Pharmacometabolomics Study of Sulfamethoxazole and Trimethoprim in Kidney Transplant Recipients: Real-World Metabolism and Urinary Excretion
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical and Pharmacometabolomics Data
2.2. Feature Selection
2.3. Metabolite Identification
3. Results
3.1. Characteristics of Study Participants
3.2. Feature Selection
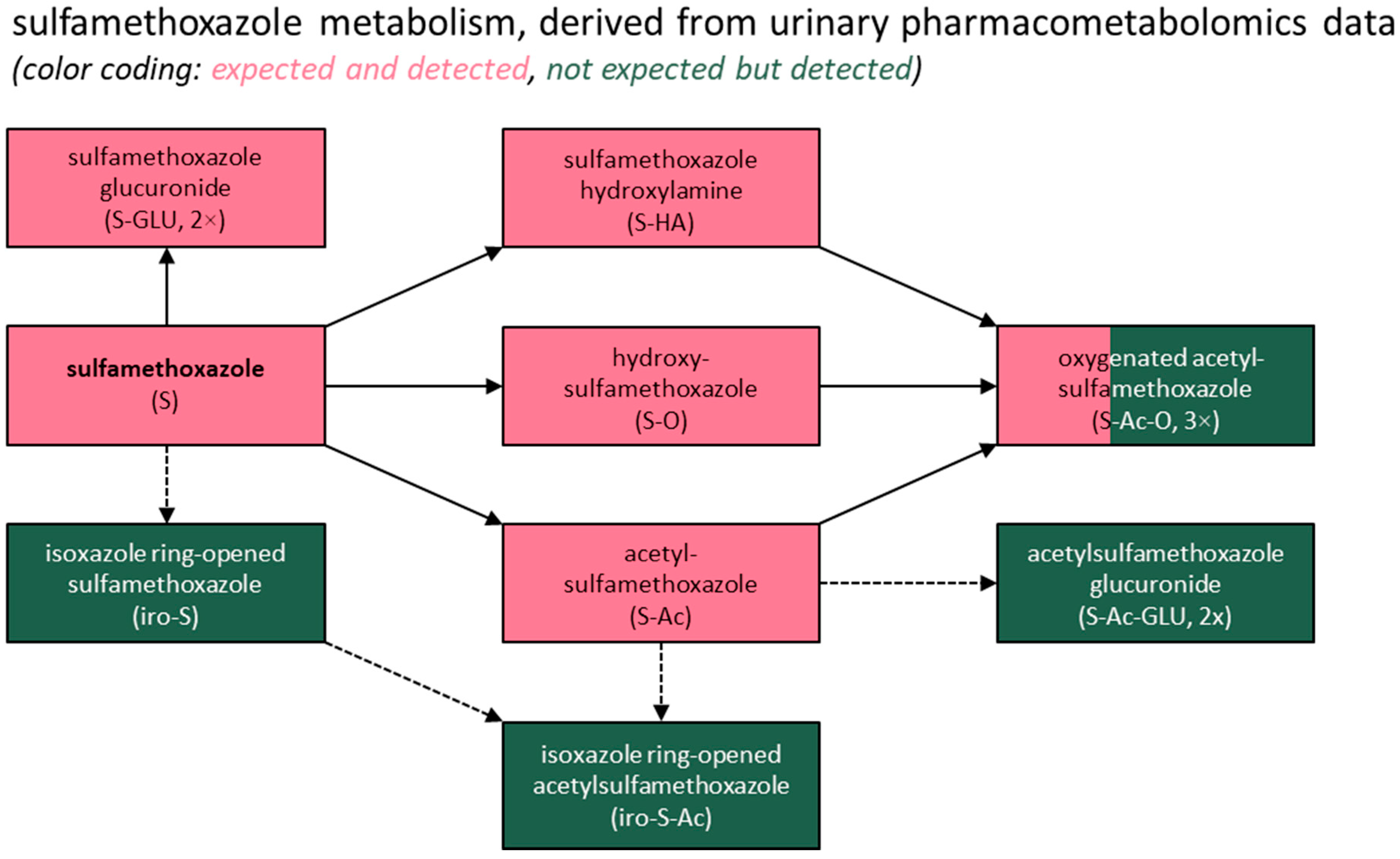
3.3. Metabolite Identification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | alanine aminotransferase |
| BMI | body mass index |
| CKD-EPI | chronic kidney disease epidemiology collaboration |
| CVID | common variable immune deficiency |
| DIA | data-independent acquisition |
| eGFR | estimated glomerular filtration rate |
| HRMS | high-resolution mass spectrometry |
| IQR | interquartile range |
| KTRs | kidney transplant recipients |
| LC | liquid chromatography |
| m/z | mass-to-charge ratio |
| NCT | national clinical trial |
| PCP | Pneumocystis jirovecii |
| PMx | pharmacometabolomics |
| PNEC | predicted no-effect concentration |
| RT | retention time |
| SWATH | sequential window acquisition of all theoretical fragment ion spectra |
| TOF | time-of-flight |
References
- Orive, G.; Lertxundi, U.; Brodin, T.; Manning, P. Greening the Pharmacy. Science (1979) 2022, 377, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Zillien, C.; Groenveld, T.; Schut, O.; Beeltje, H.; Blanco-Ania, D.; Posthuma, L.; Roex, E.; Ragas, A. Assessing City-Wide Pharmaceutical Emissions to Wastewater via Modelling and Passive Sampling. Environ. Int. 2024, 185, 108524. [Google Scholar] [CrossRef]
- Khan, U.; Bloom, R.A.; Nicell, J.A.; Laurenson, J.P. Risks Associated with the Environmental Release of Pharmaceuticals on the U.S. Food and Drug Administration “Flush List”. Sci. Total Environ. 2017, 609, 1023–1040. [Google Scholar] [CrossRef] [PubMed]
- Boxall, A.B.A. The Environmental Side Effects of Medication. EMBO Rep. 2004, 5, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Brodin, T.; Piovano, S.; Fick, J.; Klaminder, J.; Heynen, M.; Jonsson, M. Ecological Effects of Pharmaceuticals in Aquatic Systems—Impacts through Behavioural Alterations. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130580. [Google Scholar] [CrossRef]
- Marmon, P.; Owen, S.F.; Margiotta-Casaluci, L. Pharmacology-Informed Prediction of the Risk Posed to Fish by Mixtures of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) in the Environment. Environ. Int. 2021, 146, 106222. [Google Scholar] [CrossRef]
- Kidd, K.A.; Blanchfield, P.J.; Mills, K.H.; Palace, V.P.; Evans, R.E.; Lazorchak, J.M.; Flick, R.W. Collapse of a Fish Population after Exposure to a Synthetic Estrogen. Proc. Natl. Acad. Sci. USA 2007, 104, 8897–8901. [Google Scholar] [CrossRef]
- Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Ștefan, M.G.; Loghin, F. Antibiotics in the Environment: Causes and Consequences. Med. Pharm. Rep. 2020, 93, 231–240. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Zhang, T.; Fang, H.H.P. Antibiotic Resistance Genes in Water Environment. Appl. Microbiol. Biotechnol. 2009, 82, 397–414. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Vaz-Moreira, I.; Della Giustina, S.V.; Llorca, M.; Barceló, D.; Schubert, S.; Berendonk, T.U.; Michael-Kordatou, I.; Fatta-Kassinos, D.; Martinez, J.L.; et al. Antibiotic Residues in Final Effluents of European Wastewater Treatment Plants and Their Impact on the Aquatic Environment. Environ. Int. 2020, 140, 105733. [Google Scholar] [CrossRef]
- Booth, A.; Aga, D.S.; Wester, A.L. Retrospective Analysis of the Global Antibiotic Residues That Exceed the Predicted No Effect Concentration for Antimicrobial Resistance in Various Environmental Matrices. Environ. Int. 2020, 141, 105796. [Google Scholar] [CrossRef] [PubMed]
- Straub, J.O. Aquatic Environmental Risk Assessment for Human Use of the Old Antibiotic Sulfamethoxazole in Europe. Environ. Toxicol. Chem. 2016, 35, 767–779. [Google Scholar] [CrossRef]
- BIZI, M. Sulfamethoxazole Removal from Drinking Water by Activated Carbon: Kinetics and Diffusion Process. Molecules 2020, 25, 4656. [Google Scholar] [CrossRef]
- EMA. Guideline on the Environmental Risk Assessment of Medicinal Products for Human Use; EMA: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Hirsch, R.; Ternes, T.; Haberer, K.; Kratz, K.-L. Occurrence of Antibiotics in the Aquatic Environment. Sci. Total Environ. 1999, 225, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Lienert, J.; Bürki, T.; Escher, B.I. Reducing Micropollutants with Source Control: Substance Flow Analysis of 212 Pharmaceuticals in Faeces and Urine. Water Sci. Technol. 2007, 56, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Lienert, J.; Güdel, K.; Escher, B.I. Screening Method for Ecotoxicological Hazard Assessment of 42 Pharmaceuticals Considering Human Metabolism and Excretory Routes. Environ. Sci. Technol. 2007, 41, 4471–4478. [Google Scholar] [CrossRef]
- Han, E.J.; Lee, D.S. Significance of Metabolites in the Environmental Risk Assessment of Pharmaceuticals Consumed by Human. Sci. Total Environ. 2017, 592, 600–607. [Google Scholar] [CrossRef]
- Niemi, L.; Arakawa, N.; Glendell, M.; Gagkas, Z.; Gibb, S.; Anderson, C.; Pfleger, S. Co-Developing Frameworks towards Environmentally Directed Pharmaceutical Prescribing in Scotland–A Mixed Methods Study. Sci. Total Environ. 2024, 955, 176929. [Google Scholar] [CrossRef]
- Ramamoorthy, A.; Bende, G.; Chow, E.C.Y.; Dimova, H.; Hartman, N.; Jean, D.; Pahwa, S.; Ren, Y.; Shukla, C.; Yang, Y.; et al. Human Radiolabeled Mass Balance Studies Supporting the FDA Approval of New Drugs. Clin. Transl. Sci. 2022, 15, 2567–2575. [Google Scholar] [CrossRef]
