Low Metabolic Variation in Environmentally Diverse Natural Populations of Temperate Lime Trees (Tilia cordata)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Strategy and Locations
2.2. Metabolite Extraction
2.3. Metabolite Fingerprinting
2.4. Collection of Functional Trait Data
2.5. Assessment of Neutral Genetic Variation
2.6. Multivariate Data Exploration
3. Results
3.1. Metabolic Variation
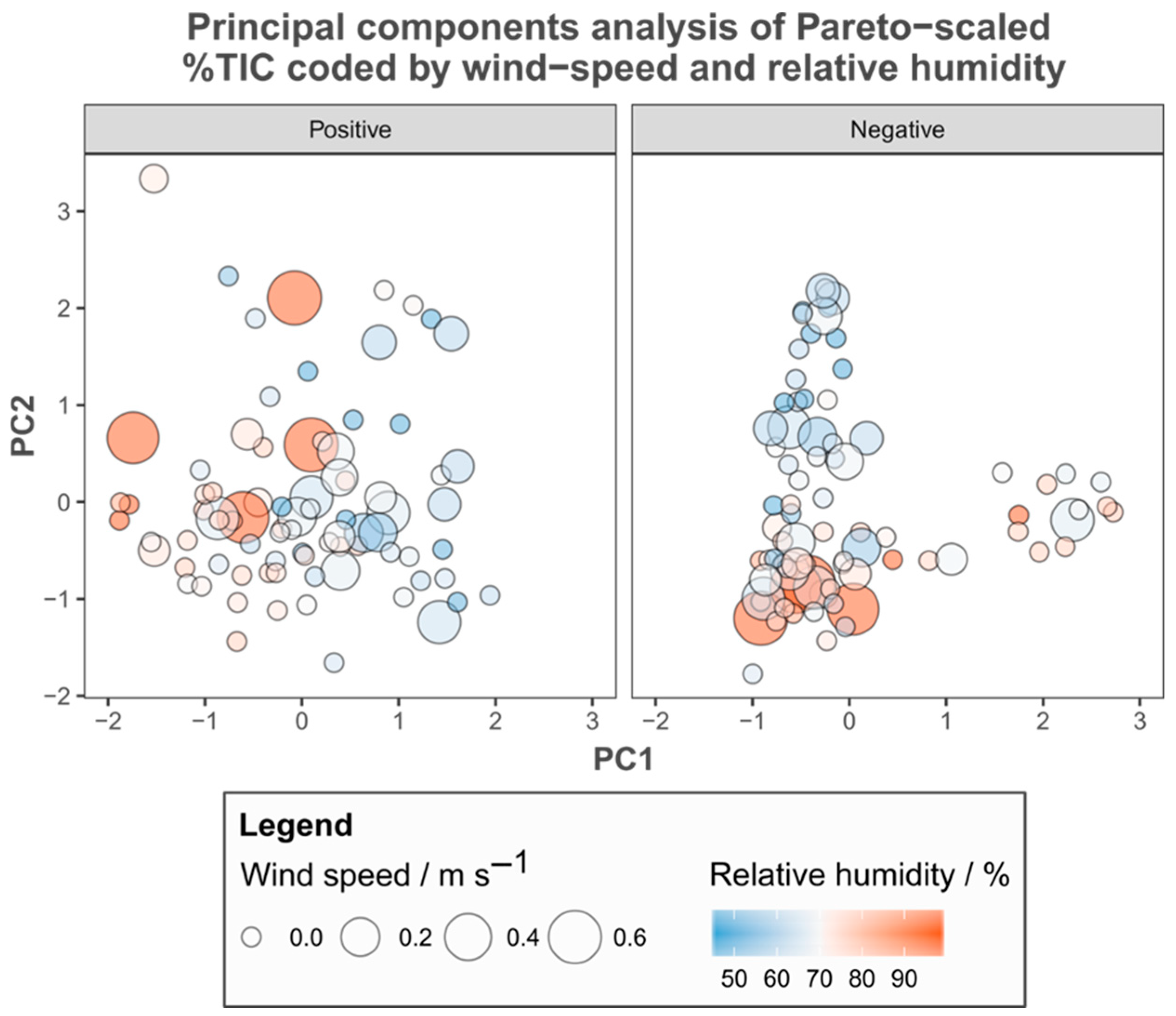
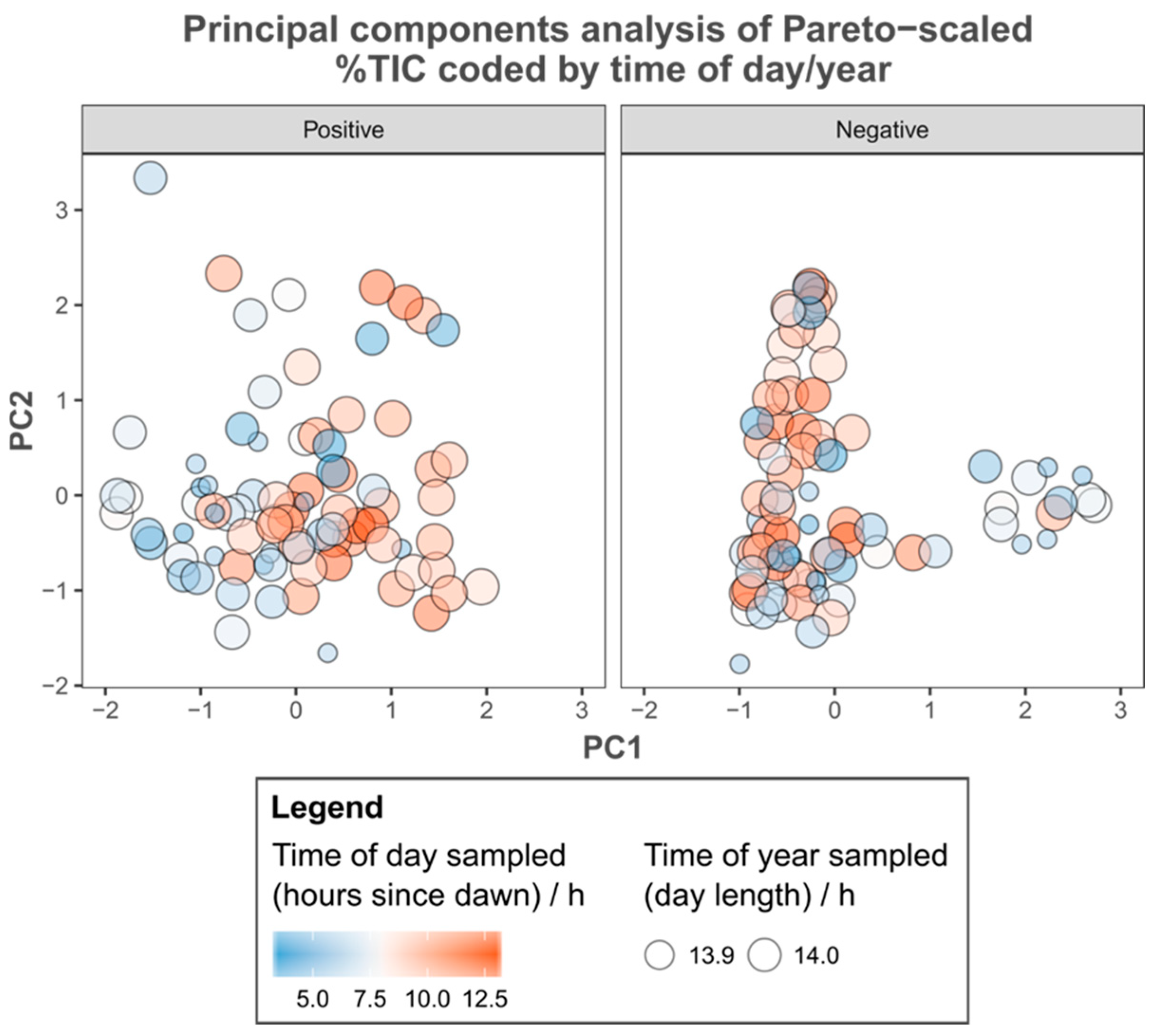
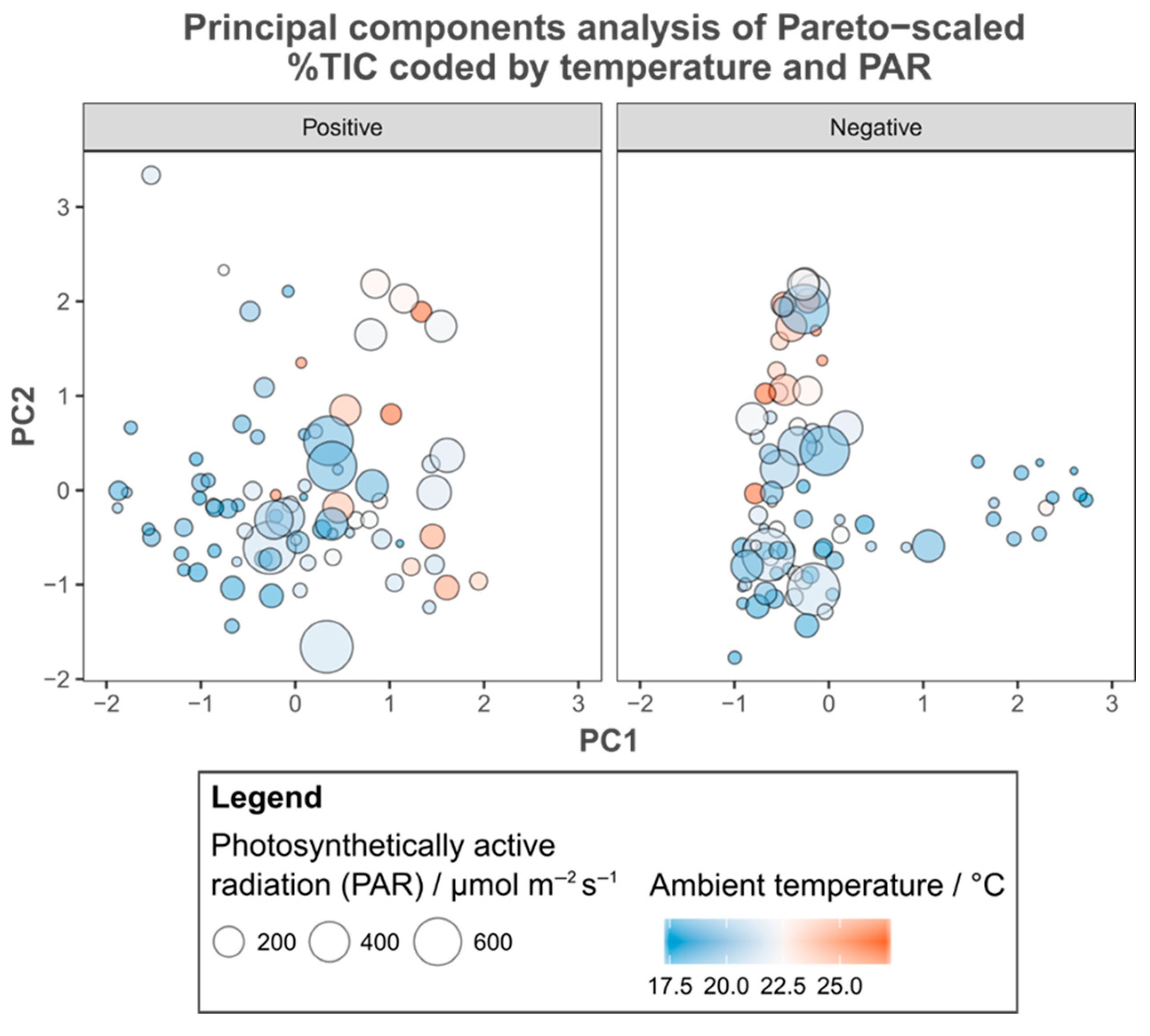
3.2. Environmental Metadata
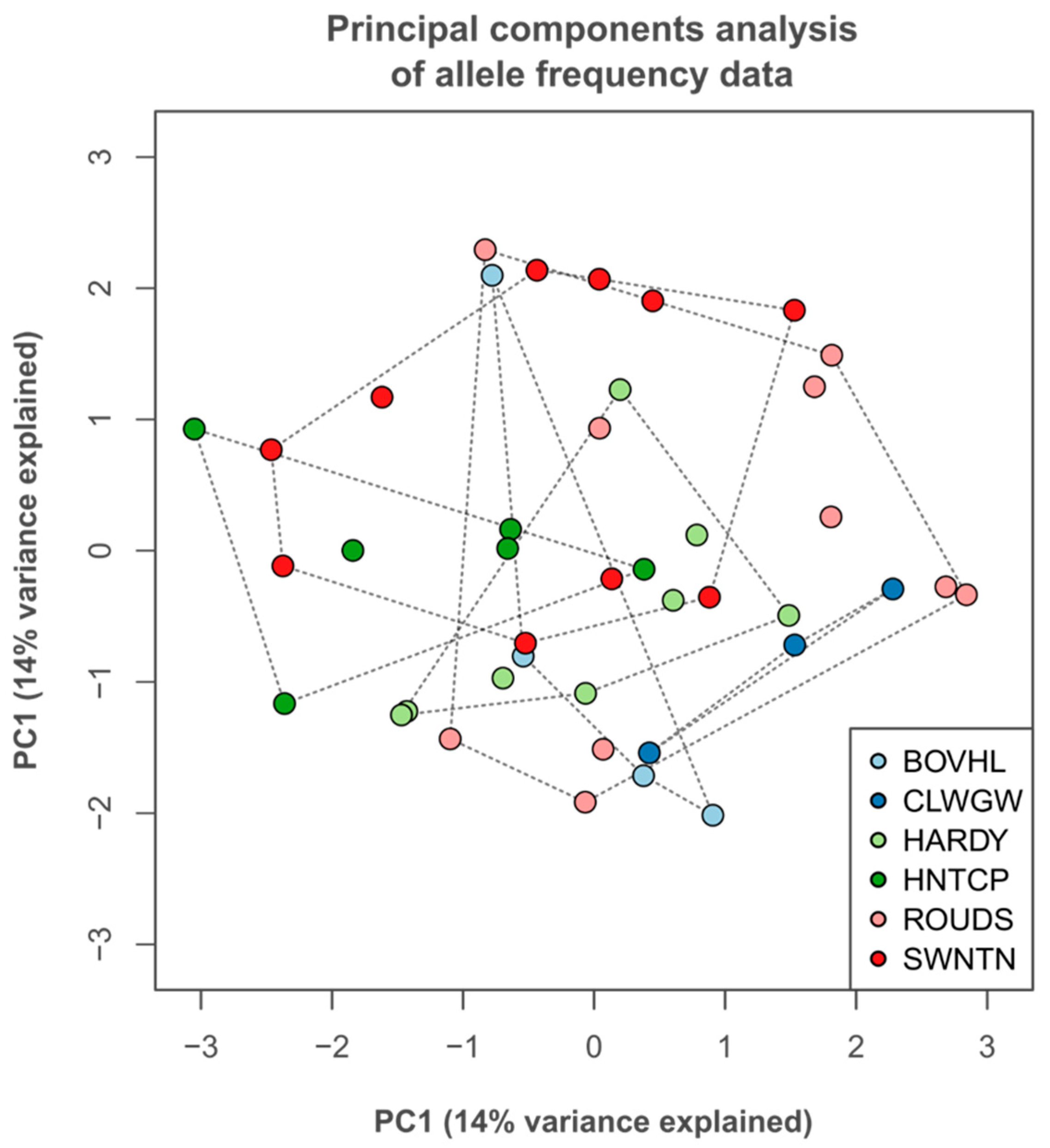
3.3. Neutral Trait Genetic Variation
3.4. Functional Trait Specific Leaf Area Variation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Aitken, S.N.; Yeaman, S.; Holliday, J.A.; Wang, T.; Curtis-McLane, S. Adaptation, migration or extirpation: Climate change outcomes for tree populations. Evol. Appl. 2008, 1, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Christmas, M.J.; Breed, M.F.; Lowe, A.J. Constraints to and conservation implications for climate change adaptation in plants. Conserv. Genet. 2015, 17, 305–320. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-C.; Hill, J.K.; Ohlemuller, R.; Roy, D.B.; Thomas, C.D. Rapid Range Shifts of Species Associated with High Levels of Climate Warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Corlett, R.T.; Westcott, D.A. Will plant movements keep up with climate change? Trends Ecol. Evol. 2013, 28, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Loarie, S.R.; Duffy, P.B.; Hamilton, H.; Asner, G.P.; Field, C.B.; Ackerly, D.D. The velocity of climate change. Nature 2009, 462, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, A.D. Unravelling phenotypic plasticity—Why should we bother? New Phytol. 2006, 170, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.P.; George, R.M.; Ooi, M.K.J.; Burrell, M.M.; Freckleton, R.P. Metabolic Niches and Plasticity of Sand-Dune Plant Communities Along a Trans-European Gradient. Metabolites 2025, 15, 217. [Google Scholar] [CrossRef] [PubMed]
