Angiotensin-Converting Enzyme Inhibitory Activity of Selected Phenolic Acids, Flavonoids, Their O-Glucosides, and Low-Molecular-Weight Phenolic Metabolites in Relation to Their Oxidation Potentials
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Stock Solutions of Standards of Phenolic Acids, Flavonoids, Their O-Glucosides, and Low-Molecular-Weight Phenolic Metabolites for ACE Inhibitory Activity Determination
2.3. Angiotensin-I-Converting Enzyme Inhibitory Assay
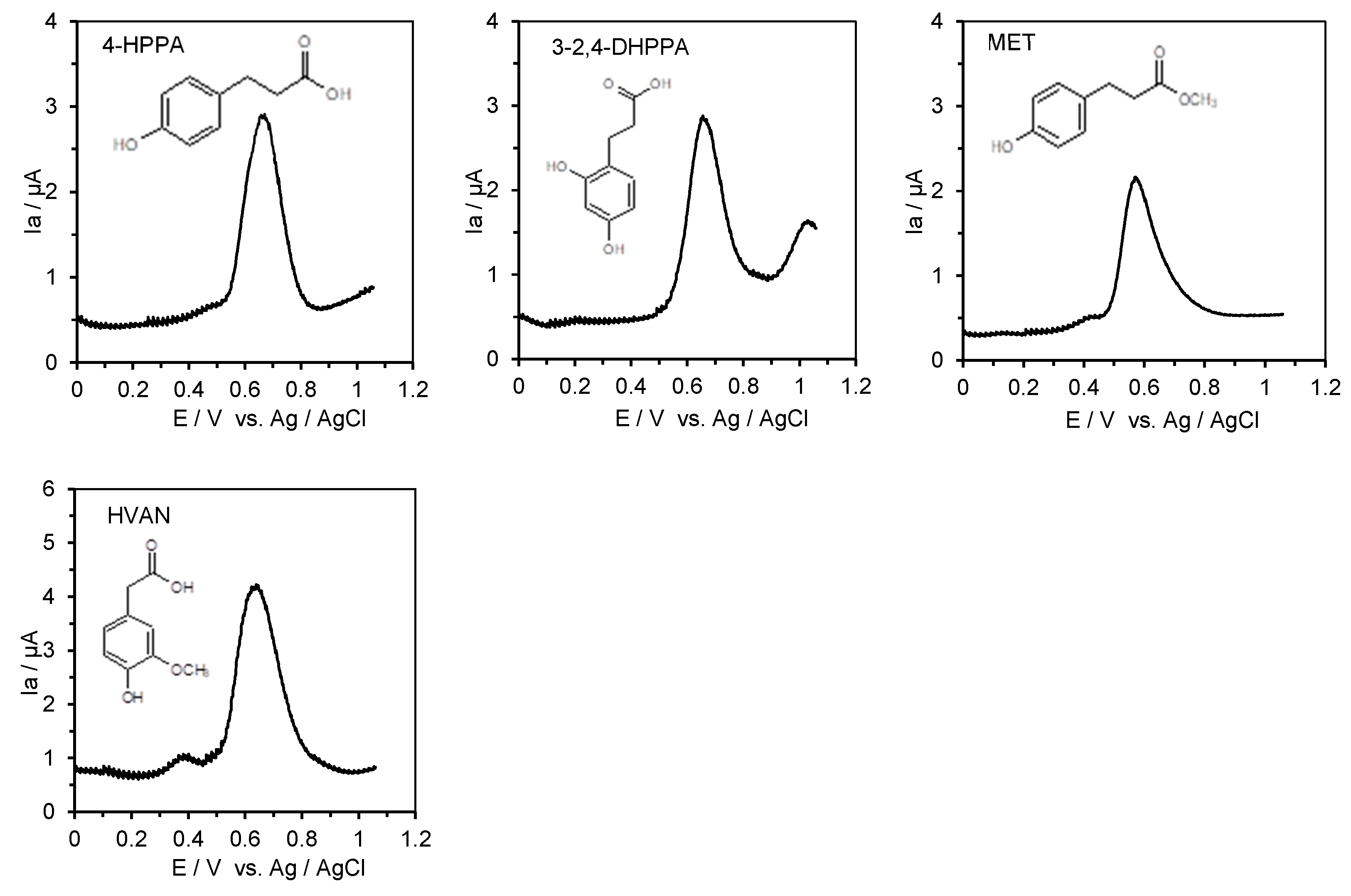
2.4. Measurement of the Anodic Oxidation Potentials of Phenolic Acids, Flavonoids, Their O-Glucosides, and Low-Molecular-Weight Phenolic Metabolites with Differential Pulse Voltammetry
2.5. Statistical Analysis
3. Results
3.1. ACE Inhibitory Activity and the First Oxidation Potential of Phenolic Acids
3.2. ACE Inhibitory Activity and the First Oxidation Potential of Flavonoids and Their O-Glucosides
3.3. ACE Inhibitory Activity and the First Oxidation Potential of Low-Molecular-Weight Phenolic Metabolites of Quercetin and Rutin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guang, C.; Philips, R.D. Plant Food-Derived Angiotensin I Converting Enzyme Inhibitory Peptides. J. Agric. Food Chem. 2009, 57, 5113–5120. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Guptha, S. Strategies for initial management of hypertension. Indian J. Med. Res. 2010, 132, 531–542. [Google Scholar] [CrossRef] [PubMed]
- López-Sendón, J.; Swedberg, K.; McMurray, J.; Tamargo, J.; Maggioni, A.P.; Dargie, H.; Tendera, M.; Waagstein, F.; Kjekshus, J.; Lechat, P.; et al. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease. The Task Force on ACE-inhibitors of the European Society of Cardiology. Eur. Heart J. 2004, 25, 1454–1470. [Google Scholar] [PubMed]
- Ondetti, M.A.; Rubin, B.; Cushman, D.W. Enzyme of the rennin angiotensin system and their inhibitors. Annu. Rev. Biochem. 1982, 51, 283–308. [Google Scholar] [CrossRef]
- Kim, S.K.; Byun, H.G.; Park, P.J.; Shahidi, F. Angiotensin I converting enzyme inhibitory peptides purified from bovine skin gelatin hydrolysate. J. Agric. Food Chem. 2001, 49, 2992–2997. [Google Scholar] [CrossRef]
