activity
4.4.4. Photoprotective Activity
4.4.5. Antimicrobial Activity
4.4.6. Other Biological Activities
4.5. Some Biological Activities of Cupuaçu (Theobroma grandiflorum Willd.) Seed Butter
4.5.1. Antioxidant Activity
4.5.2. Wound-Healing Activity
4.5.3. Emollient Activity
4.5.4. Anti-Neurodegenerative Activity
4.5.5. Photo-Protective Activity
4.5.6. Antitumoral Activity
4.5.7. Antidiabetic Activity
4.5.8. Microbiological Activity
4.6. Some Biological Activities of Pracaxi (Pentaclethra macroloba (Willd.) Kuntze) Seed Oil
4.6.1. Antioxidant Activity
4.6.2. Anti-Inflammatory Activity
4.6.3. Wound-Healing Activity
4.6.4. Antimicrobial Activity
4.6.5. Other Biological Activities
5. Nanotechnology Applications of Amazonian Oils and Butters
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribeiro, J.M.P.; Maculan, G.; de Ávila, B.O.; Morais, V.A.; Hoeckesfeld, L.; Secchi, L.; De Andrade Guerra, J.B.S.O. Deforestation by Production Displacement: Expansion of Cropland and Cattle Ranching on Amazon Forest. Environ. Dev. Sustain. 2025, 27, 1–32. [Google Scholar] [CrossRef]
- Menezes, T.C.; De Castro, G.A.B.; Fernandes, H.A.; Gutjahr, K.E.; Dantas Filho, H.A.; Da Silva, N.C.; Dantas, K.G.F. NIR-Based Classification of Vegetable Oils from Amazon Rainforest and Quantification of Adulterants. J. Food Compos. Anal. 2025, 138, 106988. [Google Scholar] [CrossRef]
- Environmental Working Group Skin Deep® Cosmetics Database. Available online: https://www.ewg.org/skindeep/ (accessed on 14 July 2025).
- Araujo-Lima, C.F.; Fernandes, A.S.; Gomes, E.M.; Oliveira, L.L.; Macedo, A.F.; Antoniassi, R.; Wilhelm, A.E.; Aiub, C.A.F.; Felzenszwalb, I. Antioxidant Activity and Genotoxic Assessment of Crabwood (Andiroba, Carapa guianensis Aublet) Seed Oils. Oxid. Med. Cell. Longev. 2018, 2018, 3246719. [Google Scholar] [CrossRef]
- Alves, A.Q.M.D.; Da Silva, V.A.J.; Góes, A.J.S.; Silva, M.S.; De Oliveira, G.G.; Bastos, I.V.G.A.; De Castro Neto, A.G.; Alves, A.J. The Fatty Acid Composition of Vegetable Oils and Their Potential Use in Wound Care. Adv. Ski. Wound Care 2019, 32, 1–8. [Google Scholar] [CrossRef]
- Fasciotti, M.; Monteiro, T.V.C.; Rocha, W.F.C.; Morais, L.R.B.; Sussulini, A.; Eberlin, M.N.; Cunha, V.S. Comprehensive Triacylglycerol Characterization of Oils and Butters of 15 Amazonian Oleaginous Species by ESI-HRMS/MS and Comparison with Common Edible Oils and Fats. Eur. J. Lipid Sci. Technol. 2020, 122, 2000019. [Google Scholar] [CrossRef]
- Dumato Ltd. Internal Analysis Report; Dumato Ltd.: Nyon, Switzerland, 2024. [Google Scholar]
- Orsavova, J.; Misurcova, L.; Ambrozova, J.V.; Vicha, R.; Mlcek, J. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Giakoumis, E.G. Analysis of 22 Vegetable Oils’ Physico-Chemical Properties and Fatty Acid Composition on a Statistical Basis, and Correlation with the Degree of Unsaturation. Renew. Energy 2018, 126, 403–419. [Google Scholar] [CrossRef]
- Maanikuu, P.M.I.; Peker, K. Medicinal and Nutritional Benefits from the Shea Tree (Vitellaria paradoxa). J. Biol. Agric. Healthc. 2017, 7, 51–57. [Google Scholar]
- Yamaguchi, K.K.d.L.; Pereira, L.F.R.; Lamarão, C.V.; Lima, E.S.; Veiga-Junior, V.F. Amazon Acai: Chemistry and Biological Activities: A Review. Food Chem. 2015, 179, 137–151. [Google Scholar] [CrossRef]
- De Almeida Magalhães, T.S.S.; De Oliveira Macedo, P.C.; Converti, A.; Neves de Lima, Á.A. The Use of Euterpe oleracea Mart. As a New Perspective for Disease Treatment and Prevention. Biomolecules 2020, 10, 813. [Google Scholar] [CrossRef]
- Favacho, H.A.S.; Oliveira, B.R.; Santos, K.C.; Medeiros, B.J.L.; Sousa, P.J.C.; Perazzo, F.F.; Carvalho, J.C.T. Anti-Inflammatory and Antinociceptive Activities of Euterpe oleracea Mart., Arecaceae, Oil. Rev. Bras. Farmacogn. 2011, 21, 105–114. [Google Scholar] [CrossRef]
- Santos, O.V.; Lemos, Y.S.; da Conceição, L.R.V.; Teixeira-Costa, B.E. Lipids from the Purple and White Açaí (Euterpe oleracea Mart) Varieties: Nutritional, Functional, and Physicochemical Properties. Front. Nutr. 2024, 11, 1385877. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.; Talcott, S.T. Chemical Composition, Antioxidant Properties, and Thermal Stability of a Phytochemical Enriched Oil from Açai (Euterpe oleracea Mart.). J. Agric. Food Chem. 2008, 56, 4631–4636. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A. Chemical Characterization, Bioactive Properties, and Pigment Stability of Polyphenolics in Açai (Euterpe oleracea Mart.). Ph.D. Dissertation, Texas A&M University, College Station, TX, USA, 2009. [Google Scholar]
- Funasaki, M.; Barroso, H.d.S.; Fernandes, V.L.A.; Menezes, I.S. Amazon Rainforest Cosmetics: Chemical Approach for Quality Control. Quím. Nova 2016, 39, 194–209. [Google Scholar] [CrossRef]
- Yamaguchi, K.K.d.L.; Dos, L.; Santarém, S.; Lamarão, C.V.; Lima, E.S.; Veiga-Junior, V.F. Avaliação in Vitro Da Atividade Fotoprotetora de Resíduos de Frutas Amazônicas. Sci. Amazon. 2016, 5, 109–116. [Google Scholar]
- Milhomem-Paixão, S.S.R.; Fascineli, M.L.; Roll, M.M.; Longo, J.P.F.; Azevedo, R.B.; Pieczarka, J.C.; Salgado, H.L.C.; Santos, A.S.; Grisolia, C.K. The Lipidome, Genotoxicity, Hematotoxicity and Antioxidant Properties of Andiroba Oil from the Brazilian Amazon. Genet. Mol. Biol. 2016, 39, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Martinborough, T. Karaba Oil (Crabwood Oil): A Literature Review; The Iwokrama International Centre for Rain Forest, Conservation and Development: Georgetown, Guyana, 2002. [Google Scholar]
- Andiroba—Carapa guianensis—Database File in the Tropical Plant Database for Herbal Remedies. Available online: https://rain-tree.com/andiroba.htm (accessed on 1 October 2024).
