Vitamin B12 and Folate in Adherent and Non-Adherent Individuals with Phenylketonuria: A Cross-Sectional Study, Systematic Review, and Meta-Analysis
Abstract
1. Introduction
2. Cross-Sectional Study
2.1. Materials and Methods
2.1.1. Study Design and Participants
2.1.2. Vitamin Assessment
2.1.3. Ethics Approval and Consent to Participate
2.1.4. Statistical Analysis
2.1.5. Bias Control
2.2. Results
3. Systematic Review and Meta-Analysis
3.1. Materials and Methods
3.1.1. Protocol and Registration
3.1.2. Inclusion and Exclusion Criteria
Division by Mean Phe Levels
- •
- For patients under 6 years of age, adherence was defined as maintaining a mean plasma Phe level of <360 μmol/L;
- •
- For patients aged 6 to 10 years, a mean plasma Phe level of <480 μmol/L;
- •
- For patients aged 10 years and older, a mean plasma Phe level of ≤600 μmol/L.
Division by Formula Consumption
- •
- Regular formula intake: participants who consistently consumed the recommended amounts of metabolic formula as part of their dietary regimen;
- •
- Irregular formula intake: participants who reported inconsistent or insufficient consumption of the metabolic formula, as defined in the study protocols or dietary records.
3.1.3. Data Collection Process, Extraction and Analysis
3.1.4. Data Item
- General information: title of the article, journal name, main author, and publication year;
- Study characteristics: study name, design, country (region), and sample size (total number of subjects and the number in each group who were included and completed the study);
- Study population characteristics: age, sex, and BMI (kg/m2);
- Description of dietary treatment: natural protein intake (g/day), protein substitute intake (g/day), total protein intake (g/day), phenylalanine intake (mg/d), annual mean/median phenylalanine levels (μmol/L), follow-up (yes or no), treatment adherence (yes or no), phenylalanine levels (μmol/L), and tyrosine levels (μmol/L);
- Main outcomes: blood or plasma levels of folate (nmol/L), folic acid (nmol/L), erythrocyte folate (nmol/L), total folate (nmol/L), and vitamin B12 (pmol/L).
3.1.5. Information Sources and Search Strategy
3.1.6. Risk of Bias of Individual Studies
- Selection: this domain assesses how well the study defines and selects participants, including the representativeness of the sample and the method of identifying cases and controls;
- Comparability: this domain evaluates how the study accounts for potential confounding factors by examining the comparability of study groups on important characteristics;
- Outcome (or exposure): this domain examines the accuracy and reliability of outcome assessment or exposure measurement.
3.1.7. Certainty of Evidence Assessment
3.1.8. Data Synthesis and Analysis
3.2. Results
3.2.1. Search Results

3.2.2. Study Characteristics
3.2.3. Risk of Bias
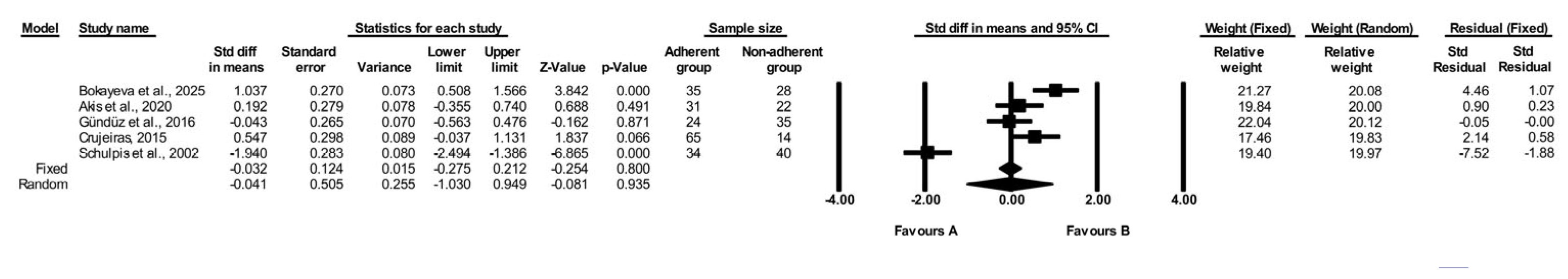
3.2.4. Comparison of Folate Levels in Adherent vs. Non-Adherent PKU Individuals
3.2.5. Comparison of Vitamin B12 Levels in Adherent vs. Non-Adherent PKU Individuals
3.2.6. Comparison of Folate Levels in Regular Intake vs. Irregular Formula Intake PKU Individuals
3.2.7. Comparison of Vitamin B12 Levels in Regular Intake vs. Irregular Intake PKU Individuals
3.2.8. Subgroup Analysis
