Postharvest NMR Metabolomic Profiling of Pomegranates Stored Under Low-Pressure Conditions: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Fruit Quality Analysis
2.2. Metabolomics Sample Preparation
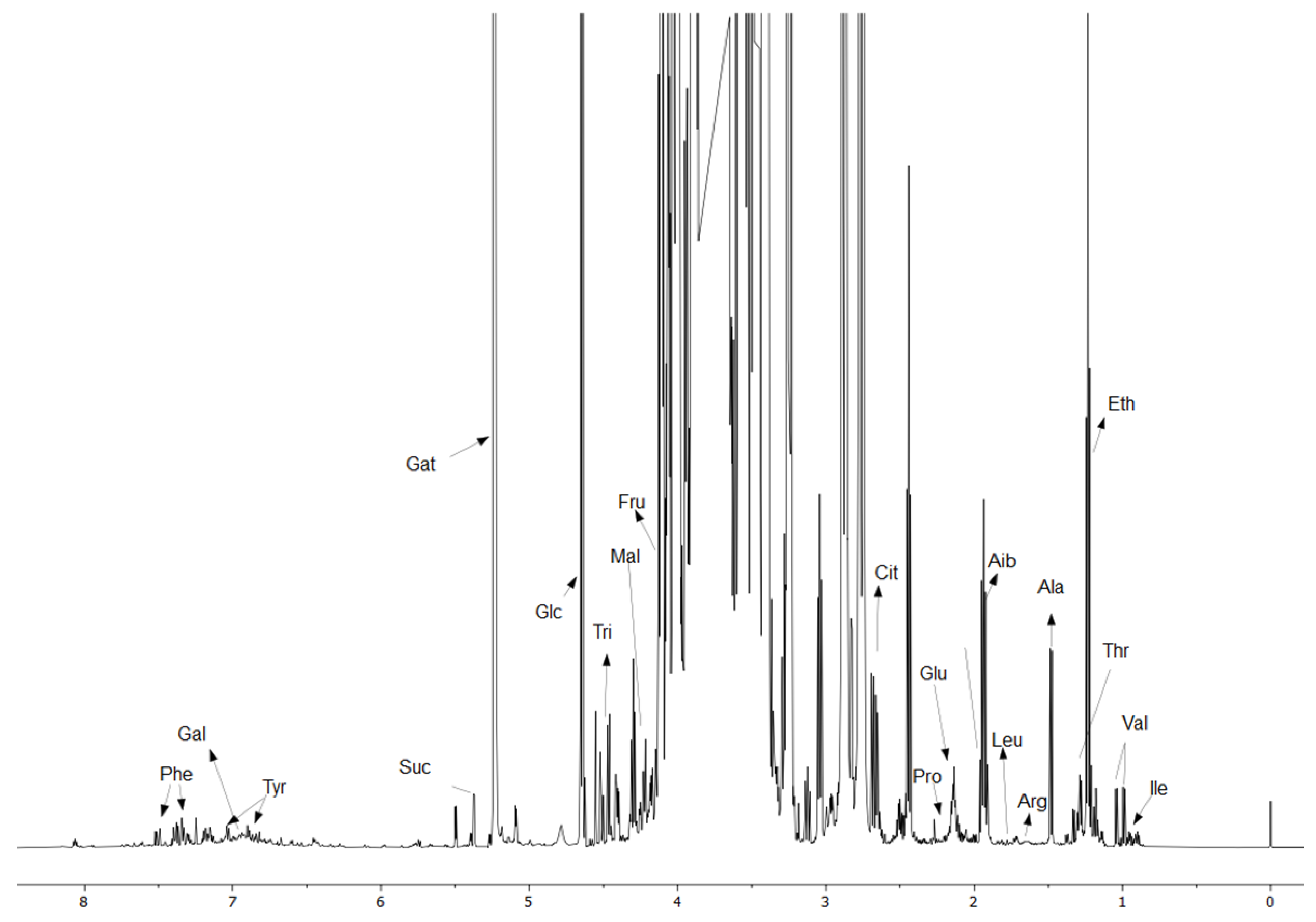
2.3. NMR Experiments
2.4. Statistical Analysis of NMR Spectroscopy Datasets
3. Results and Discussion
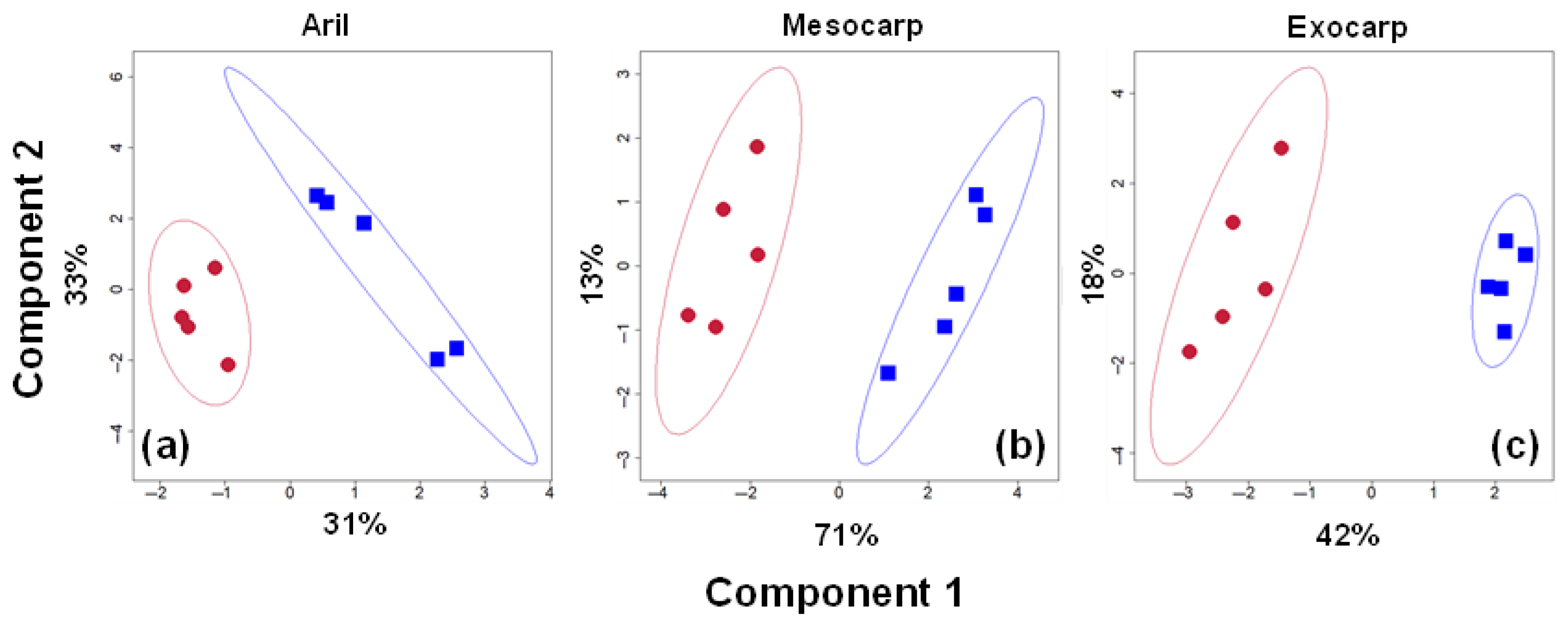
3.1. Fruit Quality and Metabolites Broadly Differentiate Between Storage Conditions
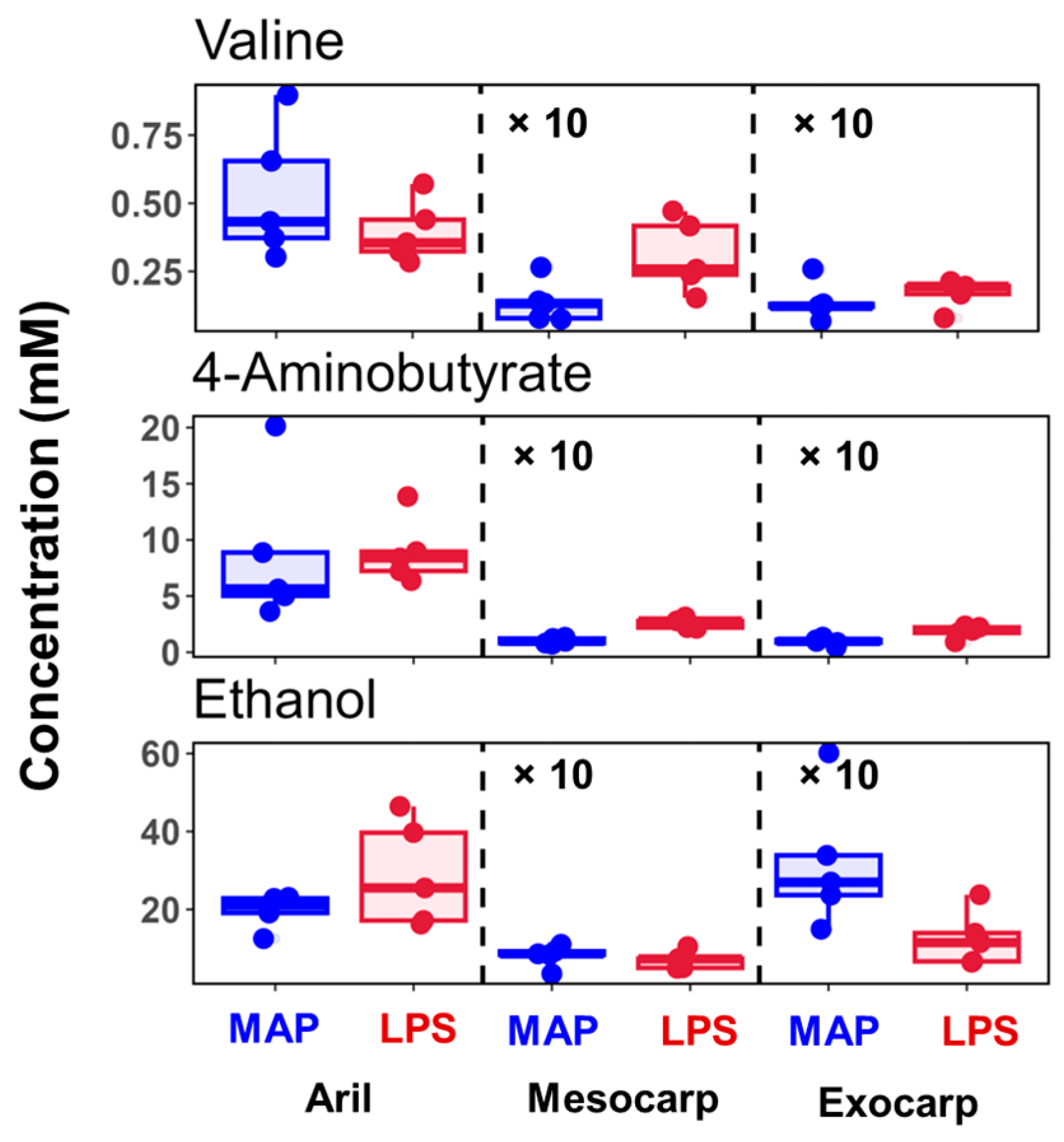
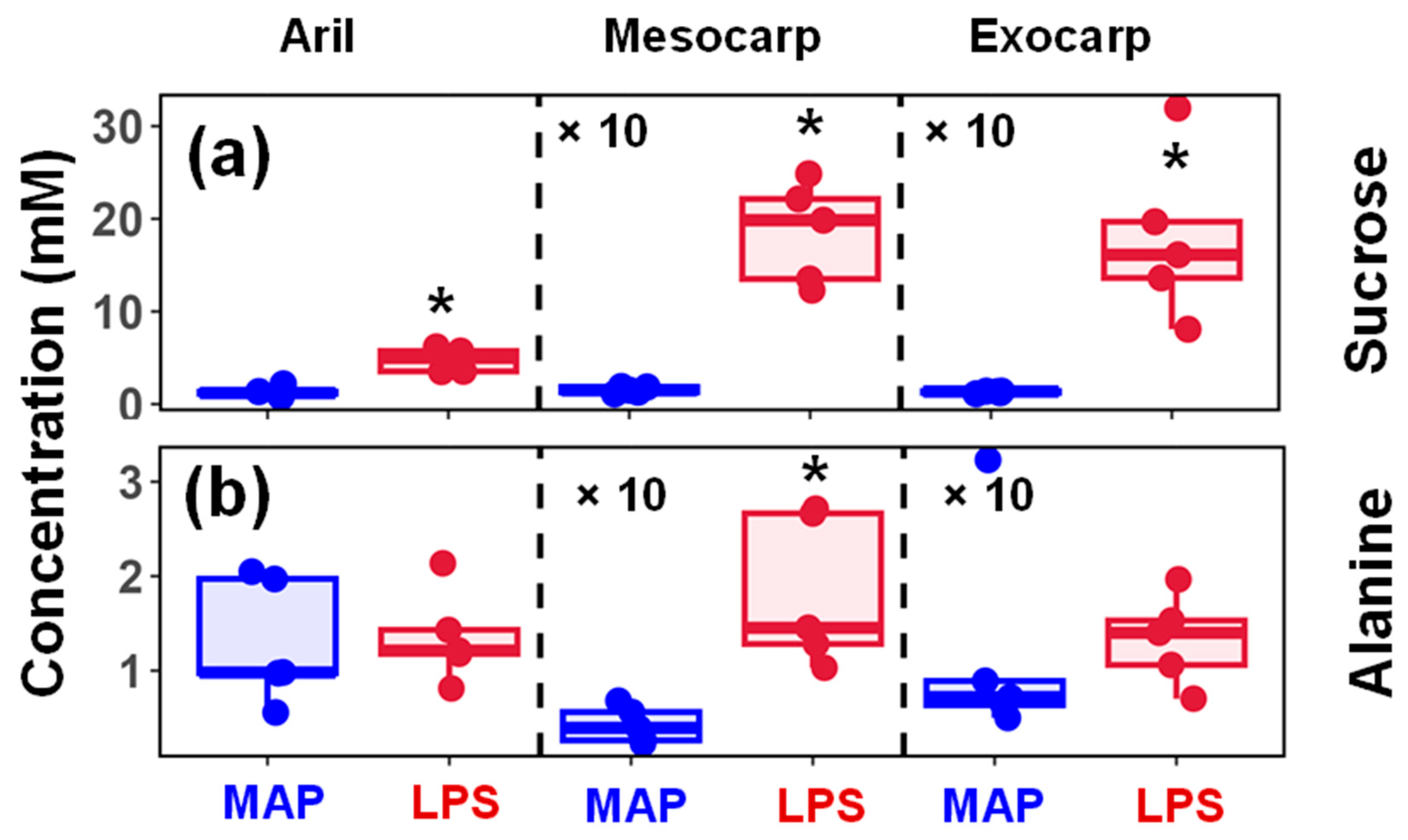
3.2. Differential Alterations of Metabolites Due to Low-Pressure Storage
3.3. Representative Changes in the Metabolites Under LPS Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

| Metabolites | Aril | Mesocarp | Exocarp | |||
|---|---|---|---|---|---|---|
| MAP (mM) | LPS (mM) | MAP (mM) | LPS (mM) | MAP (mM) | LPS (mM) | |
| 4-Aminobutyrate | 8.66 ± 5.99 | 8.96 ± 2.61 | 0.1 ± 0.02 | 0.26 ± 0.04 | 0.09 ± 0.03 | 0.18 ± 0.05 |
| Alanine | 1.31 ± 0.6 | 1.35 ± 0.44 | 0.04 ± 0.02 | 0.18 ± 0.07 | 0.12 ± 0.1 | 0.13 ± 0.04 |
| Arginine | 0.88 ± 0.41 | 0.54 ± 0.02 | 0.07 ± 0 | 0.1 ± 0.03 | 0.16 ± 0.05 | 0.2 ± 0.03 |
| Citrate | 60.83 ± 31.23 | 84.78 ± 39.11 | 17.43 ± 4.73 | 25.26 ± 6.09 | 16.51 ± 10.84 | 24.51 ± 13.8 |
| Ethanol | 19.7 ± 3.88 | 29.01 ± 12.12 | 0.81 ± 0.25 | 0.7 ± 0.2 | 3.19 ± 1.54 | 1.25 ± 0.63 |
| Fructose | 783.51 ± 339.42 | 626.91 ± 52.52 | 37.16 ± 3.68 | 41.05 ± 4.83 | 55.22 ± 8.79 | 63.46 ± 18.21 |
| Galactose | 7.44 ± 10.21 | 6.42 ± 9.59 | 0.38 ± 0.36 | 0.21 ± 0.03 | 0.72 ± 0.61 | 1.01 ± 0.92 |
| Gallate | - | - | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.11 ± 0.04 | 0.17 ± 0.07 |
| Glucose | 728.77 ± 312.72 | 639.8 ± 63.43 | 35.95 ± 3.67 | 38.99 ± 5.76 | 39.9 ± 5.26 | 51.72 ± 15.18 |
| Glutamine | 3.75 ± 2.4 | 1.82 ± 0.82 | 0.5 ± 0.22 | 0.56 ± 0.18 | 0.24 ± 0.07 | 0.31 ± 0.09 |
| Isoleucine | 0.14 ± 0.05 | 0.11 ± 0.03 | 0.01 ± 0 | 0.01 ± 0 | 0.01 ± 0 | 0.01 ± 0 |
| Leucine | 0.12 ± 0.05 | 0.1 ± 0.02 | 0.01 ± 0 | 0.01 ± 0 | 0 ± 0 | 0 ± 0 |
| Malate | 5.43 ± 3.41 | 2.68 ± 0.55 | 1.36 ± 0.14 | 2.62 ± 0.46 | 2.27 ± 0.73 | 2.44 ± 0.94 |
| Phenylalanine | 0.54 ± 0.18 | 0.41 ± 0.06 | - | - | - | - |
| Proline | 1.86 ± 0.88 | 0.84 ± 0.18 | 0.14 ± 0 | 0.15 ± 0.04 | 0.14 ± 0.09 | 0.11 ± 0.03 |
