Effect of Thermal Processing by Spray Drying on Key Ginger Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Ginger Preparation
2.2. Spray Drying
2.2.1. Materials
2.2.2. Drying Parameters
2.3. Sample Preparation for Analysis
2.4. Antioxidants and Total Polyphenols
2.5. High-Resolution Flow Infusion Electrospray Ionisation Mass Spectrometry (HR-FIE-MS)
2.6. Data Analysis
3. Results
3.1. Spray Drying Parameters
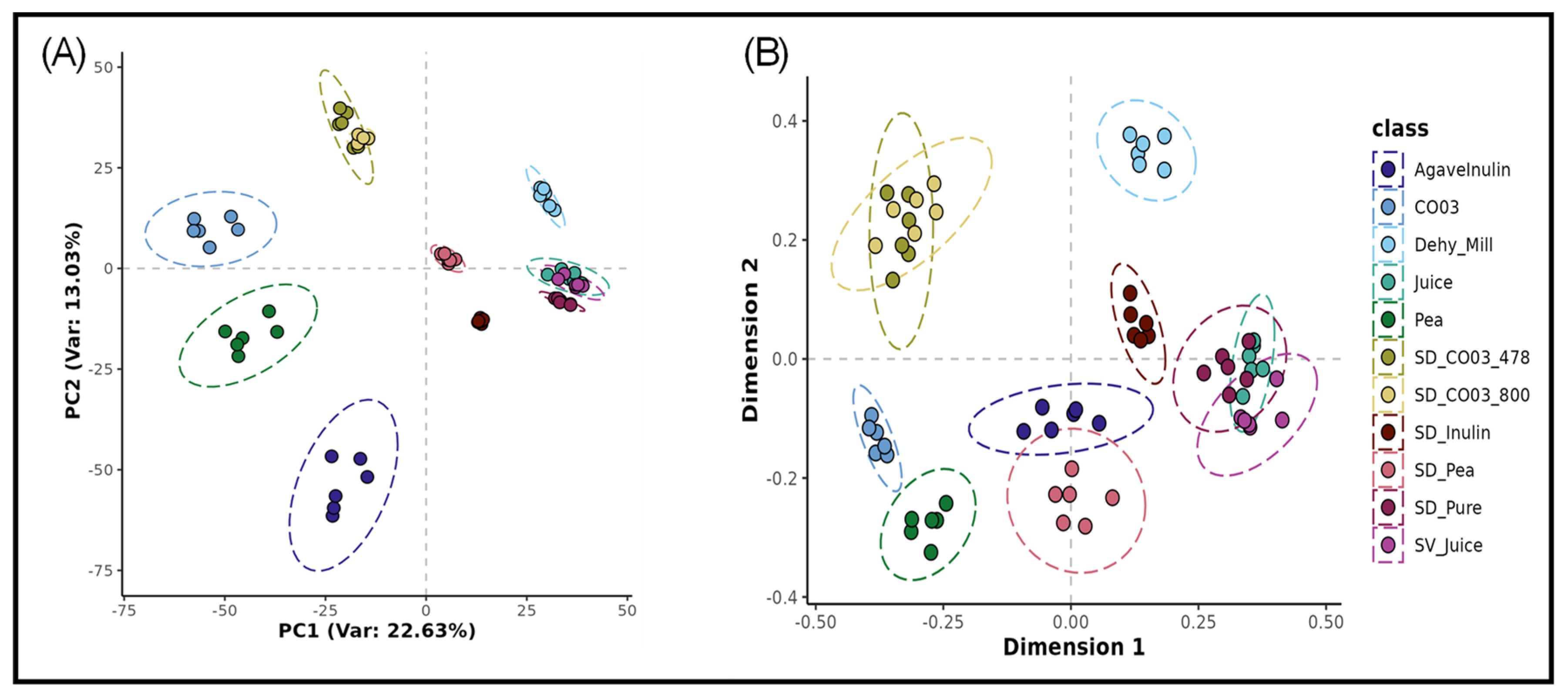
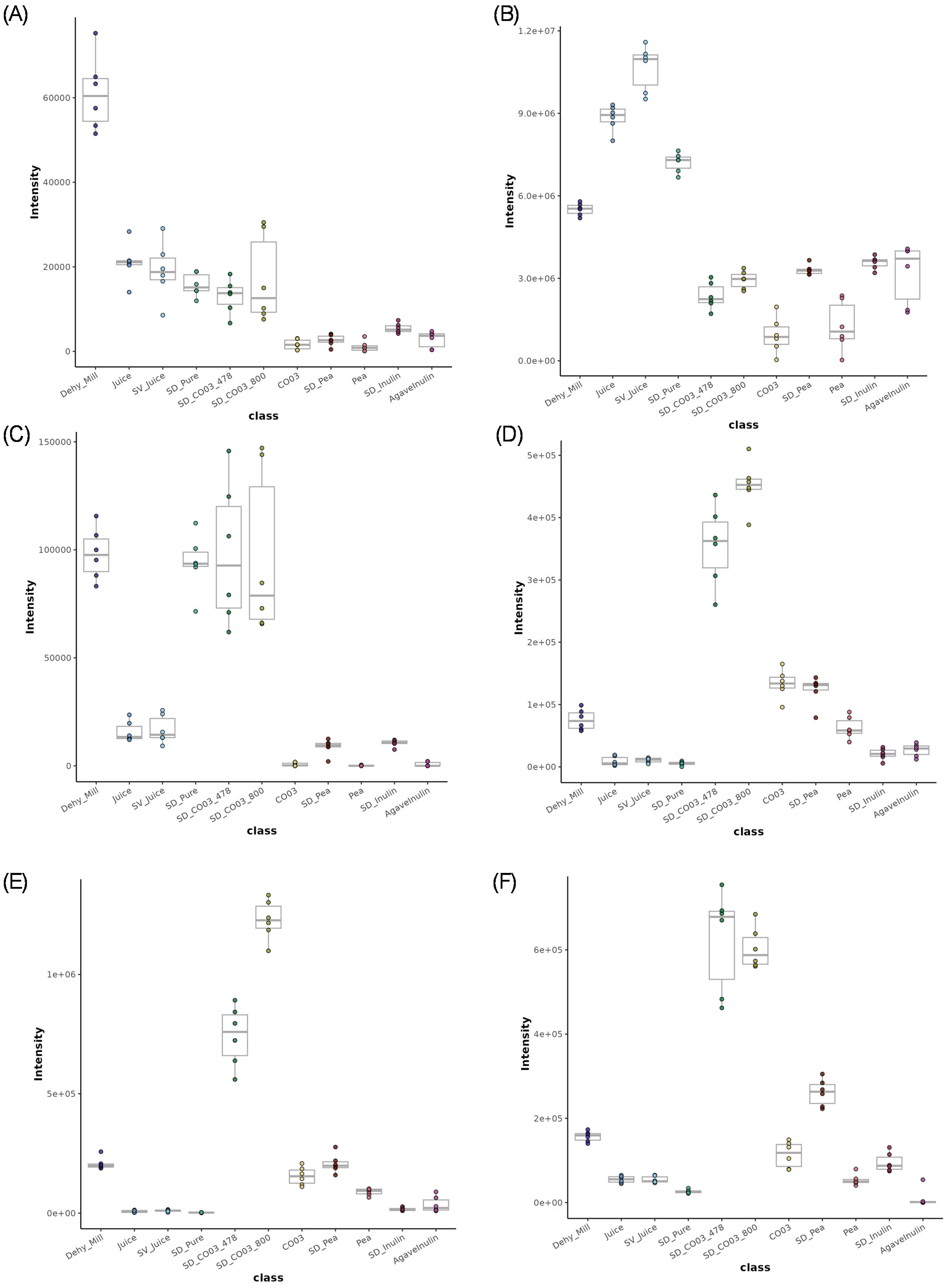
3.2. Metabolomics
3.3. Antioxidants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Clear gum | CO03 |
| master mix | MM |
| Liquid Chromatography–Mass Spectrometry | LC-MS |
| Quality control | QC |
| High resolution Flow Infusion Electrospray Ionisation Mass Spectrometry | HR-FIE-MS |
| ultra-performance liquid chromatography | UHPLC |
| Metabolomics Standards Initiative | MSI |
| Principal Component Analysis | PCA |
| Random Forest | RF |
| multi-dimensional scaling | MDS |
| False Positive Rates | FPR |
| high-temperature short-time | HTST |
References
- Gouin, S. Microencapsulation: Industrial appraisal of existing technologies and trends. Trends Food Sci. Technol. 2004, 15, 330–347. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H.B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, M.N.; Nazir, A.; Fallico, B. Ginger Bioactives: A Comprehensive Review of Health Benefits and Potential Food Applications. Antioxidants 2023, 12, 2015. [Google Scholar] [CrossRef]
- Jung, M.Y.; Lee, M.K.; Park, H.J.; Oh, E.B.; Shin, J.Y.; Park, J.S.; Choi, D.S. Heat-induced conversion of gingerols to shogaols in ginger as affected by heat type (dry or moist heat), sample type (fresh or dried), temperature and time. Food Sci. Biotechnol. 2018, 27, 687–693. [Google Scholar] [CrossRef]
- Kurniasari, H.; David, W.; Cempaka, L.; Ardiansyah, A. Effects of drying techniques on bioactivity of ginger (Zingiber officinale): A meta-analysis investigation. AIMS Agric. Food 2022, 7, 197–211. [Google Scholar] [CrossRef]
- Maghraby, Y.R.; Labib, R.M.; Sobeh, M.; Farag, M.A. Gingerols and shogaols: A multi-faceted review of their extraction, formulation, and analysis in drugs and biofluids to maximize their nutraceutical and pharmaceutical applications. Food Chem. X 2023, 20, 100947. [Google Scholar] [CrossRef]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef]
