Investigating Systemic Metabolic Effects of Betula alba Leaf Extract in Rats via Urinary Metabolomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Preparation and Characterization of Birch Extract
2.2. Treatment Protocol
2.3. Urine Output and Excretion of Na+ and K+
2.4. Metabolomic Analysis of Urine Samples
2.5. Statistical Analyses
3. Results
3.1. Characterization of Birch Extract
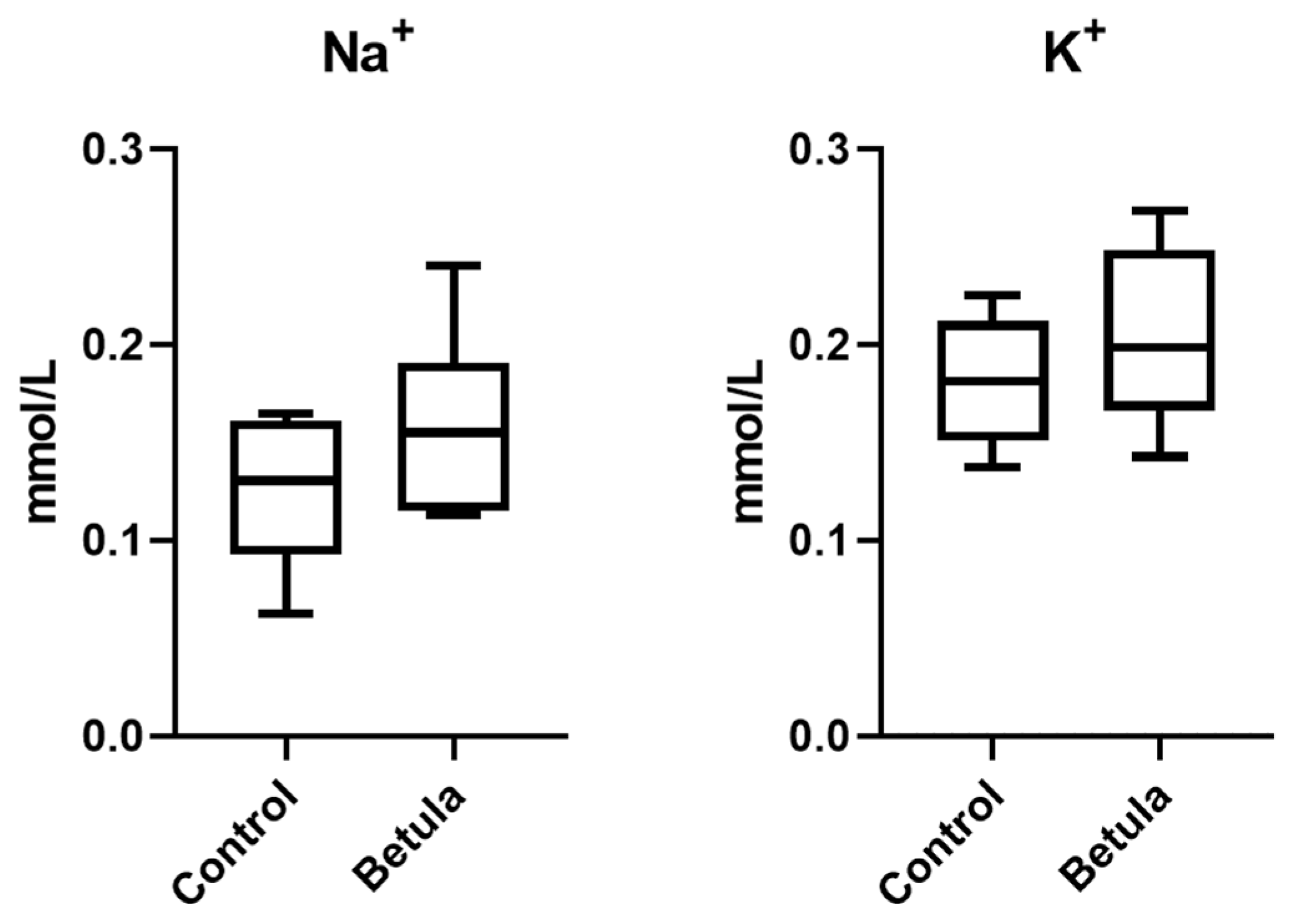
3.2. Urine Output and Excretion of Na+ and K+
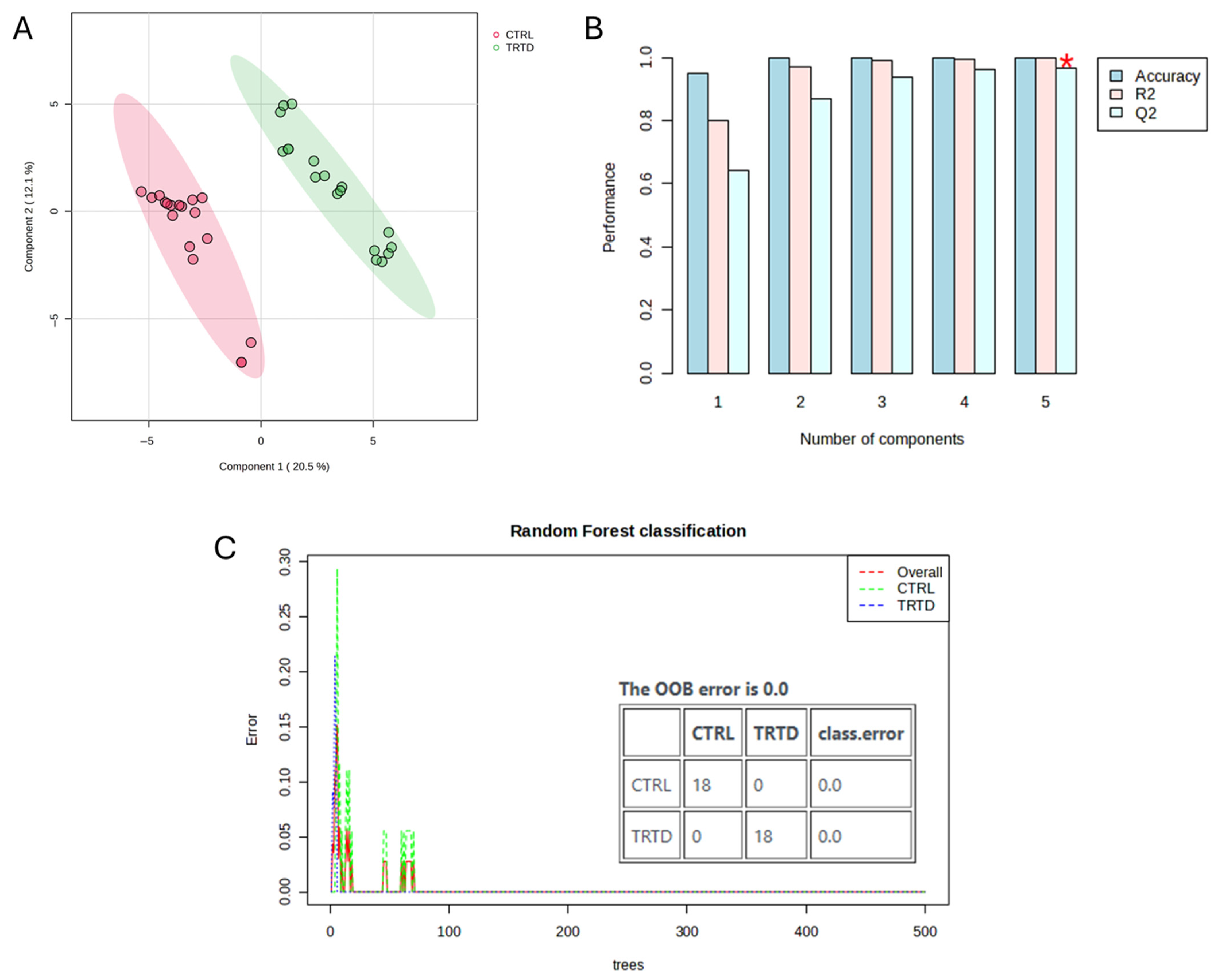
3.3. Metabolomic Analysis of Urinary Output
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rastogi, S.; Pandey, M.M.; Kumar Singh Rawat, A. Medicinal Plants of the Genus Betula—Traditional Uses and a Phytochemical–Pharmacological Review. J. Ethnopharmacol. 2015, 159, 62–83. [Google Scholar] [CrossRef]
- Rokaya, M.B.; Münzbergová, Z.; Timsina, B. Ethnobotanical Study of Medicinal Plants from the Humla District of Western Nepal. J. Ethnopharmacol. 2010, 130, 485–504. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Song, M.-J. Analysis and Recordings of Orally Transmitted Knowledge about Medicinal Plants in the Southern Mountainous Region of Korea. J. Ethnopharmacol. 2011, 134, 676–696. [Google Scholar] [CrossRef] [PubMed]
- Popowski, D.; Kruk, A.; Pawłowska, K.A.; Dolzkho, D.; Korczak, M.; Piwowarski, J.P.; Roszko, M.; Granica, S. Evaluating Birch Leaf Tea as a Functional Herbal Beverage: Beneficial Impact on the Urinary Tract, and Metabolism in Human Organism. Food Res. Int. 2024, 189, 114481. [Google Scholar] [CrossRef]
