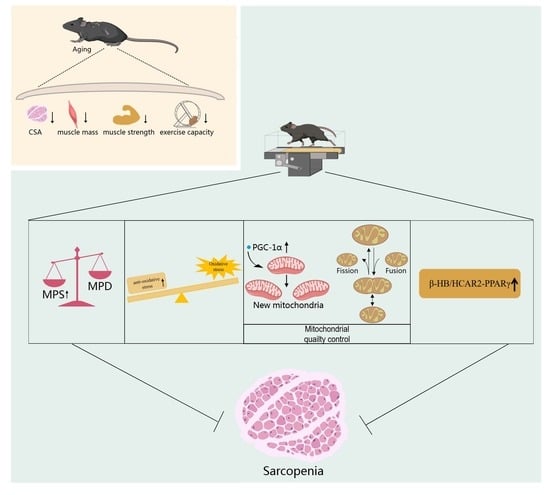
Aerobic Exercise Delays Age-Related Sarcopenia in Mice via Alleviating Imbalance in Mitochondrial Quality Control
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Study Design, and Ethics
2.2. Exercise Protocol
2.3. Mice Functional Tests
2.4. Mice Blood Indexes
2.5. Histological Examination of Gastrocnemius Muscle
2.6. CAT, GSH Activity, and MDA Content in Gastrocnemius Muscle
2.7. Total RNA Extraction and Quantitative Reverse Transcription PCR (RT-qPCR)
2.8. Western Blotting
2.9. Statistical Analysis
3. Results
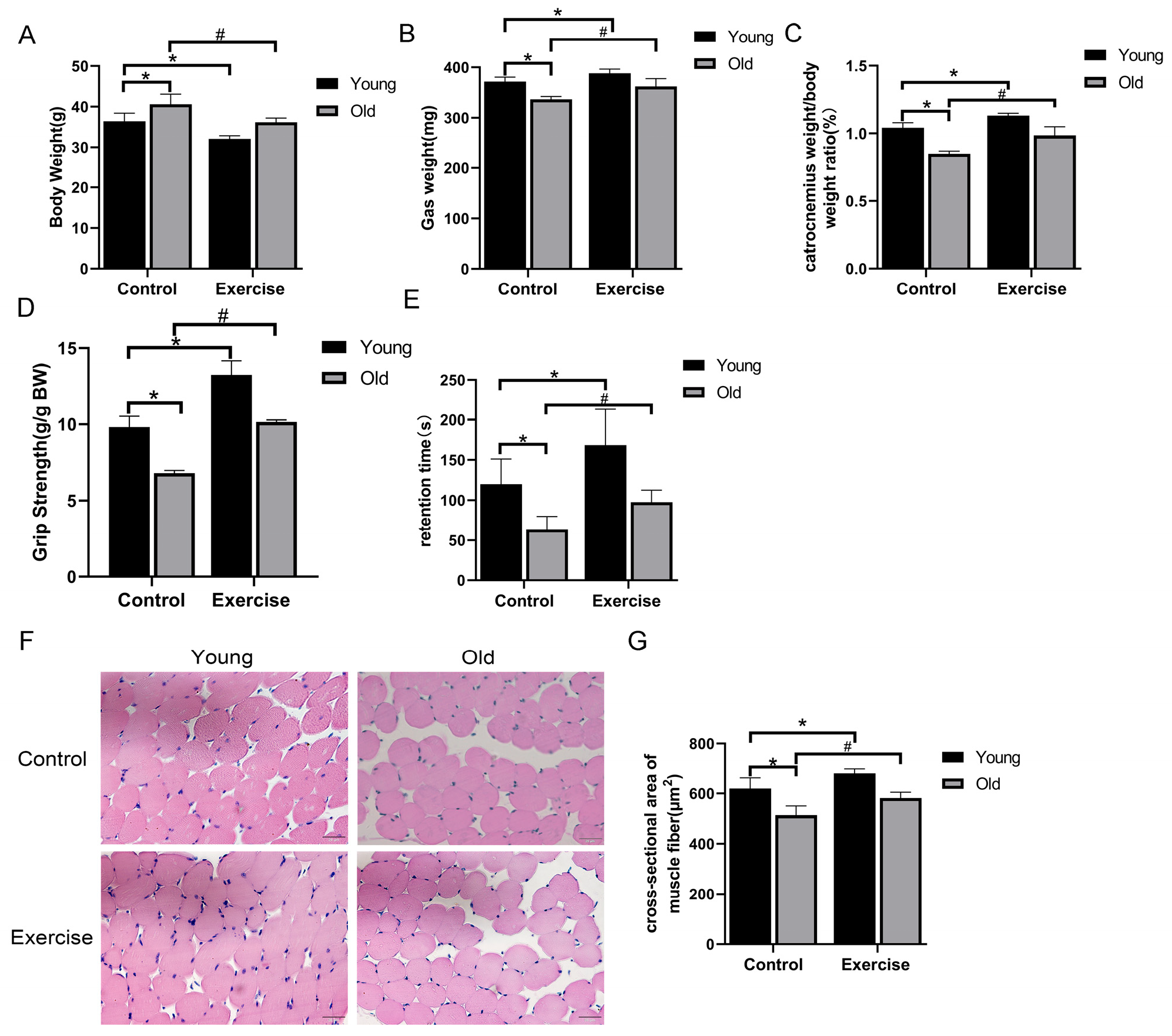
3.1. Aerobic Exercise Mitigates Age-Related Changes in Body Weight, Muscle Function, and Morphology
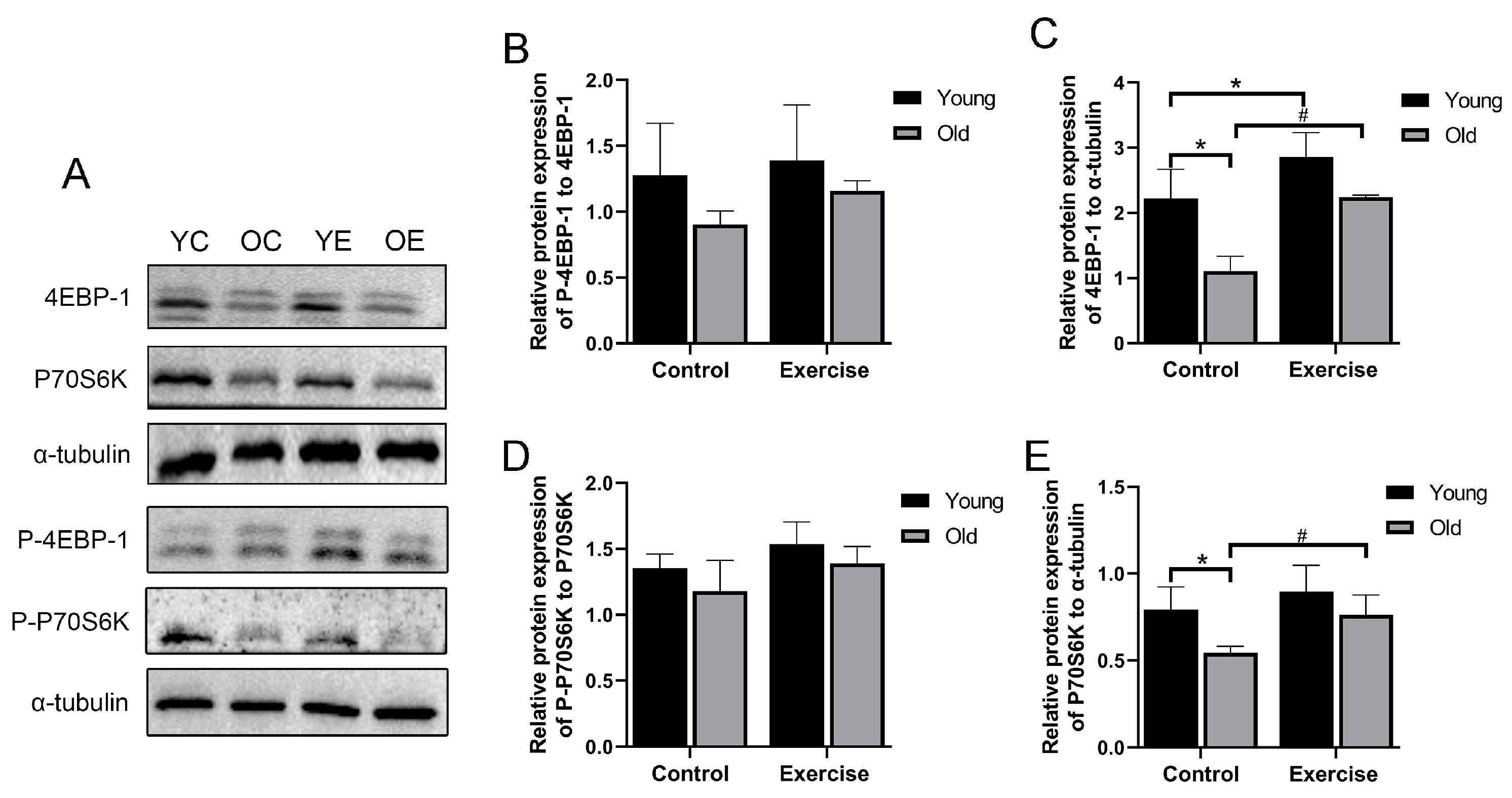
3.2. Aerobic Exercise Promotes Protein Expression of Muscle Protein Synthesis-Related Signaling Pathway Molecules in Aging Mice
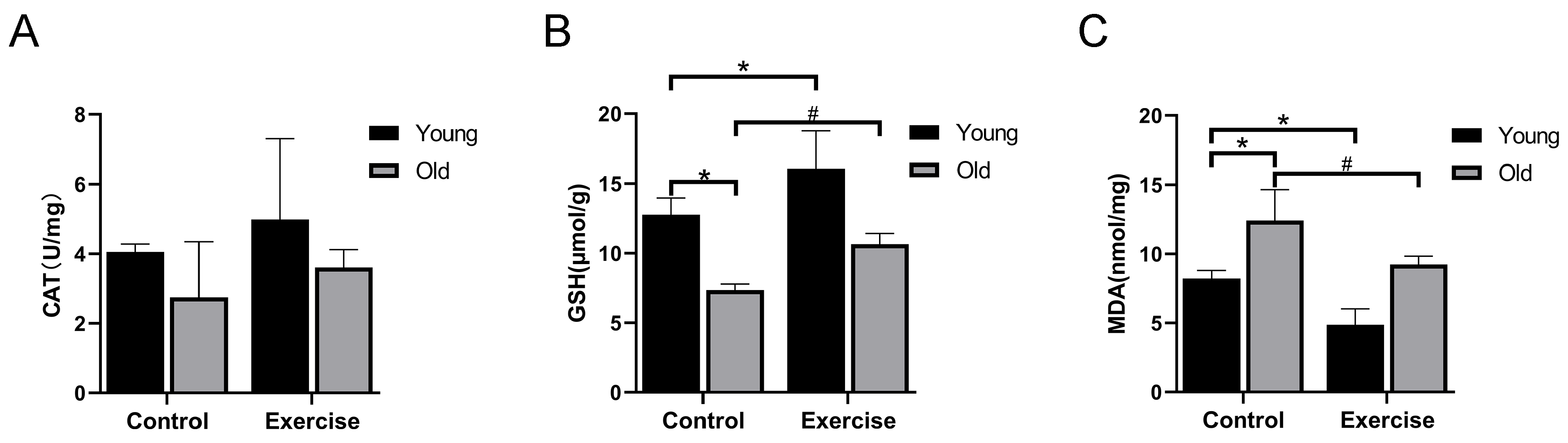
3.3. Aerobic Exercise Enhances the Antioxidant Capacity of Skeletal Muscle of Aging Mice
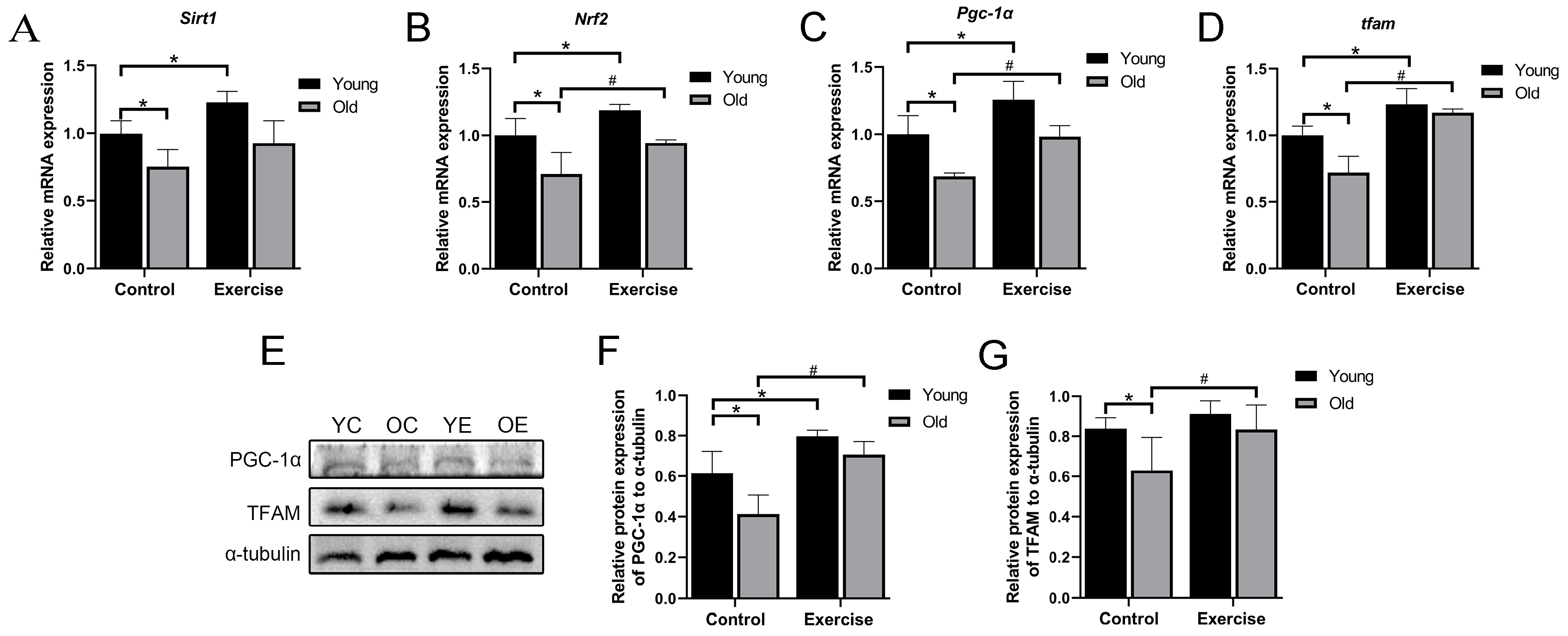
3.4. Aerobic Exercise Regulates mRNA and Protein Expression Related to Mitochondrial Biogenesis in Skeletal Muscle of Aging Mice
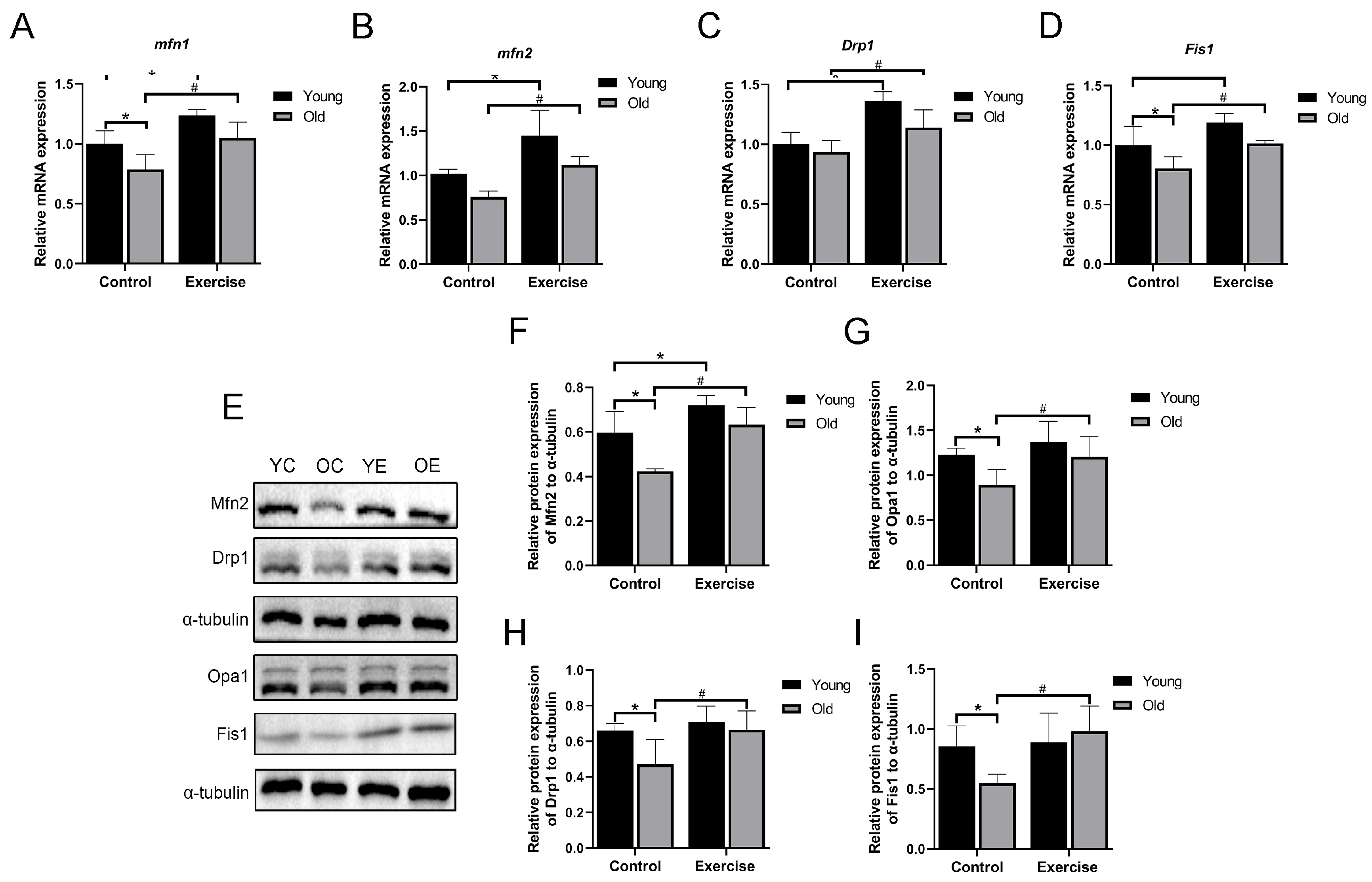
3.5. Aerobic Exercise Regulates mRNA and Protein Expression Related to Mitochondrial Dynamics in Skeletal Muscle of Aging Mice
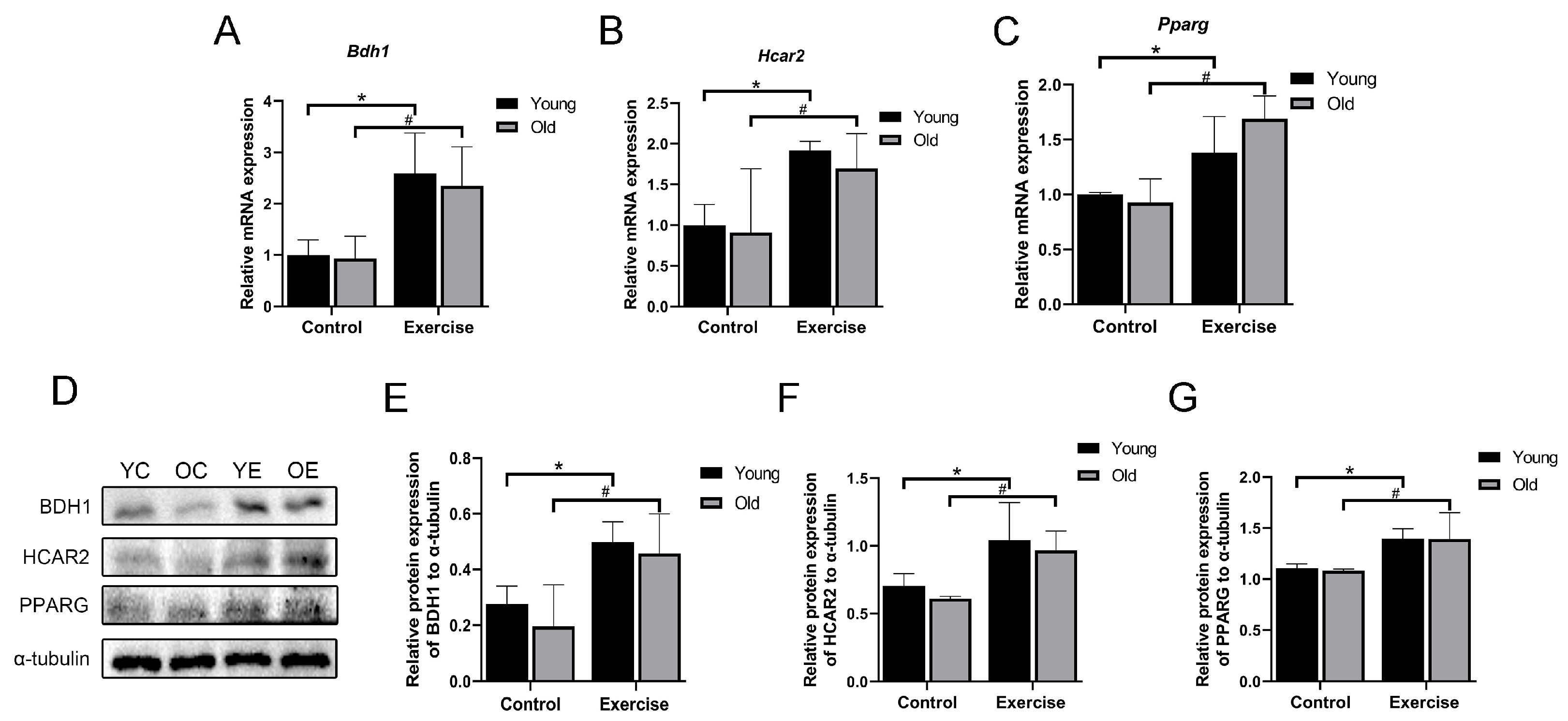
3.6. Aerobic Exercise Upregulates Blood β-HB Level in Aging Mice
3.7. Aerobic Exercise Promotes the mRNA and Protein Expression of β-HB/HCAR2-PPARG Signaling Pathway in Skeletal Muscle of Aging Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antunes, A.C.; Araujo, D.A.; Verissimo, M.T.; Amaral, T.F. Sarcopenia and hospitalisation costs in older adults: A cross-sectional study. Nutr. Diet. 2017, 74, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of muscle atrophy and hypertrophy: Implications in health and disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef] [PubMed]
- Gouspillou, G.; Sgarioto, N.; Kapchinsky, S.; Purves-Smith, F.; Norris, B.; Pion, C.H.; Barbat-Artigas, S.; Lemieux, F.; Taivassalo, T.; Morais, J.A.; et al. Increased sensitivity to mitochondrial permeability transition and myonuclear translocation of endonuclease G in atrophied muscle of physically active older humans. FASEB J. 2014, 28, 1621–1633. [Google Scholar] [CrossRef]
