Differences in the Profile of Aromatic Metabolites in the Corresponding Blood Serum and Cerebrospinal Fluid Samples of Patients with Secondary Bacterial Meningitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Desing
2.2. Blood Serum and Cerebrospinal Fluid Analysis
2.3. Statistical Analysis and Models
3. Results
3.1. Patients with Long-Term Sequelae of Severe Brain Damage
3.2. Blood Serum and Cerebrospinal Fluid Clinical and Biochemical Analysis
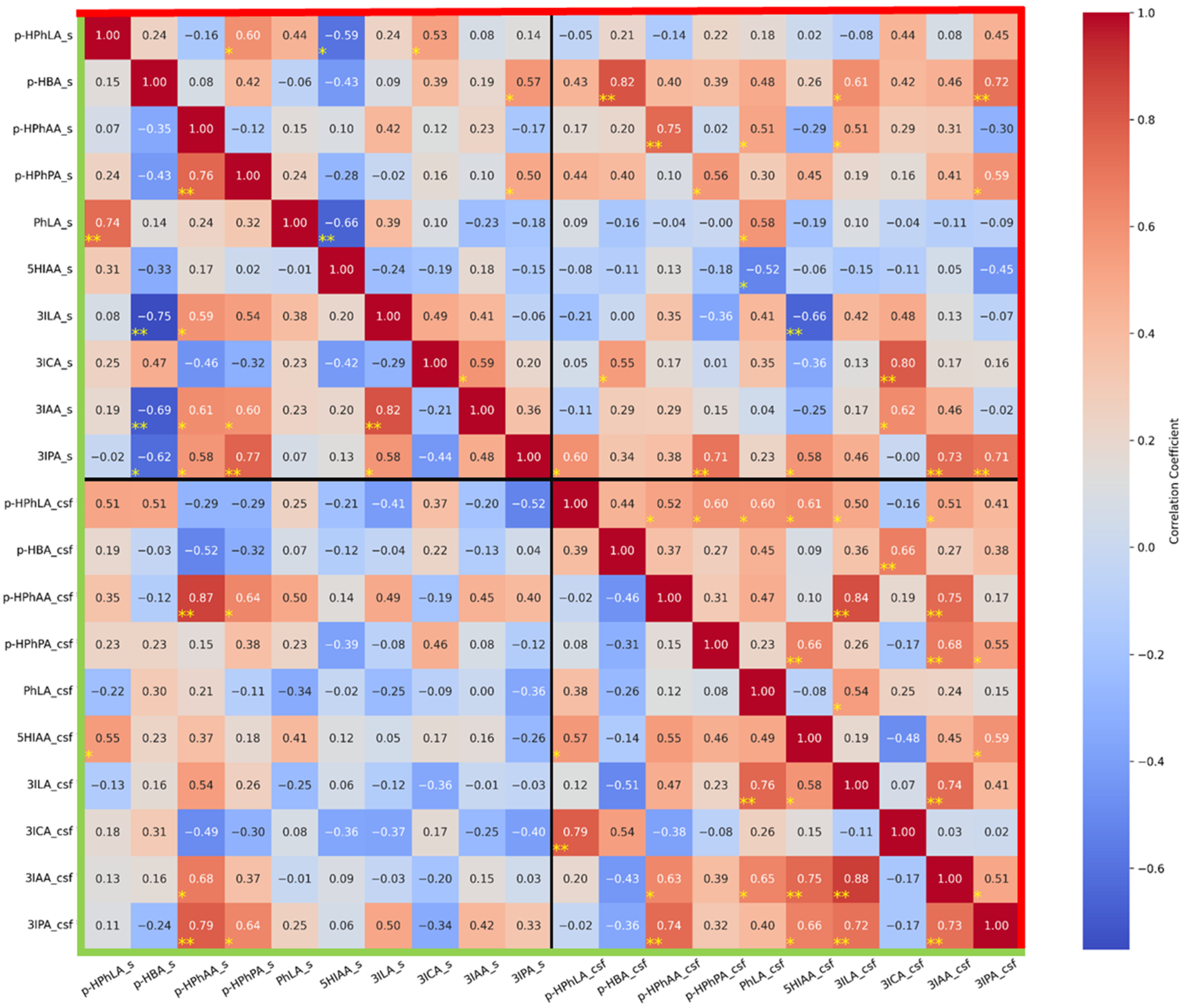
3.3. Blood Serum and Cerebrospinal Fluid Aromatic Metabolites
- (1)
- In group I without secondary meningitis, concentrations of the same metabolites in the serum and CSF correlate significantly and strongly for p-HBA (r = 0.82), p-HPhAA (r = 0.75), 3ICA (r = 0.80), and 3IPA (r = 0.70); and significantly and moderately for p-HPhPA (r = 0.56) and PhLA (r = 0.58). It is noteworthy that such a correlation was not found for p-HPhLA. In group II with secondary meningitis, the concentration of only p-HPhAA in the serum and CSF correlates significantly and strongly (r = 0.87).
- (2)
- In serum samples from group I, there were no strong and significant correlations. In serum samples from group II, there were strong negative and significant correlations between 3ILA and p-HBA (r = −0.75) and strong positive and significant correlations between PhLA and p-HPhLA (r = 0.74), p-HPhPA and p-HPhAA (r = 0.76), 3IPA and p-HPhPA (r = 0.77), and 3IAA and 3ILA (r = 0.82).