- Parish, R.C.; Miller, L.J. Adverse effects of angiotensin converting enzyme (ACE) inhibitors. An update. Drug Saf. 1992, 7, 14–31. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Sharma, R.; Baruwa, A. Pharmacological review on natural ACE inhibitors. Pharm. Lett. 2005, 2, 273–293. [Google Scholar]
- Duarte, J.; Perez-Palencia, R.; Vargas, F.; Ocete, M.A.; Perez-Vizcaino, F.; Zarzuelo, A.; Tamargo, J. Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. Brit. J. Pharmacol. 2001, 133, 117–124. [Google Scholar] [CrossRef]
- Miguel, M.; Contreras, M.M.; Recio, I.; Aleixandre, A. ACE inhibitory and antihypertensive properties of a bovine casein hydrolysate. Food Chem. 2009, 12, 211–214. [Google Scholar] [CrossRef]
- Je, J.Y.; Qian, Z.J.; Byun, H.G.; Kim, S.K. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007, 42, 840–846. [Google Scholar] [CrossRef]
- Kuba, M.; Tana, C.; Tawata, S.; Yasuda, M. Production of angiotensin I-converting enzyme inhibitory peptides from soybean protein with Monascus purpureus acid proteinase. Process Biochem. 2005, 40, 2191–2196. [Google Scholar] [CrossRef]
- Matsui, T.; Li, C.H.; Osajima, Y. Preparation and characterization novel bioactive peptides responsible for angiotensin I converting enzyme inhibition from wheat germ. J. Pept. Sci. 1999, 5, 289–297. [Google Scholar] [CrossRef]
- Torino, M.I.; Limón, R.I.; Martínez-Villaluenga, C.; Mäkinen, S.; Pihlanto, A.; Vidal-Valverde, C.; Frias, J. Antioxidant and antihypertensive properties of liquid and solid state fermented lentils. Food Chem. 2013, 136, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tian, J.; Wang, Q. ACE inhibitory and antihypertensive properties of apricot almond meal hydrolysate. Eur. Food Res. Technol. 2011, 232, 549–556. [Google Scholar] [CrossRef]
- Ren, X.; Ma, H.; Mao, S.; Zhou, H. Effects of sweeping frequency ultrasound treatment on enzymatic preparations of ACE-inhibitory peptides from zein. Eur. Food Res. Technol. 2014, 238, 435–442. [Google Scholar] [CrossRef]
- Iwaniak, A.; Minkiewicz, P.; Darewicz, M. Food-originating ACE inhibitors, including antihypertensive peptides, as preventive food components in blood pressure reduction. Compr. Rev. Food Sci. Food Saf. 2014, 13, 114–134. [Google Scholar] [CrossRef]
- Lacaille-Dubois, M.A.; Franck, U.; Wagner, H. Search for potential Angiotensin Converting Enzyme (ACE)-inhibitors from plants. Phytomedicine 2001, 8, 47–52. [Google Scholar] [CrossRef]
