Preoperative Tyrosine Levels as Predictive Biomarkers for Excessive Fat-Free Mass Loss Following Laparoscopic Sleeve Gastrectomy in Patients with Morbid Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Data Collection
2.3. Patient Classification and Outcome Measurements
2.4. Statistical Analysis
2.5. Ethics Approval
3. Results
3.1. Preoperative Patient Demographics
3.2. Postoperative Outcomes
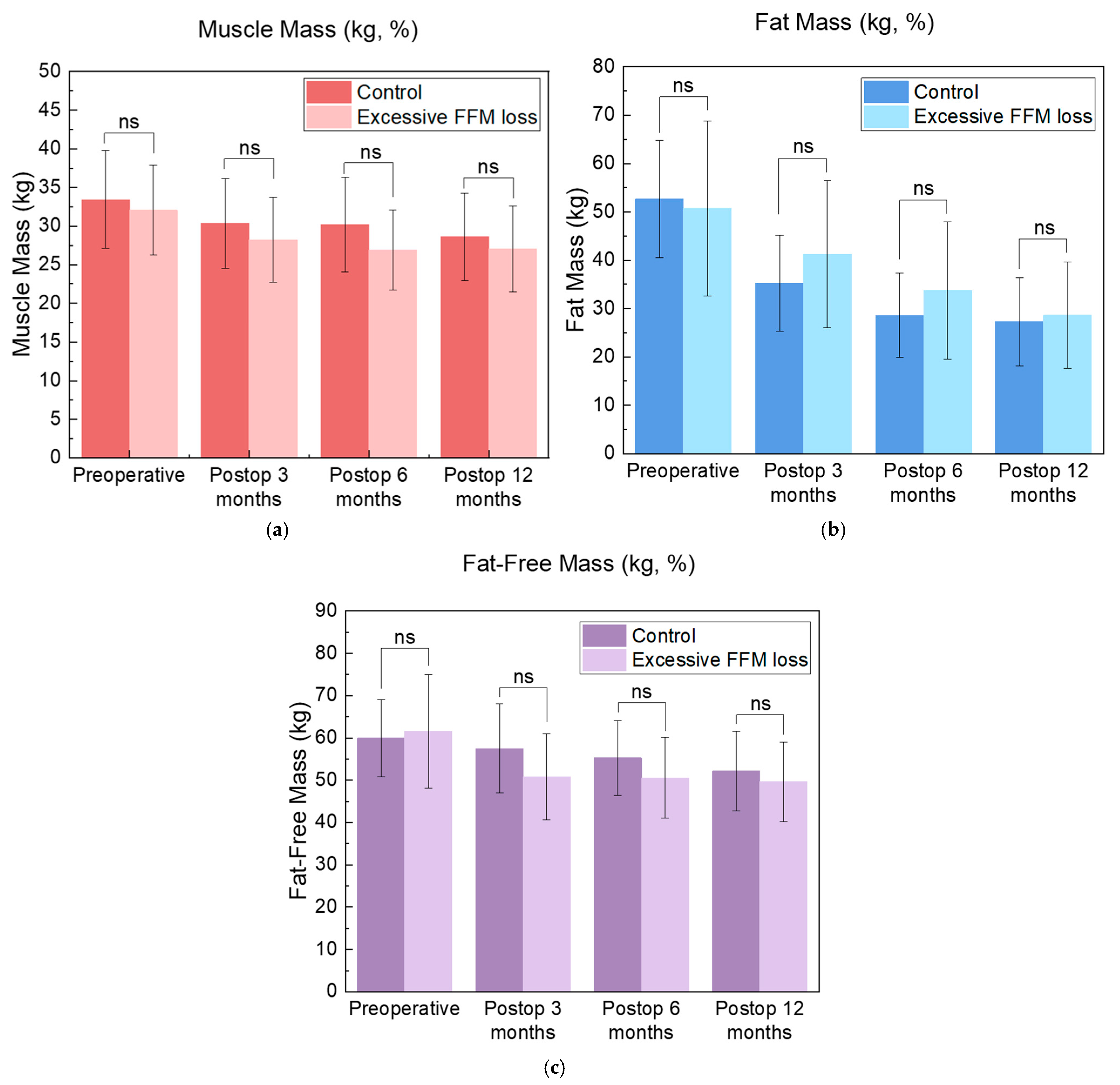
3.3. Outcomes for the Excessive FFM Loss Group
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FFM | Fat-free mass |
| BW | Body weight |
| MBS | Metabolic and bariatric surgery |
| BMI | Body mass index |
| AAMs | Amino acid metabolites |
| %FFML/BWL | Percent of fat-free mass loss relative to body weight loss |
| %TWL | Percent total weight loss |
| %EWL | Percent excess weight loss |
| ROC | Receiver operating characteristic |
| FM | Fat mass |
| Tyr | Tyrosine |
| Trp | Tryptophan |
References
- Kwon, Y.; Jang, M.; Lee, Y.; Ha, J.; Park, S. Amino Acid Metabolites and Slow Weight Loss in the Early Postoperative Period after Sleeve Gastrectomy. J. Clin. Med. 2020, 9, 2348. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Aneman, A.; Friberg, P.; Hooper, D.; Fandriks, L.; Lonroth, H.; Hunyady, B.; Mezey, E. Substantial production of dopamine in the human gastrointestinal tract. J. Clin. Endocrinol. Metab. 1997, 82, 3864–3871. [Google Scholar] [CrossRef]
- Korner, J.; Cline, G.W.; Slifstein, M.; Barba, P.; Rayat, G.R.; Febres, G.; Leibel, R.L.; Maffei, A.; Harris, P.E. A role for foregut tyrosine metabolism in glucose tolerance. Mol. Metab. 2019, 23, 37–50. [Google Scholar] [CrossRef]
- Mutch, D.M.; Fuhrmann, J.C.; Rein, D.; Wiemer, J.C.; Bouillot, J.L.; Poitou, C.; Clement, K. Metabolite profiling identifies candidate markers reflecting the clinical adaptations associated with Roux-en-Y gastric bypass surgery. PLoS ONE 2009, 4, e7905. [Google Scholar] [CrossRef] [PubMed]
- Swierczynski, J.; Sledzinski, T.; Slominska, E.; Smolenski, R.; Sledzinski, Z. Serum phenylalanine concentration as a marker of liver function in obese patients before and after bariatric surgery. Obes. Surg. 2009, 19, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Tillin, T.; Hughes, A.D.; Wang, Q.; Wurtz, P.; Ala-Korpela, M.; Sattar, N.; Forouhi, N.G.; Godsland, I.F.; Eastwood, S.V.; McKeigue, P.M.; et al. Diabetes risk and amino acid profiles: Cross-sectional and prospective analyses of ethnicity, amino acids and diabetes in a South Asian and European cohort from the SABRE (Southall And Brent REvisited) Study. Diabetologia 2015, 58, 968–979. [Google Scholar] [CrossRef]