- (3)
- In CSF samples from group I, there were strong and significant correlations between p-HPhAA and 3ILA (r = 0.84) and 3IAA (r = 0.75); 3IAA and 3ILA (r = 0.74); and a significant and moderate correlation between 3IAA and 3IPA (r = 0.51). In CSF samples from group II, there were strong and significant correlations between p-HPhLA and 3ICA (r = 0.79); 3ILA and PhLA (r = 0.76); 3IAA and 5HIAA (r = 0.75), and 3ILA (r = 0.88); 3IPA and p-HPhAA (r = 0.74), 3ILA (r = 0.72), and 3IAA (r = 0.73); and a significant and moderate correlation between p-HPhAA and 3IAA (r = 0.63).
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSF | Cerebrospinal fluid |
| p-HPhLA | 4-hydroxyphenyllactic acid |
| PhLA | 3-phenyllactic acid |
| PhPA | 3-phenylpropionic acid |
| p-HBA | 4-hydroxybenzoic acid |
| 3ICA | Indole-3-carboxylic acid |
| 3IPA | Indole-3-propionic acid |
| 3ILA | Indole-3-lactic acid |
| 3IAA | Indole-3-acetic acid |
| 5HIAA | 5-hydroxyindole-3-acetic acid |
| p-HPhAA | 4-hydroxyphenylacetic acid |
| p-HPhPA | 4-hydroxyphenylpropionic acid |
| FDA | Federal drug agency |
| ICU | Intensive care unit |
| CDC | The Centers for Disease Control |
| CNS | Central nervous system |
| PCR | Polymerase chain reaction |
| NMR | Nuclear magnetic resonance |
| BBB | Blood–brain barrier |
| ROC-AUC | Area under the receiver operator characteristic curve |
| UPLC-MS/MS | Ultra-high-pressure liquid chromatography–tandem mass spectrometry |
| GC-MS | Gas chromatography–mass spectrometry |
| CV | Coefficient of variation |
| PCA | Principal component analysis |
| SVC | Support vector classifier |
| TPR | True positive rate |
| FPR | False positive rate |
| CI | Confidence interval |
References
- Tunkel, A.R.; Hasbun, R.; Bhimraj, A.; Byers, K.; Kaplan, S.L.; Scheld, W.M.; van de Beek, D.; Bleck, T.P.; Garton, H.J.L.; Zunt, J.R. 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin. Infect. Dis. 2017, 64, e34–e65. [Google Scholar] [CrossRef]
- Savin, I.; Ershova, K.; Kurdyumova, N.; Ershova, O.; Khomenko, O.; Danilov, G.; Shifrin, M.; Zelman, V. Healthcare-Associated Ventriculitis and Meningitis in a Neuro-ICU: Incidence and Risk Factors Selected by Machine Learning Approach. J. Crit. Care 2018, 45, 95–104. [Google Scholar] [CrossRef]
- Suryaningtyas, W.; Meizikri, R.; Parenrengi, M.; Utomo, B.; Al Fauzi, A.; Bajamal, A. Risk Factors for Mortality in Patients with Bacterial Meningitis Following a Neurosurgical Procedure: A Meta-analysis. World Acad. Sci. J. 2024, 6, 59. [Google Scholar] [CrossRef]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN Surveillance Definition of Health Care-Associated Infection and Criteria for Specific Types of Infections in the Acute Care Setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). CDC/NHSN Surveillance Definitions for Specific Types of Infections. 2025. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf (accessed on 2 May 2025).
- Moorthy, R.K.; Sarkar, H.; Rajshekhar, V. Conservative Antibiotic Policy in Patients Undergoing Non-Trauma Cranial Surgery Does Not Result in Higher Rates of Postoperative Meningitis: An Audit of Nine Years of Narrow-Spectrum Prophylaxis. Br. J. Neurosurg. 2013, 27, 497–502. [Google Scholar] [CrossRef]
- Barker, F.G., 2nd. Efficacy of Prophylactic Antibiotics against Meningitis after Craniotomy: A Meta-Analysis. Neurosurgery 2007, 60, 887–894; discussion 887–894. [Google Scholar] [CrossRef] [PubMed]
- Hussein, K.; Bitterman, R.; Shofty, B.; Paul, M.; Neuberger, A. Management of Post-Neurosurgical Meningitis: Narrative Review. Clin. Microbiol. Infect. 2017, 23, 621–628. [Google Scholar] [CrossRef]
- Borowiak, A.; Safranow, K.; Sarna, A.; Łoniewska, B. Diagnostic Utility of Procalcitonin and Lactate Determination in Cerebrospinal Fluid for the Diagnosis of Neonatal Meningitis. J. Clin. Med. 2025, 14, 414. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Y.; Yang, Y.; Kong, Y.; Peng, Y. The Value of Elevated Cerebrospinal Fluid Lactate Concentrations in Post-Neurosurgical Bacterial Meningitis. BMC Neurol. 2023, 23. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.E.; Spano, M.; Davis-Hayes, C.; Salama, G.R. Lumbar Puncture Complications: A Review of Current Literature. Curr. Pain Headache Rep. 2024, 28, 803–813. [Google Scholar] [CrossRef]
