Advancing Semiochemical Tools for Mountain Pine Beetle Management: Dendroctonus ponderosae Responses to Saprophytic Fungal Volatiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Fungal Isolates and Media Preparation
2.3. Volatile Collection and Extraction
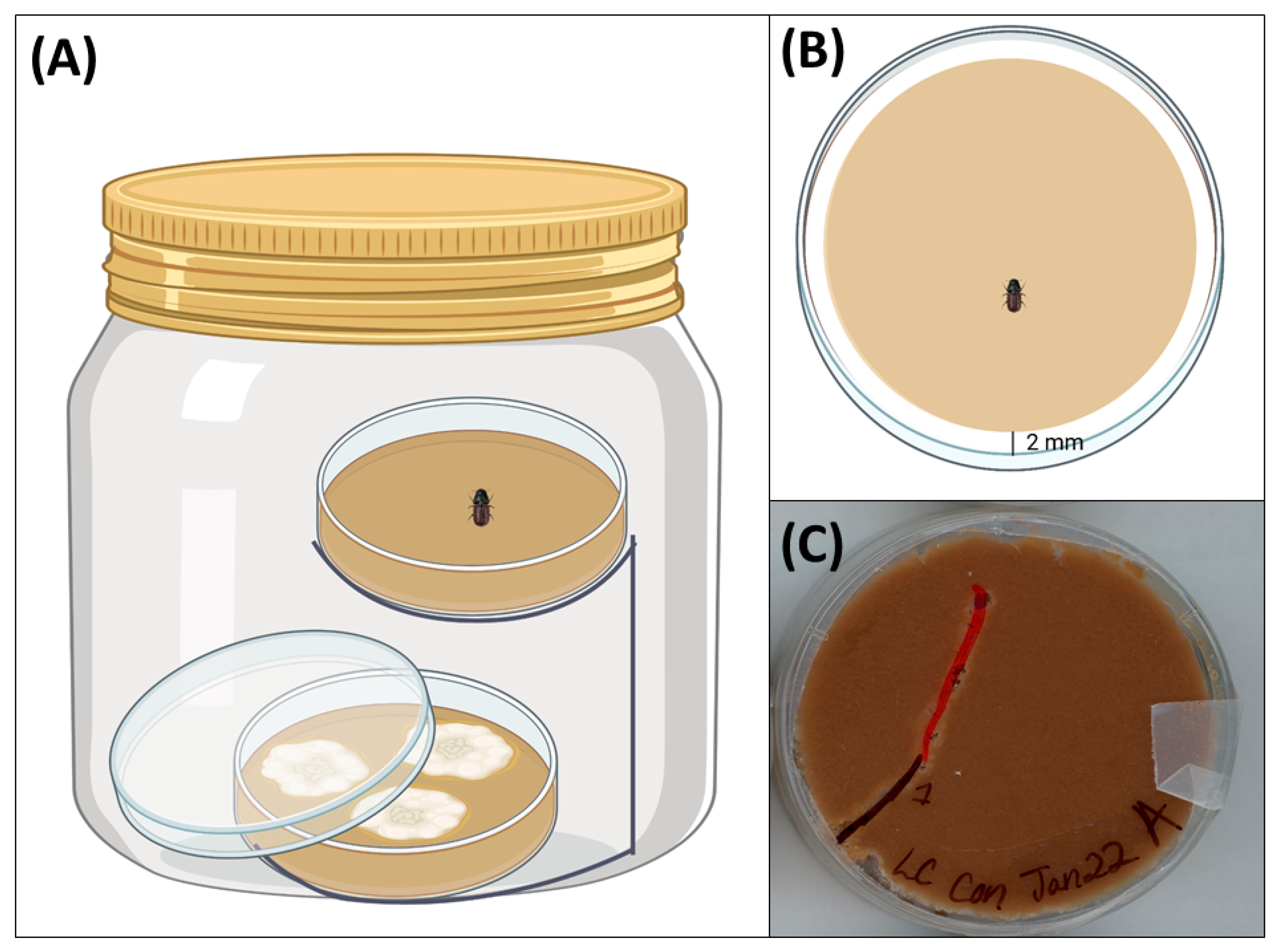
2.4. Two-Choice Olfactometer Bioassays
2.5. Feeding Bioassays
2.6. Statistical Analyses
3. Results
3.1. Volatiles Identified from Trichoderma Atroviride
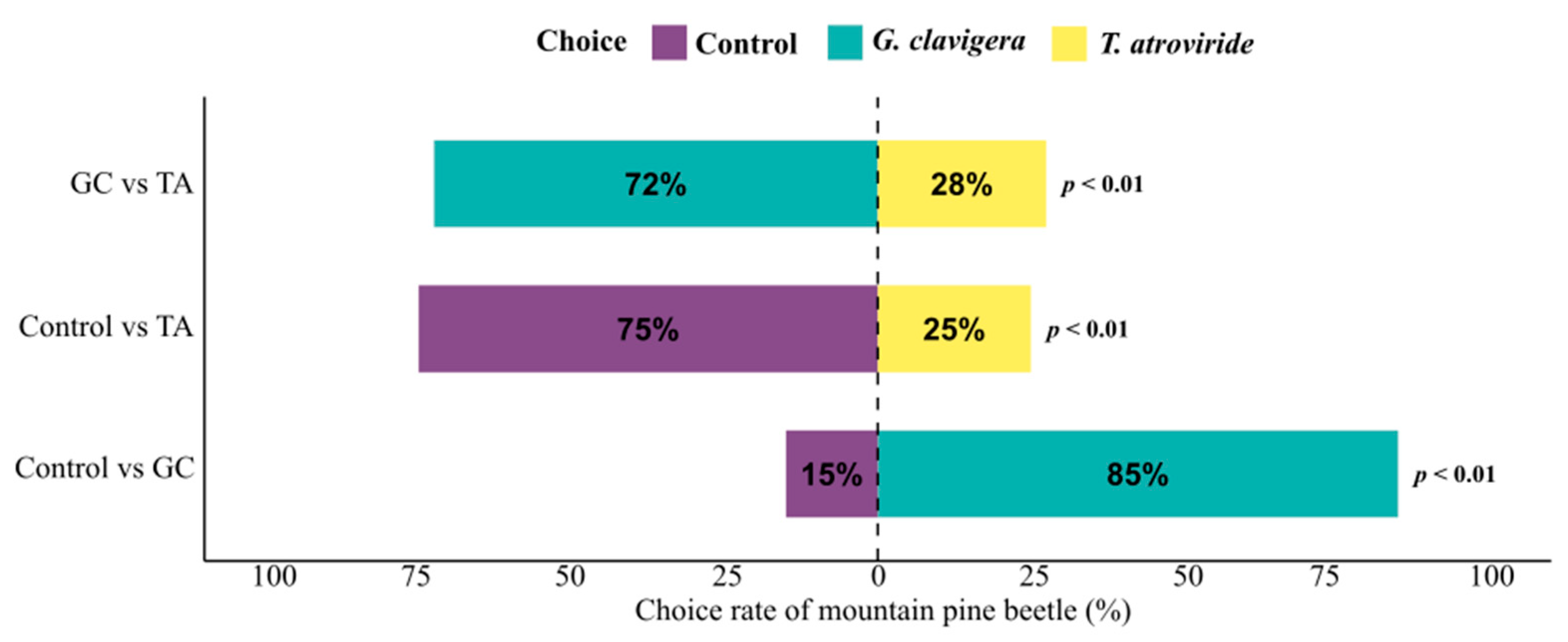
3.2. Mountain Pine Beetle Are Strongly Repelled by the FVOCs of T. atroviride
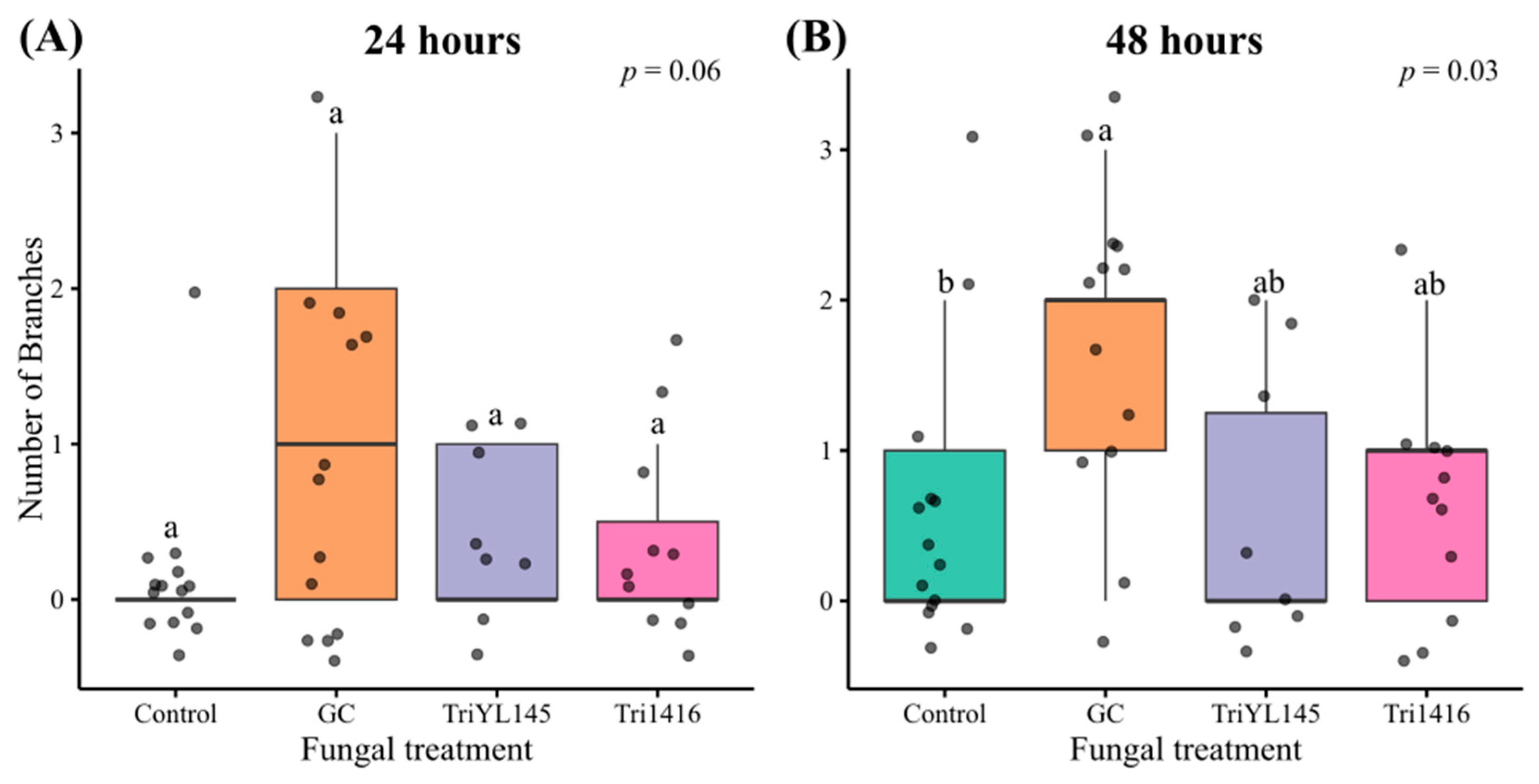
3.3. Fungal Volatiles Did Not Strongly Influence MPB Feeding Behaviour
4. Discussion
4.1. Volatiles Identified from Trichoderma Atroviride
4.2. Effects of FVOCs on MPB Attraction
4.3. Effects of FVOCs on MPB Feeding
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardoza, Y.J.; Klepzig, K.D.; Raffa, K.F. Bacteria in oral secretions of an endophytic insect inhibit antagonistic fungi. Ecol. Entomol. 2006, 31, 636–645. [Google Scholar] [CrossRef]
- Bleiker, K.P.; Six, D.L. Dietary benefits of fungal associates to an eruptive herbivore: Potential implications of multiple associates on host population dynamics. Environ. Entomol. 2007, 36, 1384–1396. [Google Scholar] [CrossRef] [PubMed]
- DiGuistini, S.; Wang, Y.; Liao, N.Y.; Taylor, G.; Tanguay, P.; Feau, N.; Henrissat, B.; Chan, S.K.; Hesse-Orce, U.; Alamouti, S.M.; et al. Genome and transcriptome analyses of the mountain pine beetle-fungal symbiont Grosmannia clavigera, a lodgepole pine pathogen. Proc. Natl. Acad. Sci. USA 2011, 108, 2504–2509. [Google Scholar] [CrossRef] [PubMed]
- Therrien, J.; Mason, C.J.; Cale, J.A.; Adams, A.; Aukema, B.H.; Currie, C.R.; Raffa, K.F.; Erbilgin, N. Bacteria influence mountain pine beetle brood development through interactions with symbiotic and antagonistic fungi: Implications for climate-driven host range expansion. Oecologia 2015, 179, 467–485. [Google Scholar] [CrossRef] [PubMed]
- Cale, J.A.; Collignon, R.M.; Klutsch, J.G.; Kanekar, S.S.; Hussain, A.; Erbilgin, N. Fungal volatiles can act as carbon sources and semiochemicals to mediate interspecific interactions among bark beetle-associated fungal symbionts. PLoS ONE 2016, 11, e0162197. [Google Scholar] [CrossRef] [PubMed]
- Zaman, R.; May, C.; Ullah, A.; Erbilgin, N. Bark beetles utilize ophiostomatoid fungi to circumvent host tree defenses. Metabolites 2023, 13, 239. [Google Scholar] [CrossRef] [PubMed]
- Crandall, L.; Zaman, R.; Duthie-Holt, M.; Jarvis, W.; Erbilgin, N. Navigating the semiochemical landscape: Attraction of subcortical beetle communities to bark beetle pheromones, fungal and host tree volatiles. Insects 2025, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Rozo, S.; Hussain, A.; Cale, J.A.; Klutsch, J.G.; Rajabzadeh, R.; Erbilgin, N. Nitrogen and ergosterol concentration varied in live jack pine phloem following inoculations with fungal associates of mountain pine beetle. Front. Microbiol. 2020, 11, 1703. [Google Scholar] [CrossRef] [PubMed]