- Oh, C.M.; Namkung, J.; Go, Y.; Shong, K.E.; Kim, K.; Kim, H.; Park, B.Y.; Lee, H.W.; Jeon, Y.H.; Song, J.; et al. Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nat. Commun. 2015, 6, 6794. [Google Scholar] [CrossRef]
- Young, R.L.; Lumsden, A.L.; Martin, A.M.; Schober, G.; Pezos, N.; Thazhath, S.S.; Isaacs, N.J.; Cvijanovic, N.; Sun, E.W.L.; Wu, T.; et al. Augmented capacity for peripheral serotonin release in human obesity. Int. J. Obes. 2018, 42, 1880–1889. [Google Scholar] [CrossRef]
- Vaz, M.; Pereira, S.S.; Monteiro, M.P. Metabolomic signatures after bariatric surgery—A systematic review. Rev. Endocr. Metab. Disord. 2022, 23, 503–519. [Google Scholar] [CrossRef]
- Chen, T.; Ni, Y.; Ma, X.; Bao, Y.; Liu, J.; Huang, F.; Hu, C.; Xie, G.; Zhao, A.; Jia, W.; et al. Branched-chain and aromatic amino acid profiles and diabetes risk in Chinese populations. Sci. Rep. 2016, 6, 20594. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Marron, M.M.; Farsijani, S.; Miljkovic, I.; Tseng, G.C.; Shah, R.V.; Murthy, V.L.; Newman, A.B. Circulating metabolomic biomarkers of 5-year body weight and composition change in a biracial cohort of community-dwelling older adults. Geroscience 2025, 47, 2593–2611. [Google Scholar] [CrossRef]
- Kraljevic, M.; Cordasco, V.; Schneider, R.; Peters, T.; Slawik, M.; Wolnerhanssen, B.; Peterli, R. Long-term Effects of Laparoscopic Sleeve Gastrectomy: What Are the Results Beyond 10 Years? Obes. Surg. 2021, 31, 3427–3433. [Google Scholar] [CrossRef]
- Huang, J.; Lucero-Prisno, D.E., 3rd; Zhang, L.; Xu, W.; Wong, S.H.; Ng, S.C.; Wong, M.C.S. Updated epidemiology of gastrointestinal cancers in East Asia. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 271–287. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, M.; Oda, I.; Matsuda, T.; Saito, Y. Epidemiological Trends and Future Perspectives of Gastric Cancer in Eastern Asia. Digestion 2022, 103, 22–28. [Google Scholar] [CrossRef]
- Nuijten, M.A.H.; Eijsvogels, T.M.H.; Monpellier, V.M.; Janssen, I.M.C.; Hazebroek, E.J.; Hopman, M.T.E. The magnitude and progress of lean body mass, fat-free mass, and skeletal muscle mass loss following bariatric surgery: A systematic review and meta-analysis. Obes. Rev. 2022, 23, e13370. [Google Scholar] [CrossRef]
