Ketogenic Metabolism in Neurodegenerative Diseases: Mechanisms of Action and Therapeutic Potential
Abstract
1. Introduction
1.1. Neurodegenerative Disorders: Shared Mechanisms
1.2. Ketogenic Metabolism and Neuroprotection
1.3. Aims of the Review
2. Ketone Bodies and Brain Metabolism
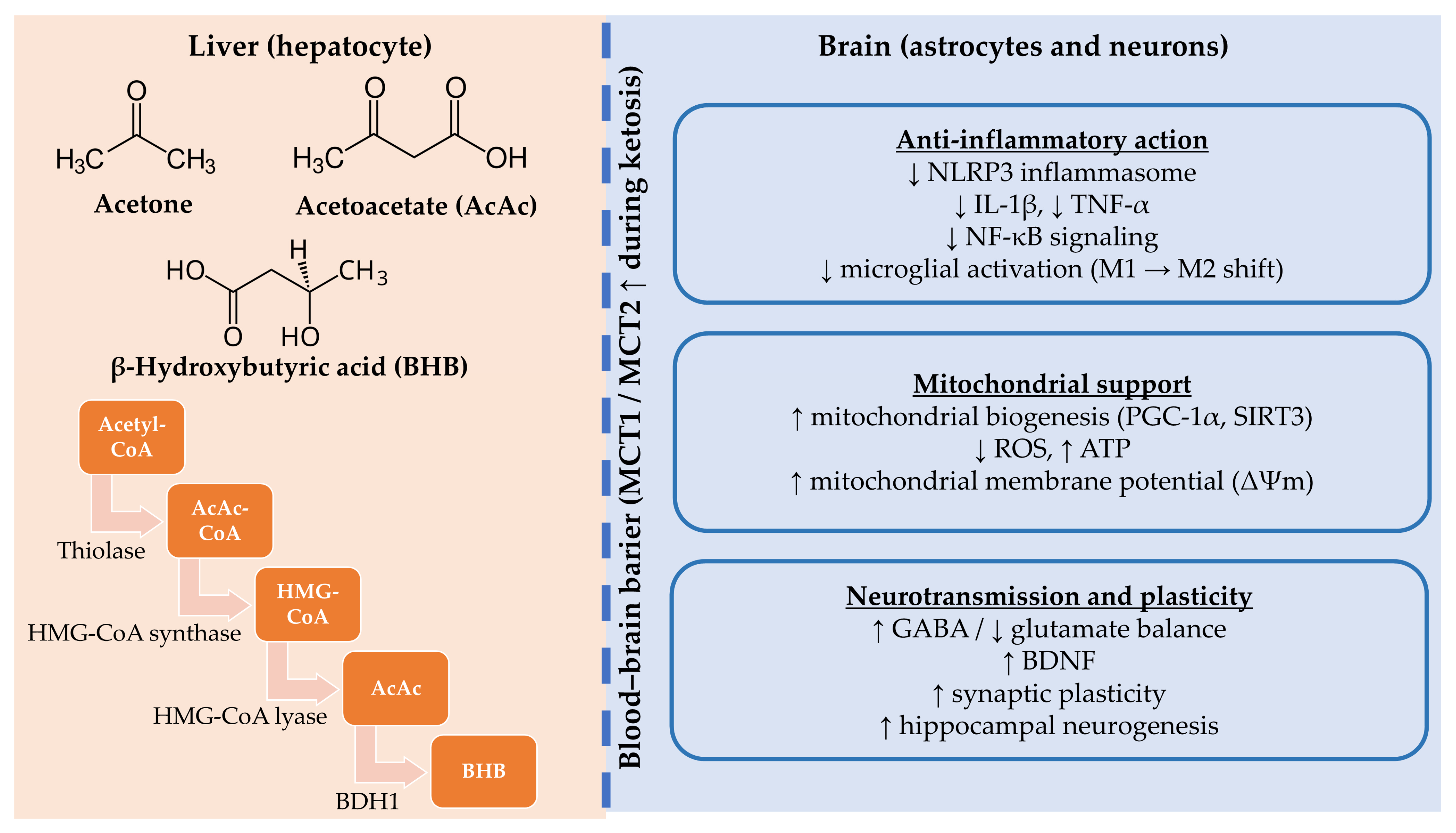
2.1. Ketogenesis and Ketone Bodies
2.2. Energy Utilization in the Brain
2.3. Metabolic and Epigenetic Effects
3. Mechanisms of Neuroprotection
3.1. Mitochondrial Enhancement and Oxidative Stress Reduction
3.2. Anti-Inflammatory and Immunomodulatory Effects
3.3. Effects on Neurotransmission and Synaptic Plasticity
3.4. Limitations of Animal Models in Translational Research
4. Clinical and Experimental Evidence
4.1. Experimental Evidence on Ketogenic Interventions in Alzheimer’s Disease
4.2. Clinical Studies on the Ketogenic Diet in Alzheimer’s Disease
4.3. Experimental Evidence on Ketogenic Interventions in Parkinson’s Disease
4.4. Clinical Studies on the Ketogenic Diet in Parkinson’s Disease
4.5. Ketogenic Strategies in Amyotrophic Lateral Sclerosis: Preclinical and Clinical Evidence
| Disorders | Study Groups | Intervention | Results | Study |
|---|---|---|---|---|
| Alzheimer’s disease | 152 mild-to-moderate AD patients | oral ketogenic compound AC-1202 | improved cognitive outcomes in APOE4 patients; no benefit in APOE4+ carriers | Henderson et al. [105] |
| Alzheimer’s disease | 26 patients with probable AD | modified ketogenic diet vs. usual diet (crossover design) | improved daily function and quality of life; no significant cognitive improvement; mild adverse effects | Phillips et al. [97] |
| Alzheimer’s disease | 83 patients with mild-to-moderate AD | ketogenic MCT drink vs. placebo | significant improvements in verbal fluency, naming, and executive function tests | Fortier et al. [135] |
| Alzheimer’s disease | MCI or early-stage AD | Modified Atkins Diet | improved episodic memory (Memory Composite Score) in participants achieving ketosis; increased energy levels; feasibility limited by adherence challenges | Brandt et al. [100] |
| Alzheimer’s disease | 413 patients with Mild-to-Moderate AD | AC-1204 | no improvement in cognitive and functional abilities in people with mild to moderate AD | Henderson et al. [96] |
| Parkinson’s disease | 14 PD patients | ketogenic diet vs. high-carbohydrate diet | enhanced cognitive performance | Krikorian et al. [116] |
| Parkinson’s disease | 7 PD patients | low-carbohydrate vs. healthy fat vs. ketogenic diet | enhanced cognition, mood, motor and nonmotor symptoms, and reduced pain and anxiety | Tidman et al. [118] |
| Parkinson’s disease | 16 PD patients | LCHF diet | improved scores on the PAS anxiety scale, no improvements on the CESD-R-20 scale for symptoms of depression in the 12 weeks, positive trends for reducing overall PD symptoms, improving biomarkers of chronic disease, and reducing anxiety in persons with PD | Tidman et al. [117] |
| Parkinson’s disease | 16 PD patients | medium-chain triglyceride-supplemented ketogenic diet | no significant improvement in motor and mobility results compared to the standard diet | Choi et al. [111] |
| Parkinson’s disease | 68 PD patients | ketogenic diet | improved VHI parameters | Koyuncu et al. [119] |
| Parkinson’s disease | 47 PD patients | modified ketogenic diet | improved non-motor symptoms (especially urinary problems, pain, fatigue, daytime sleepiness), improved cognitive impairment | Phillips et al. [115] |
| Parkinson’s disease | 7 PD patients | ketogenic diet | improved motor scores | VanItallie et al. [136] |
| Amyotrophic Lateral Sclerosis | 40 ALS patients | Mediterranean diet + coconut oil | benefits at the anthropometric level: increased percentage of muscle mass and decreased percentage of fat mass and abdominal skin folds compared to the control diet | Carrera-Juliá et al. [131] |
| Mild Cognitive Impairment | 23 older adults with MCI | ketogenic diet | improved verbal memory performance | Krikorian et al. [137] |
| Mild Cognitive Impairment | 39 participants with MCI completed | 6-month ketogenic medium-chain triglyceride supplement | minimal effects on circulating cardiometabolic and inflammatory markers, aside from a significant increase in plasma IL-8 levels of unclear clinical significance | Myette-Côté et al. [101] |
5. Therapeutic Approaches and Translational Potential
5.1. Ketogenic Diet vs. Exogenous Ketone Supplements
5.2. Potential for Combined Interventions
5.3. Barriers to Implementation
5.4. Interpretation Challenges Due to Protocol Heterogeneity
6. Limitations and Future Directions
6.1. Evidence Gaps and Methodological Issues
6.2. Personalized Approaches
6.3. Suggested Future Research
6.4. Safety Considerations and Population-Specific Risk
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 6-OHDA | 6-hydroxydopamine |
| Aβ | amyloid β |
| AC-1202 | medium-chain triglyceride composed of glycerin and caprylic acid |
| AC-1204 | caprylic triglyceride |
| AcAc | acetoacetate |
| AD | Alzheimer’s disease |
| ALS | amyotrophic lateral sclerosis |
| APOE4 | epsilon 4 variant of the apolipoprotein E gene |
| BBB | blood–brain barrier |
| BDH1 | β-hydroxybutyrate dehydrogenase 1 |
| BH4 | tetrahydrobiopterin-4 |
| BHB | β-hydroxybutyrate |
| BMI | body mass index |
| CESD-R-20 | The Centre for Epidemiological Studies Depression Scale |
| COX-2 | cyclooxygenase-2 |
| DP | the Deanna Protocol |
| ETC | electron transport chain |
| FA | fatty acid |
| GABA | gamma-aminobutyric acid |
| GFAP | glial fibrillary acidic protein |
| HMG-CoA | hydroxymethylglutaryl-CoA |
| HMGCS2 | 3-hydroxy-3-methylglutaryl-CoA synthase 2 |
| HNE | 4-hydroxy-2,3-nonenal |
| Iba1 | ionized calcium-binding adaptor molecule 1 |
| IGF-1 | insulin-like growth factor 1 |
| KBs | ketone bodies |
| KD | ketogenic diet |
| KEED | ketone ester-enriched diet |
| LCFA | long-chain fatty acid |
| LCHF diet | low-carb, high-fat diet |
| MCI | mild cognitive impairment |
| MCT | a ketogenic drink containing medium-chain triglycerides |
| MMKD | modified Mediterranean-ketogenic diet |
| MDS-UPDRS | Movement Disorder Society Unified Parkinson’s Disease Rating Scale |
| mPT | mitochondrial permeability complex |
| MT2 | metallothionein 2 |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NM | Nishimura Geriatric Rating Scale for Mental Status |
| NLRP3 | nucleotide-binding domain, leucine-rich repeat, and pyrin domain containing protein 3 (inflammasome) |
| OAA | oxaloacetate |
| OS | oxidative stress |
| PAS | Parkinson Anxiety Scale |
| PD | Parkinson’s disease |
| PGC-1α | peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| rCBF | regional cerebral blood flow |
