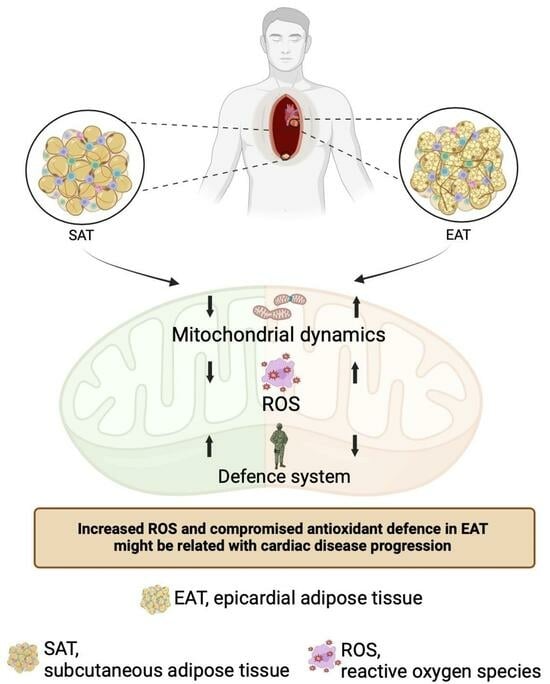
Enhanced Mitochondrial Dynamics and Reactive Oxygen Species Levels with Reduced Antioxidant Defenses in Human Epicardial Adipose Tissue
Abstract
1. Introduction
2. Materials and Methods
2.1. Adipose Tissue Donors
2.2. Adipose Tissue Gene Expression
2.3. Adipose Tissue Protein Expression
2.4. Oxidative Stress Quantification
2.4.1. Reactive Oxygen Species Evaluation
2.4.2. Lipid Peroxidation
2.4.3. Reduction and Oxidation of Glutathione
2.4.4. Glutathione Peroxidase Activation
2.4.5. Glutathione Reductase Activation
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
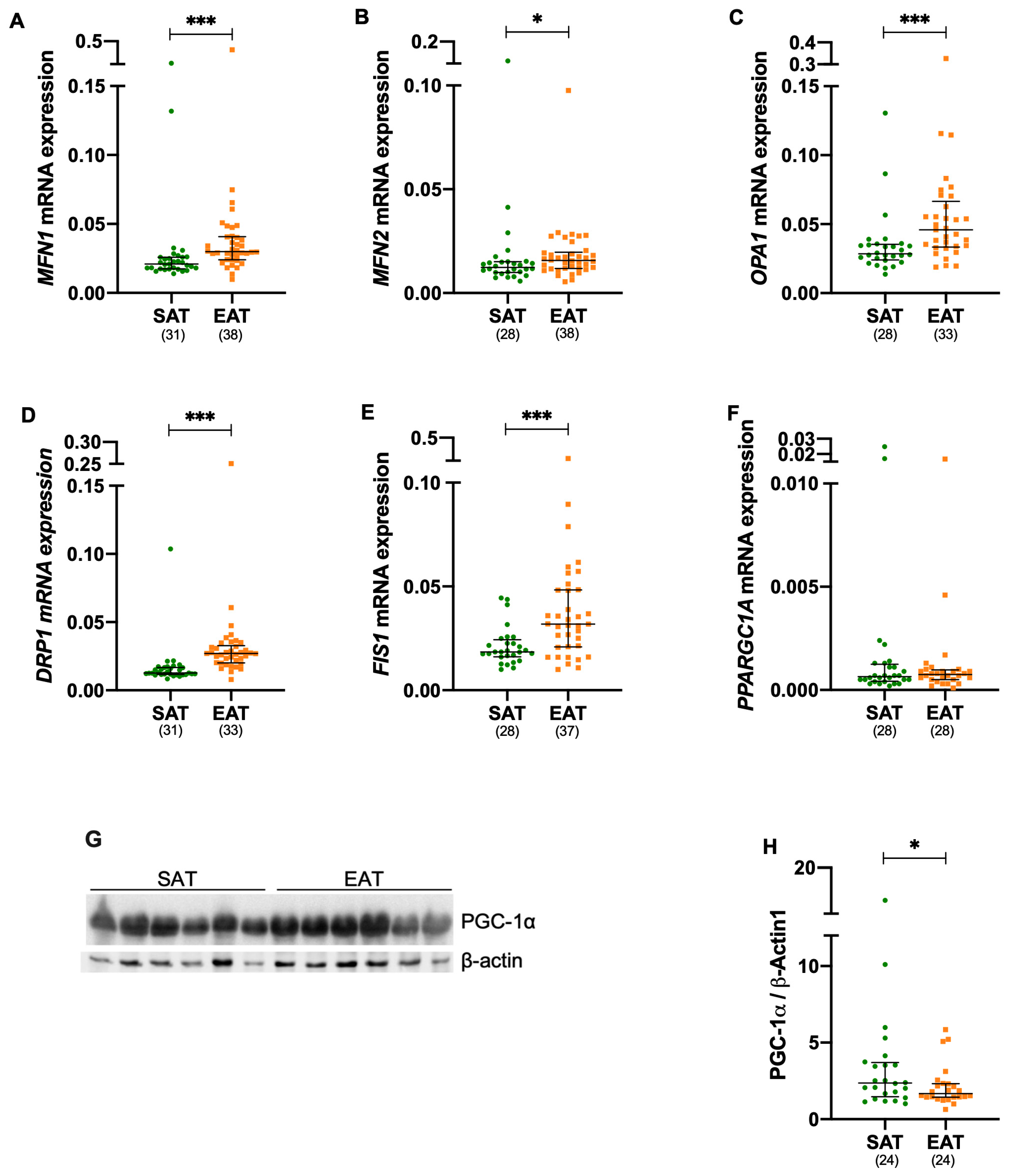
3.2. Mitochondrial Fusion and Fission Are Increased in Epicardial Adipose Tissue
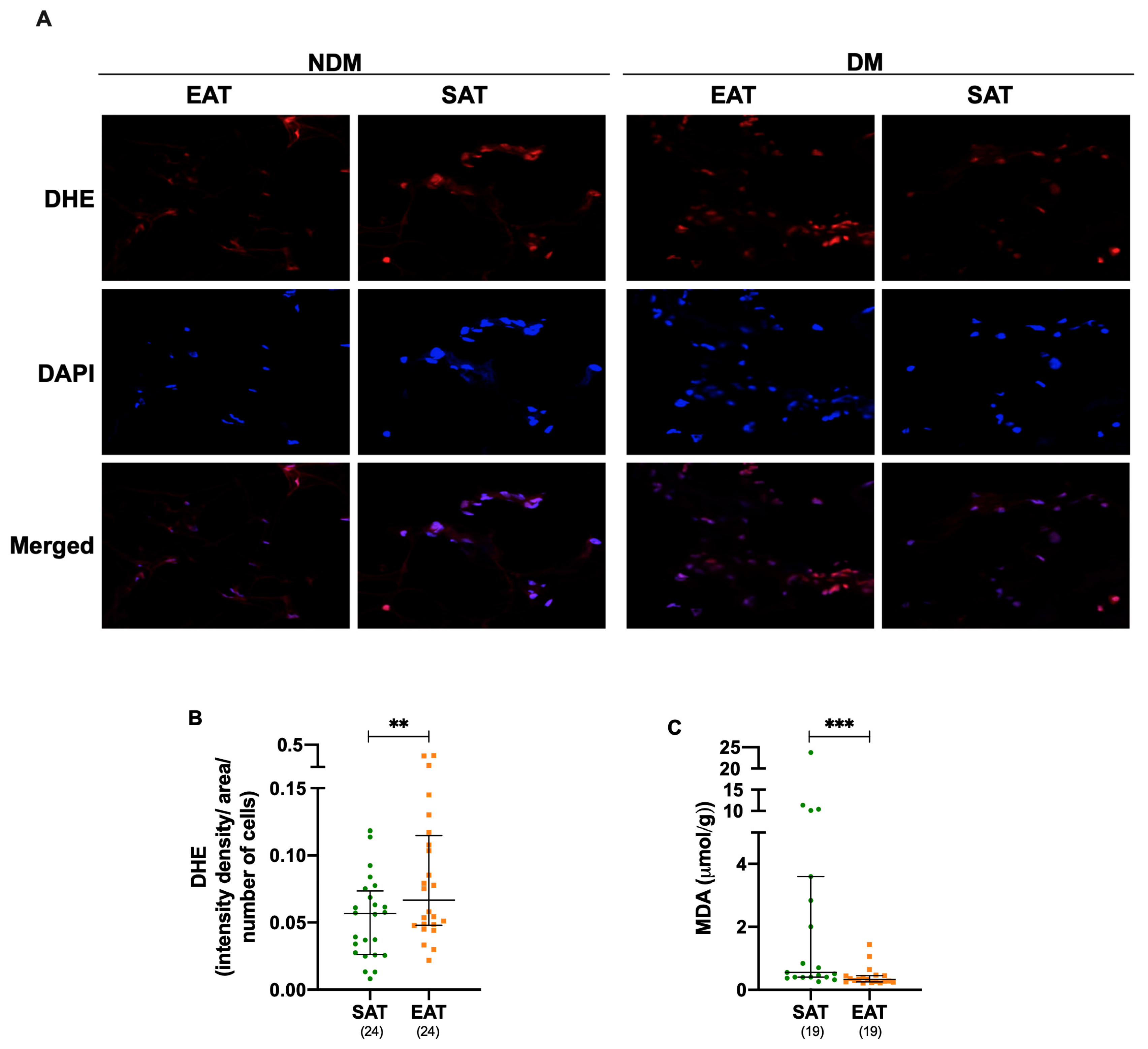
3.3. Oxidative Stress Is Increased in Epicardial Adipose Tissue Under Cardiac Disease
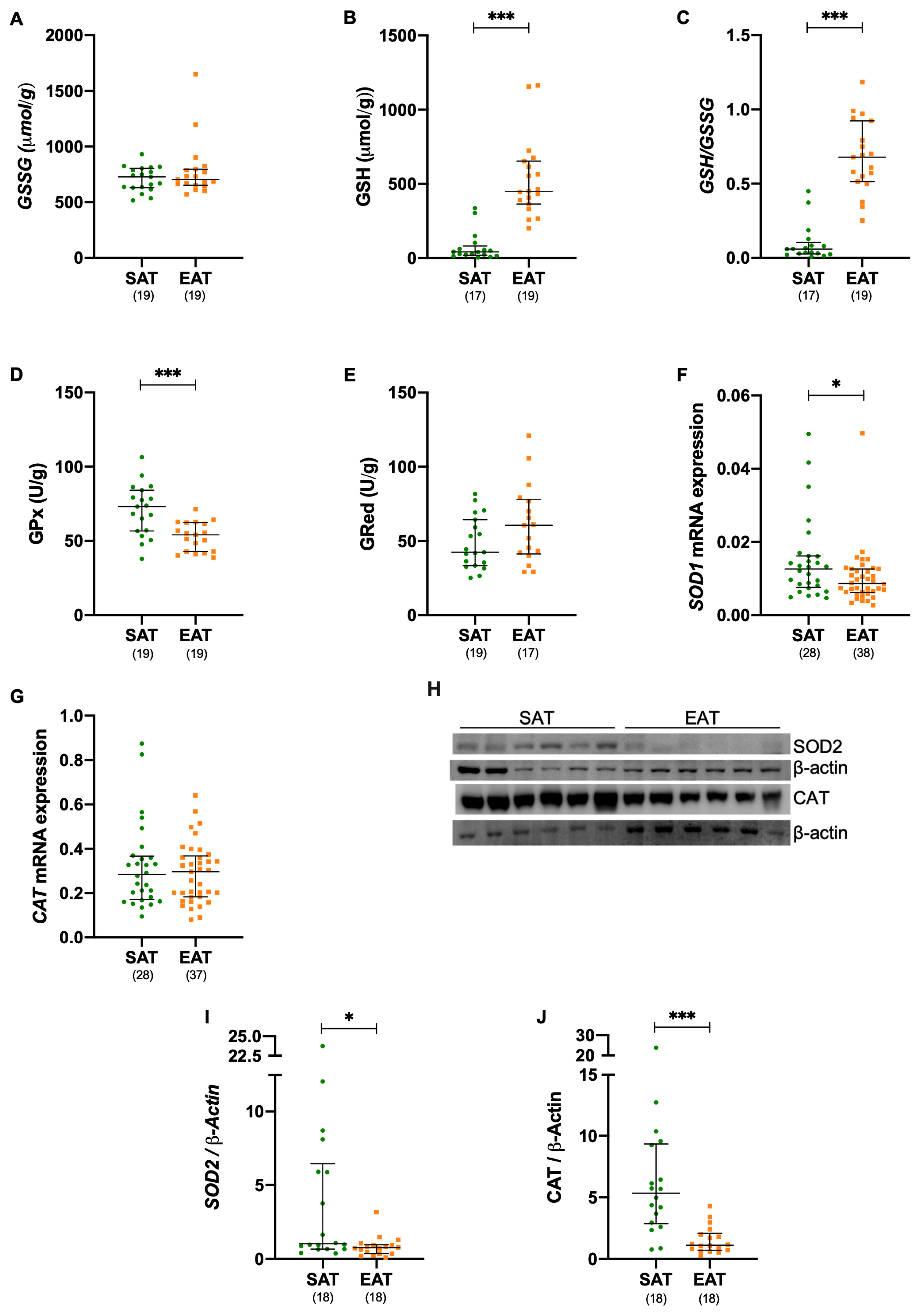
3.4. Antioxidant Defenses Are Compromised in Epicardial Adipose Tissue of Patients Elected for Cardiac Surgery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACEI | Angiotensin-converting enzyme inhibitor |
| ARB | Angiotensin II receptor blocker |
| BMI | Body mass index |
| BAT | Brown adipose tissue |
| CAT | Catalase |
| CABG | Coronary artery bypass grafting |
| CAD | Coronary artery disease |
| CVD | Cardiovascular disease |
| DHE | Dihydriethidine |
| DM | Diabetic |
| DPP-4 | Dipeptidyl peptidase-4 |
| DRP1 | Dynamin-1-like protein |
| EAT | Epicardial adipose tissue |
| FIS1 | Fission protein 1 |
| GLUT4 | Glucose transporter proteins 4 |
| GPx | Glutathione peroxidase |
| GRed | Glutathione reductase |
| GSSG | Oxidized glutathione |
| GSH | Reduced glutathione |
| MDA | Malondialdehyde |
| MNF1 | Mitofusin 1 |
| MNF2 | Mitofusin 2 |
| OCT | Optimal cutting temperature |
| OPA1 | Optic atrophy 1 |
| PGC1a | Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha |
| ROS | Reactive oxygen species |
| SAT | Subcutaneous adipose tissue |
| SOD | Superoxide dismutase |
| Ta | Annealing temperature |
| UPR | Unfold protein response |
References
