Decoding Fish Origins: How Metals and Metabolites Differentiate Wild, Cultured, and Escaped Specimens
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection and Sample Preparation
2.2. Heavy Metals Analysis by ICP-MS
2.3. Fatty Acid Analysis by GC-MS
2.4. 1H NMR Acquisition and Data Processing Parameters
2.5. Statistical Analysis
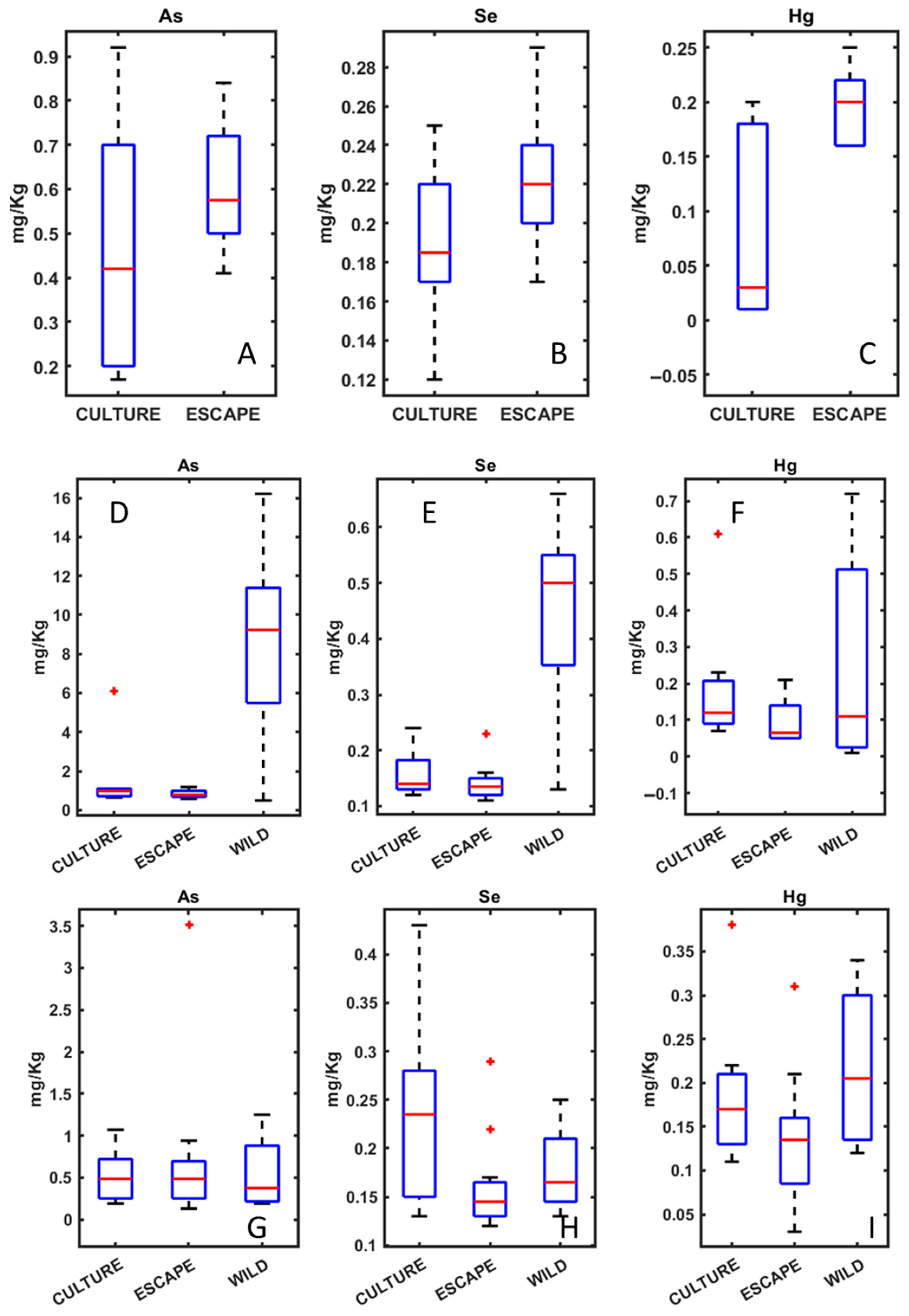
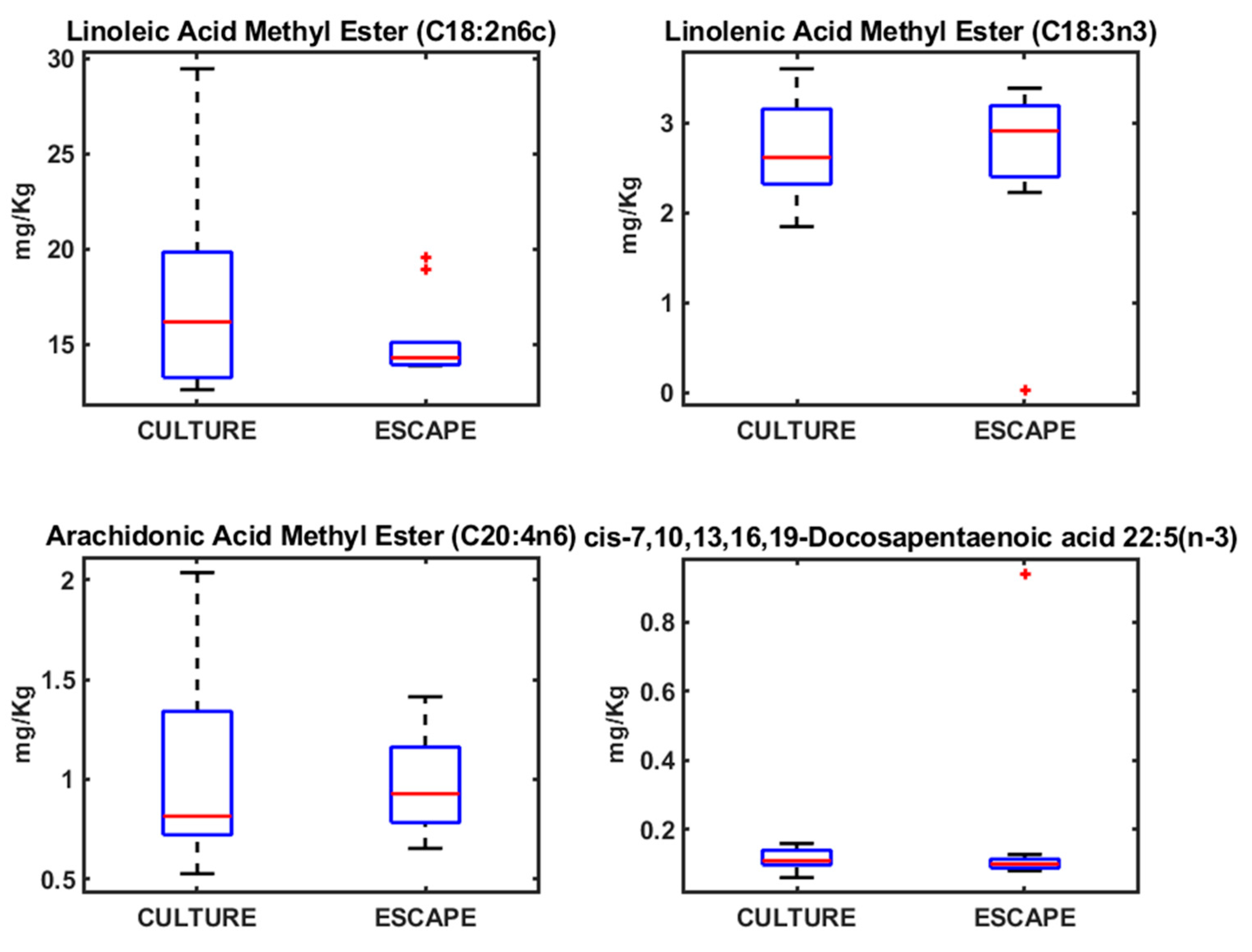
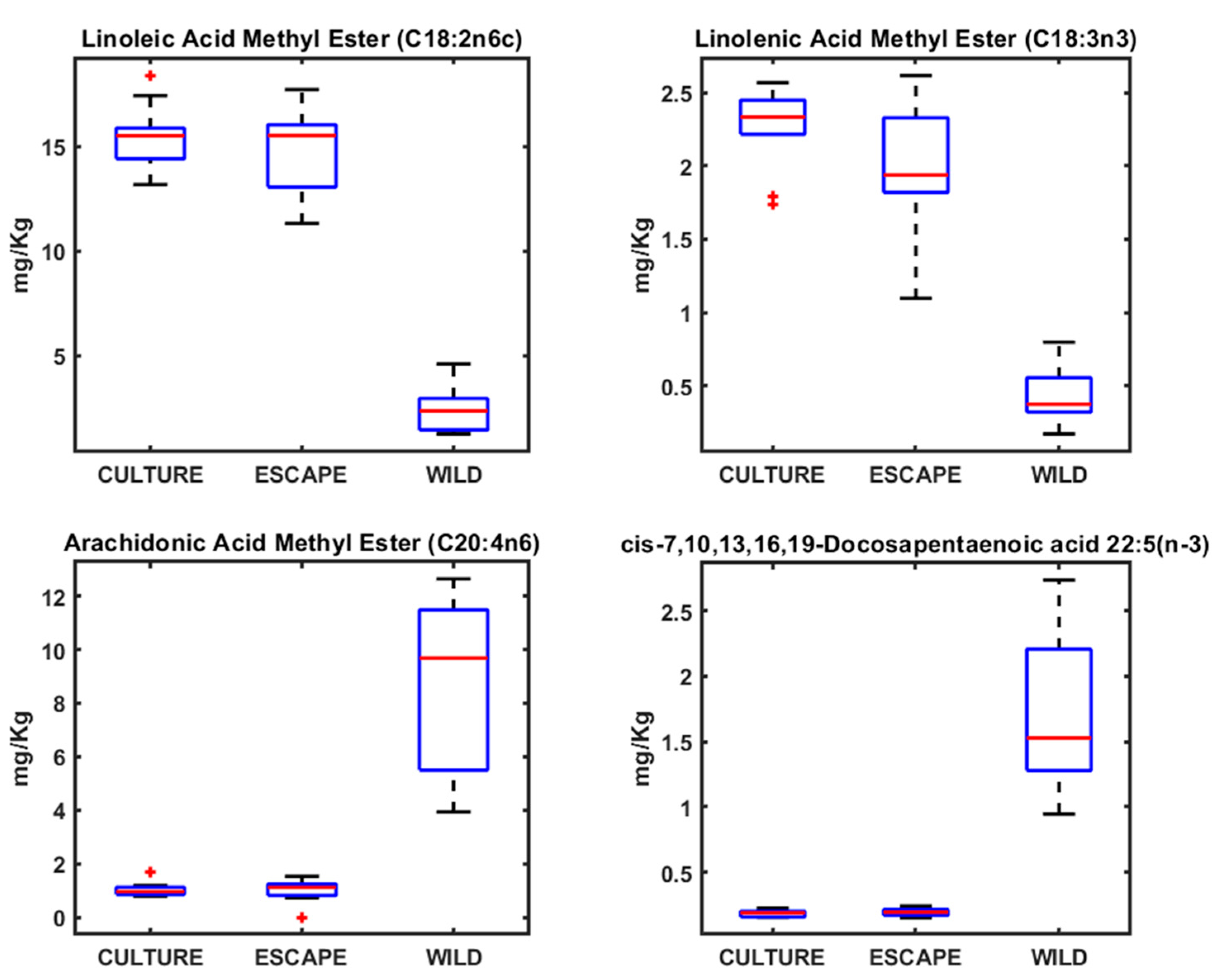
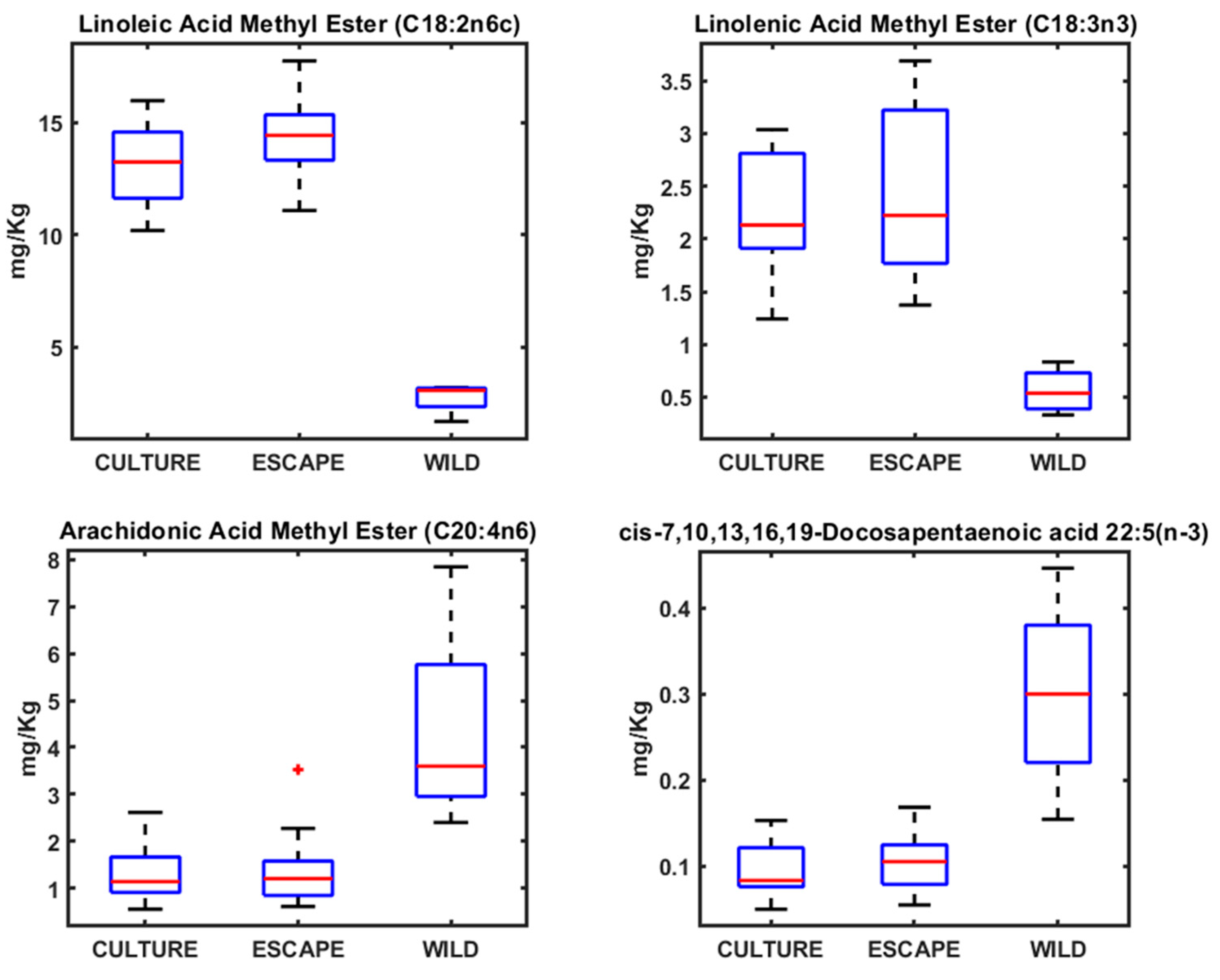
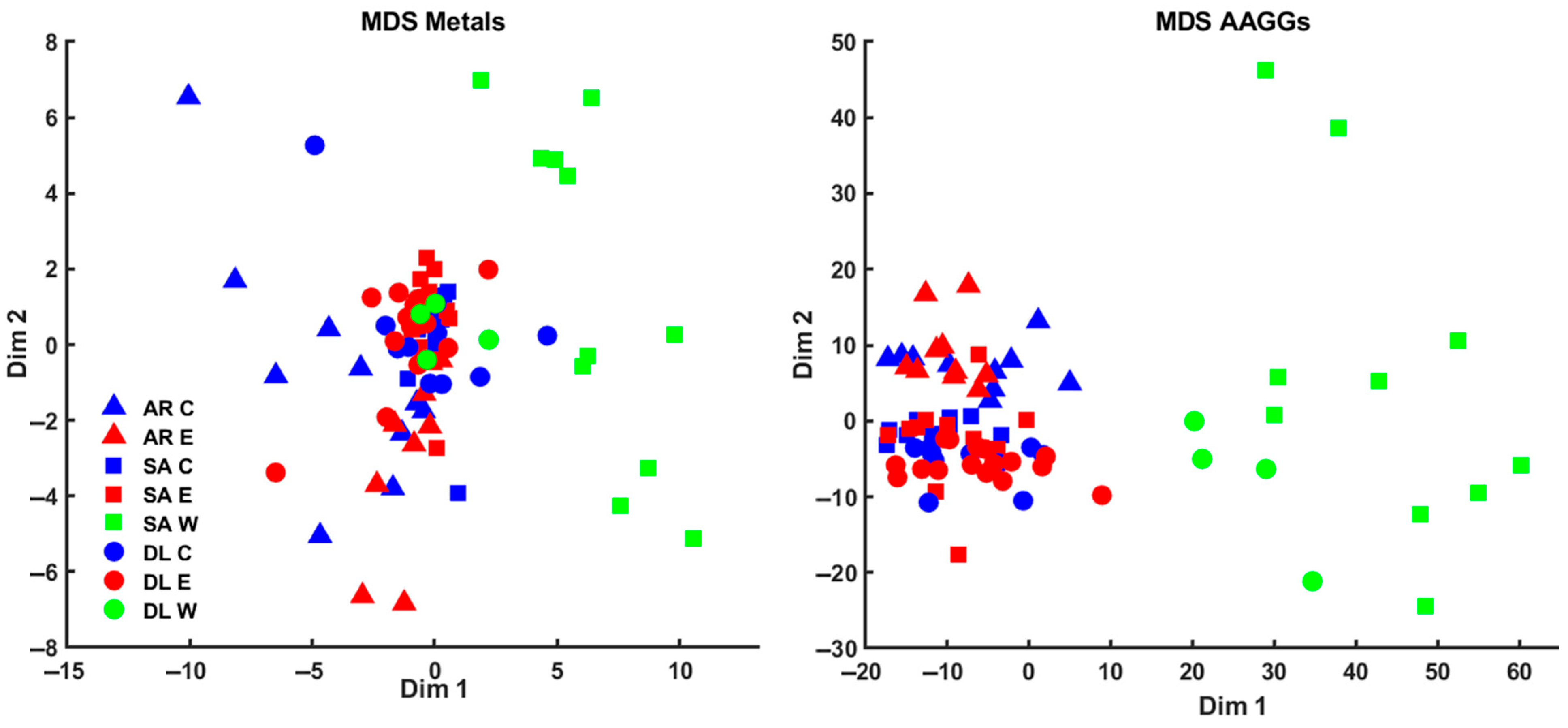
3. Results
4. Discussion
4.1. Heavy Metals Analysis in Seabream, Seabass, and Meagre
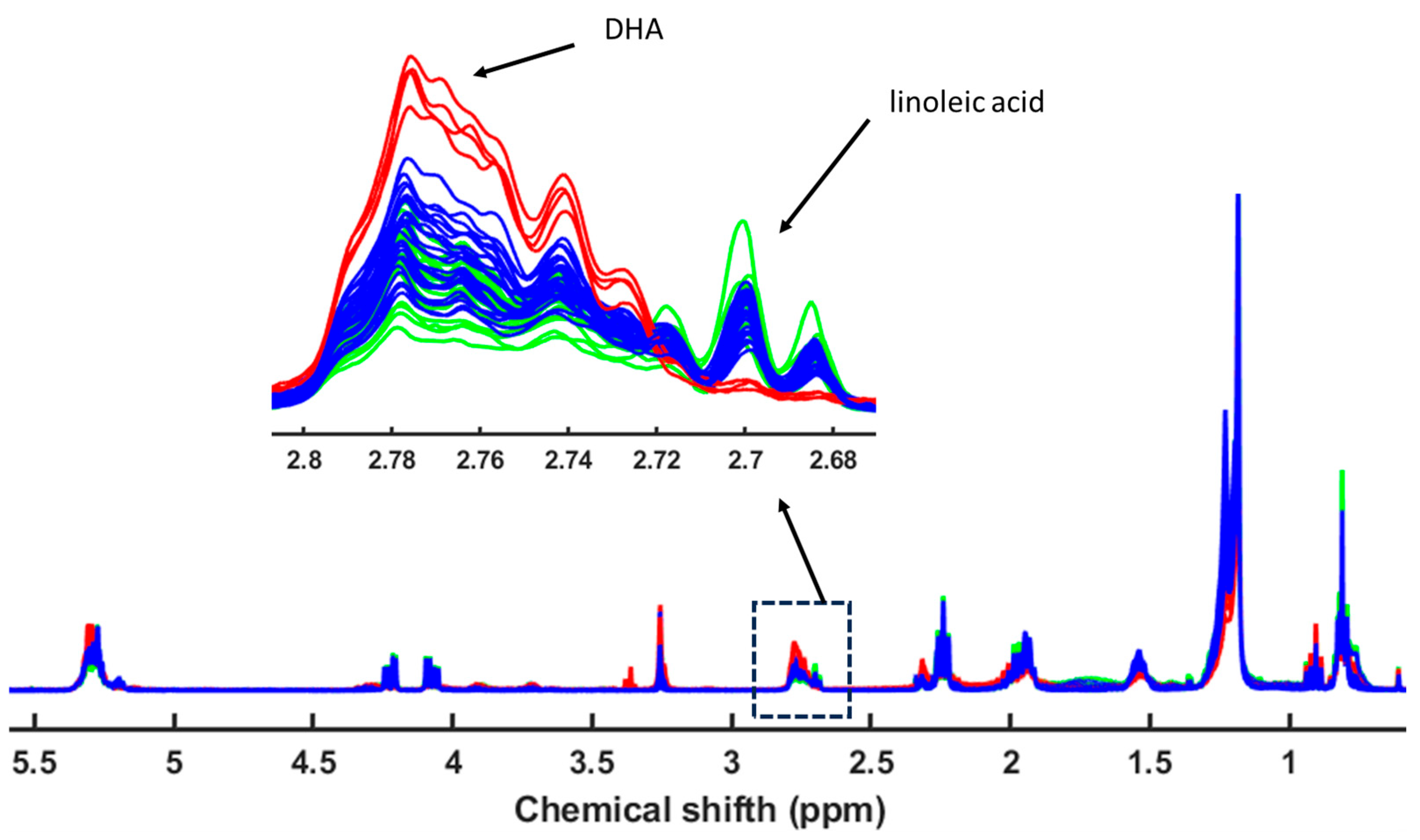
4.2. Fatty Acid Profiles in Seabream, Seabass, and Meagre
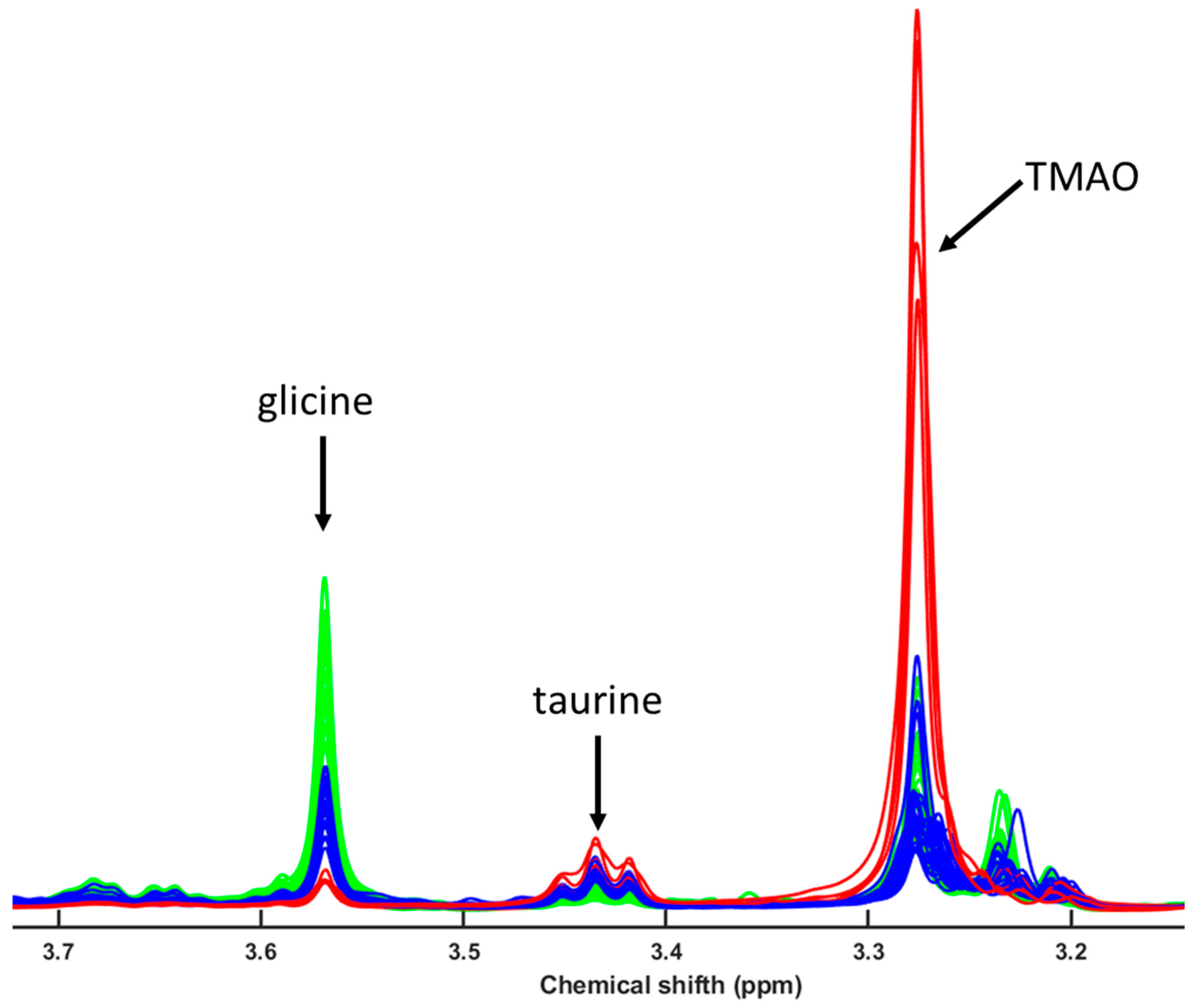
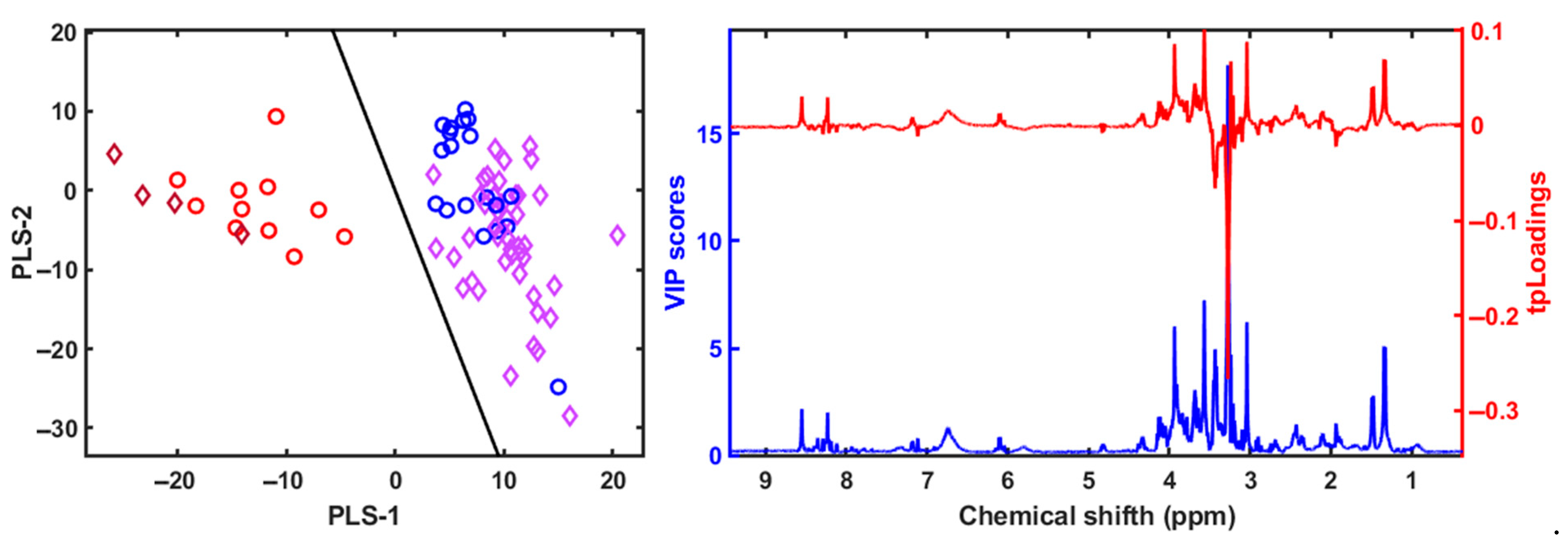
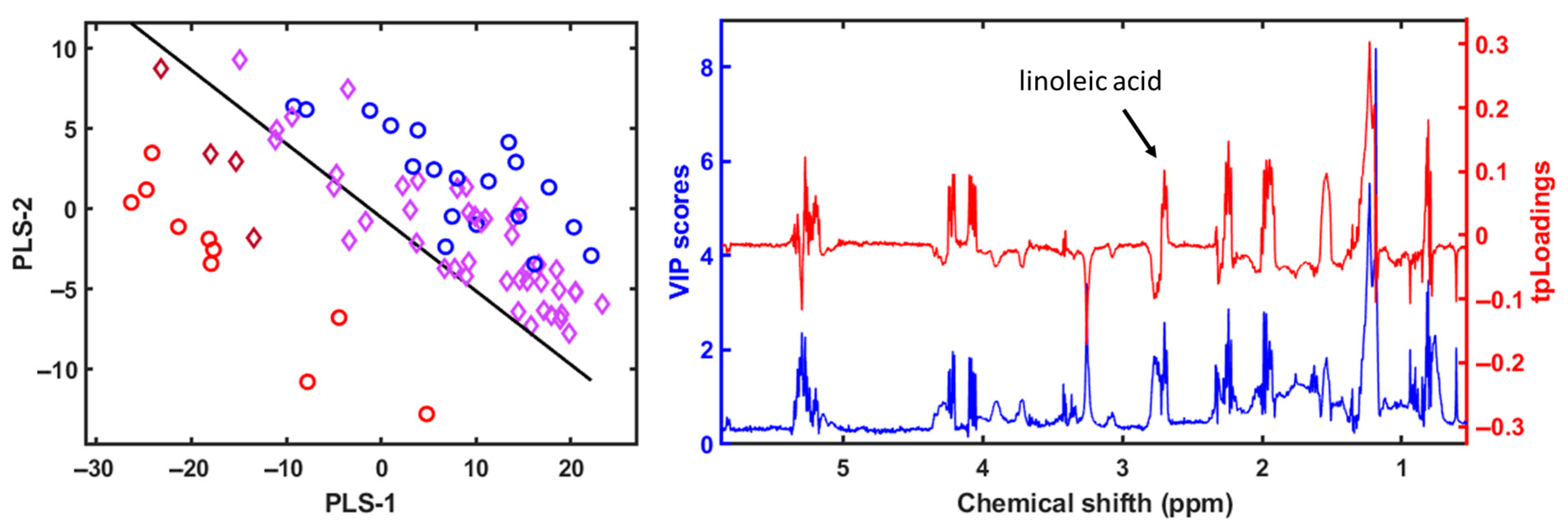
4.3. Metabolomic and Lipidomic Profiling Using NMR
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lionello, P.; Trigo, I.F.; Gil, V.; Liberato, M.L.R.; Nissen, K.M.; Pinto, J.G.; Raible, C.C.; Reale, M.; Tanzarella, A.; Trigo, R.M.; et al. Objective climatology of cyclones in the mediterranean region: A consensus view among methods with different system identification and tracking criteria. Tellus A Dyn. Meteorol. Oceanogr. 2016, 68, 29391. [Google Scholar] [CrossRef]
- Makris, C.V.; Tolika, K.; Baltikas, V.N.; Velikou, K.; Krestenitis, Y.N. The impact of climate change on the storm surges of the mediterranean sea: Coastal sea level responses to deep depression atmospheric systems. Ocean Model. 2023, 181, 102149. [Google Scholar] [CrossRef]
- Toledo-Guedes, K.; Atalah, J.; Izquierdo-Gomez, D.; Fernandez-Jover, D.; Uglem, I.; Sanchez-Jerez, P.; Arechavala-Lopez, P.; Dempster, T. Domesticating the wild through escapees of two iconic mediterranean farmed fish species. Sci. Rep. 2024, 14, 23772. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture (Sofia); FAO: Rome, Italy, 2022; 266p. [Google Scholar]
- Arechavala-Lopez, P.; Toledo-Guedes, K.; Izquierdo-Gomez, D.; Segvic-Bubic, T.; Sanchez-Jerez, P. Implications of sea bream and sea bass escapes for sustainable aquaculture management: A review of interactions, risks and consequences. Rev. Fish. Sci. Aquac. 2018, 26, 214–234. [Google Scholar] [CrossRef]
- Badaoui, W.; Marhuenda-Egea, F.C.; Valero-Rodriguez, J.M.; Sanchez-Jerez, P.; Arechavala-Lopez, P.; Toledo-Guedes, K. Metabolomic and lipidomic tools for tracing fish escapes from aquaculture facilities. ACS Food Sci. Technol. 2024, 4, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Arechavala-Lopez, P.; Valero-Rodriguez, J.M.; Penalver-Garcia, J.; Izquierdo-Gomez, D.; Sanchez-Jerez, P. Linking coastal aquaculture of meagre Argyrosomus regius and western mediterranean coastal fisheries through escapes incidents. Fish. Manag. Ecol. 2015, 22, 317–325. [Google Scholar] [CrossRef]