| SCFA | short-chain fatty acid |
| SCOT | succinyl-CoA:3-oxoacid CoA transferase |
| SOD2 | superoxide dismutase 2 |
| TCA | tricarboxylic acid |
| TH | tyrosine hydroxylase |
| TNF-α | tumor necrosis factor alpha |
| Tregs | regulatory T cells |
| TUG | Timed Up & Go test |
| VHI | Voice Handicap Index |
References
- McIsaac, T.L.; Fritz, N.E.; Quinn, L.; Muratori, L.M. Cognitive-Motor Interference in Neurodegenerative Disease: A Narrative Review and Implications for Clinical Management. Front. Psychol. 2018, 9, 2061. [Google Scholar] [CrossRef]
- Gadhave, D.G.; Sugandhi, V.V.; Jha, S.K.; Nangare, S.N.; Gupta, G.; Singh, S.K.; Dua, K.; Cho, H.; Hansbro, P.M.; Paudel, K.R. Neurodegenerative Disorders: Mechanisms of Degeneration and Therapeutic Approaches with Their Clinical Relevance. Ageing Res. Rev. 2024, 99, 102357. [Google Scholar] [CrossRef]
- van der Kant, R.; Goldstein, L.S.B.; Ossenkoppele, R. Amyloid-β-Independent Regulators of Tau Pathology in Alzheimer Disease. Nat. Rev. Neurosci. 2020, 21, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, L.; Burnham, S.C.; Dell’Agnello, G.; Dowsett, S.A.; Epelbaum, S. Diagnostic Biomarkers of Amyloid and Tau Pathology in Alzheimer’s Disease: An Overview of Tests for Clinical Practice in the United States and Europe. J. Prev. Alzheimer’s Dis. 2023, 10, 426–442. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Liu, C.; Xue, Y.; Chen, L. Changed Firing Activity of Nigra Dopaminergic Neurons in Parkinson’s Disease. Neurochem. Int. 2023, 162, 105465. [Google Scholar] [CrossRef]
- Mitra, J.; Guerrero, E.N.; Hegde, P.M.; Liachko, N.F.; Wang, H.; Vasquez, V.; Gao, J.; Pandey, A.; Paul Taylor, J.; Kraemer, B.C.; et al. Motor Neuron Disease-Associated Loss of Nuclear TDP-43 Is Linked to DNA Double-Strand Break Repair Defects. Proc. Natl. Acad. Sci. USA 2019, 116, 4696–4705. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Cui, Y.; He, C.; Yin, P.; Bai, R.; Zhu, J.; Lam, J.S.T.; Zhang, J.; Yan, R.; Zheng, X.; et al. Projections for Prevalence of Parkinson’s Disease and Its Driving Factors in 195 Countries and Territories to 2050: Modelling Study of Global Burden of Disease Study 2021. BMJ 2025, 388, e080952. [Google Scholar] [CrossRef]
- Guo, C.Y.; Sun, L.; Chen, X.P.; Zhang, D.S. Oxidative Stress, Mitochondrial Damage and Neurodegenerative Diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative Stress and Inflammation in the Pathogenesis of Neurological Disorders: Mechanisms and Implications. Acta Pharm. Sin. B 2024, 15, 15–34. [Google Scholar] [CrossRef]
- Wen, P.; Sun, Z.; Gou, F.; Wang, J.; Fan, Q.; Zhao, D.; Yang, L. Oxidative Stress and Mitochondrial Impairment: Key Drivers in Neurodegenerative Disorders. Ageing Res. Rev. 2025, 104, 102667. [Google Scholar] [CrossRef]
- Javaid, M.; Arain, F.; Javaid, M.D. Oxidative Stress in Neurodegenerative Diseases. Fundam. Princ. Oxidative Stress Metab. Reprod. Prev. Manag. 2024, 7, 167–183. [Google Scholar] [CrossRef]
- Sienes Bailo, P.; Llorente Martín, E.; Calmarza, P.; Montolio Breva, S.; Bravo Gómez, A.; Pozo Giráldez, A.; Sánchez-Pascuala Callau, J.J.; Vaquer Santamaría, J.M.; Dayaldasani Khialani, A.; Cerdá Micó, C.; et al. The Role of Oxidative Stress in Neurodegenerative Diseases and Potential Antioxidant Therapies. Adv. Lab. Med. 2022, 3, 342–350. [Google Scholar] [CrossRef]
- Addabbo, F.; Montagnani, M.; Goligorsky, M.S. Mitochondria and Reactive Oxygen Species. Hypertension 2009, 53, 885–892. [Google Scholar] [CrossRef]
- Heneka, M.T.; Golenbock, D.T.; Latz, E. Innate Immunity in Alzheimer’s Disease. Nat. Immunol. 2015, 16, 229–236. [Google Scholar] [CrossRef]
- Veech, R.L. The Therapeutic Implications of Ketone Bodies: The Effects of Ketone Bodies in Pathological Conditions: Ketosis, Ketogenic Diet, Redox States, Insulin Resistance, and Mitochondrial Metabolism. Prostaglandins Leukot. Essent. Fatty Acids 2004, 70, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Rubini, A.; Volek, J.S.; Grimaldi, K.A. Beyond Weight Loss: A Review of the Therapeutic Uses of Very-Low-Carbohydrate (Ketogenic) Diets. Eur. J. Clin. Nutr. 2013, 67, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.W.; Rothman, D.L.; Behar, K.L.; Stein, D.T.; Hetherington, H.P. Human Brain β-Hydroxybutyrate and Lactate Increase in Fasting-Induced Ketosis. J. Cereb. Blood Flow Metab. 2000, 20, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- McPherson, P.A.C.; McEneny, J. The Biochemistry of Ketogenesis and Its Role in Weight Management, Neurological Disease and Oxidative Stress. J. Physiol. Biochem. 2012, 68, 141–151. [Google Scholar] [CrossRef]
- Hwang, C.Y.; Choe, W.; Yoon, K.-S.; Ha, J.; Kim, S.S.; Yeo, E.-J.; Kang, I. Molecular Mechanisms for Ketone Body Metabolism, Signaling Functions, and Therapeutic Potential in Cancer. Nutrients 2022, 14, 4932. [Google Scholar] [CrossRef]
- Fukao, T.; Mitchell, G.; Sass, J.O.; Hori, T.; Orii, K.; Aoyama, Y. Ketone Body Metabolism and Its Defects. J. Inherit. Metab. Dis. 2014, 37, 541–551. [Google Scholar] [CrossRef]
- Jensen, N.J.; Wodschow, H.Z.; Nilsson, M.; Rungby, J. Effects of Ketone Bodies on Brain Metabolism and Function in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8767. [Google Scholar] [CrossRef]
- Puchalska, P.; Crawford, P.A. Multi-Dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.; Kempf, K.; Röhling, M.; Lenzen-Schulte, M.; Schloot, N.C.; Martin, S. Ketone Bodies: From Enemy to Friend and Guardian Angel. BMC Med. 2021, 19, 313. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.C.; Verdin, E. β-Hydroxybutyrate: A Signaling Metabolite. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.C.; Verdin, E. Ketone Bodies as Signaling Metabolites. Trends Endocrinol. Metab. 2014, 25, 42–52. [Google Scholar] [CrossRef]
- Cunnane, S.; Nugent, S.; Roy, M.; Courchesne-Loyer, A.; Croteau, E.; Tremblay, S.; Castellano, A.; Pifferi, F.; Bocti, C.; Paquet, N.; et al. Brain Fuel Metabolism, Aging, and Alzheimer’s Disease. Nutrition 2011, 27, 3–20. [Google Scholar] [CrossRef]
- Stanton, A.A. Specifically Formulated Ketogenic, Low Carbohydrate, and Carnivore Diets Can Prevent Migraine: A Perspective. Front. Nutr. 2024, 11, 1367570. [Google Scholar] [CrossRef]
- Steiner, P. Brain Fuel Utilization in the Developing Brain. Ann. Nutr. Metab. 2019, 75, 8–18. [Google Scholar] [CrossRef]
- Nakamura, M.T.; Yudell, B.E.; Loor, J.J. Regulation of Energy Metabolism by Long-Chain Fatty Acids. Prog. Lipid Res. 2014, 53, 124–144. [Google Scholar] [CrossRef]
- Rui, L. Energy Metabolism in the Liver. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2014; pp. 177–197. [Google Scholar]