- Manning, S.; Pucci, A.; Carter, N.C.; Elkalaawy, M.; Querci, G.; Magno, S.; Tamberi, A.; Finer, N.; Fiennes, A.G.; Hashemi, M.; et al. Early postoperative weight loss predicts maximal weight loss after sleeve gastrectomy and Roux-en-Y gastric bypass. Surg. Endosc. 2015, 29, 1484–1491. [Google Scholar] [CrossRef]
- Silveira, F.C.; Docherty, N.G.; Sallet, P.C.; Moraes, M.; Monclaro, T.; Arruda, E.S.M.; Pizani, C.E.; Sallet, J.A.; le Roux, C.W. Early Post-operative Weight Change After Roux-en-Y Gastric Bypass Predicts Weight Loss at 12-Month Follow-up. Obes. Surg. 2020, 30, 5020–5025. [Google Scholar] [CrossRef] [PubMed]
- Tettero, O.M.; Monpellier, V.M.; Janssen, I.M.C.; Steenhuis, I.H.M.; van Stralen, M.M. Early Postoperative Weight Loss Predicts Weight Loss up to 5 Years After Roux-En-Y Gastric Bypass, Banded Roux-En-Y Gastric Bypass, and Sleeve Gastrectomy. Obes. Surg. 2022, 32, 2891–2902. [Google Scholar] [CrossRef] [PubMed]
- Nuijten, M.A.H.; Monpellier, V.M.; Eijsvogels, T.M.H.; Janssen, I.M.C.; Hazebroek, E.J.; Hopman, M.T.E. Rate and Determinants of Excessive Fat-Free Mass Loss After Bariatric Surgery. Obes. Surg. 2020, 30, 3119–3126. [Google Scholar] [CrossRef]
- Chaston, T.B.; Dixon, J.B.; O’Brien, P.E. Changes in fat-free mass during significant weight loss: A systematic review. Int. J. Obes. 2007, 31, 743–750. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Gonzalez, M.C.; Shen, W.; Redman, L.; Thomas, D. Weight loss composition is one-fourth fat-free mass: A critical review and critique of this widely cited rule. Obes. Rev. 2014, 15, 310–321. [Google Scholar] [CrossRef]
- Prentice, A.M.; Goldberg, G.R.; Jebb, S.A.; Black, A.E.; Murgatroyd, P.R.; Diaz, E.O. Physiological responses to slimming. Proc. Nutr. Soc. 1991, 50, 441–458. [Google Scholar] [CrossRef]
- Stiegler, P.; Cunliffe, A. The role of diet and exercise for the maintenance of fat-free mass and resting metabolic rate during weight loss. Sports Med. 2006, 36, 239–262. [Google Scholar] [CrossRef] [PubMed]
- Varady, K.A. Intermittent versus daily calorie restriction: Which diet regimen is more effective for weight loss? Obes. Rev. 2011, 12, e593–e601. [Google Scholar] [CrossRef] [PubMed]
- Weinheimer, E.M.; Sands, L.P.; Campbell, W.W. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: Implications for sarcopenic obesity. Nutr. Rev. 2010, 68, 375–388. [Google Scholar] [CrossRef]
