Site-Specific Trafficking of Lipid and Polar Metabolites in Adipose and Muscle Tissue Reveals the Impact of Bariatric Surgery-Induced Weight Loss: A 6-Month Follow-Up Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants and Procedures
2.2. Materials
2.3. Metabolomics Analyses
2.4. Statistical Analyses
2.4.1. Multivariate Statistics
2.4.2. Univariate Statistics
2.5. Jaccard Similarity Traffic Analysis
3. Results
3.1. Diet and Bariatric-Induced Body Weight and Composition Changes
3.2. Multivariate Metabolomic Profiling of Diet and Bariatric Surgery-Induced Weight Loss Shows Differences in Plasma and Tissue Responses
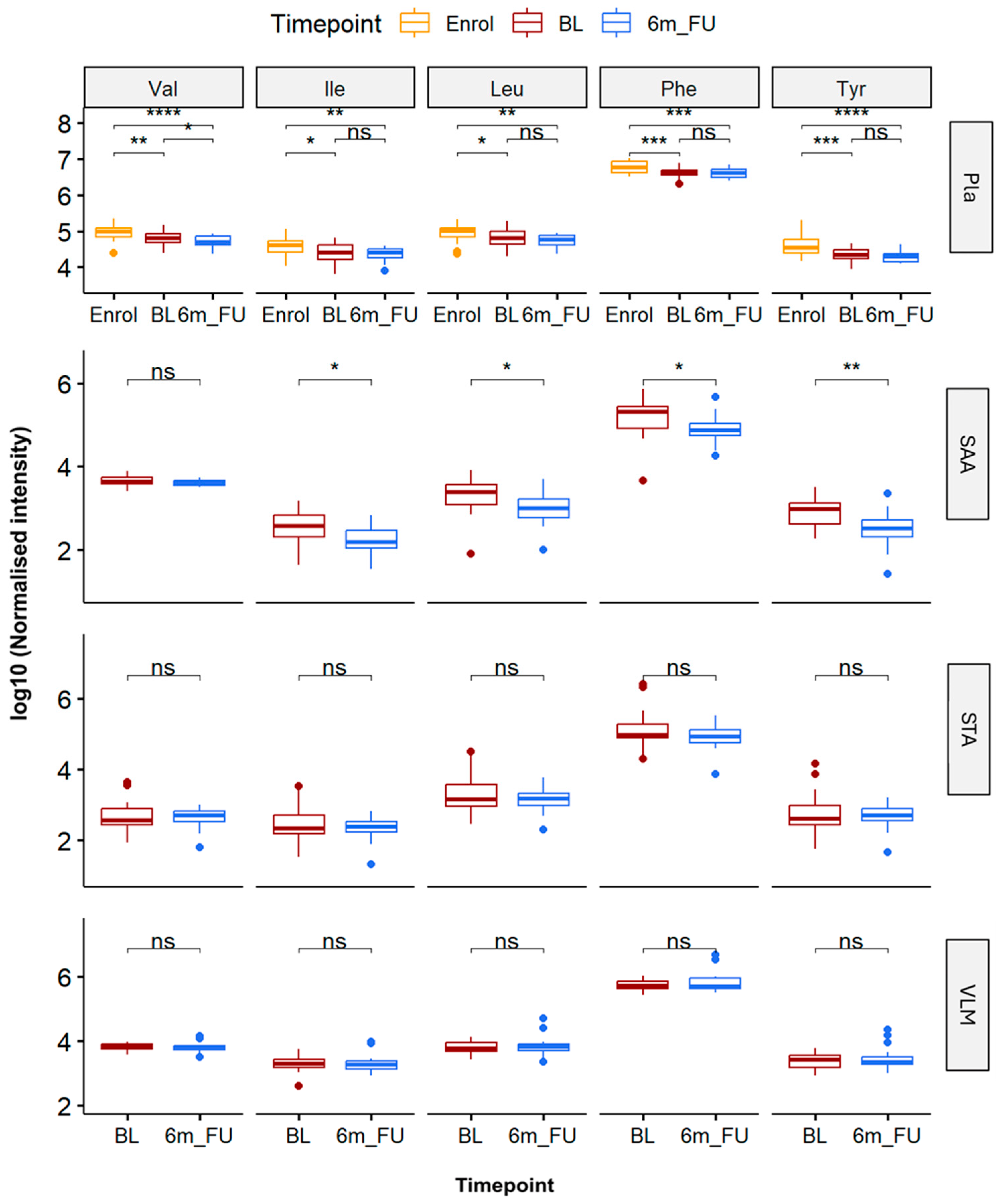
3.3. Analysis of Branched Chain and Aromatic Amino Acid Levels Within Plasma and Tissue Biopsies in Response to Bariatric Surgery Procedures
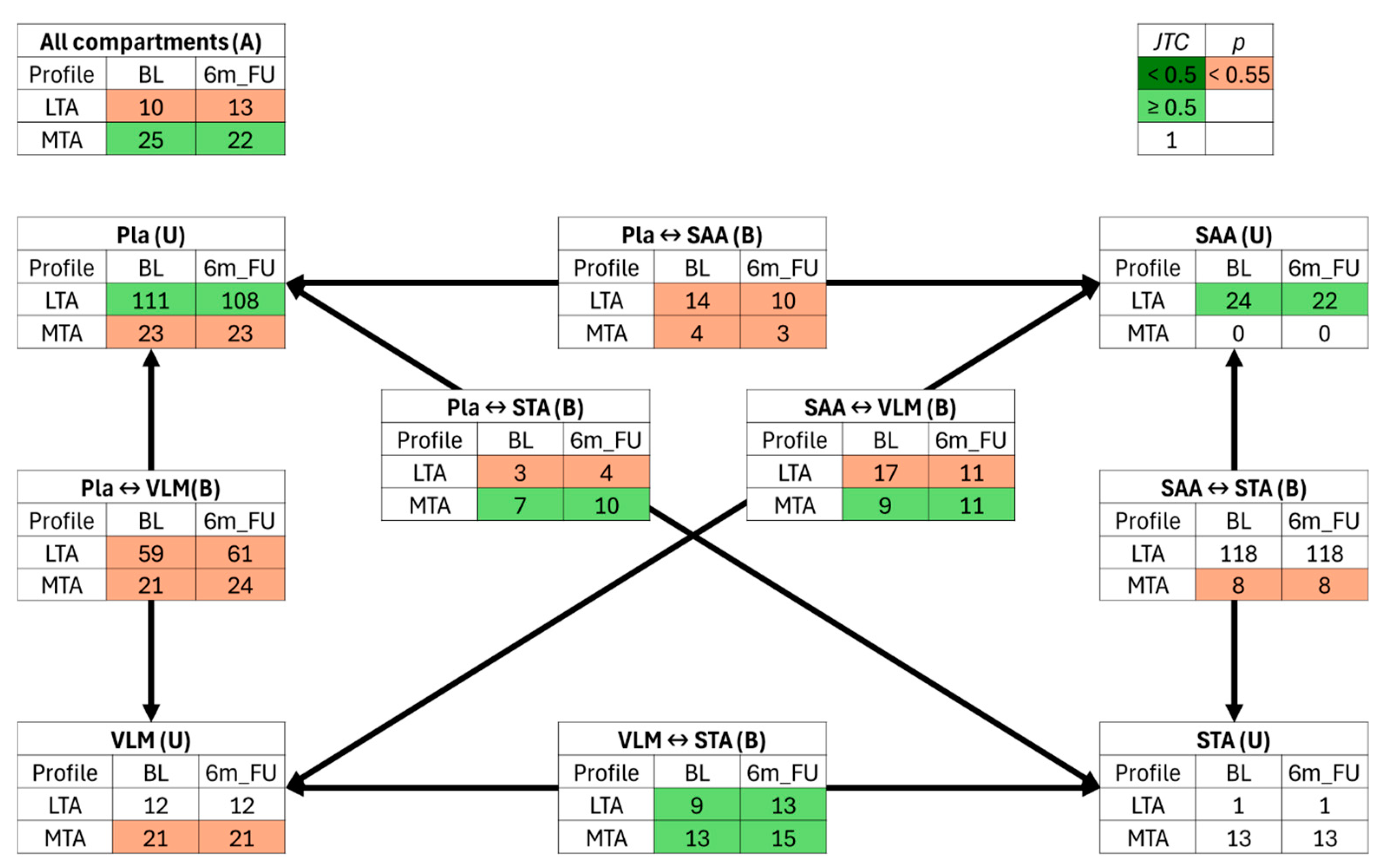
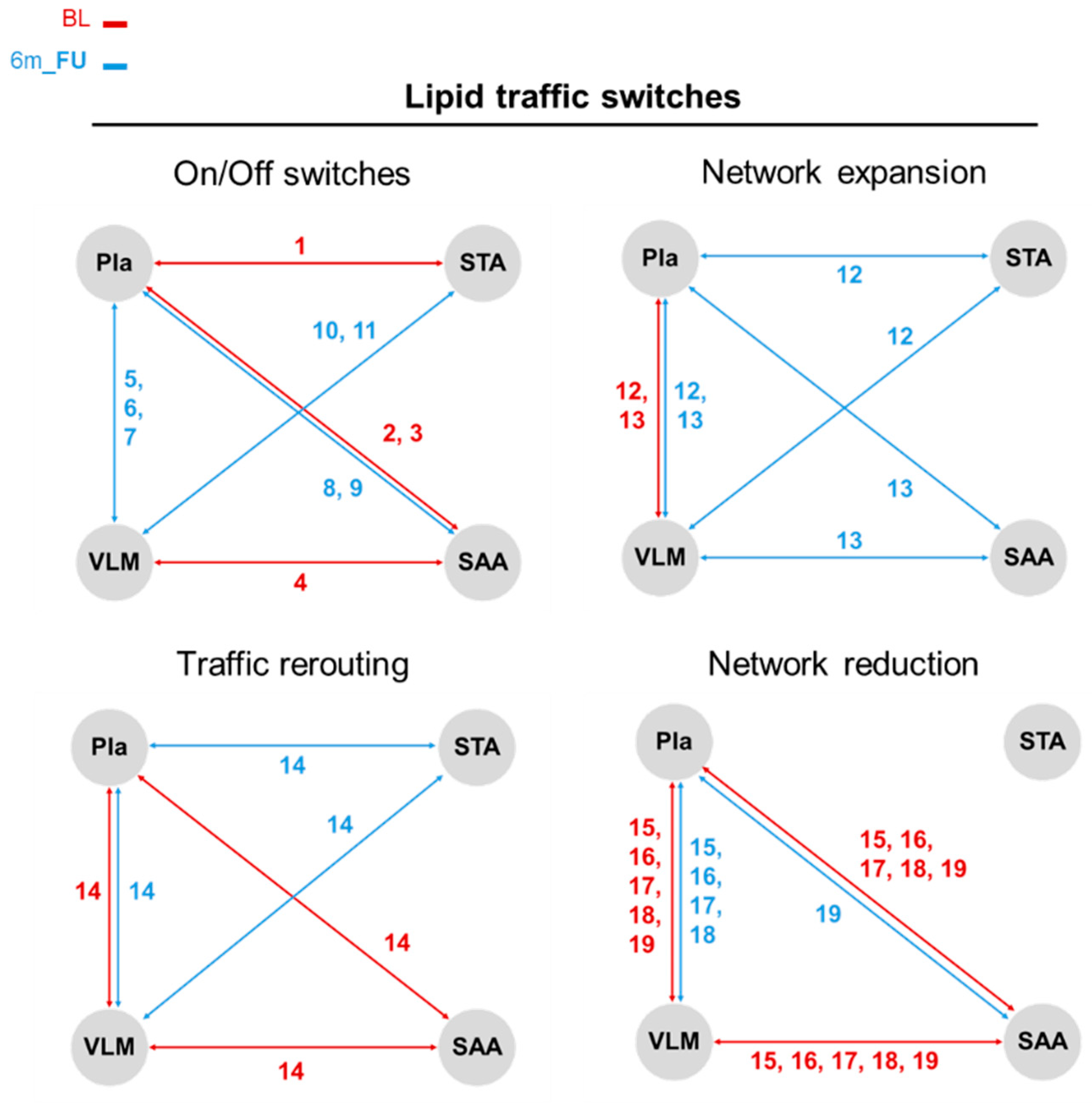
3.4. Jaccard–Tanimoto Traffic Analysis of Lipid and Polar Metabolite Changes Between Sites Identifies Changes Due to Bariatric Surgery Induced Weight Loss
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 6m_FU | 6-month follow-up |
| AAA | Aromatic amino acids |
| ADMA | Asymmetric dimethylarginine |
| BCAA | Branched chain amino acids |
| BL | Baseline |
| BMI | Body mass index |
| CID | Clinical investigation day |
| CV | Coefficient of variation |
| CV-ANOVA | Cross validation–analysis of variance |
| DSAA | Deep subcutaneous abdominal adipose |
| ENFC | Error-normalised fold change |
| Enrol | Enrolment |
| ER | Endoplasmic reticulum |
| ESI | Electrospray ionisation |
| FC | Fold change |
| IAA | Intraabdominal adipose |
| Ile | Isoleucine |
| JTC | Jaccard–Tanimoto coefficient |
| Leu | Leucine |
| LTA | Lipid traffic analysis |
| MTA | Polar metabolite traffic analysis |
| NAC | N-Acetylcarnosine |
| PC | Phosphatidylcholine |
| PCA | Principle component analysis |
| PE | Phosphatidylethanolamine |
| Phe | Phenylalanine |
| PI | Phosphatidylinositol |
| Pla | Plasma |
| PLS-DA | Partial least squares discriminant analysis |
| QC | Quality control |
| RAM | Rectus abdominis muscle |
| SAA | Subcutaneous abdominal adipose |
| SM | Sphingomyelin |
| STA | Subcutaneous thigh adipose |
| T2D | Type 2 diabetes |
| TMAO | Trimethylamine N-oxide |
| Tyr | Tyrosine |
| UHPLC-MS | Ultra-high-performance liquid chromatography–mass spectrometry |
| Val | Valine |
| VIP | Variable of importance in projection |
| VLED | Very low energy diet |
| VLM | Vastus lateralis muscle |
References