| Sucrose | 1.32 ± 0.47 | 4.79 ± 1.1 | 0.15 ± 0.03 | 1.85 ± 0.49 | 0.13 ± 0.01 | 1.79 ± 0.8 |
| Threonine | 0.32 ± 0.18 | 0.21 ± 0.04 | 0.03 ± 0 | 0.04 ± 0.01 | 0.07 ± 0.02 | 0.05 ± 0.01 |
| Trigonelline | 0.8 ± 0.41 | 0.62 ± 0.11 | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.04 ± 0.02 | 0.04 ± 0.01 |
| Tyrosine | 0.28 ± 0.13 | 0.26 ± 0.06 | - | - | - | - |
| Valine | 0.53 ± 0.22 | 0.39 ± 0.1 | 0.01 ± 0.01 | 0.03 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0 |
References
- Teksur, P.K. Alternative technologies to control postharvest diseases of pomegranate. Stewart Postharvest Rev. 2015, 11, 1–7. [Google Scholar] [CrossRef]
- Yam, K.L.; Lee, D.S. Emerging Food Packaging Technologies: Principles and Practice; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Kader, A.A.; Saltveit, M.E. Respiration and gas exchange. In Postharvest Physiology and Pathology of Vegetables; CRC Press: Boca Raton, FL, USA, 2002; pp. 31–56. [Google Scholar]
- Yam, K.L.; Lee, D.S. Design of modified atmosphere packaging for fresh produce. In Active Food Packaging; Rooney, M.L., Ed.; Springer: Boston, MA, USA, 1995; pp. 55–73. [Google Scholar] [CrossRef]
- Zhang, L.; McCarthy, M.J. Effect of controlled atmosphere storage on pomegranate quality investigated by two dimensional NMR correlation spectroscopy. LWT—Food Sci. Technol. 2013, 54, 302–306. [Google Scholar] [CrossRef]
- Burg, S.P.; Burg, E.A. Fruit Storage at Subatmospheric Pressures. Science 1966, 153, 314–315. [Google Scholar] [CrossRef]
- Dilley, D. Principles and effects of hypobaric storage of fruits and vegetables. ASHRAE Transact. 1982, 88, 1461–1477. [Google Scholar]
- Maryam, A.; Anwar, R.; Malik, A.U.; Khan, A.S.; Ali, S.; Waris, F.; Hasan, M.U.; El-Mogy, M.M. Pre-storage hypobaric treatment reduces microbial spoilage and maintains eating quality of strawberry fruits during low temperature conditions. J. Food Process. Preserv. 2022, 46, e17121. [Google Scholar] [CrossRef]
- Salas-Sanjuán, M.d.C.; Rebolloso, M.d.M.; del Moral, F.; Valenzuela, J.L. Use of Sub-Atmospheric Pressure Storage to Improve the Quality and Shelf-Life of Marmande Tomatoes cv. Rojito. Foods 2023, 12, 1197. [Google Scholar] [CrossRef]
