(-)-Epigallocatechin-3-Gallate Suppresses Hyperexcitability in Rat Primary Nociceptive Neurons Innervating Inflamed Tissues: A Comparison with Lidocaine
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparing Animals and Inducing Inflammation
2.2. Measurement of Mechanical Escape Threshold and Inflammatory Edema
2.3. Extracellular Single-Unit Electrophysiology of TG Neurons
2.4. Experimental Protocol: Electrophysiological Recording
2.5. Data Analysis and Statistics
3. Results
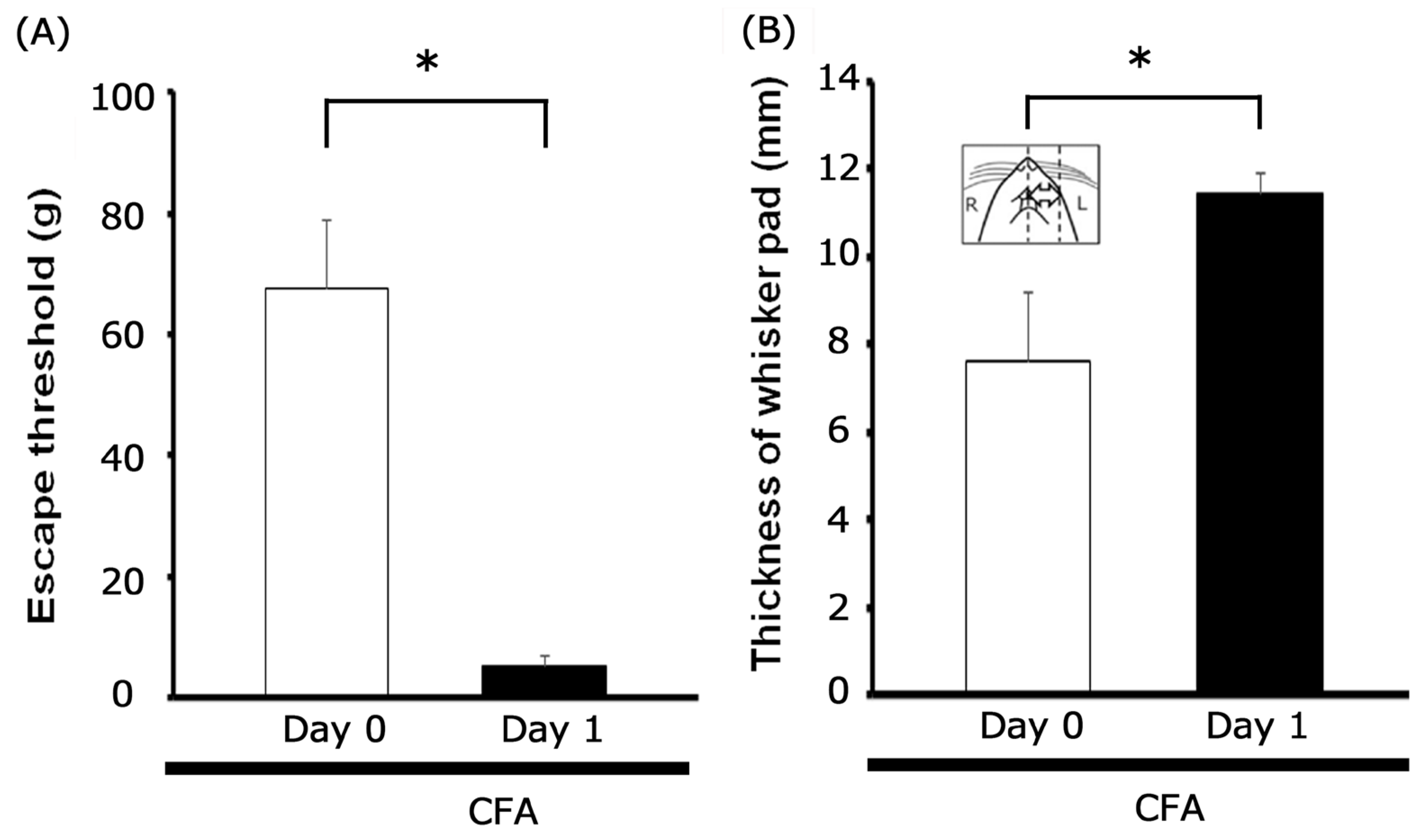
3.1. Characterization of Inflammation-Induced Hyperalgesia and Edema
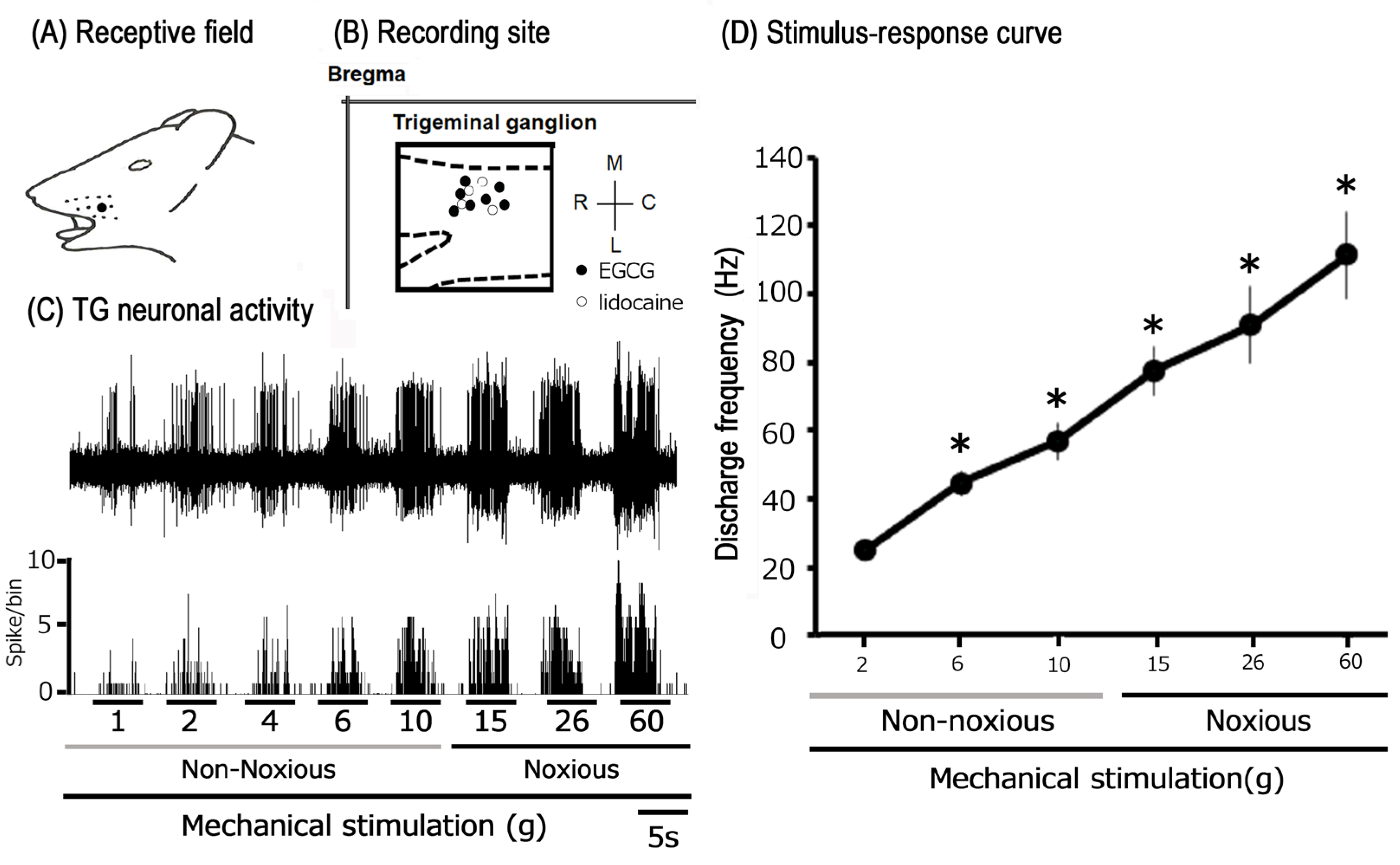
3.2. Physiological Properties of Trigeminal Ganglion Neurons Innervating Inflamed
Facial Skin
3.3. Modulation of Trigeminal Ganglion Neuronal Responses to Mechanical Stimuli by Lidocaine Following CFA-Induced Inflammation
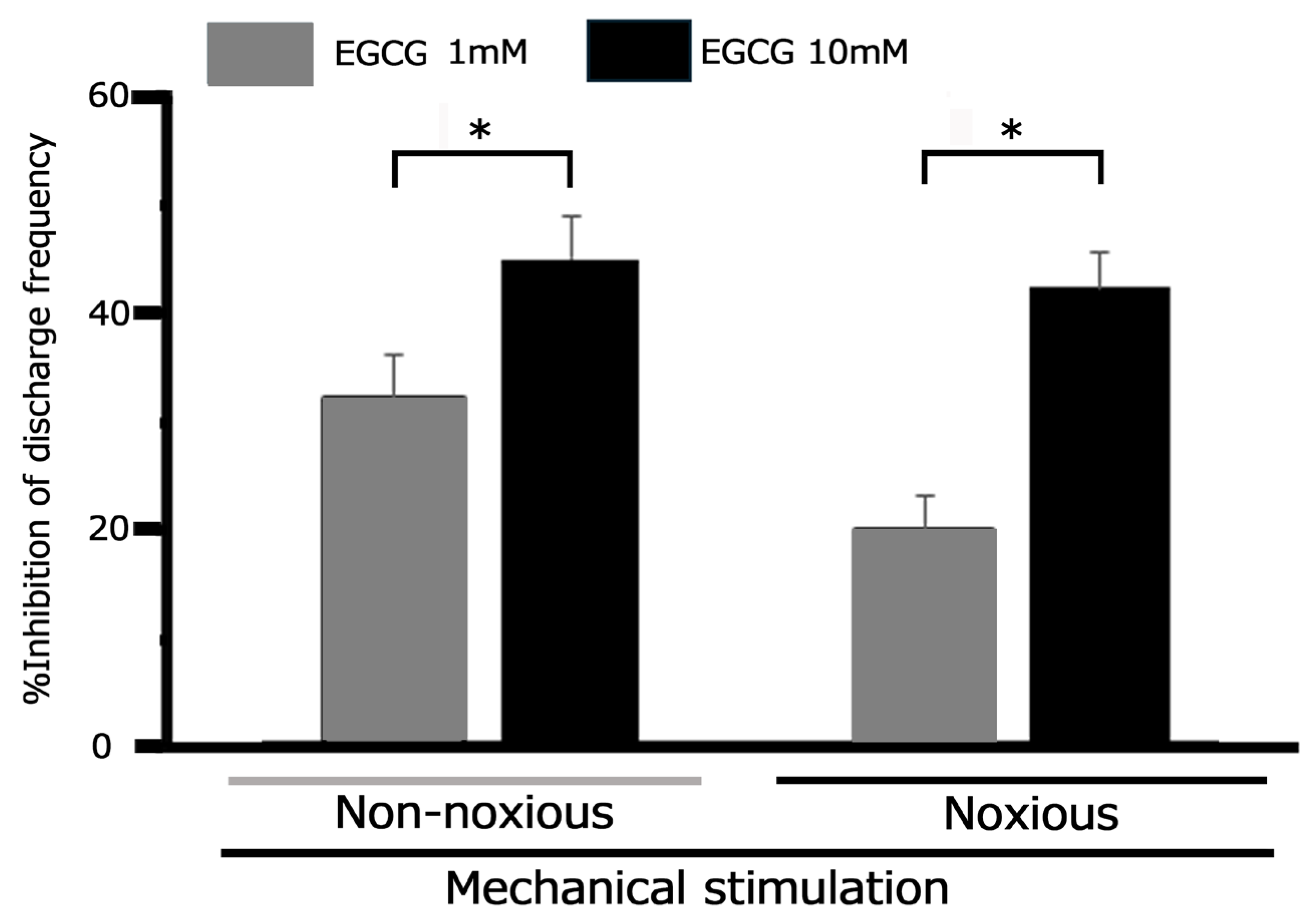
3.4. Modulation of Trigeminal Ganglion Neuronal Responses to Mechanical Stimuli by EGCG Following CFA-Induced Inflammation
3.5. Effect of EGCG on Trigeminal Ganglion Neuronal Activity Following CFA-Induced Inflammation: Response to Noxious and Non-Noxious Stimuli
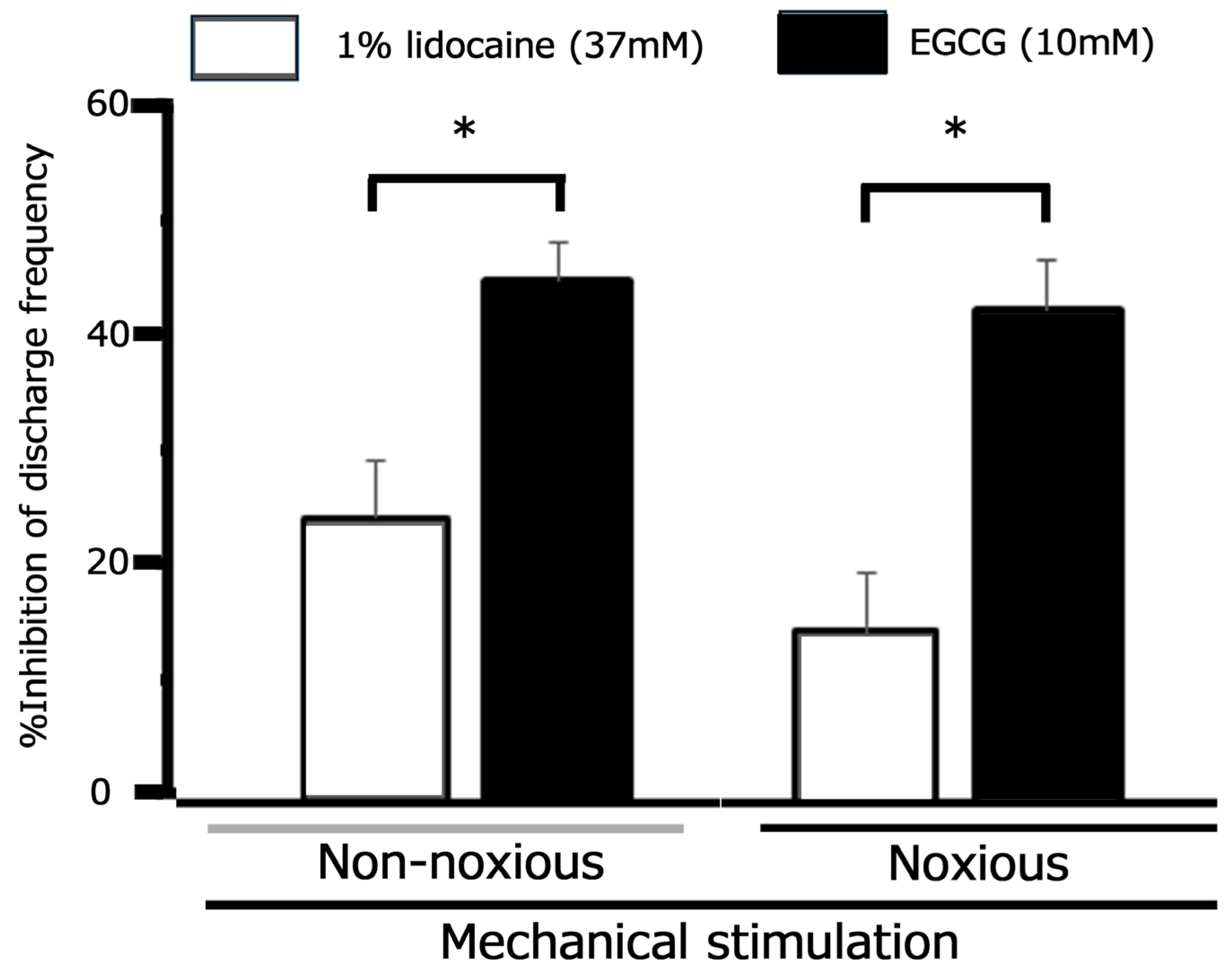
3.6. Comparative Analysis of EGCG and Lidocaine on TG Neuronal Responses to Mechanical Stimuli Following CFA-Induced Inflammation
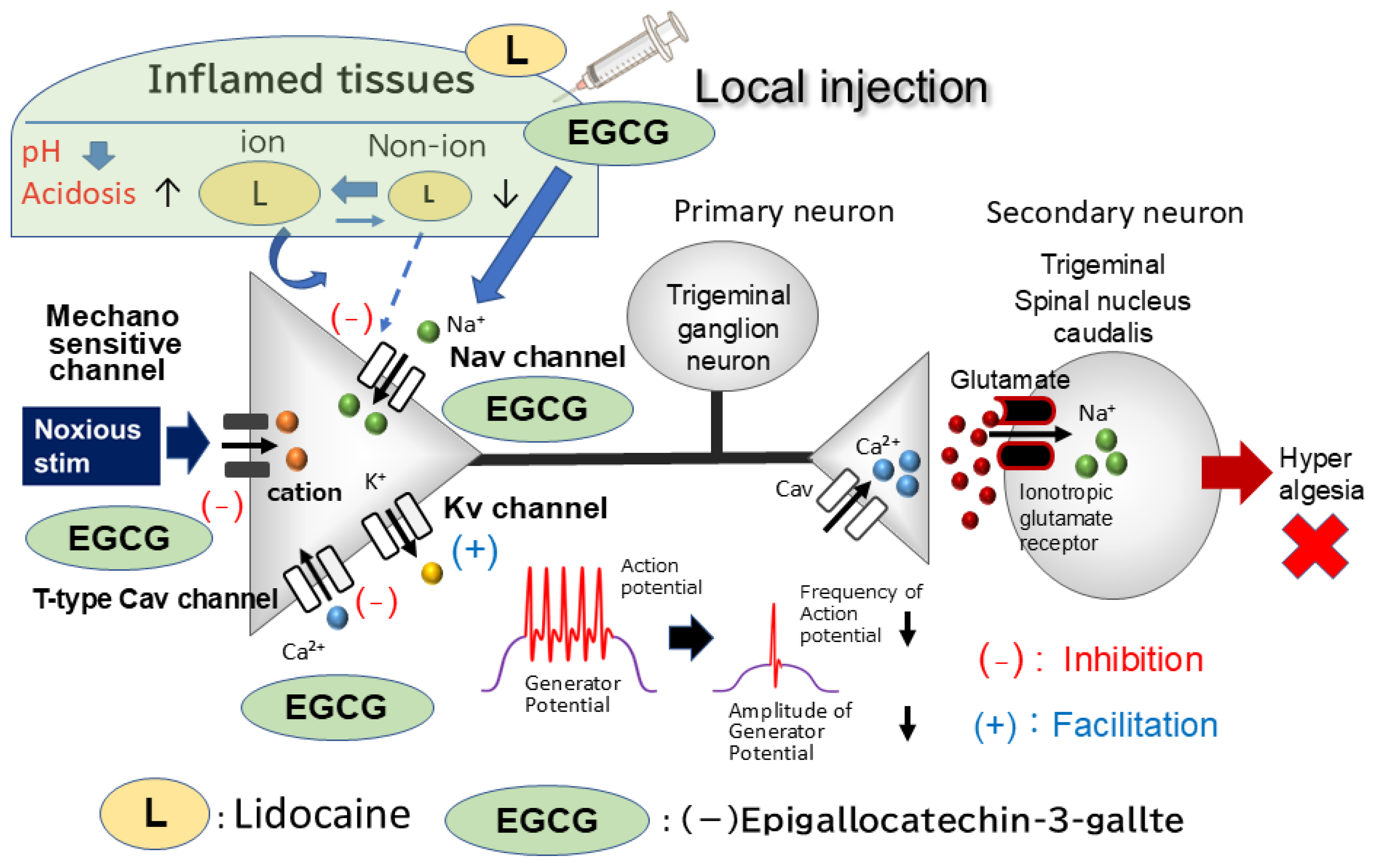
4. Discussion
4.1. Local EGCG Application Suppresses Hyperexcitability in Nociceptive Primary Trigeminal Ganglion (TG) Neurons Innervating Inflamed Tissues
4.2. Peripheral Mechanism: How Local EGCG Administration Suppresses TG Neuronal Excitability in Inflamed Tissues
4.3. Local EGCG Administration Suppresses Nociceptive Hyperexcitability in Inflamed TG Neurons: Functional Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAM | Complementary alternative medicine |
| SpVc | Spinal trigeminal nucleus caudalis |
| TG | Trigeminal ganglion |
| DRG | Dorsal root ganglion |
| WDR | Wide dynamic range |
| EGCG | (-)-epigallocatechin-3-gallate |
| ASICs | Acid sensing ionic channels |
| Nav | Voltage-gated Na |
| Kv | Voltage-gated K |
| Cav | Voltage-gated Ca |
| TTX-S | Tetrodotoxin-sensitive |
| TTX-R | Tetrodotoxin-resistant |