- Jackson, D.; Drumm, A.; McEvoy, S.; Jensen, O.; Mendiola, D.; Gabina, G.; Borg, J.A.; Papageorgiou, N.; Karakassis, I.; Black, K.D. A pan-european valuation of the extent, causes and cost of escape events from sea cage fish farming. Aquaculture 2015, 436, 21–26. [Google Scholar] [CrossRef]
- Izquierdo-Gomez, D.; Arechavala-Lopez, P.; Bayle-Sempere, J.T.; Sanchez-Jerez, P. Assessing the influence of gilthead sea bream escapees in landings of mediterranean fisheries through a scale-based methodology. Fish. Manag. Ecol. 2017, 24, 62–72. [Google Scholar] [CrossRef]
- Naylor, R.; Fang, S.; Fanzo, J. A global view of aquaculture policy. Food Policy 2023, 116, 102422. [Google Scholar] [CrossRef]
- Šegvić-Bubić, T.; Lepen, I.; Trumbić, Ž.; Ljubković, J.; Sutlović, D.; Matić-Skoko, S.; Grubišić, L.; Glamuzina, B.; Mladineo, I. Population genetic structure of reared and wild gilthead sea bream (Sparus aurata) in the Adriatic Sea inferred with microsatellite loci. Aquaculture 2011, 318, 309–315. [Google Scholar] [CrossRef]
- Jiménez-Segura, L.F.; Restrepo-Santamaria, D.; Ospina-Pabón, J.G.; Castellanos-Mejía, M.C.; Valencia-Rodríguez, D.; Galeano-Moreno, A.F.; Londoño-López, J.L.; Herrera-Pérez, J.; Medina-Ríos, V.M.; Álvarez-Bustamante, J.; et al. Fish databases for improving their conservation in Colombia. Sci. Data 2025, 12, 262. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, C.; Calagna, A.; Cammilleri, G.; Schembri, P.; Lo Monaco, D.; Ciprì, V.; Battaglia, L.; Barbera, G.; Ferrantelli, V.; Sadok, S.; et al. Risk Assessment of cadmium, lead, and mercury on human health in relation to the consumption of farmed sea bass in italy: A meta-analytical approach. Front. Mar. Sci. 2021, 8, 616488. [Google Scholar] [CrossRef]
- Salem, A.M.; El-Metwally, M.E.A. Bioaccumulation and Health risk potential of heavy metals including cancer in wild and farmed meagre Argyrosomus regius (Asso, 1801), Mediterreanean Sea, Egypt. Egypt. J. Aquat. Biol. Fish. 2024, 28, 1277–1301. [Google Scholar] [CrossRef]
- Lounas, R.; Kasmi, H.; Chernai, S.; Amarni, N.; Ghebriout, L.; Hamdi, B. Heavy metal concentrations in wild and farmed gilthead sea bream from southern mediterranean sea-human health risk assessment. Environ. Sci. Pollut. Res. Int. 2021, 28, 30732–30742. [Google Scholar] [CrossRef] [PubMed]