- Jump, A.S.; Marchant, R.; Peñuelas, J. Environmental change and the option value of genetic diversity. Trends Plant Sci. 2009, 14, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Chevin, L.-M.; Lande, R.; Mace, G.M. Adaptation, plasticity, and extinction in a changing environment: Towards a predictive theory. PLoS Biol. 2010, 8, e1000357. [Google Scholar] [CrossRef] [PubMed]
- Lande, R. Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J. Evol. Biol. 2009, 22, 1435–1446. [Google Scholar] [CrossRef] [PubMed]
- Alberto, F.J.; Aitken, S.N.; Alía, R.; González-Martínez, S.C.; Hänninen, H.; Kremer, A.; Lefèvre, F.; Lenormand, T.; Yeaman, S.; Whetten, R.; et al. Potential for evolutionary responses to climate change—evidence from tree populations. Glob. Change Biol. 2013, 19, 1645–1661. [Google Scholar] [CrossRef] [PubMed]
- Leimu, R.; Fischer, M. A meta-analysis of local adaptation in plants. PLoS ONE 2008, 3, e4010. [Google Scholar] [CrossRef] [PubMed]
- Barker, C.; Ashton, M.; Ashton, P.A. Segregation of Tilia cordata and T. platyphyllos (Malvaceae) along environmental gradients. New J. Bot. 2015, 5, 157–163. [Google Scholar] [CrossRef]
- Logan, S.A.; Phuekvilai, P.; Wolff, K. Ancient woodlands in the limelight: Delineation and genetic structure of ancient woodland species Tilia cordata and Tilia platyphyllos (Tiliaceae) in the UK. Tree Genet. 2015, 11, 52. [Google Scholar] [CrossRef]
- Mittell, E.A.; Nakagawa, S.; Hadfield, J.D. Are molecular markers useful predictors of adaptive potential? Ecol. Lett. 2015, 18, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.H.; Frankham, R. How closely correlated are molecular and quantitative measures of genetic variation? A meta-analysis. Evolution 