- Agbulu, V.; Zaman, R.; Ishangulyyeva, G.; Cahill, J.F.; Erbilgin, N. Host defense metabolites alter the interactions between a bark beetle and its symbiotic fungi. Microb. Ecol. 2022, 84, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Six, D.L.; Paine, T.D. Effects of mycangial fungi and host tree species on progeny survival and emergence of Dendroctonus ponderosae (Coleoptera: Scolytidae). Environ. Entomol. 1998, 27, 1393–1401. [Google Scholar] [CrossRef]
- Ruangwong, O.U.; Wonglom, P.; Suwannarach, N.; Kumla, J.; Thaochan, N.; Chomnunti, P.; Pitija, K.; Sunpapao, A. Volatile organic compound from Trichoderma asperelloides TSU1: Impact on plant pathogenic fungi. J. Fungi 2021, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Gugliuzzo, A.; Aiello, D.; Biondi, A.; Giurdanella, G.; Siscaro, G.; Zappalà, L.; Vitale, A.; Garzia, G.T.; Polizzi, G. Microbial mutualism suppression by Trichoderma and Bacillus species for controlling the invasive ambrosia beetle Xylosandrus compactus. Biol. Control 2022, 170, 104929. [Google Scholar] [CrossRef]
- Lee, S.; Yap, M.; Behringer, G.; Hung, R.; Bennett, J.W. Volatile organic compounds emitted by Trichoderma species mediate plant growth. Fungal Biol. Biotechnol. 2016, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Nahrung, H.F.; Lawson, S.A.; Hayes, R.A. How effective are push–pull semiochemicals as deterrents for bark beetles? A global meta-analysis of thirty years of research. Insects 2023, 14, 812. [Google Scholar] [CrossRef] [PubMed]
- Erbilgin, N.; Christiansen, E.; Krokene, P. A host monoterpene influences Ips typographus responses (Coleoptera: Curculionidae, Scolytinae) to its aggregation pheromone: Implications for host colonization of bark beetles. Agric. For. Entomol. 2007, 9, 135–140. [Google Scholar] [CrossRef]
- Progar, R.A.; Blackford, D.C.; Cluck, D.R.; Costello, S.; Dunning, L.B.; Eager, T.; Jorgensen, C.L.; Munson, A.S.; Steed, B.; Rinella, M.J. Population densities and tree diameter effects associated with verbenone treatments to reduce mountain pine beetle-caused mortality of lodgepole pine. J. Econ. Entomol. 2013, 106, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Wilson, I.M.; Borden, J.H.; Gries, R.; Gries, G. Green leaf volatiles as antiaggregants for the mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Scolytidae). J. Chem. Ecol. 1996, 22, 1861–1875. [Google Scholar] [CrossRef] [PubMed]
- Borden, J.H.; Wilson, I.M.; Gries, R.; Chong, L.J.; Pierce, H.D., Jr.; Gries, G. Volatiles from the bark of trembling aspen, Populus tremuloides Michx.(Salicaceae) disrupt secondary attraction by the mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Scolytidae). Chemoecology 1998, 8, 69–75. [Google Scholar] [CrossRef]
- Borden, J.H.; Birmingham, A.L.; Burleigh, J.S. Evaluation of the push-pull tactic against the mountain pine beetle using verbenone and non-host volatiles in combination with pheromone-baited trees. For. Chron. 2006, 82, 579–590. [Google Scholar] [CrossRef]
- Gillette, N.E.; Munson, A.S. Semiochemical sabotage: Behavorial chemicals for protection of western conifers from bark beetles. In The Western Bark Beetle Research Group: A Unique Collaboration With Forest Health Protection, Proceedings of the 2007 Society of American Foresters Conference, Portland, OR, USA, 23–28 October 2007; Hayes, J.L., Lundquist, J.E., Eds.; Department of Agriculture, Forest Service, Pacific Northwest Research Station: Corvallis, OR, USA, 2009; pp. 85–109. [Google Scholar]