- Wanzeler, A.M.V.; Júnior, S.M.A.; Gomes, J.T.; Gouveia, E.H.H.; Henriques, H.Y.B.; Chaves, R.H.; Soares, B.M.; Salgado, H.L.C.; Santos, A.S.; Tuji, F.M. Therapeutic Effect of Andiroba Oil (Carapa guianensis Aubl.) against Oral Mucositis: An Experimental Study in Golden Syrian Hamsters. Clin. Oral Investig. 2018, 22, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Melo, K.M.; Fascineli, M.L.; Milhomem-Paixão, S.S.R.; Grisolia, C.K.; Santos, A.S.; Salgado, H.L.C.; Muehlmann, L.A.; Azevedo, R.B.; Pieczarka, J.C.; Nagamachi, C.Y. Evaluation of the Genotoxic and Antigenotoxic Effects of Andiroba (Carapa guianensis Aublet) Oil and Nanoemulsion on Swiss Mice. J. Nanomater. 2018, 2018, 4706057. [Google Scholar] [CrossRef]
- Silva dos Reis, A.; Santos, A.S. Relevances of Ethnobotanical, Chemical and Biotechnological Aspects of Andiroba (Carapa Spp.) As a Source of Applications in Strategic Sectors. Review. Rev. Gest. Soc. Ambient. 2024, 18, e03660. [Google Scholar] [CrossRef]
- Ribeiro, C.D.B.; Costa, P.A.d.; Lima, S.R.V.d.; Silva, M.T.d. O uso medicinal de Carapa guianensis Abul. (Andiroba). Res. Soc. Dev. 2021, 10, e391101522815. [Google Scholar] [CrossRef]
- Arcanjo, D.D.R.; Costa-Júnior, J.S.d.; Moura, L.H.P.; Ferraz, A.B.F.; Rossatto, R.R.; David, J.M.; Oliveira, A.P.d. Garcinielliptone FC, a Polyisoprenylated Benzophenone from Platonia insignis Mart., Promotes Vasorelaxant Effect on Rat Mesenteric Artery. Nat. Prod. Res. 2014, 28, 923–927. [Google Scholar] [CrossRef]
- Ribeiro, D.C.; Russo, H.M.; Fraige, K.; Zeraik, M.L.; Nogueira, C.R.; Da Silva, P.B.; Codo, A.C.; Calixto, G.M.F.; De Medeiros, A.I.; Chorilli, M.; et al. Bioactive Bioflavonoids from Platonia insignis (Bacuri) Residues as Added Value Compounds. J. Braz. Chem. Soc. 2021, 32, 786–799. [Google Scholar] [CrossRef]
- Albuquerque, S.R.S. Obtenção e Caracterização Do Óleo Da Espécie Mauritia flexuosa, L. f. Na Região Do Baixo Acre: Análises Físico-Químicas e Espectroscópicas. Ph.D. Dissertation, Universidade Federal do Acre, Rio Branco, Brazil, 2013. [Google Scholar]
- Barboza, N.L.; Cruz, J.M.d.A.; Corrêa, R.F.; Lamarão, C.V.; Lima, A.R.; Inada, N.M.; Sanches, E.A.; Bezerra, J.d.A.; Campelo, P.H. Buriti (Mauritia flexuosa, L. f.): An Amazonian Fruit with Potential Health Benefits. Food Res. Int. 2022, 159, 111654. [Google Scholar] [CrossRef]
- Speranza, P.; Leão, K.M.M.; Gomes, T.S.N.; Reis, L.V.C.; Rodrigues, A.P.; Macedo, J.A.; Ribeiro, A.P.B.; Macedo, G.A. Improving the Chemical Properties of Buriti Oil (Mauritia flexuosa, L.) by Enzymatic Interesterification. Grasas Aceites 2018, 69, e282. [Google Scholar] [CrossRef]
- Freitas, M.L.F.; Chisté, R.C.; Polachini, T.C.; Sardella, L.a.C.Z.; Aranha, C.P.M.; Ribeiro, A.P.B.; Nicoletti, V.R. Quality Characteristics and Thermal Behavior of Buriti (Mauritia flexuosa L.) Oil. Grasas Aceites 2017, 68, e220. [Google Scholar] [CrossRef]
- Cohen, K.d.O.; Jackix, M.d.N.H. Características químicas e física da gordura de cupuaçu e da manteiga de cacau, 1st ed.; Embrapa Cerrados: Planaltina, Brazil, 2009. [Google Scholar]
- Leonardi, B.; Arauz, L.; Baruque-Ramos, J. Chemical Characterization of Amazonian Non-Polar Vegetal Extracts (Buriti, Tucumã, Brazil Nut, Cupuaçu, and Cocoa) by Infrared Spectroscopy (FTIR) and Gas Chromatography (GC-FID). Infarma Ciênc. Farm. 2019, 31, 163–176. [Google Scholar] [CrossRef]
- Archambault, J.-C.; Bonté, F. Vegetable Fats in Cosmeticology. Rev. Boliv. Quím. 2021, 38, 68–79. [Google Scholar] [CrossRef]
- Pugliese, A.G.; Tomas-Barberan, F.A.; Truchado, P.; Genovese, M.I. Flavonoids, Proanthocyanidins, Vitamin C, and Antioxidant Activity of Theobroma grandiflorum (Cupuassu) Pulp and Seeds. J. Agric. Food Chem. 2013, 61, 2720–2728. [Google Scholar] [CrossRef]
- Yang, H.; Protiva, P.; Cui, B.; Ma, C.; Baggett, S.; Hequet, V.; Mori, S.; Weinstein, I.B.; Kennelly, E.J. New Bioactive Polyphenols from Theobroma grandiflorum (“Cupuaçu”). J. Nat. Prod. 2003, 66, 1501–1504. [Google Scholar] [CrossRef]
- Barros, H.R.d.M.; García-Villalba, R.; Tomás-Barberán, F.A.; Genovese, M.I. Evaluation of the Distribution and Metabolism of Polyphenols Derived from Cupuassu (Theobroma grandiflorum) in Mice Gastrointestinal Tract by UPLC-ESI-QTOF. J. Funct. Foods 2016, 22, 477–489. [Google Scholar] [CrossRef]
- Da Silva, C.V.A.; Salimo, Z.M.; De Souza, T.A.; Reyes, D.E.; Bassicheto, M.C.; De Medeiros, L.S.; Sartim, M.A.; De Carvalho, J.C.; Gonçalves, J.F.C.; Monteiro, W.M.; et al. Cupuaçu (Theobroma grandiflorum): A Multifunctional Amazonian Fruit with Extensive Benefits. Food Res. Int. 2024, 192, 114729. [Google Scholar] [CrossRef]
- Teixeira, R.d.S.; Rocha, P.R.; Polonini, H.C.; Brandão, M.A.F.; Chaves, M.d.G.A.M.; Raposo, N.R.B. Mushroom Tyrosinase Inhibitory Activity and Major Fatty Acid Constituents of Amazonian Native Flora Oils. Braz. J. Pharm. Sci. 2012, 48, 399–404. [Google Scholar] [CrossRef]
- Nobre Lamarão, M.L.; Ferreira, L.M.d.M.C.; Gyles Lynch, D.; Morais, L.R.B.; Silva-Júnior, J.O.C.; Ribeiro-Costa, R.M. Pentaclethra Macroloba: A Review of the Biological, Pharmacological, Phytochemical, Cosmetic, Nutritional and Biofuel Potential of This Amazonian Plant. Plants 2023, 12, 1330. [Google Scholar] [CrossRef]
- Eberhart, B.D.S.; Komiyama, C.M.; Burbarelli, M.F.D.C.; Heiss, V.A.R.C.; Garcia, R.G.; Felix, G.A.; Cardoso, C.A.L.; Borges, R.; Braz, P.H.; Teodoro, C.R.; et al. Characterization and Subchronic Oral Toxicity of Pentaclethra macroloba (Pracaxi) Oil in Rattus norvegicus (Lin. Wistar). Toxicon 2023, 230, 107151. [Google Scholar] [CrossRef]
- Dos Santos Costa, M.N.F.; Muniz, M.A.P.; Negrão, C.A.B.; Da Costa, C.E.F.; Lamarão, M.L.N.; Morais, L.; Silva Júnior, J.O.C.; Ribeiro Costa, R.M. Characterization of Pentaclethra macroloba Oil. J. Therm. Anal. Calorim. 2014, 115, 2269–2275. [Google Scholar] [CrossRef]
