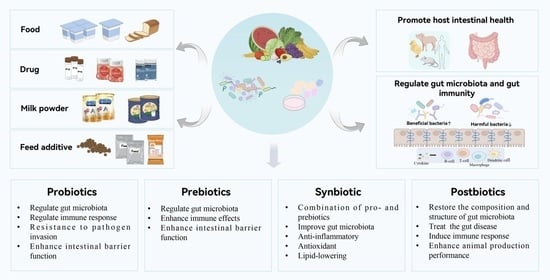
The Role of Probiotics, Prebiotics, Synbiotics, and Postbiotics in Livestock and Poultry Gut Health: A Review
Abstract
1. Introduction
2. Probiotics and Gut Health
2.1. Lactobacillus
2.2. Bifidobacteria
2.3. Saccharomyces
3. Prebiotics and Gut Health
3.1. Fructooligosaccharides
3.2. Isomalto-Oligosaccharides
3.3. Galactooligosaccharides
3.4. Inulin
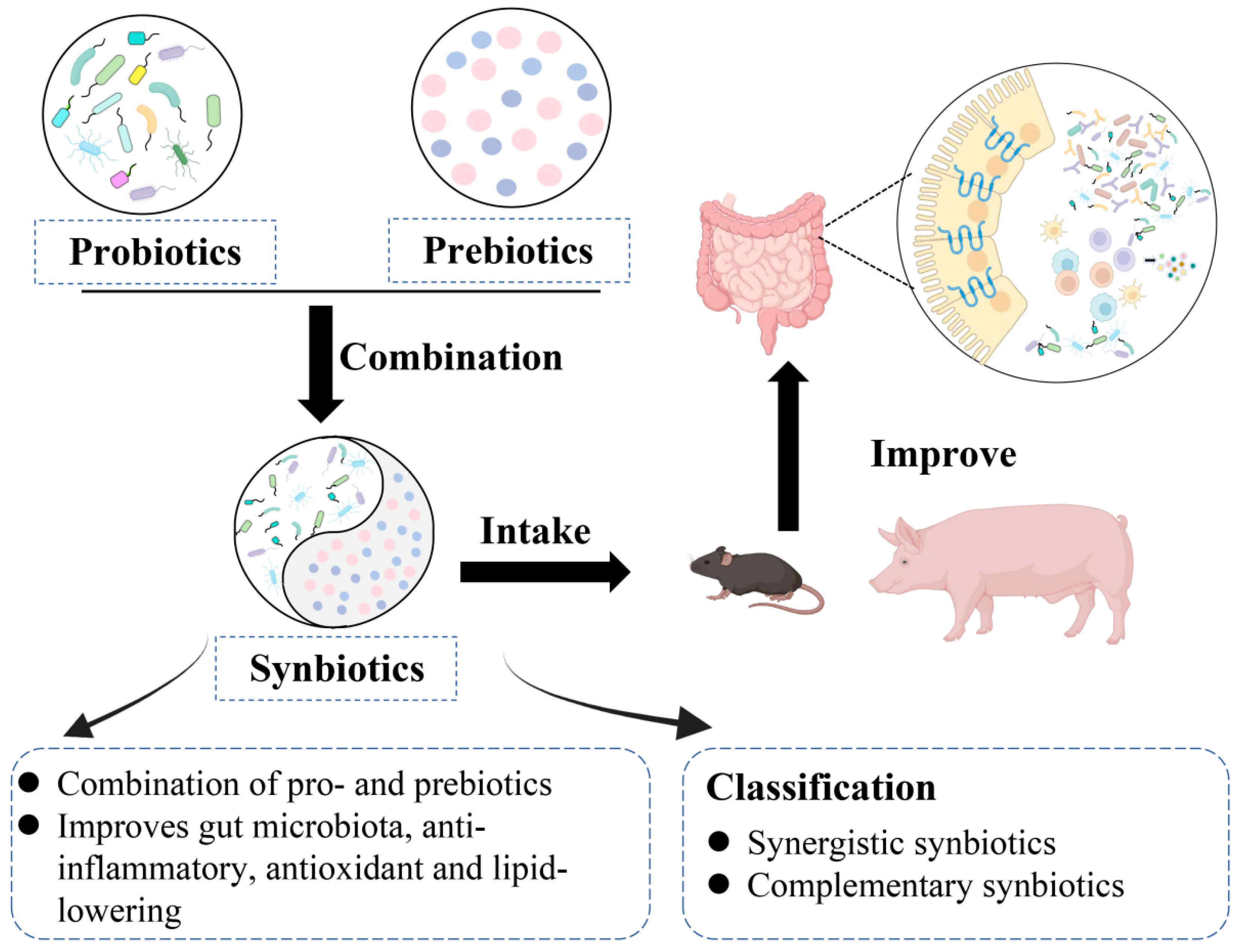
4. Synbiotics and Gut Health
5. Postbiotics and Gut Health
| The Active Ingredients of the Postbiotics | Source Strain | Research Object | Intervention Time | Outcomes | References |
|---|---|---|---|---|---|
| Cell-Free Supernatants | Lactobacillus plantarum TL1, RG11, RG14, RS5, and RI11 | Wean piglets | 5 weeks | ↑ Lactobacillus and SCFAs | [182] |
| Lactobacillus plantarum RI11, RS5, and UL4 | One-day-old fifty-two Cobb 500 male chicks | 21 days | ↓ Salmonella; ↑ Bifidobacterium and Lactobacillus, IgM and IgG | [186] | |
| Lactobacillus plantarum RG14 | Newly weaned lambs | 60 days | ↑ IL-6, TJP-1, CLDN-1, and CLDN-4; ↓ IL-1β, IL-10, and TNF | [187] | |
| Saccharomyces cerevisiae PTCC 5269 | SW480 colon cancer cells | 48 h | ↓ Listeria monocytogenes, Streptococcus mutans, Salmonella typhi, and E. coli | [188] | |
| Exopolysaccharides | Lactobacillus plantarum NCU116 | Eight-week-old C57BL/6 male mice | 7 days | ↑ Claudin-1, Occludin, and ZO-1 ↓ TNF-α, IFN-γ, and IL-6 | [189] |
| Lactobacillus delbrueckii subsp. delbrueckii TUA4408L | Porcine gut epitheliocytes | 48 h | ↓ IL-6, IL-8, and MCP-1 | [190] | |
| Inactivated cells | Enterococcus faecalis strain EC-12 | Newly hatched chicks | 15 days | ↑ Total IgA and total IgG ↓ Vancomycin-resistant enterococci (VRE) colonization of the gut | [182] |
| Streptococcus salivarius M18 | Human CRC epithelial cell lines HCT-116 and SW-480 (ATCC) | Indicated period of time | ↓ Pseudomonas aeruginosa, Klebsiella pneumonia | [191] | |
| Pichia kudriavzevii FZ12 | Weaned piglets | 3, 6, and 12 days | ↑ Beneficial bacteria, promoted growth performance, improved gut health performance | [192] | |
| Bacillus subtilis ACCC 11025 | 480-day-old broilers | 21 and 42 days | ↓ Salmonella; ↑ Lactobacillus bacteria | [193] | |
| Bacteriocin | Lactobacillus gasseri | Different bacteria | / | ↓ Listeria monocytogenes, Bacillus cereus, and Staphylococcus aureus | [194] |
| Short-chain fatty acid | Roseburia intestinalis | Eight-week-old GF male ApoE−/− mice | 18 weeks | ↓ Lipopolysaccharide and TNF-α | [195] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GM | Gut microbiota |
| PPSP | Probiotics, Prebiotics, Synbiotics, and Postbiotics |
| FAO | Food and Agriculture Organization |
| WHO | World Health Organization |
| GIT | Gastrointestinal tract |
| E. coli | Escherichia coli |
| LGG | Lactobacillus rhamnosus GG |
| GRAS | Generally recognized as safe |
| TLR-2 | Toll-like receptor 2 |
| COX-2 | Cyclooxygenase-2 |
| ISAPP | International Society for the Science of Probiotics and Prebiotics |
| FOS | Fructooligosaccharides |
| XOS | Xylo-oligosaccharide |
| IMO | Isomalto-oligosaccharides |
| GOS | Galactooligosaccharides |
| APEC | Avian pathogenic E. coli |
| UC | Ulcerative colitis |
| DP | Degrees of polymerization |
| gRNAs | Guide RNAs |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| NGS | Next-generation sequencing |
| SCFAs | Short-chain fatty acids |
References
- Okaiyeto, S.A.; Sutar, P.P.; Chen, C.; Ni, J.; Zhang, C.; Xiao, H. Antibiotic resistant bacteriainfood systems: Current status, resistance mechanisms, and mitigationstrategies. Agric. Commun. 2024, 2, 100027. [Google Scholar]
- Deng, Z.C.; Wang, J.; Wang, J.; Yan, Y.Q.; Huang, Y.X.; Chen, C.Q.; Sun, L.H.; Liu, M. Tannic acid extracted from gallnut improves intestinal health with regulation of redox homeostasis and gut microbiota of weaned piglets. Anim. Res. One Health 2024, 2, 16–27. [Google Scholar] [CrossRef]
- Al-Khalaifa, H.; Al-Nasser, A.; Al-Surayee, T.; Al-Kandari, S.; Al-Enzi, N.; Al-Sharrah, T.; Ragheb, G.; Al-Qalaf, S.; Mohammed, A. Effect of dietary probiotics and prebiotics on the performance of broiler chickens. Poult. Sci. 2019, 98, 4465–4479. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2014, 14, 182. [Google Scholar] [CrossRef] [PubMed]
- Darby, E.; Trampari, E.; Siasat, P.A.; Gaya, M.; Alav, I.; Webber, M.; Blair, J. Molecular mechanisms of antibiotic resistance revisited. Nat. Rev. Microbiol. 2022, 21, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Chukwudi, P.; Umeugokwe, P.I.; Ikeh, N.E.; Amaefule, B.C. The effects of organic acids on broiler chicken nutrition: A review. Anim. Res. One Health 2025, 3, 43–53. [Google Scholar] [CrossRef]
- Chen, P.; Xu, T.; Zhang, C.; Tong, X.; Shaukat, A.; He, Y.; Liu, K.; Huang, S. Effects of Probiotics and Gut Microbiota on Bone Metabolism in Chickens: A Review. Metabolites 2022, 12, 1000. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Liu, N.; Huang, S.; Zhang, C. A Comprehensive Review of Licorice: The Preparation, Chemical Composition, Bioactivities and Its Applications. Am. J. Chin. Med. 2024, 52, 667–716. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.W.; Abbas, S.M.; Saeed, M.A.; Farooq, U.; Waseem, M.; Adil, M.; Javed, M.R.; Haq, I.U.; Osei Tutu, C. Environmental and Nutritional Value of Fruit and Vegetable Peels as Animal Feed: A Comprehensive Review. Anim. Res. One Health 2025, 3, 149–164. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health benefits of heat-killed (Tyndallized) probiotics: An overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Zhou, D.D.; Gan, R.Y.; Huang, S.Y.; Zhao, C.; Shang, A.; Xu, X.Y.; Li, H.B. Effects and mechanisms of probiotics, prebiotics, synbiotics, and postbiotics on metabolic diseases targeting gut microbiota: A narrative review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Rehman, M.U.; He, Y.F.; Li, A.Y.; Jian, F.C.; Zhang, L.X.; Huang, S.C. Exploring the interplay between Eimeria spp. infection and the host: Understanding the dynamics of gut barrier function. Vet. Q. 2025, 45, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Poorniammal, R.; Dufossé, L. Microbial Metabolites: A Sustainable Approach to Combat Plant Pests. Metabolites 2025, 15, 418. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.N.; Yue, T.J.; Ding, W.L.; Xu, B.W.; Li, A.Y.; Huang, S.C. Gut-X Axis and Its Role in Poultry Bone Health: A Review. Microorganisms 2025, 13, 757. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.T.; Chen, P.; Zhang, C.D.; Shaukat, A.; Lin, L.X.; Yue, K.; Ding, W.L.; Tong, X.S.; Liu, K.L.; He, Y.F.; et al. Gut microbiome dysregulation drives bone damage in broiler tibial dyschondroplasia by disrupting glucose homeostasis. npj Biofilms Microbiomes 2023, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, F.; Yang, K.; Han, S.; Jian, S.; Deng, B. Dihydromyricetin alleviates intestinal inflammation by changing intestinal microbial metabolites and inhibiting the expression of the MyD88/NF-κB signaling pathway. Anim. Res. One Health 2023, 1, 219–232. [Google Scholar] [CrossRef]
- Huang, S.; Lin, L.; Wang, S.; Ding, W.; Zhang, C.; Shaukat, A.; Xu, B.; Yue, K.; Zhang, C.; Liu, F. Total Flavonoids of Rhizoma Drynariae Mitigates Aflatoxin B1-Induced Liver Toxicity in Chickens via Microbiota-Gut-Liver Axis Interaction Mechanisms. Antioxidants 2023, 12, 819. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.; He, Y.F.; Chen, P.; Liu, K.L.; Shaukat, A. Gut microbiota as a target in the bone health of livestock and poultry: Roles of short-chain fatty acids. Anim. Dis. 2023, 3, 23. [Google Scholar] [CrossRef]