- Vithu, P.; Moses, J. Hypobaric storage of horticultural products: A review. In Engineering Practices for Agricultural Production and Water Conservation; Academic Press: Cambridge, MA, USA, 2017; pp. 155–170. [Google Scholar]
- Leszczuk, A.; Kutyrieva-Nowak, N.; Nowak, A.; Nosalewicz, A.; Zdunek, A. Low oxygen environment effect on the tomato cell wall composition during the fruit ripening process. BMC Plant Biol. 2024, 24, 503. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, S.; Salamanca, M.C.; Sierra, C.A.; Palomeque, L.A.; Castellanos, D.A. Determination of changes in physicochemical and sensory characteristics of purple passion fruit with the application of different packaging systems, including an ethylene scavenger additive. J. Food Sci. 2021, 86, 1372–1383. [Google Scholar] [CrossRef] [PubMed]
- Burg, S.P. Postharvest Physiology and Hypobaric Storage of Fresh Produce; Cabi: Wallingford, UK, 2004. [Google Scholar]
- Gao, H.Y.; Chen, H.J.; Chen, W.X.; Yang, Y.T.; Song, L.L.; Jiang, Y.M.; Zheng, Y.H. Effect of hypobaric storage on physiological and quality attributes of loquat fruit at low temperature. In Proceedings of the IV International Conference on Managing Quality in Chains—The Integrated View on Fruits and Vegetables Quality, Bangkok, Thailand, 7–10 August 2006; pp. 269–274. [Google Scholar]
- Kan, A.; Wang, N.; Lin, L.; Sun, X.; Zhang, L. Experimental investigation of hypobaric storage effect on freshness-keeping of strawberries. J. Food Compos. Anal. 2025, 137, 106989. [Google Scholar] [CrossRef]
- Li, W.-x.; Zhang, M. Effects of combined hypobaric and atmosphere cold storage on the preservation of honey peach. Int. Agrophys. 2005, 19, 231–236. [Google Scholar]
- Romanazzi, G.; Nigro, F.; Ippolito, A. Short hypobaric treatments potentiate the effect of chitosan in reducing storage decay of sweet cherries. Postharvest Biol. Technol. 2003, 29, 73–80. [Google Scholar] [CrossRef]
- Anthony, B.M.; Kim, Y.-K.; Minas, I.S. Evaluating low pressure storage for prolonging the postharvest life of blueberries. Sci. Hortic. 2024, 337, 113535. [Google Scholar] [CrossRef]
- Anthony, B.M.; Kim, Y.K. RipeLocker: Low pressure storage to extend the storage life of horticultural commodities. In Proceedings of the VII International Conference Postharvest Unlimited, Wageningen, The Netherlands, 14–17 May 2023; pp. 271–276. [Google Scholar]