| CFA | Freund’s adjuvant |
| K-current | Slow-inactivating sustained K |
| A-current | Fast-activating transient |
| TMJ | Temporomandibular joint |
References
- Konvicka, J.J.; Meyer, T.A.; McDavid, A.J.; Roberson, C.R. Complementary/alternative medicine use among chronic pain clinic patients. J. Perianesth. Nurs. 2008, 23, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.K.; Mihaliak, K.; Kroenke, K.; Bradley, J.; Tierney, W.M.; Weinberger, M. Use of complementary therapies for arthritis among patients of rheumatologists. Ann. Intern. Med. 1999, 131, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.I.; Genao, I.; Chen, I.; Mechaber, A.J.; Wood, J.A.; Faselis, C.J.; Kurz, J.; Menon, M.; O’Rorke, J.; Panda, M.; et al. Complementary and alternative medicine use by primary care patients with chronic pain. Pain Med. 2008, 9, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E. Complementary medicine. Curr. Opin. Rheumatol. 2003, 15, 151–155. [Google Scholar] [CrossRef]
- Shir, Y.; Raja, S.N.; Weissman, C.S.; Campbell, J.N.; Seltzer, Z. Consumption of soy diet before nerve injury preempts the development of neuro pathic pain in rats. Anesthesiology 2001, 95, 1238–1244. [Google Scholar] [CrossRef]
- Iwata, K.; Takeda, M.; Oh, S.B.; Shinoda, M. Neurophysiology of Orofacial Pain. In Contemporary Oral Medicine; Farah, C.S., Bal asubramaniam, R., McCullough, M.J., Eds.; Springer International: Zurich, Switzerland, 2017; pp. 1749–1773. [Google Scholar]
- Takeda, M.; Matsumoto, S.; Sessle, B.J.; Shinoda, M.; Iwata, K. Peripheral and central mechanisms of trigeminal neuropathic and inflammatory pain. J. Oral Biosci. 2011, 53, 318–322. [Google Scholar] [CrossRef]
- Sessle, B.J. Chronic orofacial pain: Models, mechanisms, and Genetic and related environmental influences. Int. J. Mol. Sci. 2021, 22, 7112. [Google Scholar] [CrossRef]
- Sessle, B.J. Peripheral and central mechanisms of orofacial pain and their clinical correlates. Minerva Anestesiol. 2005, 71, 117–136. [Google Scholar]
- Shinoda, M.; Suzuro, H.; Iwata, K.; Hayashi, Y. Plastic changes in nociceptive pathways contributing to persistent orofacial pain. J. Oral Biosci. 2022, 64, 263–270. [Google Scholar] [CrossRef]