- Gouspillou, G.; Bourdel-Marchasson, I.; Rouland, R.; Calmettes, G.; Franconi, J.M.; Deschodt-Arsac, V.; Diolez, P. Alteration of mitochondrial oxidative phosphorylation in aged skeletal muscle involves modification of adenine nucleotide translocator. Biochim. Biophys. Acta 2010, 1797, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.C.; Verdin, E. β-Hydroxybutyrate: A Signaling Metabolite. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef]
- Deng, Y.; Xie, M.; Li, Q.; Xu, X.; Ou, W.; Zhang, Y.; Xiao, H.; Yu, H.; Zheng, Y.; Liang, Y.; et al. Targeting Mitochondria-Inflammation Circuit by β-Hydroxybutyrate Mitigates HFpEF. Circ. Res. 2021, 128, 232–245. [Google Scholar] [CrossRef]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef]
- Veech, R.L. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 309–319. [Google Scholar] [CrossRef]
- Veech, R.L.; Bradshaw, P.C.; Clarke, K.; Curtis, W.; Pawlosky, R.; King, M.T. Ketone bodies mimic the life span extending properties of caloric restriction. IUBMB Life 2017, 69, 305–314. [Google Scholar] [CrossRef]
- Spigoni, V.; Cinquegrani, G.; Iannozzi, N.T.; Frigeri, G.; Maggiolo, G.; Maggi, M.; Parello, V.; Dei Cas, A. Activation of G protein-coupled receptors by ketone bodies: Clinical implication of the ketogenic diet in metabolic disorders. Front. Endocrinol. 2022, 13, 972890. [Google Scholar] [CrossRef]
- Chen, L.; So, W.Y.; Li, S.Y.; Cheng, Q.; Boucher, B.J.; Leung, P.S. Niacin-induced hyperglycemia is partially mediated via niacin receptor GPR109a in pancreatic islets. Mol. Cell Endocrinol. 2015, 404, 56–66. [Google Scholar] [CrossRef]
- Manickam, R.; Duszka, K.; Wahli, W. PPARs and Microbiota in Skeletal Muscle Health and Wasting. Int. J. Mol. Sci. 2020, 21, 8056. [Google Scholar] [CrossRef]
- Crossland, H.; Constantin-Teodosiu, D.; Greenhaff, P.L. The Regulatory Roles of PPARs in Skeletal Muscle Fuel Metabolism and Inflammation: Impact of PPAR Agonism on Muscle in Chronic Disease, Contraction and Sepsis. Int. J. Mol. Sci. 2021, 22, 9775. [Google Scholar] [CrossRef]
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2012, 2, 1143–1211. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, H.; Zeng, Z.; Lv, J.; Huang, J.; Wu, X.; Wang, M.; Xu, J.; Fan, J.; Chen, N. MicroRNA profiling of different exercise interventions for alleviating skeletal muscle atrophy in naturally aging rats. J. Cachexia Sarcopenia Muscle 2023, 14, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, M.M.; Halling, J.F.; Moller, H.D.; Plomgaard, P.; Regenberg, B.; Ringholm, S.; Pilegaard, H. Regulation of apoptosis and autophagy in mouse and human skeletal muscle with aging and lifelong exercise training. Exp. Gerontol. 2018, 111, 141–153. [Google Scholar] [CrossRef]