2001, 55, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Jacob, D.; Petersen, J.; Eggert, B.; Alias, A.; Christensen, O.B.; Bouwer, L.M.; Braun, A.; Colette, A.; Déqué, M.; Georgievski, G.; et al. EURO-CORDEX: New high-resolution climate change projections for European impact research. Reg. Environ. Change 2014, 14, 563–578. [Google Scholar] [CrossRef]
- Attorre, F.; Alfò, M.; De Sanctis, M.; Francesconi, F.; Valenti, R.; Vitale, M.; Bruno, F. Evaluating the effects of climate change on tree species abundance and distribution in the Italian peninsula. Appl. Veg. Sci. 2011, 14, 242–255. [Google Scholar] [CrossRef]
- Barker, C.; Davis, M.L.; Ashton, P. Reproductive strategy of a temperate canopy tree Tilia cordata Mill. (Malvaceae) is related to temperature during flowering and density of recent recruits. Tree Genet. Gen. 2022, 18, 22. [Google Scholar] [CrossRef]
- Ramírez-Valiente, J.A.; Sánchez-Gómez, D.; Aranda, I.; Valladares, F. Phenotypic plasticity and local adaptation in leaf ecophysiological traits of 13 contrasting cork oak populations under different water availabilities. Tree Physiol. 2010, 30, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Scheepens, J.F.; Frei, E.S.; Stöcklin, J. Genotypic and environmental variation in specific leaf area in a widespread Alpine plant after transplantation to different altitudes. Oecologia 2010, 164, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Oleksyk, T.K.; Smith, M.W.; O’Brien, S.J. Genome-wide scans for footprints of natural selection. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Kosmides, A.K.; Kamisoglu, K.; Calvano, S.E.; Corbett, S.A.; Androulakis, I.P. Metabolomic fingerprinting: Challenges and opportunities. Crit. Rev. Biomed. Eng. 2013, 41, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Bundy, J.G.; Davey, M.P.; Viant, M.R. Environmental metabolomics: A critical review and future perspectives. Metabolomics 2009, 5, 3–21. [Google Scholar] [CrossRef]