3.2.9. Certainty of Evidence Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BH4 | Tetrahydrobiopterin |
| BMI | Body Mass Index |
| CI | Confidence Interval |
| GRADE | Grading of Recommendations, Assessment, Development, and Evaluation |
| NA | Not Analysed |
| NI | No Information |
| NOS | Newcastle–Ottawa Scale |
| PAH | Phenylalanine Hydroxylase |
| Phe | Phenylalanine |
| PKU | Phenylketonuria |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SD | Standard Deviation |
| SMD | Standardised Mean Difference |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| Tyr | Tyrosine |
References
- van Spronsen, F.J.; van Wegberg, A.M.; Ahring, K.; Bélanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. Key European Guidelines for the Diagnosis and Management of Patients with Phenylketonuria. Lancet Diabetes Endocrinol. 2017, 5, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Hillert, A.; Anikster, Y.; Belanger-Quintana, A.; Burlina, A.; Burton, B.K.; Carducci, C.; Chiesa, A.E.; Christodoulou, J.; Đorđević, M.; Desviat, L.R.; et al. The Genetic Landscape and Epidemiology of Phenylketonuria. Am. J. Hum. Genet. 2020, 107, 234–250. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.; van Wegberg, A.M.J.; Ahring, K.; Beblo, S.; Bélanger-Quintana, A.; Burlina, A.; Campistol, J.; Coşkun, T.; Feillet, F.; Giżewska, M.; et al. PKU Dietary Handbook to Accompany PKU Guidelines. Orphanet J. Rare Dis. 2020, 15, 171. [Google Scholar] [CrossRef]
- Macdonald, A.; Davies, P.; Daly, A.; Hopkins, V.; Hall, S.K.; Asplin, D.; Hendriksz, C.; Chakrapani, A. Does Maternal Knowledge and Parent Education Affect Blood Phenylalanine Control in Phenylketonuria? J. Hum. Nutr. Diet. 2008, 21, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Walkowiak, D.; Bukowska-Posadzy, A.; Kałużny, Ł.; Ołtarzewski, M.; Staszewski, R.; Musielak, M.; Walkowiak, J. Therapy Compliance in Children with Phenylketonuria Younger than 5 Years: A Cohort Study. Adv. Clin. Exp. Med. 2019, 28, 1385–1391. [Google Scholar] [CrossRef]
- Jurecki, E.R.; Cederbaum, S.; Kopesky, J.; Perry, K.; Rohr, F.; Sanchez-Valle, A.; Viau, K.S.; Sheinin, M.Y.; Cohen-Pfeffer, J.L. Adherence to Clinic Recommendations among Patients with Phenylketonuria in the United States. Mol. Genet. Metab. 2017, 120, 190–197. [Google Scholar] [CrossRef]
- Walter, J.H.; White, F.J. Blood Phenylalanine Control in Adolescents with Phenylketonuria. Int. J. Adolesc. Med. Health 2004, 16, 41–45. [Google Scholar] [CrossRef]
- Crone, M.R.; van Spronsen, F.J.; Oudshoorn, K.; Bekhof, J.; van Rijn, G.; Verkerk, P.H. Behavioural Factors Related to Metabolic Control in Patients with Phenylketonuria. J. Inherit. Metab. Dis. 2005, 28, 627–637. [Google Scholar] [CrossRef]
- Vieira, T.A.; Nalin, T.; Krug, B.C.; Bittar, C.M.; Netto, C.B.O.; Schwartz, I.V.D. Adherence to Treatment of Phenylketonuria: A Study in Southern Brazilian Patients. J. Inborn Errors Metab. Screen. 2015, 3, 2326409815579861. [Google Scholar] [CrossRef]
- Kumar Dalei, S.; Adlakha, N. Food Regime for Phenylketonuria: Presenting Complications and Possible Solutions. J. Multidiscip. Healthc. 2022, 15, 125–136. [Google Scholar] [CrossRef]