- Bashir, B.; Fahmy, A.A.; Raza, F.; Banerjee, M. Non-Diabetic Ketoacidosis: A Case Series and Literature Review. Postgrad. Med. J. 2021, 97, 667–671. [Google Scholar] [CrossRef]
- Demers-Marcil, S.; Coles, J.P. Cerebral Metabolic Derangements Following Traumatic Brain Injury. Curr. Opin. Anaesthesiol. 2022, 35, 562–569. [Google Scholar] [CrossRef]
- Ramezani, M.; Fernando, M.; Eslick, S.; Asih, P.R.; Shadfar, S.; Bandara, E.M.S.; Hillebrandt, H.; Meghwar, S.; Shahriari, M.; Chatterjee, P.; et al. Ketone Bodies Mediate Alterations in Brain Energy Metabolism and Biomarkers of Alzheimer’s Disease. Front. Neurosci. 2023, 17, 1297984. [Google Scholar] [CrossRef]
- Kapogiannis, D.; Avgerinos, K.I. Brain Glucose and Ketone Utilization in Brain Aging and Neurodegenerative Diseases. Int. Rev. Neurobiol. 2020, 154, 79–110. [Google Scholar]
- Monda, A.; La Torre, M.E.; Messina, A.; Di Maio, G.; Monda, V.; Moscatelli, F.; De Stefano, M.; La Marra, M.; Di Padova, M.; Dipace, A.; et al. Exploring the Ketogenic Diet’s Potential in Reducing Neuroinflammation and Modulating Immune Responses. Front. Immunol. 2024, 15, 1425816. [Google Scholar] [CrossRef]
- Simeone, T.A.; Simeone, K.A.; Rho, J.M. Ketone Bodies as Anti-Seizure Agents. Neurochem. Res. 2017, 42, 2011–2018. [Google Scholar] [CrossRef]
- He, Y.; Cheng, X.; Zhou, T.; Li, D.; Peng, J.; Xu, Y.; Huang, W. β-Hydroxybutyrate as an Epigenetic Modifier: Underlying Mechanisms and Implications. Heliyon 2023, 9, e21098. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. β-Hydroxybutyrate: Much More than a Metabolite. Diabetes Res. Clin. Pract. 2014, 106, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of Oxidative Stress by β-Hydroxybutyrate, an Endogenous Histone Deacetylase Inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef]
- Wood, T.R.; Stubbs, B.J.; Juul, S.E. Exogenous Ketone Bodies as Promising Neuroprotective Agents for Developmental Brain Injury. Dev. Neurosci. 2018, 40, 451–462. [Google Scholar] [CrossRef]
- Huang, H.; Xu, K.; Lardelli, M. Targeting C99-Mediated Metabolic Disruptions with Ketone Therapy in Alzheimer’s Disease. arXiv 2025, arXiv:2502.11395. [Google Scholar]
- Bandera-Merchan, B.; Boughanem, H.; Crujeiras, A.B.; Macias-Gonzalez, M.; Tinahones, F.J. Ketotherapy as an Epigenetic Modifier in Cancer. Rev. Endocr. Metab. Disord. 2020, 21, 509–519. [Google Scholar] [CrossRef]
- Dyńka, D.; Kowalcze, K.; Charuta, A.; Paziewska, A. The Ketogenic Diet and Cardiovascular Diseases. Nutrients 2023, 15, 3368. [Google Scholar] [CrossRef]
- Rehman, M.U.; Wali, A.F.; Ahmad, A.; Shakeel, S.; Rasool, S.; Ali, R.; Rashid, S.M.; Madkhali, H.; Ganaie, M.A.; Khan, R. Neuroprotective Strategies for Neurological Disorders by Natural Products: An Update. Curr. Neuropharmacol. 2018, 17, 247–267. [Google Scholar] [CrossRef]
- Yang, H.M. Mitochondrial Dysfunction in Neurodegenerative Diseases. Cells 2025, 14, 276. [Google Scholar] [CrossRef]
- Tsang, C.K.; Liu, Y.; Thomas, J.; Zhang, Y.; Zheng, X.F.S. Superoxide Dismutase 1 Acts as a Nuclear Transcription Factor to Regulate Oxidative Stress Resistance. Nat. Commun. 2014, 5, 3446. [Google Scholar] [CrossRef]
- Palma, F.R.; He, C.; Danes, J.M.; Paviani, V.; Coelho, D.R.; Gantner, B.N.; Bonini, M.G. Mitochondrial Superoxide Dismutase: What the Established, the Intriguing, and the Novel Reveal about a Key Cellular Redox Switch. Antioxid. Redox Signal. 2020, 32, 701–714. [Google Scholar] [CrossRef]
- Spanier, G.; Xu, H.; Xia, N.; Tobias, S.; Deng, S.; Wojnowski, L.; Forstermann, U.; Li, H. Resveratrol Reduces Endothelial Oxidative Stress by Modulating the Gene Expression of Superoxide Dismutase 1 (SOD1), Glutathione Peroxidase 1 (GPx1) and NADPH Oxidase Subunit (Nox4). J. Physiol. Pharmacol. 2009, 60 (Suppl. 4), 111–116. [Google Scholar]
- He, X.; Yablonskiy, D.A. Quantitative BOLD: Mapping of Human Cerebral Deoxygenated Blood Volume and Oxygen Extraction Fraction: Default State. Magn. Reson. Med. 2007, 57, 115–126. [Google Scholar] [CrossRef]
- Petronczki, M.; Matos, J.; Mori, S.; Gregan, J.; Bogdanova, A.; Schwickart, M.; Mechtler, K.; Shirahige, K.; Zachariae, W.; Nasmyth, K. Monopolar Attachment of Sister Kinetochores at Meiosis I Requires Casein Kinase 1. Cell 2006, 126, 1049–1064. [Google Scholar] [CrossRef]
- Hao, J.; Madigan, M.C.; Khatri, A.; Power, C.A.; Hung, T.T.; Beretov, J.; Chang, L.; Xiao, W.; Cozzi, P.J.; Graham, P.H.; et al. In Vitro and in Vivo Prostate Cancer Metastasis and Chemoresistance Can Be Modulated by Expression of Either CD44 or CD147. PLoS ONE 2012, 7, e40716. [Google Scholar] [CrossRef]
- Johnson, P.L.; Truitt, W.A.; Fitz, S.D.; Lowry, C.A.; Anantha, S. Neural Pathways Underlying Lactate-Induced Panic. Neuropsychopharmacology 2008, 9, 2093–2107. [Google Scholar] [CrossRef]
- Dobkowski, J.; Kijak, M.; Sazanovich, I.V.; Waluk, J. Solvent-Controlled Excited State Relaxation Path of 4-Acetyl-4′-(Dimethylamino)Biphenyl. J. Phys. Chem. B 2015, 119, 7294–7307. [Google Scholar] [CrossRef] [PubMed]
- Cantero-Fortiz, Y.; Boada, M. The Role of Inflammation in Neurological Disorders: A Brief Overview of Multiple Sclerosis, Alzheimer’s, and Parkinson’s Disease’. Front. Neurol. 2024, 15, 1439125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of Neuroinflammation in Neurodegeneration Development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in Neurodegenerative Diseases: Mechanism and Potential Therapeutic Targets; Springer: New York, NY, USA, 2023; Volume 8, ISBN 4139202301588. [Google Scholar]
- Ageeva, T.; Rizvanov, A.; Mukhamedshina, Y. NF-ΚB and JAK/STAT Signaling Pathways as Crucial Regulators of Neuroinflammation and Astrocyte Modulation in Spinal Cord Injury. Cells 2024, 13, 581. [Google Scholar] [CrossRef] [PubMed]
- Eo, H.; Kim, S.; Jung, U.J.; Kim, S.R. Alpha-Synuclein and Microglia in Parkinson’s Disease: From Pathogenesis to Therapeutic Prospects. J. Clin. Med. 2024, 13, 7243. [Google Scholar] [CrossRef]
- Machhi, J.; Kevadiya, B.D.; Muhammad, I.K.; Herskovitz, J.; Olson, K.E.; Mosley, R.L.; Gendelman, H.E. Harnessing Regulatory T Cell Neuroprotective Activities for Treatment of Neurodegenerative Disorders. Mol. Neurodegener. 2020, 15, 32. [Google Scholar] [CrossRef]
- Ermakov, A.V.; Verkhovskii, R.A.; Babushkina, I.V.; Trushina, D.B.; Inozemtseva, O.A.; Lukyanets, E.A.; Ulyanov, V.J.; Gorin, D.A.; Belyakov, S.; Antipina, M.N. In Vitro Bioeffects of Polyelectrolyte Multilayer Microcapsules Post-Loaded with Water-Soluble Cationic Photosensitizer. Pharmaceutics 2020, 12, 610. [Google Scholar] [CrossRef]