- Paoli, A.; Cancellara, P.; Pompei, P.; Moro, T. Ketogenic Diet and Skeletal Muscle Hypertrophy: A Frenemy Relationship? J. Hum. Kinet. 2019, 68, 233–247. [Google Scholar] [CrossRef]
- Takahara, S.; Soni, S.; Phaterpekar, K.; Kim, T.T.; Maayah, Z.H.; Levasseur, J.L.; Silver, H.L.; Freed, D.H.; Ferdaoussi, M.; Dyck, J.R.B. Chronic exogenous ketone supplementation blunts the decline of cardiac function in the failing heart. ESC Heart Fail. 2021, 8, 5606–5612. [Google Scholar] [CrossRef]
- Khariv, V.; Pang, K.; Servatius, R.J.; David, B.T.; Goodus, M.T.; Beck, K.D.; Heary, R.F.; Elkabes, S. Toll-like receptor 9 deficiency impacts sensory and motor behaviors. Brain Behav. Immun. 2013, 32, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Graber, T.G.; Maroto, R.; Fry, C.S.; Brightwell, C.R.; Rasmussen, B.B. Measuring Exercise Capacity and Physical Function in Adult and Older Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Bai, L.; Lu, Z.; Gu, R.; Zhao, D.; Yan, F.; Bai, J. TRPV4 contributes to ER stress and inflammation: Implications for Parkinson’s disease. J. Neuro Inflamm. 2022, 19, 26. [Google Scholar] [CrossRef]
- Yang, Q.; Chan, P. Skeletal Muscle Metabolic Alternation Develops Sarcopenia. Aging Dis. 2022, 13, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.W.; Ding, K.H.; Pennington, C.; Chao, Y.J.; Wu, Y.D.; Howard, B.; Immel, D.; Borlongan, C.; McNeil, P.L.; Bollag, W.B.; et al. Age-related loss of muscle mass and bone strength in mice is associated with a decline in physical activity and serum leptin. Bone 2006, 39, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Sayed, R.K.; de Leonardis, E.C.; Guerrero-Martinez, J.A.; Rahim, I.; Mokhtar, D.M.; Saleh, A.M.; Abdalla, K.E.; Pozo, M.J.; Escames, G.; Lopez, L.C.; et al. Identification of morphological markers of sarcopenia at early stage of aging in skeletal muscle of mice. Exp. Gerontol. 2016, 83, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, H.; Zeng, Z.; Wu, L.; Zhang, Y.; Guo, Y.; Lv, J.; Wang, C.; Fan, J.; Chen, N. Lifelong Aerobic Exercise Alleviates Sarcopenia by Activating Autophagy and Inhibiting Protein Degradation via the AMPK/PGC-1alpha Signaling Pathway. Metabolites 2021, 11, 323. [Google Scholar] [CrossRef]