- Shikov, A.N.; Narkevich, I.A.; Flisyuk, E.V.; Luzhanin, V.G.; Pozharitskaya, O.N. Medicinal Plants from the 14th Edition of the Russian Pharmacopoeia, Recent Updates. J. Ethnopharmacol. 2021, 268, 113685. [Google Scholar] [CrossRef] [PubMed]
- Paniagua-Zambrana, N.Y.; Bussmann, R.W.; Kikvidze, Z.; Khojimatov, O.K. Betula pendula Roth Betula pubescens Ehrh. Betulacae. In Ethnobotany of the Mountain Regions of Eastern Europe: Carpathians; Bussmann, R.W., Paniagua-Zambrana, N.Y., Kikvidze, Z., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 1–16. ISBN 978-3-030-98744-2. [Google Scholar]
- Assessment Report on Betula pendula Roth and/or Betula pubescens Ehrh. as Well as Hybrids of Both Species, Folium; European Medicines Agency: Amsterdam, The Netherlands, 2014.
- Agostoni, C.; Bresson, J.-L.; Fairweather-Tait, S.; Flynn, A.; Golly, I.; Korhonen, H.; Lagiou, P.; Løvik, M.; Marchelli, R.; Martin, A.; et al. Scientific Opinion on the Substantiation of Health Claims Related to Various Food(s)/Food Constituent(s) Claiming an Increase in Renal Water Elimination, “Kidneys Health”, “Urinary Health”, “Bladder Health”, “Health of Lower Urinary Tract”, “Blood Health”, “Elimination”, “Urinary System Benefits” and/or “Supports/Promotes the Excretory Function of the Kidney”, and Treatment/Prevention of Renal Gravel/Kidney Stones and Urinary Tract Infections. EFSA J. 2010, 8, 1742. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R.; Rousi, M.; Bryant, J.; Sorsa, S.; Keinänen, M.; Sikanen, H. Chemical Diversity of Several Betulaceae Species: Comparison of Phenolics and Terpenoids in Northern Birch Stems. Trees 1996, 11, 16–22. [Google Scholar] [CrossRef]
- Weston, R.J.; Smith, G.J. Sesquiterpenes from the Inner Bark of the Silver Birch and the Paper Birch. Nat. Prod. Commun. 2012, 7, 1934578X1200700202. [Google Scholar] [CrossRef]