- Balakumar, P.; Maung-U, K.; Jagadeesh, G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol. Res. 2016, 113, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Whaley-Connell, A.; Sowers, J.R. Diabetic cardiomyopathy: A hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia 2018, 61, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.X.; Ma, X.N.; Guan, C.H.; Li, Y.D.; Mauricio, D.; Fu, S.B. Cardiovascular disease in type 2 diabetes mellitus: Progress toward personalized management. Cardiovasc. Diabetol. 2022, 21, 74. [Google Scholar] [CrossRef] [PubMed]
- Marchington, J.M.; Mattacks, C.A.; Pond, C.M. Adipose tissue in the mammalian heart and pericardium; structure, fetal development and biochemical properties. Comp. Biochem. Physiol. 1989, 94, 225–232. [Google Scholar]
- Gaborit, B.; Sengenes, C.; Ancel, P.; Jacquier, A.; Dutour, A. Role of Epicardial Adipose Tissue in Health and Disease: A Matter of Fat? Compr. Physiol. 2017, 7, 1051–1082. [Google Scholar] [CrossRef] [PubMed]
- Sacks, H.S.; Fain, J.N.; Bahouth, S.W.; Ojha, S.; Frontini, A.; Budge, H.; Cinti, S.; Symonds, M.E. Adult epicardial fat exhibits beige features. J. Clin. Endocrinol. Metab. 2013, 98, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Chechi, K.; Blanchard, P.G.; Mathieu, P.; Deshaies, Y.; Richard, D. Brown fat like gene expression in the epicardial fat depot correlates with circulating HDL-cholesterol and triglycerides in patients with coronary artery disease. Int. J. Cardiol. 2013, 167, 2264–2270. [Google Scholar] [CrossRef] [PubMed]
- Burgeiro, A.; Fonseca, A.C.; Espinoza, D.; Carvalho, L.; Lourenço, N.; Antunes, M.; Carvalho, E. Proteostasis in epicardial versus subcutaneous adipose tissue in heart failure subjects with and without diabetes. Biochim. Biophys. Acta. Mol. Basis Dis. 2018, 1864, 2183–2198. [Google Scholar] [CrossRef] [PubMed]
- Burgeiro, A.; Fuhrmann, A.; Cherian, S.; Espinoza, D.; Jarak, I.; Carvalho, R.A.; Loureiro, M.; Patrício, M.; Antunes, M.; Carvalho, E. Glucose uptake and lipid metabolism are impaired in epicardial adipose tissue from heart failure patients with or without diabetes. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E550–E564. [Google Scholar] [CrossRef] [PubMed]