- Nijdam, F.B.; Hof, M.A.J.; Blokzijl, H.; Bakker, S.J.L.; Hak, E.; Hopfgartner, G.; Klont, F.; Investigators, T. Pharmacometabolomics Enables Real-World Drug Metabolism Sciences. Metabolites 2025, 15, 39. [Google Scholar] [CrossRef]
- Elinder, C.-G.; Andersson, J.; Bolinder, G.; Tydén, G. Effectiveness of Low-Dose Cotrimoxazole Prophylaxis against Pneumocystis Carinii Pneumonia after Renal and/or Pancreas Transplantation. Transpl. Int. 1992, 5, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Bright, P.D.; Rooney, N.; Virgo, P.F.; Lock, R.J.; Johnston, S.L.; Unsworth, D.J. Laboratory Clues to Immunodeficiency; Missed Chances for Early Diagnosis? J. Clin. Pathol. 2015, 68, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Goto, N.; Oka, S. Pneumocystis Jirovecii Pneumonia in Kidney Transplantation. Transpl. Infect. Dis. 2011, 13, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Eisenga, M.F.; Gomes-Neto, A.W.; van Londen, M.; Ziengs, A.L.; Douwes, R.M.; Stam, S.P.; Osté, M.C.J.; Knobbe, T.J.; Hessels, N.R.; Buunk, A.M.; et al. Rationale and Design of TransplantLines: A Prospective Cohort Study and Biobank of Solid Organ Transplant Recipients. BMJ Open 2018, 8, e024502. [Google Scholar] [CrossRef]
- Venkataramanan, R.; Habucky, K.; Burckart, G.J.; Ptachcinski, R.J. Clinical Pharmacokinetics in Organ Transplant Patients. Clin. Pharmacokinet. 1989, 16, 134–161. [Google Scholar] [CrossRef]
- Hopfgartner, G.; Tonoli, D.; Varesio, E. High-Resolution Mass Spectrometry for Integrated Qualitative and Quantitative Analysis of Pharmaceuticals in Biological Matrices. Anal. Bioanal. Chem. 2012, 402, 2587–2596. [Google Scholar] [CrossRef]
- Gillet, L.C.; Navarro, P.; Tate, S.; Röst, H.; Selevsek, N.; Reiter, L.; Bonner, R.; Aebersold, R. Targeted Data Extraction of the MS/MS Spectra Generated by Data-Independent Acquisition: A New Concept for Consistent and Accurate Proteome Analysis. Mol. Cell Proteom. 2012, 11, O111.016717. [Google Scholar] [CrossRef]
- Rozman, B. Clinical Pharmacokinetics of Leflunomide. Clin. Pharmacokinet. 2002, 41, 421–430. [Google Scholar] [CrossRef]
- Koopman, J.; Grimme, S. From QCEIMS to QCxMS: A Tool to Routinely Calculate CID Mass Spectra Using Molecular Dynamics. J. Am. Soc. Mass. Spectrom. 2021, 32, 1735–1751. [Google Scholar] [CrossRef]
- Rieder, J. Metabolism and Techniques for Assay of Trimethoprim and Sulfamethoxazole. J. Infect. Dis. 1973, 128, S567–S573. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, D.; Ray, K.; Zhu, M. Mass Defect Filter Technique and Its Applications to Drug Metabolite Identification by High-Resolution Mass Spectrometry. J. Mass Spectrom. 2009, 44, 999–1016. [Google Scholar] [CrossRef] [PubMed]
- Mohatt, J.L.; Hu, L.; Finneran, K.T.; Strathmann, T.J. Microbially Mediated Abiotic Transformation of the Antimicrobial Agent Sulfamethoxazole under Iron-Reducing Soil Conditions. Environ. Sci. Technol. 2011, 45, 4793–4801. [Google Scholar] [CrossRef]