- Rivas-Ubach, A.; Gargallo-Garriga, A.; Sardans, J.; Oravec, M.; Mateu-Castell, L.; Pérez-Trujillo, M.; Parella, T.; Ogaya, R.; Urban, O.; Peñuelas, J. Drought enhances folivory by shifting foliar metabolomes in Quercus ilex trees. New Phytol. 2014, 202, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Gargallo-Garriga, A.; Pérez-Trujillo, M.; Parella, T.J.; Seco, R.; Filella, I.; Peñuelas, J. Metabolic responses of Quercus ilex seedlings to wounding analysed with nuclear magnetic resonance profiling. Plant Biol. 2014, 16, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.P.; Burrell, M.M.; Woodward, F.I.; Quick, W.P. Population-specific metabolic phenotypes of Arabidopsis lyrata ssp. petraea. New Phytol. 2008, 177, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.P.; Norman, L.; Sterk, P.; Huete-Ortega, M.; Bunbury, F.; Kin Wai Loh, B.; Stockton, S.; Peck, L.S.; Convey, P.; Newsham, K.K.; et al. Snow algae communities in Antarctica—metabolic and taxonomic composition. New Phytol. 2019, 222, 1242–1255. [Google Scholar] [CrossRef] [PubMed]
- Field, K.J.; Lake, J.A. Environmental metabolomics links genotype to phenotype and predicts genotype abundance in wild plant populations. Physiol. Plant. 2011, 142, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Kunin, W.E.; Vergeer, P.; Kenta, T.; Davey, M.P.; Burke, T.; Woodward, F.I.; Quick, P.; Mannarelli, M.-E.; Watson-Haigh, N.S.; Butlin, R. Variation at range margins across multiple spatial scales: Environmental temperature, population genetics and metabolomic phenotype. Proc. Biol. Sci. 2009, 276, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Halket, J.M.; Waterman, D.; Przyborowska, A.M.; Patel, R.K.P.; Fraser, P.D.; Bramley, P.M. Chemical derivatization and mass spectral libraries in metabolic profiling