- Mandel, S.; Weinreb, O.; Amit, T.; Youdim, M.B. Cell signaling pathways in the neuroprotective actions of the green tea poly phenol (-)-epigallocatechin-3-gallate: Implication of neurodegenerative diseases. J. Neurochem. 2004, 88, 1555–1569. [Google Scholar] [CrossRef]
- Komori, A.; Yatsunami, J.; Okabe, S.; Abe, S.; Hara, K.; Suganuma, M.; Kim, S.J.; Fujiki, H. Anticarcinogenic activity of green tea polyphenols. Jpn. J. Clin. Oncol. 1993, 23, 186–190. [Google Scholar] [PubMed]
- Kuroda, Y.; Hara, Y. Antimutagenic and anticarcinogenic activity of tea polyphenols. Mutat. Res. 1999, 436, 69–97. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Leonard, Y.J.; Ding, M.; Vallyaythan, V.; Castranova, V.; Rojanasakul, Y.; Dong, Z. Antioxidant properties of (-)-epigal locatechin-3-gallate and inhibition of Cr(VI)-induced DNA damage and Cr(IV)- or TPA-stimulated Nf-kappa B activation. Mol. Cell Biochem. 2000, 206, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Valcic, S.; Burr, J.A.; Timmermann, B.N.; Liebler, D.C. Antioxidant chemistry of green tea catechins. New oxidation products of (-)-epigallocatechin-3-gallate and (-)-epigallocatechin from their reactions with peroxyl radicals. Chem. Res. Toxicol. 2000, 13, 801–810. [Google Scholar] [CrossRef]
- Dona, M.; Dell’Aica, I.; Calabrese, F.; Benelli, R.; Morini, M.; Albini, A.; Garbisa, S. Neutrophil restraint by green tea: Inhibition of inflammation, associated angiogenesis and pulmonary fibrosis. J. Immunol. 2003, 170, 4335–4341. [Google Scholar] [CrossRef]
- Homma, T.; Hirai, K.; Hara, Y.; Katayama, Y. Tea catechin (-)-epigallocatechin gallate, causes membrane depolarizations of myenteric neurons in the guinea-pig small intestine. Neurosci. Lett. 2001, 309, 93–96. [Google Scholar] [CrossRef]
- Bae, J.H.; Mun, K.C.; Park, W.K.; Lee, S.R.; Suh, S.I.; Baek, W.K.; Yin, M.B.; Kwon, T.K.; Song, D.K. EGCG attenuates AMPA-induced intracellular calcium increase in hippocampal neurons. Biochem. Biophys. Res. Commun. 2002, 290, 1506–1512. [Google Scholar]
- Katayama, Y.; Homma, T.; Hara, Y.; Hirai, K. Tea catechin, (-)-epigallocatechin gallate, facilitates cholinergic ganglion transmission in the myenteric plexus of the guinea-pig small intestine. Neurosci. Lett. 2002, 319, 63–66. [Google Scholar] [CrossRef]