- Gurman, P.; Miranda, O.R.; Clayton, K.; Rosen, Y.; Elman, N.M. Clinical Applications of Biomedical Microdevices for Controlled Drug Delivery. Mayo Clin. Proc. 2015, 90, 93–108. [Google Scholar] [CrossRef]
- Lee, C.Y.; Lee, S.J.; Shen, C.C.; Yeh, C.T.; Chang, C.C.; Lo, Y.M. Application of Flexible Micro Temperature Sensor in Oxidative Steam Reforming by a Methanol Micro Reformer. Sensors 2011, 11, 2246–2256. [Google Scholar] [CrossRef]
- Winkler, N.S.; Raza, S.; Mackesy, M.; Birdwell, R.L. Breast Density: Clinical Implications and Assessment Methods. Radiographics 2015, 35, 316–324. [Google Scholar] [CrossRef]
- Sumithran, P.; Prendergast, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Ketosis and Appetite-Mediating Nutrients and Hormones after Weight Loss. Eur. J. Clin. Nutr. 2013, 67, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Bockorny, K.; Youssef-Morgan, C.M. Entrepreneurs’ Courage, Psychological Capital, and Life Satisfaction. Front. Psychol. 2019, 10, 789. [Google Scholar] [CrossRef] [PubMed]
- Brandão, P.; Marques, C.; Burke, A.J.; Pineiro, M. The Application of Isatin-Based Multicomponent-Reactions in the Quest for New Bioactive and Druglike Molecules. Eur. J. Med. Chem. 2021, 211, 113102. [Google Scholar] [CrossRef] [PubMed]
- Sears, C.L.; Garrett, W.S. Microbes, Microbiota, and Colon Cancer. Cell Host Microbe 2014, 15, 317–328. [Google Scholar] [CrossRef]
- Lau, C.G.; Takeuchi, K.; Rodenas-Ruano, A.; Takayasu, Y.; Murphy, J.; Bennett, M.V.I.; Zukin, R.S. Regulation of NMDA Receptor Ca2+ Signalling and Synaptic Plasticity. Biochem. Soc. Trans. 2009, 37, 1369–1374. [Google Scholar] [CrossRef]
- Magdaleno Roman, J.Y.; Chapa González, C. Glutamate and Excitotoxicity in Central Nervous System Disorders: Ionotropic Glutamate Receptors as a Target for Neuroprotection. Neuroprotection 2024, 2, 137–150. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, A.K.Y.; Ip, N.Y. Synaptic Dysfunction in Alzheimer’s Disease: Mechanisms and Therapeutic Strategies. Pharmacol. Ther. 2019, 195, 186–198. [Google Scholar] [CrossRef]
- Sharma, J.C.; Ross, I.N.; Rascol, O.; Brooks, D. Relationship between Weight, Levodopa and Dyskinesia: The Significance of Levodopa Dose per Kilogram Body Weight. Eur. J. Neurol. 2008, 15, 493–496. [Google Scholar] [CrossRef]
- Reiter, M.; Tscholakoff, D.; Kopsa, W.; Stain, M.; Bucek, R.A. Idiopathic Subclavian Vein Thrombosis: Anatomical Findings by Magnetic Resonance Imaging and Correlation with Clinical Provocation Tests. J. Thromb. Haemost. 2007, 5, 628–630. [Google Scholar] [CrossRef]
- Xu, C.; Zuo, Z.; Liu, K.; Jia, H.; Zhang, Y.; Luo, H. Transcriptome Analysis of the Tan Sheep Testes: Differential Expression of Antioxidant Enzyme-Related Genes and Proteins in Response to Dietary Vitamin E Supplementation. Gene 2016, 579, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, K.; Grabowski, M.; Przybyła, M.; Pondel, N.; Barski, J.J.; Nowacka-Chmielewska, M.; Liśkiewicz, D. Ketogenic Diet and Behavior: Insights from Experimental Studies. Front. Nutr. 2024, 11, 1322509. [Google Scholar] [CrossRef] [PubMed]
- Grochowska, K.; Przeliorz, A. The Effect of the Ketogenic Diet on the Therapy of Neurodegenerative Diseases and Its Impact on Improving Cognitive Functions. Dement. Geriatr. Cogn. Disord. Extra 2022, 12, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Peng, Y.; Shen, Q.; Chen, K.; Fang, B.; Li, W. Effects of Ketogenic Diet on Cognitive Function of Patients with Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Nutr. Health Aging 2024, 28, 100306. [Google Scholar] [CrossRef]
- Yin, P.; Li, S.; Li, X.-J.; Yang, W. New Pathogenic Insights from Large Animal Models of Neurodegenerative Diseases. Protein Cell 2022, 13, 707–720. [Google Scholar] [CrossRef]
- Evangelisti, C.; Ramadan, S.; Orlacchio, A.; Panza, E. Experimental Cell Models for Investigating Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 9747. [Google Scholar] [CrossRef]
- Jhuo, C.; Chen, C.; Tzen, J.T.C.; Chen, W. Teaghrelin protected dopaminergic neurons in MPTP-induced Parkinson’s disease animal model by promoting PINK1/Parkin-mediated mitophagy and AMPK/SIRT1/PGC1-α-mediated mitochondrial biogenesis. Environ. Toxicol. 2024, 39, 4022–4034. [Google Scholar] [CrossRef]
- Barilar, J.O.; Knezovic, A.; Perhoc, A.B.; Homolak, J.; Riederer, P.; Salkovic-Petrisic, M. Shared Cerebral Metabolic Pathology in Non-Transgenic Animal Models of Alzheimer’s and Parkinson’s Disease. J. Neural Transm. 2020, 127, 231–250. [Google Scholar] [CrossRef]
- Jiang, L.; Roberts, R.; Wong, M.; Zhang, L.; Webber, C.J.; Libera, J.; Wang, Z.; Kilci, A.; Jenkins, M.; Ortiz, A.R.; et al. β-Amyloid Accumulation Enhances Microtubule Associated Protein Tau Pathology in an APPNL-G-F/MAPTP301S Mouse Model of Alzheimer’s Disease. Front. Neurosci. 2024, 18, 1372297. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Van der Auwera, I.; Wera, S.; Van Leuven, F.; Henderson, S.T. A Ketogenic Diet Reduces Amyloid Beta 40 and 42 in a Mouse Model of Alzheimer’s Disease. Nutr. Metab. 2005, 2, 28. [Google Scholar] [CrossRef]
- Beckett, T.L.; Studzinski, C.M.; Keller, J.N.; Paul Murphy, M.; Niedowicz, D.M. A Ketogenic Diet Improves Motor Performance but Does Not Affect β-Amyloid Levels in a Mouse Model of Alzheimer’s Disease. Brain Res. 2013, 1505, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Brownlow, M.L.; Benner, L.; D’Agostino, D.; Gordon, M.N.; Morgan, D. Ketogenic Diet Improves Motor Performance but Not Cognition in Two Mouse Models of Alzheimer’s Pathology. PLoS ONE 2013, 8, e75713. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Zhang, T.; Wu, X.; Yi Qiu, J. Ketone Production by Ketogenic Diet and by Intermittent Fasting Has Different Effects on the Gut Microbiota and Disease Progression in an Alzheimer’s Disease Rat Model. J. Clin. Biochem. Nutr. 2020, 67, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Kashiwaya, Y.; Bergman, C.; Lee, J.H.; Wan, R.; King, M.T.; Mughal, M.R.; Okun, E.; Clarke, K.; Mattson, M.P.; Veech, R.L. A Ketone Ester Diet Exhibits Anxiolytic and Cognition-Sparing Properties, and Lessens Amyloid and Tau Pathologies in a Mouse Model of Alzheimer’s Disease. Neurobiol. Aging 2013, 34, 1530–1539. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, C.; Wu, J.; Liu, P.; Deng, X.; Zhang, Y.; Peng, B.; Zhu, Y. Ketogenic Diet Ameliorates Cognitive Impairment and Neuroinflammation in a Mouse Model of Alzheimer’s Disease. CNS Neurosci. Ther. 2022, 28, 580–592. [Google Scholar] [CrossRef]
- Shippy, D.C.; Wilhelm, C.; Viharkumar, P.A.; Raife, T.J.; Ulland, T.K. β-Hydroxybutyrate Inhibits Inflammasome Activation to Attenuate Alzheimer’s Disease Pathology. J. Neuroinflamm. 2020, 17, 280. [Google Scholar] [CrossRef]