- Progar, R.A.; Fettig, C.J.; Munson, A.S.; Mortenson, L.A.; Snyder, C.L.; Kegley, S.J.; Cluck, D.R.; Steed, B.E.; Mafra-Neto, A.; Rinella, M.J. Comparisons of efficiency of two formulations of verbenone (4, 6, 6-trimethylbicyclo [3.1.1] hept-3-en-2-one) for protecting whitebark pine, Pinus albicaulis (Pinales: Pinaceae) from mountain pine beetle (Colopetera: Curculionidae). J. Econ. Entomol. 2021, 114, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Fettig, C.J.; Munson, A.S. Efficacy of verbenone and a blend of verbenone and nonhost volatiles for protecting lodgepole pine from mountain pine beetle (Coleoptera: Curculionidae). Agric. For. Entomol. 2020, 22, 373–378. [Google Scholar] [CrossRef]
- Frühbrodt, T.; Schebeck, M.; Andersson, M.N.; Holighaus, G.; Kreuzwieser, J.; Burzlaff, T.; Delb, H.; Biedermann, P.H.W. Verbenone—The universal bark beetle repellent? Its origin, effects, and ecological roles. J. Pest Sci. 2024, 97, 35–71. [Google Scholar] [CrossRef]
- Pureswaran, D.S.; Borden, J.H. New repellent semiochemicals for three species of Dendroctonus (Coleoptera: Scolytidae). Chemoecology 2004, 14, 67–75. [Google Scholar] [CrossRef]
- Pureswaran, D.S.; Gries, R.; Borden, J.H.; Pierce, H.D., Jr. Dynamics of pheromone production and communication in the mountain pine beetle, Dendroctonus ponderosae Hopkins, and the pine engraver, Ips pini (Say) (Coleoptera: Scolytidae). Chemoecology 2000, 10, 153–168. [Google Scholar] [CrossRef]
- Sullivan, B.T.; Dalusky, M.J.; Wakarchuk, D.; Berisford, C.W. Field evaluations of potential aggregation inhibitors for the southern pine beetle, Dendroctonus frontalis (Coleoptera: Curculionidae). J. Entomol. Sci. 2007, 42, 139–149. [Google Scholar] [CrossRef]
- Kandasamy, D.; Zaman, R.; Nakamura, Y.; Zhao, T.; Hartmann, H.; Andersson, M.N.; Hammerbacher, A.; Gershenzon, J. Conifer-killing bark beetles locate fungal symbionts by detecting volatile fungal metabolites of host tree resin monoterpenes. PLoS Biol. 2023, 21, e3001887. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, D.; Gershenzon, J.; Hammerbacher, A. Volatile organic compounds emitted by fungal associates of conifer bark beetles and their potential in bark beetle control. J. Chem. Ecol. 2016, 42, 952–969. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, R.W.; Cronin, J.T.; Klepzig, K.D.; Moser, J.C.; Ayres, M.P. Antagonisms, mutualisms and commensalisms affect outbreak dynamics of the southern pine beetle. Oecologia 2006, 147, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Wallin, K.F.; Raffa, K.F. Influences of host chemicals and internal physiology on the multiple steps of postlanding host acceptance behavior of Ips pini (Coleoptera: Scolytidae). Environ. Entomol. 2000, 29, 442–453. [Google Scholar] [CrossRef]
- Ullah, A.; Klutsch, J.G.; Erbilgin, N. Production of complementary defense metabolites reflects a co-evolutionary arms race between a host plant and a mutualistic bark beetle-fungal complex. Plant Cell Environ. 2021, 44, 3064–3077. [Google Scholar] [CrossRef] [PubMed]