- Omar, A.S.; ElShawarby, A.; Singh, R. Early Monitoring of Ventriculostomy-Related Infections with Procalcitonin in Patients with Ventricular Drains. J. Clin. Monit. Comput. 2015, 29, 759–765. [Google Scholar] [CrossRef]
- Alnomasy, S.F.; Alotaibi, B.S.; Mujamammi, A.H.; Hassan, E.A.; Ali, M.E. Microbial Aspects and Potential Markers for Differentiation between Bacterial and Viral Meningitis among Adult Patients. PLoS ONE 2021, 16, e0251518. [Google Scholar] [CrossRef]
- Lin, Q.; Huang, E.; Fan, K.; Zhang, Z.; Shangguan, H.; Zhang, W.; Fang, W.; Ou, Q.; Liu, X. Cerebrospinal Fluid Neutrophil Gelatinase-Associated Lipocalin as a Novel Biomarker for Postneurosurgical Bacterial Meningitis: A Prospective Observational Cohort Study. Neurosurgery 2024, 95, 1418–1428. [Google Scholar] [CrossRef]
- Jiang, X.; Li, F.; Mei, J.; Wu, T.; Zhu, J.; Li, Z.; Wu, Z.; Jiang, H.; Li, N.; Lei, L. Brain Immune Cell Infiltration and Serum Metabolomic Characteristics Reveal That Lauric Acid Promotes Immune Cell Infiltration in Brain and Streptococcus Suis Meningitis in Mice. Mol. Neurobiol. 2024, 61, 9302–9319. [Google Scholar] [CrossRef] [PubMed]
- Himmelreich, U.; Malik, R.; Kühn, T.; Daniel, H.-M.; Somorjai, R.L.; Dolenko, B.; Sorrell, T.C. Rapid Etiological Classification of Meningitis by NMR Spectroscopy Based on Metabolite Profiles and Host Response. PLoS ONE 2009, 4, e5328. [Google Scholar] [CrossRef]
- Gordon, S.M.; Srinivasan, L.; Taylor, D.M.; Master, S.R.; Tremoglie, M.A.; Hankeova, A.; Flannery, D.D.; Abbasi, S.; Fitzgerald, J.C.; Harris, M.C. Derivation of a Metabolic Signature Associated with Bacterial Meningitis in Infants. Pediatr. Res. 2020, 88, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Hartonen, M.; Mattila, I.; Ruskeepää, A.-L.; Orešič, M.; Hyötyläinen, T. Characterization of Cerebrospinal Fluid by Comprehensive Two-Dimensional Gas Chromatography Coupled to Time-of-Flight Mass Spectrometry. J. Chromatogr. A 2013, 1293, 142–149. [Google Scholar] [CrossRef]
- Rogachev, A.D.; Alemasov, N.A.; Ivanisenko, V.A.; Ivanisenko, N.V.; Gaisler, E.V.; Oleshko, O.S.; Cheresiz, S.V.; Mishinov, S.V.; Stupak, V.V.; Pokrovsky, A.G. Correlation of Metabolic Profiles of Plasma and Cerebrospinal Fluid of High-Grade Glioma Patients. Metabolites 2021, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Murgia, F.; Lorefice, L.; Poddighe, S.; Fenu, G.; Secci, M.A.; Marrosu, M.G.; Cocco, E.; Atzori, L. Multi-Platform Characterization of Cerebrospinal Fluid and Serum Metabolome of Patients Affected by Relapsing–Remitting and Primary Progressive Multiple Sclerosis. J. Clin. Med. 2020, 9, 863. [Google Scholar] [CrossRef]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A Gut Bacterial Pathway Metabolizes Aromatic Amino Acids into Nine Circulating Metabolites. Nature 2017, 551, 648–652. [Google Scholar] [CrossRef]
- Pautova, A.K.; Samokhin, A.S.; Beloborodova, N.V.; Revelsky, A.I. Multivariate Prognostic Model for Predicting the Outcome of Critically Ill Patients Using the Aromatic Metabolites Detected by Gas Chromatography-Mass Spectrometry. Molecules 2022, 27, 4784. [Google Scholar] [CrossRef]
- Pautova, A.K.; Sergeev, A.A.; Beloborodova, N.V. Prospects for Monitoring Aromatic and Mitochondrial Metabolites Using Gas Chromatography–Mass Spectrometry during Extracorporeal Blood Purification in Patients with Sepsis. J. Anal. Chem. 2024, 79, 1951–1955. [Google Scholar] [CrossRef]
- Grin, O.O.; Beloborodova, N.V.; Grekova, M.S.; Pautova, A.K.; Charchyan, E.R.; Akselrod, B.A.; Dymova, O.V.; Rizun, L.I.; Eremenko, A.A.; Babaev, M.A. Prediction of Local Infectious and Inflammatory Complications after Reconstructive Surgery of Aorta. Gen. Reanimatol. 2025, 21, 4–14. [Google Scholar] [CrossRef]
- Pautova, A.K.; Meglei, A.Y.; Chernevskaya, E.A.; Alexandrova, I.A.; Beloborodova, N.V. 4-Hydroxyphenyllactic Acid in Cerebrospinal Fluid as a Possible Marker of Post-Neurosurgical Meningitis: Retrospective Study. J. Pers. Med. 2022, 12, 399. [Google Scholar] [CrossRef] [PubMed]
- FDA. Bioanalytical Method Validation Guidance Industry Biopharmaceutics Bioanalytical Method Validation Guidance Industry Biopharmaceutics Contains Nonbinding Recommendations. 2018. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 2 May 2025).