- Xu, F.; Li, G. Metabolomics reveals the effect of hypobaric treatment on energy metabolism in vibration-injured ‘Huangguan’ pears. Food Chem. 2023, 400, 134057. [Google Scholar] [CrossRef] [PubMed]
- Jurenka, J.S. Therapeutic applications of pomegranate (Punica granatum L.): A review. Altern. Med. Rev. 2008, 13, 128–144. [Google Scholar]
- Puneeth, H.R.; Chandra, S.S.P. A review on potential therapeutic properties of Pomegranate (Punica granatum L.). Plant Sci. Today 2020, 7, 9–16. [Google Scholar] [CrossRef]
- Basu, A.; Penugonda, K. Pomegranate juice: A heart-healthy fruit juice. Nutr. Rev. 2009, 67, 49–56. [Google Scholar] [CrossRef]
- Malik, A.; Afaq, F.; Sarfaraz, S.; Adhami, V.M.; Syed, D.N.; Mukhtar, H. Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 14813–14818. [Google Scholar] [CrossRef]
- Sumner, M.D.; Elliott-Eller, M.; Weidner, G.; Daubenmier, J.J.; Chew, M.H.; Marlin, R.; Raisin, C.J.; Ornish, D. Effects of pomegranate juice consumption on myocardial perfusion in patients with coronary heart disease. Am. J. Cardiol. 2005, 96, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J. Pomegranate and its many functional components as related to human health: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [CrossRef]
- Magangana, T.P.; Makunga, N.P.; Fawole, O.A.; Stander, M.A.; Opara, U.L. Antioxidant, Antimicrobial, and Metabolomic Characterization of Blanched Pomegranate Peel Extracts: Effect of Cultivar. Molecules 2022, 27, 2979. [Google Scholar] [CrossRef]
- Montgomery, K.H.; Elhabashy, A.; Del Carmen Reynoso Rivas, M.; Brar, G.; Krishnan, V.V. NMR metabolomics as a complementary tool to brix-acid tests for navel orange quality control of long-term cold storage. Sci. Rep. 2024, 14, 30078. [Google Scholar] [CrossRef]
- Augustijn, D.; de Groot, H.J.M.; Alia, A. HR-MAS NMR Applications in Plant Metabolomics. Molecules 2021, 26, 931. [Google Scholar] [CrossRef]
- Tang, F.; Hatzakis, E. NMR-Based Analysis of Pomegranate Juice Using Untargeted Metabolomics Coupled with Nested and Quantitative Approaches. Anal. Chem. 2020, 92, 11177–11185. [Google Scholar] [CrossRef]