- Ansaldo, A.M.; Montecucco, F.; Sahebkar, A.; Dallegri, F.; Carbone, F. Epicardial adipose tissue and cardiovascular diseases. Int. J. Cardiol. 2019, 278, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.B.; Hausenloy, D.J. Mitochondrial morphology and cardiovascular disease. Cardiovasc. Res. 2010, 88, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Lesnefsky, E.J.; Chen, Q.; Hoppel, C.L. Mitochondrial Metabolism in Aging Heart. Circ. Res. 2016, 118, 1593–1611. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. Mitochondrial genetic medicine. Nat. Genet. 2018, 50, 1642–1649. [Google Scholar] [CrossRef] [PubMed]
- Bombicino, S.S.; Iglesias, D.E.; Mikusic, I.A.R.; D’Annunzio, V.; Gelpi, R.J.; Boveris, A.; Valdez, L.B. Diabetes impairs heart mitochondrial function without changes in resting cardiac performance. Int. J. Biochem. Cell Biol. 2016, 81, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-R.; Zweier, J.L. Cardiac mitochondria and reactive oxygen species generation. Circ. Res. 2014, 114, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, H.N. Targeting mitochondrial dysfunction in the treatment of heart failure. Expert Rev. Cardiovasc. Ther. 2016, 14, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Lkhagva, B.; Kao, Y.H.; Lee, T.I.; Lee, T.W.; Cheng, W.L.; Chen, Y.J. Activation of Class I histone deacetylases contributes to mitochondrial dysfunction in cardiomyocytes with altered complex activities. Epigenetics 2018, 13, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Houston, B. A comprehensive review of the bioenergetics of fatty acid and glucose metabolism in the healthy and failing heart in nondiabetic condition. Heart Fail. Rev. 2017, 22, 825–842. [Google Scholar] [CrossRef] [PubMed]
- Antunes, P.E.; de Oliveira, J.F.; Antunes, M.J. Risk-prediction for postoperative major morbidity in coronary surgery. Eur. J. Cardiothorac Surg. 2009, 35, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Pastore, A.; Federici, G.; Bertini, E.; Piemonte, F. Analysis of glutathione: Implication in redox and detoxification. Clin. Chim. Acta 2003, 333, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Goldberg, D.; Spooner, R. 1983 Assay of glutathione reductase. In Methods of Enzymatic Analysis, 3rd ed.; Bergmeyer, H.U., Ed.; Verlog Cheme: Deerfiled Beach, FL, USA, 1986; Volume 3, pp. 256–258. [Google Scholar]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World, J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.; Jansson, P.A.; Nagaev, I.; Wenthzel, A.M.; Smith, U. Insulin resistance with low cellular IRS-1 expression is also associated with low GLUT4 expression and impaired insulin-stimulated glucose transport. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2001, 15, 1101–1103. [Google Scholar]