- EMEA/CHMP, ICH Guideline M10 Bioanalytical Method Validation Study Sample Analysis—Committee Medicinal Products Human Use (CHMP). European Medicines Agency 2022. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-m10-bioanalytical-method-validation-step-5_en.pdf (accessed on 2 May 2025).
- Sobolev, P.D.; Burnakova, N.A.; Beloborodova, N.V.; Revelsky, A.I.; Pautova, A.K. Analysis of 4-Hydroxyphenyllactic Acid and Other Diagnostically Important Metabolites of α-Amino Acids in Human Blood Serum Using a Validated and Sensitive Ultra-High-Pressure Liquid Chromatography-Tandem Mass Spectrometry Method. Metabolites 2023, 13, 1128. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, P.D.; Burnakova, N.A.; Revelsky, A.I.; Zakharchenko, V.E.; Beloborodova, N.V.; Pautova, A.K. A Sensitive Method for the Profiling of Phenyl- and Indole-Containing Metabolites in Blood Serum and Cerebrospinal Fluid Samples of Patients with Severe Brain Damage Using Ultra-High-Pressure Liquid Chromatography-Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 2025, 260, 116803. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Org, N.; Gramfort, A.; Michel, V.; Fr, L.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; et al. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Guyon, I.; Weston, J.; Barnhill, S.; Vapnik, V. Gene Selection for Cancer Classification using Support Vector Machines. Mach. Learn. 2002, 46, 389–422. [Google Scholar]
- Kursa, M.; Rudnicki, W. Feature Selection Boruta Package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef]
- Raschka, S. Model Evaluation, Model Selection, and Algorithm Selection in Machine Learning. arXiv 2018, arXiv:1811.12808. [Google Scholar]
- Likhvantsev, V.V.; Berikashvili, L.B.; Yadgarov, M.Y.; Yakovlev, A.A.; Kuzovlev, A.N. The Tri-Steps Model of Critical Conditions in Intensive Care: Introducing a New Paradigm for Chronic Critical Illness. J. Clin. Med. 2024, 13, 3683. [Google Scholar] [CrossRef]
- Grechko, A.V.; Gurkova, M.M.; Zhdanova, M.A.; Zurabov, A.Y.; Zurabov, F.M.; Kuzovlev, A.N.; Petrova, M.V.; Polyakov, P.A.; Cheboksarov, D.V.; Chernevskaya, E.A.; et al. Prevention of Nosocomial Pneumonia Recurrence Using a Bacteriophage Cocktail in Intensive Care Unit. Anesteziol. Reanimatol. 2024, 2, 39–48. [Google Scholar] [CrossRef]
- Li, Z.; Wu, X.; Yu, J.; Wu, X.; Du, Z.; Sun, Y.; Yuan, Q.; Hu, J. Empirical Combination Antibiotic Therapy Improves the Outcome of Nosocomial Meningitis or Ventriculitis in Neuro-Critical Care Unit Patients. Surg. Infect. 2016, 17, 465–472. [Google Scholar] [CrossRef]