- Lenas, D.; Triantafillou, D.; Chatziantoniou, S.; Nathanailides, C. Fatty acid profile of wild and farmed gilthead sea bream (Sparus aurata). J. Für Verbraucherschutz Und Leb. 2011, 6, 435–440. [Google Scholar] [CrossRef]
- Lindon, J.C.; Nicholson, J.K.; Holmes, E. The Handbook of Metabonomics and Metabolomics; Elsevier Science: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Southam, A.; Lange, A.; Al-Salhi, R.; Hill, E.; Tyler, C.; Viant, M. Distinguishing between the metabolome and xenobiotic exposome in environmental field samples analysed by direct-infusion mass spectrometry based metabolomics and lipidomics. Metabolomics 2014, 10, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Griboff, J.; Wunderlin, D.A.; Monferran, M.V. Metals, As and Se determination by inductively coupled plasma-mass spectrometry (ICP-MS) in edible fish collected from three eutrophic reservoirs. Their consumption represents a risk for human health? Microchem. J. 2017, 130, 236–244. [Google Scholar] [CrossRef]
- O’Fallon, J.V.; Busboom, J.R.; Nelson, M.L.; Gaskins, C.T. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. J. Anim. Sci. 2007, 85, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Li, H.D.; Xu, Q.S.; Liang, Y.Z. libPLS: An integrated library for partial least squares regression and linear discriminant analysis. Chemom. Intell. Lab. Syst. 2018, 176, 34–43. [Google Scholar] [CrossRef]
- Benedito-Palos, L.; Navarro, J.C.; Sitjà-Bobadilla, A.; Gordon Bell, J.; Kaushik, S.; Pérez-Sánchez, J. High levels of vegetable oils in plant protein-rich diets fed to gilthead sea bream (Sparus aurata L.): Growth performance, muscle fatty acid profiles and histological alterations of target tissues. Br. J. Nutr. 2008, 100, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Robin, J.H.; Vincent, B. Microparticulate diets as first food for gilthead sea bream larva (Sparus aurata): Study of fatty acid incorporation. Aquaculture 2003, 225, 463–474. [Google Scholar]
- Marhuenda-Egea, F.C.; Sanchez-Jerez, P. Metabolomic insights into wild and farmed gilthead seabream (Sparus aurata): Lipid composition, freshness indicators, and environmental adaptations. Molecules 2025, 30, 770. [Google Scholar] [CrossRef] [PubMed]
- Melis, R.; Sanna, R.; Braca, A.; Bonaglini, E.; Cappuccinelli, R.; Slawski, H.; Roggio, T.; Uzzau, S.; Anedda, R. Molecular details on gilthead sea bream (Sparus aurata) sensitivity to low water temperatures from 1H NMR metabolomics. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 204, 129–136. [Google Scholar] [CrossRef]
- Martinez, I.; Bathen, T.; Standal, I.; Halvorsen, J.; Aursand, M.; Gribbestad, I.; Axelson, D. Bioactive compounds in cod (Gadus morhua) products and suitability of H-1 NMR metabolite profiling for classification of the products using multivariate data analyses. J. Agric. Food Chem. 2005, 53, 6889–6895. [Google Scholar] [CrossRef]