- Cani, P. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Liu, K.L.; Yue, T.J.; Lu, Y.N.; Li, S.Y.; Jian, F.C.; Huang, S.C. Plants, plant-derived compounds, probiotics, and postbiotics as green agents to fight against poultry coccidiosis: A review. Anim. Res. One Health 2024, 1–21. [Google Scholar] [CrossRef]
- Brown, A.; Valiere, A. Probiotics and medical nutrition therapy. Nutr. Clin. Care 2004, 7, 56–68. [Google Scholar] [PubMed]
- Liu, C.; Ma, N.; Feng, Y.; Zhou, M.; Li, H.; Zhang, X.; Ma, X. From probiotics to postbiotics: Concepts and applications. Anim. Res. One Health 2023, 1, 92–114. [Google Scholar] [CrossRef]
- Sarowska, J.; Choroszy-Król, I.; Regulska-Ilow, B.; Frej-Mądrzak, M.; Jama-Kmiecik, A. The therapeutic effect of probiotic bacteria on gastrointestinal diseases. Adv. Clin. Exp. Med. 2013, 22, 759–766. [Google Scholar] [PubMed]
- Draper, K.; Ley, C.; Parsonnet, J. Probiotic guidelines and physician practice: A cross-sectional survey and overview of the literature. Benef. Microbes 2017, 8, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Fata, G.; Weber, P.; Mohajeri, H. Probiotics and the gut immune system: Indirect regulation. Probiotics Antimicrob. Proteins 2018, 10, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Metchnikoff, E. The Prolongation of Life: Optimistic Studies; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Žuntar, I.; Petric, Z.; Kovačević, D.B.; Putnik, P. Safety of probiotics: Functional fruit beverages and nutraceuticals. Foods 2020, 9, 947. [Google Scholar] [CrossRef] [PubMed]
- Hotel, A.A. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. In Proceedings of the Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria, Córdoba, Argentina, 1–4 October 2001. [Google Scholar]
- Patel, S. Probiotic supplements might not be universally-effective and safe: A review. Biomed. Pharmacother. 2019, 111, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Henry, K.; Hagen, K.; Gordonpour, M.; Tompkins, T.; Sherman, P. Surface-layer protein extracts from Lactobacillus helveticus inhibit enterohaemorrhagic Escherichia coli O157:H7 adhesion to epithelial cells. Cell Microbiol. 2007, 9, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Jacobus, N.V.; Deneke, C.; Gorbach, S.L. Antimicrobial substance from a human Lactobacillus strain. Agents Chemother. 1987, 31, 1231–1233. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Tsen, H.Y.; Lin, C.L.; Yu, B.; Chen, C.S. Oral administration of a combination of select lactic acid bacteria strains to reduce the Salmonella invasion and inflammation of broiler chicks. Poult. Sci. 2012, 91, 2139–2147. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Chang, S.Y.; Bogere, P.; Won, K.; Choi, J.-Y.; Choi, Y.-J.; Lee, H.K.; Hur, J.; Park, B.-Y.; Kim, Y.; et al. Beneficial roles of probiotics on the modulation of gut microbiota and immune response in pigs. PLoS ONE 2019, 14, e0220843. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, Z.; Wang, Y.; Li, L.; Jia, H.; Zhang, L. Compound probiotics can improve intestinal health by affecting the gut microbiota of broilers. J. Anim. Sci. 2023, 101, skad388. [Google Scholar] [CrossRef] [PubMed]
- Lessard, M.; Dupuis, M.; Gagnon, N.; Nadeau, E.; Matte, J.; Goulet, J.; Fairbrother, J. Administration of Pediococcus acidilactici or Saccharomyces cerevisiae boulardii modulates development of porcine mucosal immunity and reduces intestinal bacterial translocation after Escherichia coli challenge. J. Anim. Sci. 2008, 87, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Brousseau, J.P.; Talbot, G.; Beaudoin, F.; Lauzon, K.; Roy, D.; Lessard, M. Effects of probiotics Pediococcus acidilactici strain MA18/5M and Saccharomyces cerevisiae subsp. boulardii strain SB-CNCM I-1079 on fecal and intestinal microbiota of nursing and weanling piglets. J. Anim. Sci. 2015, 93, 5313–5326. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Choi, J.W.; Jhun, J.; Kwon, J.Y.; Lee, B.-I.; Yang, C.W.; Park, S.-H.; Cho, M.-L. Lactobacillus acidophilus Improves Intestinal Inflammation in an Acute Colitis Mouse Model by Regulation of Th17 and Treg Cell Balance and Fibrosis Development. J. Med. Food 2018, 21, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; He, J.; Gao, N.; Lu, X.; Li, M.; Wu, X.; Liu, Z.; Jin, Y.; Liu, J.; Xu, J.; et al. Probiotics may delay the progression of nonalcoholic fatty liver disease by restoring the gut microbiota structure and improving intestinal endotoxemia. Sci. Rep. 2017, 7, 45176. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Ping, L.; Zhang, K.; Tang, H.; Liu, J.; Liu, D.; Zhao, L.; Evivie, S.; Li, B.; Huo, G. Immunomodulatory effects of the mixed Lactobacillus plantarum on lipopolysaccharide-induced intestinal injury in mice. Food Funct. 2022, 13, 4914–4929. [Google Scholar] [CrossRef] [PubMed]
- Brisbin, J.T.; Gong, J.; Orouji, S.; Esufali, J.; Mallick, A.I.; Parvizi, P.; Shewen, P.E.; Sharif, S. Oral treatment of chickens with lactobacilli influences elicitation of immune responses. Clin. Vaccine Immunol. 2011, 18, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Engström Jakobsson, H.; Holmén larsson, J.; Schütte, A.; Ermund, A.; Rodriguez-Pineiro, A.; Arike, L.; Wising, C.; Svensson, F.; Bäckhed, F.; et al. Normalization of host intestinal mucus layers requires long-term microbial colonization. Cell Host Microbe 2015, 18, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xu, C.; Xu, Z.; Chen, X.; Gao, F.; Lin, T.; Yang, P.; Kan, S.; Yin, Y.; Chen, D. Selenium-enriched Bifidobacterium longum DD98 significantly improves the efficacy of Mesalazine and Cyclosporin A in colitis mice. Food Biosci. 2024, 61, 104297. [Google Scholar] [CrossRef]
- Li, W.; Kai, L.; Jiang, Z.; He, H.; Yang, M.; Su, W.; Wang, Y.; Jin, M.; Lu, Z. Bifidobacterium longum, Lactobacillus plantarum and Pediococcus acidilactici reversed ETEC-inducing intestinal inflammation in mice. Microorganisms 2022, 10, 2350. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Tomosada, Y.; Villena, J.; Chiba, E.; Shimazu, T.; Aso, H.; Iwabuchi, N.; Xiao, J.-Z.; Saito, T.; Kitazawa, H. Bifidobacterium breve MCC-117 induces tolerance in porcine intestinal epithelial cells: Study of the echanisms involved in the immunoregulatory effect. Biosci. Microbiota Food Health 2014, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Moore, R.J.; Stanley, D.; Chousalkar, K.K. The gut microbiota of laying hens and its manipulation with prebiotics and probiotics to enhance gut health and food safety. Appl. Environ. Microbiol. 2020, 86, e00600–e00620. [Google Scholar] [CrossRef] [PubMed]
- Cuaycal, A.E.; Torrez Lamberti, M.F.; Lorca, G.L.; Gonzalez, C.F. Lactobacillus johnsonii N6.2 Phospholipids Induce T Cell Anergy upon Cognate Dendritic Cell Interactions. Metabolites 2025, 15, 284. [Google Scholar] [CrossRef] [PubMed]
- Un-Nisa, A.; Khan, A.; Zakria, M.; Siraj, S.; Ullah, S.; Tipu, M.K.; Ikram, M.; Kim, M. Updates on the role of probiotics against different health issues: Focus on Lactobacillus. Int. J. Mol. Sci. 2022, 24, 142. [Google Scholar] [CrossRef] [PubMed]