- Kwon, Y.; Jang, M.; Lee, Y.; Ha, J.; Park, S. Metabolomic Analysis of the Improvements in Insulin Secretion and Resistance After Sleeve Gastrectomy: Implications of the Novel Biomarkers. Obes. Surg. 2021, 31, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Sucher, K.; Hollenbeck, C.B. Comparison of ideal body weight equations and published height-weight tables with body mass index tables for healthy adults in the United States. Nutr. Clin. Pract. 2006, 21, 312–319. [Google Scholar] [CrossRef]
- Mitchell, W.K.; Williams, J.; Atherton, P.; Larvin, M.; Lund, J.; Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol. 2012, 3, 260. [Google Scholar] [CrossRef]
- Eksteen, G.; Vanuytsel, T.; Vangoitsenhoven, R.; Mertens, A.; Lannoo, M.; De Leus, E.; Van der Schueren, B.; Matthys, C. Sarcopenia, Muscle Mass and Protein Intake in Adults Older Than 65 Years After Earlier Bariatric Surgery. J. Cachexia Sarcopenia Muscle 2025, 16, e13839. [Google Scholar] [CrossRef]
- Courcoulas, A.P.; Daigle, C.R.; Arterburn, D.E. Long term outcomes of metabolic/bariatric surgery in adults. BMJ 2023, 383, e071027. [Google Scholar] [CrossRef]
- Jabbour, G.; Salman, A. Bariatric Surgery in Adults with Obesity: The Impact on Performance, Metabolism, and Health Indices. Obes. Surg. 2021, 31, 1767–1789. [Google Scholar] [CrossRef]
- Mikkola, T.M.; Salonen, M.K.; Kajantie, E.; Kautiainen, H.; Eriksson, J.G. Associations of Fat and Lean Body Mass with Circulating Amino Acids in Older Men and Women. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Alba, D.L.; Wu, L.; Cawthon, P.M.; Mulligan, K.; Lang, T.; Patel, S.; King, N.J.; Carter, J.T.; Rogers, S.J.; Posselt, A.M.; et al. Changes in Lean Mass, Absolute and Relative Muscle Strength, and Physical Performance After Gastric Bypass Surgery. J. Clin. Endocrinol. Metab. 2019, 104, 711–720. [Google Scholar] [CrossRef]
- Lamarca, F.; Melendez-Araujo, M.S.; Porto de Toledo, I.; Dutra, E.S.; de Carvalho, K.M.B. Relative Energy Expenditure Decreases during the First Year after Bariatric Surgery: A Systematic Review and Meta-Analysis. Obes. Surg. 2019, 29, 2648–2659. [Google Scholar] [CrossRef] [PubMed]
- Turicchi, J.; O’Driscoll, R.; Finlayson, G.; Duarte, C.; Hopkins, M.; Martins, N.; Michalowska, J.; Larsen, T.M.; van Baak, M.A.; Astrup, A.; et al. Associations between the proportion of fat-free mass loss during weight loss, changes in appetite, and subsequent weight change: Results from a randomized 2-stage dietary intervention trial. Am. J. Clin. Nutr. 2020, 111, 536–544. [Google Scholar] [CrossRef]