- Zhang, L.; McCarthy, M.J. Assessment of pomegranate postharvest quality using nuclear magnetic resonance. Postharvest Biol. Technol. 2013, 77, 59–66. [Google Scholar] [CrossRef]
- Hasanpour, M.; Saberi, S.; Iranshahi, M. Metabolic Profiling and Untargeted 1H-NMR-Based Metabolomics Study of Different Iranian Pomegranate (Punica granatum) Ecotypes. Planta Med. 2020, 86, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Baghel, R.S.; Keren-Keiserman, A.; Ginzberg, I. Metabolic changes in pomegranate fruit skin following cold storage promote chilling injury of the peel. Sci. Rep. 2021, 11, 9141. [Google Scholar] [CrossRef] [PubMed]
- Al Shoffe, Y.; Shah, A.S.; Nock, J.F.; Watkins, C.B. Acetaldehyde and Ethanol Metabolism during Conditioning and Air Storage of ‘Honeycrisp’ Apples. HortScience 2018, 53, 1347–1351. [Google Scholar] [CrossRef]
- Montgomery, K.H.; Brar, G.; Krishnan, V.V. Metabolomics Study at the Postharvest Conditions of Cold Storage and Fungicide (Imazalil Sulfate) Treatment in Navel Oranges and Clementine Mandarins. ACS Agric. Sci. Technol. 2022, 2, 79–89. [Google Scholar] [CrossRef]
- Wilkop, T.E.; Wang, M.; Heringer, A.; Singh, J.; Zakharov, F.; Krishnan, V.V.; Drakakaki, G. NMR spectroscopy analysis reveals differential metabolic responses in arabidopsis roots and leaves treated with a cytokinesis inhibitor. PLoS ONE 2020, 15, e0241627. [Google Scholar] [CrossRef]
- Ernst, R.R.; Anderson, W.A. Application of Fourier Transform Spectroscopy to Magnetic Resonance. Rev. Sci. Instrum. 1966, 37, 93–102. [Google Scholar] [CrossRef]
- Ernst, R.R.; Bodenhausen, G.; Wokaun, A. Principles of Nuclear Magnetic Resonance in One and Two Dimensions; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Krishnan, V.V.; Ravindran, R.; Wun, T.; Luciw, P.A.; Khan, I.H.; Janatpour, K. Multiplexed measurements of immunomodulator levels in peripheral blood of healthy subjects: Effects of analytical variables based on anticoagulants, age, and gender. Cytom. B Clin. Cytom. 2014, 86, 426–435. [Google Scholar] [CrossRef]
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Hasnaoui, N.; Jbir, R.; Mars, M.; Trifi, M.; Kamal-Eldin, A.; Melgarjo, P.; Hernandez, F. Organic Acids, Sugars, and Anthocyanins Contents in Juices of Tunisian Pomegranate Fruits. Int. J. Food Prop. 2011, 14, 741–757. [Google Scholar] [CrossRef]