- Yancey, P.H.; Samerotte, A.L.; Brand, G.L.; Drazen, J.C. Increasing contents of TMAO with depth within species as well as among species of teleost fish. Integr. Comp. Biol. 2004, 44, 669. [Google Scholar]
- Houston, S.J.S.; Karalazos, V.; Tinsley, J.; Betancor, M.B.; Martin, S.A.M.; Tocher, D.R.; Monroig, O. The compositional and metabolic responses of gilthead seabream (Sparus aurata) to a gradient of dietary fish oil and associated n-3 long-chain PUFA content. Br. J. Nutr. 2017, 118, 1010–1022. [Google Scholar] [CrossRef]
- Zhang, W.; Miao, A.-J.; Wang, N.-X.; Li, C.; Sha, J.; Jia, J.; Alessi, D.S.; Yan, B.; Ok, Y.S. Arsenic bioaccumulation and biotransformation in aquatic organisms. Environ. Int. 2022, 163, 107221. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Sohn, M. Aquatic arsenic: Toxicity, speciation, transformations, and remediation. Environ. Int. 2009, 35, 743–759. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef]
- Byeon, E.; Kang, H.-M.; Yoon, C.; Lee, J.-S. Toxicity mechanisms of arsenic compounds in aquatic organisms. Aquat. Toxicol. 2021, 237, 105901. [Google Scholar] [CrossRef] [PubMed]
- El Hanafi, K.; Fernández-Bautista, T.; Ouerdane, L.; Corns, W.T.; Bueno, M.; Fontagné-Dicharry, S.; Amouroux, D.; Pedrero, Z. Exploring mercury detoxification in fish: The role of selenium from tuna byproduct diets for sustainable aquaculture. J. Hazard. Mater. 2024, 480, 135779. [Google Scholar] [CrossRef] [PubMed]
- Spiller, H.A. Rethinking mercury: The role of selenium in the pathophysiology of mercury toxicity. Clin. Toxicol. 2018, 56, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Greani, S.; Lourkisti, R.; Berti, L.; Marchand, B.; Giannettini, J.; Santini, J.; Quilichini, Y. Effect of chronic arsenic exposure under environmental conditions on bioaccumulation, oxidative stress, and antioxidant enzymatic defenses in wild trout Salmo trutta (Pisces, Teleostei). Ecotoxicology 2017, 26, 930–941. [Google Scholar] [CrossRef]
- Magellan, K.; Barral-Fraga, L.; Rovira, M.; Srean, P.; Urrea, G.; García-Berthou, E.; Guasch, H. Behavioural and physical effects of arsenic exposure in fish are aggravated by aquatic algae. Aquat. Toxicol. 2014, 156, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Kakakhel, M.A.; Narwal, N.; Kataria, N.; Johari, S.A.; Zaheer Ud Din, S.; Jiang, Z.; Khoo, K.S.; Xiaotao, S. Deciphering the dysbiosis caused in the fish microbiota by emerging contaminants and its mitigation strategies-A review. Environ. Res. 2023, 237, 117002. [Google Scholar] [CrossRef] [PubMed]