- Jeong, H.S.; Kim, Y.S.; Park, J.S. Modulation of neuronal activity by EGCG. Brain Res. 2005, 1047, 267–279. [Google Scholar] [CrossRef]
- Wallace, C.H.R.; Baczko, I.; Jones, L.; Fercho, M.; Light, P.E. Inhibition of cardiac voltage-gated sodium channels by grape pol yphenols. Br. J. Pharmacol. 2006, 149, 657–665. [Google Scholar] [CrossRef]
- Campos-Toimil, M.; Orallo, F. Effect of (-)-epigallocatechin-3-gallate in Ca2+-permeable non-selective cation channels and voltage-operated Ca2+ channels in vascular smooth muscle cells. Life Sci. 2007, 80, 2147–2153. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lim, J.-M.; Kim, S.S.; Park, M.; Song, J.-H. Effects of (-)-epigallocatechin-3-gallate on Na(+) currents in rat dorsal root ganglion neurons. Eur. J. Pharmacol. 2009, 604, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Cheng, H.; Ji, J.; Incardona, J.; Rampe, D. In vitro electrocardiographic and cardiac ion channel effects of (-)-epigallo catechin-3-gallate, the main catechin of green tea. J. Pharmacol. Exp. Ther. 2010, 334, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.G.; Li, W.G.; Zhu, J.J.; Huang, C.; Han, S.L.; Jiang, Q.; Xu, T.L.; Liu, J.H. Subtype-selective inhibition of acid-sensing ion channel 3 by a natural flavonoid. CNS Neurosci. Ther. 2019, 25, 47–56. [Google Scholar] [CrossRef]
- Redford, K.E.; Rognant, S.; Jepps, T.A.; Abbott, G.W. KCNQ5 potassium channel activation underlies vasodilation by tea. Cell Physiol. Biochem. 2012, 30, 46–64. [Google Scholar]
- Utugi, S.; Chida, R.; Yamaguchi, S.; Sashide, Y.; Takeda, M. Local Administration of (-)-Epigallocatechin-3-Gallate as a Local Anesthetic Agent Inhibits the Excitability of Rat Nociceptive Primary Sensory Neurons. Cells 2025, 14, 52. [Google Scholar] [CrossRef]
- Borzan, J.; Zhao, C.; Meyer, R.A.; Raja, S.N. A role for acid-sensing ion channel 3, but not acid-sensing ion channel 2, in sensing dynamic mechanical stimuli. Anesthesiology 2010, 113, 647–654. [Google Scholar] [CrossRef]
- Kang, S.; Jang, J.H.; Price, M.P.; Gautam, M.; Benson, C.J.; Gong, H.; Welsh, M.J.; Brennan, T.J.; Matsunami, H. Simultaneous disruption of mouse ASIC1a, ASIC2 and ASIC3 genes enhances cutaneous mechanosensitivity. PLoS ONE 2012, 7, e35225. [Google Scholar] [CrossRef]