- Pawlosky, R.J.; Kashiwaya, Y.; King, M.T.; Veech, R.L. A Dietary Ketone Ester Normalizes Abnormal Behavior in a Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1044. [Google Scholar] [CrossRef]
- Llorente-Folch, I.; Düssmann, H.; Watters, O.; Connolly, N.M.C.; Prehn, J.H.M. Ketone Body β-Hydroxybutyrate (BHB) Preserves Mitochondrial Bioenergetics. Sci. Rep. 2023, 13, 19664. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, G.; Kwok, L.Y.; Sun, Z. Gut Microbiome-Targeted Therapies for Alzheimer’s Disease. Gut Microbes 2023, 15, 2271613. [Google Scholar] [CrossRef]
- Abe, S.; Ezaki, O.; Suzuki, M. Medium-Chain Triglycerides in Combination with Leucine and Vitamin D Benefit Cognition in Frail Elderly Adults: A Randomized Controlled Trial. J. Nutr. Sci. Vitaminol. 2017, 63, 133–140. [Google Scholar] [CrossRef]
- Abe, S.; Ezaki, O.; Suzuki, M. Medium-Chain Triglycerides (8:0 and 10:0) Increase Mini-Mental State Examination (MMSE) Score in Frail Elderly Adults in a Randomized Controlled Trial. J. Nutr. 2020, 150, 2383–2390. [Google Scholar] [CrossRef]
- Juby, A.G.; Blackburn, T.E.; Mager, D.R. Use of Medium Chain Triglyceride (MCT) Oil in Subjects with Alzheimer’s Disease: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study, with an Open-Label Extension. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12259. [Google Scholar] [CrossRef]
- Henderson, S.T.; Morimoto, B.H.; Cummings, J.L.; Farlow, M.R.; Walker, J. A Placebo-Controlled, Parallel-Group, Randomized Clinical Trial of AC-1204 in Mild-to-Moderate Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 75, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.C.L.; Deprez, L.M.; Mortimer, G.M.N.; Murtagh, D.K.J.; McCoy, S.; Mylchreest, R.; Gilbertson, L.J.; Clark, K.M.; Simpson, P.V.; McManus, E.J.; et al. Randomized Crossover Trial of a Modified Ketogenic Diet in Alzheimer’s Disease. Alzheimer’s Res. Ther. 2021, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.K.; Sullivan, D.K.; Mahnken, J.D.; Burns, J.M.; Swerdlow, R.H. Feasibility and Efficacy Data from a Ketogenic Diet Intervention in Alzheimer’s Disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Reger, M.A.; Henderson, S.T.; Hale, C.; Cholerton, B.; Baker, L.D.; Watson, G.S.; Hyde, K.; Chapman, D.; Craft, S. Effects of β-Hydroxybutyrate on Cognition in Memory-Impaired Adults. Neurobiol. Aging 2004, 25, 311–314. [Google Scholar] [CrossRef]
- Brandt, J.; Buchholz, A.; Henry-Barron, B.; Vizthum, D.; Avramopoulos, D.; Cervenka, M.C. Preliminary Report on the Feasibility and Efficacy of the Modified Atkins Diet for Treatment of Mild Cognitive Impairment and Early Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 68, 969–981. [Google Scholar] [CrossRef]
- Myette-Côté, É.; St-Pierre, V.; Beaulieu, S.; Castellano, C.-A.A.; Fortier, M.; Plourde, M.; Bocti, C.; Fulop, T.; Cunnane, S.C. The Effect of a 6-Month Ketogenic Medium-Chain Triglyceride Supplement on Plasma Cardiometabolic and Inflammatory Markers in Mild Cognitive Impairment. Prostaglandins Leukot. Essent. Fat. Acids 2021, 169, 102236. [Google Scholar] [CrossRef]
- Krolak-Salmon, P.; Swerdlow, R.H.; Mastain, T.; Dive-Pouletty, C.; Pooley, N.; Kisomi, M. Efficacy and Safety of Exogenous Ketones in People with Mild Neurocognitive Disorder and Alzheimer’s Disease: A Systematic Literature Review. Nutr. Rev. 2025, 83, e1034–e1048. [Google Scholar] [CrossRef]
- Shabbir, I.; Liu, K.; Riaz, B.; Rahim, M.F.; Zhong, S.; Aweya, J.J.; Cheong, K.L. Investigating the Therapeutic Potential of the Ketogenic Diet in Modulating Neurodegenerative Pathophysiology: An Interdisciplinary Approach. Nutrients 2025, 17, 1268. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, Y.; Zhang, X.; Liu, L.; Zhou, B.; Mo, R.; Li, Y.; Li, H.; Li, F.; Tao, Y.; et al. Medium-Chain Triglycerides Improved Cognition and Lipid Metabolomics in Mild to Moderate Alzheimer’s Disease Patients with APOE4−/−: A Double-Blind, Randomized, Placebo-Controlled Crossover Trial. Clin. Nutr. 2020, 39, 2092–2105. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.T.; Vogel, J.L.; Barr, L.J.; Garvin, F.; Jones, J.J.; Costantini, L.C. Study of the Ketogenic Agent AC-1202 in Mild to Moderate Alzheimer’s Disease: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. Nutr. Metab. 2009, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Homma, D.; Katoh, S.; Tokuoka, H.; Ichinose, H. The Role of Tetrahydrobiopterin and Catecholamines in the Developmental Regulation of Tyrosine Hydroxylase Level in the Brain. J. Neurochem. 2013, 126, 70–81. [Google Scholar] [CrossRef]
- Lovenberg, W.; Levine, R.A.; Robinson, D.S.; Ebert, M.; Williams, A.C.; Calne, D.B. Hydroxylase Cofactor Activity in Cerebrospinal Fluid of Normal Subjects and Patients with Parkinson’s Disease. Science 1979, 204, 624–626. [Google Scholar] [CrossRef]
- Mahajan, V.R.; Nadel, J.A.; King, M.T.; Pawlosky, R.J.; Davis, M.I.; Veech, R.L.; Lovinger, D.M.; Salinas, A.G. Ketone Ester–Enriched Diet Ameliorates Motor and Dopamine Release Deficits in MitoPark Mice. Eur. J. Neurosci. 2024, 60, 6875–6890. [Google Scholar] [CrossRef]
- Zhu, Y.; Tang, X.; Cheng, Z.; Dong, Q.; Ruan, G. The Anti-Inflammatory Effect of Preventive Intervention with Ketogenic Diet Mediated by the Histone Acetylation of MGluR5 Promotor Region in Rat Parkinson’s Disease Model: A Dual-Tracer PET Study. Park. Dis. 2022, 2022, 3506213. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, B. Neuroprotective and Anti-Inflammatory Activities of Ketogenic Diet on MPTP-Induced Neurotoxicity. J. Mol. Neurosci. 2010, 42, 145–153. [Google Scholar] [CrossRef]
- Choi, A.H.; Delgado, M.; Chen, K.Y.; Chung, S.T.; Courville, A.; Turner, S.A.; Yang, S.; Airaghi, K.; Dustin, I.; McGurrin, P.; et al. A Randomized Feasibility Trial of Medium Chain Triglyceride-Supplemented Ketogenic Diet in People with Parkinson’s Disease. BMC Neurol. 2024, 24, 106. [Google Scholar] [CrossRef]
- Kuter, K.Z.; Olech, Ł.; Głowacka, U.; Paleczna, M. Increased Beta-Hydroxybutyrate Level Is Not Sufficient for the Neuroprotective Effect of Long-Term Ketogenic Diet in an Animal Model of Early Parkinson’s Disease. Exploration of Brain and Liver Energy Metabolism Markers. Int. J. Mol. Sci. 2021, 22, 7556. [Google Scholar] [CrossRef]