- Dhokte, S.; Czaja, K. Visceral adipose tissue: The hidden culprit for type 2 diabetes. Nutrients 2024, 16, 1015. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. An overview of obesity-related complications: The epidemiological evidence linking body weight and other markers of obesity to adverse health outcomes. Diabetes Obes. Metab. 2025, 27 (Suppl. 2), 3–19. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J. The causal role of ectopic fat deposition in the pathogenesis of metabolic syndrome. Int. J. Mol. Sci. 2024, 25, 13238. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Y.; Mao, J.; Yuan, Y.; Luo, P.; Wang, G.; Zhou, S. Features, functions, and associated diseases of visceral and ectopic fat: A comprehensive review. Obesity 2025, 33, 825–838. [Google Scholar] [CrossRef]
- Ding, L.; Fan, Y.; Li, H.; Zhang, Y.; Qi, D.; Tang, S.; Cui, J.; He, Q.; Zhuo, C.; Liu, M. Comparative effectiveness of bariatric surgeries in patients with obesity and type 2 diabetes mellitus: A network meta-analysis of randomized controlled trials. Obes. Rev. 2020, 21, e13030. [Google Scholar] [CrossRef]
- Topart, P. Obesity surgery: Which procedure should we choose and why? J. Visc. Surg. 2023, 160, S30–S37. [Google Scholar] [CrossRef] [PubMed]
- Holderbaum, M.; Casagrande, D.S.; Sussenbach, S.; Buss, C. Effects of very low calorie diets on liver size and weight loss in the preoperative period of bariatric surgery: A systematic review. Surg. Obes. Relat. Dis. 2018, 14, 237–244. [Google Scholar] [CrossRef]
- McKechnie, T.; Lee, Y.; Dionne, J.; Doumouras, A.; Parpia, S.; Bhandari, M.; Eskicioglu, C. Very low energy diets prior to bariatric surgery may reduce postoperative morbidity: A systematic review and meta-analysis of randomized controlled trials. Front. Nutr. 2023, 10, 1211575. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Hruby, A.; Toledo, E.; Clish, C.B.; Martínez-González, M.A.; Salas-Salvadó, J.; Hu, F.B. Metabolomics in prediabetes and diabetes: A systematic review and meta-analysis. Diabetes Care 2016, 39, 833–846. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Pastor-Villaescusa, B.; Gil, A. Are we close to defining a metabolomic signature of human obesity? A systematic review of metabolomics studies. Metabolomics 2019, 15, 93. [Google Scholar] [CrossRef]