- Zieliński, H.; Honke, J.; Topolska, J.; Bączek, N.; Piskuła, M.K.; Wiczkowski, W.; Wronkowska, M. ACE inhibitory properties and phenolics profile of fermented flours and of baked and digested biscuits from buckwheat. Foods 2020, 9, 847. [Google Scholar] [CrossRef]
- Galleano, M.; Pechanova, O.; Fraga, C.G. Hypertension, nitric oxide, oxidants, and dietary polyphenols. Curr. Pharm. Biotechnol. 2010, 11, 837–848. [Google Scholar] [CrossRef]
- Cordova, A.C.; Sumpio, B.J.; Sumpio, B.E. Perfecting the plate: Adding cardioprotective compounds to the diet. Am. Col. Surg. 2012, 214, 97–114. [Google Scholar] [CrossRef]
- Pan, M.-H.; Lai, C.-S.; Dushenkov, S.; Ho, C.-T. Modulation of inflammatory genes by natural dietary bioactive compounds. J. Agric. Food Chem. 2009, 57, 4467–4477. [Google Scholar] [CrossRef] [PubMed]
- Kroon, P.A.; Clifford, M.N.; Crozier, A.; Day, A.J.; Donovan, J.L.; Manach, C.; Williamson, G. How should we assess the effects of exposure to dietary polyphenols in vitro? Am. J. Clin. Nutr. 2004, 80, 15–21. [Google Scholar] [CrossRef]
- Simons, A.L.; Renouf, M.; Hendrich, S.; Murphy, P.A. Human gut microbial degradation of flavonoids: Structure–function relationships. J. Agric. Food Chem. 2005, 3, 4258–4263. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Macia, A.; Romero, M.-P.; Reguant, J.; Ortega, N.; Motilva, M.-J. Metabolic pathway of the colon metabolism of flavonoids (flavonols, flavones and flavanones) and phenolic acids. Food Chem. 2011, 130, 383–393. [Google Scholar] [CrossRef]
- Terao, J. Potential role of quercetin glycosides as anti-atherosclerotic food-derived factors for human health. Antioxidants 2023, 12, 258. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Brown, N.M.; Lydeking-Olsen, E. The clinical importance of the metabolite equol—A clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar] [CrossRef]
- Gonthier, M.; Cheynier, V.; Donovan, J.L.; Manach, C.; Morand, C.; Mila, I. Microbial aromatic acid metabolites formed in the gut account for a major fraction of the polyphenols excreted in urine of rats fed red wine polyphenols. J. Nutr. 2003, 133, 461–467. [Google Scholar] [CrossRef]