- Tanaka, T.; Nishimura, A.; Nishiyama, K.; Goto, T.; Numaga-Tomita, T.; Nishida, M. Mitochondrial dynamics in exercise physiology. Pflug. Arch. 2020, 472, 137–153. [Google Scholar] [CrossRef]
- Liang, J.; Zeng, Z.; Zhang, Y.; Chen, N. Regulatory role of exercise-induced autophagy for sarcopenia. Exp. Gerontol. 2020, 130, 110789. [Google Scholar] [CrossRef]
- Wang, Q.; Lan, X.; Ke, H.; Xu, S.; Huang, C.; Wang, J.; Wang, X.; Huang, T.; Wu, X.; Chen, M.; et al. Histone β-hydroxybutyrylation is critical in reversal of sarcopenia. Aging Cell 2024, 23, e14284. [Google Scholar] [CrossRef]
- Taggart, A.K.; Kero, J.; Gan, X.; Cai, T.Q.; Cheng, K.; Ippolito, M.; Ren, N.; Kaplan, R.; Wu, K.; Wu, T.J.; et al. (D)-β-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem. 2005, 280, 26649–26652. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef]
- Crane, J.D.; Macneil, L.G.; Tarnopolsky, M.A. Long-term aerobic exercise is associated with greater muscle strength throughout the life span. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Cartee, G.D.; Hepple, R.T.; Bamman, M.M.; Zierath, J.R. Exercise Promotes Healthy Aging of Skeletal Muscle. Cell Metab. 2016, 23, 1034–1047. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Pierzchalski, P.; Szczepanik, M.; Bonior, J.; Zoladz, J.A. Multifactorial Mechanism of Sarcopenia and Sarcopenic Obesity. Role of Physical Exercise, Microbiota and Myokines. Cells 2022, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, C.; Xie, L.; Niu, Y.; Fu, L. Aerobic Exercise Improves Mitochondrial Function in Sarcopenia Mice Through Sestrin2 in an AMPKalpha2-Dependent Manner. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1161–1168. [Google Scholar] [CrossRef]
- Jiao, J.; Demontis, F. Skeletal muscle autophagy and its role in sarcopenia and organismal aging. Curr. Opin. Pharmacol. 2017, 34, 1–6. [Google Scholar] [CrossRef]
- Serova, M.; Didry-Barca, B.; Deloux, R.; Foucault, A.S.; Veillet, S.; Lafont, R.; Dilda, P.J.; Latil, M. BIO101 stimulates myoblast differentiation and improves muscle function in adult and old mice. J. Cachexia Sarcopenia Muscle 2024, 15, 55–66. [Google Scholar] [CrossRef]