- Krueger, D.A. Composition of Pomegranate Juice. J. AOAC Int. 2019, 95, 163–168. [Google Scholar] [CrossRef]
- Tezcan, F.; Uzaşçı, S.; Uyar, G.; Öztekin, N.; Erim, F.B. Determination of amino acids in pomegranate juices and fingerprint for adulteration with apple juices. Food Chem. 2013, 141, 1187–1191. [Google Scholar] [CrossRef]
- Brizzolara, S.; Manganaris, G.A.; Fotopoulos, V.; Watkins, C.B.; Tonutti, P. Primary Metabolism in Fresh Fruits During Storage. Front. Plant Sci. 2020, 11, 80. [Google Scholar] [CrossRef]
- Borsani, J.; Budde, C.O.; Porrini, L.; Lauxmann, M.A.; Lombardo, V.A.; Murray, R.; Andreo, C.S.; Drincovich, M.F.; Lara, M.V. Carbon metabolism of peach fruit after harvest: Changes in enzymes involved in organic acid and sugar level modifications. J. Exp. Bot. 2009, 60, 1823–1837. [Google Scholar] [CrossRef]
- Fugate, K.K.; Eide, J.D.; Lafta, A.M.; Tehseen, M.M.; Chu, C.; Khan, M.F.R.; Finger, F.L. Transcriptomic and metabolomic changes in postharvest sugarbeet roots reveal widespread metabolic changes in storage and identify genes potentially responsible for respiratory sucrose loss. Front. Plant Sci. 2024, 15, 1320705. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Shao, X.; Gong, Y.; Zhu, Y.; Wang, H.; Zhang, X.; Yu, D.; Yu, F.; Qiu, Z.; Lu, H. The metabolism of soluble carbohydrates related to chilling injury in peach fruit exposed to cold stress. Postharvest Biol. Technol. 2013, 86, 53–61. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Fukao, T.; Gibbs, D.J.; Holdsworth, M.J.; Lee, S.C.; Licausi, F.; Perata, P.; Voesenek, L.A.; van Dongen, J.T. Making sense of low oxygen sensing. Trends Plant Sci. 2012, 17, 129–138. [Google Scholar] [CrossRef]
- Cukrov, D.; Zermiani, M.; Brizzolara, S.; Cestaro, A.; Licausi, F.; Luchinat, C.; Santucci, C.; Tenori, L.; Van Veen, H.; Zuccolo, A.; et al. Extreme hypoxic conditions induce selective molecular responses and metabolic reset in detached apple fruit. Front. Plant Sci. 2016, 7, 146. [Google Scholar] [CrossRef] [PubMed]