- Chernevskaya, E.; Klimenko, N.; Pautova, A.; Buyakova, I.; Tyakht, A.; Beloborodova, N. Host-Microbiome Interactions Mediated by Phenolic Metabolites in Chronically Critically Ill Patients. Metabolites 2021, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Keszthelyi, D.; Troost, F.J.; Masclee, A.A.M. Understanding the Role of Tryptophan and Serotonin Metabolism in Gastrointestinal Function. Neurogastroenterol. Motil. 2009, 21, 1239–1249. [Google Scholar] [CrossRef]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics Analysis Reveals Large Effects of Gut Microflora on Mammalian Blood Metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef]
- Gertsman, I.; Gangoiti, J.A.; Nyhan, W.L.; Barshop, B.A. Perturbations of Tyrosine Metabolism Promote the Indolepyruvate Pathway via Tryptophan in Host and Microbiome. Mol. Genet. Metab. 2015, 114, 431–437. [Google Scholar] [CrossRef]
- Nemet, I.; Li, X.S.; Haghikia, A.; Li, L.; Wilcox, J.; Romano, K.A.; Buffa, J.A.; Witkowski, M.; Demuth, I.; König, M.; et al. Atlas of Gut Microbe-Derived Products from Aromatic Amino Acids and Risk of Cardiovascular Morbidity and Mortality. Eur. Heart J. 2023, 44, 3085–3096. [Google Scholar] [CrossRef]
- Aziz-Zadeh, L.; Ringold, S.M.; Jayashankar, A.; Kilroy, E.; Butera, C.; Jacobs, J.P.; Tanartkit, S.; Mahurkar-Joshi, S.; Bhatt, R.R.; Dapretto, M.; et al. Relationships between Brain Activity, Tryptophan-Related Gut Metabolites, and Autism Symptomatology. Nat. Commun. 2025, 16, 3465. [Google Scholar] [CrossRef] [PubMed]
- Kinra, M.; Joseph, A.; Nampoothiri, M.; Arora, D.; Mudgal, J. Inhibition of NLRP3-Inflammasome Mediated IL-1β Release by Phenylpropanoic Acid Derivatives: In-Silico and in-Vitro Approach. Eur. J. Pharm. Sci. 2021, 157, 105637. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Devotta, H.; Li, J.; Lunjani, N.; Sadlier, C.; Lavelle, A.; Albrich, W.C.; Walter, J.; O’Toole, P.W.; O’Mahony, L. Dysrupted Microbial Tryptophan Metabolism Associates with SARS-CoV-2 Acute Inflammatory Responses and Long COVID. Gut Microbes 2024, 16, 2429754. [Google Scholar] [CrossRef]
- Wei, W.; Liu, Y.; Hou, Y.; Cao, S.; Chen, Z.; Zhang, Y.; Cai, X.; Yan, Q.; Li, Z.; Yuan, Y.; et al. Psychological Stress-Induced Microbial Metabolite Indole-3-Acetate Disrupts Intestinal Cell Lineage Commitment. Cell Metab. 2024, 36, 466–483.e7. [Google Scholar] [CrossRef]
- Krishnan, S.; Ding, Y.; Saedi, N.; Choi, M.; Sridharan, G.V.; Sherr, D.H.; Yarmush, M.L.; Alaniz, R.C.; Jayaraman, A.; Lee, K. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep. 2018, 23, 1099–1111. [Google Scholar] [CrossRef]
- Ehrlich, A.M.; Pacheco, A.R.; Henrick, B.M.; Taft, D.; Xu, G.; Huda, M.N.; Mishchuk, D.; Goodson, M.L.; Slupsky, C.; Barile, D.; et al. Indole-3-Lactic Acid Associated with Bifidobacterium-Dominated Microbiota Significantly Decreases Inflammation in Intestinal Epithelial Cells. BMC Microbiol. 2020, 20, 357. [Google Scholar] [CrossRef]