- Xia, Z.; Cholewa, J.; Zhao, Y.; Yang, Y.Q.; Shang, H.Y.; Guimaraes-Ferreira, L.; Naimo, M.A.; Su, Q.S.; Zanchi, N.E. Hypertrophy-Promoting Effects of Leucine Supplementation and Moderate Intensity Aerobic Exercise in Pre-Senescent Mice. Nutrients 2016, 8, 246. [Google Scholar] [CrossRef]
- Rommel, C.; Bodine, S.C.; Clarke, B.A.; Rossman, R.; Nunez, L.; Stitt, T.N.; Yancopoulos, G.D.; Glass, D.J. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat. Cell Biol. 2001, 3, 1009–1013. [Google Scholar] [CrossRef]
- Kumar, V.; Selby, A.; Rankin, D.; Patel, R.; Atherton, P.; Hildebrandt, W.; Williams, J.; Smith, K.; Seynnes, O.; Hiscock, N.; et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J. Physiol. 2009, 587, 211–217. [Google Scholar] [CrossRef]
- Mayhew, D.L.; Kim, J.S.; Cross, J.M.; Ferrando, A.A.; Bamman, M.M. Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans. J. Appl. Physiol. 2009, 107, 1655–1662. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Zhou, Y.; Ni, G.; Su, Q.; Chen, Z.; Chen, Z.; Li, J.; Chen, X.; Hou, X.; et al. Association of LEPR and ANKK1 Gene Polymorphisms with Weight Gain in Epilepsy Patients Receiving Valproic Acid. Int. J. Neuropsychopharmacol. 2015, 18, pyv021. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berni, R.; Romi, M.; Cantini, C.; Hausman, J.F.; Guerriero, G.; Cai, G. Functional Molecules in Locally-Adapted Crops: The Case Study of Tomatoes, Onions, and Sweet Cherry Fruits from Tuscany in Italy. Front. Plant Sci. 2018, 9, 1983. [Google Scholar] [CrossRef]
- Altenhofer, S.; Radermacher, K.A.; Kleikers, P.W.; Wingler, K.; Schmidt, H.H. Evolution of NADPH Oxidase Inhibitors: Selectivity and Mechanisms for Target Engagement. Antioxid. Redox Signal. 2015, 23, 406–427. [Google Scholar] [CrossRef] [PubMed]
- Bowen, T.S.; Schuler, G.; Adams, V. Skeletal muscle wasting in cachexia and sarcopenia: Molecular pathophysiology and impact of exercise training. J. Cachexia Sarcopenia Muscle 2015, 6, 197–207. [Google Scholar] [CrossRef]
- Ding, Q.; Yang, D.; Zhang, W.; Lu, Y.; Zhang, M.; Wang, L.; Li, X.; Zhou, L.; Wu, Q.; Pan, W.; et al. Antioxidant and anti-aging activities of the polysaccharide TLH-3 from Tricholoma lobayense. Int. J. Biol. Macromol. 2016, 85, 133–140. [Google Scholar] [CrossRef]
- Yuan, S.; Yang, Y.; Li, J.; Tan, X.; Cao, Y.; Li, S.; Hong, H.D.; Liu, L.; Zhang, Q. Ganoderma lucidum Rhodiola compound preparation prevent D-galactose-induced immune impairment and oxidative stress in aging rat model. Sci. Rep. 2020, 10, 19244. [Google Scholar] [CrossRef]