- Ruiz-Canela, M.; Hruby, A.; Clish, C.B.; Liang, L.; Martínez-González, M.A.; Hu, F.B. Comprehensive metabolomic profiling and incident cardiovascular disease: A systematic review. J. Am. Heart Assoc. 2017, 6, e005705. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, C.; Hould, F.S.; Lebel, S.; Biron, S.; Brochu, G.; Tchernof, A. Omental and subcutaneous adipose tissue steroid levels in obese men. Steroids 2006, 71, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Hellmuth, C.; Demmelmair, H.; Schmitt, I.; Peissner, W.; Blüher, M.; Koletzko, B. Association between plasma nonesterified fatty acids species and adipose tissue fatty acid composition. PLoS ONE 2013, 8, e74927. [Google Scholar] [CrossRef]
- Walker, G.E.; Verti, B.; Marzullo, P.; Savia, G.; Mencarelli, M.; Zurleni, F.; Liuzzi, A.; Di Blasio, A.M. Deep subcutaneous adipose tissue: A distinct abdominal adipose depot. Obesity 2007, 15, 1933–1943. [Google Scholar] [CrossRef] [PubMed]
- Soeters, M.R.; Sauerwein, H.P.; Duran, M.; Wanders, R.J.; Ackermans, M.T.; Fliers, E.; Houten, S.M.; Serlie, M.J. Muscle acylcarnitines during short-term fasting in lean healthy men. Clin. Sci. 2009, 116, 585–592. [Google Scholar] [CrossRef]
- van der Kolk, B.W.; Pirinen, E.; Nicoll, R.; Pietiläinen, K.H.; Heinonen, S. Subcutaneous adipose tissue and skeletal muscle mitochondria following weight loss. Trends Endocrinol. Metab. 2025, 36, 339–363. [Google Scholar] [CrossRef]
- Wu, Z.E.; Kruger, M.C.; Cooper, G.J.S.; Poppitt, S.D.; Fraser, K. Tissue-specific sample dilution: An important parameter to optimise prior to untargeted LC-MS metabolomics. Metabolites 2019, 9, 124. [Google Scholar] [CrossRef]
- Wu, Z.E.; Kruger, M.C.; Cooper, G.J.S.; Sequeira, I.R.; Mcgill, A.T.; Poppitt, S.D.; Fraser, K. Dissecting the relationship between plasma and tissue metabolome in a cohort of women with obesity: Analysis of subcutaneous and visceral adipose, muscle, and liver. FASEB J. 2022, 36, e22371. [Google Scholar] [CrossRef]
- Neinast, M.D.; Jang, C.; Hui, S.; Murashige, D.S.; Chu, Q.; Morscher, R.J.; Li, X.; Zhan, L.; White, E.; Anthony, T.G.; et al. Quantitative Analysis of the Whole-Body Metabolic Fate of Branched-Chain Amino Acids. Cell Metab. 2019, 29, 417–429.e414. [Google Scholar] [CrossRef]
- Samad, F.; Hester, K.D.; Yang, G.; Hannun, Y.A.; Bielawski, J. Altered adipose and plasma sphingolipid metabolism in obesity. Diabetes 2006, 55, 2579–2587. [Google Scholar] [CrossRef]
- Psychogios, N.; Hau, D.D.; Peng, J.; Guo, A.C.; Mandal, R.; Bouatra, S.; Sinelnikov, I.; Krishnamurthy, R.; Eisner, R.; Gautam, B.; et al. The human serum metabolome. PLoS ONE 2011, 6, e16957. [Google Scholar] [CrossRef] [PubMed]
- Quehenberger, O.; Dennis, E.A. The human plasma lipidome. N. Engl. J. Med. 2011, 365, 1812–1823. [Google Scholar] [CrossRef]