- Jayamohananan, H.; Manoj Kumar, M.K.; P, A.T. 5-HIAA as a Potential Biological Marker for Neurological and Psychiatric Disorders. Adv. Pharm. Bull. 2019, 9, 374–381. [Google Scholar] [CrossRef]
- Zaragozá, R. Transport of Amino Acids Across the Blood-Brain Barrier. Front. Physiol. 2020, 11, 973. [Google Scholar] [CrossRef] [PubMed]
- Westholm, D.E.; Rumbley, J.N.; Salo, D.R.; Rich, T.P.; Anderson, G.W. Organic anion-transporting polypeptides at the blood-brain and blood-cerebrospinal fluid barriers. Curr. Top. Dev. Biol. 2007, 80, 135–170. [Google Scholar]
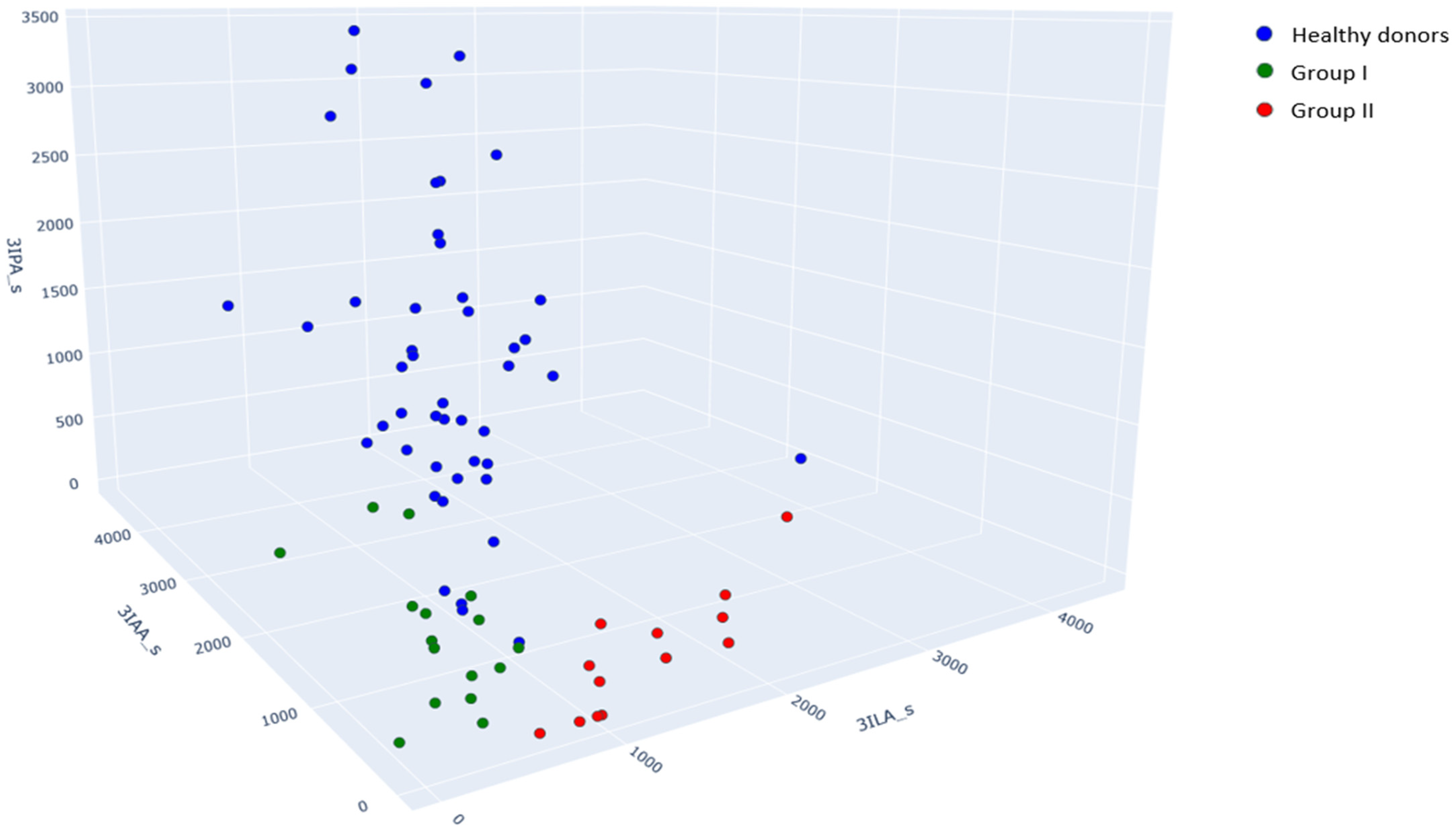

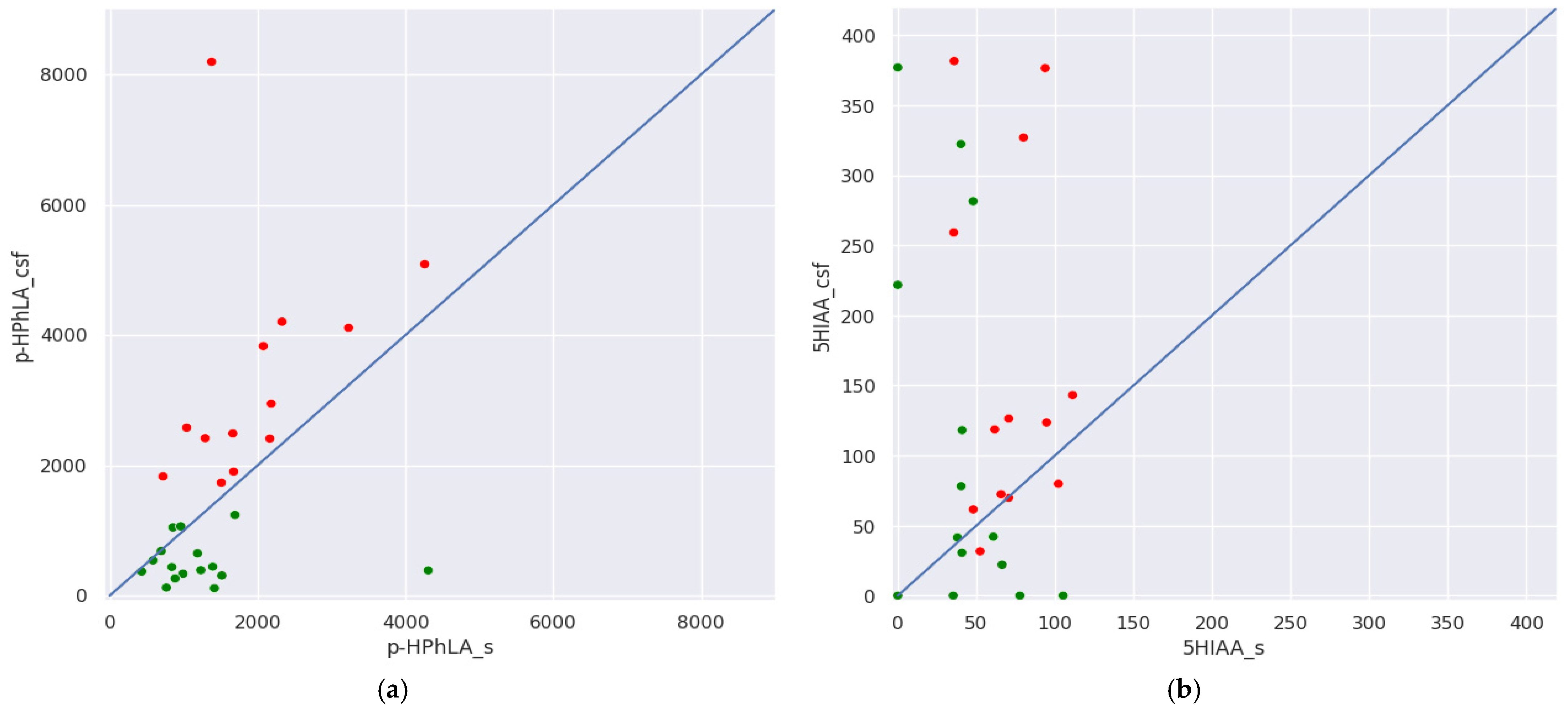
| Parameter | Group I. Patients Without Secondary Bacterial Meningitis (N = 11) | Group II. Patients with Secondary Bacterial Meningitis (N = 4) |
|---|---|---|
| Age, years | 55 (38, 62) | 58 (45, 64) |
| Sex | 8 men, 3 women | 2 men, 2 women |
| Length of stay at the time of sample collection, days | 17 (13, 24) | 31 (16, 60) |
| Depressed consciousness | 10 | 2 |
| Elevated body temperature, >38 °C | 8 | 4 |
| Deaths | 0 | 4 |
| Primary Diagnoses | ||
| Stroke | 6 | 2 |
| Cerebral hematoma | 7 | 2 |
| Traumatic brain injury | 7 | 1 |
| Stroke | 6 | 2 |
| Surgical Risk Factors of Bacterial Meningitis | ||
| Ventriculoperitoneal shunt | 5 | 1 |
| Invasive monitoring of intracranial pressure | 6 | 3 |
| Decompressive trepanation | 6 | 1 |
| Mechanical ventilation and tracheostomy | 8 | 3 |
| Concomitant Diseases | ||
| Diabetes | 2 | 1 |
| Hypertension | 8 | 3 |
| Ischemic heart disease | 8 | 3 |
| Gastrointestinal disorders | 8 | 2 |
| Infectious Complications | ||
| Meningitis | 0 | 4 |
| Pneumonia | 8 | 4 |
| Urogenital tract infections | 5 | 2 |
| Results of Microbiological Analysis | ||
| CSF | Staphylococcus epidermidis: 3 | Klebsiella pneumonia: 4 Candida parapsilosis: 1 |
| Bronchoalveolar lavage | Klebsiella pneumonia: 7 Pseudomonas spp.: 1 Acinetobacter spp.: 2 Stenotrophomonas maltophilia: 1 | Klebsiella pneumonia: 2 Acinetobacter spp.: 2 |
| Blood | Staphylococcus spp.: 3 Acinetobacter spp.: 1 | Klebsiella pneumonia: 1 |
| Urine | Klebsiella pneumonia: 1 Candida albicans: 6 Escherichia coli: 8 | Candida albicans: 1 Proteus spp.: 1 |
| Parameter | Reference Value | Group I. Samples (n = 16) from Patients Without Secondary Bacterial Meningitis | Group II. Samples (n = 13) from Patients with Secondary Bacterial Meningitis | p-Value * |
|---|---|---|---|---|
| Blood | ||||
| Leukocytes, 109 | 4.0–9.0 | 10.6 (7.8, 11.4) | 11.5 (8.6, 16.1) | 0.25 |
| Hemoglobin, g/L | 130–160 | 119.5 (100.2, 129.2) | 90.0 (81.0, 104.0) | 0.02 |
| Hematocrit, % | 35.0–50.0 | 35.2 (29.9, 38.2) | 28.8 (24.1, 31.7) | 0.03 |
| Platelets, 109 | 180–320 | 286 (255, 337) | 194 (147, 276) | 0.25 |
| Total protein, g/L | 66.0–88.0 | 61.0 (54.9, 65.6) | 51.1 (50.4, 52.2) | 0.03 |
| Glucose, mmol/L | 3.9–6.4 | 5.8 (5.6, 6.2) | 7.6 (5.4, 10.1) | 0.03 |