- Promdam, N.; Panichayupakaranant, P. [6]-Gingerol: A narrative review of its beneficial effect on human health. Food Chem. Adv. 2022, 1, 100043. [Google Scholar] [CrossRef]
- Bischoff-Kont, I.; Fürst, R. Benefits of Ginger and Its Constituent 6-Shogaol in Inhibiting Inflammatory Processes. Pharmaceuticals 2021, 14, 571. [Google Scholar] [CrossRef]
- Jangam, S.; Thorat, B. Optimization of Spray Drying of Ginger Extract. Dry. Technol. 2010, 28, 1426–1434. [Google Scholar] [CrossRef]
- Simon-Brown, K.; Solval, K.M.; Chotiko, A.; Alfaro, L.; Reyes, V.; Liu, C.; Sathivel, S. Microencapsulation of ginger (Zingiber officinale) extract by spray drying technology. Lebensm. Wiss. Technol. 2016, 70, 119–125. [Google Scholar] [CrossRef]
- Lloyd, A.J.; Warren-Walker, A.; Finch, J.; Harper, J.; Bennet, K.; Watson, A.; Beckmann, M. Chemical Diversity of UK Grown Tea Explored Using Metabolomics and Machine Learning. Metabolites 2025, 15, 52. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, L.; Zheng, X.; Huang, Q.; Farag, M.A.; Zhu, R.; Zhao, C. Emerging applications of metabolomics in food science and future trends. Food Chem X 2022, 16, 100500. [Google Scholar] [CrossRef]
- Utpott, M.; Rodrigues, E.; Rios, A.d.O.; Mercali, G.D.; Flôres, S.H. Metabolomics: An analytical technique for food processing evaluation. Food Chem. 2022, 366, 130685. [Google Scholar] [CrossRef]
- Wongsa, P.; Khampa, N.; Horadee, S.; Chaiwarith, J.; Rattanapanone, N. Quality and bioactive compounds of blends of Arabica and Robusta spray-dried coffee. Food Chem. 2019, 283, 579–587. [Google Scholar] [CrossRef]
- Zorzenon, M.R.T.; Formigoni, M.; da Silva, S.B.; Hodas, F.; Piovan, S.; Ciotta, S.R.; Costa, S.C. Spray drying encapsulation of stevia extract with maltodextrin and evaluation of the physicochemical and functional properties of produced powders. J. Food Sci. 2020, 85, 3590–3600. [Google Scholar] [CrossRef]
- Jensen, S.K. Improved Bligh and Dyer extraction procedure. Lipid Technol. 2008, 20, 280–281. [Google Scholar] [CrossRef]
- Catchpole, G.S.; Beckmann, M.; Enot, D.P.; Mondhe, M.; Zywicki, B.; Taylor, J.; Draper, J. Hierarchical metabolomics demonstrates substantial compositional similarity between genetically modified and conventional potato crops. Proc. Natl. Acad. Sci. USA 2005, 102, 14458–14462. [Google Scholar] [CrossRef]