- Anwar, Z.; Ye, C.; Wang, M.; Zeng, S.; Gao, M.; Guo, S.; Kakakhel, M.A.; Hu, B.; Zhao, G.; Hong, Y. Effects of dietary selenium on growth performance, antioxidant status, and gut microbial diversity of zebrafish (Danio rerio). Aquac. Rep. 2024, 37, 102276. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M. Selenium and mercury molar ratios in saltwater fish from New Jersey: Individual and species variability complicate use in human health fish consumption advisories. Environ. Res. 2012, 114, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Ralston, N.V.C. Selenium health benefit values as seafood safety criteria. EcoHealth 2008, 5, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Ralston, N.V.; Blackwell, J.L., 3rd; Raymond, L.J. Importance of molar ratios in selenium-dependent protection against methylmercury toxicity. Biol. Trace Elem. Res. 2007, 119, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ai, Q.; Mai, K.; Xu, W.; Wang, J.; Ma, H.; Zhang, W.; Wang, X.; Liufu, Z. Effects of dietary arachidonic acid on growth performance, survival, immune response and tissue fatty acid composition of juvenile Japanese seabass, Lateolabrax japonicus. Aquaculture 2010, 307, 75–82. [Google Scholar] [CrossRef]
- Calder, P.C. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins, Leukot. Essent. Fat. Acids 2008, 79, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Furne, M.; Holen, E.; Araujo, P.; Lie, K.K.; Moren, M. Cytokine gene expression and prostaglandin production in head kidney leukocytes isolated from Atlantic cod (Gadus morhua) added different levels of arachidonic acid and eicosapentaenoic acid. Fish Shellfish Immunol. 2013, 34, 770–777. [Google Scholar]
- Hardy, R.W.; Lee, C.-s. Aquaculture feed and seafood quality. Bull. Fish. Res. Dev. Agency 2010, 31, 43–50. [Google Scholar]
- Tocher, D.R. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Tocher, D.R.; Bendiksen, E.Å.; Campbell, P.J.; Bell, J.G. The role of phospholipids in nutrition and metabolism of teleost fish. Aquaculture 2008, 280, 21–34. [Google Scholar] [CrossRef]
- Du, Z.Y.; Monroig, Ó.; Rodríguez, C.; Garrido, D.; Tocher, D.R. Lipid metabolism in fish and shellfish. In Nutrition and Physiology of Fish and Shellfish: Feed Regulation, Metabolism, and Digestion; Academic Press: San Diego, CA, USA, 2025; pp. 303–347. [Google Scholar] [CrossRef]
- Turchini, G.M.; Francis, D.S.; Du, Z.Y.; Olsen, R.E.; Ringø, E.; Tocher, D.R. The lipids. In Fish Nutrition; Hardy, R.W., Kaushik, S.J., Eds.; Academic Press: San Diego, CA, USA, 2021; pp. 303–467. ISBN 978-0-12-819587-1. [Google Scholar] [CrossRef]