- Gonthier, M.; Donovan, J.L.; Texier, O.; Felgines, C.; Remesy, C.; Scalbert, A. Metabolism of dietary procyanidins in rats. Free Radic. Biol. Med. 2003, 35, 837–844. [Google Scholar] [CrossRef]
- Duenas, M.; Surco-Laos, F.; Gonzalez-Manzano, S.; Gonzalez-Paramas, A.M.; Santos-Buelga, C. Antioxidant properties of major metabolites of quercetin. Eur. Food Res. Technol. 2011, 232, 103–111. [Google Scholar] [CrossRef]
- Pashikanti, S.; de Alba, D.R.; Boissonneault, G.A.; Cervantes-Laurean, D. Rutin metabolites: Novel inhibitors of nonoxidative advanced glycation end products. Free Radic. Biol. Med. 2010, 48, 656–663. [Google Scholar] [CrossRef]
- Gross, M.; Pfeiffer, M.; Martini, M.; Campbell, D.; Slavin, J.; Potter, J. The quantitation of metabolites of quercetin flavonols in human urine. Cancer Epidemiol. Biomarkers Prev. 1996, 5, 711–720. [Google Scholar] [PubMed]
- Jaganath, I.B.; Mullen, W.; Lean, M.E.J.; Edwards, C.A.; Crozier, A. In vitro catabolism of rutin by human fecal bacteria and the antioxidant capacity of its catabolites. Free Radic. Biol. Med. 2009, 47, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Persson, I.-A.-L.; Josefsson, M.; Persson, K.; Andersson, R.G.G. Tea flavanols inhibit angiotensin-converting enzyme activity and increase nitric oxide production in human endothelial cells. J. Pharm. Pharmacol. 2006, 58, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Paiva, L.; Lima, E.; Marcone, M.; Baptista, J. Angiotensin I-converting enzyme (ACE) inhibition and biological activities of green and black tea samples from Azorean Camellia sinensis. J. Funct. Foods 2023, 107, 105701. [Google Scholar] [CrossRef]
- Shukor, N.A.; Camp, J.V.; Gonzales, G.B.; Staljanssens, D.; Struijs, K.; Zotti, M.J.; Raes, K.; Smagghe, G. Angiotensin-Converting Enzyme Inhibitory Effects by Plant Phenolic Compounds: A Study of Structure Activity Relationships. J. Agric. Food Chem. 2013, 61, 11832–11839. [Google Scholar] [CrossRef]
- Chevion, S.; Roberts, M.A.; Chevion, M. The use of cyclic voltammetry for the evaluation of antioxidant capacity. Free Radic. Biol. Med. 2000, 28, 860–870. [Google Scholar] [CrossRef]
- Magalhaes, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef]