by GC/MS and LC/MS/MS. J. Exp. Bot. 2005, 56, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Verpoorte, R. Sample preparation for plant metabolomics. Phytochem. Anal. 2010, 21, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Gosling, P. Raising trees and shrubs from seed. In Foresty Commision Practice Guide; Forestry Commission: Edinburgh, UK, 2007; ISBN 978-0-85538-736-5. [Google Scholar]
- Pigott, C.D. Tilia cordata Miller. J. Ecol. 1991, 79, 1147–1207. [Google Scholar] [CrossRef]
- Pigott, C.D.; Huntley, J.P. Factors controlling the distribution of Tilia cordata at the northern limits of its geographical range. III. Nature and causes of seed sterility. New Phytol. 1981, 8, 817–839. [Google Scholar] [CrossRef]
- Linhart, Y.B.; Grant, M.C. Evolutionary significance of local genetic differentiation in plants. Annu. Rev. Ecol. Syst. 1996, 27, 237–277. [Google Scholar] [CrossRef]
- Gibon, Y.; Usadel, B.; Blaesing, O.E.; Kamlage, B.; Hoehne, M.; Trethewey, R.; Stitt, M. Integration of metabolite with transcript and enzyme activity profiling during diurnal cycles in Arabidopsis rosettes. Genome Biol. 2006, 7, R76. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.A.H.; Maguire, M.L.; Griffin, J.L.; Dias, D.A.; Spurgeon, D.J.; Svendsen, C. Metabolomics and its use in ecology. Austral Ecol. 2013, 38, 713–720. [Google Scholar] [CrossRef]
- Brunetti, C.; George, R.M.; Tattini, M.; Field, K.J.; Davey, M.P. Metabolomics in plant environmental physiology. J. Exp. Bot. 2013, 64, 4011–4020. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Ter Steege, H.; Morgan, H.D.; Van Der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 4, 335–380. [Google Scholar] [CrossRef]
- Lavorel, S.; Garnier, E. Predicting changes in community composition and ecosystem functioning from plant traits: Revisiting the Holy Grail. Funct. Ecol. 2002, 16, 545–556. [Google Scholar] [CrossRef]