- Bae, H.; Lam, K.; Jang, C. Metabolic flux between organs measured by arteriovenous metabolite gradients. Exp. Mol. Med. 2022, 54, 1354–1366. [Google Scholar] [CrossRef] [PubMed]
- Chung, N.C.; Miasojedow, B.; Startek, M.; Gambin, A. Jaccard/Tanimoto similarity test and estimation methods for biological presence-absence data. BMC Bioinform. 2019, 20, 644. [Google Scholar] [CrossRef]
- Furse, S.; Watkins, A.J.; Hojat, N.; Smith, J.; Williams, H.E.L.; Chiarugi, D.; Koulman, A. Lipid Traffic Analysis reveals the impact of high paternal carbohydrate intake on offsprings’ lipid metabolism. Commun. Biol. 2021, 4, 163. [Google Scholar] [CrossRef]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- van der Kloet, F.M.; Bobeldijk, I.; Verheij, E.R.; Jellema, R.H. Analytical error reduction using single point calibration for accurate and precise metabolomic phenotyping. J. Proteome Res. 2009, 8, 5132–5141. [Google Scholar] [CrossRef]
- Furse, S.; de Kroon, A.I. Phosphatidylcholine’s functions beyond that of a membrane brick. Mol. Membr. Biol. 2015, 32, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Jaccard, P. The distribution of the flora in the alpine zone. New Phytol. 1912, 11, 37–50. [Google Scholar] [CrossRef]
- Rahman, S.A.; Cuesta, S.M.; Furnham, N.; Holliday, G.L.; Thornton, J.M. EC-BLAST: A tool to automatically search and compare enzyme reactions. Nat. Methods 2014, 11, 171–174. [Google Scholar] [CrossRef]
- Furse, S.; White, S.L.; Meek, C.L.; Jenkins, B.; Petry, C.J.; Vieira, M.C.; Ozanne, S.E.; Dunger, D.B.; Poston, L.; Koulman, A. Altered triglyceride and phospholipid metabolism predates the diagnosis of gestational diabetes in obese pregnancy. Mol. Omics 2019, 15, 420–430. [Google Scholar] [CrossRef]
- Wu, Z.E.; Fraser, K.; Kruger, M.C.; Sequeira, I.R.; Yip, W.; Lu, L.W.; Plank, L.D.; Murphy, R.; Cooper, G.J.S.; Martin, J.-C.; et al. Metabolomic signatures for visceral adiposity and dysglycaemia in Asian Chinese and Caucasian European adults: The cross-sectional TOFI_Asia study. Nutr. Metab. 2020, 17, 95. [Google Scholar] [CrossRef]
- Chen, T.; Ni, Y.; Ma, X.; Bao, Y.; Liu, J.; Huang, F.; Hu, C.; Xie, G.; Zhao, A.; Jia, W.; et al. Branched-chain and aromatic amino acid profiles and diabetes risk in Chinese populations. Sci. Rep. 2016, 6, 20594. [Google Scholar] [CrossRef]
- Magkos, F.; Hjorth, M.F.; Astrup, A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 545–555. [Google Scholar] [CrossRef]