- Jia, Y.; Khanal, S.K.; Zhang, H.; Chen, G.-H.; Lu, H. Sulfamethoxazole Degradation in Anaerobic Sulfate-Reducing Bacteria Sludge System. Water Res. 2017, 119, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.-Y.; Birkigt, J.; Richnow, H.H.; Adrian, L. Anaerobic Transformation and Detoxification of Sulfamethoxazole by Sulfate-Reducing Enrichments and Desulfovibrio Vulgaris. Environ. Sci. Technol. 2021, 55, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Stravs, M.A.; Hollender, J. How Wastewater Reflects Human Metabolism─Suspect Screening of Pharmaceutical Metabolites in Wastewater Influent. Environ. Sci. Technol. 2024, 58, 9828–9839. [Google Scholar] [CrossRef]
- Straub, J. An Environmental Risk Assessment for Human-Use Trimethoprim in European Surface Waters. Antibiotics 2013, 2, 115–162. [Google Scholar] [CrossRef]
- Mpatani, F.M.; Aryee, A.A.; Kani, A.N.; Han, R.; Li, Z.; Dovi, E.; Qu, L. A Review of Treatment Techniques Applied for Selective Removal of Emerging Pollutant-Trimethoprim from Aqueous Systems. J. Clean. Prod. 2021, 308, 127359. [Google Scholar] [CrossRef]
- Göbel, A.; Thomsen, A.; McArdell, C.S.; Joss, A.; Giger, W. Occurrence and Sorption Behavior of Sulfonamides, Macrolides, and Trimethoprim in Activated Sludge Treatment. Environ. Sci. Technol. 2005, 39, 3981–3989. [Google Scholar] [CrossRef]
- Bonvin, F.; Omlin, J.; Rutler, R.; Schweizer, W.B.; Alaimo, P.J.; Strathmann, T.J.; McNeill, K.; Kohn, T. Direct Photolysis of Human Metabolites of the Antibiotic Sulfamethoxazole: Evidence for Abiotic Back-Transformation. Environ. Sci. Technol. 2013, 47, 6746–6755. [Google Scholar] [CrossRef]
- Wang, J.; Gardinali, P.R. Identification of Phase II Pharmaceutical Metabolites in Reclaimed Water Using High Resolution Benchtop Orbitrap Mass Spectrometry. Chemosphere 2014, 107, 65–73. [Google Scholar] [CrossRef]
- Geng, C.; Bergheaud, V.; Garnier, P.; Zhu, Y.-G.; Haudin, C.-S. Impact of Sludge Treatments on the Extractability and Fate of Acetyl Sulfamethoxazole Residues in Amended Soils. Chemosphere 2018, 194, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.J.M.; Homem, V.; Alves, A.; Vilar, V.J.P.; Manaia, C.M.; Nunes, O.C. Insights on Sulfamethoxazole Bio-Transformation by Environmental Proteobacteria Isolates. J. Hazard. Mater. 2018, 358, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Klont, F.; Kremer, D.; Gomes Neto, A.W.; Berger, S.P.; Touw, D.J.; Hak, E.; Bonner, R.; Bakker, S.J.L.; Hopfgartner, G. Metabolomics Data Complemented Drug Use Information in Epidemiological Databases: Pilot Study of Potential Kidney Donors. J. Clin. Epidemiol. 2021, 135, 10–16. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed Minimum Reporting Standards for Chemical Analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]