- Nuijten, M.A.H.; Tettero, O.M.; Wolf, R.J.; Bakker, E.A.; Eijsvogels, T.M.H.; Monpellier, V.M.; Hazebroek, E.J.; Janssen, I.M.C.; Hopman, M.T.E. Changes in Physical Activity in Relation to Body Composition, Fitness and Quality of Life after Primary Bariatric Surgery: A Two-Year Follow-Up Study. Obes. Surg. 2021, 31, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Zaaria, M.; Pasleau, F.; Reginster, J.Y.; Bruyere, O. Health Outcomes of Sarcopenia: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169548. [Google Scholar] [CrossRef]
- Pacifico, J.; Geerlings, M.A.J.; Reijnierse, E.M.; Phassouliotis, C.; Lim, W.K.; Maier, A.B. Prevalence of sarcopenia as a comorbid disease: A systematic review and meta-analysis. Exp. Gerontol. 2020, 131, 110801. [Google Scholar] [CrossRef]
- Voican, C.S.; Lebrun, A.; Maitre, S.; Lainas, P.; Lamouri, K.; Njike-Nakseu, M.; Gaillard, M.; Tranchart, H.; Balian, A.; Dagher, I.; et al. Predictive score of sarcopenia occurrence one year after bariatric surgery in severely obese patients. PLoS ONE 2018, 13, e0197248. [Google Scholar] [CrossRef]
- Prado, C.M.; Wells, J.C.; Smith, S.R.; Stephan, B.C.; Siervo, M. Sarcopenic obesity: A Critical appraisal of the current evidence. Clin. Nutr. 2012, 31, 583–601. [Google Scholar] [CrossRef]
- Mendes, C.; Carvalho, M.; Bravo, J.; Martins, S.; Raimundo, A. Exercise Interventions for the Prevention of Sarcopenia After Bariatric Surgery: A Systematic Review. J. Sci. Sport. Exerc. 2025, 7, 142–160. [Google Scholar] [CrossRef]
- Mattsson, S.; Thomas, B.J. Development of methods for body composition studies. Phys. Med. Biol. 2006, 51, R203–R228. [Google Scholar] [CrossRef]
- Combest, T.M.; Howard, R.S.; Andrews, A.M. Comparison of Circumference Body Composition Measurements and Eight-Point Bioelectrical Impedance Analysis to Dual Energy X-Ray Absorptiometry to Measure Body Fat Percentage. Mil. Med. 2017, 182, e1908–e1912. [Google Scholar] [CrossRef][Green Version]
- Yang, S.W.; Kim, T.H.; Choi, H.M. The reproducibility and validity verification for body composition measuring devices using bioelectrical impedance analysis in Korean adults. J. Exerc. Rehabil. 2018, 14, 621–627. [Google Scholar] [CrossRef]
- Bellicha, A.; Ciangura, C.; Roda, C.; Torcivia, A.; Aron-Wisnewsky, J.; Poitou, C.; Oppert, J.M. Effect of exercise training after bariatric surgery: A 5-year follow-up study of a randomized controlled trial. PLoS ONE 2022, 17, e0271561. [Google Scholar] [CrossRef]
- Romeijn, M.M.; Holthuijsen, D.D.B.; Kolen, A.M.; Janssen, L.; Schep, G.; van Dielen, F.M.H.; Leclercq, W.K.G. The effect of additional protein on lean body mass preservation in post-bariatric surgery patients: A systematic review. Nutr. J. 2021, 20, 27. [Google Scholar] [CrossRef]