- Parker, D.; Beckmann, M.; Enot, D.P.; Overy, D.P.; Rios, Z.C.; Gilbert, M.; Draper, J. Rice blast infection of Brachypodium distachyon as a model system to study dynamic host/pathogen interactions. Nat. Protoc. 2008, 3, 435–445. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Gonzalez-Manzano, S.; Dueñas, M.; Gonzalez-Paramas, A.M. Extraction and isolation of phenolic compounds. Methods Mol. Biol. 2012, 864, 427–464. [Google Scholar]
- Lloyd, A.J.; Willis, N.D.; Wilson, T.; Zubair, H.; Chambers, E.; Garcia-Perez, I.; Draper, J. Addressing the pitfalls when designing intervention studies to discover and validate biomarkers of habitual dietary intake. Metabolomics 2019, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.J.; Nash, R.J.; Taylor, A.; Warren-Walker, A.; Davies, C.; Watson, A.; Holt, N. Daily consumption of traditional kombucha influences the urinary metabolome in a health human cohort. Metabolites 2025. [Google Scholar]
- Lloyd, A.J.; Beckmann, M.; Fave, G.; Mathers, J.C.; Draper, J. Proline betaine and its biotransformation products in fasting urine samples are potential biomarkers of habitual citrus fruit consumption. Br. J. Nutr. 2011, 106, 812–824. [Google Scholar] [CrossRef]
- Lloyd, A.J.; Beckmann, M.; Haldar, S.; Seal, C.; Brandt, K.; Draper, J. Data-driven strategy for the discovery of potential urinary biomarkers of habitual dietary exposure. Am. J. Clin. Nutr. 2013, 97, 377–389. [Google Scholar] [CrossRef]
- Lloyd, A.J.; Beckmann, M.; Tailliart, K.; Brown, W.Y.; Draper, J.; Allaway, D. Characterisation of the main drivers of intra- and inter- breed variability in the plasma metabolome of dogs. Metabolomics 2016, 12, 72. [Google Scholar] [CrossRef][Green Version]
- Martens, L.; Chambers, M.; Sturm, M.; Kessner, D.; Levander, F.; Shofstahl, J.; Deutsch, E.W. mzML—A Community Standard for Mass Spectrometry Data. Mol. Cell. Proteomics. 2011, 10, R110.000133. [Google Scholar] [CrossRef]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Mallick, P. A Cross-platform Toolkit for Mass Spectrometry and Proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef]