- Walkowiak, D.; Domaradzki, J.; Mozrzymas, R.; Korycińska-Chaaban, D.; Duś-Żuchowska, M.; Didycz, B.; Mikołuć, B.; Walkowiak, J. Professional Activity, Gender and Disease-Related Emotions: The Impact on Parents’ Experiences in Caring for Children with Phenylketonuria. Mol. Genet. Metab. Rep. 2023, 36, 100992. [Google Scholar] [CrossRef] [PubMed]
- Blau, N.; van Spronsen, F.J.; Levy, H.L. Phenylketonuria. Lancet 2010, 376, 1417–1427. [Google Scholar] [CrossRef]
- Bokayeva, K.; Jamka, M.; Walkowiak, D.; Duś-Żuchowska, M.; Herzig, K.-H.; Walkowiak, J. Vitamin Status in Patients with Phenylketonuria: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 5065. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.; Rocha, J.C.; van Rijn, M.; Feillet, F. Nutrition in Phenylketonuria. Mol. Genet. Metab. 2011, 104, S10–S18. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh, M.; Sohrab, G.; Alaei, M.; Eini-Zinab, H.; Mohammadpour-Ahranjani, B.; Rastgoo, S.; Namkhah, Z. Growth and Nutritional Status of Phenylketonuric Children and Adolescents. BMC Pediatr. 2022, 22, 664. [Google Scholar] [CrossRef]
- Evans, S.; Daly, A.; MacDonald, J.; Preece, M.A.; Santra, S.; Vijay, S.; Chakrapani, A.; MacDonald, A. The Micronutrient Status of Patients with Phenylketonuria on Dietary Treatment: An Ongoing Challenge. Ann. Nutr. Metab. 2014, 65, 42–48. [Google Scholar] [CrossRef]
- Stølen, L.H.; Lilje, R.; Jørgensen, J.V.; Bliksrud, Y.T.; Almaas, R. High Dietary Folic Acid and High Plasma Folate in Children and Adults with Phenylketonuria. JIMD Rep. 2013, 13, 83–90. [Google Scholar] [CrossRef]
- Kose, E.; Arslan, N. Vitamin/Mineral and Micronutrient Status in Patients with Classical Phenylketonuria. Clin. Nutr. 2019, 38, 197–203. [Google Scholar] [CrossRef]
- Hoss, G.R.W.; Sperb-Ludwig, F.; Tonon, T.; Poloni, S.; Behringer, S.; Blom, H.J.; Maillot, F.; Schwartz, I.V.D. Homocysteine and Methylmalonic Acid in Phenylketonuria Patients. Genet. Mol. Biol. 2023, 46, e20230103. [Google Scholar] [CrossRef]
- Schulpis, K.H.; Karikas, G.A.; Papakonstantinou, E. Homocysteine and Other Vascular Risk Factors in Patients with Phenylketonuria on a Diet. Acta Paediatr. 2002, 91, 905–909. [Google Scholar] [CrossRef]
- Hochuli, M.; Bollhalder, S.; Thierer, C.; Refardt, J.; Gerber, P.; Baumgartner, M.R. Effects of Inadequate Amino Acid Mixture Intake on Nutrient Supply of Adult Patients with Phenylketonuria. Ann. Nutr. Metab. 2017, 71, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Gündüz, M.; Çakar, S.; Kuyum, P.; Makay, B.; Arslan, N. Comparison of Atherogenic Risk Factors among Poorly Controlled and Well-Controlled Adolescent Phenylketonuria Patients. Cardiol. Young 2016, 26, 901–908. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.K.; Walkowiak, J. Study Designs in Medical Research and Their Key Characteristics. J. Med. Sci. 2023, 92, e928. [Google Scholar] [CrossRef]
- Akış, M.; Kant, M.; Işık, İ.; Kısa, P.T.; Köse, E.; Arslan, N.; İşlekel, H. Functional Vitamin B12 Deficiency in Phenylketonuria Patients and Healthy Controls: An Evaluation with Combined Indicator of Vitamin B12 Status as a Biochemical Index. Ann. Clin. Biochem. 2020, 57, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Malone, H.E.; Nicholl, H.; Coyne, I. Fundamentals of Estimating Sample Size. Nurse Res. 2016, 23, 21–25. [Google Scholar] [CrossRef]