- Sun, M.F.; Shen, Y.Q. Dysbiosis of Gut Microbiota and Microbial Metabolites in Parkinson’s Disease. Ageing Res. Rev. 2018, 45, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wang, X.; Zhang, H.; Yin, J.; Zhao, P.; Yin, Q.; Wang, Z. Ketogenic Diet Protects MPTP-Induced Mouse Model of Parkinson’s Disease via Altering Gut Microbiota and Metabolites. MedComm 2023, 4, e268. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.C.L.; Murtagh, D.K.J.; Gilbertson, L.J.; Asztely, F.J.S.; Lynch, C.D.P. Low-Fat versus Ketogenic Diet in Parkinson’s Disease: A Pilot Randomized Controlled Trial. Mov. Disord. 2018, 33, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Shidler, M.D.; Summer, S.S.; Sullivan, P.G.; Duker, A.P.; Isaacson, R.S.; Espay, A.J. Nutritional Ketosis for Mild Cognitive Impairment in Parkinson’s Disease: A Controlled Pilot Trial. Clin. Park. Relat. Disord. 2019, 1, 41–47. [Google Scholar] [CrossRef]
- Tidman, M.M.; White, D.; White, T. Effects of an Low Carbohydrate/Healthy Fat/Ketogenic Diet on Biomarkers of Health and Symptoms, Anxiety and Depression in Parkinson’s Disease: A Pilot Study. Neurodegener. Dis. Manag. 2022, 12, 57–66. [Google Scholar] [CrossRef]
- Tidman, M.M.; White, D.R.; White, T.A. Impact of a Keto Diet on Symptoms of Parkinson’s Disease, Biomarkers, Depression, Anxiety and Quality of Life: A Longitudinal Study. Neurodegener. Dis. Manag. 2024, 14, 97–110. [Google Scholar] [CrossRef]
- Koyuncu, H.; Fidan, V.; Toktas, H.; Binay, O.; Celik, H. Effect of Ketogenic Diet versus Regular Diet on Voice Quality of Patients with Parkinson’s Disease. Acta Neurol. Belg. 2021, 121, 1729–1732. [Google Scholar] [CrossRef]
- Szelechowski, M.; Amoedo, N.; Obre, E.; Léger, C.; Allard, L.; Bonneu, M.; Claverol, S.; Lacombe, D.; Oliet, S.; Chevallier, S.; et al. Metabolic Reprogramming in Amyotrophic Lateral Sclerosis. Sci. Rep. 2018, 8, 3953. [Google Scholar] [CrossRef]
- Nabakhteh, S.; Lotfi, A.; Afsartaha, A.; Khodadadi, E.S.; Abdolghaderi, S.; Mohammadpour, M.; Shokri, Y.; Kiani, P.; Ehtiati, S.; Khakshournia, S.; et al. Nutritional Interventions in Amyotrophic Lateral Sclerosis: From Ketogenic Diet and Neuroprotective Nutrients to the Microbiota-Gut-Brain Axis Regulation. Mol. Neurobiol. 2025, 62, 9216–9239. [Google Scholar] [CrossRef]
- Herrmann, C.; Uzelac, Z.; Michels, S.; Weber, A.; Richter, L.; Elmas, Z.; Jagodzinski, L.; Wurster, C.; Schuster, J.; Dreyhaupt, J.; et al. Alterations of Fat and Ketone Body Metabolism in ALS and SMA—A Prospective Observational Study. Eur. J. Neurol. 2025, 32, e70132. [Google Scholar] [CrossRef]
- Zhao, Z.; Lange, D.J.; Voustianiouk, A.; MacGrogan, D.; Ho, L.; Suh, J.; Humala, N.; Thiyagarajan, M.; Wang, J.; Pasinetti, G.M. A Ketogenic Diet as a Potential Novel Therapeutic Intervention in Amyotrophic Lateral Sclerosis. BMC Neurosci. 2006, 7, 29. [Google Scholar] [CrossRef]
- Zhao, W.; Varghese, M.; Vempati, P.; Dzhun, A.; Cheng, A.; Wang, J.; Lange, D.; Bilski, A.; Faravelli, I.; Pasinetti, G.M. Caprylic Triglyceride as a Novel Therapeutic Approach to Effectively Improve the Performance and Attenuate the Symptoms Due to the Motor Neuron Loss in ALS Disease. PLoS ONE 2012, 7, e49191. [Google Scholar] [CrossRef]
- Ari, C.; Poff, A.M.; Held, H.E.; Landon, C.S.; Goldhagen, C.R.; Mavromates, N.; D’Agostino, D.P. Metabolic Therapy with Deanna Protocol Supplementation Delays Disease Progression and Extends Survival in Amyotrophic Lateral Sclerosis (ALS) Mouse Model. PLoS ONE 2014, 9, e103526. [Google Scholar] [CrossRef] [PubMed]
- Tefera, T.W.; Wong, Y.; Barkl-Luke, M.E.; Ngo, S.T.; Thomas, N.K.; McDonald, T.S.; Borges, K. Triheptanoin Protects Motor Neurons and Delays the Onset of Motor Symptoms in a Mouse Model of Amyotrophic Lateral Sclerosis. PLoS ONE 2016, 11, e0161816. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov Safety and Tolerability of the Ketogenic Diet in Amyotrophic Lateral Sclerosis (ALS). Available online: https://clinicaltrials.gov/study/NCT01016522 (accessed on 22 May 2025).
- Okamoto, K.; Kihira, T.; Kondo, T.; Kobashi, G.; Washio, M.; Sasaki, S.; Yokoyama, T.; Miyake, Y.; Sakamoto, N.; Inaba, Y.; et al. Nutritional Status and Risk of Amyotrophic Lateral Sclerosis in Japan. Amyotroph. Lateral Scler. 2007, 8, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Huang, Y.; Zhu, Y.; Jin, Q. Association between Dietary Patterns and the Prognosis of Amyotrophic Lateral Sclerosis in China: A Cross-Sectional Study. Front. Nutr. 2024, 11, 1437521. [Google Scholar] [CrossRef]
- Carrera-Juliá, S.; Obrador, E.; López-Blanch, R.; Oriol-Caballo, M.; Moreno-Murciano, P.; Estrela, J.M. Ketogenic Effect of Coconut Oil in ALS Patients. Front. Nutr. 2024, 11, 1429498. [Google Scholar] [CrossRef]
- Carrera-Juliá, S.; Estrela, J.M.; Zacarés, M.; Navarro, M.Á.; Vega-Bello, M.J.; de la Rubia Ortí, J.E.; Moreno, M.L.; Drehmer, E. Effect of the Mediterranean Diet Supplemented with Nicotinamide Riboside and Pterostilbene and/or Coconut Oil on Anthropometric Variables in Amyotrophic Lateral Sclerosis. A Pilot Study. Front. Nutr. 2023, 10, 1232184. [Google Scholar] [CrossRef]
- Norgren, J.; Kåreholt, I.; Sindi, S. Is There Evidence of a Ketogenic Effect of Coconut Oil? Commentary: Effect of the Mediterranean Diet Supplemented with Nicotinamide Riboside and Pterostilbene and/or Coconut Oil on Anthropometric Variables in Amyotrophic Lateral Sclerosis. A Pilot Study. Front. Nutr. 2024, 10, 1333933. [Google Scholar] [CrossRef]
- Song, W.; Song, Y.; Kincaid, B.; Bossy, B.; Bossy-Wetzel, E. Mutant SOD1G93A Triggers Mitochondrial Fragmentation in Spinal Cord Motor Neurons: Neuroprotection by SIRT3 and PGC-1α. Neurobiol. Dis. 2013, 51, 72–81. [Google Scholar] [CrossRef]
- Shimazu, T.; Hirschey, M.D.; Hua, L.; Dittenhafer-Reed, K.E.; Schwer, B.; Lombard, D.B.; Li, Y.; Bunkenborg, J.; Alt, F.W.; Denu, J.M.; et al. SIRT3 Deacetylates Mitochondrial 3-Hydroxy-3-Methylglutaryl CoA Synthase 2 and Regulates Ketone Body Production. Cell Metab. 2010, 12, 654–661. [Google Scholar] [CrossRef]
- Fortier, M.; Castellano, C.A.; St-Pierre, V.; Myette-Côté, É.; Langlois, F.; Roy, M.; Morin, M.C.; Bocti, C.; Fulop, T.; Godin, J.P.; et al. A Ketogenic Drink Improves Cognition in Mild Cognitive Impairment: Results of a 6-Month RCT. Alzheimer’s Dement. 2021, 17, 543–552. [Google Scholar] [CrossRef]
- VanItallie, T.B.; Nonas, C.; Di Rocco, A.; Boyar, K.; Hyams, K.; Heymsfield, S.B. Treatment of Parkinson Disease with Diet-Induced Hyperketonemia: A Feasibility Study. Neurology 2005, 64, 728–730. [Google Scholar] [CrossRef]
- Krikorian, R.; Shidler, M.D.; Dangelo, K.; Couch, S.C.; Benoit, S.C.; Clegg, D.J. Dietary Ketosis Enhances Memory in Mild Cognitive Impairment. Neurobiol. Aging 2012, 33, 425.e19–425.e27. [Google Scholar] [CrossRef]
- Zarnowska, I.M. Therapeutic Use of the Ketogenic Diet in Refractory Epilepsy: What We Know and What Still Needs to Be Learned. Nutrients 2020, 12, 2616. [Google Scholar] [CrossRef]