- Mack, D.; Michail, S.; Wei, S.; McDougall, L.; Hollingsworth, M. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. 1999, 276, G941–G950. [Google Scholar] [PubMed]
- Yang, J.; Qian, K.; Wang, C.; Wu, Y. Roles of probiotic Lactobacilli inclusion in helping piglets establish healthy intestinal inter-environment for pathogen defense. Probiotics Antimicrob. Proteins 2018, 10, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ren, X.; Wang, S.; Liu, R.; Shi, B.; Dong, H.; Wu, Q. Microbial interventions in yak colibacillosis: Lactobacillus-mediated regulation of intestinal barrier. Front. Cell Infect. Microbiol. 2024, 14, 1337439. [Google Scholar] [CrossRef] [PubMed]
- Tellez, G.; Pixley, C.; Wolfenden, R.E.; Layton, S.L.; Hargis, B.M. Probiotics/direct fed microbials for Salmonella control in poultry. Food Res. Int. 2012, 45, 628–633. [Google Scholar] [CrossRef]
- Jiao, Y. Anti-inflammatory capacity of Lactobacillus rhamnosus GG in monophasic variant Salmonella infected piglets is correlated with impeding NLRP6-mediated host inflammatory responses. Vet. Microbiol. 2017, 210, 91–100. [Google Scholar]
- He, F.; Jin, X.; Wang, C.; Hu, J.; Su, S.; Zhao, L.; Geng, T.; Zhao, Y.; Pan, L.; Bao, N.; et al. Lactobacillus rhamnosus GG ATCC53103 and Lactobacillus plantarum JL01 improved nitrogen metabolism in weaned piglets by regulating the intestinal flora structure and portal vein metabolites. Front. Microbiol. 2023, 14, 1200594. [Google Scholar] [CrossRef] [PubMed]
- Geng, T.; He, F.; Su, S.; Sun, K.; Zhao, L.; Zhao, Y.; Bao, N.; Pan, L.; Sun, H. Probiotics Lactobacillus rhamnosus GG ATCC53103 and Lactobacillus plantarum JL01 induce cytokine alterations by the production of TCDA, DHA, and succinic and palmitic acids, and enhance immunity of weaned piglets. Res. Vet. Sci. 2021, 137, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Ma, K.; Li, J.; Ren, Z.; Zhang, J.; Shan, A. Lactobacillus rhamnosus GG ameliorates DON-induced intestinal damage depending on the enrichment of beneficial bacteria in weaned piglets. J. Anim. Sci. Biotechnol. 2022, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, X.; Liu, X.; Zhao, X.; Liu, S.; Li, Y.; Zhang, Y. Growth, health, rumen fermentation, and bacterial community of Holstein calves fed Lactobacillus rhamnosus GG during the preweaning stage1. J. Anim. Sci. 2019, 97, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Hou, C.; Zeng, X.; Qiao, S. The use of lactic Acid bacteria as a probiotic in Swine diets. Pathogens 2015, 4, 34–45. [Google Scholar] [CrossRef] [PubMed]
- He, B.-L.; Xiong, Y.; Hu, T.-G.; Zong, M.-H.; Wu, H. Bifidobacterium spp. as functional foods: A review of current status, challenges, and strategies. Crit. Rev. Food Sci. Nutr. 2022, 63, 8048–8065. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.Z.; Wang, X.; Yan, X. Effects of Aeriscardovia aeriphila on growth performance, antioxidant functions, immune responses, and gut microbiota in broiler chickens. J. Zhejiang Univ. Sci. B 2023, 24, 1014–1026. [Google Scholar] [CrossRef] [PubMed]
- El-Moneim, A.; El-Wardany, I.; Abu-Taleb, A.M.; Wakwak, M.M.; Ebeid, T.A.; Saleh, A.A. Assessment of in ovo administration of Bifidobacterium bifidum and Bifidobacterium longum on performance, ileal histomorphometry, blood hematological, and biochemical parameters of broilers. Probiotics Antimicrob. Proteins 2020, 12, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Yang, J. Correlations of gastrointestinal hormones with inflammation and intestinal flora in patients with gastric cancer. J. BUON 2019, 24, 1595–1600. [Google Scholar] [PubMed]
- Turroni, F.; Özcan, E.; Milani, C.; Mancabelli, L.; Viappiani, A.; Van Sinderen, D.; Sela, D.; Ventura, M. Glycan cross-feeding activities between bifidobacteria under in vitro conditions. Front. Microbiol. 2015, 6, 1030. [Google Scholar] [CrossRef] [PubMed]
- Zhi, T.; Ma, A.; Liu, X.; Chen, Z.; Li, S.; Jia, Y. Dietary Supplementation of Brevibacillus laterosporus S62-9 Improves Broiler Growth and Immunity by Regulating Cecal Microbiota and Metabolites. Probiotics Antimicrob. Proteins 2024, 16, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Bao, C.; Wang, J.; Zang, J.; Cao, Y. Administration of Saccharomyces boulardii mafic-1701 improves feed conversion ratio, promotes antioxidant capacity, alleviates intestinal inflammation and modulates gut microbiota in weaned piglets. J. Anim. Sci. Biotechnol. 2020, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Shruthi, B.; Nagaraj, D.; Somashekaraiah, R.; Gunduraj, A.; Divyashree, S.; Sreenivasa, M. Exploring biotechnological and functional characteristics of probiotic yeasts: A review. Biotechnol. Rep. 2022, 34, e00716. [Google Scholar] [CrossRef] [PubMed]
- Abid, R.; Waseem, H.; Ali, J.; Ghazanfar, S.; Ali, G.; Elasbali, A.; Alharethi, S. Probiotic Yeast Saccharomyces: Back to Nature to Improve Human Health. J. Fungi 2022, 8, 444. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, S.; Ganapathy, S.; Mitra, M.; Neha Joshi, D.; Veligandla, K.; Kotak, B.; Rathod, R. Unique Properties of Yeast Probiotic Saccharomyces boulardii CNCM I-745: A Narrative Review. Cureus 2023, 15, e46314. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Siedlecka, K.; Ruszkowski, J.; Fic, M.; Folwarski, M.; Makarewicz, W. Saccharomyces boulardii CNCM I-745: A non-bacterial microorganism used as probiotic agent in supporting treatment of selected diseases. Curr. Microbiol. 2020, 77, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, E.; Cotter, P.; Stanton, C.; Ross, R.; Hill, C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: Bacteriocins and conjugated linoleic acid. Int. J. Food Microbiol. 2011, 152, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, B.; Ross, R.; Jin, Y.; Stanton, C.; Zhao, J.; Zhang, H.; Chen, W. Orally administered CLA ameliorates DSS-induced colitis in mice via intestinal barrier improvement, oxidative stress reduction, inflammatory cytokine and gut microbiota modulation. J. Agric. Food Chem. 2019, 67, 13282–13298. [Google Scholar] [CrossRef] [PubMed]
- Roy Sarkar, S.; Mazumder, P.; Chatterjee, K.; Sarkar, A.; Adhikary, M.; Mukhopadhyay, K.; Banerjee, S. Saccharomyces boulardii ameliorates gut dysbiosis associated cognitive decline. Physiol. Behav. 2021, 236, 113411. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Man, F.; Lasa, J. Impact of Saccharomyces boulardii CNCM I-745 on bacterial overgrowth and composition of intestinal microbiota in IBS-D patients: Results of a randomized pilot study. Dig. Dis. 2023, 41, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: A door to the body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef] [PubMed]
- Tomičić, Z.M.; Čolović, R.R.; Čabarkapa, I.S.; Vukmirović, Đ.M.; Đuragić, O.M.; Tomičić, R.M. Beneficial properties of probiotic yeast Saccharomyces boulardii. Food Feed. Res. 2016, 43, 109–110. [Google Scholar] [CrossRef]
- Mondal, O.; Khanna, D.; Panwar, S.; Negi, S.; Basu, S. Systematic review on therapeutic applications of yeast ‘Saccharomyces’. Int. J. Sci. Res. Sci. Technol. 2021, 8, 174–197. [Google Scholar] [CrossRef]