| Albumin, g/L | 34.0–50.0 | 35.8 (32.8, 38.5) | 28.7 (25.5, 31.2) | 0.03 |
| Creatinine, μmol/L | 53.0–115.0 | 72.3 (55.2, 80.7) | 53.9 (49.8, 66.4) | 0.25 |
| Urea, mmol/L | 3.0–9.2 | 4.3 (3.1, 5.1) | 6.5 (5.2, 12.1) | 0.01 |
| C-reactive protein, mg/L | 0.0–5.0 | 21.1 (0.7, 73.6) | 31.6 (21.2, 66.5) | 0.49 |
| International normalized ratio | 0.8–1.2 | 10.6 (7.8, 11.4) | 11.5 (8.6, 16.1) | 0.75 |
| Activated partial thromboplastin time, sec | 25.4–36.9 | 119.5 (100.2, 129.2) | 90.0 (81.0, 104.0) | 0.05 |
| Cerebrospinal Fluid | ||||
| Leukocyte count, cells/mm3 | 2–8 | 12 (4, 20) >300: n = 0 <300: n = 16 | 1586 (244, 2133) >300: n = 9 <300: n = 4 | 0.1 |
| Neutrophils, % | 3–5 | 47 (26, 62) >80: n = 0 <80: n = 16 | 91 (88, 93) >80: n = 11 <80: n = 2 | 0.03 |
| Glucose, mmol/L | 2.8–3.9 | 3.8 (2.9, 4.2) <2.7: n = 5 >2.7: n = 11 | 1.3 (0.5, 3.7) <2.7: n = 9 >2.7: n = 4 | 0.99 |
| Protein, g/L | 0.1–0.3 | 0.9 (0.5, 1.1) >1.0: n = 5 <1.0: n = 11 | 1.7 (1.0, 5.9) >1.0: n = 10 <1.0: n = 3 | 0.02 |
| Lymphocytes, % | 90–95 | 69 (39, 84) | 6 (5, 9) | <0.001 |
| Aromatic Metabolite | Serum Samples from Healthy Donors (n = 48) | Group I. Serum Samples (n = 16) from Patients Without Secondary Bacterial Meningitis | p-Value * | Group II. Serum Samples (n = 13) from Patients with Secondary Bacterial Meningitis | p-Value * |
|---|---|---|---|---|---|
| 4-Hydroxyphenyllactic acid (p-HPhLA) | 1212 (959, 1557) | 975 (821, 1398) | 0.89 | 1676 (1378, 2184) | <0.001 |
| 4-Hydroxybenzoic acid (p-HBA) | 18 (16, 24) | 12,020 (8408, 21,565) | <0.001 | 10,300 (7523, 19,520) | <0.001 |
| 4-Hydroxyphenylacetic acid (p-HPhAA) | 316 (0, 461) | 1238 (479, 1918) | <0.001 | 2636 (500, 5512) | <0.001 |
| 3-Phenylpropionic acid (PhPA) | 458 (269, 724) | <250 | - | <250 | - |
| 4-Hydroxyphenylpropionic acid (p-HPhPA) | 9 (<7.5, 14) | 36 (16, 58) | <0.001 | <7.5 (<7.5, 10) | 0.35 |
| 3-Phenyllactic acid (PhLA) | 315 (249, 391) | 2445 (1713, 3856) | <0.001 | 5602 (4610, 8132) | <0.001 |
| 5-Hydroxyindole-3-acetic acid (5HIAA) | 78 (64, 93) | 40 (20, 51) | 0.43 | 71 (52, 94) | 0.73 |
| Indole-3-lactic acid (3ILA) | 1068 (839, 1272) | 595 (433, 676) | <0.001 | 1547 (957, 1996) | 0.27 |
| Indole-3-carboxylic acid (3ICA) | 22 (18, 26) | 33 (29, 39) | <0.001 | 31 (26, 42) | <0.001 |
| Indole-3-acetic acid (3IAA) | 1823 (1513, 2377) | 928 (673, 1161) | <0.001 | 362 (200, 624) | <0.001 |
| Indole-3-propionic acid (3IPA) | 1362 (773, 2087) | <200 (<200, 245) | - | <200 | - |
| Aromatic Metabolite | Biological Sample | Group I. Samples (n = 16) from Patients Without Secondary Bacterial Meningitis | Group II. Samples (n = 13) from Patients with Secondary Bacterial Meningitis | p-Value * |
|---|---|---|---|---|
| 4-Hydroxyphenyllactic acid (p-HPhLA) | Serum | 975 (821, 1398) | 1676 (1378, 2184) | 0.13 |