- Hou, P.Y.; Bi, K.S.; Geng, L.L.; Zhao, X.; Meng, X.; Ma, B.J.; Zeng, Y.; Wang, X.F.; Chen, X.H. Toxic Effects of Euphorbia Pekinensis Rupr. and Development of a Validated UPLC/MS/MS Method for Profiling of Urine Metabolic Changes. Anal. Methods 2013, 5, 953–960. [Google Scholar] [CrossRef]
- Quinn, R.A.; Melnik, A.V.; Vrbanac, A.; Fu, T.; Patras, K.A.; Christy, M.P.; Bodai, Z.; Belda-Ferre, P.; Tripathi, A.; Chung, L.K.; et al. Global Chemical Effects of the Microbiome Include New Bile-Acid Conjugations. Nature 2020, 579, 123–129. [Google Scholar] [CrossRef]
- Peron, G.; Pellizzaro, A.; Brun, P.; Schievano, E.; Mammi, S.; Sut, S.; Castagliuolo, I.; Dall’Acqua, S. Antiadhesive Activity and Metabolomics Analysis of Rat Urine after Cranberry (Vaccinium macrocarpon Aiton) Administration. J. Agric. Food Chem. 2017, 65, 5657–5667. [Google Scholar] [CrossRef] [PubMed]
- Dragićević, A.; Kitic, D.; Pavlovic, D. Biological Activity of the Birch Leaf and Bark. Lek. Sirovine 2022, 42, 89–95. [Google Scholar] [CrossRef]
- Rickling Karl-Werner, B.G. Saponins in the Leaves of Birch? Hemolytic Dammarane Triterpenoid Esters of Betula pendula. Planta Med. 1993, 59, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Chumak, O.; Bezrukavyi, Y.; Maloshtan, L.; Maloshtan, A. Studies of the Anti-Inflammatory and Diuretic Properties of Tablets with Dense Extract of Betula pendula Leaves. Dan. Sci. J. 2019, 1, 46–48. [Google Scholar]
- Arumugham, V.B.; Shahin, M.H. Therapeutic Uses of Diuretic Agents; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557838/ (accessed on 25 June 2025).