- Sunesson, A.L.; Vaes, W.; Nilsson, C.; Blomquist, G.; Andersson, B.; Carlson, R. Identification of volatile metabolites from five fungal species cultivated on two media. Appl. Environ. Microbiol. 1995, 61, 2911–2918. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, R.; Hackett, C.; Bruce, A.; Kundzewicz, A. Effect of substrate composition on production of volatile organic compounds from Trichoderma spp. inhibitory to wood decay fungi. Int. Biodeterior. Biodegrad. 1997, 39, 199–205. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Bellemain, E.; Carlsen, T.; Brochmann, C.; Taberlet, P.; Kauserud, H. ITS as an environmental DNA barcode for fungi: An in silico approach reveals potential PCR biases. BMC Microbiol. 2010, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- White, T.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR-Protocols a Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninski, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Zaman, R.; Shah, A.; Shah, A.; Ullah, A.; Ishangulyyeva, G.; Erbilgin, N. Unraveling the multifaceted effects of climatic factors on mountain pine beetle and its interaction with fungal symbionts. Glob. Change Biol. 2024, 30, e17207. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.Z.; Fan, F.L.; Li, Y.L.; Liu, X.D.; Lu, Y.Q.; Song, A. Noncontact inhibitory of volatile organic compounds from rice root bacteria on Rhizopus microsporus. Sci. Agric. Sin. 2020, 53, 1986–1996. [Google Scholar] [CrossRef]
- Humphris, S.N.; Wheatley, R.E.; Bruce, A. The effects of specific volatile organic compounds produced by Trichoderma. Fungal Biol. Biotechnol. 2001, 55, 233–237. [Google Scholar] [CrossRef]
- Jeleń, H.; Błaszczyk, L.; Chełkowski, J.; Rogowicz, K.; Strakowska, J. Formation of 6-n-pentyl-2H-pyran-2-one (6-PAP) and other volatiles by different Trichoderma species. Mycol. Prog. 2014, 13, 589–600. [Google Scholar] [CrossRef]
- Garnica-Vergara, A.; Barrera-Ortiz, S.; Muñoz-Parra, E.; Raya-González, J.; Méndez-Bravo, A.; Macías-Rodríguez, L.; Ruiz-Herrera, L.F.; López-Bucio, J. The volatile 6-pentyl-2H-pyran-2-one from Trichoderma atroviride regulates Arabidopsis thaliana root. New Phytol. 2016, 209, 1496–1512. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.P.; Gries, R.; Borden, J.H.; Pierce, H.D., Jr. A survey of antennal responses by five species of coniferophagous bark beetles (Coleoptera: Scolytidae) to bark volatiles of six species of angiosperm trees. Chemoecology 2000, 10, 103–113. [Google Scholar] [CrossRef]
- Unelius, C.R.; Schiebe, C.; Bohman, B.; Andersson, M.N.; Schlyter, F. Non-host volatile blend optimization for forest protection against the European spruce bark beetle, Ips typographus. PLoS ONE 2014, 9, e85381. [Google Scholar] [CrossRef] [PubMed]
- Brand, J.M.; Schultz, J.; Barras, S.J.; Edson, L.J.; Payne, T.L.; Hedden, R.L. Bark-beetle pheromones: Enhancement of Dendroctonus frontalis (Coleoptera: Scolytidae) aggregation pheromone by yeast metabolites in laboratory bioassays. J. Chem. Ecol. 1977, 3, 657–666. [Google Scholar] [CrossRef]