| Variable | At 3 Months | At 12 Months | At 24 Months |
|---|---|---|---|
| Age (years), median (IQR) | 58 (48–65) | ||
| Female (%) | 34 | ||
| Living kidney donation (%) | 70 | ||
| BMI (kg/m2), median (IQR) | 27 (24–29) | 27 (24–30) | 27 (24–30) |
| Serum albumin (g/L), median (IQR) | 44 (42–46) | 44 (42–46) | 44 (42–46) |
| Serum ALT (U/L), median (IQR) | 20 (15–26) | 20 (16–26) | 20 (16–26) |
| eGFR (mL/min/1.73 m2), median (IQR) | 48 (38–58) | 51 (40–59) | 53 (41–63) |
| Self-reported PCP prophylactic agent use | |||
| Cotrimoxazole (%) | 91 | 7 | 3 |
| Trimethoprim (%) | 1 a | 1 | 1 |
| Atovaquone (%) | 6 a | 0 | 0 |
| Pentamidine (%) | 1 | 0 | 0 |
| Analytical evidence of (presumed) drug exposure | |||
| Sulfamethoxazole (%) | 93 | 6 | 3 |
| Trimethoprim (%) | 96 | 14 | 6 |
| Self-reported immunosuppressant use | |||
| Cyclosporine (%) | 2 | 6 | 6 |
| Tacrolimus (%) | 98 | 93 | 93 |
| Azathioprine (%) | 3 | 4 | 5 |
| Mycophenolate/mycophenolate mofetil (%) | 92 | 90 | 85 |
| Everolimus (%) | 4 | 4 | 4 |
| Sirolimus (%) | 0 | 0 | 0 |
| Prednisolone (%) | 99 | 99 | 99 |
| Features with Even m/z Values | Features with Odd m/z Values | ||||||
|---|---|---|---|---|---|---|---|
| m/z | RT (min) | Rel. Median (%) a | p Value | m/z | RT (min) | Rel. Median (%) a | p Value |
| 254.058 b | 6.6 | 49.1 | 1.0 × 10−149 | 291.144 c | 5.5 | 100.0 | 6.5 × 10−189 |
| 258.089 | 3.6 | 5.3 | 3.6 × 10−142 | 307.138 | 6.0 | 7.0 | 3.1 × 10−126 |
| 270.052 | 4.4 | 4.1 | 7.8 × 10−106 | 307.139 | 6.9 | 4.7 | 6.2 × 10−107 |
| 270.053 | 6.5 | 2.6 | 1.7 × 10−70 | 307.139 | 4.8 | 2.8 | 2.6 × 10−122 |
| 296.068 | 7.8 | 185.4 | 3.5 × 10−306 | 357.083 | 4.3 | 4.5 | 5.8 × 10−116 |
| 300.100 | 5.1 | 11.8 | 1.7 × 10−158 | 357.084 | 3.2 | 1.1 | 3.1 × 10−90 |
| 312.063 | 7.1 | 5.4 | 5.0 × 10−143 | 453.157 | 4.1 | 14.0 | 5.7 × 10−115 |
| 312.063 | 8.5 | 1.3 | 3.4 × 10−120 | 453.158 | 3.2 | 6.1 | 2.4 × 10−132 |
| 312.064 | 6.0 | 21.3 | 2.3 × 10−133 | 467.175 | 4.8 | 10.1 | 2.1 × 10−131 |
| 430.088 | 5.4 | 35.2 | 9.2 × 10−173 | 483.168 | 5.2 | 2.5 | 5.0 × 10−83 |
| 430.088 | 3.3 | 1.0 | 2.5 × 10−142 | ||||
| 472.099 | 4.5 | 4.5 | 4.4 × 10−120 | ||||
| 472.099 | 6.9 | 6.2 | 7.8 × 10−126 | ||||
| Identity a,b | Abbreviation | Molecular Formula | m/z | RT (min) | Median (IQR; Range) Metabolite Abundance c in 152 KTRs (%) |
|---|---|---|---|---|---|
| Sulfamethoxazole | S | C10H11N3O3S | 254.06 | 6.6 | 16.5 (11.1–20.9; 4.6–34.6) |
| Isoxazole ring-opened sulfamethoxazole | iro-S | C10H15N3O3S | 258.09 | 3.6 | 1.6 (1.2–2.1; 0.0–6.3) |
| Hydroxysulfamethoxazole or | S-O or S-HA | C10H11N3O4S | 270.05 | 4.4 | 1.2 (0.7–1.8; 0.2–3.5) |
| sulfamethoxazole hydroxylamine | 6.5 | 0.8 (0.4–1.4; 0.0–3.5) | |||
| Acetylsulfamethoxazole | S-Ac | C12H13N3O4S | 296.07 | 7.8 | 59.2 (56.0–63.1; 39.4–80.6) |
| Isoxazole ring-opened acetylsulfamethoxazole | iro-S-Ac | C12H17N3O4S | 300.10 | 5.1 | 3.0 (2.2–3.8; 0.1–10.1) |