- Guixà-González, R.; Javanainen, M.; Gómez-Soler, M.; Cordobilla, B.; Domingo, J.C.; Sanz, F.; Pastor, M.; Ciruela, F.; Martinez-Seara, H.; Selent, J. Membrane omega-3 fatty acids modulate the oligomerisation kinetics of adenosine A2A and dopamine D2 receptors. Sci. Rep. 2016, 6, 19839. [Google Scholar] [CrossRef] [PubMed]
- Mejri, S.C.; Tremblay, R.; Audet, C.; Wills, P.S.; Riche, M. Essential fatty acid requirements in tropical and cold-water marine fish larvae and juveniles. Front. Mar. Sci. 2021, 8, 680003. [Google Scholar] [CrossRef]
- Wang, S.; Wang, M.; Zhang, H.; Yan, X.; Guo, H.; You, C.; Tocher, D.R.; Chen, C.; Li, Y. Long-chain polyunsaturated fatty acid metabolism in carnivorous marine teleosts: Insight into the profile of endogenous biosynthesis in golden pompano Trachinotus ovatus. Aquac. Res. 2020, 51, 623–635. [Google Scholar] [CrossRef]
- Baccouch, R.; Rascol, E.; Stoklosa, K.; Alves, I.D. The role of the lipid environment in the activity of G protein coupled receptors. Biophys. Chem. 2022, 285, 106794. [Google Scholar] [CrossRef] [PubMed]
- De Santis, A.; Varela, Y.; Sot, J.; D’Errico, G.; Goñi, F.M.; Alonso, A. Omega-3 polyunsaturated fatty acids do not fluidify bilayers in the liquid-crystalline state. Sci. Rep. 2018, 8, 16240. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.J.Y.; Gabriel, F.; Tandale, A.; Nietlispach, D. Structure and dynamics of gpcrs in lipid membranes: Physical principles and experimental approaches. Molecules 2020, 25, 4729. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, D.; Prasanna, X.; Mohole, M.; Chattopadhyay, A. Exploring GPCR–lipid interactions by molecular dynamics simulations: Excitements, challenges, and the way forward. J. Phys. Chem. B 2018, 122, 5727–5737. [Google Scholar] [CrossRef] [PubMed]
- Solé-Jiménez, P.; Naya-Català, F.; Piazzon, M.C.; Estensoro, I.; Calduch-Giner, J.À.; Sitjà-Bobadilla, A.; Van Mullem, D.; Pérez-Sánchez, J. Reshaping of gut microbiota in gilthead sea bream fed microbial and processed animal proteins as the main dietary protein source. Front. Mar. Sci. 2021, 8, 705041. [Google Scholar] [CrossRef]
- Quero, G.M.; Piredda, R.; Basili, M.; Maricchiolo, G.; Mirto, S.; Manini, E.; Seyfarth, A.M.; Candela, M.; Luna, G.M. Host-associated and environmental microbiomes in an open-sea mediterranean gilthead sea bream fish farm. Microb. Ecol. 2023, 86, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Bockus, A.B.; Seibel, B.A. Trimethylamine oxide accumulation as a function of depth in Hawaiian mid-water fishes. Deep Sea Res. Part I: Oceanogr. Res. Pap. 2016, 112, 37–44. [Google Scholar] [CrossRef]