- Carvalho, E.; Eliasson, B.; Wesslau, C.; Smith, U. Impaired phosphorylation and insulin-stimulated translocation to the plasma membrane of protein kinase B/Akt in adipocytes from type II diabetic subjects. Diabetologia 2000, 43, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Amilburu, V.; Jong-Raadsen, S.; Bakkers, J.; Spaink, H.P.; Marín-Juez, R. GLUT12 deficiency during early development results in heart failure and a diabetic phenotype in zebrafish. J. Endocrinol. 2015, 224, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Song, R.; Feng, Y.; Guo, J.; Chen, Y.; Zhang, Y.; Chen, T.; Wang, Y.; Huang, Y.; Li, C.Y.; et al. Upregulation of MG53 induces diabetic cardiomyopathy through transcriptional activation of peroxisome proliferation-activated receptor α. Circulation 2015, 131, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Tilokani, L.; Nagashima, S.; Paupe, V.; Prudent, J. Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem. 2018, 62, 341–360. [Google Scholar] [CrossRef] [PubMed]
- Saotome, M.; Ikoma, T.; Hasan, P.; Maekawa, Y. Cardiac insulin resistance in heart failure: The role of mitochondrial dynamics. Int. J. Mol. Sci. 2019, 20, 3552. [Google Scholar] [CrossRef] [PubMed]
- Otera, H.; Wang, C.; Cleland, M.M.; Setoguchi, K.; Yokota, S.; Youle, R.J.; Mihara, K. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J. Cell Biol. 2010, 191, 1141–1158. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef] [PubMed]
- Javadov, S.; Rajapurohitam, V.; Kilić, A.; Hunter, J.C.; Zeidan, A.; Said Faruq, N.; Escobales, N.; Karmazyn, M. Expression of mitochondrial fusion-fission proteins during post-infarction remodeling: The effect of NHE-1 inhibition. Basic Res. Cardiol. 