- Cole, T.J.; Lobstein, T. Extended International (IOTF) Body Mass Index Cut-Offs for Thinness, Overweight and Obesity. Pediatr. Obes. 2012, 7, 284–294. [Google Scholar] [CrossRef]
- Vockley, J.; Andersson, H.C.; Antshel, K.M.; Braverman, N.E.; Burton, B.K.; Frazier, D.M.; Mitchell, J.; Smith, W.E.; Thompson, B.H.; Berry, S.A.; et al. Phenylalanine Hydroxylase Deficiency: Diagnosis and Management Guideline. Genet. Med. 2014, 16, 188–200. [Google Scholar] [CrossRef]
- Sawicka-Gutaj, N.; Gruszczyński, D.; Guzik, P.; Mostowska, A.; Walkowiak, J. Publication Ethics of Human Studies in the Light of the Declaration of Helsinki—A Mini-Review. J. Med. Sci. 2022, 91, e700. [Google Scholar] [CrossRef]
- Posit Team. RStudio: Integrated Development Environment for R. Posit Software; PBC: Boston, MA, USA, 2024. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.5 (Updated August 2024). 2024. Cochrane. Available online: https://training.cochrane.org/handbook (accessed on 15 December 2024).
- Bokayeva, K.; Jamka, M.; Walkowiak, D.; Walkowiak, J.; Herzig, K.-H. Vitamin B12 and Folate Comparison in Adherent and Non-Adherent Individuals with PKU. PROSPERO: International Prospective Register of Systematic Reviews. Available online: https://www.crd.york.ac.uk/PROSPERO/view/CRD420250650808 (accessed on 4 March 2025).
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analyses. Ottawa: Ottawa Hospital Research Institute. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 1 February 2025).
- Modesti, P.A.; Reboldi, G.; Cappuccio, F.P.; Agyemang, C.; Remuzzi, G.; Rapi, S.; Perruolo, E.; Parati, G. ESH Working Group on CV Risk in Low Resource Settings Panethnic Differences in Blood Pressure in Europe: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0147601. [Google Scholar] [CrossRef] [PubMed]
- Puhan, M.A.; Schünemann, H.J.; Murad, M.H.; Li, T.; Brignardello-Petersen, R.; Singh, J.A.; Kessels, A.G.; Guyatt, G.H. A GRADE Working Group Approach for Rating the Quality of Treatment Effect Estimates from Network Meta-Analysis. BMJ 2014, 349, g5630. [Google Scholar] [CrossRef]
- Crujeiras, V.; Aldámiz-Echevarría, L.; Dalmau, J.; Vitoria, I.; Andrade, F.; Roca, I.; Leis, R.; Fermandez-Marmiesse, A.; Couce, M.L. Micronutrient in Hyperphenylalaninemia. Data Brief. 2015, 4, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.; White, F.J.; Cleary, M.A.; Wraith, E.; Lam, W.K.; Walter, J.H. Increased Risk of Vitamin B12 Deficiency in Patients with Phenylketonuria on an Unrestricted or Relaxed Diet. J. Pediatr. 2000, 136, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Agurto, E.; Leal-Witt, M.J.; Arias, C.; Cabello, J.F.; Bunout, D.; Cornejo, V. Muscle and Bone Health in Young Chilean Adults with Phenylketonuria and Different Degrees of Compliance with the Phenylalanine Restricted Diet. Nutrients 2023, 15, 2939. [Google Scholar] [CrossRef]
- Watanabe, F.; Bito, T. Vitamin B12 Sources and Microbial Interaction. Exp. Biol. Med. 2018, 243, 148–158. [Google Scholar] [CrossRef]
- Vitamin B12: Fact Sheet for Health Professionals. NIH Office of Dietary Supplements. Available online: https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/ (accessed on 14 February 2025).