- Finch, J.P.; Wilson, T.; Lyons, L.; Phillips, H.; Beckmann, M.; Draper, J. Spectral binning as an approach to post-acquisition processing of high resolution FIE-MS metabolome fingerprinting data. Metabolomics 2022, 18, 64. [Google Scholar] [CrossRef]
- Draper, J.; Enot, D.P.; Parker, D.; Beckmann, M.; Snowdon, S.; Lin, W.; Zubair, H. Metabolite signal identification in accurate mass metabolomics data with MZedDB, an interactive m/z annotation tool utilising predicted ionisation behaviour ‘rules’. BMC Bioinform. 2009, 10, 227. [Google Scholar] [CrossRef]
- Salek, R.M.; Steinbeck, C.; Viant, M.R.; Goodacre, R.; Dunn, W.B. The role of reporting standards for metabolite annotation and identification in metabolomic studies. Gigascience 2013, 2, 13. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Lefranc-Millot, C.; Perreau, C.; Chentouf, A.; Caron, J.; Ramnath, M.; Dugardin, C.; Furger, C. Antioxidative Properties of Plant-Based Protein Isolates: NUTRALYS® Pea Protein. Curr. Dev. Nutr. 2022, 6, 305. [Google Scholar] [CrossRef]
- Díaz-Montes, E. Advances in the Preservation of Plant-Based Pigments via Spray Drying—A Systematic Review. Processes 2025, 13, 663. [Google Scholar] [CrossRef]
- Zagórska, J.; Czernicka-Boś, L.; Kukula-Koch, W.; Szalak, R.; Koch, W. Impact of Thermal Processing on the Composition of Secondary Metabolites of Ginger Rhizome—A Review. Foods 2022, 11, 3484. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.-C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef]
- Monteiro, C.A. Nutrition and health. The issue is not food, nor nutrients, so much as processing. Public Health Nutr. 2009, 12, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Braesco, V.; Souchon, I.; Sauvant, P.; Haurogné, T.; Maillot, M.; Féart, C.; Darmon, N. Ultra-processed foods: How functional is the NOVA system? Eur. J. Clin. Nutr. 2022, 76, 1245–1253. [Google Scholar] [CrossRef]
- Trumbo, P.R.; Bleiweiss-Sande, R.; Campbell, J.K.; Decker, E.; Drewnowski, A.; Erdman, J.W.; Yu, L. Toward a science-based classification of processed foods to support meaningful research and effective health policies. Front. Nutr. 2024, 11, 1389601. [Google Scholar] [CrossRef]
- Azhar, M.D.; Hashib, S.A.; Ibrahim, U.K.; Rahman, N.A. Development of carrier material for food applications in spray drying technology: An overview. Mater. Today Proc. 2021, 47, 1371–1375. [Google Scholar] [CrossRef]