- Zielińska, D.; Zieliński, H. Antioxidant activity of flavone C-glucosides determined by updated analytical strategies. Food Chem. 2011, 124, 672–678. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.M.; Enache, T.A.; Gil, E.S.; Oliveira-Brett, A.M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef]
- Zielińska, D.; Zieliński, H.; Piskuła, M.K. An Electrochemical Determination of the Total Reducing Capacity of Wheat, Spelt, and Rye Breads. Antioxidants 2022, 11, 1438. [Google Scholar] [CrossRef]
- Sochor, J.; Dobes, J.; Krystofova, O.; Ruttkay-Nedecky, B.; Babula, P.; Pohanka, M.; Jurikova, T.; Zitka, O.; Adam, V.; Klejdus, B.; et al. Electrochemistry as a Tool for Studying Antioxidant Properties. Inter.J. Electroch. Sci. 2013, 8, 8464–8489. [Google Scholar] [CrossRef]
- Alam, M.W.; Najeeb, J.; Naeem, S.; Usman, S.M.; Nahvi, I.; Alismail, F.; Abuzir, A.; Farhan, M.; Allah Nawaz, A. Electrochemical methodologies for investigating the antioxidant potential of plant and fruit extracts: A review. Antioxidants 2022, 11, 1205. [Google Scholar] [CrossRef] [PubMed]
- Zlatić, G.; Arapović, A.; Martinović, I.; Martinović Bevanda, A.; Bošković, P.; Prkić, A.; Paut, A.; Vukušić, T. Antioxidant Capacity of Herzegovinian Wildflowers Evaluated by UV—VIS and Cyclic Voltammetry Analysis. Molecules 2022, 27, 5466. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.A.; Custer, L.J.; Arakaki, C.; Murphy, S.P. Vitamin C and flavonoids levels of fruits and vegetables consumed in Hawai. J. Food Compos. Anal. 2004, 17, 1–35. [Google Scholar] [CrossRef]
- Sentandreu, M.Á.; Toldrá, F. A rapid, simple and sensitive fluorescence method for the assay of angiotensin I converting enzyme. Food Chem. 2006, 97, 546–554. [Google Scholar] [CrossRef]
- Cosio, M.S.; Buratti, S.; Mannino, S.; Benedetti, S. Use of an electrochemical method to evaluate the antioxidant activity of herb extracts from the Labiatae family. Food Chem. 2006, 97, 725–731. [Google Scholar] [CrossRef]
- Blasco, A.J.; Rogerio, M.C.; Gonzalez, M.C.; Escarpa, A. “Electrochemical Index” as a screening method to determine “total polyphenolics” in foods: A proposal. Anal. Chim. Acta 2005, 539, 237–244. [Google Scholar] [CrossRef]
- Rechner, A.R.; Kuhnle, G.; Bremner, P.; Hubbard, G.P.; Moore, K.P.; Rice-Evans, C.A. The metabolic fate of dietary polyphenols in humans. Free Radic. Biol. Med. 2002, 33, 220–235. [Google Scholar] [CrossRef]