- Goodacre, R.; Broadhurst, D.; Smilde, A.K.; Kristal, B.S.; Baker, J.D.; Beger, R.; Bessant, C.; Connor, S.; Capuani, G.; Craig, A.; et al. Proposed minimum reporting standards for data analysis in metabolomics. Metabolomics 2007, 3, 231–241. [Google Scholar] [CrossRef]
- Bivand, R.; Lewin-Koh, N. Maptools: Tools for Reading and Handling Spatial Objects. 2017. Available online: https://r-forge.r-project.org/projects/maptools/ (accessed on 15 July 2025).
- Kanehisa Laboratories, KEGG: Kyoto Encyclopedia of Genes and Genomes [WWW Document]. URL. 2017. Available online: http://www.genome.jp/kegg/ (accessed on 15 July 2025).
- van den Berg, R.A.; Hoefsloot, H.C.J.; Westerhuis, J.A.; Smilde, A.K.; van der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genomics 2006, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, X.; Lu, X.; Lin, X.; Xu, G. A data preprocessing strategy for metabolomics to reduce the mask effect in data analysis. Front. Mol. Biosci. 2015, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Worley, B.; Powers, R. Multivariate Analysis in Metabolomics. Curr. Metabolomics 2013, 1, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, M.S. Properties of Sufficiency and Statistical Tests. Proc. R. Soc. A Math. Phys. Eng. Sci. 1937, 160, 268–282. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple Comparisons Using Rank Sums. Technometrics 1964, 6, 241. [Google Scholar] [CrossRef]
- Barker, C. Doctoral Thesis Ecological, Genetic and Metabolic Variation in Populations of Tilia Cordata. Edge Hill University, UK. 2018. Available online: https://research.edgehill.ac.uk/en/studentTheses/ecological-genetic-and-metabolic-variation-in-populations-of-tili (accessed on 15 July 2025).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. 2017. Available online: http://CRAN.Rproject.org/package=vegan (accessed on 15 July 2025).