- Gedek, B.R. Adherence of Escherichia coli serogroup O 157 and the Salmonella typhimurium mutant DT 104 to the surface of Saccharomyces boulardii. Mycoses 1999, 42, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Tasteyre, A.; Barc, M.-C.; Karjalainen, T.; Bourlioux, P.; Collignon, A. Inhibition of in vitro cell adherence of Clostridium difficile by Saccharomyces boulardii. Microb. Pathog. 2002, 32, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Ma, T.; Steele, M.; Guan, L.L. Varied microbial community assembly and specialization patterns driven by early life microbiome perturbation and modulation in young ruminants. ISME Commun. 2024, 4, ycae044. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; An, K.; Li, P.; Li, L.; Xia, Z. Dietary Saccharomyces cerevisiae improves intestinal flora structure and barrier function of Pekin ducks. Poult. Sci. 2023, 102, 101940. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Gong, L.; Zhang, X.; Wang, Y.; Wang, Y.; Wang, B.; Li, Y.; Li, W. Effect of Saccharomyces boulardii and Bacillus subtilis B10 on gut microbiota modulation in broilers. Anim. Nutr. 2018, 4, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Chousalkar, K.K.; Gast, R.K.; Martelli, F.; Pande, V. Review of egg-related salmonellosis and reduction strategies in United States, Australia, United Kingdom and New Zealand. Crit. Rev. Microbiol. 2017, 44, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Ricke, S.; Gast, R. Producing Safe Eggs: Microbial Ecology of Salmonella; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.; Scott, K.P.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Farias, D.d.P.; de Araújo, F.F.; Neri-Numa, I.A.; Pastore, G.M. Prebiotics: Trends in food, health and technological applications. Trends Food Sci. Technol. 2019, 93, 23–35. [Google Scholar] [CrossRef]
- Ni, M.; Wang, Z.; Li, Z.; Chen, M.; He, H.; Cai, H.; Chen, Z.; Li, M.; Xu, H. Dietary supplement of sodium butyrate improves the growth performance and intestinal health by targeting Wnt/β-catenin signaling pathway in rabbits. Anim. Res. One Health 2024, 1–16. [Google Scholar] [CrossRef]
- Chu, X.; Xing, H.; Chao, M.; Xie, P.; Jiang, L. Gut Microbiota Modulation in Osteoporosis: Probiotics, Prebiotics, and Natural Compounds. Metabolites 2025, 15, 301. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Probiotics, prebiotics and synbiotics—A promising strategy in prevention and treatment of cardiovascular diseases? Int. J. Mol. Sci. 2020, 21, 9737. [Google Scholar] [CrossRef] [PubMed]
- Nicolucci, A.C.; Reimer, R.A. Prebiotics as a modulator of gut microbiota in paediatric obesity. Pediatr. Obes. 2017, 12, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.; Gibson, G.R.; Delzenne, N. The biochemistry of oligofructose, a nondigestible fiber: An approach to calculate its caloric value. Nutr. Rev. 1993, 51, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Korczak, R.; Slavin, J. Fructooligosaccharides and appetite. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Caetano, B.F.R.; De Moura, N.A.; Almeida, A.P.S.; Dias, M.C.; Sivieri, K.; Barbisan, L.F. Yacon (Smallanthus sonchifolius) as a food supplement: Health-promoting benefits of fructooligosaccharides. Nutrients 2016, 8, 436. [Google Scholar] [CrossRef] [PubMed]
- Wernimont, S.; Northington, R.; Kullen, M.J.; Yao, M.; Bettler, J. Effect of an α-Lactalbumin-enriched infant formula supplemented with oligofructose on fecal microbiota, stool characteristics, and hydration status: A randomized, double-blind, controlled trial. Clin. Pediatr. 2015, 54, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Beatty, E.R.; Wang, X.; Cummings, J.H. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 1995, 108, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Flores-Gallegos, A.C.; Contreras-Esquivel, J.C.; Morlett-Chávez, J.A.; Aguilar, C.N.; Rodríguez-Herrera, R. Comparative study of fungal strains for thermostable inulinase production. J. Biosci. Bioeng. 2015, 119, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W. Fructooligosaccharides—Occurrence, preparation, and application. Enzyme Microb. Technol. 1996, 19, 107–117. [Google Scholar] [CrossRef]
- Johnson-Henry, K.C.; Pinnell, L.J.; Waskow, A.M.; Irrazabal, T.; Martin, A.; Hausner, M.; Sherman, P.M. Short-chain fructo-oligosaccharide and inulin modulate inflammatory responses and microbial communities in Caco2-bbe cells and in a mouse model of intestinal injury. J. Nutr. 2014, 144, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Delgado, G.T.C.; Tamashiro, W.M.S.C.; Pastore, G.M. Immunomodulatory effects of fructans. Food Res. Int. 2010, 43, 1231–1236. [Google Scholar] [CrossRef]
- Peshev, D.; Van den Ende, W. Fructans: Prebiotics and immunomodulators. J. Funct. Food 2014, 8, 348–357. [Google Scholar] [CrossRef]
- Vos, A.P.; van Esch, B.C.; Stahl, B.; M’Rabet, L.; Folkerts, G.; Nijkamp, F.P.; Garssen, J. Dietary supplementation with specific oligosaccharide mixtures decreases parameters of allergic asthma in mice. Int. Immunopharmacol. 2007, 7, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Su, B.; Li, F.; Li, J.; Nie, J.; Xiong, W.; Luo, J.; Huang, S.; Zhou, T.; Liang, X.; et al. Maternal or post-weaning dietary fructo-oligosaccharide supplementation reduces stillbirth rate of sows and diarrhea of weaned piglets. Anim. Nutr. 2024, 17, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, L.; Chen, D.; Yu, B.; Zheng, P.; Mao, X.; Huang, Z.; Yu, J.; Luo, J.; Yan, H.; et al. Dietary supplementation of fructo-oligosaccharides alleviates enterotoxigenic E. coli-induced disruption of intestinal epithelium in a weaned piglet model. Br. J. Nutr. 2022, 128, 1526–1534. [Google Scholar]
- Berghaus, R.D.; Thayer, S.G.; Law, B.F.; Mild, R.M.; Hofacre, C.L.; Singer, R.S. Enumeration of Salmonella and Campylobacter spp. in environmental farm samples and processing plant carcass rinses from commercial broiler chicken flocks. Appl. Environ. Microbiol. 2013, 79, 4106–4114. [Google Scholar] [CrossRef] [PubMed]
- Clavijo, V.; Flórez, M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: A review. Poult. Sci. 2018, 97, 1006–1021. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Cao, Q.Q.; Shaukat, A.; Zhang, C.; Huang, S.C. Insights into the evaluation, influential factors and improvement strategies for poultry meat quality: A review. npj Sci. Food. 2024, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Ricke, S.C.; Dunkley, C.S.; Durant, J.A. A review on development of novel strategies for controlling Salmonella enteritidis colonization in laying hens: Fiber-based molt diets1 1Presented as part of the tomorrow’s Poultry: Sustainability and safety keynote symposium at the Poultry Science Association’s annual meeting in Athens, Georgia. Poult. Sci. 2013, 92, 502–525. [Google Scholar] [PubMed]
- Hu, Y.; Ketabi, A.; Buchko, A. Metabolism of isomalto-oligosaccharides by Lactobacillus reuteri and bifidobacteria. Lett. Appl. Microbiol. 2013, 57, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gu, X.; Wang, J.; Liao, S.; Duan, Y.; Li, H.; Song, Z.; He, X.; Fan, Z. Effects of dietary isomaltooligosaccharide levels on the gut microbiota, immune function of sows, and the diarrhea rate of their offspring. Front. Microbiol. 2021, 11, 588986. [Google Scholar] [CrossRef] [PubMed]