- Diab, H.; Limami, A.M. Reconfiguration of N metabolism upon hypoxia stress and recovery: Roles of alanine aminotransferase (AlaAT) and glutamate dehydrogenase (GDH). Plants 2016, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Hatoum, D.; Hertog, M.L.; Geeraerd, A.H.; Nicolai, B.M. Effect of browning related pre-and postharvest factors on the ‘Braeburn’apple metabolome during CA storage. Postharvest Biol. Technol. 2016, 111, 106–116. [Google Scholar] [CrossRef]
- Vandendriessche, T.; Schäfer, H.; Verlinden, B.E.; Humpfer, E.; Hertog, M.L.; Nicolaï, B.M. High-throughput NMR based metabolic profiling of Braeburn apple in relation to internal browning. Postharvest Biol. Technol. 2013, 80, 18–24. [Google Scholar] [CrossRef]
- Narsai, R.; Rocha, M.; Geigenberger, P.; Whelan, J.; van Dongen, J.T. Comparative analysis between plant species of transcriptional and metabolic responses to hypoxia. New Phytol. 2011, 190, 472. [Google Scholar] [CrossRef]
- Deewatthanawong, R.; Nock, J.F.; Watkins, C.B. γ-Aminobutyric acid (GABA) accumulation in four strawberry cultivars in response to elevated CO2 storage. Postharvest Biol. Technol. 2010, 57, 92–96. [Google Scholar] [CrossRef]
- Deewatthanawong, R.; Rowell, P.; Watkins, C.B. γ-Aminobutyric acid (GABA) metabolism in CO2 treated tomatoes. Postharvest Biol. Technol. 2010, 57, 97–105. [Google Scholar] [CrossRef]
- Pedreschi, R.; Franck, C.; Lammertyn, J.; Erban, A.; Kopka, J.; Hertog, M.; Verlinden, B.; Nicolaï, B. Metabolic profiling of ‘Conference’pears under low oxygen stress. Postharvest Biol. Technol. 2009, 51, 123–130. [Google Scholar] [CrossRef]
- Anthony, B.M.; Chaparro, J.M.; Prenni, J.E.; Minas, I.S. Early metabolic priming under differing carbon sufficiency conditions influences peach fruit quality development. Plant Physiol. Biochem. 2020, 157, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Anthony, B.M.; Chaparro, J.M.; Prenni, J.E.; Minas, I.S. Carbon sufficiency boosts phenylpropanoid biosynthesis early in peach fruit development priming superior fruit quality. Plant Physiol. Biochem. 2023, 196, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Anthony, B.M.; Chaparro, J.M.; Sterle, D.G.; Prenni, J.E.; Minas, I.S. Metabolic signatures of the true physiological impact of canopy light environment on peach fruit quality. Environ. Exp. Bot. 2021, 191, 104630. [Google Scholar] [CrossRef]
| Time Point | Storage Treatment | pH | SSC a (%) | TA b (%) | SSC/TA Ratio | Ethanol (%) | Weight Loss (%) |