2011, 106, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Islam, H.; Edgett, B.A.; Gurd, B.J. Coordination of mitochondrial biogenesis by PGC-1α in human skeletal muscle: A re-evaluation. Metabolism 2018, 79, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Ost, M.; Keipert, S.; Klaus, S. Targeted mitochondrial uncoupling beyond UCP1–The fine line between death and metabolic health. Biochimie 2017, 134, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Naryzhnaya, N.V.; Koshelskaya, O.A.; Kologrivova, I.V.; Suslova, T.E.; Kharitonova, O.A.; Andreev, S.L.; Gorbunov, A.S.; Kurbatov, B.K.; Boshchenko, A.A. Production of Reactive Oxygen Species by Epicardial Adipocytes Is Associated with an Increase in Postprandial Glycemia, Postprandial Insulin, and a Decrease in Serum Adiponectin in Patients with Severe Coronary Atherosclerosis. Biomedicines 2022, 10, 2054. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, A.A.; Chen, L.; Malik, Z.A. Heart failure and mitochondrial dysfunction: The role of mitochondrial fission/fusion abnormalities and new therapeutic strategies. J Cardiovasc. Pharmacol. 2014, 63, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Yang, Y.; Zhao, H.; Li, X.; Yang, D.; Li, D.; Yang, Y. The Role of Mitochondrial Functional Proteins in ROS Production in Ischemic Heart Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 5470457. [Google Scholar] [CrossRef] [PubMed]
- Emelyanova, L.; Ashary, Z.; Cosic, M.; Negmadjanov, U.; Ross, G.; Rizvi, F.; Olet, S.; Kress, D.; Sra, J.; Tajik, A.J.; et al. Selective downregulation of mitochondrial electron transport chain activity and increased oxidative stress in human atrial fibrillation. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H54–H63. [Google Scholar] [CrossRef] [PubMed]
- Oyewole, A.O.; Birch-Machin, M.A. Mitochondria-targeted antioxidants. FASEB J. 2015, 29, 4766–4771. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, M.; Khemka, V.K.; Chatterjee, G.; Ganguly, A.; Mukhopadhyay, S.; Chakrabarti, S. Enhanced ROS production and oxidative damage in subcutaneous white adipose tissue mitochondria in obese and type 2 diabetes subjects. Mol. Cell. Biochem. 2015, 399, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.B.; Shah, S.; Verma, S.; Oudit, G.Y. Epicardial adipose tissue as a metabolic transducer: Role in heart failure and coronary artery disease. Heart Fail. Rev. 2017, 22, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Yin, Y.; Ma, X.; Zhang, J.; Pan, W.; Tan, M.; Zhao, Y.; Yang, T.; Jiang, T.; Li, H. Glutathione