- Henriques, H.K.F.; Kattah, F.M.; Piccolo, M.S.; dos Santos, E.A.; de Araújo Ventura, L.H.D.; Cerqueira, F.R.; Vieira, C.M.A.F.; Leite, J.I.A. The Role of Whey Protein in Maintaining Fat-Free Mass and Promoting Fat Loss After 18 Months of Bariatric Surgery. Obesities 2025, 5, 42. [Google Scholar] [CrossRef]
- Hopkins, M.; Gibbons, C.; Blundell, J. Fat-free mass and resting metabolic rate are determinants of energy intake: Implications for a theory of appetite control. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2023, 378, 20220213. [Google Scholar] [CrossRef]
- Middleton, A.; Fritz, S.L.; Lusardi, M. Walking speed: The functional vital sign. J. Aging Phys. Act. 2015, 23, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Whitson, H.E.; Arnold, A.M.; Yee, L.M.; Mukamal, K.J.; Kizer, J.R.; Djousse, L.; Ix, J.H.; Siscovick, D.; Tracy, R.P.; Thielke, S.M.; et al. Serum carboxymethyl-lysine, disability, and frailty in older persons: The Cardiovascular Health Study. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 710–716. [Google Scholar] [CrossRef] [PubMed]

| Preoperative Values (n = 40) | |
|---|---|
| Age (years) | 37.4 ± 11.3 |
| Sex | |
| Male (n, %) | 13 (32.5) |
| Female (n, %) | 27 (67.5) |
| Concurrent MD (n, %) | 29 (72.5) |
| DM (n, %) | 16 (40%) |
| HTN (n, %) | 24 (60%) |
| DL (n, %) | 16 (40%) |
| BMI (kg/m2) | 40.81 ± 6.37 |
| BW (kg) | 112.4 ± 21.4 |
| FM (kg) | 51.5 ± 16.0 |
| MM (kg) | 32.6 ± 6.00 |
| FFM (kg) | 61.0 ± 11.9 |
| FFM/BW (%) | 54.7 ± 8.3 |
| %FFML/BWL | 37.6 ± 28.8 |
| Preoperative (n = 40) | Postoperative | p-Value | |||
|---|---|---|---|---|---|
| 3 m (n = 40) | 6 m (n = 40) | 12 m (n = 32) | |||
| BMI (kg/m2) | 40.8 ± 6.4 | 33.5 ± 5.8 | 30.5± 5.2 | 29.1 ± 5.1 | <0.001 ** |
| BW (kg) | 112.4 ± 21.4 | 92.3 ± 18.9 | 84.0 ± 17.0 | 80.3 ± 16.9 | <0.001 ** |
| FM (kg) | 51.5 ± 16.0 | 38.5 ± 14.0 | 31.8 ± 12.4 | 28.1 ± 10.1 | <0.001 ** |
| MM (kg) | 32.6 ± 6.0 | 29.5 ± 5.6 | 28.1 ± 5.6 | 27.7 ± 5.6 | <0.001 ** |
| FFM (kg) | 61.0 ± 11.9 | 53.3 ± 10.7 | 52.6 ± 9.6 | 50.9 ± 9.4 | <0.001 ** |
| FFM/BW (%) | 54.7 ± 8.2 | 58.4 ± 8.4 | 63.3 ± 8.2 | 64.9 ± 7.9 | ≤0.001 ** |
| %TWL | - | 18.0 ± 5.0 | 25.2 ± 6.5 | 28.2 ± 8.2 | <0.001 ** |
| %EWL | - | 40.1 ± 14.1 | 55.8 ± 17.2 | 69.2 ± 35.8 | <0.001 ** |
| %FFML/BWL | - | 37.6 ± 28.8 | 30.0 ± 22.2 | 33.3± 22.9 | a 0.013 * b 0.455 c 0.188 |
| Control (n = 15) | Excessive FFM Loss (n = 25) | p-Value | |
|---|---|---|---|
| Age (years) | 40.3 ± 10.7 | 35.7 ± 11.5 | 0.280 |