- Banov, D.; Banov, F.; Bassani, A.S. Case Series: The Effectiveness of Fatty Acids from Pracaxi Oil in a Topical Silicone Base for Scar and Wound Therapy. Dermatol. Ther. 2014, 4, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Silva de Freitas, J.S.d.; Santos, I.L.; Da Silva, L.H.M. Propriedades e Potencialidades de Fontes Amazônicas Oleaginosas: Tucumã e Pracaxi. Contrib. LAS Cienc. Soc. 2024, 17, e6562. [Google Scholar] [CrossRef]
- Bizzo, H.R.; Antoniassi, R.J.N. Fixed Oils and Antimicrobial Effects. In Recent Progress in Medicinal Plants: Fixed Oils and Fats; Govil, J.N., Ed.; Studium Press LLC: Houston, TX, USA, 2012; Volume 33, pp. 273–285. [Google Scholar]
- Rufino, M.S.M.; Pérez-Jiménez, J.; Arranz, S.; Alves, R.E.; De Brito, E.S.; Oliveira, M.S.P.; Saura-Calixto, F. Açaí (Euterpe oleraceae) ‘BRS Pará’: A Tropical Fruit Source of Antioxidant Dietary Fiber and High Antioxidant Capacity Oil. Food Res. Int. 2011, 44, 2100–2106. [Google Scholar] [CrossRef]
- De Almeida Magalhães, T.S.S.; De Oliveira Macedo, P.C.; Da Costa, É.C.P.; De Aragão Tavares, E.; Da Silva, V.C.; Guerra, G.C.B.; Pereira, J.R.; De Araújo Moura Lemos, T.M.; De Negreiros, M.M.F.; De Oliveira Rocha, H.A.; et al. Increase in the Antioxidant and Anti-Inflammatory Activity of Euterpe oleracea Martius Oil Complexed in β-Cyclodextrin and Hydroxypropyl-β-Cyclodextrin. Int. J. Mol. Sci. 2021, 22, 11524. [Google Scholar] [CrossRef]
- Dos Santos, D.D.S.; Klauck, V.; Campigotto, G.; Alba, D.F.; Dos Reis, J.H.; Gebert, R.R.; Souza, C.F.; Baldissera, M.D.; Schogor, A.L.B.; Santos, I.D.; et al. Benefits of the Inclusion of Açai Oil in the Diet of Dairy Sheep in Heat Stress on Health and Milk Production and Quality. J. Therm. Biol. 2019, 84, 250–258. [Google Scholar] [CrossRef]
- Borges, K.R.A.; Wolff, L.A.S.; Da Silva, M.A.C.N.; De Carvalho Silva, A.K.; Campos, C.D.L.; Souza, F.S.; Teles, A.M.; Vale, A.Á.M.; Pascoa, H.; Lima, E.M.; et al. Açaí (Euterpe oleracea Mart.) Seed Oil and Its Nanoemulsion: Chemical Characterisation, Toxicity Evaluation, Antioxidant and Anticancer Activities. Curr. Issues Mol. Biol. 2024, 46, 3763–3793. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Magalhães, T.S.S.; De Oliveira Macedo, P.C.; Kawashima Pacheco, S.Y.; Silva, S.S.d.; Barbosa, E.G.; Pereira, R.R.; Costa, R.M.R.; Silva Junior, J.O.C.; Da Silva Ferreira, M.A.; De Almeida, J.C.; et al. Development and Evaluation of Antimicrobial and Modulatory Activity of Inclusion Complex of Euterpe oleracea Mart Oil and β-Cyclodextrin or HP-β-Cyclodextrin. Int. J. Mol. Sci. 2020, 21, 942. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.A.C.N.; Tessmann, J.W.; Borges, K.R.A.; Wolff, L.A.S.; Botelho, F.D.; Vieira, L.A.; Morgado-Diaz, J.A.; Franca, T.C.C.; Barbosa, M.d.C.L.; Nascimento, M.d.D.S.B.; et al. Açaí (Euterpe oleracea Mart.) Seed Oil Exerts a Cytotoxic Role over Colorectal Cancer Cells: Insights of Annexin A2 Regulation and Molecular Modeling. Metabolites 2023, 13, 789. [Google Scholar] [CrossRef]
- Monge-Fuentes, V.; Muehlmann, L.A.; Longo, J.P.F.; Silva, J.R.; Fascineli, M.L.; Souza, P.; Faria, F.; Degterev, I.A.; Rodriguez, A.; Carneiro, F.P.; et al. Photodynamic Therapy Mediated by Acai Oil (Euterpe oleracea Martius) in Nanoemulsion: A Potential Treatment for Melanoma. J. Photochem. Photobiol. 2017, 166, 301–310. [Google Scholar] [CrossRef]
- Souza, B.S.F.E.; Carvalho, H.O.; Taglialegna, T.; Barros, A.S.A.; Da Cunha, E.L.; Ferreira, I.M.; Keita, H.; Navarrete, A.; Carvalho, J.C.T. Effect of Euterpe oleracea Mart. (Açaí) Oil on Dyslipidemia Caused by Cocos nucifera L. Saturated Fat in Wistar Rats. J. Med. Food 2017, 20, 830–837. [Google Scholar] [CrossRef]
- Melhorança Filho, A.L.; Pereira, M.R.R. Atividade antimicrobiana de óleos extraídos de açaí e de pupunha sobre o desenvolvimento de Pseudomonas aeruginosa e Staphylococcus aureus. Biosci. J. 2012, 28, 598–603. [Google Scholar]
- Da Silva, V.P.; Oliveira, R.R.; Figueiredo, M.R. Isolation of Limonoids from Seeds of Carapa guianensis Aublet (Meliaceae) by High-Speed Countercurrent Chromatography. Phytochem. Anal. 2009, 20, 77–81. [Google Scholar] [CrossRef]
- Fonseca, A.S.A.; Monteiro, I.S.; Santos, C.R.; Carneiro, M.L.B.; Morais, S.S.; Araújo, P.L.; Santana, T.F.; Joanitti, G.A. Effects of Andiroba Oil (Carapa guianensis Aublet) on the Immune System in Inflammation and Wound Healing: A Scoping Review. J. Ethnopharmacol. 2024, 327, 118004. [Google Scholar] [CrossRef]
- Penido, C.; Conte, F.P.; Chagas, M.S.S.; Rodrigues, C.a.B.; Pereira, J.F.G.; Henriques, M.G.M.O. Antiinflammatory Effects of Natural Tetranortriterpenoids Isolated from Carapa guianensis Aublet on Zymosan-Induced Arthritis in Mice. Inflamm. Res. 2006, 55, 457–464. [Google Scholar] [CrossRef]
- Brito, M.V.H.; Figueiredo, R.C.; Tavares, M.L.C.; Silveira, T.S.; Cantanhêde, G. Efeito Dos Óleos de Andiroba e Copaíba Na Miosite Induzida Em Ratos. Rev. Para. Med. 2006, 20, 17–24. [Google Scholar] [CrossRef]
- Chicaro, C.F. Análise Da Expressão Da Proteína NF-KappaB Antes e Depois Do Tratamento Com Dexametasona e Os Óleos de Copaíba e Andiroba Em Cultura de Células de Carcinoma Epidermóide Bucal. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2009. [Google Scholar]
- Nayak, B.S.; Kanhai, J.; Milne, D.M.; Pereira, L.P.; Swanston, W.H. Experimental Evaluation of Ethanolic Extract of Carapa guianensis L. Leaf for Its Wound Healing Activity Using Three Wound Models. Evid. Based Complement. Alternat. Med. 2011, 2011, 419612. [Google Scholar] [CrossRef]
- Nayak, B.S.; Kanhai, J.; Milne, D.M.; Swanston, W.H.; Mayers, S.; Eversley, M.; Rao, A.V.C. Investigation of the Wound Healing Activity of Carapa guianensis L. (Meliaceae) Bark Extract in Rats Using Excision, Incision, and Dead Space Wound Models. J. Med. Food 2010, 13, 1141–1146. [Google Scholar] [CrossRef]