| Recipe | Carrier Material | Ginger Juice (g) | Carrier (g) | Water Added (g) | Inlet Temp (°C) | Outlet Temp (°C) |
|---|---|---|---|---|---|---|
| SD_pure | None | 137 | N/A | 100 | 170 | 88 |
| SD_CO03_478 | Modified Starch (CO03) | 137 | 137 | 478 | 160 | 80 |
| SD_CO03_800 | Modified Starch (CO03) | 137 | 137 | 800 | 180 | 90 |
| SD_pea | Pea Protein | 137 | 37 | 400 | 180 | 90 |
| SD_Inulin | Inulin | 137 | 39.36 | 450 | 170 | 82 |
| Sample Description | Antioxidant Capacity (µmol Trolox Equivalents) | Total Phenolic Content (mM) |
|---|---|---|
| Ginger dehydrated and milled (Dehy_mill) | 1936.25 | 0.693 |
| Ginger Juice (Juice) | 696.25 | 0.179 |
| Speed Vac Ginger Juice (SV_juice) | not available | not available |
| Spray-Dried Pure Ginger Juice (SD_Pure) | 431.46 | 0.713 |
| Spray-Dried Ginger Juice, Clear Gum CO03 1:1, 478 mL Water (SD_CO03_478) | 382.29 | 0.065 |
| Spray-Dried Ginger Juice, Clear Gum CO03 1:1, 800 mL Water (SD_CO03_800) | 377.29 | 0.051 |
| Cleargum CO03 (CO03) | 0 | 0.012 |
| Spray-Dried Ginger Juice, Pea Protein (SD_pea) | 220.21 | 0.075 |
| Pea Protein (Pea) | 87.08 | 0.033 |
| Spray-Dried Ginger Juice, Inulin 3:1 (SD_Inulin) | 549.17 | 0.158 |
| Agave Inulin (AgaveInulin) | 18.96 | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warren-Walker, A.; Beckmann, M.; Watson, A.; McAllister, S.; Lloyd, A.J. Effect of Thermal Processing by Spray Drying on Key Ginger Compounds. Metabolites 2025, 15, 350. https://doi.org/10.3390/metabo15060350
Warren-Walker A, Beckmann M, Watson A, McAllister S, Lloyd AJ. Effect of Thermal Processing by Spray Drying on Key Ginger Compounds. Metabolites. 2025; 15(6):350. https://doi.org/10.3390/metabo15060350
Chicago/Turabian StyleWarren-Walker, Alina, Manfred Beckmann, Alison Watson, Steffan McAllister, and Amanda J. Lloyd. 2025. "Effect of Thermal Processing by Spray Drying on Key Ginger Compounds" Metabolites 15, no. 6: 350. https://doi.org/10.3390/metabo15060350
APA StyleWarren-Walker, A., Beckmann, M., Watson, A., McAllister, S., & Lloyd, A. J. (2025). Effect of Thermal Processing by Spray Drying on Key Ginger Compounds. Metabolites, 15(6), 350. https://doi.org/10.3390/metabo15060350