| Sex | 0.029 * | ||
| Male (n, %) | 8 (53.3) | 5 (20.0) | |
| Female (n, %) | 7 (46.7) | 20 (80.0) | |
| Concurrent MD (n, %) | 11 (73.3) | 18 (72.0) | 0.927 |
| DM (n, %) | 7 (46.7) | 9 (36.0) | 0.527 |
| HTN (n, %) | 10 (66.7) | 14 (56.0) | 0.740 |
| DL (n, %) | 8 (53.3) | 8 (32.0) | 0.205 |
| BMI (kg/m2) | 33.4 ± 5.0 | 33.6 ± 6.3 | 0.956 |
| BW (kg) | 92.7 ± 16.5 | 92.0 ± 20.5 | 0.783 |
| FM (kg) | 35.2 ± 9.9 | 40.5 ± 15.8 | 0.376 |
| MM (kg) | 30.4 ± 5.8 | 29.0 ± 5.5 | 0.543 |
| FFM (kg) | 57.5 ± 10.5 | 50.8 ± 10.2 | 0.106 |
| FFM/BW (%) | 66.1 ± 8.7 | 64.1 ± 7.4 | 0.505 |
| Control (n = 15) | Excessive FFM Loss (n = 25) | p-Value | |
|---|---|---|---|
| %FFML/BWL | |||
| 3 m | 12.9 ± 12.2 | 52.5 ± 25.5 | <0.001 ** |
| 6 m | 16.8 ± 12.5 | 38.4 ± 22.8 | <0.001 ** |
| 12 m | 25.7 ± 17.1 | 38.5 ± 25.2 | 0.040 * |
| %TWL | |||
| 3 m | 17.6 ± 5.1 | 18.2 ± 5.2 | 0.740 |
| 6 m | 25.3 ± 6.5 | 25.2 ± 6.7 | 0.946 |
| 12 m | 27.4 ± 8.3 | 28.7 ± 8.3 | 0.788 |
| %EWL | |||
| 3 m | 39.9 ± 14.1 | 40.3 ± 14.3 | 0.938 |
| 6 m | 56.5 ± 15.9 | 55.3 ± 18.2 | 0.834 |
| 12 m | 60.8 ± 18.2 | 74.2 ± 42.6 | 0.258 |
| Control (n = 15) | Excessive FFM Loss (n = 25) | p-Value | |
|---|---|---|---|
| Phe | 68.9 ± 11.8 | 68.69 ± 10.6 | 0.761 |
| Trp | 59.6 ± 9.0 | 52.30 ± 9.6 | 0.015 * |
| Tyr | 64.2 ± 12.6 | 54.17 ± 11.5 | 0.025 * |
| 5-HT | 0.186 ± 0.157 | 0.279 ± 0.228 | 0.182 |
| 5-HTP | 0.018 ± 0.008 | 0.0176 ± 0.006 | 0.804 |
| 5-HIAA | 0.040 ± 0.013 | 0.0384 ± 0.018 | 0.406 |
| L-DOPA | 0.644 ± 0.302 | 0.515 ± 0.251 | 0.112 |
| Model 1 | ||||
|---|---|---|---|---|
| Step 1 | B (s.e.) | p-value | Odds Ratio | 95% CI |
| Trp | −0.084 (0.040) | 0.033 * | 0.919 | 0.850–0.993 |
| Nagelkerke R2 = 0.173, p = 0.020 *, HL = 0.766, −2LL = 47.487 | ||||
| Tyr | −0.071 (0.031) | 0.022 * | 0.932 | 0.877–0.990 |
| Nagelkerke R2 = 0.528, p = 0.012 *, HL = 0.920, −2LL = 46.633 | ||||
| Model 2 | ||||
| Step 1 | B (s.e.) | p-value | Odds Ratio | 95% CI |
| Trp | −0.090 (0.037) | 0.027 * | 0.914 | 0.845–0.990 |
| Age | −0.049 (0.037) | 0.189 | 0.952 | 0.885–1.024 |
| BMI | −0.013 (0.063) | 0.833 | 0.987 | 0.873–1.116 |
| FFM | −0.011 (0.035) | 0.744 | 0.989 | 0.923–1.059 |
| Nagelkerke R2 = 0.229, p = 0.118, HL = 0.538, −2LL = 45.557 | ||||
| Step 1 | B (s.e.) | p-value | Odds Ratio | 95% CI |
| Tyr | −0.076 (0.032) | 0.018 * | 0.927 | 0.870–0.987 |
| Age | −0.051 (0.038) | 0.180 | 0.950 | 0.882–1.024 |
| BMI | −0.007 (0.062) | 0.915 | 0.993 | 0.879–1.122 |
| FFM | −0.010 (0.036) | 0.772 | 0.990 | 0.922–1.062 |