- Kwan, K.Y.; Glazer, J.M.; Corey, D.P.; Rice, F.L.; Stucky, C.L. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J. Neurosci. 2009, 29, 4808–4819. [Google Scholar] [CrossRef] [PubMed]
- Price, M.P.; McIlwrath, S.L.; Xie, J.; Cheng, C.; Qiao, J.; E Tarr, D.; A Sluka, K.; Brennan, T.J.; Lewin, G.R.; Welsh, M.J. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron 2001, 32, 1071–1083. [Google Scholar] [CrossRef]
- Punnia-Moorthy, A. Evaluation of pH changes in inflammation of the subcutaneous air pouch lining in the rat, induced by carrageenan, dextran and Staphylococcus aureus. J. Oral Pathol. 1987, 16, 36–44. [Google Scholar] [CrossRef] [PubMed]
- López, A.B.; Diago, M.P. Failure of locoregional anesthesia in dental practice. Review of the literature. Med. Oral Patol. Oral Cir. Bucal 2006, 11, E510–E513. [Google Scholar]
- Fu, H.; Fang, P.; Zhou, H.; Zhou, J.; Yu, X.; Ni, M.; Zheng, J.; Jin, Y.; Chen, J.; Wang, F.; et al. Acid-sensing ion channels in trigeminal ganglion neurons innervating the orofacial region contribute to orofacial inflammatory pain. Clin. Exp. Pharmacol. Physiol. 2016, 43, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Sashide, Y.; Toyota, R.; Takeda, M. Local administration of the phytochemical, quercetin, attenuates the hyperexcitability of rat nociceptive primary sensory neurons following inflammation comparable to lidocaine. J. Pain 2024, 25, 755–765. [Google Scholar] [CrossRef]
- Toyota, R.; Itou, H.; Sashide, Y.; Takeda, M. Suppression of the excitability of rat nociceptive primary sensory neurons follow ing local administration of the phytochemical quercetin. J. Pain 2023, 24, 540–549. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 2nd ed.; Academic Press: New York, NY, USA, 1986. [Google Scholar]
- Nakagawa, K.; Takeda, M.; Tsuboi, Y.; Kondo, M.; Kitagawa, J.; Matsumoto, S.; Kobayashi, A.; Sessle, B.J.; Shinoda, M.; Iwata, K. Alternation of primary afferent activity following inferior alveolar nerve transec tion in rats. Mol. Pain 2010, 6, 9. [Google Scholar] [CrossRef]