- She, P.; Reid, T.M.; Bronson, S.K.; Vary, T.C.; Hajnal, A.; Lynch, C.J.; Hutson, S.M. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 2007, 6, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Park, Y.S.; Cho, C.; Lee, H.; Park, J.; Park, D.J.; Lee, J.H.; Lee, H.J.; Ha, T.K.; Kim, Y.J.; et al. Short-term changes in the serum metabolome after laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. Metabolomics 2021, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Maltais-Payette, I.; Lajeunesse-Trempe, F.; Nadeau, M.; Bouvet-Bouchard, L.; Hould, F.S.; Biertho, L.; Tchernof, A. Circulating amino acid changes three years after bariatric surgery. Metabolites 2025, 15, 297. [Google Scholar] [CrossRef] [PubMed]
- Lappa, D.; Meijnikman, A.S.; Krautkramer, K.A.; Olsson, L.M.; Aydin, Ö.; Van Rijswijk, A.S.; Acherman, Y.I.Z.; De Brauw, M.L.; Tremaroli, V.; Olofsson, L.E.; et al. Self-organized metabotyping of obese individuals identifies clusters responding differently to bariatric surgery. PLoS ONE 2023, 18, e0279335. [Google Scholar] [CrossRef]
- Vizioli, C.; Jaime-Lara, R.B.; Franks, A.T.; Ortiz, R.; Joseph, P.V. Untargeted metabolomic approach shows no differences in subcutaneous adipose tissue of diabetic and non-diabetic subjects undergoing bariatric surgery: An exploratory study. Biol. Res. Nurs. 2021, 23, 109–118. [Google Scholar] [CrossRef]
- Bagheri, M.; Tanriverdi, K.; Iafrati, M.D.; Mosley, J.D.; Freedman, J.E.; Ferguson, J.F. Characterization of the plasma metabolome and lipidome in response to sleeve gastrectomy and gastric bypass surgeries reveals molecular patterns of surgical weight loss. Metabolism 2024, 158, 155955. [Google Scholar] [CrossRef]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of omega-3 fatty acids on the gut microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef]
- Jayapala, H.P.S.; Lim, S.Y. N-3 Polyunsaturated fatty acids and gut microbiota. Comb. Chem. High Throughput Screen. 2023, 26, 892–905. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, B.; West, J.A.; Koulman, A. A review of odd-chain fatty acid metabolism and the role of pentadecanoic Acid (c15:0) and heptadecanoic Acid (c17:0) in health and disease. Molecules 2015, 20, 2425–2444. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, B.J.; Seyssel, K.; Chiu, S.; Pan, P.H.; Lin, S.Y.; Stanley, E.; Ament, Z.; West, J.A.; Summerhill, K.; Griffin, J.L.; et al. Odd chain fatty acids; New insights of the relationship between the gut microbiota, dietary intake, biosynthesis and glucose intolerance. Sci. Rep. 2017, 7, 44845. [Google Scholar] [CrossRef] [PubMed]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Shi, W.; Yang, Z.; Fu, P.; Yang, Y. Metabolite 2-aminoadipic acid: Implications for metabolic disorders and therapeutic opportunities. Front. Pharmacol. 2025, 16, 1569020. [Google Scholar] [CrossRef]