- Zielińska, D.; Zieliński, H.; Laparra-Llopis, J.M.; Honke, J.; Giménez-Bastida, J.A. Caffeic acid modulates processes associated with intestinal inflammation. Nutrients 2021, 13, 554. [Google Scholar] [CrossRef]
- Zielińska, D.; Laparra-Llopis, J.M.; Zieliński, H.; Szawara-Nowak, D.; Gimenez Bastida, J.A. Role of Apple Phytochemicals, Phloretin and Phloridzin, in Modulating Processes Related to Intestinal Inflammation. Nutrients 2019, 11, 1173. [Google Scholar] [CrossRef]
- Guerrero, L.; Castillo, J.; Quiñones, M.; Garcia-Vallvé, S.; Arola, L.; Pujadas, G.; Muguerza, B.; Lu, J.R. Inhibition of angiotensin-converting enzyme activity by flavonoids: Structure-activity relationship studies. PLoS ONE 2012, 7, e49493. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Bastida, J.A.; Zieliński, H.; Piskuła, M.K.; Zielińska, D.; Szawara-Nowak, D. Buckwheat bioactive compounds, their derived phenolic metabolites and their health benefits. Mol. Nutr. Food Res. 2017, 61, 1600475. [Google Scholar] [CrossRef] [PubMed]
- Aura, A. Microbial metabolism of dietary phenolic compounds in the colon. Phytochem. Rev. 2008, 7, 407–429. [Google Scholar] [CrossRef]
- Selma, M.V.; Espin, J.C.; Tomas-Barberan, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Ojeda, D.; Jimenez-Ferrer, E.; Zamilpa, A.; Herrera-Arellano, A.; Tortoriello, J.; Alvarez, L. Inhibition of angiotensin convertin enzyme (ACE) activity by the anthocyanins delphinidin- and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. J. Ethnopharmacol. 2010, 127, 7–10. [Google Scholar] [CrossRef]
- Dong, J.; Xu, X.; Liang, Y.; Head, R.; Bennett, L. Inhibition of angiotensin converting enzyme (ACE) activity by polyphenols from tea (Camellia sinensis) and links to processing method. Food Funct. 2011, 2, 310–319. [Google Scholar] [CrossRef]
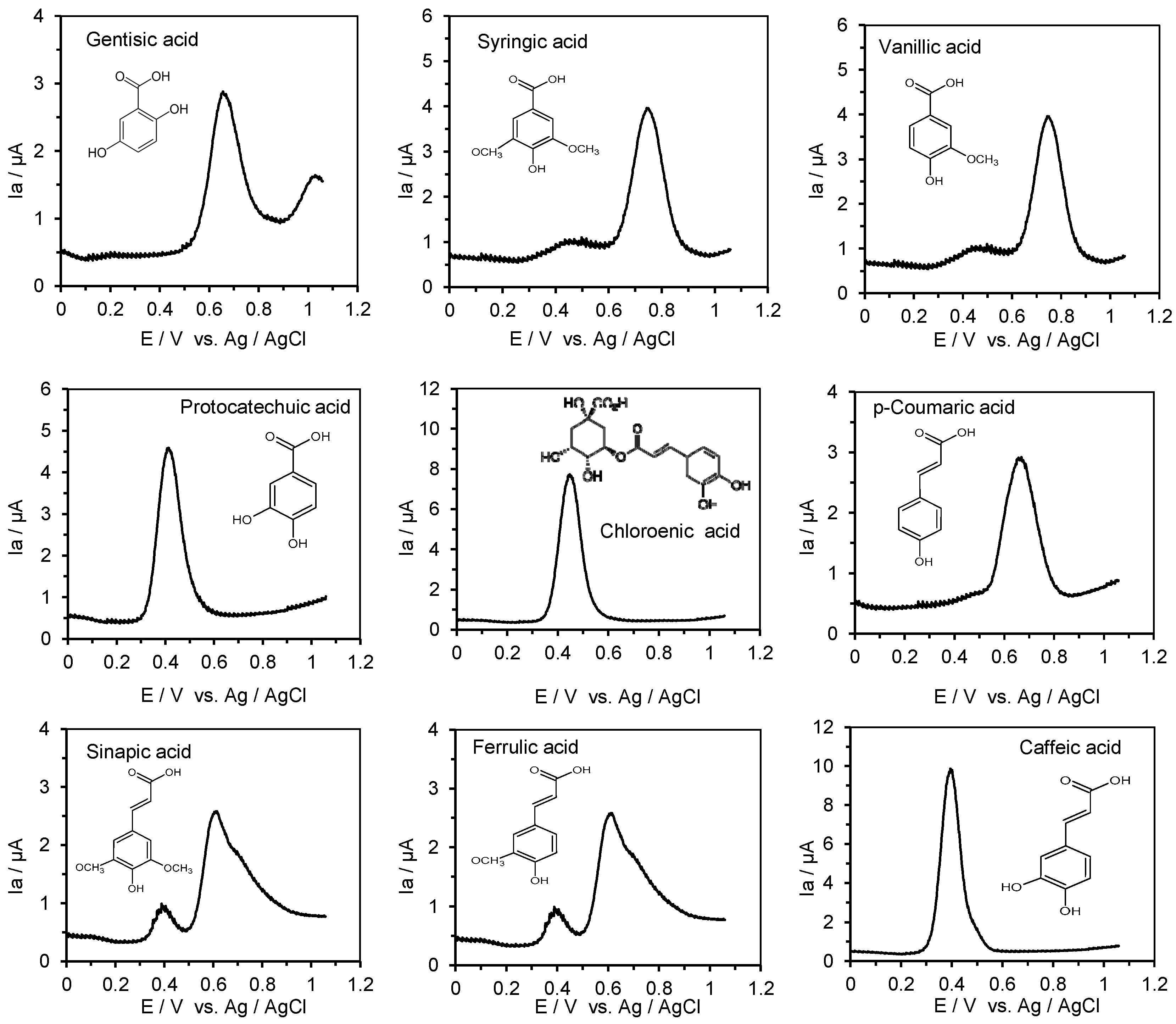
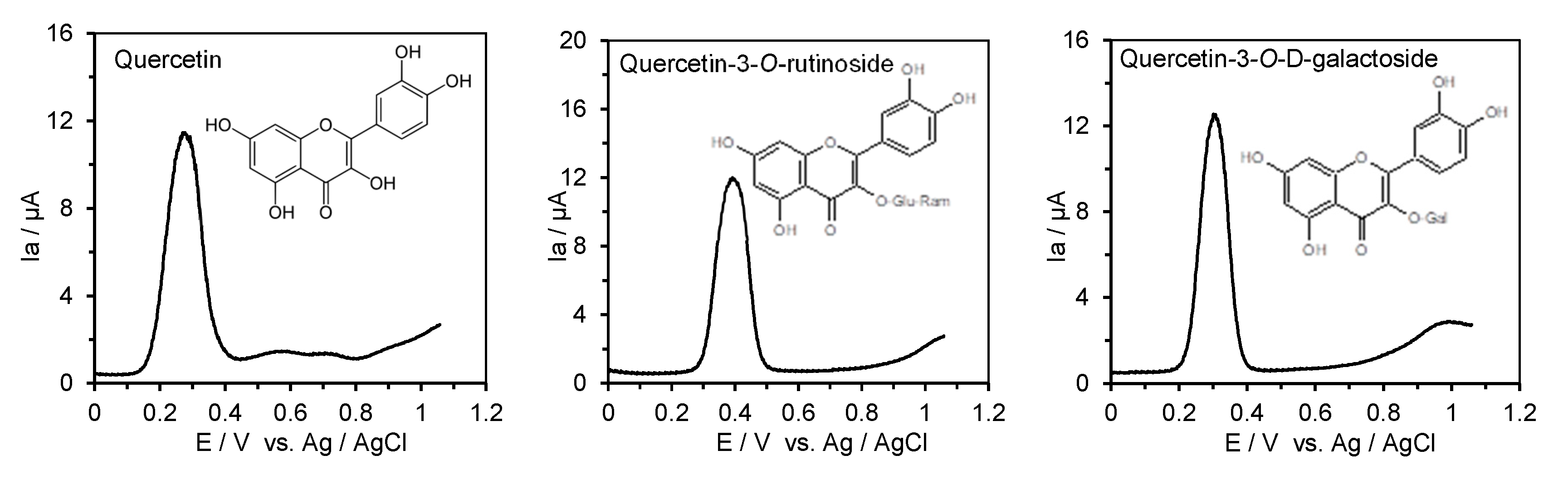
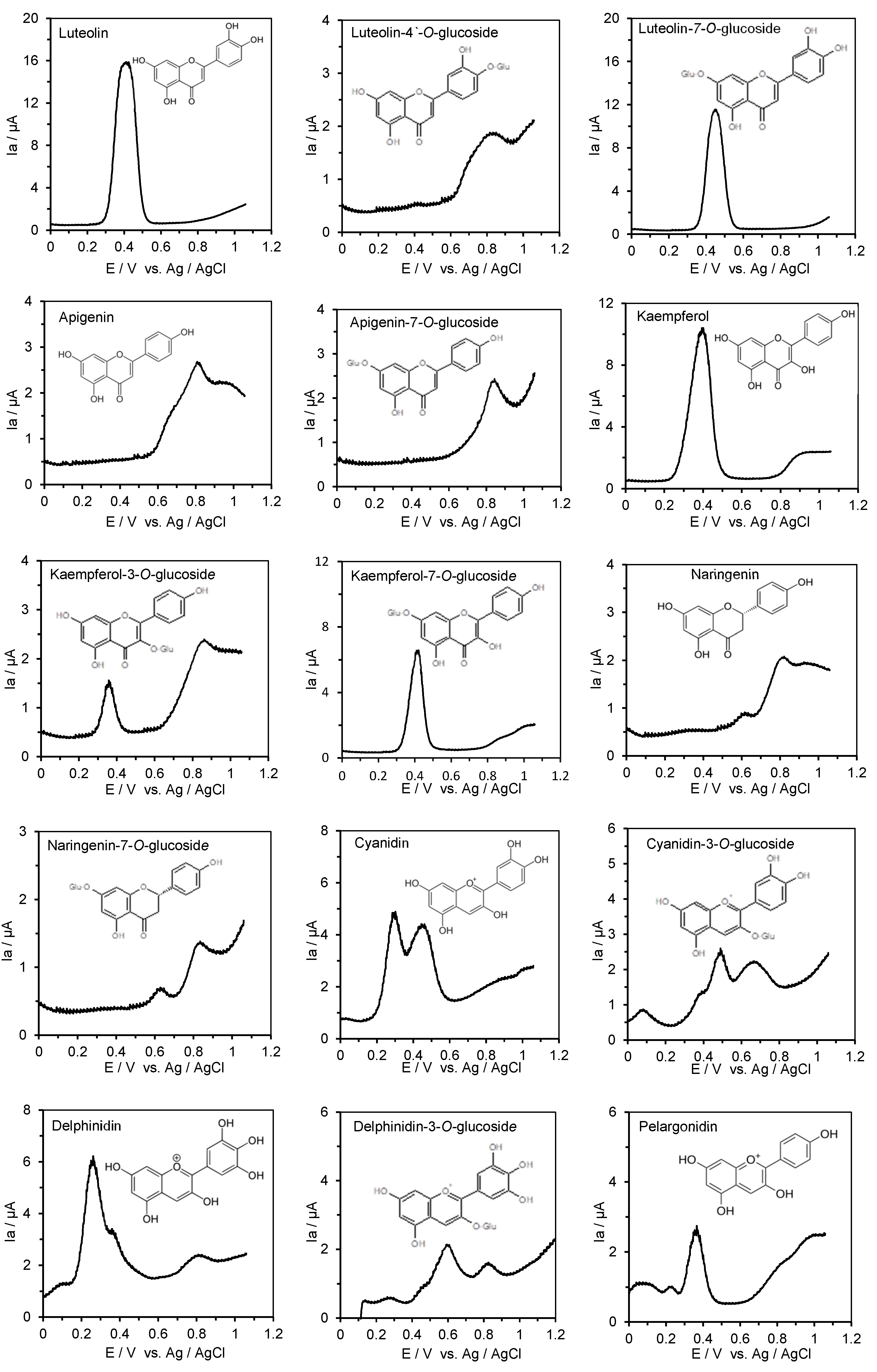

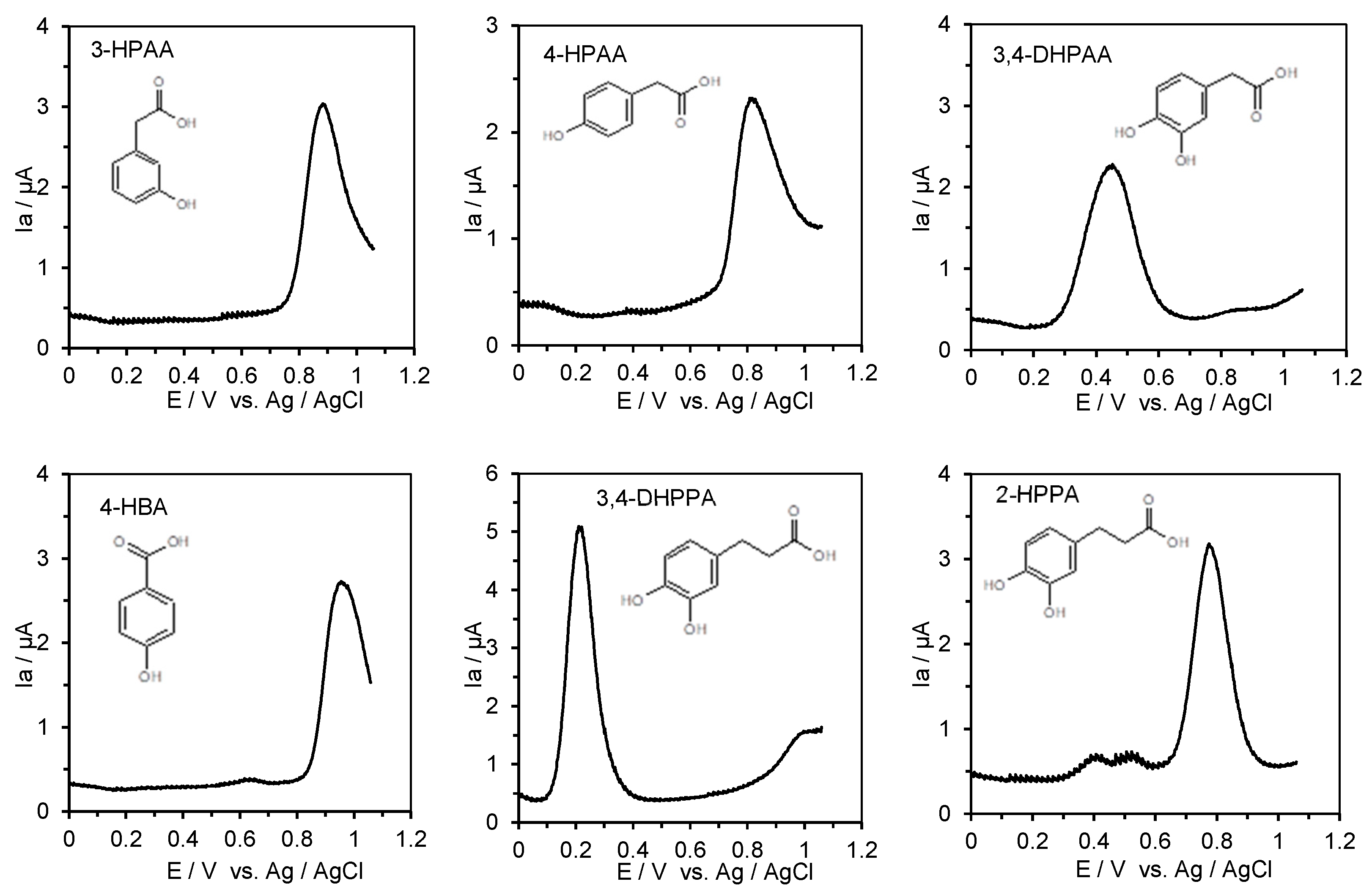
| Phenolic Acid | IC50 Value (µM) | Equation of the Linear Regression | (mV) |
|---|---|---|---|
| Benzoic acid derivatives | |||
| Gentisic acid | 834.49 ± 9.26 g | y = 0.038x + 18.373 | 622 ± 8 c |
| Syringic acid | 2756.62 ± 36.34 d | y = 0.007x + 30.428 | 731 ± 9 a |