| Oxygenated acetylsulfamethoxazole | S-Ac-O | C12H13N3O5S | 312.06 | 6.0 | 5.3 (3.4–7.0; 1.0–24.1) |
| 7.1 | 1.4 (0.9–1.9; 0.3–3.5) | ||||
| 8.5 | 0.3 (0.2–0.4; 0.0–0.9) | ||||
| Sulfamethoxazole glucuronide | S-GLU | C16H19N3O9S | 430.09 | 3.3 | 0.2 (0.1–0.2; 0.0–0.5) |
| 5.4 | 6.7 (5.2–8.3; 0.8–14.7) | ||||
| Acetylsulfamethoxazole glucuronide | S-Ac-GLU | C18H21N3O10S | 472.10 | 4.5 | 0.7 (0.5–1.1; 0.2–3.8) |
| 6.9 | 1.1 (0.8–1.5; 0.1–5.3) |
| Identity a,b | Abbreviation | Molecular Formula | m/z | RT (min) | Median (IQR; Range) Metabolite Abundance c in 156 KTRs (%) |
|---|---|---|---|---|---|
| Trimethoprim | T | C14H18N4O3 | 291.14 | 5.5 | 74.6 (68.2–79.4; 54.3–90.8) |
| Oxygenated trimethoprim | T-O | C14H18N4O4 | 307.14 | 4.8 | 1.7 (1.3–2.2; 0.6–4.6) |
| 6.0 | 4.2 (3.2–5.3; 0.9–7.7) | ||||
| 6.9 | 2.9 (2.2–3.8; 0.9–6.5) | ||||
| Demethyl trimethoprim sulfate | T-DM-SUL | C13H16N4O6S | 357.08 | 3.2 | 0.5 (0.4–0.8; 0.1–15.4) |
| 4.3 | 2.4 (1.8–3.4; 0.7–5.5) | ||||
| Demethyl trimethoprim glucuronide | T-DM-GLU | C19H24N4O9 | 453.16 | 3.2 | 2.3 (1.7–3.2; 0.6–5.1) |
| 4.1 | 5.7 (4.2–7.3; 1.5–15.6) | ||||
| Trimethoprim glucuronide | T-GLU | C20H26N4O9 | 467.17 | 4.8 | 3.7 (2.8–4.5; 0.5–8.7) |
| 5.2 | 0.4 (0.3–0.6; 0.1–1.2) | ||||
| 5.7 | 0.1 (0.1–0.1; 0.0–1.3) | ||||
| Oxygenated trimethoprim glucuronide | T-O-GLU | C20H26N4O10 | 483.17 | 5.2 | 0.9 (0.6–1.3; 0.1–3.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hof, M.A.J.; de Haan, H.; Stepanovic, S.; Bakker, S.J.L.; Hak, E.; Hopfgartner, G.; Klont, F.; Investigators, T. Pharmacometabolomics Study of Sulfamethoxazole and Trimethoprim in Kidney Transplant Recipients: Real-World Metabolism and Urinary Excretion. Metabolites 2025, 15, 473. https://doi.org/10.3390/metabo15070473
Hof MAJ, de Haan H, Stepanovic S, Bakker SJL, Hak E, Hopfgartner G, Klont F, Investigators T. Pharmacometabolomics Study of Sulfamethoxazole and Trimethoprim in Kidney Transplant Recipients: Real-World Metabolism and Urinary Excretion. Metabolites. 2025; 15(7):473. https://doi.org/10.3390/metabo15070473
Chicago/Turabian StyleHof, Marieke A. J., Hessel de Haan, Stepan Stepanovic, Stephan J. L. Bakker, Eelko Hak, Gérard Hopfgartner, Frank Klont, and TransplantLines Investigators. 2025. "Pharmacometabolomics Study of Sulfamethoxazole and Trimethoprim in Kidney Transplant Recipients: Real-World Metabolism and Urinary Excretion" Metabolites 15, no. 7: 473. https://doi.org/10.3390/metabo15070473
APA StyleHof, M. A. J., de Haan, H., Stepanovic, S., Bakker, S. J. L., Hak, E., Hopfgartner, G., Klont, F., & Investigators, T. (2025). Pharmacometabolomics Study of Sulfamethoxazole and Trimethoprim in Kidney Transplant Recipients: Real-World Metabolism and Urinary Excretion. Metabolites, 15(7), 473. https://doi.org/10.3390/metabo15070473