- Bell, R.; Mandalia, R. Diuretics and the Kidney. BJA Educ. 2022, 22, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Raff, H. CORT, Cort, B, Corticosterone, and Now Cortistatin: Enough Already! Endocrinology 2016, 157, 3307–3308. [Google Scholar] [CrossRef]
- Shipston, M.J. Glucocorticoid Action in the Anterior Pituitary Gland: Insights from Corticotroph Physiology. Curr. Opin. Endocr. Metab. Res. 2022, 25, 100358. [Google Scholar] [CrossRef]
- Usa, K.; Singh, R.J.; Netzel, B.C.; Liu, Y.; Raff, H.; Liang, M. Renal Interstitial Corticosterone and 11-Dehydrocorticosterone in Conscious Rats. Am. J. Physiol. Ren. Physiol. 2007, 293, F186–F192. [Google Scholar] [CrossRef]
- Huebbe, P.; Nikolai, S.; Schloesser, A.; Herebian, D.; Campbell, G.; Glüer, C.-C.; Zeyner, A.; Demetrowitsch, T.; Schwarz, K.; Metges, C.; et al. An Extract from the Atlantic Brown Algae Saccorhiza Polyschides Counteracts Diet-Induced Obesity in Mice via a Gut Related Multifactorial Mechanisms. Oncotarget 2017, 8, 73501–73515. [Google Scholar] [CrossRef]
- Qin, S.; Tian, J.; Zhao, Y.; Wang, L.; Wang, J.; Liu, S.; Meng, J.; Wang, F.; Liu, C.; Han, J.; et al. Gardenia Extract Protects against Intrahepatic Cholestasis by Regulating Bile Acid Enterohepatic Circulation. J. Ethnopharmacol. 2024, 319, 117083. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Wang, W. Bile Acid Signaling in Renal Water Regulation. Am. J. Physiol. Ren. Physiol. 2019, 317, F73–F76. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, H.; Tang, J. Interplay between Bile Acids and Intestinal Microbiota: Regulatory Mechanisms and Therapeutic Potential for Infections. Pathogens 2024, 13, 702. [Google Scholar] [CrossRef] [PubMed]
- Trottier, J.; Białek, A.; Caron, P.; Straka, R.J.; Milkiewicz, P.; Barbier, O. Profiling Circulating and Urinary Bile Acids in Patients with Biliary Obstruction before and after Biliary Stenting. PLoS ONE 2011, 6, e22094. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.-U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.J.; Jiang, C.; Patterson, A.D. An Intestinal Microbiota-Farnesoid X Receptor Axis Modulates Metabolic Disease. Gastroenterology 2016, 151, 845–859. [Google Scholar] [CrossRef]