| Vanillic acid | 4108.26 ± 48.28 c | y = 0.010x + 7.68549 | 748 ± 10 a |
| Protocatechuic acid | 6234.53 ± 42.02 b | y = 0.007x + 6.358 | 417 ± 12 e |
| Cinnamic acid derivatives | |||
| Chlorogenic acid | 134.2 ± 2.96 j | y = 0.098x + 36.822 | 443 ± 9 d |
| p-Coumaric acid | 314.89 ± 9.80 i | y = 0.022x + 43.041 | 672 ± 10 b |
| Sinapic acid | 493.11 ± 6.14 h | y = 0.038x + 31.262 | 618 ± 7 c |
| Ferulic acid | 979.58 ± 13.55 f | y = 0.019x + 31.290 | 611 ± 6 c |
| Caffeic acid | 9105.1 ± 58.2 a | y = 0.004x + 15.401 | 397 ± 8 e |
| Compound | IC50 Value (µM) | Equation of the Linear Regression | (mV) |
|---|---|---|---|
| Quercetin | 120.54 ± 1.56 m | y = 0.322x + 11.355 | 330 ± 4 g |
| Rutin (quercetin-O-3-rutinoside) | 59.29 ± 0.48 t | y = 0.383x + 27.285 | 389 ± 4 g |
| Hyperoside (quercetin -3-O-D-galactoside | 157.24 ± 1.45 n | y = 0.185x + 20.990 | 307 ± 3 h |
| Luteolin | 77.10 ± 2.55 s | y = 0.242x + 31.364 | 407 ± 5 f |
| Luteolin-4′-O-glucoside | 88.93 ± 1.68 r | y = 0.236x + 29.039 | 828 ± 5 b |
| Luteolin-7-O-glucoside | 61.50 ± 1.62 t | y = 0.207x + 37.282 | 445 ± 3 e |
| Apigenin | 336.81 ± 3.72 d | y = 0.080x + 22.954 | 810 ± 6 c |
| Apigenin-7-O-glucoside | 218.44 ± 2.53 g | y = 0.080x + 22.954 | 841 ± 6 ab |
| Kaempferol | 131.95 ± 2.47 k | y = 0.121x + 34.008 | 394 ± 4 g |
| Kaempferol-3-O-glucoside | 110.27 ± 3.56 o | y = 0.113x + 37.540 | 359 ± 3 fg |
| Kaempferol-7-O-glucoside | 97.70 ± 2.08 p | y = 0.122x + 38.101 | 419 ± 6 f |
| Naringenin | 390.75 ± 3.96 a | y = 0.079x + 19.287 | 819 ± 7 bc |
| Naringenin-7-O-glucoside | 357.17 ± 1.99 c | y = 0.109x + 11.033 | 830 ± 8 ab |
| Cyanidin | 176.22 ± 2.38 j | y = 0.142x + 25.065 | 295 ± 3 h |
| Cyanidin-3-O-glucoside | 185.38 ± 2.58 i | y = 0.137x + 24.696 | 373 ± 4 g |
| Delphinidin | 247.72 ± 1.85 f | y = 0.135x + 16.657 | 259 ± 3 i |
| Delphinidin-3-O-glucoside | 208.78 ± 1.33 h | y = 0.139x + 21.001 | 453 ± 4 e |
| Pelargonidin | 292.68 ± 3.40 e | y = 0.123x + 13.883 | 228 ± 5 j |
| Pelargonidin-3-O-glucoside | 376.88 ± 2.61 b | y = 0.116x + 6.433 | 473 ± 5 d |
| Phenolic Acid | IC50 Value (µM) | Equation of the Linear Regression | (mV) |
|---|---|---|---|
| 3-hydroxyphenylacetic acid (3-HPAA) | 678.86 ± 7.70 f | y = 0.052x + 14.971 | 883 ± 11 b |
| 4-hydroxyphenylacetic acid (4-HPAA) | 1390.67 ± 10.18 b | y = 0.027x + 12.730 | 815 ± 12 c |