system enhancement for cardiac protection: Pharmacological options against oxidative stress and ferroptosis. Cell Death Dis. 2023, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Walton, P.A.; Brees, C.; Lismont, C.; Apanasets, O.; Fransen, M. The peroxisomal import receptor PEX5 functions as a stress sensor, retaining catalase in the cytosol in times of oxidative stress. Biochim. Biophys. Acta. Mol. Cell Res. 2017, 1864, 1833–1843. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Somoza, A.; Teijeira-Fernández, E.; Luis Fernández, Á.; Ramón González-Juanatey, J.; Eiras, S. Proteomic analysis of epicardial and subcutaneous adipose tissue reveals differences in proteins involved in oxidative stress. Am. J. Physiol.-Heart Circ. Physiol. 2010, 299, H202–H209. [Google Scholar] [CrossRef] [PubMed]
- Rapti, K.; Diokmetzidou, A.; Kloukina, I.; Milner, D.J.; Varela, A.; Davos, C.H.; Capetanaki, Y. Opposite effects of catalase and MnSOD ectopic expression on stress induced defects and mortality in the desmin deficient cardiomyopathy model. Free. Radic. Biol. Med. 2017, 110, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Saotome, M.; Nobuhara, M.; Sakamoto, A.; Urushida, T.; Katoh, H.; Satoh, H.; Funaki, M.; Hayashi, H. Roles of mitochondrial fragmentation and reactive oxygen species in mitochondrial dysfunction and myocardial insulin resistance. Exp. Cell Res. 2014, 323, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, Z.; Nagy, A.; Jahner, K.; Szabados, E. Impact of Selected Glucagon-like Peptide-1 Receptor Agonists on Serum Lipids, Adipose Tissue, and Muscle Metabolism—A Narrative Review. Int. J. Mol. Sci. 2024, 25, 8214. [Google Scholar] [CrossRef] [PubMed]
- Lauritsen, K.M.; Voigt, J.H.; Pedersen, S.B.; Hansen, T.K.; Møller, N.; Jessen, N.; Gormsen, L.C.; Søndergaard, E. Effects of SGLT2 inhibition on lipid transport in adipose tissue in type 2 diabetes. Endocr. Connect. 2022, 11, e210558. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, M.; Torres, G.; Wu, S.; Ouyang, C.; Xie, Z.; Zou, M.H. Metformin suppresses diabetes-accelerated atherosclerosis via the inhibition of Drp1-mediated mitochondrial fission. Diabetes 2017, 66, 193–205. [Google Scholar] [CrossRef] [PubMed]
| Name | Sequence (5′-3′) | Ta (°C) |