| Nagelkerke R2 = 0.258, p = 0.079, HL = 0.721, −2LL = 44.545 | ||||
| Model 3 | ||||
| Step 1 | B (s.e.) | p-value | Odds Ratio | 95% CI |
| Trp | −0.071 (0.042) | 0.093 | 0.931 | 0.857–1.012 |
| Sex (Male) | −2.748 (1.261) | 0.029 * | 0.064 | 0.005–0.758 |
| Age | −0.030 (0.040) | 0.452 | 0.970 | 0.897–1.049 |
| BMI | 0.010 (0.068) | 0.882 | 1.010 | 0.884–1.154 |
| FFM | 0.061 (0.052) | 0.234 | 1.063 | 0.961–1.177 |
| Nagelkerke R2 = 0.390, p = 0.019 **, HL = 0.434, −2LL = 39.422 | ||||
| Step 1 | B (s.e.) | p-value | Odds Ratio | 95% CI |
| Tyr | −0.105 (0.041) | 0.012 * | 0.901 | 0.830–0.977 |
| Sex (Male) | −3.990 (1.513) | 0.008 ** | 0.018 | 0.001–0.359 |
| Age | −0.029 (0.043) | 0.508 | 0.972 | 0.893–1.058 |
| BMI | 0.021 (0.072) | 0.768 | 1.022 | 0.886–1.177 |
| FFM | 0.083 (0.053) | 0.120 | 1.086 | 0.979–1.206 |
| Nagelkerke R2 = 0.528, p = 0.001 **, HL = 0.502, −2LL = 33.335 | ||||
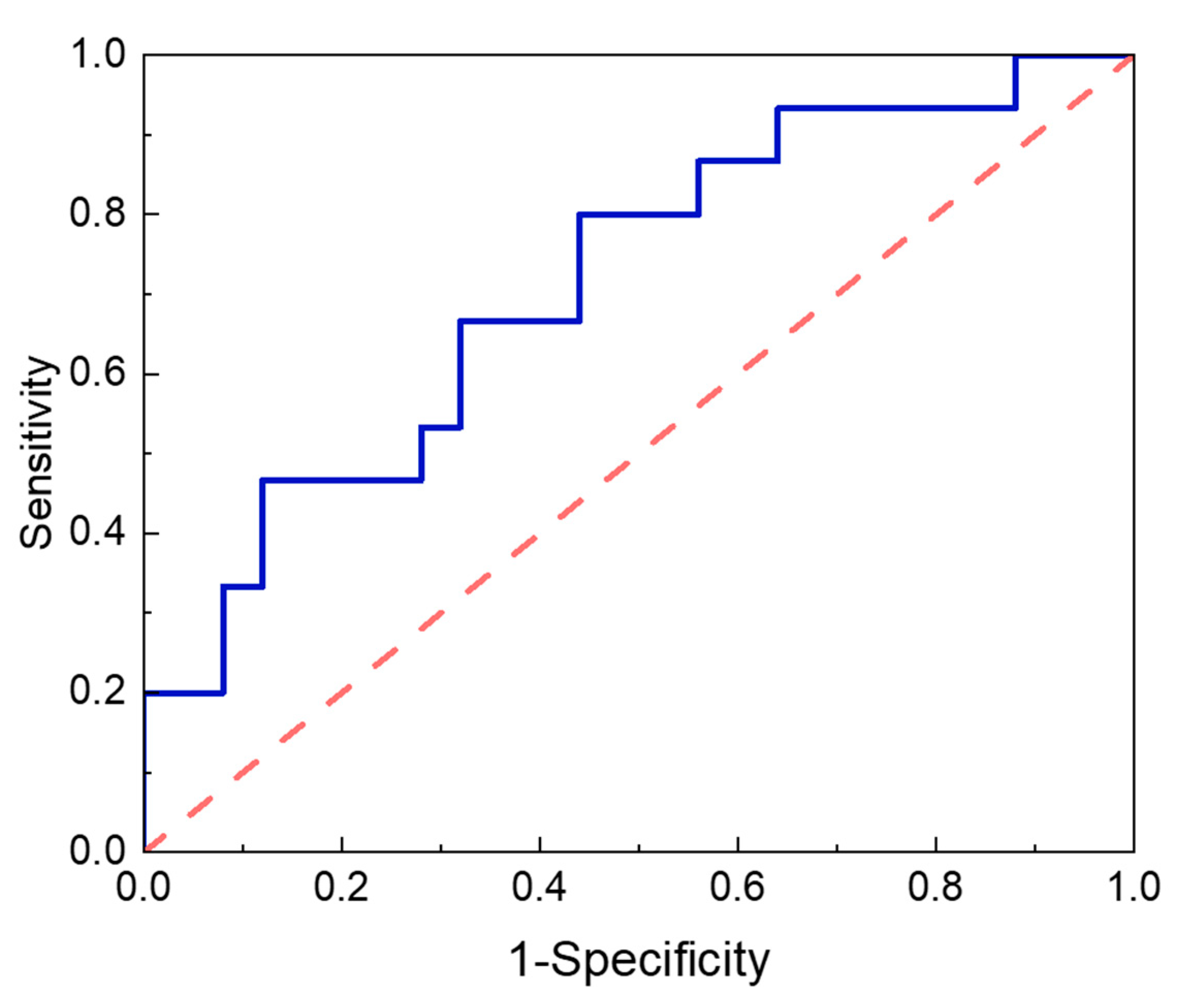
| AUC a | SE b | 95% Confidence Interval c | p-Value d | Cut-Off e | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Tyr | 0.715 | 0.084 | 0.550 | 0.880 | 0.025 * | ≥54.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, I.; Seo, E.; Kwon, Y.; Lee, C.M.; Kim, N.H.; Kim, J.-H.; Choi, S.I.; Park, S. Preoperative Tyrosine Levels as Predictive Biomarkers for Excessive Fat-Free Mass Loss Following Laparoscopic Sleeve Gastrectomy in Patients with Morbid Obesity. Metabolites 2025, 15, 543. https://doi.org/10.3390/metabo15080543
Lee I, Seo E, Kwon Y, Lee CM, Kim NH, Kim J-H, Choi SI, Park S. Preoperative Tyrosine Levels as Predictive Biomarkers for Excessive Fat-Free Mass Loss Following Laparoscopic Sleeve Gastrectomy in Patients with Morbid Obesity. Metabolites. 2025; 15(8):543. https://doi.org/10.3390/metabo15080543
Chicago/Turabian StyleLee, Inyoung, Eunhye Seo, Yeongkeun Kwon, Chang Min Lee, Nam Hoon Kim, Jong-Han Kim, Sung Il Choi, and Sungsoo Park. 2025. "Preoperative Tyrosine Levels as Predictive Biomarkers for Excessive Fat-Free Mass Loss Following Laparoscopic Sleeve Gastrectomy in Patients with Morbid Obesity" Metabolites 15, no. 8: 543. https://doi.org/10.3390/metabo15080543
APA StyleLee, I., Seo, E., Kwon, Y., Lee, C. M., Kim, N. H., Kim, J.-H., Choi, S. I., & Park, S. (2025). Preoperative Tyrosine Levels as Predictive Biomarkers for Excessive Fat-Free Mass Loss Following Laparoscopic Sleeve Gastrectomy in Patients with Morbid Obesity. Metabolites, 15(8), 543. https://doi.org/10.3390/metabo15080543