- Souza, B.A.A.; Braga, L.A.; Lopes, L.R.O.; Ribeiro Júnior, R.F.G.; Nascimento, L.N.S.; Cavalcante, L.C.d.C.; Monteiro, A.M.; Couteiro, R.P.; Yasojima, E.Y.; Hamoy, M. Effects of Andiroba Oil (Carapa guianensis) on Wound Healing in Alloxan-Diabetic Rats. Int. Arch. Med. 2017, 10, 1–6. [Google Scholar] [CrossRef]
- Capela, I.L.B.; Campos, J.S.; Lima, J.P.M.; Silva, P.I.C.; Marialva, E.L.T.; Quintarios, R.R.D.; Yamaguchi, S.S.; Corrêa, R.D.R.; Conceição, A.B.D.D.; Souza, A.J.S.; et al. Os efeitos da fotobiomodulação (PBMT) e do òleo de andiroba (Carapa guianensis) sobre a lesão muscular aguda em ratas: Um estudo piloto. Rev. Cent. Pesqui. Av. Qual. Vida 2020, 13, 1–10. [Google Scholar] [CrossRef]
- Mu, Y.-M.; Yanase, T.; Nishi, Y.; Tanaka, A.; Saito, M.; Jin, C.-H.; Mukasa, C.; Okabe, T.; Nomura, M.; Goto, K.; et al. Saturated FFAs, Palmitic Acid and Stearic Acid, Induce Apoptosis in Human Granulosa Cells. Endocrinology 2001, 142, 3590–3597. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.H.; Mu, Y.M.; Wang, B.A.; Li, X.L.; Lu, J.M.; Li, J.Y.; Pan, C.Y.; Yanase, T.; Nawata, H. Saturated Free Fatty Acids, Palmitic Acid and Stearic Acid, Induce Apoptosis by Stimulation of Ceramide Generation in Rat Testicular Leydig Cell. Biochem. Biophys. Res. Commun. 2003, 303, 1002–1007. [Google Scholar] [CrossRef]
- Cury-Boaventura, M.F.; Pompéia, C.; Curi, R. Comparative Toxicity of Oleic Acid and Linoleic Acid on Jurkat Cells. Clin. Nutr. 2004, 23, 721–732. [Google Scholar] [CrossRef]
- Porfírio-Dias, C.L.; Melo, K.M.; Bastos, C.E.M.C.; Ferreira, T.A.A.; Azevedo, L.F.C.; Salgado, H.L.; Santos, A.S.; Rissino, J.D.; Nagamachi, C.Y.; Pieczarka, J.C. Andiroba Oil (Carapa guianensis Aubl) Shows Cytotoxicity but No Mutagenicity in the ACPP02 Gastric Cancer Cell Line. J. Appl. Toxicol. 2020, 40, 1060–1066. [Google Scholar] [CrossRef]
- Penido, C.; Costa, K.A.; Pennaforte, R.J.; Costa, M.F.S.; Pereira, J.F.G.; Siani, A.C.; Henriques, M.G.M.O. Anti-Allergic Effects of Natural Tetranortriterpenoids Isolated from Carapa guianensis Aublet on Allergen-Induced Vascular Permeability and Hyperalgesia. Inflamm. Res. 2005, 54, 295–303. [Google Scholar] [CrossRef]
- Penido, C.; Costa, K.A.; de Souza Costa, M.F.; Pereira, J.D.F.G.; Siani, A.C.; De Oliveira, M.D.G.M. Inhibition of Allergen-Induced Eosinophil Recruitment by Natural Tetranortriterpenoids Is Mediated by the Suppression of IL-5, CCL11/Eotaxin and NFκB Activation. Int. Immunopharmacol. 2006, 6, 109–121. [Google Scholar] [CrossRef]
- Hammer, M.L.A.; Johns, E.A. Tapping an Amazônian Plethora: Four Medicinal Plants of Marajó Island, Pará (Brazil). J. Ethnopharmacol. 1993, 40, 53–75. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.A.; Scussel, V.M. Characteristics and Effects of the Amazonian Andiroba (Carapa guianensis Aubl.) Oil against Living Organisms—A Review. J. Biotechnol. Biochem. 2020, 6, 31–47. [Google Scholar]
- Henriques, M.d.G.; Penido, C. The Therapeutic Properties of Carapa guianensis. Curr. Pharm. Des. 2014, 20, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Machado, I.R.; Mendes, K.R. Ethnobotanical, Medical, Therapeutical and Pharmacological Study of Carapa guianensis Aublet: A Review. Biodivers. Bras. 2021, 11, 1–24. [Google Scholar] [CrossRef]
- Dos Santos, A.O.; Ueda-Nakamura, T.; Dias Filho, B.P.; Veiga Junior, V.F.; Pinto, A.C.; Nakamura, C.V. Antimicrobial Activity of Brazilian Copaiba Oils Obtained from Different Species of the Copaifera Genus. Mem. Inst. Oswaldo Cruz 2008, 103, 277–281. [Google Scholar] [CrossRef]
- Silva, O.S.; Prophiro, J.S.; Nogared, J.C.; Kanis, L.; Emerick, S.; Blazius, R.D.; Romão, P.R.T. Larvicidal Effect of Andiroba Oil, Carapa guianensis (Meliaceae) against Aedes Aegypti. J. Am. Mosq. Control Assoc. 2006, 22, 699–701. [Google Scholar] [CrossRef]
- Mendonça, F.A.C.; Da Silva, K.F.S.; Dos Santos, K.K.; Ribeiro Junior, K.A.L.; Sant’Ana, A.E.G. Activities of Some Brazilian Plants against Larvae of the Mosquito Aedes Aegypti. Fitoterapia 2005, 76, 629–636. [Google Scholar] [CrossRef]
- Farias, M.P.O.; Sousa, D.P.; Arruda, A.C.; Wanderley, A.G.; Teixeira, W.C.; Alves, L.C.; Faustino, M.a.G. Potencial acaricida do óleo de andiroba Carapa guianensis Aubl. sobre fêmeas adultas ingurgitadas de Anocentor nitens Neumann, 1897 e Rhipicephalus sanguineus Latreille, 1806. Arq. Bras. Med. Vet. Zootec. 2009, 61, 877–882. [Google Scholar] [CrossRef]
- Freire, D.d.C.B.; Brito-Filha, C.R.d.C.; Carvalho-Zilse, G.A. Efeito dos óleos vegetais de andiroba (Carapa sp.) e Copaíba (Copaifera sp.) sobre forídeo, pragas de colméias, (Diptera: Phoridae) na Amazônia Central. Acta Amazon. 2006, 36, 365–368. [Google Scholar] [CrossRef]
- Miranda Júnior, R.N.C.; Dolabela, M.F.; Da Silva, M.N.; Póvoa, M.M.; Maia, J.G.S. Antiplasmodial Activity of the Andiroba (Carapa guianensis Aubl., Meliaceae) Oil and Its Limonoid-Rich Fraction. J. Ethnopharmacol. 2012, 142, 679–683. [Google Scholar] [CrossRef]
- Barros, F.N.d.; Farias, M.P.O.; Tavares, J.P.C.; Alves, L.C.; Faustino, M.A.d.G. In Vitro Efficacy of Oil from the Seed of Carapa guianensis (Andiroba) in the Control of Felicola subrostratus. Rev. Bras. Farmacogn. 2012, 22, 1130–1133. [Google Scholar] [CrossRef]
- Brito, M.V.H.; Brazão, R.V.; Siqueira, R.B.P.; Santos, M.T.d. Efeito Do Óleo de Andiroba Em Cultura de Staphylococcus aureus e Escherichia coli: Estudo In Vitro. Rev. Para. Med. 2001, 15, 36–40. [Google Scholar]
- Miranda Júnior, R.N.C. Avaliação Da Atividade Antiplasmódica In Vitro Dos Óleos de Andiroba (Carapa guianensis Aubl.) e Pimenta-de-Macaco (Piper aduncum L.). Master’s Dissertation, Universidade Federal do Pará, Belém, Brazil, 2010. [Google Scholar]