- Xu, W.Y.; Shen, Y.; Zhu, H.; Gao, J.; Zhang, C.; Tang, L.; Lu, S.Y.; Shen, C.L.; Zhang, H.X.; Li, Z.; et al. 2-Aminoadipic acid protects against obesity and diabetes. J. Endocrinol. 2019, 243, 111–123. [Google Scholar] [CrossRef]
- Patle, R.; Dubb, S.; Alaghband-Zadeh, J.; Sherwood, R.A.; Tam, F.; Frankel, A.; Moniz, C.; Bueter, M.; Vincent, R.P.; le Roux, C.W. Improved blood pressure, nitric oxide and asymmetric dimethylarginine are independent after bariatric surgery. Ann. Clin. Biochem. 2012, 49, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Poirier, J.; Cloteau, C.; Aguesse, A.; Billot, X.; Thévenot, E.; Krempf, M.; Valéro, R.; Maraninchi, M.; Croyal, M. Bariatric surgery improves the atherogenic profile of circulating methylarginines in obese patients: Results from a pilot study. Metabolites 2021, 11, 759. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Jorga, J. Guanidinoacetic acid in human nutrition: Beyond creatine synthesis. Food Sci. Nutr. 2023, 11, 1606–1611. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.Y.; Huang, Z.H.; Fang, A.P.; Li, S.Y.; Huang, R.Z.; Chen, Y.M.; Huang, B.X.; Zhu, H.L. Correlation between serum trimethylamine-N-oxide and body fat distribution in middle-aged and older adults: A prospective cohort study. Nutr. J. 2024, 23, 70. [Google Scholar] [CrossRef]
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-Oxide: The Good, the Bad and the Unknown. Toxins 2016, 8, 326. [Google Scholar] [CrossRef]
- Dumas, M.-E.; Rothwell, A.R.; Hoyles, L.; Aranias, T.; Chilloux, J.; Calderari, S.; Noll, E.M.; Péan, N.; Boulangé, C.L.; Blancher, C.; et al. Microbial-host co-metabolites are prodromal markers predicting phenotypic heterogeneity in behavior, obesity, and impaired glucose tolerance. Cell Rep. 2017, 20, 136–148. [Google Scholar] [CrossRef]
- Krueger, E.S.; Lloyd, T.S.; Tessem, J.S. The accumulation and molecular effects of trimethylamine N-oxide on metabolic tissues: It’s not all bad. Nutrients 2021, 13, 2873. [Google Scholar] [CrossRef] [PubMed]
- Milam, N.; Miranda, E.; Funai, k. Effect of N-Acetyl-Carnosine on Body Weight and Composition. Physiology 2024, 39, 2302. [Google Scholar] [CrossRef]