- Sadowska-Krepa, E.; Rzetecki, A.; Zajac-Gawlak, I.; Nawrat-Szoltysik, A.; Rozpara, M.; Mikulakova, W.; Stanek, A.; Palka, T. Comparison of selected prooxidant-antioxidant balance and bone metabolism indicators and BDNF levels between older women with different levels of physical activity. BMC Geriatr. 2023, 23, 489. [Google Scholar] [CrossRef]
- Xie, G.; Xu, Z.; Li, F.; Kong, M.; Wang, P.; Shao, Y. Aerobic Exercise Ameliorates Cognitive Disorder and Declined Oxidative Stress via Modulating the Nrf2 Signaling Pathway in D-galactose Induced Aging Mouse Model. Neurochem. Res. 2024, 49, 2408–2422. [Google Scholar] [CrossRef]
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef]
- Joseph, A.M.; Adhihetty, P.J.; Buford, T.W.; Wohlgemuth, S.E.; Lees, H.A.; Nguyen, L.M.; Aranda, J.M.; Sandesara, B.D.; Pahor, M.; Manini, T.M.; et al. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell 2012, 11, 801–809. [Google Scholar] [CrossRef]
- Sebastian, D.; Sorianello, E.; Segales, J.; Irazoki, A.; Ruiz-Bonilla, V.; Sala, D.; Planet, E.; Berenguer-Llergo, A.; Munoz, J.P.; Sanchez-Feutrie, M.; et al. Mfn2 deficiency links age-related sarcopenia and impaired autophagy to activation of an adaptive mitophagy pathway. EMBO J. 2016, 35, 1677–1693. [Google Scholar] [CrossRef] [PubMed]
- Tezze, C.; Romanello, V.; Desbats, M.A.; Fadini, G.P.; Albiero, M.; Favaro, G.; Ciciliot, S.; Soriano, M.E.; Morbidoni, V.; Cerqua, C.; et al. Age-Associated Loss of OPA1 in Muscle Impacts Muscle Mass, Metabolic Homeostasis, Systemic Inflammation, and Epithelial Senescence. Cell Metab. 2017, 25, 1374–1389.e6. [Google Scholar] [CrossRef]
- Wang, L.; Gao, J.; Liu, J.; Siedlak, S.L.; Torres, S.; Fujioka, H.; Huntley, M.L.; Jiang, Y.; Ji, H.; Yan, T.; et al. Mitofusin 2 Regulates Axonal Transport of Calpastatin to Prevent Neuromuscular Synaptic Elimination in Skeletal Muscles. Cell Metab. 2018, 28, 400–414.e8. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Vermulst, M.; Wang, Y.E.; Chomyn, A.; Prolla, T.A.; McCaffery, J.M.; Chan, D.C. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 2010, 141, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Distefano, G.; Standley, R.A.; Dube, J.J.; Carnero, E.A.; Ritov, V.B.; Stefanovic-Racic, M.; Toledo, F.G.; Piva, S.R.; Goodpaster, B.H.; Coen, P.M. Chronological Age Does not Influence Ex-vivo Mitochondrial Respiration and Quality Control in Skeletal Muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 535–542. [Google Scholar] [CrossRef]