- Ambrozin, A.R.; Leite, A.C.; Bueno, F.C.; Vieira, P.C.; Fernandes, J.B.; Bueno, O.C.; Silva, M.; Pagnocca, F.C.; Hebling, M.J.A.; Bacci Jr, M. Limonoids from Andiroba Oil and Cedrela Fissilis and Their Insecticidal Activity. J. Braz. Chem. Soc. 2006, 17, 542–547. [Google Scholar] [CrossRef]
- Golynski, A.A. Controle de Helmintos de Frangos de Corte Utilizando as Plantas Mentha Piperita, Carapa guianensis, Artemisia Absinthium e Chenopodium Ambrosioides. Master’s Dissertation, Universidade Federal Rural do Rio de Janeiro, Seropédica, Brazil, 2003. [Google Scholar]
- Dantas, E.P.M. Prospecção de biocida em plantas amazônicas e exóticas, visando seu uso racional. Master’s Dissertation, Universidade Federal do Para, Belém, Brazil, 2009. [Google Scholar]
- Ribas, J.; Carreño, A.M. Evaluation of the Use of Repellent against Mosquito Bite by Military Personnel in the Amazon Basin. An. Bras. Dermatol. 2010, 85, 33–38. [Google Scholar] [CrossRef]
- Miot, H.A.; Batistella, R.F.; Batista, K.d.A.; Volpato, D.E.C.; Augusto, L.S.T.; Madeira, N.G.; Haddad Jr, V.; Miot, L.D.B. Comparative Study of the Topical Effectiveness of the Andiroba Oil (Carapa guianensis) and DEET 50% as Repellent for Aedes Sp. Rev. Inst. Med. Trop. São Paulo 2004, 46, 253–256. [Google Scholar] [CrossRef]
- Lustosa, A.K.M.F.; Coêlho, A.G.; Santos, A.A.d.; Barros, Y.S.O.; Rodrigues, K.A.d.F.; Amorim, L.V.; Alves, M.M.d.M.; Carvalho, A.L.M.; Mendes, A.N.; Carvalho, F.A.d.A.; et al. Formulações tópicas à base de manteiga das sementes de Platonia insignis Mart. para o tratamento de lesões relacionadas à leishmaniose cutânea experimental. Res. Soc. Dev. 2021, 10, e52310413665. [Google Scholar] [CrossRef]
- Dos Santos Júnior, R.Q.; Soares, L.C.; Filho, A.L.M.M.; Sousa, K.A.d.; Santos, Í.M.S.P.; Júnior, J.S.d.C.; Saffi, J. Estudo histológico da cicatrização de feridas cutâneas utilizando a banha de bacuri (Platonia insignis Mart.). ConScientiae Saúde 2010, 9, 575–581. [Google Scholar] [CrossRef]
- Cavalcante, A.N.; Feitosa, C.M.; Lima, L.K.F.; Sousa Júnior, R.S.; Alencar, A.G.S. Pharmacological Properties of Extracts and Compounds Isolated from Platonia insignis Mart.—A Perspective for Developing Phytomedicines. In Ethnobotany—Application of Medicinal Plants, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 277–290. ISBN 978-0-429-45313-7. [Google Scholar]
- Lima, G.d.M.; Brito, A.K.d.S.; Farias, L.M.d.; Rodrigues, L.A.R.L.; Pereira, C.F.d.C.; Lima, S.K.R.; Frota, K.d.M.G.; Rizzo, M.d.S.; Nunes, P.H.M.; Lucarini, M.; et al. Effects of “Bacuri” Seed Butter (Platonia insignis Mart.) on Metabolic Parameters in Hamsters with Diet-Induced Hypercholesterolemia. Evid. Based Complement. Alternat. Med. 2021, 2021, 5584965. [Google Scholar] [CrossRef]
- Lustosa, A.K.M.F.; Bezerra, É.A.; Rodrigues, K.A.F.; Amorim, L.V.; Lima-Neto, J.S.; Araújo, B.Q.; Costa-Júnior, J.S.d.; Mendes, A.N.; Carvalho, F.A.A.; Arcanjo, D.D.R.; et al. Antileishmanial Effect of Fruit Seeds from Platonia insignis against Macrophage-Internalized Amastigote Forms of Leishmania amazonensis. Rev. Cuba. Plantas Med. 2018, 23, 1–17. [Google Scholar]
- Coêlho, E.d.S.; Lopes, G.L.N.; Pinheiro, I.M.; Holanda, J.N.P.d.; Alves, M.M.d.M.; Nogueira, N.C.; Carvalho, F.A.d.A.; Carvalho, A.L.M. Emulgel Based on Amphotericin B and Bacuri Butter (Platonia insignis Mart.) for the Treatment of Cutaneous Leishmaniasis: Characterization and In Vitro Assays. Drug Dev. Ind. Pharm. 2018, 44, 1713–1723. [Google Scholar] [CrossRef]
- Costa Júnior, J.S.; Ferraz, A.B.F.; Filho, B.A.B.; Feitosa, C.M.; Citó, A.M.G.L.; Freitas, R.M.; Saffi, J. Evaluation of Antioxidant Effects in Vitro of Garcinielliptone FC (GFC) Isolated from Platonia insignis Mart. J. Med. Plants Res. 2011, 5, 293–299. [Google Scholar]
- Costa Júnior, J.S.; Almeida, A.A.C.d.; Costa, J.P.; Citó, A.M.d.G.L.; Saffi, J.; Freitas, R.M.d. Superoxide Dismutase and Catalase Activities in Rat Hippocampus Pretreated with Garcinielliptone FC from Platonia insignis. Pharm. Biol. 2012, 50, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Costa Júnior, J.S.; Ferraz, A.B.F.; Sousa, T.O.; Silva, R.A.C.; Lima, S.G.D.; Feitosa, C.M.; Citó, A.M.G.L.; Cavalcante, A.A.C.M.; Freitas, R.M.; Sperotto, A.R.M.; et al. Investigation of Biological Activities of Dichloromethane and Ethyl Acetate Fractions of Platonia insignis Mart. Seed. Basic Clin. Pharmacol. Toxicol. 2013, 112, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, A.d.N.; Lima, L.K.F.; Araújo, C.M.; Santos, F.P.D.S.; Nascimento, M.O.D.; Sousa, J.M.D.C.E.; Rai, M.; Feitosa, C.M. Toxicity, Cytotoxicity, Mutagenicity and In Vitro Antioxidant Models of 2-Oleyl-1,3-Dipalmitoyl-Glycerol Isolated from the Hexane Extract of Platonia insignis MART Seeds. Toxicol. Rep. 2020, 7, 209–216. [Google Scholar] [CrossRef]
- Costa Júnior, J.S.; De Almeida, A.A.C.; Tomé, A.d.R.; Citó, A.M.d.G.L.; Saffi, J.; Freitas, R.M.d. Evaluation of Possible Antioxidant and Anticonvulsant Effects of the Ethyl Acetate Fraction from Platonia insignis Mart. (Bacuri) on Epilepsy Models. Epilepsy Behav. 2011, 22, 678–684. [Google Scholar] [CrossRef]
- Coêlho, A.G.; De Almeida, J.O.C.S.; Santos, A.A.d.; Santos, W.R.P.d.; Da Rocha Sousa, L.; Viana, N.R.; Batista, F.A.; De Sousa Brito Neta, M.; Santos, A.S.; Da Silva, S.W.; et al. Solid Lipid Nanoparticles from Platonia insignis Seeds, a Brazilian Amazon Fruit: Characterization, In Vitro and In Vivo Toxicological and Antioxidant Activities. J. Compos. Sci. 2023, 7, 368. [Google Scholar] [CrossRef]
- Costa Júnior, J.S.; De Almeida, A.A.C.; Ferraz, A.d.B.F.; Rossatto, R.R.; Silva, T.G.; Silva, P.B.N.; Militão, G.C.G.; Citó, A.M.d.G.L.; Santana, L.C.L.R.; Carvalho, F.A.d.A.; et al. Cytotoxic and Leishmanicidal Properties of Garcinielliptone FC, a Prenylated Benzophenone from Platonia insignis. Nat. Prod. Res. 2013, 27, 470–474. [Google Scholar] [CrossRef]