|---|---|---|---|---|---|---|---|
| 0 d (Initial) | Initial | 3.35 ± 0.04 b | 17.1 ± 0.47 a | 1.09 ± 0.01 a | 15.7 ± 0.58 a | 0.01 ± 0.00 b | - |
| 212 d (Opening) | LPS | 3.67 ± 0.07 a | 15.9 ± 0.34 b | 0.84 ± 0.06 b | 18.9 ± 1.24 abc | 0.44 ± 0.06 a | 0.6 ± 1.4 b |
| MAP | 3.72 ± 0.04 a | 15.6 ± 0.54 b | 0.72 ± 0.05 b | 21.8 ± 1.42 a | 0.23 ± 0.13 ab | 3.6 ± 1.8 a | |
| +15 d (Shelf Life) | LPS | 3.63 ± 0.09 a | 15.6 ± 0.64 b | 0.88 ± 0.10 b | 17.9 ± 2.21 bc | 0.50 ± 0.23 a | - |
| MAP | 3.60 ± 0.09 a | 15.2 ± 0.63 b | 0.74 ± 0.08 b | 20.8 ± 2.54 ab | 0.31 ± 0.23 ab | - | |
| Significance | * | * | * | * | * | * | |
| Metabolites | Aril | Mesocarp | Exocarp |
|---|---|---|---|
| a FC | a FC | a FC | |
| Sucrose | 1.9 | 3.58 | 3.65 |
| Proline | −1.04 | −0.02 | −0.22 |
| Malate | −0.79 | 0.93 | 0.08 |
| Glutamine | −0.91 | 0.21 | 0.39 |
| Arginine | −0.59 | 0.43 | 0.34 |
| Alanine | 0.15 | 2.12 | 0.45 |
| 4-Aminobutyrate | 0.28 | 1.39 | 0.96 |
| Valine | −0.36 | 1.2 | 0.33 |
| Isoleucine | −0.35 | 1.12 | 0.59 |
| Leucine | −0.05 | 0.89 | −0.08 |
| Ethanol | 0.46 | −0.18 | −1.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montgomery, K.H.; Elhabashy, A.; Anthony, B.M.; Kim, Y.-K.; Krishnan, V.V. Postharvest NMR Metabolomic Profiling of Pomegranates Stored Under Low-Pressure Conditions: A Pilot Study. Metabolites 2025, 15, 507. https://doi.org/10.3390/metabo15080507
Montgomery KH, Elhabashy A, Anthony BM, Kim Y-K, Krishnan VV. Postharvest NMR Metabolomic Profiling of Pomegranates Stored Under Low-Pressure Conditions: A Pilot Study. Metabolites. 2025; 15(8):507. https://doi.org/10.3390/metabo15080507
Chicago/Turabian StyleMontgomery, Keeton H., Aya Elhabashy, Brendon M. Anthony, Yong-Ki Kim, and Viswanathan V. Krishnan. 2025. "Postharvest NMR Metabolomic Profiling of Pomegranates Stored Under Low-Pressure Conditions: A Pilot Study" Metabolites 15, no. 8: 507. https://doi.org/10.3390/metabo15080507
APA StyleMontgomery, K. H., Elhabashy, A., Anthony, B. M., Kim, Y.-K., & Krishnan, V. V. (2025). Postharvest NMR Metabolomic Profiling of Pomegranates Stored Under Low-Pressure Conditions: A Pilot Study. Metabolites, 15(8), 507. https://doi.org/10.3390/metabo15080507