- Hvas, A.M.; Nexo, E.; Nielsen, J.B. Vitamin B12 and Vitamin B6 Supplementation Is Needed among Adults with Phenylketonuria (PKU). J. Inherit. Metab. Dis. 2006, 29, 47–53. [Google Scholar] [CrossRef]
- Vugteveen, I.; Hoeksma, M.; Monsen, A.-L.B.; Fokkema, M.R.; Reijngoud, D.-J.; van Rijn, M.; van Spronsen, F.J. Serum Vitamin B12 Concentrations within Reference Values Do Not Exclude Functional Vitamin B12 Deficiency in PKU Patients of Various Ages. Mol. Genet. Metab. 2011, 102, 13–17. [Google Scholar] [CrossRef]
- Hanley, W.; Feigenbaum, A.; Clarke, J.; Schoonheyt, W.; Austin, V. Vitamin B12 Deficiency in Adolescents and Young Adults with Phenylketonuria. Lancet 1993, 342, 997. [Google Scholar] [CrossRef]
- Crujeiras, V.; Aldámiz-Echevarría, L.; Dalmau, J.; Vitoria, I.; Andrade, F.; Roca, I.; Leis, R.; Fernandez-Marmiesse, A.; Couce, M.L. Vitamin and Mineral Status in Patients with Hyperphenylalaninemia. Mol. Genet. Metab. 2015, 115, 145–150. [Google Scholar] [CrossRef]
- Vitamin B-12. Available online: https://www.mayoclinic.org/drugs-supplements-vitamin-b12/art-20363663 (accessed on 7 April 2025).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Scientific Opinion on Dietary Reference Values for Folate. EFSA J. 2014, 12, 3893. [CrossRef]
- National Institutes of Health Folate Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/ (accessed on 12 February 2025).
- Rocha, J.C.; MacDonald, A. Dietary Intervention in the Management of Phenylketonuria: Current Perspectives. Pediatr. Health Med. Ther. 2016, 7, 155–163. [Google Scholar] [CrossRef]
- Procházková, D.; Jarkovský, J.; Haňková, Z.; Konečná, P.; Benáková, H.; Vinohradská, H.; Mikušková, A. Long-Term Treatment for Hyperphenylalaninemia and Phenylketonuria: A Risk for Nutritional Vitamin B12 Deficiency? J. Pediatr. Endocrinol. Metab. 2015, 28, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Huemer, M.; Födinger, M.; Bodamer, O.A.; Mühl, A.; Herle, M.; Weigmann, C.; Ulmer, H.; Stöckler-Ipsiroglu, S.; Möslinger, D. Total Homocysteine, B-Vitamins and Genetic Polymorphisms in Patients with Classical Phenylketonuria. Mol. Genet. Metab. 2008, 94, 46–51. [Google Scholar] [CrossRef]
- Zielińska, M.; Żółkowska, J.; Przybylska-Kruszewska, A.; Gładysz, D.; Korycińska-Chaaban, D.; Nowacka, M.; Hozyasz, K.K. High plasma folate in patients with phenylketonuria. Pol. Merkur. Lekarski 2016, 40, 223–229. [Google Scholar] [PubMed]
- Wiig, I.; Motzfeldt, K.; Løken, E.B.; Kase, B.F. Nutritional Consequences of Adhering to a Low Phenylalanine Diet for Late-Treated Adults with PKU: Low Phe Diet for Adults with PKU. JIMD Rep. 2013, 7, 109–116. [Google Scholar] [CrossRef]