|---|---|---|
| β-actin | Forward: AACTACCTTCAACTCCATC Reverse: TGATCTTGATCTTCATTGTG | 60 |
| CAT | Forward: AACTTCACTGAGGTCCAC Reverse: ATCGCATTCTTAGGCTTCT | 60 |
| DRP1 | Forward: AAGAAGAGTGTAACTGATT Reverse: AGAAGAGACTGATACTGA | 52 |
| FIS1 | Forward: CAATGATGACATCCGTAA Reverse: AGGTAGAAGACGTAATCC | 52 |
| MFN1 | Forward: ATAATGGCAGAACCTGTT Reverse: GGATTCTTATATGTTGCTTCA | 55 |
| MFN2 | Forward: CAGAAGAGAACTCAGAATC Reverse: CTTGACTGTGACGATAGA | 52 |
| OPA1 | Forward: TGTATTCTGAAGTTCTTGATGT Reverse: ATCTCCAACCACAACAAC | 55 |
| PGC1A | Forward: GAGGAATATCAGCACGAGAGG Reverse: ACTTCAAAACGGTCCCTCAG | 60 |
| SOD1 | Forward: ATGGCCCAATAAACATTC Reverse: CTATACAAATCTTCCAAGTGA | 60 |
| NDM | DM | p-Value a | NCAD | CAD | p-Value b | |
|---|---|---|---|---|---|---|
| N | 68 | 60 | 67 | 61 | ||
| Male (M) | 55 (81%) | 44 (73%) | 0.31 | 44 (66%) | 55 (90%) | 0.001 |
| Age (years) | 67.0 (60.0–76.0) | 71.5 (65.0–76.8) | 0.052 | 71.0 (63.0–77.0) | 68.0 (62.0–74.0) | 0.17 |
| Cardiovascular risk factors | ||||||
| Diabetes Mellitus | 31 (46%) | 29 (49%) | 0.88 | |||
| Hypertension | 43 (63%) | 49 (82%) | 0.021 | 42 (63%) | 45 (74%) | 0.015 |
| Dyslipidemia | 47 (69%) | 46 (77%) | 0.34 | 40 (60%) | 53 (87%) | ≤0.001 |
| Smoking | ||||||
| Nonsmoker | 45 (66%) | 39 (65%) | 0.89 | 50 (75%) | 34 (56%) | 0.025 |
| Ex-smoker | 21 (35%) | 16 (27%) | 0.60 | 16 (24%) | 22 (34%) | 0.08 |
| Recent smoker history | 0 (0%) | 2 (3%) | 0.13 | 0 (0%) | 2 (3%) | 0.14 |
| Active smoker | 2 (3%) | 3 (5%) | 0.54 | 2 (3%) | 3 (2%) | 0.71 |
| BMI | 26.52 ± 0.35 | 27.31 ± 0.29 | 0.030 | 27.0 (25.0–29.0) | 28.0 (26.0–29.0) | 0.23 |
| Family history of heart disease | 8 (12%) | 7 (12%) | 0.97 | 1 (2%) | 14 (23%) | ≤0.001 |
| Medication | ||||||
| Antiplatelet | 35 (51%) | 37 (62%) | 0.25 | 22 (33%) | 50 (82%) | ≤0.001 |
| Antiarrhythmic | 6 (9%) | 10 (17%) | 0.18 | 14 (21%) | 4 (7%) | 0.052 |
| Anticoagulant | 10 (15%) | 11 (18%) | 0.55 | 13 (14%) | 8 (13%) | 0.39 |
| Insulin | 0 (0%) | 11 (18%) | ≤0.001 | 4 (6%) | 7 (11%) | 0.27 |
| Oral antidiabetic | ||||||
| Biguanide | 0 (0%) | 20 (33%) | ≤0.001 | 15 (21%) | 5 (8%) | 0.027 |
| DPP4 inhibitor | 0 (0%) | 9 (15%) | ≤0.001 | 6 (9%) | 3 (5%) | 0.37 |
| DPP4 inhibitor + Biguanide | 0 (0%) | 14 (23%) | ≤0.001 | 6 (9%) | 8 (13%) | 0.45 |