- Kohmoto, T.; Fukui, F.; Takaku, H.; Machida, Y.; Arai, M.; Mitsuoka, T. Effect of isomalto-oligosaccharides on human fecal flora. Bifidobact. Microflora 1988, 7, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Ketabi, A.; Dieleman, L. Influence of isomalto-oligosaccharides on intestinal microbiota in rats. J. Appl. Microbiol. 2011, 110, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Thitaram, S.N.; Chung, C.H.; Day, D.F.; Hinton, A.; Bailey, J.S.; Siragusa, G.R. Isomaltooligosaccharide increases cecal Bifidobacterium population in young broiler chickens. Poult. Sci. 2005, 84, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Pan, L.; Shang, Q.H.; Ma, X.K.; Long, S.F.; Xu, Y.T.; Piao, X.S. Effects of isomalto-oligosaccharides as potential prebiotics on performance, immune function and gut microbiota in weaned pigs. Anim. Feed. Sci. Technol. 2017, 230, 126–135. [Google Scholar] [CrossRef]
- Wang, X.X.; Song, P.X.; Wu, H.; Xue, J.X.; Zhong, X.; Zhang, L.Y. Effects of graded levels of isomaltooligosaccharides on the performance, immune function and intestinal status of weaned pigs. Asian-Australas J. Anim. Sci. 2016, 29, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.-H.; Tseng, Y.-H.; Kuo, Y.-W.; Lee, M.-C.; Chen, H.-L. Long-term supplementation of isomalto-oligosaccharides improved colonic microflora profile, bowel function, and blood cholesterol levels in constipated elderly people—A placebo-controlled, diet-controlled trial. Nutrition 2011, 27, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Tarabees, R.; Gafar, K.M.; El-Sayed, M.S.; Shehata, A.A.; Ahmed, M. Effects of dietary supplementation of probiotic mix and prebiotic on growth performance, cecal microbiota composition, and protection against wscherichia coli O78 in broiler chickens. Probiotics Antimicrob. Proteins 2019, 11, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Pi, G.; Wang, J.; Song, W.; Li, Y.; Yang, H. Effects of isomalto-oligosaccharides and herbal extracts on growth performance, serum biochemical profiles and intestinal bacterial populations in early-weaned piglets. J. Anim. Physiol. Anim. Nutr. 2022, 106, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Ding, W.L.; Xu, B.W.; Rehman, M.U.; Liu, K.L.; He, Y.F.; Li, S.Y.; Jian, F.C.; Huang, S.C. Aflatoxin B1 as a complicit in intestinal damage caused by Eimeria ovinoidalis in lambs: Novel insights to reveal parasite-gut battle. Sci. Total Environ. 2024, 947, 174539. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Borewicz, K.; Richter, B.; van der Zaal, P.H.; Smidt, H.; Buwalda, P.L.; Schols, H.A. In vitro fermentation behavior of isomalto/malto-polysaccharides using human fecal inoculum indicates prebiotic potential. Mol. Nutr. Food Res. 2018, 62, e1800232. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Lei, Q.; Yin, H.; Hu, T.; Wang, S.; Dong, K.; Pan, H.; Liu, Y.; Lin, Q.; Cao, Z. In vitro effects of prebiotics and synbiotics on apis cerana gut microbiota. Pol. J. Microbiol. 2021, 70, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Koleva, P.; Ketabi, A.; Valcheva, R.; Gänzle, M.G.; Dieleman, L.A. Dieleman chemically defined diet alters the protective properties of fructo-oligosaccharides and isomalto-oligosaccharides in HLA-B27 transgenic rats. PLoS ONE 2014, 9, e111717. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Katayama, T. Consumption of non-digestible oligosaccharides elevates colonic alkaline phosphatase activity by up-regulating the expression of IAP-I, with increased mucins and microbial fermentation in rats fed a high-fat diet. Br. J. Nutr. 2018, 121, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Logtenberg, M.; Akkerman, R.; Hobe, R.; Donners, K.; van Leeuwen, S.; Hermes, G.; de Haan, B.; Faas, M.; Buwalda, P.; Zoetendal, E.; et al. Structure-specific fermentation of galacto-oligosaccharides, isomalto-oligosaccharides and isomalto/malto-polysaccharides by infant fecal microbiota and impact on dendritic cell cytokine responses. Mol. Nutr. Food Res. 2021, 65, 2001077. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.; Bhattacharjee, C.; Chowdhury, R. Synthesis and separation of galacto-oligosaccharides using membrane bioreactor. Desalination 2013, 316, 31–41. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Gosling, A.; Stevens, G.W.; Barber, A.R.; Kentish, S.E.; Gras, S.L. Effect of the substrate concentration and water activity on the yield and rate of the transfer reaction of β-galactosidase from Bacillus circulans. J. Agric. Food Chem. 2011, 59, 3366–3372. [Google Scholar] [CrossRef] [PubMed]
- Krumbeck, J.A.; Rasmussen, H.E.; Hutkins, R.W.; Clarke, J.; Shawron, K.; Keshavarzian, A.; Walter, J. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 2018, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Fai, A.; Pastore, G. Galactooligosaccharides: Production, health benefits, application to foods and perspectives. Sci. Agropecu. 2010, 6, 69–81. [Google Scholar]
- Huang, S.C.; Liu, K.L.; Chen, P.; Xu, B.W.; Ding, W.L.; Yue, T.J.; Lu, Y.N.; Li, S.Y.; Li, J.K.; Jian, F.C. New insights into the combined effects of aflatoxin B1 and Eimeria ovinoidalis on uterine function by disrupting the gut-blood-reproductive axis in sheep. Microbiome 2024, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- Sawada, N.; Murata, M.; Kikuchi, K.; Osanai, M.; Tobioka, H.; Kojima, T.; Chiba, H. Tight junctions and human diseases. Med. Electron. Microsc. 2003, 36, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, H.; Jin, Y.; Xiao, Z.; Umar Yaqoob, M.; Lin, Y.; Chen, H.; Wang, M. Galactooligosaccharides as a protective agent for intestinal barrier and its regulatory functions for intestinal microbiota. Food Res. Int. 2022, 155, 111003. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Prabhu, P.N.; Benefiel, A.C.; Miller, M.J.; Chow, J.; Davis, S.R.; Gaskins, H.R. Galacto-oligosaccharides may directly enhance intestinal barrier function through the modulation of goblet cells. Mol. Nutr. Food Res. 2015, 59, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Holmes, Z.C.; Tang, H.; Liu, C.; Bush, A.; Neubert, B.C.; Jiao, Y.; Covington, M.; Cardona, D.M.; Kirtley, M.C.; Chen, B.J.; et al. Prebiotic galactooligosaccharides interact with mouse gut microbiota to attenuate acute graft-versus-host disease. Blood 2022, 140, 2300–2304. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Liang, L.; Connerton, P.L.; Connerton, I.F.; Mellits, K.H. Galacto-oligosaccharides fed during gestation increase Rotavirus A specific antibodies in sow colostrum, modulate the microbiome, and reduce infectivity in neonatal piglets in a commercial farm setting. Front. Vet. Sci. 2023, 10, 1118302. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Akbari, P.; Difilippo, E.; Schols, H.A.; Ulfman, L.H.; Schoterman, M.H.; Garssen, J.; Fink-Gremmels, J.; Braber, S. The piglet as a model for studying dietary components in infant diets: Effects of galacto-oligosaccharides on intestinal functions. Br. J. Nutr. 2016, 115, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.M.; De Silva, A.; Walton, G.E.; Gibson, G.R. A review on the use of prebiotics in ulcerative colitis. Trends Microbiol. 2024, 32, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.Q.; Wang, L.Y.; Yang, X.Y.; Xu, Y.J.; Fan, G.; Fan, Y.G.; Ren, J.N.; An, Q.; Li, X. Inulin: Properties and health benefits. Food Funct. 2023, 14, 2948–2968. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Guo, H.; Liang, Y.; Zhou, C.; Liu, Z.; Li, K.; Niu, F.; Zhai, X.; Wang, L. The physiological functions and pharmaceutical applications of inulin: A review. Carbohydr. Polym. 2020, 246, 116589. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Vajdi, M.; Khorvash, F.; Rouhani, M.H.; Ghavami, A.; Clark, C.C.T.; Askari, G. Effect of inulin supplementation on clinical symptoms, inflammatory and oxidative stress markers in women with migraine: Study protocol for a randomized clinical trial. Trials 2023, 24, 722. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Ji, G.; Zhang, L. Immunomodulatory effects of inulin and its intestinal metabolites. Front. Immunol. 2023, 14, 1224092. [Google Scholar] [CrossRef] [PubMed]