- Robert, M.; Rocha, J.C.; van Rijn, M.; Ahring, K.; Bélanger-Quintana, A.; MacDonald, A.; Dokoupil, K.; Gokmen Ozel, H.; Lammardo, A.M.; Goyens, P.; et al. Micronutrient Status in Phenylketonuria. Mol. Genet. Metab. 2013, 110, S6–S17. [Google Scholar] [CrossRef]
- Fardous, A.M.; Heydari, A.R. Uncovering the Hidden Dangers and Molecular Mechanisms of Excess Folate: A Narrative Review. Nutrients 2023, 15, 4699. [Google Scholar] [CrossRef]








| Age Group | Females (ng/mL) | Males (ng/mL) |
|---|---|---|
| 12–13 years | 1–10 | 1.5–11 |
| 13–18 years | 1.2–7.1 | 1.2–8.8 |
| 18–20 years | 3.8–16 | |
| >20 years | 3.9–26.8 | |
| Parameter | Adherent PKU Group Median (Q1–Q3); Mean ± SD | Non-Adherent PKU Group Median (Q1–Q3); Mean ± SD | p-Value |
|---|---|---|---|
| n | 35 | 28 | |
| Age (years) | 19.00 (14.05–28.25); 20.88 ± 7.33 | 22.75 (17.00–29.78); 24.01 ± 8.38 | 0.109 1 |
| Sex (n (%)) | |||
| Female | 23 (65.7%) | 14 (50.0%) | 0.208 2 |
| Male | 12 (34.3%) | 14 (50.0%) | |
| BMI (kg/m2) BMI-IOTF corrected (kg/m2) | 21.90 (19.82–24.09); 21.71 ± 3.35 23.46 (20.18–24.77); 22.68 ± 3.69 | 23.25 (20.62–29.65); 24.78 ± 5.42 23.56 (21.09–29.78); 25.11 ± 5.22 | 0.067 1 0.142 1 |
| Phe mean (mg/dL) | 7.62 (5.79–8.93); 7.09 ± 2.13 | 15.42 (12.92–17.88); 15.62 ± 3.53 | <0.0001 3* |
| Phe median (mg/dL) | 6.80 (5.22–8.66); 6.72 ± 2.30 | 15.09 (12.46–17.62); 15.47 ± 3.48 | <0.0001 3* |
| Abnormal values (%) | 23.7 (4.5–33.3); 20.9 ± 15.1 | 100 (79.0–100); 88.9 ± 14.9 | 0.0001 1* |
| Vitamin B12 (pg/mL) | 732.0 (576.5–919.0); 767.6 ± 264.5 95% CI (676.78–858.52) | 491.5 (369.2–675.8); 524.7 ± 216.4 95% CI (440.76–608.59) | <0.001 4* |
| Folate (ng/mL) | 13.40 (12.35–14.60); 13.44 ± 1.96 95% CI (12.80–14.12) | 10.05 (7.97–12.90); 10.62 ± 3.36 95% CI (9.32–11.93) | <0.001 3* |
| Parameter | Regular PKU Group Median (Q1–Q3); Mean ± SD | Irregular PKU Group Median (Q1–Q3); Mean ± SD | p-Value |
|---|---|---|---|
| n | 38 | 25 | |
| Age (years) | 19.05 (14.1–27.4); 20.82 ± 7.25 | 24.2 (17–29.6); 24.48 ± 8.49 | 0.087 1 |
| Sex (n (%)) | |||
| Female | 25 (65.8%) | 12 (52.0%) | 0.161 2 |
| Male | 13 (34.2%) | 13 (48.0%) | |
| BMI (kg/m2) BMI-IOTF corrected (kg/m2) | 21.65 (19.81–23.55); 21.80 ± 3.60 23.47 (21.24–24.78); 23.39 ± 4.15 | 24.35 (21–29.65); 25.01 ± 5.35 25.21 (21.75–28.88); 25.94 ± 5.39 | 0.012 3* 0.222 4 |
| Phe mean (mg/dL) | 8.17 (6.02–9.59); 8.16 ± 3.17 | 16.66 (11.65–18.7); 15.02 ± 4.76 | <0.0001 3* |