- Harriott, A.M.; Gold, M.S. Contribution of primary afferent channels to neuropathic pain. Curr. Pain Headache Rep. 2009, 13, 197–207. [Google Scholar] [CrossRef]
- Hucho, T.; Levine, J.D. Signaling pathways in sensitization: Toward a nociceptor cell biology. Neuron 2007, 55, 365–376. [Google Scholar] [CrossRef]
- Scholz, J.; Woolf, C.J. Can we conquer pain? Nat. Neurosci. 2002, 5, 1062–1067. [Google Scholar] [CrossRef]
- Beyak, M.J.; Vanner, S. Inflammation-induced hyperexcitability of nociceptive gastrointestinal DRG neurons: Role of voltage gated ion channels. Neurogastroenterol. Motil. 2005, 17, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Dang, K.; Bielefeldt, K.; Gebhart, G.F. Gastric ulcers reduce A-type potassium currents in rat gastric sensory ganglion neurons. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G573–G579. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.A.; Stewart, T.M.R.; Hill, C.; Vanner, S.J. TNBS ileitis evokes hyperexcitability and changes in ionic membrane properties of nociceptive DRG neurons. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G1045–G1051. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.R.; Gebhart, G.F. Characterization of a model of chronic orofacial hyperalgesia in the rat: Contribution of NA(V) 1.8. J. Pain 2008, 9, 522–531. [Google Scholar] [CrossRef]
- Stewart, T.; Beyak, M.J.; Vanner, S. Ileitis modulates potassium and sodium currents in guinea pig dorsal root ganglia sensory neurons. J. Physiol. 2003, 552, 797–807. [Google Scholar] [CrossRef]
- Yoshimura, N.; De Groat, W.C. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J. Neurosci. 1999, 19, 4644–4653. [Google Scholar] [CrossRef]
- Guo, L.; Zhao, L.; Ming, P.; Hong, L.; Liu, A.; Li, R. Sumatriptan inhibits the electrophysiological activity of ASICs in rat trigeminal ganglion neurons. Eur. J. Pharmacol. 2018, 841, 98–103. [Google Scholar] [CrossRef]
- Meng, Q.; Fang, P.; Hu, Z.; Ling, Y.; Liu, H. Mechanotransduction of trigeminal ganglion neurons innervating inner walls of rat anterior eye chambers. Am. J. Physiol. Cell Physiol. 2015, 309, C1–C10. [Google Scholar] [CrossRef]
- A Black, J.; Liu, S.; Tanaka, M.; Cummins, T.R.; Waxman, S.G. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain 2004, 108, 237–247. [Google Scholar] [CrossRef]