- Birgersson, G.; Schlyter, F.; Löfqvist, J.; Bergström, G. Quantitative variation of pheromone components in the spruce bark beetle Ips typographus from different attack phases. J. Chem. Ecol. 1984, 10, 1029–1055. [Google Scholar] [CrossRef] [PubMed]
- Jirošová, A.; Modlinger, R.; Hradecký, J.; Ramakrishnan, R.; Beránková, K.; Kandasamy, D. Ophiostomatoid fungi synergize attraction of the Eurasian spruce bark beetle, Ips typographus to its aggregation pheromone in field traps. Front. Microbiol. 2022, 13, 980251. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Rajarao, G.K.; Nordenhem, H.; Nordlander, G.; Borg-Karlson, A.K. Penicillium expansum volatiles reduce pine weevil attraction to host plants. J. Chem. Ecol. 2013, 39, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Schiebe, C.; Birgersson, G.; Jankuvova, J.; Brodelius, P.E.; Schlyter, F.; Hansson, B.S. Styrene, (+)-trans-(1R,4S,5S)-4-thujanol and oxygenated monoterpenes related to host stress elicit strong electrophysiological responses in the bark beetle Ips typographus. J. Chem. Ecol. 2019, 45, 474–489. [Google Scholar] [CrossRef] [PubMed]
- Gnanasunderam, C.; Young, H.; Hutchins, R. Defensive secretions of New Zealand tenebrionids V. Presence of methyl ketones in Uloma tenebrionoides (Coleoptera: Tenebrionidae). J. Chem. Ecol. 1985, 11, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Germinara, G.S.; De Cristofaro, A.; Rotundo, G. Bioactivity of short-chain aliphatic ketones against adults of the granary weevil, Sitophilus granarius (L.). Pest Manag. Sci. 2012, 68, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.K.; Blum, M.S.; Roncadori, R.W. Antifungal properties of the insect alarm pheromones, citral, 2-heptanone, and 4-methyl-3-heptanone. Mycologia 1975, 67, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Molander, M.A.; Eriksson, B.; Winde, I.B.; Zou, Y.; Millar, J.G.; Larsson, M.C. The aggregation-sex pheromones of the cerambycid beetles Anaglyptus mysticus and Xylotrechus antilope ssp. antilope: New model species for insect conservation through pheromone-based monitoring. Chemoecology 2019, 29, 111–124. [Google Scholar] [CrossRef]
- Axelsson, K.; Konstanzer, V.; Rajarao, G.K.; Terenius, O.; Seriot, L.; Nordenhem, H.; Nordlander, G.; Borg-Karlson, A.K. Antifeedants produced by bacteria associated with the gut of the pine weevil Hylobius abietis. Microb. Ecol. 2017, 74, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhou, Y.; Jiang, D.; Lü, Y.; Liu, Y.; Yu, M.; Zhang, A.; Yan, S. Evaluation of trap efficiency for the Asian longhorned beetle, Anoplophora glabripennis. J. For. Res. 2023, 34, 1133–1144. [Google Scholar] [CrossRef]
- Lyu, F.; Hai, X.; Wang, Z. A review of the host plant location and recognition mechanisms of Asian longhorn beetle. Insects 2023, 14, 292. [Google Scholar] [CrossRef] [PubMed]
- Monte, E. Understanding Trichoderma: Between biotechnology and microbial ecology. Int. Microbiol. 2001, 4, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Rashad, Y.M.; Moussa, T.A.A. Biocontrol agents for fungal plant diseases management. In Cottage Industry of Biocontrol Agents and Their Applications; El-Wakeil, N., Saleh, M., Abu-hashim, M., Eds.; Springer: Cham, Germany, 2020; pp. 337–363. [Google Scholar] [CrossRef]