- Lustosa, A.K.M.F.; Silva, F.V.; Silva, E.R.S.; Mendes, A.; Sousa, L.d.R.; Carvalho, A.L.; Oliveira, R.C.M.; Carvalho, F.A.A.; Arcanjo, D.D.R.; Citó, A.M.G.L. Lecithin-Based Organogel for an Industrialized Butter from Platonia insignis Mart. Seeds and Its Anti- Inflammatory Potential: Formulation and Preclinical Studies. J. Glob. Innov. 2020, 2, eJGI000011. [Google Scholar]
- Da Silva, A.P.S.C.L.; Lopes, J.S.L.; Vieira, P.S.; Pinheiro, E.E.A.; Da Silva, M.L.G.; Silva Filho, J.C.C.L.; Da Costa Júnior, J.S.; David, J.M.; De Freitas, R.M. Behavioral and Neurochemical Studies in Mice Pretreated with Garcinielliptone FC in Pilocarpine-Induced Seizures. Pharmacol. Biochem. Behav. 2014, 124, 305–310. [Google Scholar] [CrossRef]
- Lima Nascimento, J.; Coelho, A.G.; Barros, Y.S.O.; Oliveira, I.S.; Da Silva, F.V.; Viana, A.F.S.C.; Araújo, B.Q.; Rocha, M.S.; De Andrade, F.C.P.; Barbosa, C.O.; et al. Production and Characterization of a β-Cyclodextrin Inclusion Complex with Platonia insignis Seed Extract as a Proposal for a Gastroprotective System. Appl. Sci. 2023, 13, 58. [Google Scholar] [CrossRef]
- Lustosa, A.K.M.F.; Arcanjo, D.D.R.; Ribeiro, R.G.; Rodrigues, K.A.F.; Passos, F.F.B.; Piauilino, C.A.; Silva-Filho, J.C.; Araújo, B.Q.; Lima-Neto, J.S.; Costa-Júnior, J.S.; et al. Immunomodulatory and Toxicological Evaluation of the Fruit Seeds from Platonia insignis, a Native Species from Brazilian Amazon Rainforest. Rev. Bras. Farmacogn. 2016, 26, 77–82. [Google Scholar] [CrossRef]
- Silva, A.P.; Silva, M.P.; Oliveira, C.G.; Monteiro, D.C.; Pinto, P.L.; Mendonça, R.Z.; Costa Júnior, J.S.; Freitas, R.M.; De Moraes, J. Garcinielliptone FC: Antiparasitic Activity without Cytotoxicity to Mammalian Cells. Toxicol. In Vitro 2015, 29, 681–687. [Google Scholar] [CrossRef]
- Leão, K.M.M.; Reis, L.V.C.; Speranza, P.; Rodrigues, A.P.; Ribeiro, A.P.B.; Macedo, J.A.; Macedo, G.A. Physicochemical Characterization and Antimicrobial Activity in Novel Systems Containing Buriti Oil and Structured Lipids Nanoemulsions. Biotechnol. Rep. 2019, 24, e00365. [Google Scholar] [CrossRef] [PubMed]
- Morais, N.d.S.; Passos, T.S.; Ramos, G.R.; Ferreira, V.A.F.; Moreira, S.M.G.; Filho, G.P.C.; Barreto, A.P.G.; Leite, P.I.P.; Almeida, R.S.d.; Paulo, C.L.R.; et al. Nanoencapsulation of Buriti Oil (Mauritia flexuosa L.f.) in Porcine Gelatin Enhances the Antioxidant Potential and Improves the Effect on the Antibiotic Activity Modulation. PLoS ONE 2022, 17, e0265649. [Google Scholar] [CrossRef] [PubMed]
- Serra, J.L.; Rodrigues, A.M.C.; Freitas, R.A.; Meirelles, A.J.A.; Darnet, S.H.; Silva, L.H.M. Alternative Sources of Oils and Fats from Amazonian Plants: Fatty Acids, Methyl Tocols, Total Carotenoids and Chemical Composition. Food Res. Int. 2019, 116, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.V.d.C.; Leão, K.M.; Speranza, P.; Ribeiro, A.P.B.; Macedo, G.A.; Macedo, J.A. Evaluation of Nanostructured Lipid Carriers Produced with Interesterified Buriti Oil. Food Technol. Biotechnol. 2020, 58, 284–294. [Google Scholar] [CrossRef]
- Reis-Mansur, M.C.P.P.; Firmino Gomes, C.C.; Nigro, F.; Ricci-Júnior, E.; de Freitas, Z.M.F.; Dos Santos, E.P. Nanotechnology as a Tool for Optimizing Topical Photoprotective Formulations Containing Buriti Oil (Mauritia flexuosa) and Dry Aloe Vera Extracts: Stability and Cytotoxicity Evaluations. Pharmaceuticals 2023, 16, 292. [Google Scholar] [CrossRef]
- De Souza Aquino, J.; Batista, K.S.; Araujo-Silva, G.; Dos Santos, D.C.; De Brito, N.J.N.; López, J.A.; Da Silva, J.A.; Das Graças Almeida, M.; Pincheira, C.G.; Magnani, M.; et al. Antioxidant and Lipid-Lowering Effects of Buriti Oil (Mauritia flexuosa L.) Administered to Iron-Overloaded Rats. Molecules 2023, 28, 2585. [Google Scholar] [CrossRef]
- Ferreira, M.O.G.; Lima, I.S.; Ribeiro, A.B.; Lobo, A.O.; Rizzo, M.S.; Osajima, J.A.; Estevinho, L.M.; Silva-Filho, E.C. Biocompatible Gels of Chitosan–Buriti Oil for Potential Wound Healing Applications. Materials 2020, 13, 1977. [Google Scholar] [CrossRef]
- Parente, L.B.; Gellen, L.F.A.; Welter, Á.; Castro, I.P.M.d.; Panontin, J.F. Análise in vitro da atividade antioxidante e determinação do FPS de formulações dermocosméticas contendo óleo de buriti (Mauritia flexuosa L.). Res. Soc. Dev. 2022, 11, e328111032917. [Google Scholar] [CrossRef]
- Castro, G.M.M.A.; Passos, T.S.; Nascimento, S.S.d.C.; Medeiros, I.; Araújo, N.K.; Maciel, B.L.L.; Padilha, C.E.; Ramalho, A.M.Z.; Sousa Júnior, F.C.; De Assis, C.F. Gelatin Nanoparticles Enable Water Dispersibility and Potentialize the Antimicrobial Activity of Buriti (Mauritia flexuosa) Oil. BMC Biotechnol. 2020, 20, 55. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, K.; Takeda, S.; Nakamura, S.; Matsuda, H.; Shimoda, H. Estrogenic and Antiandrogenic Activities of Methoxyflavans Isolated from the Fruit of Mauritia flexuosa (Moriche Palm). J. Food Biochem. 2021, 45, e13583. [Google Scholar] [CrossRef] [PubMed]
- Sano, K.; Kawanobe, H.; Someya, T. Effect of Cupuassu Butter on Human Skin Cells. Data Brief 2018, 21, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, G.N.; Matos, B.N.; Da Silva Brito, G.F.; Da Cunha Miranda, T.; Alencar-Silva, T.; Sodré, F.F.; Gelfuso, G.M.; Cunha-Filho, M.; Carvalho, J.L.; Da Silva, J.K.d.R.; et al. Skin Regenerative Potential of Cupuaçu Seed Extract (Theobroma grandiflorum), a Native Fruit from the Amazon: Development of a Topical Formulation Based on Chitosan-Coated Nanocapsules. Pharmaceutics 2022, 14, 207. [Google Scholar] [CrossRef]
- Benlloch-Tinoco, M.; Nuñez Ramírez, J.M.; García, P.; Gentile, P.; Girón-Hernández, J. Theobroma Genus: Exploring the Therapeutic Potential of T. grandiflorum and T. bicolor in Biomedicine. Food Biosci. 2024, 61, 104755. [Google Scholar] [CrossRef]