- Chou, J.; Liu, R.; Yu, J.; Liu, X.; Zhao, X.; Li, Y.; Liu, L.; Sun, C. Fasting serum α-hydroxybutyrate and pyroglutamic acid as important metabolites for detecting isolated post-challenge diabetes based on organic acid profiles. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1100–1101, 6–16. [Google Scholar] [CrossRef]
- Molinaro, A.; Bel Lassen, P.; Henricsson, M.; Wu, H.; Adriouch, S.; Belda, E.; Chakaroun, R.; Nielsen, T.; Bergh, P.O.; Rouault, C.; et al. Imidazole propionate is increased in diabetes and associated with dietary patterns and altered microbial ecology. Nat. Commun. 2020, 11, 5881. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, W.; Li, Y.; Liu, Y.; Liu, Y. Imidazole propionate in type 2 diabetes mellitus and cardiovascular diseases: A mini review. Front. Immunol. 2024, 15-2024, 1454210. [Google Scholar] [CrossRef]
- Yoshinari, O.; Igarashi, K. Anti-diabetic effect of pyroglutamic acid in type 2 diabetic Goto-Kakizaki rats and KK-Ay mice. Br. J. Nutr. 2011, 106, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Pekař, M.; Pekařová, A.; Bužga, M.; Holéczy, P.; Soltes, M. The risk of sarcopenia 24 months after bariatric surgery—Assessment by dual energy X-ray absorptiometry (DEXA): A prospective study. Wideochir Inne Tech. Maloinwazyjne 2020, 15, 583–587. [Google Scholar] [CrossRef]
- Reis, M.G.; Guimarães, G.; Moreira, L.F.; Siqueira Veloso de Andrade Carvalho, L.; Tianeze de Castro, C.; Adrielle Lima Vieira, R.; Sernizon Guimarães, N. Weight regain after bariatric surgery: A systematic review and meta-analysis of observational studies. Obes. Med. 2024, 45, 100528. [Google Scholar] [CrossRef]
- Bellicha, A.; van Baak, M.A.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Busetto, L.; Carraça, E.V.; Dicker, D.; Encantado, J.; Ermolao, A.; et al. Effect of exercise training before and after bariatric surgery: A systematic review and meta-analysis. Obes. Rev. 2021, 22 (Suppl. 4), e13296. [Google Scholar] [CrossRef] [PubMed]
- Qadhi, A.H.; Almuqati, A.H.; Alamro, N.S.; Azhri, A.S.; Azzeh, F.S.; Azhar, W.F.; Alyamani, R.A.; Almohmadi, N.H.; Alkholy, S.O.; Alhassani, W.E.; et al. The effect of bariatric surgery on dietary behaviour, dietary recommendation adherence, and micronutrient deficiencies one year after surgery. Prev. Med. Rep. 2023, 35, 102343. [Google Scholar] [CrossRef] [PubMed]
- Rakusanova, S.; Fiehn, O.; Cajka, T. Toward building mass spectrometry-based metabolomics and lipidomics atlases for biological and clinical research. TrAC Trends Anal. Chem. 2023, 158, 116825. [Google Scholar] [CrossRef]


| Enrol | BL | 6m_FU | |
|---|---|---|---|