- Cummins, T.R.; Dib-Hajj, S.D.; Black, J.A.; Akopian, A.N.; Wood, J.N.; Waxman, S.G. A novel persistent tetrodotoxin-resistant sodium current in SNS-null and wild-type small primary sensory neurons. J. Neurosci. 1999, 19, RC43. [Google Scholar] [CrossRef]
- Takeda, M.; Tanimoto, T.; Ikeda, M.; Nasu, M.; Kadoi, J.; Yoshida, S.; Matsumoto, S. Enhanced excitability of rat trigeminal root ganglion neurons via decrease in A-type potassium currents following temporomandibular joint inflammation. Neuroscience 2006, 138, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Tsuboi, Y.; Kitagawa, J.; Nakagawa, K.; Iwata, K.; Matsumoto, S. Potassium channels as a potential therapeutic target for trigeminal neuropathic and inflammatory pain. Mol. Pain 2011, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Hara, N.; Takeda, M.; Takahashi, M.; Matsumoto, S. Iontophoretic application of an A-type potassium channel blocker to the trigeminal ganglion neurons enhances the excitability of Aδ- and C-neurons innervating the temporomandibular joint. Neurosci. Res. 2012, 74, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Granados-Soto, V.; Argüelles, C.F.; Ortiz, M.I. The peripheral antinociceptive effect of resveratrol is associated with acti vation of potassium channels. Neuropharmacology 2002, 43, 917–923. [Google Scholar] [CrossRef]
- Jacus, M.O.; Uebele, V.N.; Renger, J.J.; Todorovic, S.M. Presynaptic Cav3.2 channels regulate excitatory neurotransmission in nociceptive dorsal horn neurons. J. Neurosci. 2012, 32, 9374–9382. [Google Scholar] [CrossRef]
- Todorovic, S.M.; Jevtovic-Todorovic, V. Regulation of T- type calcium channels in the peripheral pain pathway. Channels 2007, 1, 238–245. [Google Scholar] [CrossRef][Green Version]
- Gambeta, E.; Chichorro, J.G.; Zamponi, G.W. Trigeminal neuralgia: An overview from pathophysiology to pharmacological treatments. Mol. Pain 2020, 16, 1–18. [Google Scholar] [CrossRef]
- Gadotti, V.M.; Caballero, A.G.; Berger, N.D.; Gladding, C.M.; Chen, L.; Pfeifer, T.A.; Zamponi, G.W. Small organic molecule disruptors of Cav3.2-USP5 interactions reverse in flammatory and neuropathic pain. Mol. Pain 2015, 11, 12. [Google Scholar] [CrossRef]