- Barker, M.; Rayens, W. Partial least squares for discrimination. J. Chemom. 2003, 17, 166–173. [Google Scholar] [CrossRef]
- Le Cao, K.-A.; Rohart, F.; Gonzalez, I.; Gautier, B.; Dejean, S.; Bartolo, F.; Monget, P.; Coquery, J.; Yao, F.Z.; Liquet, B. mixOmics: Omics Data Integration Project. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference and Prediction, 2nd ed.; Springer Series in Statistics: New York, NY, USA, 2001. [Google Scholar] [CrossRef]
- Bowcock, A.M.; Ruiz-Linares, A.; Tomfohrde, J.; Minch, E.; Kidd, J.R.; Cavalli-Sforza, L.L. High resolution of human evolutionary trees with polymorphic microsatellites. Nature 1994, 368, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Legendre, P.; Fortin, M.-J. Comparison of the Mantel test and alternative approaches for detecting complex multivariate relationships in the spatial analysis of genetic data. Mol. Ecol. Resour. 2010, 10, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Meirmans, P.G. Seven common mistakes in population genetics and how to avoid them. Mol. Ecol. 2015, 24, 3223–3231. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, R.I.; Fady, B.; Vendramin, G.G.; Dawson, I.K.; Fleming, R.A.; Sáenz-Romero, C.; Lindig-Cisneros, R.A.; Murdock, T.; Vinceti, B.; Navarro, C.M.; et al. The role of forest genetic resources in responding to biotic and abiotic factors in the context of anthropogenic climate change. For. Ecol. Manag. 2014, 333, 76–87. [Google Scholar] [CrossRef]
- Stepansky, A.; Leustek, T. Histidine biosynthesis in plants. Amino Acids 2006, 30, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, H. Pharmacology of estrogens and progestogens: Influence of different routes of administration. Climacteric 2005, 8, 3–63. [Google Scholar] [CrossRef] [PubMed]
- Coward, L.; Barnes, N.C.; Setchell, K.D.R.; Barnes, S. Genistein, Daidzein, and Their β-Glycoside Conjugates: Antitumor Isoflavones in Soybean Foods from American and Asian Diets. J. Agric. Food Chem. 1993, 41, 1961–1967. [Google Scholar] [CrossRef]
- Negri, G.; Santi, D.; Tabach, R. Flavonol glycosides found in hydroethanolic extracts from Tilia cordata, a species utilized as anxiolytics. Brazilian J. Med. Plants 2013, 15, 217–224. [Google Scholar] [CrossRef]
- Macel, M.; van Dam, N.M.; Keurentjes, J.J.B. Metabolomics: The chemistry between ecology and genetics. Mol. Ecol. Resour. 2010, 10, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.G. Introduction to Plant Physiology, 1st ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1995; ISBN 13: 9780471545477. [Google Scholar]
- Hew, C.S.; Krotkov, G.; Canvin, D.T. Effects of Temperature on Photosynthesis and CO2 Evolution in Light and Darkness by Green Leaves. Plant Physiol. 1969, 44, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Sage, R.F.; Kubien, D.S. The temperature response of C3 and C4 photosynthesis. Plant Cell Environ. 2007, 30, 1086–1106. [Google Scholar] [CrossRef] [PubMed]
- Hernández, I.; Alegre, L.; Van Breusegem, F.; Munné-Bosch, S. How relevant are flavonoids as antioxidants in plants? Trends Plant Sci. 2009, 14, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Paiva, N.L. Stress-Induced Phenylpropanoid Metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, B.L.; Edrada-Ebel, R.A.; Da Costa, F.B. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: A model for environmental metabolomics of plants. Sci. Rep. 2016, 6, 29265. [Google Scholar] [CrossRef] [PubMed]
- Winkel-Shirley, B. It takes a garden. How work on diverse plant species has contributed to an understanding of flavonoid metabolism. Plant Physiol. 2001, 127, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Benning, C.; Stitt, M. Physiology and metabolism: Reacting to the full complexity of metabolic pathways in a postgenomic era. Curr. Opin. Plant Biol. 2004, 7, 231–234. [Google Scholar] [CrossRef]
- Met Office. Climate summaries [WWW Document]. URL; 2017. Available online: https://www.metoffice.gov.uk/climate/uk/summaries (accessed on 15 July 2025).
- Hulshof, C.M.; Violle, C.; Spasojevic, M.J.; Mcgill, B.; Damschen, E.; Harrison, S.; Enquist, B.J. Intra-specific and inter-specific variation in specific leaf area reveal the importance of abiotic and biotic drivers of species diversity across elevation and latitude. J. Veg. Sci. 2013, 24, 921–931. [Google Scholar] [CrossRef]
- Li, B.; Suzuki, J.-I.; Hara, T. Latitudinal variation in plant size and relative growth rate in Arabidopsis thaliana. Oecologia 1998, 115, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Robson, T.M.; Sánchez-Gómez, D.; Cano, F.J.; Aranda, I. Variation in functional leaf traits among beech provenances during a Spanish summer reflects the differences in their origin. Tree Genet. Genomes 2012, 8, 1111–1121. [Google Scholar] [CrossRef]