- Fleck, C.A.; Newman, M. Advanced Skin Care—A Novel Ingredient. J. Am. Coll. Clin. Wound Spec. 2012, 4, 92–94. [Google Scholar] [CrossRef][Green Version]
- Aparicio-Álvarez, C.M.; Espinoza-Salazar, C.E.; Del Carpio-Jiménez, C. In Vitro Antioxidant Activity and in Vivo Photoprotective Effect of Theobroma grandiflorum Butter Emulgels on Skin of Mice Exposed to UVB Irradiation. Front. Sustain. 2023, 3, 682178. [Google Scholar] [CrossRef]
- Yanes, C.V.I.; Filho, A.A.d.M.; Ribeiro, P.R.E.; Melo, A.C.G.R.; Takahashi, J.A.; Ferraz, V.P.; Mozombite, D.M.S.; Dos Santos, R.C. Inhibition of Acetylcholinesterase and Fatty Acid Composition in Theobroma grandiflorum Seeds. Orbital Electron. J. Chem. 2017, 9, 127–130. [Google Scholar] [CrossRef]
- Pinent, M.; Castell-Auví, A.; Genovese, M.I.; Serrano, J.; Casanova, A.; Blay, M.; Ardévol, A. Antioxidant Effects of Proanthocyanidin-Rich Natural Extracts from Grape Seed and Cupuassu on Gastrointestinal Mucosa. J. Sci. Food Agric. 2016, 96, 178–182. [Google Scholar] [CrossRef]
- Andrade, J.K.S.; Barros, R.G.C.; Pereira, U.C.; Gualberto, N.C.; De Oliveira, C.S.; Shanmugam, S.; Narain, N. α-Amylase Inhibition, Cytotoxicity and Influence of the In Vitro Gastrointestinal Digestion on the Bioaccessibility of Phenolic Compounds in the Peel and Seed of Theobroma grandiflorum. Food Chem. 2022, 373, 131494. [Google Scholar] [CrossRef]
- Bastos, I.S. Avaliação Da Atividade Antibacteriana, Antifúngica e Antimalárica de Extratos, Frações e Composto Obtidos de Plantas Da Região Amazônica. Master’s Dissertation, Universidade Federal do Amazonas, Manaus, Brazil, 2015. [Google Scholar]
- Teixeira, G.L.; Maciel, L.G.; Gonçalves, C.B.; Mazzutti, S.; Ferreira, S.R.S.; Block, J.M. Composition, Thermal Behavior and Antioxidant Activity of Pracaxi (Pentaclethra macroloba) Seed Oil Obtained by Supercritical CO2. Biocatal. Agric. Biotechnol. 2020, 24, 101521. [Google Scholar] [CrossRef]
- Cardoso, C.R.B.; Souza, M.A.; Ferro, E.A.V.; Favoreto, S.; Pena, J.D.O. Influence of Topical Administration of N-3 and n-6 Essential and n-9 Nonessential Fatty Acids on the Healing of Cutaneous Wounds. Wound Repair Regen. 2004, 12, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.L.A.; Cunha, E.A.; Matias, F.O.; Garcia, P.G.; Danopoulos, P.; Swikidisa, R.; Pinheiro, V.A.; Nogueira, R.J.L. Antimicrobial Activity of Copaiba (Copaifera officinalis) and Pracaxi (Pentaclethra macroloba) Oils against Staphylococcus aureus: Importance in Compounding for Wound Care. Int. J. Pharm. Compd. 2016, 20, 58–62. [Google Scholar] [PubMed]
- Ruthig, D.J.; Meckling-Gill, K.A. Both (n-3) and (n-6) Fatty Acids Stimulate Wound Healing in the Rat Intestinal Epithelial Cell Line, IEC-6. J. Nutr. 1999, 129, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.V.; Banov, F.; Banov, D. Use of a Topical Anhydrous Silicone Base Containing Fatty Acids from Pracaxi Oil in a Patient with a Diabetic Ulcer. SAGE Open Med. Case Rep. 2015, 3, 1–4. [Google Scholar] [CrossRef]
- Leal, I.C.R.; Júnior, I.I.; Pereira, E.M.; Laport, M.d.S.; Kuster, R.M.; Dos Santos, K.R.N. Pentaclethra macroloba Tannins Fractions Active against Methicillin-Resistant Staphylococcal and Gram-Negative Strains Showing Selective Toxicity. Rev. Bras. Farmacogn. 2011, 21, 991–999. [Google Scholar] [CrossRef]
- Huh, Y.; Amoa, L.; Misra, R.; Kakarla, S.; Vinagolu-Baur, J. Pracaxi Oil as a Natural Remedy for Hyperpigmentation: Analyzing Its Efficacy in Melasma and Post-Inflammatory Hyperpigmentation. Am. J. Clin. Med. Res. 2024, 4. [Google Scholar] [CrossRef]
- Monoolein. Biological Lipid. MedChemExpress. Available online: https://www.medchemexpress.com/monoolein.html (accessed on 15 May 2025).
- Laurindo, L.F.; Barbalho, S.M.; Araujo, A.C.; Guiger, E.L.; Mondal, A.; Bachtel, G.; Bishayee, A. Açaí (Euterpe oleracea Mart.) in Health and Disease: A Critical Review. Nutrients 2023, 15, 989. [Google Scholar] [CrossRef]
- Costa-Silva, J.H.; Lima, C.R.; Silva, E.J.R.; Araújo, A.V.; Fraga, M.C.C.A.; Ribeiro e Ribeiro, A.; Arruda, A.C.; Lafayette, S.S.L.; Wanderley, A.G. Acute and Subacute Toxicity of the Carapa guianensis Aublet (Meliaceae) Seed Oil. J. Ethnopharmacol. 2008, 116, 495–500. [Google Scholar] [CrossRef]
- Farias, M.P.O.; Sousa, D.P.; Arruda, A.C.; Arruda, M.; Wanderley, A.G.; Alves, L.; Da Gloria Faustino, M. Eficácia In Vitro Do Óleo Da Carapa guianensis Aubl. (Andiroba) No Controle de Boophilus Microplus (Acari: Ixodidae). Rev. Bras. Plantas Med. 2007, 9, 68–71. [Google Scholar]
- Teixeira, R.K.C.; Houat, A.d.P.; Costa, F.L.d.S.; Saraiva Filho, J.C.d.P.; Yasojima, E.Y.; Brito, M.V.H. Efeito do óleo de andiroba na sobrevida de camundongos submetidos à sepse abdominal. Rev. Soc. Bras. Clín. Méd. 2012, 10, 407–409. [Google Scholar]
- Mendes, M.C.S.; Oliveira, G.A.L.d.; Lacerda, J.S.; Junior, L.M.R.; Silva, M.L.d.G.d.; Coelho, M.L.; Tome, A.d.R.; Junior, J.S.d.C.; Ferraz, A.d.B.F.; David, J.M.; et al. Evaluation of the Cicatrizant Activity of a Semisolid Pharmaceutical Formulation Obtained from Platonia insignis Mart. Afr. J. Pharm. Pharmacol. 2015, 9, 154–164. [Google Scholar] [CrossRef]
- Feitosa, C.M.; Santos, P.R.P.d.; Freitas, R.M.d.; Rodrigues, A.M.X.; De, G.A.L.O.; Soares da Costa, J., Jr.; Cavalcante, A.d.N. Ensaios pré-clínicos em ratos tratados com 1,3-diestearil-2-oleil-glicerol, constituinte isolado de Platonia insiginis. ConSci. Saúde 2015, 14, 555–567. [Google Scholar] [CrossRef]
- Zanatta, C.F.; Mitjans, M.; Urgatondo, V.; Rocha-Filho, P.A.; Vinardell, M.P. Photoprotective Potential of Emulsions Formulated with Buriti Oil (Mauritia flexuosa) against UV Irradiation on Keratinocytes and Fibroblasts Cell Lines. Food Chem. Toxicol. 2010, 48, 70–75. [Google Scholar] [CrossRef]