- El-Benawy, N.M.; Abdel-Fattah, G.M.; Ghoneem, K.M.; Shabana, Y.M. Antimicrobial activities of Trichoderma atroviride against common bean seed-borne Macrophomina phaseolina and Rhizoctonia solani. Egypt. J. Basic Appl. Sci. 2020, 7, 267–280. [Google Scholar] [CrossRef]
- Parker, S.R.; Cutler, H.G.; Jacyno, J.M.; Hill, R.A. Biological activity of 6-pentyl-2 H-pyran-2-one and its analogs. J. Agric. Food Chem. 1997, 45, 2774–2776. [Google Scholar] [CrossRef]
- Zhou, H.; Tao, N.; Jia, L. Antifungal activity of citral, octanal and α-terpineol against Geotrichum citri-aurantii. Food Control 2014, 37, 277–283. [Google Scholar] [CrossRef]
- Di Francesco, A.; Ugolini, L.; Lazzeri, L.; Mari, M. Production of volatile organic compounds by Aureobasidium pullulans as a potential mechanism of action against postharvest fruit pathogens. Biol. Control 2015, 81, 8–14. [Google Scholar] [CrossRef]
- Jin, X.; Guo, L.; Jin, B.; Zhu, S.; Mei, X.; Wu, J.; Liu, T.; He, X. Inhibitory mechanism of 6-Pentyl-2H-pyran-2-one secreted by Trichoderma atroviride T2 against Cylindrocarpon destructans. Pestic. Biochem. Physiol. 2020, 170, 104683. [Google Scholar] [CrossRef] [PubMed]
- Yalage Don, S.M.; Schmidtke, L.M.; Gambetta, J.M.; Steel, C.C. Aureobasidium pullulans volatilome identified by a novel, quantitative approach employing SPME-GC-MS, suppressed Botrytis cinerea and Alternaria alternata in vitro. Sci. Rep. 2020, 10, 4498. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhu, X.; Xie, Y.; Liang, J. Antifungal properties and mechanisms of three volatile aldehydes (octanal, nonanal and decanal) on Aspergillus flavus. Grain Oil Sci. Technol. 2021, 4, 131–140. [Google Scholar] [CrossRef]
- Borden, J.H.; Ryker, L.C.; Chong, L.J.; Pierce, H.D., Jr.; Johnston, B.D.; Oehlschlager, A.C. Response of the mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Scolytidae), to five semiochemicals in British Columbia lodgepole pine forests. Can. J. For. Res. 1987, 17, 118–128. [Google Scholar] [CrossRef]
- Miller, D.R.; Borden, J.H. Dose-dependent and species-specific responses of pine bark beetles (Coleoptera: Scolytidae) to monoterpenes in association with pheromones. Can. Entomol. 2000, 132, 183–195. [Google Scholar] [CrossRef]
- Zhao, T.; Krokene, P.; Hu, J.; Christiansen, E.; Björklund, N.; Långström, B.; Solheim, H.; Borg-Karlson, A.K. Induced terpene accumulation in Norway spruce inhibits bark beetle colonization in a dose-dependent manner. PLoS ONE 2011, 6, e26649. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.R. Ethanol: Dose-dependent flight responses of bark and woodboring beetles, and associated species of Coleoptera. Environ. Entomol. 2025, 10, nvaf032. [Google Scholar] [CrossRef] [PubMed]
- Hung, R.; Lee, S.; Bennett, J.W. Fungal volatile organic compounds and their role in ecosystems. Appl. Microbiol. Biotechnol. 2015, 99, 3395–3405. [Google Scholar] [CrossRef] [PubMed]
- El Jaddaoui, I.; Rangel, D.E.; Bennett, J.W. Fungal volatiles have physiological properties. Fungal Biol. 2023, 127, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Guo, H.; Zhang, K.; Zhao, M.; Ruan, J.; Chen, J. Trichoderma and its role in biological control of plant fungal and nematode disease. Front. Microbiol. 2023, 14, 1160551. [Google Scholar] [CrossRef] [PubMed]