- Aranda, I.; Sánchez-Gómez, D.; de Miguel, M.; Mancha, J.A.; Guevara, M.A.; Cadahía, E.; Fernández de Simón, M.B. Fagus sylvatica L. provenances maintain different leaf metabolic profiles and functional response. Acta Oecologica 2017, 82, 1–9. [Google Scholar] [CrossRef]
- Yin, X.; Kropff, M.J.; Stam, P. The role of ecophysiological models in QTL analysis: The example of specific leaf area in barley. Heredity 1999, 82, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Steege, M.W.; den Ouden, F.M.; Lambers, H.; Stam, P.; Peeters, A.J.M. Genetic and physiological architecture of early vigor in Aegilops tauschii, the D-genome donor of hexaploid wheat. A quantitative trait loci analysis. Plant Physiol. 2005, 139, 1078–1094. [Google Scholar] [CrossRef] [PubMed]



| Mode | Component | Mass Bin | Rank | Loading | Putative Identification | Amino Acid Metabolism | Synthesis of Secondary Metabolites | Cell Signalling | Cell Membrane Metabolism | Carbohydrate Metabolism | Citric Acid Cycle | Purine Metabolism | Pyrimidine Metabolism |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | PC1 | 285 | 1 | −0.42 | Various (10) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| 381 | 2 | 0.29 | Oligosaccharides; galactinol; panthetheine 4′-PO4 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| 266 | 3 | 0.26 | Amino acids (3), nucleosides (2) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| 447.2 | 4 | −0.24 | Various (10) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| 593.2 | 5 | −0.22 | - | ||||||||||
| 615.2 | 6 | −0.20 | - | ||||||||||
| 222 | 7 | 0.20 | Amino acids (5), amino sugars (3) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| 286 | 8 | −0.18 | Pyridoxal-PO4; pyrodoxine-PO4; linamarin | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| 594.2 | 9 | −0.14 | - | ||||||||||
| 447 | 10 | −0.13 | CDP-ethanolamine; Khellol glucoside | ✓ | |||||||||
| PC2 | 266 | 1 | −0.27 | Amino acids (3), nucleosides (2) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| 631.2 | 2 | −0.25 | Diosmin; reserpine | ||||||||||
| 381 | 3 | 0.22 | Oligosaccharides; galactinol; panthetheine 4′-PO4 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| 222 | 4 | −0.22 | Amino acids (5), amino sugars (3) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| 285 | 5 | −0.20 | Various (10) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| 417 | 6 | 0.19 | - | ||||||||||
| 365.2 | 7 | 0.16 | Oligosaccharides; XMP; GA44; galactinol; ajmaline | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| 631 | 8 | −0.15 | - | ||||||||||
| 411 | 9 | −0.15 | 2′-Deoxyuridine 5′-diphosphate | ✓ | |||||||||
| 184 | 10 | 0.12 | Amino acids (4); selenophosphate | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Mode | Component | Mass Bin | Rank | Loading | Putative Identification | Amino Acid Metabolism | Synthesis of Secondary Metabolites | Cell Signalling | Cell Membrane Metabolism | Carbohydrate Metabolism | Citric Acid Cycle | Purine Metabolism | Pyrimidine Metabolism |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative | PC1 | 268.4 | 1 | −0.32 | - | ||||||||
| 282.4 | 2 | −0.27 | - | ||||||||||
| 267.4 | 3 | −0.19 | - | ||||||||||
| 264.6 | 4 | 0.17 | - | ||||||||||
| 292.6 | 5 | 0.16 | - | ||||||||||
| 324.6 | 6 | 0.15 | - | ||||||||||
| 576.4 | 7 | −0.15 | Phosphoribosyl-formimino-AICAR-phosphate | ✓ | ✓ | ||||||||
| 192.4 | 8 | −0.14 | - | ||||||||||
| 283.4 | 9 | −0.13 | - | ||||||||||
| 269.4 | 10 | −0.13 | Estrone | ✓ | |||||||||
| PC2 | 282.4 | 1 | −0.46 | - | |||||||||
| 192.6 | 2 | −0.24 | - | ||||||||||
| 576.4 | 3 | −0.21 | Phosphoribosyl-formimino-AICAR-phosphate | ✓ | ✓ | ||||||||
| 268.4 | 4 | −0.20 | - | ||||||||||
| 300.4 | 5 | 0.14 | - | ||||||||||
| 284.4 | 6 | 0.13 | - | ||||||||||
| 242.4 | 7 | 0.13 | - | ||||||||||
| 254.4 | 8 | 0.11 | - | ||||||||||
| 577.4 | 9 | −0.11 | - | ||||||||||
| 590.4 | 10 | −0.11 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barker, C.; Ashton, P.; Davey, M.P. Low Metabolic Variation in Environmentally Diverse Natural Populations of Temperate Lime Trees (Tilia cordata). Metabolites 2025, 15, 509. https://doi.org/10.3390/metabo15080509
Barker C, Ashton P, Davey MP. Low Metabolic Variation in Environmentally Diverse Natural Populations of Temperate Lime Trees (Tilia cordata). Metabolites. 2025; 15(8):509. https://doi.org/10.3390/metabo15080509
Chicago/Turabian StyleBarker, Carl, Paul Ashton, and Matthew P. Davey. 2025. "Low Metabolic Variation in Environmentally Diverse Natural Populations of Temperate Lime Trees (Tilia cordata)" Metabolites 15, no. 8: 509. https://doi.org/10.3390/metabo15080509
APA StyleBarker, C., Ashton, P., & Davey, M. P. (2025). Low Metabolic Variation in Environmentally Diverse Natural Populations of Temperate Lime Trees (Tilia cordata). Metabolites, 15(8), 509. https://doi.org/10.3390/metabo15080509