- Ramírez-Mejía, M.; Castillo-Castañeda, S.; Pal, S.; Qi, X.; Méndez-Sánchez, N. The Multifaceted Role of Bilirubin in Liver Disease: A Literature Review. J. Clin. Transl. Hepatol. 2024, 12, 939–948. [Google Scholar] [CrossRef]
- Yang, G.; Shufan, G.; Rashim, S.; Sumit, B.; Katherine, S.; Ming, Z.; Jiong, L.; Yifan, T.; Chenning, Z.; Jinbao, W.; et al. Glucuronidation: Driving Factors and Their Impact on Glucuronide Disposition. Drug Metab. Rev. 2017, 49, 105–138. [Google Scholar] [CrossRef]
- Wen, H.; Yang, H.; An, Y.J.; Kim, J.M.; Lee, D.H.; Jin, X.; Park, S.; Min, K.-J.; Park, S. Enhanced Phase II Detoxification Contributes to Beneficial Effects of Dietary Restriction as Revealed by Multi-Platform Metabolomics Studies. Mol. Cell. Proteom. 2013, 12, 575–586. [Google Scholar] [CrossRef]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular Mechanism of PPARα Action and Its Impact on Lipid Metabolism, Inflammation and Fibrosis in Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef]
- Khodorova, N.V.; Rietman, A.; Rutledge, D.N.; Schwarz, J.; Piedcoq, J.; Pilard, S.; Siebelink, E.; Kok, F.J.; Tomé, D.; Mensink, M.; et al. Urinary Medium-Chained Acyl-Carnitines Sign High Caloric Intake Whereas Short-Chained Acyl-Carnitines Sign High -Protein Diet within a High-Fat, Hypercaloric Diet in a Randomized Crossover Design Dietary Trial. Nutrients 2021, 13, 1191. [Google Scholar] [CrossRef]
- Peron, G.; Sut, S.; Dal Ben, S.; Voinovich, D.; Dall’Acqua, S. Untargeted UPLC-MS Metabolomics Reveals Multiple Changes of Urine Composition in Healthy Adult Volunteers after Consumption of Curcuma longa L. Extract. Food Res. Int. 2020, 127, 108730. [Google Scholar] [CrossRef] [PubMed]
- Mortishire-Smith, B.J.; Becker, S.M.; Simeone, A.; Melidis, L.; Balasubramanian, S. A Photoredox Reaction for the Selective Modification of 5-Carboxycytosine in DNA. J. Am. Chem. Soc. 2023, 145, 10505–10511. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Mo, J.; Lu, M.; Wang, H. Detection of Human Urinary 5-Hydroxymethylcytosine by Stable Isotope Dilution HPLC-MS/MS Analysis. Anal. Chem. 2015, 87, 1846–1852. [Google Scholar] [CrossRef] [PubMed]
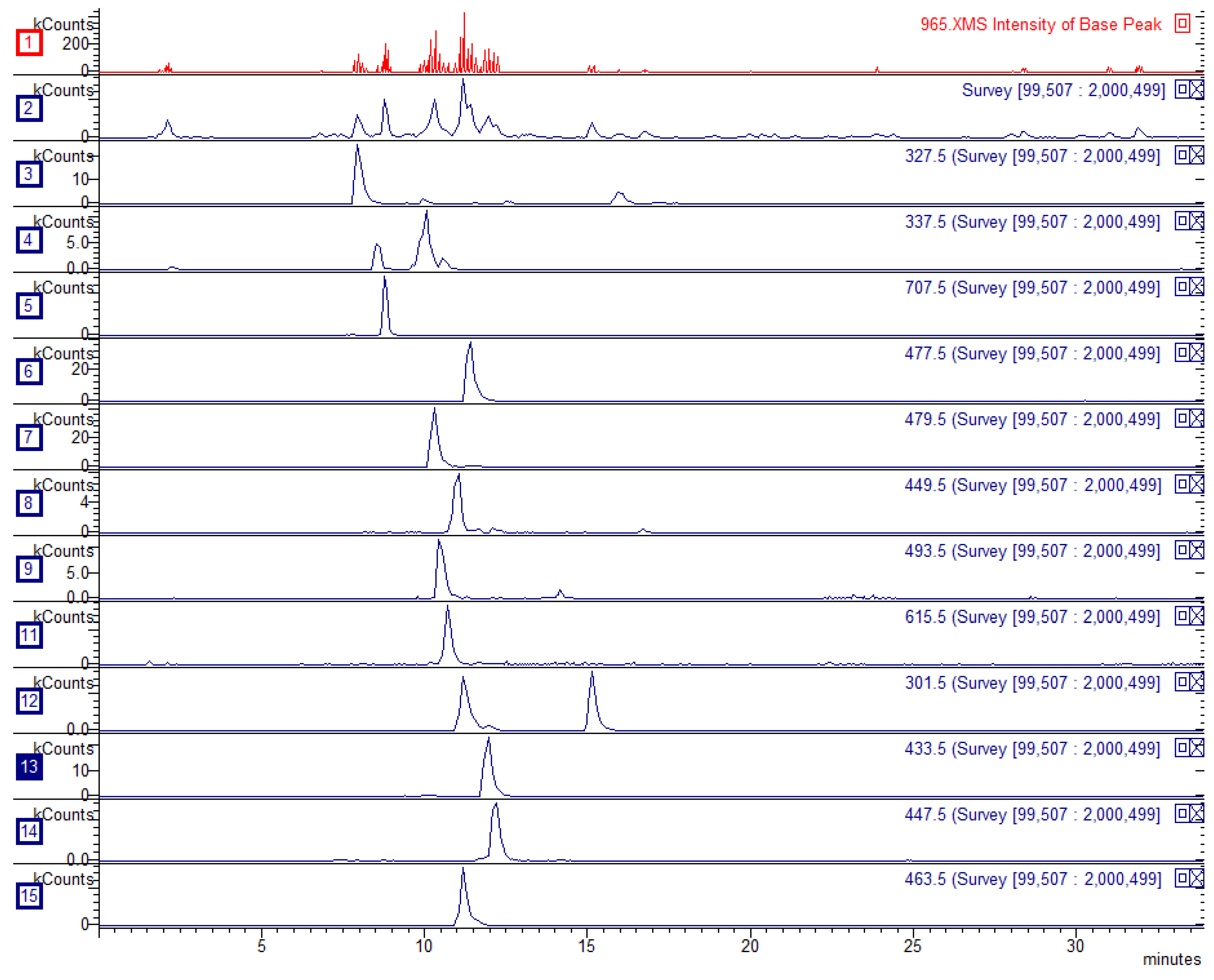

| RT (min) | Identification | [M−H]− | Fragments | w/w % |