- Faitg, J.; Leduc-Gaudet, J.P.; Reynaud, O.; Ferland, G.; Gaudreau, P.; Gouspillou, G. Effects of Aging and Caloric Restriction on Fiber Type Composition, Mitochondrial Morphology and Dynamics in Rat Oxidative and Glycolytic Muscles. Front. Physiol. 2019, 10, 420. [Google Scholar] [CrossRef]
- Yeo, D.; Kang, C.; Gomez-Cabrera, M.C.; Vina, J.; Ji, L.L. Intensified mitophagy in skeletal muscle with aging is downregulated by PGC-1alpha overexpression In Vivo. Free Radic. Biol. Med. 2019, 130, 361–368. [Google Scholar] [CrossRef]
- Senior, T. Home and away. Br. J. Gen. Pract. 2016, 66, 266. [Google Scholar] [CrossRef]
- Mujawar, S.Y.; Vaigankar, D.C.; Dubey, S.K. Biological characterization of Bacillus flexus strain SSAI1 transforming highly toxic arsenite to less toxic arsenate mediated by periplasmic arsenite oxidase enzyme encoded by aioAB genes. Biometals 2021, 34, 895–907. [Google Scholar] [CrossRef]
- Koutnik, A.P.; D’Agostino, D.P.; Egan, B. Anticatabolic Effects of Ketone Bodies in Skeletal Muscle. Trends Endocrinol. Metab. 2019, 30, 227–229. [Google Scholar] [CrossRef]
- Stubbs, B.J.; Koutnik, A.P.; Volek, J.S.; Newman, J.C. From bedside to battlefield: Intersection of ketone body mechanisms in geroscience with military resilience. Geroscience 2021, 43, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Morales, P.; Tapia, E.; Pedraza-Chaverri, J. β-Hydroxybutyrate: A signaling metabolite in starvation response? Cell. Signal. 2016, 28, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.K.; Kim, D.H.; Bang, E.; Choi, Y.J.; Chung, H.Y. β-Hydroxybutyrate Suppresses Lipid Accumulation in Aged Liver through GPR109A-mediated Signaling. Aging Dis. 2020, 11, 777–790. [Google Scholar] [CrossRef]
- Kovacs, Z.; Brunner, B.; Ari, C. Beneficial Effects of Exogenous Ketogenic Supplements on Aging Processes and Age-Related Neurodegenerative Diseases. Nutrients 2021, 13, 2197. [Google Scholar] [CrossRef] [PubMed]
- Petridou, A.; Tsalouhidou, S.; Tsalis, G.; Schulz, T.; Michna, H.; Mougios, V. Long-term exercise increases the DNA binding activity of peroxisome proliferator-activated receptor gamma in rat adipose tissue. Metabolism 2007, 56, 1029–1036. [Google Scholar] [CrossRef]