- Ahmad, Y.; Seo, D.S.; Jang, Y. Metabolic Effects of Ketogenic Diets: Exploring Whole-Body Metabolism in Connection with Adipose Tissue and Other Metabolic Organs. Int. J. Mol. Sci. 2024, 25, 7076. [Google Scholar] [CrossRef]
- Omori, N.E.; Malys, M.K.; Woo, G.; Mansor, L. Exogenous Ketone Bodies and the Ketogenic Diet as a Treatment Option for Neurodevelopmental Disorders. Front. Nutr. 2024, 11, 1485280. [Google Scholar] [CrossRef]
- Luong, T.V.; Pedersen, M.G.B.; Abild, C.B.; Cunnane, S.C.; Croteau, E.; Lauritsen, K.M.; Kjærulff, M.L.G.; Tolbod, L.P.; Møller, N.; Søndergaard, E.; et al. A Ketogenic Diet Lowers Myocardial Fatty Acid Oxidation but Does Not Affect Oxygen Consumption: A Study in Overweight Humans. Obesity 2024, 32, 506–516. [Google Scholar] [CrossRef]
- Selvaraj, S.; Margulies, K.B.; Dugyala, S.; Schubert, E.; Tierney, A.; Arany, Z.; Pryma, D.A.; Shah, S.H.; Rame, J.E.; Kelly, D.P.; et al. Comparison of Exogenous Ketone Administration Versus Dietary Carbohydrate Restriction on Myocardial Glucose Suppression: A Crossover Clinical Trial. J. Nucl. Med. 2022, 63, 770–776. [Google Scholar] [CrossRef]
- Kansakar, U.; Nieves Garcia, C.; Santulli, G.; Gambardella, J.; Mone, P.; Jankauskas, S.S.; Lombardi, A. Exogenous Ketones in Cardiovascular Disease and Diabetes: From Bench to Bedside. J. Clin. Med. 2024, 13, 7391. [Google Scholar] [CrossRef]
- Mohammed, H.; Hernando-Herraez, I.; Savino, A.; Scialdone, A.; Macaulay, I.; Mulas, C.; Chandra, T.; Voet, T.; Dean, W.; Nichols, J.; et al. Single-Cell Landscape of Transcriptional Heterogeneity and Cell Fate Decisions during Mouse Early Gastrulation. Cell Rep. 2017, 20, 1215–1228. [Google Scholar] [CrossRef]
- Lobach, Y.N.; Luferenko, E.D.; Shevel, V.N. Radiation Protection Performance for the Dismantling of the WWR-M Primary Cooling Circuit. Radiat. Prot. Dosimetry 2014, 162, 416–420. [Google Scholar] [CrossRef]
- Wang, L.; Brooks, A.N.; Fan, J.; Wan, Y.; Gambe, R.; Li, S.; Hergert, S.; Yin, S.; Freeman, S.S.; Levin, J.Z.; et al. Transcriptomic Characterization of SF3B1 Mutation Reveals Its Pleiotropic Effects in Chronic Lymphocytic Leukemia. Cancer Cell 2016, 30, 750–763. [Google Scholar] [CrossRef]
- Zanaboni, M.P.; Pasca, L.; Geraci, M.A.; Varesio, C.; Guglielmetti, M.; Tagliabue, A.; Grumi, S.; De Giorgis, V. Case Report: KETOLAND the Psychoeducation Program for Ketogenic Diet. Front. Psychiatry 2023, 14, 1155717. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Guo, J.; Zhang, P.; Xu, Z.; Peng, K.; Dong, X.; Zhao, L. Efficacy and Safety of Modified Medium-Chain Triglyceride Ketogenic Diet in Patients with Drug-Resistant Epilepsy. Acta Epileptol. 2024, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Rogawski, M.A. A Fatty Acid in the MCT Ketogenic Diet for Epilepsy Treatment Blocks AMPA Receptors. Brain 2016, 139, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-Y.; Liu, H.-W.; Hung, T.-M. The Ketogenic Effect of Medium-Chain Triacylglycerides. Front. Nutr. 2021, 8, 747284. [Google Scholar] [CrossRef]
- Harvey, C.J.D.C.; Schofield, G.M.; Williden, M.; McQuillan, J.A. The Effect of Medium Chain Triglycerides on Time to Nutritional Ketosis and Symptoms of Keto-Induction in Healthy Adults: A Randomised Controlled Clinical Trial. J. Nutr. Metab. 2018, 2018, 2630565. [Google Scholar] [CrossRef]
- Saris, C.G.J.; Timmers, S. Ketogenic Diets and Ketone Suplementation: A Strategy for Therapeutic Intervention. Front. Nutr. 2022, 9, 947567. [Google Scholar] [CrossRef]
- Malinowska, D.; Żendzian-Piotrowska, M. Ketogenic Diet: A Review of Composition Diversity, Mechanism of Action and Clinical Application. J. Nutr. Metab. 2024, 2024, 6666171. [Google Scholar] [CrossRef]
- Khodabakhshi, A.; Akbari, M.E.; Mirzaei, H.R.; Mehrad-Majd, H.; Kalamian, M.; Davoodi, S.H. Feasibility, Safety, and Beneficial Effects of MCT-Based Ketogenic Diet for Breast Cancer Treatment: A Randomized Controlled Trial Study. Nutr. Cancer 2020, 72, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Rai, R.; Singh, D.; Vohora, D. Octanoic Acid a Major Component of Widely Consumed Medium-Chain Triglyceride Ketogenic Diet Is Detrimental to Bone. Sci. Rep. 2021, 11, 7003. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.J.D.C.; Schofield, G.M.; Williden, M. The Use of Nutritional Supplements to Induce Ketosis and Reduce Symptoms Associated with Keto-Induction: A Narrative Review. PeerJ 2018, 6, e4488. [Google Scholar] [CrossRef] [PubMed]
- Szendi, K.; Murányi, E.; Hunter, N.; Németh, B. Methodological Challenges and Confounders in Research on the Effects of Ketogenic Diets: A Literature Review of Meta-Analyses. Foods 2024, 13, 248. [Google Scholar] [CrossRef]
- Lemke, G. Biology of the TAM Receptors. Cold Spring Harb. Perspect. Biol. 2013, 5, a009076. [Google Scholar] [CrossRef]
- Laney, D.F.; Keshavamurthy, J.H.; Agarwal, S. Dural Ectasia as an Incidental Finding on MRI in a Patient with Marfan Syndrome. Postgrad. Med. J. 2017, 93, 237. [Google Scholar] [CrossRef]
- Siva, N. New Gene Biomarker Identified for Indolent Prostate Cancer. Lancet Oncol. 2013, 14, e446. [Google Scholar] [CrossRef]
- Bij de Vaate, A.J.M.; van Doorninck, C.E.M.; Visser, M.; van der Slikke, J.W.; Brölmann, H.A.M. Endometrial Aspiration before or after Saline Infusion Sonography and the Effect on Specimen Quality: A Randomized Study. J. Minim. Invasive Gynecol. 2008, 15, 580–583. [Google Scholar] [CrossRef]
- Zaheer-ul-Haq; Lodhi, M.A.; Ahmad Nawaz, S.; Iqbal, S.; Mohammed Khan, K.; Rode, B.M.; Atta-ur-Rahman; Choudhary, M.I. 3D-QSAR CoMFA Studies on Bis-Coumarine Analogues as Urease Inhibitors: A Strategic Design in Anti-Urease Agents. Bioorg. Med. Chem. 2008, 16, 3456–3461. [Google Scholar] [CrossRef]
- Sarkhoo, E.; Udo, E.E.; Boswihi, S.S.; Monecke, S.; Mueller, E.; Ehricht, R. The Dissemination and Molecular Characterization of Clonal Complex 361 (CC361) Methicillin-Resistant Staphylococcus Aureus (MRSA) in Kuwait Hospitals. Front. Microbiol. 2021, 12, 658772. [Google Scholar] [CrossRef]
- Birceanu, O.; Chowdhury, M.J.; Gillis, P.L.; McGeer, J.C.; Wood, C.M.; Wilkie, M.P. Modes of Metal Toxicity and Impaired Branchial Ionoregulation in Rainbow Trout Exposed to Mixtures of Pb and Cd in Soft Water. Aquat. Toxicol. 2008, 89, 222–231. [Google Scholar] [CrossRef]
- Pellegrini-Giampietro, D.E.; Mannaioni, G.; Bagetta, G. Post-Ischemic Brain Damage: The Endocannabinoid System in the Mechanisms of Neuronal Death. FEBS J. 2009, 276, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Keich, U.; Kertesz-Farkas, A.; Noble, W.S. Improved False Discovery Rate Estimation Procedure for Shotgun Proteomics. J. Proteome Res. 2015, 14, 3148–3161. [Google Scholar] [CrossRef] [PubMed]