|---|---|---|---|---|
| 7.6 | Betuloside | 327 | 147 119 | 0.22 |
| 8.2 | Coumaroylquinic acid | 337 | 191 163 127 | 0.04 |
| 8.4 | Chlorogenic acid | 353 | 191 | 0.28 |
| 9.5 | Coumaroylquinic acid isomer | 337 | 163 | 0.11 |
| 9.7 | Myricetin glucoside | 479 | 316 271 243 | 0.32 |
| 9.9 | Myricetin glucuronide | 493 | 317 179 151 | 0.10 |
| 10.1 | Quercetin galloyl glucoside | 615 | 463 301 271 179 | 0.05 |
| 10.7 | Myricetin-pentoside | 449 | 316 271 179 | 0.07 |
| 10.8 | Hyperoside | 463 | 301 151 179 | 0.53 |
| 11.0 | Quercetin-glucuronide | 477 | 301 151 179 | 0.36 |
| 11.5 | Quercetin-pentoside | 433 | 301 151 179 | 0.21 |
| 11.8 | Quercetin-rhamnoside | 447 | 301 | 0.15 |
| 14.7 | Quercetin | 301 | 179 151 107 | 0.13 |
| Total polyphenols | 2.73 |
| RT (min) | m/z | log2(FC) * | FDR-p | Formula | Tentative Identification (Adduct Type) | Main Fragments |
|---|---|---|---|---|---|---|
| 3.4 | 370.2215 | −3.2262 | <0.0001 | C16H31NO4 | Nonanoylcarnitine ([M+H+HCOONa]+) | 243.1599 85.0262 |
| 1.1 | 156.0401 | 4.204 | <0.0001 | C5H5N3O3 | Cytosine-5-carboxylic acid | 138.0298 |
| 5.6 | 430.2962 | 1.7467 | 0.0001327 | C26H43NO6 | Glycocholic acid ([M+H−2H2O]+) | 466.3172 |
| 5.6 | 466.3172 | 1.506 | 0.0001327 | C26H43NO6 | Glycocholic acid | 412.2855 448.3066 |
| 5.6 | 448.3064 | 2.2355 | 0.00017282 | C26H43NO6 | Glycocholic acid ([M+H−H2O]+) | 412.2854 |
| 5.6 | 412.2856 | 1.6676 | 0.00017282 | C24H34O3 | 3-Oxo-4,6-choladienoic acid ([M+H+ACN]+) | 371.2551 |
| 1.5 | 158.1166 | −1.4373 | 0.00017282 | C8H15NO2 | Pipecolic acid betaine | 128.0686 |
| 5.6 | 931.6222 | 3.555 | 0.00057128 | C26H43NO6 | Glycocholic acid ([2M+H]+) | 466.3172 |
| 7 | 332.3315 | 1.7681 | 0.00057128 | C23H46NO2 | Oleoylcholine ([M+H−2H2O]+) | ND |
| 6.2 | 817.5814 | 1.1926 | 0.00057128 | C24H40O5 | Alpha-muricholic acid ([2M+H]+) | ND |
| 6.2 | 355.2634 | 1.0807 | 0.00057128 | C24H35O2 | Cholic acid fragment | ND |
| 1.1 | 118.0858 | 2.7488 | 0.0018969 | C5H11NO2 | Betaine | ND |
| 3.6 | 338.0892 | 2.2069 | 0.0024467 | C15H15NO8 | 2,8-Dihydroxyquinoline-beta-D-glucuronide | 162.0558 134.0598 |
| 4.1 | 211.1421 | −1.2327 | 0.0026492 | C9H21N2O2 | Trimethyllysine | ND |
| 4.1 | 461.1088 | 1.2708 | 0.0030694 | C22H22O12 | Unknown | 353.0505 |