- Jeninga, E.H.; Bugge, A.; Nielsen, R.; Kersten, S.; Hamers, N.; Dani, C.; Wabitsch, M.; Berger, R.; Stunnenberg, H.G.; Mandrup, S.; et al. Peroxisome proliferator-activated receptor gamma regulates expression of the anti-lipolytic G-protein-coupled receptor 81 (GPR81/Gpr81). J. Biol. Chem. 2009, 284, 26385–26393. [Google Scholar] [CrossRef]

| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Nox2 | TGATCCTGCTGCCAGTGTGTC | GTGAGGTTCCTGTCCAGTTGTCTTC |
| Nrf2 | TGAGATCTGATGCCTTCTTCTTGCC | CACGACGAGTGTACCTGGGAGTAGC |
| Sod1 | TATGGGGACAATACACAAGGCT | CGGGCCACCATGTTTCTTAGA |
| Sirt1 | TGATTGGCACCGATCCTCG | CCACAGCGTCATATCATCCAG |
| Pgc-1α | GAAAGGGCCAAACAGAGAGA | GTAAATCACACGGCGCTCTT |
| tfam | AACACCCAGATGCAAAACTTTCA | GACTTGGAGTTAGCTGCTCTTT |
| Mfn1 | ATGGCAGAAACGGTATCTCCA | GCCCTCAGTAACAAACTCCAGT |
| Mfn2 | AGAACTGGACCCGGTTACCA | CACTTCGCTGATACCCCTGA |
| Drp1 | GAAGTGGTGCAGTGGAAATGAC | GTTTCTATTGGGAACCACTGCC |
| Fis1 | AGAGCACGCAATTTGAATATGCC | ATAGTCCCGCTGTTCCTCTTT |
| Bdh1 | CACCGGAGTGTGTGTAAGGC | CTCGTCTGAACCCGTAGCTC |
| Hcar2 | CTGGAGGTTCGGAGGCATC | TCGCCATTTTTGGTCATCATGT |
| Pparg | CCAGGTGACCCTCCTCAAGT | CTGCAGCAGGTTGTCTTGGA |
| β-actin | ACCACACCTTCTACAATGAG | ACGACCAGAGGCATACAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, D.; Wang, L.; Gao, H.; Wang, Z.; Li, K.; Ma, X.; Zhao, L.; Xiao, W. Aerobic Exercise Delays Age-Related Sarcopenia in Mice via Alleviating Imbalance in Mitochondrial Quality Control. Metabolites 2025, 15, 472. https://doi.org/10.3390/metabo15070472
Zhu D, Wang L, Gao H, Wang Z, Li K, Ma X, Zhao L, Xiao W. Aerobic Exercise Delays Age-Related Sarcopenia in Mice via Alleviating Imbalance in Mitochondrial Quality Control. Metabolites. 2025; 15(7):472. https://doi.org/10.3390/metabo15070472
Chicago/Turabian StyleZhu, Danlin, Lian Wang, Haoyang Gao, Ze Wang, Ke Li, Xiaotong Ma, Linlin Zhao, and Weihua Xiao. 2025. "Aerobic Exercise Delays Age-Related Sarcopenia in Mice via Alleviating Imbalance in Mitochondrial Quality Control" Metabolites 15, no. 7: 472. https://doi.org/10.3390/metabo15070472
APA StyleZhu, D., Wang, L., Gao, H., Wang, Z., Li, K., Ma, X., Zhao, L., & Xiao, W. (2025). Aerobic Exercise Delays Age-Related Sarcopenia in Mice via Alleviating Imbalance in Mitochondrial Quality Control. Metabolites, 15(7), 472. https://doi.org/10.3390/metabo15070472