| Anti-Attractant Compounds | Source of Compounds | Function | References | |
|---|---|---|---|---|
| Non-host volatiles | Verbenone with a blend of non-host volatiles (1-Hexanol, E-2- & Z-3-hexen-1-ol and benzyl alcohol) | Non-host volatiles from aspen bark | Significantly reduced catches on baited trees and funnel traps. Did not affect clerid predators. | [17,18,19] |
| Con-specific volatiles | Verbenone | MPBs | Most effective known anti-attractant for MPBs and other bark beetle species. Mixed results in some applications. | [16,20,21,22,23] |
| 1-Octen-3-ol | Female MPBs | Decreased response to pheromones. | [24] | |
| Phenylethyl alcohol | Male MPBs | Decreased response to pheromones. | [25,26] | |
| Hetero-specific volatiles | 3-methyl-2-cyclohexen-1-one | Anti-aggregation pheromone of D. pseudotsugae and D. rufipennis | Decreased response to pheromones. | [24] |
| Isolate Source | Isolate Label | Species | Identity | Growth on PDA (Days) | Growth on Diet Media (Days) |
|---|---|---|---|---|---|
| NFC | Trichoderma 1416 | T. atroviride | 100% | 3 | 4 |
| Lodgepole pine phloem | Trichoderma YL145 | T. atroviride | 100% | 3 | 4 |
| Lab collection | Grosmannia Clavigera EL035 | G. clavigera | 100% | N/A | 5 |
| Volatile Compounds | PDA | PS |
|---|---|---|
| Mean (SE) | Mean (SE) | |
| 2-Methyl-1-butanol | 375.06 (92.5) | 21.71 (3.7) |
| 3-Methyl-1-butanol | 1126.21 (325.4) | 343.25 (55.8) |
| Acetoin | 38.92 (8.9) | 10.95 (3.9) |
| 1-Hexanol | 2.12 (1.2) | 3.37 (1.3) |
| 6-Pentyl-2H-pyran-2-one | 167.29 (35.6) | 2.37 (1.2) |
| Ethyl acetate | 33.59 (8.1) | 0.00 (0.0) |
| 2-Methyl-1-propanol | 691.99 (122.7) | 0.00 (0.0) |
| Isobutyl acetate | 11.97 (2.9) | 0.00 (0.0) |
| Octanal | 0.81 (0.5) | 0.00 (0.0) |
| Phenylethyl alcohol | 83.58 (17.1) | 0.00 (0.0) |
| 2-Methyl-2-butanol | 0.00 (0.0) | 1.38 (0.7) |
| Styrene | 0.00 (0.0) | 0.61 (0.4) |
| 2-Pentanone | 0.00 (0.0) | 46.21 (6.9) |
| 2-Furanmethanol | 0.00 (0.0) | 4.19 (1.2) |
| 2-Heptanone | 0.00 (0.0) | 16.11 (2.5) |
| 2-Octanone | 0.00 (0.0) | 0.75 (0.2) |
| 5-Methyl-2-hexanone | 0.00 (0.0) | 0.58 (0.2) |
| 4-(1-Methylethyl)-cyclohexanol | 0.00 (0.0) | 5.41 (2.1) |
| 2-Pentanol | 0.00 (0.0) | 17.74 (3.5) |
| Treatment Combination | Df | p-Value | |
|---|---|---|---|
| Control vs. G. clavigera | 1 | 9.8 | 0.002 * |
| Control vs. T. atroviride | 1 | 10 | 0.002 * |
| G. clavigera vs. T. atroviride | 1 | 8.1 | 0.004 * |
| Time | Measurement | Df | p-Value | |
|---|---|---|---|---|
| 24 h | Number of branches | 3 | 7.55 | 0.06 |
| Longest tunnel segment (mm) | 3 | 0.88 | 0.83 | |
| Total tunneling length (mm) | 3 | 4.63 | 0.20 | |
| 48 h | Number of branches | 3 | 9.09 | 0.03 * |
| Longest tunnel segment (mm) | 3 | 4.36 | 0.23 | |
| Total tunneling length (mm) | 3 | 5.43 | 0.14 |
| Identified FVOCs | Present in PDA | Present in PS | Previous Findings | References |
|---|---|---|---|---|
| 2-Methyl-1-butanol | x | x | Detected from MPB symbiotic fungi. Synergized attraction of MPB to its aggregation pheromones. Detected from Trichoderma sp. Inhibited wood decay fungal growth at high concentrations. | [6,7,39] |