| Phe median (mg/dL) | 7.86 (5.93–9.78); 7.92 ± 3.28 | 15.91 (11.36–17.73); 14.70 ± 5.04 | <0.0001 3* |
| Abnormal values (%) | 32.3 (6.1–40.1); 32.7 ± 30.2 | 100 (66.7–100); 79.0 ± 28.7 | 0.0001 1* |
| Vitamin B12 (pg/mL) | 721.5 (613.5–905); 746.7 ± 228.4 95% CI (671.61–821.76) | 449 (358–548); 527.4 ± 281.9 95% CI (411.05–643.75) | 0.0001 1* |
| Folate (ng/mL) | 13.45 (12.4–14.6); 13.32 ± 2.25 95% CI (12.58–14.06) | 10.1 (8.1–12.7); 10.48 ± 3.23 95% CI (9.15–11.81) | <0.001 3* |
| Author | Year | Country (Region) | Groups | n Included | n Completed | Age [Years] 1 | BMI [kg/m2] 1 | Sex [% of Women] |
|---|---|---|---|---|---|---|---|---|
| Bokayeva et al. | 2025 | Poland | Adherent | 35 | 35 | 20.9 ± 7.3 | 21.7 ± 3.4 | 65.7 |
| Non-adherent | 28 | 28 | 24.0 ± 8.4 | 24.8 ± 5.4 | 50 | |||
| Regular | 38 | 38 | 20.82 ± 7.25 | 21.80 ± 3.60 | 65.8% | |||
| Irregular | 25 | 25 | 24.48 ± 8.49 | 25.01 ± 5.35 | 52.0% | |||
| Rojas-Agurto et al. [39] | 2023 | Chile | Regular 2 | 10 | 10 | 23.5 (19–26) 4 | 24.3 (22.4–28.5) 4 | 50 |
| Irregular 3 | 14 | 14 | 22.5 (18.5–25.5) 4 | 26.7 (24–29.9) 4 | 36 | |||
| Akış et al. [25] | 2020 | Turkey | Adherent 5 | 31 | 31 | 9.5 (5.0–18.0) 7 | NI | 38 |
| Non-adherent 6 | 22 | 22 | ||||||
| Hochuli et al. [21] | 2017 | Switzerland | Regular 8 | 15 | 15 | 32.0 ± 12.0 | 24.6 ± 4.3 | 53 |
| Irregular 9 | 5 | 5 | 39.0 ± 8.4 | 20.6 ± 2.1 | 20 | |||
| Gündüz et al. [22] | 2016 | Turkey | Adherent 10 | 24 | 24 | 13.1 ± 2.4 | 19.1 ± 2.1 | 33 |
| Non-adherent 11 | 35 | 35 | 14.1 ± 2.9 | 18.9 ± 1.9 | 54 | |||
| Crujeiras et al. [37] | 2015 | Spain | Adherent | 69 | 69 12 | 10.9 (1–92) 13 | NI | 47.8% 50% |
| Non-adherent | 14 | 14 | 23.5 (3–30) 13 | |||||
| Schulpis et al. [20] | 2002 | Greece | Adherent 10 | 34 | 34 | 6.8 ± 1.5 | NI | NI |
| Non-adherent 11 | 40 | 40 | 8.0 ± 3.2 | |||||
| Robinson et al. [38] | 2000 | The United Kingdom | Regular 14 | 22 | 22 16 | 24.0 18 | NI | NI |
| Irregular 15 | 61 | 61 17 | 21.0 18 |
| Author | Year | Groups | Phe Intake [mg/d] | Mean/Median Phe Levels 1 | Medical Control | Last Phe Level [μmol/L] 1 | Last Tyr Level [μmol/L] 1 |
|---|---|---|---|---|---|---|---|
| Bokayeva et al. | 2025 | Adherent | NI | 7.09 ± 2.13 mg/dL | Yes | NI | NI |
| Non-adherent | 15.62 ± 3.53 mg/dL | Yes | |||||
| Regular | NI | 8.16 ± 3.17 mg/dL | Yes | NI | NI | ||
| Irregular | 15.02 ± 4.76 mg/dL | Yes | |||||
| Rojas-Agurto et al. [39] | 2023 | Regular 2 | 600 (400–800) 4 | NI | Yes | 260.3 (170.0–642.0) 4 | 46.60 (33.1–49.7) 4 |