- Levran, N.; Levek, N.; Sher, B.; Gruber, N.; Afek, A.; Monsonego-Ornan, E.; Pinhas-Hamiel, O. The Impact of a Low-Carbohydrate Diet on Micronutrient Intake and Status in Adolescents with Type 1 Diabetes. Nutrients 2023, 15, 1418. [Google Scholar] [CrossRef] [PubMed]
- Kenig, S.; Petelin, A.; Poklar Vatovec, T.; Mohorko, N.; Jenko-Pražnikar, Z. Assessment of Micronutrients in a 12-Wk Ketogenic Diet in Obese Adults. Nutrition 2019, 67–68, 110522. [Google Scholar] [CrossRef]
- Dias, C.; Duarte-Ribeiro, F.; Pipa, S.; Mota, M. A Rare and Potentially Catastrophic Infection: Primary Intestinal Aspergillosis—Case Report in an HIV Patient. Case Rep. Infect. Dis. 2018, 2018, 3269847. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Jiang, L.R.; Tan, J.Y.; Guo, L.N.; Wang, X.L.; Dong, W.; Wang, W.B.; Sun, J.K.; Song, B. Alkaloids Constituents from the Roots of Phragmites Australis (Cav.) Trin. Ex Steud. with Their Cytotoxic Activities. Nat. Prod. Res. 2022, 36, 1454–1459. [Google Scholar] [CrossRef]
- Gao, X.; Chen, Z.; Liu, M.; Jia, Y.M.; Yang, N.; Yao, Z.; Feng, X.M.; Xu, Y.; Wang, G. Effects of Short-Term Levothyroxine Therapy on Myocardial Injuries in Patients with Severe Overt Hypothyroidism: Evidence from a Cardiac MRI Study. J. Magn. Reson. Imaging 2017, 46, 897–904. [Google Scholar] [CrossRef]
- Fulghum, K.; Salathe, S.F.; Davis, X.; Thyfault, J.P.; Puchalska, P.; Crawford, P.A. Ketone Body Metabolism and Cardiometabolic Implications for Cognitive Health. npj Metab. Health Dis. 2024, 2, 29. [Google Scholar] [CrossRef]
- Mentzelou, M.; Dakanalis, A.; Vasios, G.K.; Gialeli, M.; Papadopoulou, S.K.; Giaginis, C. The Relationship of Ketogenic Diet with Neurodegenerative and Psychiatric Diseases: A Scoping Review from Basic Research to Clinical Practice. Nutrients 2023, 15, 2270. [Google Scholar] [CrossRef]
- Huang, L.; Li, H.; Zhong, J.; Yang, L.; Chen, G.; Wang, D.; Zheng, G.; Han, H.; Han, X.; Long, Y.; et al. Efficacy and Safety of the Ketogenic Diet for Mitochondrial Disease with Epilepsy: A Prospective, Open-Labeled, Controlled Study. Front. Neurol. 2022, 13, 880944. [Google Scholar] [CrossRef]
- Qiao, X.; Ye, Z.; Wen, J.; Lin, S.; Cao, D.; Chen, L.; Zou, D.; Zou, H.; Zhang, M.; Chen, Z.; et al. Exploring Physiological Beta-Hydroxybutyrate Level in Children Treated with the Classical Ketogenic Diet for Drug-Resistant Epilepsy. Acta Epileptol. 2025, 7, 10. [Google Scholar] [CrossRef]
- He, F.; Ye, L.; Miao, P.; Zhou, J.; Ding, Y.; Wang, S. Long-term Ketogenic Diet Therapy Improves Mitochondrial Encephalopathy with Lactic Acidosis and Stroke-like Episodes (MELAS): A Case Report. CNS Neurosci. Ther. 2023, 29, 2717–2720. [Google Scholar] [CrossRef] [PubMed]
- Wesół-Kucharska, D.; Greczan, M.; Witulska, K.; Piekutowska-Abramczuk, D.; Ciara, E.; Kowalski, P.; Rokicki, D. Improvement of Cardiomyopathy after Ketogenic Diet in a Patient with Leigh Syndrome Caused by MTND5 Mutation. Res. Sq. 2021, 1–14. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kim, W.; Na, J.-H.; Lee, Y.-M. Nutritional Intervention Through Ketogenic Diet in GLUT1 Deficiency Syndrome. Clin. Nutr. Res. 2023, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- Sandu, C.; Burloiu, C.M.; Barca, D.G.; Magureanu, S.A.; Craiu, D.C. Ketogenic Diet in Patients with GLUT1 Deficiency Syndrome. Maedica J. Clin. Med. 2019, 14, 93–97. [Google Scholar] [CrossRef]
- Murofushi, Y.; Hayakawa, I.; Abe, Y.; Ohto, T.; Murayama, K.; Suzuki, H.; Takenouchi, T.; Kosaki, K.; Kubota, M. Ketogenic Diet for KARS-Related Mitochondrial Dysfunction and Progressive Leukodystrophy. Neuropediatrics 2022, 53, 065–068. [Google Scholar] [CrossRef]
- Schoenen, S.; Verbeeck, J.; Koletzko, L.; Brambilla, I.; Kuchenbuch, M.; Dirani, M.; Zimmermann, G.; Dette, H.; Hilgers, R.-D.; Molenberghs, G.; et al. Istore: A Project on Innovative Statistical Methodologies to Improve Rare Diseases Clinical Trials in Limited Populations. Orphanet J. Rare Dis. 2024, 19, 96. [Google Scholar] [CrossRef]
- Almodallal, Y.; Cook, K.; Lammert, L.M.; Lee, M.; Le-Rademacher, J.G.; Jatoi, A. Can Older Patients Adopt and Maintain a Ketogenic Diet? An Observational Study in Support of Clinical Trials in Older Patients. Medicine 2021, 100, e28033. [Google Scholar] [CrossRef]
- Patikorn, C.; Saidoung, P.; Pham, T.; Phisalprapa, P.; Lee, Y.Y.; Varady, K.A.; Veettil, S.K.; Chaiyakunapruk, N. Effects of Ketogenic Diet on Health Outcomes: An Umbrella Review of Meta-Analyses of Randomized Clinical Trials. BMC Med. 2023, 21, 196. [Google Scholar] [CrossRef]
- Crosby, L.; Davis, B.; Joshi, S.; Jardine, M.; Paul, J.; Neola, M.; Barnard, N.D. Ketogenic Diets and Chronic Disease: Weighing the Benefits Against the Risks. Front. Nutr. 2021, 8, 702802. [Google Scholar] [CrossRef]
- Khdher, S.; Mohammed, S.; Muhammed, K.; Ismael, A. The Significant Impact of High-Fat, Low-Carbohydrate Ketogenic Diet on Serum Lipid Profile and Atherosclerotic Cardiovascular Disease Risk in Overweight and Obese Adults. Cureus 2024, 16, e57920. [Google Scholar] [CrossRef]
- Martino, F.K.; Zattarin, A.; Cinquini, C.; Toniazzo, S.; Francini Pesenti, F.; Stefanelli, L.F.; Cacciapuoti, M.; Bettin, E.; Calò, L.A.; Spinella, P. Low-Protein Diet in Elderly Patients with Chronic Kidney Disease Stage 4 and 5 in Conservative Management: Focus on Sarcopenia Development. Nutrients 2024, 16, 1498. [Google Scholar] [CrossRef]
- Rana, A.M.; Mansoor, K.; Assad, S.; Abouzid, M.; Ogu, I.; Bandak, G.S.I. Severe Metabolic Acidosis: A Case of Triple Hit with Ketogenic Diet, Vinegar, and Metformin in an Obese Patient. Case Rep. Nephrol. 2020, 2020, 8861978. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawłowska, M.; Kruszka, J.; Porzych, M.; Garbarek, J.; Nuszkiewicz, J. Ketogenic Metabolism in Neurodegenerative Diseases: Mechanisms of Action and Therapeutic Potential. Metabolites 2025, 15, 508. https://doi.org/10.3390/metabo15080508
Pawłowska M, Kruszka J, Porzych M, Garbarek J, Nuszkiewicz J. Ketogenic Metabolism in Neurodegenerative Diseases: Mechanisms of Action and Therapeutic Potential. Metabolites. 2025; 15(8):508. https://doi.org/10.3390/metabo15080508
Chicago/Turabian StylePawłowska, Marta, Joanna Kruszka, Marta Porzych, Jakub Garbarek, and Jarosław Nuszkiewicz. 2025. "Ketogenic Metabolism in Neurodegenerative Diseases: Mechanisms of Action and Therapeutic Potential" Metabolites 15, no. 8: 508. https://doi.org/10.3390/metabo15080508
APA StylePawłowska, M., Kruszka, J., Porzych, M., Garbarek, J., & Nuszkiewicz, J. (2025). Ketogenic Metabolism in Neurodegenerative Diseases: Mechanisms of Action and Therapeutic Potential. Metabolites, 15(8), 508. https://doi.org/10.3390/metabo15080508