| Plasma | 24 † | 28 | 26 * |
| SAA | - | 19 | 21 |
| STA | - | 25 | 19 |
| VLM | - | 22 | 18 |
| Number | Lipids |
|---|---|
| 1 | SM(d16:0/25:1) |
| 2 | SM(d18:2/16:0) |
| 3 | SM(d17:1/16:0) |
| 4 | PE(16:0p/18:2) |
| 5 | PC(16:0e/22:6) |
| 6 | PE(18:0/20:3) |
| 7 | SM(d20:0/18:1) |
| 8 | SM(d16:1/16:0) |
| 9 | SM(d22:1/16:0) |
| 10 | PE(16:0p/20:5) |
| 11 | PG(18:1/18:1) |
| 12 | PE(18:0p/20:4) |
| 13 | PC(16:0/20:5) |
| 14 | Pl(16:0/20:4) |
| 15 | PC(18:0/22:6) |
| 16 | SM(d16:0/24:2) |
| 17 | SM(d16:0/27:2) |
| 18 | SM(d18:1/18:0) |
| 19 | PC(18:0/18:1) |
| No. | Polar Metabolites |
|---|---|
| 1 | Acetylcarnosine |
| 2 | Guanidoacetic acid |
| 3 | Guanine |
| 4 | 4-Imidazolone-5-propionic acid |
| 5 | Pyroglutamic acid |
| 6 | Trimethylamine N-oxide |
| 7 | 2-Aminooctanoic acid |
| 8 | Asymmetric dimethylarginine |
| 9 | N6,N6,N6-Trimethyl-L-lysine |
| 10 | Aminoadipic acid |
| 11 | Methionine sulfoxide |
| 12 | L-Acetylcarnitine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joblin-Mills, A.; Wu, Z.E.; Cooper, G.J.S.; Sequeira-Bisson, I.R.; Miles-Chan, J.L.; McGill, A.-T.; Poppitt, S.D.; Fraser, K. Site-Specific Trafficking of Lipid and Polar Metabolites in Adipose and Muscle Tissue Reveals the Impact of Bariatric Surgery-Induced Weight Loss: A 6-Month Follow-Up Study. Metabolites 2025, 15, 525. https://doi.org/10.3390/metabo15080525
Joblin-Mills A, Wu ZE, Cooper GJS, Sequeira-Bisson IR, Miles-Chan JL, McGill A-T, Poppitt SD, Fraser K. Site-Specific Trafficking of Lipid and Polar Metabolites in Adipose and Muscle Tissue Reveals the Impact of Bariatric Surgery-Induced Weight Loss: A 6-Month Follow-Up Study. Metabolites. 2025; 15(8):525. https://doi.org/10.3390/metabo15080525
Chicago/Turabian StyleJoblin-Mills, Aidan, Zhanxuan E. Wu, Garth J. S. Cooper, Ivana R. Sequeira-Bisson, Jennifer L. Miles-Chan, Anne-Thea McGill, Sally D. Poppitt, and Karl Fraser. 2025. "Site-Specific Trafficking of Lipid and Polar Metabolites in Adipose and Muscle Tissue Reveals the Impact of Bariatric Surgery-Induced Weight Loss: A 6-Month Follow-Up Study" Metabolites 15, no. 8: 525. https://doi.org/10.3390/metabo15080525
APA StyleJoblin-Mills, A., Wu, Z. E., Cooper, G. J. S., Sequeira-Bisson, I. R., Miles-Chan, J. L., McGill, A.-T., Poppitt, S. D., & Fraser, K. (2025). Site-Specific Trafficking of Lipid and Polar Metabolites in Adipose and Muscle Tissue Reveals the Impact of Bariatric Surgery-Induced Weight Loss: A 6-Month Follow-Up Study. Metabolites, 15(8), 525. https://doi.org/10.3390/metabo15080525