| 3,4-dihydroxyphenylacetic acid (3,4-DHPAA) | 672.82 ± 4.93 f | y = 0.048x + 18.041 | 432 ± 6 g |
| 4-hydroxybenzoic acid (4-HBA) | 788.32 ± 11.08 d | y = 0.039x + 18.546 | 961 ± 11 a |
| 3-(3,4-dihydroxyphenyl)propionic acid (3,4-DHPPA) | 612.04 ± 6.51 g | y = 0.054x +16.705 | 218 ± 3 h |
| 3-(2-hydroxyphenyl)propionic acid(2-HPPA) | 932.92 ± 6.42 c | y = 0.044x + 8.952 | 781 ± 9 d |
| 3-(4-hydroxyphenyl)propionic acid (4-HPPA) | 490.65 ± 4.45 h | y = 0.053x + 24.143 | 664 ± 8 e |
| 3-(2,4-dihydroxyphenyl)propionic acid(3-2,4-DHPPA) | 744.66 ± 8.33 e | y = 0.048x + 14.033 | 659 ± 8 e |
| 3-(4-hydroxyphenyl)propionic methyl ester (MET) | 726.02 ± 5.71 e | y = 0.045x + 17.692 | 574 ± 5 f |
| 2-(3-methoxy-4-hydroxyphenyl)acetic acid (homovanillic acid) (HVA) | 1460.54 ± 10.80 a | y = 0.019x + 10.253 | 643 ± 5 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska, D.; Starowicz, M.; Wronkowska, M.; Zieliński, H. Angiotensin-Converting Enzyme Inhibitory Activity of Selected Phenolic Acids, Flavonoids, Their O-Glucosides, and Low-Molecular-Weight Phenolic Metabolites in Relation to Their Oxidation Potentials. Metabolites 2025, 15, 443. https://doi.org/10.3390/metabo15070443
Zielińska D, Starowicz M, Wronkowska M, Zieliński H. Angiotensin-Converting Enzyme Inhibitory Activity of Selected Phenolic Acids, Flavonoids, Their O-Glucosides, and Low-Molecular-Weight Phenolic Metabolites in Relation to Their Oxidation Potentials. Metabolites. 2025; 15(7):443. https://doi.org/10.3390/metabo15070443
Chicago/Turabian StyleZielińska, Danuta, Małgorzata Starowicz, Małgorzata Wronkowska, and Henryk Zieliński. 2025. "Angiotensin-Converting Enzyme Inhibitory Activity of Selected Phenolic Acids, Flavonoids, Their O-Glucosides, and Low-Molecular-Weight Phenolic Metabolites in Relation to Their Oxidation Potentials" Metabolites 15, no. 7: 443. https://doi.org/10.3390/metabo15070443
APA StyleZielińska, D., Starowicz, M., Wronkowska, M., & Zieliński, H. (2025). Angiotensin-Converting Enzyme Inhibitory Activity of Selected Phenolic Acids, Flavonoids, Their O-Glucosides, and Low-Molecular-Weight Phenolic Metabolites in Relation to Their Oxidation Potentials. Metabolites, 15(7), 443. https://doi.org/10.3390/metabo15070443