- Illippangama, A.U.; Jayasena, D.D.; Jo, C.; Mudannayake, D.C. Inulin as a functional ingredient and their applications in meat products. Carbohydr. Polym. 2022, 275, 118706. [Google Scholar] [CrossRef] [PubMed]
- Le Bastard, Q.; Chapelet, G.; Javaudin, F.; Lepelletier, D.; Batard, E.; Montassier, E. The effects of inulin on gut microbial composition: A systematic review of evidence from human studies. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Schroyen, M.; Leblois, J.; Wavreille, J.; Soyeurt, H.; Bindelle, J.; Everaert, N. Effects of inulin supplementation to piglets in the suckling period on growth performance, postileal microbial and immunological traits in the suckling period and three weeks after weaning. Arch. Anim. Nutr. 2018, 72, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Qin, S.; Zhai, S.; Gao, Y.; Li, L. Inulin with different degrees of polymerization modulates composition of intestinal microbiota in mice. FEMS Microbiol. Lett. 2017, 364, fnx075. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Su, H.; Lv, Y.; Tao, H.; Jiang, Y.; Ni, Z.; Peng, L.; Chen, X. Inulin intervention attenuates hepatic steatosis in rats via modulating gut microbiota and maintaining intestinal barrier function. Food Res. Int. 2023, 163, 112309. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yu, Y.; Li, H.; Ding, X.; Li, X.; Jing, X.; Chen, J.; Liu, G.; Lin, Y.; Jiang, C.; et al. Inulin supplementation ameliorates hyperuricemia and modulates gut microbiota in Uox-knockout mice. Eur. J. Nutr. 2021, 60, 2217–2230. [Google Scholar] [CrossRef] [PubMed]
- Hamasalim, H.J. Synbiotic as feed additives relating to animal health and performance. Adv. Microbiol. 2016, 6, 288–302. [Google Scholar] [CrossRef]
- Sergeev, I.N.; Aljutaily, T.; Walton, G.; Huarte, E. Effects of synbiotic supplement on human gut microbiota, body composition and weight loss in obesity. Nutrients 2020, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhu, Q.; Kong, X.; Song, M.; Azad, M.A.K.; Xiong, L.; Zheng, Y.; He, Q. Dietary probiotics or synbiotics supplementation during gestation, lactation, and nursery periods modifies colonic microbiota, antioxidant capacity, and immune function in weaned piglets. Front. Vet. Sci. 2020, 7, 597832. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, S.; Yin, J.; Peng, X.; King, L.; Li, L.; Xu, Z.; Zhou, L.; Peng, Z.; Ze, X.; et al. Effect of synbiotic supplementation on immune parameters and gut microbiota in healthy adults: A double-blind randomized controlled trial. Gut Microbes 2023, 15, 2247025. [Google Scholar] [CrossRef] [PubMed]
- Kolida, S.; Gibson, G.R. Synbiotics in health and disease. Annu. Rev. Food Sci. Technol. 2011, 2, 373–393. [Google Scholar] [CrossRef] [PubMed]
- Gomez Quintero, D.F.; Kok, C.R.; Hutkins, R. The future of synbiotics: Rational formulation and design. Front. Microbiol. 2022, 13, 919725. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.S.; Lee, J.Y.; Kim, Y.T.; Kim, S.H.; Lee, J.H. Cancer-protective effect of a synbiotic combination between Lactobacillus gasseri 505 and a Cudrania tricuspidata leaf extract on colitis-associated colorectal cancer. Gut Microbes 2020, 12, 1785803. [Google Scholar] [CrossRef] [PubMed]
- Krumbeck, J.A.; Walter, J.; Hutkins, R.W. Synbiotics for improved human health: Recent developments, challenges, and opportunities. Annu. Rev. Food Sci. Technol. 2018, 9, 451–479. [Google Scholar] [CrossRef] [PubMed]
- Morshedi, M.; Saghafi-Asl, M.; Hosseinifard, E.S. The potential therapeutic effects of the gut microbiome manipulation by synbiotic containing-Lactobacillus plantarum on neuropsychological performance of diabetic rats. J. Transl. Med. 2020, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Cheng, Y.; Chen, Y.; Wang, S.; Zhao, H.; Wen, C.; Zhou, Y. Dietary supplementation with synbiotics improves growth performance, antioxidant status, immune function, and intestinal barrier function in broilers subjected to cyclic heat stress. Environ. Sci. Pollut. Res. Int. 2023, 30, 18026–18038. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Tao, F.; Hu, Y.; Li, Z.; Zhang, Y.; Deng, B.; Zhan, X. Positive effects of a Clostridium butyricum-based compound probiotic on growth performance, immune responses, intestinal morphology, hypothalamic neurotransmitters, and colonic microbiota in weaned piglets. Food Funct. 2019, 10, 2926–2934. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Gao, Q.; Zhang, W.; Zhu, Q.; Tang, W.; Blachier, F.; Ding, H.; Kong, X. Supplementing synbiotic in sows’ diets modifies beneficially blood parameters and colonic microbiota composition and metabolic activity in suckling piglets. Front. Vet. Sci. 2020, 7, 575685. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Marcial-Coba, M.S.; Pjaca, A.S.; Andersen, C.J.; Knøchel, S.; Nielsen, D.S. Dried date paste as carrier of the proposed probiotic Bacillus coagulans BC4 and viability assessment during storage and simulated gastric passage. LWT 2019, 99, 197–201. [Google Scholar] [CrossRef]
- Tsilingiri, K.; Rescigno, M. Postbiotics: What else? Benef. Microbes 2013, 4, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ding, Y.; Wang, S.; Jiang, L. Gut Microbiota Dysbiosis and Its Impact on Type 2 Diabetes: From Pathogenesis to Therapeutic Strategies. Metabolites 2025, 15, 397. [Google Scholar] [CrossRef] [PubMed]
- Moludi, J.; Alizadeh, M.; Lotfi Yagin, N.; Pasdar, Y.; Nachvak, S.M.; Abdollahzad, H.; Sadeghpour Tabaei, A. New insights on atherosclerosis: A cross-talk between endocannabinoid systems with gut microbiota. J. Cardiovasc. Thorac. Res. 2018, 10, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Mantziari, A.; Salminen, S.; Szajewska, H.; Malagón-Rojas, J.N. Postbiotics against pathogens commonly involved in pediatric infectious diseases. Microorganisms 2020, 8, 1510. [Google Scholar] [CrossRef] [PubMed]
- Mani-López, E.; Arrioja-Bretón, D.; López-Malo, A. The impacts of antimicrobial and antifungal activity of cell-free supernatants from lactic acid bacteria in vitro and foods. Compr. Rev. Food Sci. Food Saf. 2022, 21, 604–641. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zeng, Q.; Puthiyakunnon, S.; Zeng, Z.; Yang, W.; Qiu, J.; Du, L.; Boddu, S.; Wu, T.; Cai, D.; et al. Lactobacillus rhamnosus GG supernatant enhance neonatal resistance to systemic Escherichia coli K1 infection by accelerating development of intestinal defense. Sci. Rep. 2017, 7, 43305. [Google Scholar] [CrossRef] [PubMed]