| 5.8 | 405.2627 | 2.4142 | 0.0051249 | C24H36O5 | 7a,12a-Dihydroxy-3-oxo-4-cholenoic acid | 387.2562 369.2414 |
| 3.6 | 675.1685 | 2.635 | 0.012798 | C15H15NO8 | 2,8-Dihydroxyquinoline-beta-D-glucuronide ([2M+H]+) | 338.0891 |
| 4.4 | 447.0927 | 1.2704 | 0.016324 | C21H18O11 | Genistein 5-O-glucuronide | 271.0601 |
| 6.3 | 329.2110 | 1.0332 | 0.017429 | C21H30O4 | Corticosterone ([M+H−H2O]+) | ND |
| 4.4 | 345.2061 | 1.3707 | 0.020438 | C21H28O4 | 11-Dehydrocorticosterone | ND |
| 5.9 | 281.1041 | −1.339 | 0.02119 | C17H25NO6 | Deca-2,5,8-trienedioylcarnitine | 85.0277 |
| 4.8 | 299.1292 | −1.2686 | 0.02119 | C19H14N4 | Bilin | 273.1166 |
| 1.9 | 244.116 | −1.4018 | 0.021233 | NA | Unknown | NA |
| 1.7 | 268.1049 | 1.6417 | 0.023064 | C10H13N5O4 | Adenosine | 136.0623 |
| 3.7 | 340.1041 | 3.0114 | 0.026528 | C15H18NO8 | Hydroxy-methoxyindole glucuronide | 164.0703 |
| 5.6 | 929.5964 | −1.7314 | 0.028311 | C49H89N2O10PS | PC(LTE4/P-18:0) | ND |
| 4.3 | 164.0711 | 2.583 | 0.032292 | C9H9NO2 | 3-Methyldioxyindole | 146.0593 118.0655 |
| 1.6 | 483.2411 | −1.4317 | 0.039056 | NA | Unknown | NA |
| 3.7 | 164.0711 | 2.7965 | 0.041092 | C9H9NO2 | 3-Methyldioxyindole isomer | 146.0593 118.0655 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peron, G.; Yerkassymova, A.; Zengin, G.; Dall’Acqua, S. Investigating Systemic Metabolic Effects of Betula alba Leaf Extract in Rats via Urinary Metabolomics. Metabolites 2025, 15, 471. https://doi.org/10.3390/metabo15070471
Peron G, Yerkassymova A, Zengin G, Dall’Acqua S. Investigating Systemic Metabolic Effects of Betula alba Leaf Extract in Rats via Urinary Metabolomics. Metabolites. 2025; 15(7):471. https://doi.org/10.3390/metabo15070471
Chicago/Turabian StylePeron, Gregorio, Alina Yerkassymova, Gokhan Zengin, and Stefano Dall’Acqua. 2025. "Investigating Systemic Metabolic Effects of Betula alba Leaf Extract in Rats via Urinary Metabolomics" Metabolites 15, no. 7: 471. https://doi.org/10.3390/metabo15070471
APA StylePeron, G., Yerkassymova, A., Zengin, G., & Dall’Acqua, S. (2025). Investigating Systemic Metabolic Effects of Betula alba Leaf Extract in Rats via Urinary Metabolomics. Metabolites, 15(7), 471. https://doi.org/10.3390/metabo15070471