- Samerotte, A.L.; Drazen, J.C.; Brand, G.L.; Seibel, B.A.; Yancey, P.H. Correlation of trimethylamine oxide and habitat depth within and among species of teleost fish: An analysis of causation. Physiol. Biochem. Zool. 2007, 80, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Kunkel-Patterson, A.; Fiess, J.C.; Mathias, L.; Samerotte, A.; Hirano, T.; Yancey, P.H. Effects of salinity and temperature on osmolyte composition in tilapia. Integr. Comp. Biol. 2005, 45, 1156. [Google Scholar]
- Treberg, J.R.; Driedzic, W.R. Maintenance and accumulation of trimethylamine oxide by winter skate (Leucoraja ocellata): Reliance on low whole animal losses rather than synthesis. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2006, 291, R1790–R1798. [Google Scholar] [CrossRef] [PubMed]
- Yancey, P.H. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 2005, 208, 2819–2830. [Google Scholar] [CrossRef] [PubMed]
- Sampath, W.W.H.A.; Rathnayake, R.M.D.S.; Yang, M.; Zhang, W.; Mai, K. Roles of dietary taurine in fish nutrition. Mar. Life Sci. Technol. 2020, 2, 360–375. [Google Scholar] [CrossRef]
- Shen, G.; Wang, S.; Dong, J.; Feng, J.; Xu, J.; Xia, F.; Wang, X.; Ye, J. Metabolic effect of dietary taurine supplementation on grouper (Epinephelus coioides): A H-1-NMR-based metabolomics study. Molecules 2019, 24, 2253. [Google Scholar] [CrossRef] [PubMed]
- Salze, G.P.; Davis, D.A. Taurine: A critical nutrient for future fish feeds. Aquaculture 2015, 437, 215–229. [Google Scholar] [CrossRef]
- Pinto, W.; Ronnestad, I.; Dinis, M.T.; Aragao, C. Taurine and Fish Development: Insights for the Aquaculture Industry. In Taurine 8, Vol 2: Nutrition and Metabolism, Protective Role, and Role in Reproduction, Development, and Differentiation; ElIdrissi, A., Lamoreaux, W.J., Eds.; Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2013; Volume 776, pp. 329–334. [Google Scholar]
- Shen, G.P.; Ding, Z.N.; Dai, T.; Feng, J.H.; Dong, J.Y.; Xia, F.; Xu, J.J.; Ye, J.D. Effect of dietary taurine supplementation on metabolome variation in plasma of Nile tilapia. Animal 2021, 15, 100167. [Google Scholar] [CrossRef] [PubMed]
- Schrama, D.; Cerqueira, M.; Raposo, C.S.; Rosa da Costa, A.M.; Wulff, T.; Gonçalves, A.; Camacho, C.; Colen, R.; Fonseca, F.; Rodrigues, P.M. Dietary creatine supplementation in gilthead seabream (Sparus aurata): Comparative proteomics analysis on fish allergens, muscle quality, and liver. Front. Physiol. 2018, 9, 1844. [Google Scholar] [CrossRef] [PubMed]
- Teles, A.; Guzmán-Villanueva, L.; Hernández-de Dios, M.A.; Maldonado-García, M.; Tovar-Ramírez, D. Taurine enhances antioxidant enzyme activity and immune response in Seriola rivoliana juveniles after lipopolysaccharide injection. Fishes 2024, 10, 225. [Google Scholar] [CrossRef]