- Baby, A.R.; Maciel, C.P.M.; Salgado-Santos, I.M.N.; Dias, T.C.d.S.; Kaneko, T.M.; Consiglieri, V.O.; Velasco, M.V.R. Uso de extratos de plantas em produtos cosméticos. Cosmet. Toilet. 2005, 17, 78–82. [Google Scholar]
- Rodrigues, M.Á.V.; Marangon, C.A.; Leite, P.M.F.; Da Martins, V.C.A.; Nitschke, M.; De Plepis, A.M.G. Emulsões de quitosana/gelatina com óleos de andiroba e de pracaxi: Avaliação da atividade antimicrobiana sobre Staphylococcus aureus. In Ciências Tecnológicas, Exatas e da Terra e seu Alto Grau de Aplicabilidade; Atena Editora: Ponta Grossa, Brazil, 2020; pp. 206–215. ISBN 978-65-86002-63-8. [Google Scholar]
- Escaramele, L.R.; Amorim, L.M.; Martin, M.L.B.; Garcia, A.d.F.S.R. Uso do óleo vegetal de Pracaxi como silicone natural na haste capilar. Braz. J. Nat. Sci. 2020, 3, 514. [Google Scholar] [CrossRef]





| Octonoic | Decanoic | Lauric | Myristic | Palmitic | Palmitoleic | Stearic | Oleic | Linoleic | Linolenic | Arachidic | Behenic | Lignoceric | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C8:0 | C10:0 | C12:0 | C14:0 | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 | C22:0 | C24:0 | ||
| Açai oil Euterpe oleracea | 2 | 1 | 25 | 4 | 2 | 57 | 9 | 1 | [6] | |||||
| Andiroba oil Carapa guianensis | 27 | 1 | 9 | 52 | 9 | 2 | [6] | |||||||
| Bacuri butter Platonia insignis | 1 | 2 | 60 | 7 | 1 | 28 | 2 | [6] | ||||||
| Buriti oil Mauritia flexuosa | 17 | 2 | 75 | 5 | 1 | [7] | ||||||||
| Cupuaçu butter Theobroma grandiflorum | 9 | 36 | 43 | 2 | 7 | 2 | [7] | |||||||
| Pracaxi oil Pentaclethra macroloba | 2 | 4 | 52 | 11 | 1 | 17 | 11 | [6] |
| Octonoic | Decanoic | Lauric | Myristic | Palmitic | Palmitoleic | Stearic | Oleic | Linoleic | Linolenic | Arachidic | Behenic | Lignoceric | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C8:0 | C10:0 | C12:0 | C14:0 | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 | C22:0 | C24:0 | ||
| Almond oil Prunus amygdalus dulcis | 7 | 2 | 67 | 23 | [8] | |||||||||
| Castor oil Ricinus communis | 1 | 1 | 1 | 88 * | 5 | [9] | ||||||||
| Coconut oil Cocos nucifera | 6 | 6 | 47 | 19 | 9 | 3 | 7 | 2 | [9] | |||||
| Olive oil Olea europaea | 11 | 1 | 2 | 75 | 10 | 1 | [9] | |||||||
| Shea butter Vitellaria paradoxa | 4 | 41 | 46 | 7 | 1 | [10] | ||||||||
| Sunflower oil Helianthus annuus | 17 | 1 | 13 | 60 | 8 | [8] |
| Plant/Oil Origin | Type of Biological Activity | References |
|---|---|---|
| Açai Euterpe oleracea fruit oil | Antioxidant | [15,16,46,47,48,49] |
| Anti-inflammatory Antinociceptive | [13,47,48,50] | |
| Antitumoral | [49,51,52] | |
| Antihypercholesterolemic | [53] | |
| Antimicrobial: Staphylococcus aureus | [54] | |
| Andiroba Carapa guianensis seed oil | Antioxidant | [4,19,55] |
| Anti-inflammatory | [4,5,22,25,55,56,57,58,59,60,61] | |
| Wound and tissue healing | [5,56,62,63] | |
| Antitumoral | [59,64,65,66,67] | |
| Anti-allergic | [68,69] | |
| Analgesic, anti-rheumatism, anti-arthritis | [70] | |
| Antimicrobial | [4,55,71,72,73,74] | |
| Antiparasitic | [4,22,71,75,76,77,78,79,80,81,82,83,84,85,86,87] | |
| Bacuri Platonia insignis seed butter | Wound healing | [88,89,90] |
| Cardioprotective | [91] | |
| Hypolipidemic | [91] | |
| Antimicrobial: anti-Leishmaniasis | [88,92,93] | |
| Bacuri Platonia insignis seed extract | Antioxidant | [25,90,94,95,96,97,98,99,100] |
| Anti-glycant | [27] | |
| Nitric oxide inhibitor | [27] | |
| Anti-inflammatory | [27,88,96,101] | |
| Anti-neurodegenerative: neuroprotective and central nervous system stimulator | [94,95,96,98] | |
| Anti-epileptic and anticonvulsant | [90,95,98,102] | |
| Gastroprotective | [103] | |
| Immunomodulatory | [104] | |
| Vasorelaxant | [26] | |
| Antiparasitic: anti-schistosomiasis | [90,96,100,105] | |
| Buriti Mauritia flexuosa fruit oil | Antioxidant | [106,107,108,109,110,111] |
| Anti-inflammatory | [112] | |
| Wound healing | [29,112] | |
| Photoprotective | [109,110,112,113] | |
| Antimicrobial activity: Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Staphylococcus aureus | [106,107,112,114] | |
| Antifungal, antithrombotic, anti-proliferative, antidiabetic, prebiotic action | [29] | |
| Buriti Mauritia flexuosa pulp powder | Estrogenic and antiandrogenic | [115] |
| Cupuaçu Theobroma grandiflorum seed butter | Wound healing | [116,117,118] |
| Emollient effect | [36,119] | |
| Photoprotective | [118,120] | |
| Anti-neurodegenerative | [118,121] | |
| Cupuaçu Theobroma grandiflorum seed extract | Antioxidant | [35,36,38,118,122,123] |
| Antitumoral | [36,118] | |
| Antidiabetic | [118,123] | |
| Antimicrobial: Plasmodium falciparum | [38,124] | |
| Pracaxi Pentaclethra macroloba seed oil | Antioxidant | [41,108,125] |
| Anti-inflammatory | [43,126,127,128] | |
| Wound healing | [43,126,128,129] | |
| Against ulcers, stretch marks | [42,43] | |
| Antimicrobial | [130] | |
| Melanogenese regulation | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merckling-Almeida da Silva, L.; Merckling, N.; Bigi, E.; Cunha de Melo, K.; Popa, I. Review of Biological Activities of Some Rare Oils from Amazonian Plants. Metabolites 2025, 15, 554. https://doi.org/10.3390/metabo15080554
Merckling-Almeida da Silva L, Merckling N, Bigi E, Cunha de Melo K, Popa I. Review of Biological Activities of Some Rare Oils from Amazonian Plants. Metabolites. 2025; 15(8):554. https://doi.org/10.3390/metabo15080554
Chicago/Turabian StyleMerckling-Almeida da Silva, Luana, Nicolas Merckling, Enrico Bigi, Katiane Cunha de Melo, and Iuliana Popa. 2025. "Review of Biological Activities of Some Rare Oils from Amazonian Plants" Metabolites 15, no. 8: 554. https://doi.org/10.3390/metabo15080554
APA StyleMerckling-Almeida da Silva, L., Merckling, N., Bigi, E., Cunha de Melo, K., & Popa, I. (2025). Review of Biological Activities of Some Rare Oils from Amazonian Plants. Metabolites, 15(8), 554. https://doi.org/10.3390/metabo15080554