| 6-Pentyl-2H-pyran-2-one | x | x | Detected from FVOC of Trichoderma atroviride. Inhibited growth of several phytopathogenetic fungi. | [40,41] |
| 3-Methyl-1-butanol | x | x | Detected from T. atroviride. Detected from MPB symbiotic fungi. | [6,13] |
| Acetoin | x | x | Detected from T. atroviride. Detected from MPB symbiotic fungi. | [6,13] |
| 1-Hexanol | x | x | Detected from of Trichoderma sp. Antennal response in MPBs. When included in a blend of GLVs, it acted as an anti-attractant for MPBs and Ips typographus. | [17,18,33,42,43] |
| Phenylethyl alcohol | x | Decreased MPB response to pheromones. A pheromone component of I. typographus. Acetate ester 2-phenylethyl acetate produced by several bark beetle symbionts and found to attract Dendroctonus frontalis. Decreased D. frontalis attraction to pheromones. Highest concentration in the FVOC profile of Leptographium longicalvatum compared to other MPB symbiotic fungi. | [6,25,26,28,44,45,46,47] | |
| 2-Methyl-2-butanol | x | Detected from MPB symbiotic fungi. | [6] | |
| Styrene | x | Strong antennal response in I. typographus. Induced defense chemical of Picea abies. Extracted from Penicillium expansum found in Hylobius abietis frass and reduced H. abietis attraction to host tissues. | [47,48] | |
| 2-Pentanone | x | Component of defensive secretions from Uloma tenebrionoides. Reduced Sitophilus granarius orientation towards food source (wheat grains). | [49,50] | |
| 2-Heptanone | x | Produced in trace amounts by male Anaglyptus mysticus (Cerambycidae) beetles. Reduced S. granarius orientation towards food source (wheat grains). Alarm pheromone in various insects, including ants. | [50,51,52] | |
| 2-Octanone | x | Volatile from Rahnella aquatilis bacteria isolated from H. abietis guts with antifeedant properties for the beetle. | [53] | |
| 2-Pentanol | x | Green leaf volatile and increased Anoplophora glabripennis attraction to pheromones in field experiments. | [54,55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crandall, L.; Zaman, R.; Ishangulyyeva, G.; Erbilgin, N. Advancing Semiochemical Tools for Mountain Pine Beetle Management: Dendroctonus ponderosae Responses to Saprophytic Fungal Volatiles. Metabolites 2025, 15, 488. https://doi.org/10.3390/metabo15070488
Crandall L, Zaman R, Ishangulyyeva G, Erbilgin N. Advancing Semiochemical Tools for Mountain Pine Beetle Management: Dendroctonus ponderosae Responses to Saprophytic Fungal Volatiles. Metabolites. 2025; 15(7):488. https://doi.org/10.3390/metabo15070488
Chicago/Turabian StyleCrandall, Leah, Rashaduz Zaman, Guncha Ishangulyyeva, and Nadir Erbilgin. 2025. "Advancing Semiochemical Tools for Mountain Pine Beetle Management: Dendroctonus ponderosae Responses to Saprophytic Fungal Volatiles" Metabolites 15, no. 7: 488. https://doi.org/10.3390/metabo15070488
APA StyleCrandall, L., Zaman, R., Ishangulyyeva, G., & Erbilgin, N. (2025). Advancing Semiochemical Tools for Mountain Pine Beetle Management: Dendroctonus ponderosae Responses to Saprophytic Fungal Volatiles. Metabolites, 15(7), 488. https://doi.org/10.3390/metabo15070488