- Warda, A.K.; de Almeida Bettio, P.H.; Hueston, C.M.; Di Benedetto, G.; Clooney, A.G.; Hill, C. Oral Administration of Heat-Treated Lactobacilli Modifies the Murine Microbiome and Reduces Citrobacter Induced Colitis. Front. Microbiol. 2020, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Loh, T.C.; Thanh, N.T.; Foo, H.L.; Hair-Bejo, M.; Azhar, B.K. Feeding of different levels of metabolite combinations produced by Lactobacillus plantarum on growth performance, fecal microflora, volatile fatty acids and villi height in broilers. Anim. Sci. J. 2010, 81, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Rosyidah, M.R.; Loh, T.; Foo, H.L.; Cheng, X.F.; Bejo, M.H. Effect of feeding metabolites and acidifier on growth performance, faecal characteristics and microflora in broiler chickens. J. Anim. Vet. Adv. 2011, 10, 2758–2764. [Google Scholar]
- Kareem, K.Y.; Loh, T.C.; Foo, H.L.; Asmara, S.A.; Akit, H. Influence of postbiotic RG14 and inulin combination on cecal microbiota, organic acid concentration, and cytokine expression in broiler chickens. Poult. Sci. 2017, 96, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Ozma, M.A.; Abbasi, A.; Akrami, S.; Lahouty, M.; Shahbazi, N.; Ganbarov, K.; Pagliano, P.; Sabahi, S.; Köse, Ş.; Yousefi, M.; et al. Postbiotics as the key mediators of the gut microbiota-host interactions. Infez. Med. 2022, 30, 180–193. [Google Scholar] [PubMed]
- Lee, C.; Kim, B.G.; Kim, J.H.; Chun, J.; Im, J.P.; Kim, J.S. Sodium butyrate inhibits the NF-kappa B signaling pathway and histone deacetylation, and attenuates experimental colitis in an IL-10 independent manner. Int. Immunopharmacol. 2017, 51, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Pandey, H.; Jain, D.; Tang, D.W.T.; Wong, S.H.; Lal, D. Gut microbiota in pathophysiology, diagnosis, and therapeutics of inflammatory bowel disease. Intest. Res. 2024, 22, 15–43. [Google Scholar] [CrossRef] [PubMed]
- Thananimit, S.; Pahumunto, N.; Teanpaisan, R. Characterization of short chain fatty acids produced by selected potential probiotic Lactobacillus strains. Biomolecules 2022, 12, 1829. [Google Scholar] [CrossRef] [PubMed]
- Pupa, P.; Apiwatsiri, P.; Sirichokchatchawan, W.; Pirarat, N.; Maison, T.; Koontanatechanon, A.; Prapasarakul, N. Use of Lactobacillus plantarum (strains 22F and 25F) and Pediococcus acidilactici (strain 72N) as replacements for antibiotic-growth promotants in pigs. Sci. Rep. 2021, 11, 12028. [Google Scholar] [CrossRef] [PubMed]
- Thu, T.V.; Loh, T.C.; Foo, H.L.; Yaakub, H.; Bejo, M.H. Effects of liquid metabolite combinations produced by Lactobacillus plantarum on growth performance, faeces characteristics, intestinal morphology and diarrhoea incidence in postweaning piglets. Trop. Anim. Health Prod. 2011, 43, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Tsukahara, T.; Bukawa, W.; Matsubara, N.; Ushida, K. Cell preparation of Enterococcus faecalis strain EC-12 prevents vancomycin-resistant enterococci colonization in the cecum of newly hatched chicks. Poult. Sci. 2006, 85, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.N.; Kogut, M.H.; Genovese, K.; He, H.; Kazemi, S.; Arsenault, R.J. Administration of a postbiotic causes immunomodulatory responses in broiler gut and reduces disease pathogenesis following challenge. Microorganisms 2019, 7, 268. [Google Scholar] [CrossRef] [PubMed]
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Izuddin, W.I.; Awad, E.A.; Idrus, Z.; Samsudin, A.A.; Mustapha, N.M. Dietary supplementation of postbiotics mitigates adverse impacts of heat stress on antioxidant enzyme activity, total antioxidant, lipid peroxidation, physiological stress indicators, lipid profile and meat quality in broilers. Animals 2020, 10, 982. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Sawant, S.; Hauff, K.; Hampp, G. Validated postbiotic screening confirms presence of physiologically-active metabolites, such as short-chain fatty acids, amino acids and vitamins in Hylak® Forte. Probiotics Antimicrob. Proteins 2019, 11, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Izuddin, W.I.; Loh, T.C.; Foo, H.L.; Samsudin, A.A.; Humam, A.M. Postbiotic L. plantarum RG14 improves ruminal epithelium growth, immune status and upregulates the intestinal barrier function in post-weaning lambs. Sci. Rep. 2019, 9, 9938. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, H.; Abbasi, A.; Sabahi, S.; Akrami, S.; Yousefi-Avarvand, A. Assessing the potential biological activities of postbiotics derived from Saccharomyces cerevisiae: An In vitro study. Probiotics Antimicrob. Proteins. 2023, 16, 1348–1364. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Qi, W.; Hong, T.; Xiong, T.; Gong, D.; Xie, M.; Nie, S. Exopolysaccharides from Lactobacillus plantarum NCU116 regulate intestinal barrier function via STAT3 signaling pathway. J. Agric. Food Chem. 2018, 66, 9719–9727. [Google Scholar] [CrossRef] [PubMed]
- Wachi, S.; Kanmani, P.; Tomosada, Y.; Kobayashi, H.; Yuri, T.; Egusa, S.; Shimazu, T.; Suda, Y.; Aso, H.; Sugawara, M.; et al. Lactobacillus delbrueckii TUA4408L and its extracellular polysaccharides attenuate enterotoxigenic Escherichia coli-induced inflammatory response in porcine intestinal epitheliocytes via Toll-like receptor-2 and 4. Mol. Nutr. Food Res. 2014, 58, 2080–2093. [Google Scholar] [CrossRef] [PubMed]
- Karaçam, S.; Tunçer, S. Exploiting the acidic extracellular pH: Evaluation of streptococcus salivarius M18 postbiotics to target cancer cells. Probiotics Antimicrob. Proteins 2022, 14, 995–1011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, Q.; Wang, J.; Tan, H.; Jin, X.; Fan, Y.; Liu, J.; Zhao, S.; Zheng, J.; Peng, N. Postbiotics from Pichia kudriavzevii promote intestinal health performance through regulation of Limosilactobacillus reuteri in weaned piglets. Food Funct. 2023, 14, 3463–3474. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Fan, X.; Xu, S.; Gao, S.; Wang, T.; Chen, Z.; Li, D. Effects of dietary supplementation of postbiotic derived from Bacillus subtilis ACCC 11025 on growth performance, meat yield, meat quality, excreta bacteria, and excreta ammonia emission of broiler chicks. Poult. Sci. 2024, 103, 103444. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Malik, R.K.; Kaushik, J.K.; Singroha, G. Gassericin A: A circular bacteriocin produced by lactic acid bacteria Lactobacillus gasseri. World J. Microbiol. Biotechnol. 2013, 29, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, K.; Krautkramer, K.A.; Org, E.; Romano, K.A.; Kerby, R.L.; Vivas, E.I.; Mehrabian, M.; Denu, J.M.; Bäckhed, F.; Lusis, A.J.; et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat. Microbiol. 2018, 3, 1461–1471. [Google Scholar] [CrossRef] [PubMed]


| Probiotic Strains | Research Object | Disease | Intervention Time | Therapeutic Outcome | References |
|---|---|---|---|---|---|
| Lactobacillus helveticus | HEp-2 and T84 epithelial cells | / | 18 h | Decreased pathogen adherence and attaching-effacing lesions in addition to preserving the barrier function of monolayers. | [33] |
| Lactobacillus rhamnosus GG | Weaned pig | The monophasic variant Salmonella | 7 days | Removed or reduced the residence of pathogenic bacteria, produce substances that can antagonize food-borne pathogenic bacteria, and also directly participate in the repair of damage to the intestinal mucosal barrier. | [34] |
| Lactobacillus acidophilus SW, Lactobacillus fermentum 33, Lactobacillus plantarum L05, and Enterococcus faecium TM39 | Newly hatched Arbor Acres broiler chicks | Salmonella | 3 days | Up-regulated the ratio of Firmicutes/Bacteroidetes and increased the proportion of genus of Clostridiales. | [35] |