| Sulfonylurea | 0 (0%) | 15 (25%) | ≤0.001 | 7 (10%) | 8 (13%) | 0.64 |
| Diuretic | 34 (50%) | 30 (50%) | >0.99 | 37 (55%) | 27 (44%) | 0.22 |
| ACEI | 22 (32%) | 25 (22%) | 0.28 | 24 (36%) | 23 (38%) | 0.83 |
| ARB | 16 (24%) | 13 (22%) | 0.84 | 15 (22%) | 14 (23%) | 0.98 |
| β blocker | 45 (66%) | 30 (50%) | 0.10 | 32 (47%) | 43 (70%) | 0.015 |
| Calcium channel blocker | 9 (13%) | 10 (17%) | 0.57 | 5 (8%) | 14 (23%) | 0.014 |
| Electrolyte—KCl | 8 (12%) | 8 (13%) | 0.79 | 10 (15%) | 6 (10%) | 0.39 |
| Statins | 41 (60%) | 49 (82%) | 0.008 | 38 (57%) | 52 (85%) | ≤0.001 |
| Vasodilator | 13 (19%) | 13 (22%) | 0.72 | 7 (10%) | 19 (31%) | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgeiro, A.; Santos, D.; Fonseca, A.C.R.G.; Baldeiras, I.; Leal, E.C.; Moura, J.; Costa-Nunes, J.; Seraphim, P.M.; Oliveira, A.; Canotilho, A.; et al. Enhanced Mitochondrial Dynamics and Reactive Oxygen Species Levels with Reduced Antioxidant Defenses in Human Epicardial Adipose Tissue. Metabolites 2025, 15, 481. https://doi.org/10.3390/metabo15070481
Burgeiro A, Santos D, Fonseca ACRG, Baldeiras I, Leal EC, Moura J, Costa-Nunes J, Seraphim PM, Oliveira A, Canotilho A, et al. Enhanced Mitochondrial Dynamics and Reactive Oxygen Species Levels with Reduced Antioxidant Defenses in Human Epicardial Adipose Tissue. Metabolites. 2025; 15(7):481. https://doi.org/10.3390/metabo15070481
Chicago/Turabian StyleBurgeiro, Ana, Diana Santos, Ana Catarina R. G. Fonseca, Inês Baldeiras, Ermelindo C. Leal, João Moura, João Costa-Nunes, Patrícia Monteiro Seraphim, Aryane Oliveira, António Canotilho, and et al. 2025. "Enhanced Mitochondrial Dynamics and Reactive Oxygen Species Levels with Reduced Antioxidant Defenses in Human Epicardial Adipose Tissue" Metabolites 15, no. 7: 481. https://doi.org/10.3390/metabo15070481
APA StyleBurgeiro, A., Santos, D., Fonseca, A. C. R. G., Baldeiras, I., Leal, E. C., Moura, J., Costa-Nunes, J., Seraphim, P. M., Oliveira, A., Canotilho, A., Coutinho, G., Prieto, D., Antunes, P., Antunes, M., & Carvalho, E. (2025). Enhanced Mitochondrial Dynamics and Reactive Oxygen Species Levels with Reduced Antioxidant Defenses in Human Epicardial Adipose Tissue. Metabolites, 15(7), 481. https://doi.org/10.3390/metabo15070481