| CSF | 415 (329, 658) | 2578 (2410, 4111) | <0.001 | |
| 4-Hydroxybenzoic acid (p-HBA) | Serum | 12,020 (8408, 21,565) | 10,300 (7523, 19,520) | 0.76 |
| CSF | 32 (26, 53) | 42 (24, 99) | 0.09 | |
| 4-Hydroxyphenylacetic acid (p-HPhAA) | Serum | 1238 (479, 1918) | 2636 (500, 5512) | 0.09 |
| CSF | 141 (66, 280) | 1827 (362, 3894) | <0.001 | |
| 3-Phenylpropionic acid (PhPA) | Serum | <250 | <250 | - |
| CSF | <25 | <25 | - | |
| 4-Hydroxyphenylpropionic acid (p-HPhPA) | Serum | 36 (16, 58) | <7.5 (<7.5, 10) | 0.04 |
| CSF | <7.5 (<7.5, 2) | <7.5 | - | |
| 3-Phenyllactic acid (PhLA) | Serum | 2445 (1713, 3856) | 5602 (4610, 8132) | 0.10 |
| CSF | 50 (35, 69) | 572 (431, 678) | 0.03 | |
| 5-Hydroxyindole-3-acetic acid (5HIAA) | Serum | 40 (20, 51) | 71 (52, 94) | 0.01 |
| CSF | 36 (20, 144) | 124 (72, 259) | 0.27 | |
| Indole-3-lactic acid (3ILA) | Serum | 595 (433, 676) | 1547 (957, 1996) | <0.001 |
| CSF | 8 (3, 14) | 156 (64, 476) | 0.02 | |
| Indole-3-carboxylic acid (3ICA) | Serum | 33 (29, 39) | 31 (26, 42) | 0.70 |
| CSF | 11 (7, 13) | 13 (12, 20) | 0.12 | |
| Indole-3-acetic acid (3IAA) | Serum | 928 (673, 1161) | 362 (200, 624) | 0.04 |
| CSF | 28 (21, 51) | 90 (37, 148) | 0.05 | |
| Indole-3-propionic acid (3IPA) | Serum | <200 (<200, 245) | <200 | 0.38 |
| CSF | 3 (2, 6) | 4 (2, 4) | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pautova, A.K.; Meinarovich, P.A.; Zakharchenko, V.E.; Sobolev, P.D.; Burnakova, N.A.; Beloborodova, N.V. Differences in the Profile of Aromatic Metabolites in the Corresponding Blood Serum and Cerebrospinal Fluid Samples of Patients with Secondary Bacterial Meningitis. Metabolites 2025, 15, 527. https://doi.org/10.3390/metabo15080527
Pautova AK, Meinarovich PA, Zakharchenko VE, Sobolev PD, Burnakova NA, Beloborodova NV. Differences in the Profile of Aromatic Metabolites in the Corresponding Blood Serum and Cerebrospinal Fluid Samples of Patients with Secondary Bacterial Meningitis. Metabolites. 2025; 15(8):527. https://doi.org/10.3390/metabo15080527
Chicago/Turabian StylePautova, Alisa K., Peter A. Meinarovich, Vladislav E. Zakharchenko, Pavel D. Sobolev, Natalia A. Burnakova, and Natalia V. Beloborodova. 2025. "Differences in the Profile of Aromatic Metabolites in the Corresponding Blood Serum and Cerebrospinal Fluid Samples of Patients with Secondary Bacterial Meningitis" Metabolites 15, no. 8: 527. https://doi.org/10.3390/metabo15080527
APA StylePautova, A. K., Meinarovich, P. A., Zakharchenko, V. E., Sobolev, P. D., Burnakova, N. A., & Beloborodova, N. V. (2025). Differences in the Profile of Aromatic Metabolites in the Corresponding Blood Serum and Cerebrospinal Fluid Samples of Patients with Secondary Bacterial Meningitis. Metabolites, 15(8), 527. https://doi.org/10.3390/metabo15080527