| Lactobacillus plantarum JDFM LP11 | Six female three-way crossbred piglets | / | 4 weeks | Increased the population of lactic acid bacteria in feces and enhanced the development of villi in the small intestine. | [36] |
| Lactobacillus, Lactococcus and Bifidobacterium | One-day-old Arbor Acres broilers | / | 42 days | Increased the relative abundance of Bacteroidales, Rikenellaceae and Alistipes, enhanced the cecal microbiota, and decreased the relative abundance of harmful microorganisms. | [37] |
| Saccharomyces cerevisiae var. boulardii | Eighteen-day-old pigs | Enterotoxigenic E. coli infection | 18, 24, 42 and 52 days | Reduced the adhesion of enterotoxigenic E. coli to mesenteric lymph nodes. | [38] |
| Saccharomyces cerevisiae subsp. boulardii SB-CNCM I-1079 | Wean piglets | / | 37 days | Promoted the establishment of Porphyromonadaceae and Ruminococcaceae in the colon and influenced the intestinal and colonic microbiota. | [39] |
| Lactobacillus acidopilus | Eight-week-old male C57BL/6 mice | Inflammatory bowel disease | 7 days | Suppressed IL-6, TNF-α, IL-1β, and IL-17; modulated the balance between Th17 and Treg cells | [40] |
| Bifidobacterium infantis | Eight-week-old SPF male SD rats | Nonalcoholic fatty liver disease | 12 weeks | Improved gut microbiota structure and liver pathology; downregulated serum LPS and liver TLR4. | [41] |
| Lactobacillus plantarum KLDS1.0318, Lactobacillus plantarum KLDS1.0344, Lactobacillus plantarum KLDS1.0386 and Lactobacillus plantarum WCSF1 | Six-week-old, SPF female 70 BALB/c mice | LPS-induced intestinal injury | 15 days | Decreased TNF-α, IL-6 and IL-12 levels, increased the number of CD4+ T cells and IgA plasma cells and the expression Claudin1 and Occludin, and increased the relative abundance of Lactobacillus, Lachnoclostridium, and Desulfovibrio. | [42] |
| Lactobacillus acidophilus, Lactobacillus reuteri and Lactobacillus salivarius | Newly hatched female commercial broiler chicks | / | 4, 5, and 6 weeks | Enhanced the antibody levels and cell-mediated immunity. | [43] |
| Bifidobacterium dentis | Eight–sixteen-week-old GF female mice | / | 17 days | Limited the interaction of harmful microbiota with epithelial cells in the intestinal lumen and inhibited the growth of E. coli, Clostridium difficile, Salmonella, Helicobacter pylori, and Listeria. | [44] |
| Bifidobacterium longum DD98 | Six–eight-week-old C57BL/6 male mice | Ulcerative colitis | 14 days | Improved the diversity of gut microbiota, promoted the proliferation of Trichoderma, Lactobacillus, and Prevotella, enriched the intestinal population of Bacteroides and Clostridium leptum, and enhanced butyric acid metabolism. | [45] |
| Bifidobacterium longum subsp. Infantis LR655210.1 | Four-week-old male C57BL/6 mice | Enterotoxigenic E. coli K88-induced diarrhea | 14 days | Recovered weight and colon length to a certain extent and down-regulated the levels of IL-6 and TNF-α. | [46] |
| Bifidobacterium shortum MCC-117 | Porcine intestinal epithelial (PIE) cells | Enterotoxigenic E. coli-induced inflammation | 48 h | Stimulated the expression of TLR-2 and COX-2 in the ileal epithelium and blocked cytokine-induced apoptosis. | [47] |
| Lactobacillus suspension or Lactobacillus plantarum | Layer | C. jejuni- and Saccharomyces Enteritidis-induced infections | 4 days | Enhanced gut colonization resistance to C. jejuni and upregulated IL-6, IL-10, and TLR4 in ileum. | [48] |
| Category | Efficacy | Safety | Key Application Challenges | Recommended Species |
|---|---|---|---|---|
| Probiotics (e.g., Lactobacillus, Bifidobacterium, Saccharomyces) | 1. Modulate gut microbiota 2. Enhance gut barrier 3. Boost immunity 4. Species/strain-specific effects | Generally recognized as safe (GRAS) | 1. Low survival during feed processing/storage 2. Variable colonization in the gut 3. Potential antibiotic resistance gene transfer | Pigs, poultry, calves |
| Prebiotics (e.g., FOS, IMO, GOS, inulin) | 1. Selective growth of beneficial bacteria (e.g., Bifidobacterium) 2. SCFAs production 3. Immune modulation | Safe, non-digestible | 1. Dose-dependent effects 2. Fermentation may cause bloating 3. Variable efficacy across diets | Poultry, weaned piglets |
| Synbiotics (probiotic + prebiotic) | 1. Enhanced probiotic survival 2. Synergistic microbiota modulation 3. Improved growth performance | Safe if components are GRAS | 1. Optimal pairing required 2. Higher cost 3. Stability during processing | Broilers, sows, piglets |
| Postbiotics (e.g., inactivated cells, metabolites, SCFAs) | 1. Stable under processing 2. Anti-inflammatory/antibacterial effects 3. No live bacteria risks | High safety (no viability concerns) | 1. Limited long-term studies 2. Production scalability 3. Regulatory ambiguity | Poultry, piglets |
| Category | Main Mechanism | Key Metabolites/Components | |
|---|---|---|---|
| Probiotics | 1. Competitively inhibit pathogen colonization 2. Enhance intestinal barrier (e.g., up-regulate occludin) 3. Modulate immunity (e.g., promote sIgA secretion) | Lactic acid, acetic acid, bacteriocins | |
| Probiotics | 1. Selectively promote proliferation of beneficial bacteria 2. Fermentation to produce SCFAs 3. Reduce intestinal pH to inhibit pathogens | Short-chain fatty acids (SCFAs), oligosaccharides (e.g., FOS, GOS) | |
| Synergistic | 1. Probiotics and prebiotics synergy 2. Increased probiotic survival rate 3. Enhanced metabolite production | SCFAs, vitamins | |
| Postbiotics | Inactivated cells | 1. Physical adsorption of pathogens (cell wall binding) 2. Immunomodulation (TLR2/4 activation) 3. Competitive occupancy | Peptidoglycan, lipophosphatidic acid |
| Short-chain fatty acids | 1. Energy supply (colonic cell butyric acid utilization) 2. pH lowering inhibition of pathogens 3. Regulate Treg differentiation | Acetic acid, propionate, butyrate | |
| Antimicrobial peptides | 1. Directly cleave pathogen cell membranes 2. Inhibit biofilm formation Bacteriocins | Bacteriocin, defensins | |
| Exopolysaccharides | 1. Physical barriers to protect probiotics 2. Induction of immune tolerance (e.g., IL-10 upregulation) 3. Binding of heavy metals/toxins | β-glucan, hyaluronic acid | |
| Extracellular vesicles | 1. Delivery of nucleic acids/proteins to regulate host cells 2. Cross-species signaling | miRNA, functional enzymes | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, T.; Lu, Y.; Ding, W.; Xu, B.; Zhang, C.; Li, L.; Jian, F.; Huang, S. The Role of Probiotics, Prebiotics, Synbiotics, and Postbiotics in Livestock and Poultry Gut Health: A Review. Metabolites 2025, 15, 478. https://doi.org/10.3390/metabo15070478
Yue T, Lu Y, Ding W, Xu B, Zhang C, Li L, Jian F, Huang S. The Role of Probiotics, Prebiotics, Synbiotics, and Postbiotics in Livestock and Poultry Gut Health: A Review. Metabolites. 2025; 15(7):478. https://doi.org/10.3390/metabo15070478
Chicago/Turabian StyleYue, Taojing, Yanan Lu, Wenli Ding, Bowen Xu, Cai Zhang, Lei Li, Fuchun Jian, and Shucheng Huang. 2025. "The Role of Probiotics, Prebiotics, Synbiotics, and Postbiotics in Livestock and Poultry Gut Health: A Review" Metabolites 15, no. 7: 478. https://doi.org/10.3390/metabo15070478
APA StyleYue, T., Lu, Y., Ding, W., Xu, B., Zhang, C., Li, L., Jian, F., & Huang, S. (2025). The Role of Probiotics, Prebiotics, Synbiotics, and Postbiotics in Livestock and Poultry Gut Health: A Review. Metabolites, 15(7), 478. https://doi.org/10.3390/metabo15070478