- Gambeta, E.; Gandini, M.A.; Souza, I.A.; Zamponi, G.W. Cav 3.2 calcium channels contribute to trigeminal neuralgia. Pain 2022, 163, 2315–2325. [Google Scholar] [CrossRef]
- Ali, M.Y.; Gadotti, V.M.; Huang, S.; Garcia-Caballero, A.; Antunes, F.T.; Jung, H.A.; Choi, J.S.; Zamponi, G.W. Icariside, Aa prenyl- flavonol, alleviates inflammatory and neuropathic pain by inhib iting T-type calcium channels and USP5-cav3.2 interactions. ACS Chem. Neurosci. 2023, 14, 1859–1869. [Google Scholar] [CrossRef]
- Miller, R.D. Local anesthetics. In Basic and Clinical Pharmacology; Katzung, B.G., Ed.; Lange Medical Books/McGraw-Hill: New York, NY, USA, 1996; pp. 425–433. [Google Scholar]
- Imbe, H.; Iwata, K.; Zhou, Q.-Q.; Zou, S.; Dubner, R.; Ren, K. Orofacial deep and cutaneous tissue inflammation and trigeminal neuronal activation. Cells Tissues Organs 2001, 169, 238–247. [Google Scholar] [CrossRef]
- Ragsdale, D.S.; McPhee, J.C.; Scheuer, T.; Catterall, W.A. Molecular determinants of state-dependent block of Na+ channel by local anes thetics. Science 1994, 265, 1724–1728. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Utugi, S.; Sashide, Y.; Takeda, M. (-)-Epigallocatechin-3-Gallate Suppresses Hyperexcitability in Rat Primary Nociceptive Neurons Innervating Inflamed Tissues: A Comparison with Lidocaine. Metabolites 2025, 15, 439. https://doi.org/10.3390/metabo15070439
Utugi S, Sashide Y, Takeda M. (-)-Epigallocatechin-3-Gallate Suppresses Hyperexcitability in Rat Primary Nociceptive Neurons Innervating Inflamed Tissues: A Comparison with Lidocaine. Metabolites. 2025; 15(7):439. https://doi.org/10.3390/metabo15070439
Chicago/Turabian StyleUtugi, Syogo, Yukito Sashide, and Mamoru Takeda. 2025. "(-)-Epigallocatechin-3-Gallate Suppresses Hyperexcitability in Rat Primary Nociceptive Neurons Innervating Inflamed Tissues: A Comparison with Lidocaine" Metabolites 15, no. 7: 439. https://doi.org/10.3390/metabo15070439
APA StyleUtugi, S., Sashide, Y., & Takeda, M. (2025). (-)-Epigallocatechin-3-Gallate Suppresses Hyperexcitability in Rat Primary Nociceptive Neurons Innervating Inflamed Tissues: A Comparison with Lidocaine. Metabolites, 15(7), 439. https://doi.org/10.3390/metabo15070439