- Stubhaug, I.; Tocher, D.R.; Bell, J.G.; Dick, J.R.; Torstensen, B.E. Fatty acid metabolism in Atlantic salmon (Salmo salar L.) hepatocytes and influence of dietary vegetable oil. Biochim. Et Biophys. Acta Mol. Cell Biol. Lipids 2005, 1734, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.S.; Obach, A.; Arantzamendi, L.; Montero, D.; Robaina, L.; Rosenlund, G. Dietary lipid sources for seabream and seabass: Growth performance, tissue composition and flesh quality. Aquac. Nutr. 2003, 9, 397–407. [Google Scholar] [CrossRef]
| Species | Total Weight (g) | Gutted Weight (g) | Total Length (cm) | Furcal Length (cm) |
|---|---|---|---|---|
| Cultured Seabream | 504.96 ± 113.17 | 461.61 ± 101.32 | 30.91 ± 3.81 | 26.14 ± 2.82 |
| Escaped Seabream | 606.39 ± 299.26 | 563.05 ± 273.57 | 30.90 ± 3.66 | 27.33 ± 3.76 |
| Wild Seabream | 347.69 ± 263.24 | 320.77 ± 250.79 | 28.26 ± 5.13 | 23.29 ± 4.70 |
| Cultured Seabass | 618.33 ± 212.59 | 563.66 ± 218.34 | 38.30 ± 4.40 | 33.22 ± 4.00 |
| Escaped Seabass | 869.64 ± 354.98 | 753.36 ± 320.69 | 41.81 ± 5.34 | 36.83 ± 4.63 |
| Wild Seabass | 597.08 ± 227.84 | 535.27 ± 211.42 | 37.38 ± 5.32 | 32.00 ± 4.27 |
| Cultured Meagre | 1274.93 ± 374.01 | 1198.78 ± 338.38 | 49.92 ± 4.47 | 45.34 ± 4.08 |
| Escaped Meagre | 1364.59 ± 389.41 | 1204.92 ± 528.79 | 52.58 ± 5.05 | 48.14 ± 4.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badaoui, W.; Toledo-Guedes, K.; Valero-Rodriguez, J.M.; Villar-Montalt, A.; Marhuenda-Egea, F.C. Decoding Fish Origins: How Metals and Metabolites Differentiate Wild, Cultured, and Escaped Specimens. Metabolites 2025, 15, 490. https://doi.org/10.3390/metabo15070490
Badaoui W, Toledo-Guedes K, Valero-Rodriguez JM, Villar-Montalt A, Marhuenda-Egea FC. Decoding Fish Origins: How Metals and Metabolites Differentiate Wild, Cultured, and Escaped Specimens. Metabolites. 2025; 15(7):490. https://doi.org/10.3390/metabo15070490
Chicago/Turabian StyleBadaoui, Warda, Kilian Toledo-Guedes, Juan Manuel Valero-Rodriguez, Adrian Villar-Montalt, and Frutos C. Marhuenda-Egea. 2025. "Decoding Fish Origins: How Metals and Metabolites Differentiate Wild, Cultured, and Escaped Specimens" Metabolites 15, no. 7: 490. https://doi.org/10.3390/metabo15070490
APA StyleBadaoui, W., Toledo-Guedes, K., Valero-Rodriguez, J. M., Villar-Montalt, A., & Marhuenda-Egea, F. C. (2025). Decoding Fish Origins: How Metals and Metabolites Differentiate Wild, Cultured, and Escaped Specimens. Metabolites, 15(7), 490. https://doi.org/10.3390/metabo15070490