| Irregular 3 | 1200 (500–1700) 4 | No | 781.0 (636.0–1035.1) 4 | 35.90 (33.1–55.2) 4 | |||
| Akış et al. [25] | 2020 | Adherent 5 | NI | NI | Yes | 357.19 (121.10–514.60) 7 | NI |
| Non-adherent 6 | Yes | 696.21 (441.90–1035.20) 7 | |||||
| Hochuli et al. [21] | 2017 | Regular 8 | NI | NI | NI | 650 ± 283 | NI |
| Irregular 9 | 760 ± 350 | ||||||
| Gündüz et al. [22] | 2016 | Adherent 10 | 300–900 12 | NI | NI | 306.1 ± 78.0 | NI |
| Non-adherent 11 | 720.8 ± 196.7 | ||||||
| Crujeiras et al. [37] | 2015 | Adherent | NI | 276.6 ± 133.4 μmol/L (4.57 ± 2.20 mg/dL) | NI | NI | NI |
| Non-adherent | 867.1 ± 273.7 μmol/L (14.32 ± 4.52 mg/dL) | ||||||
| Schulpis et al. [20] | 2002 | Adherent 10 | NI | NI | NI | 192 ± 115 | NI |
| Non-adherent 11 | 599 ± 16 | ||||||
| Robinson et al. [38] | 2000 | Regular 13 | NI | NI | Yes | NI | NI |
| Irregular 14 | Yes |
| Study (First Author) | Selection | Comparability | Outcome | Overall Score | ||||
|---|---|---|---|---|---|---|---|---|
| Representativeness of the Sample | Sample Size | Non- Respondents | Ascertainment of Exposure | Based on Design and Analysis | Assessment of Outcome | Statistical Test | ||
| Bokayeva et al., 2025 | + | + | + | + + | + + | ++ | + | 10 |
| Rojas-Agurto et al. [39], 2023 | + | + + | + + | 5 | ||||
| Akış et al. [25], 2020 | + | + + | + + | 5 | ||||
| Hochuli et al. [21], 2017 | + | + + | + | 4 | ||||
| Gündüz et al. [22], 2016 | + | + + | + + | 5 | ||||
| Crujeiras et al. [37], 2015 | + | + + | 3 | |||||
| Schulpis et al. [20], 2002 | + | + + | 3 | |||||
| Robinson et al. [38], 2000 | 0 | |||||||
| Author | Year | Groups | Folate 1 | Vitamin B12 1 |
|---|---|---|---|---|
| Bokayeva et al. | 2025 | Adherent | 13.4 ± 1.96 2,3 | 767.6 ± 264.5 2,4 |
| Non-adherent | 10.63 ± 3.36 2,3 | 524.7 ± 216.4 2,4 | ||
| Regular | 13.32 ± 2.25 2,3 | 746.7 ± 228.4 2,4 | ||
| Irregular | 10.48 ± 3.23 2,3 | 527.4 ± 281.9 2,4 | ||
| Rojas-Agurto et al. [39] | 2023 | Regular 5 | 25.69 ± 7.58 2,3,7,8 | 706.4 ± 330.4 2,8,9 |
| Irregular 6 | 23.68 ± 7.19 2,3,7,8 | 383.4 ± 253.2 2,8,9 | ||
| Akış et al. [25] | 2020 | Adherent 10 | 37.2 ± 9.5 2,12 | 282.2 ± 128.5 2,13 |
| Non-Adherent 11 | 35.3 ± 10.4 2,12 | 318.9 ± 123.2 2,13 | ||
| Hochuli et al. [21] | 2017 | Regular 14 | 98 ± 290 2,7,16 | 540 ± 208 2,9 |
| Irregular 15 | 14 ± 3 2,7,16 | 251 ± 75 2,9 | ||
| Gündüz et al. [22] | 2016 | Adherent 17 | 32.4 ± 9.7 2,7,12 | 256.5 ± 139.3 2,13 |
| Non-Adherent 18 | 32.8 ± 9.0 2,7,12 | 308.8 ± 119.1 2,13 | ||
| Crujeiras et al. [37] | 2015 | Adherent | 19.8 ± 7.0 3,7,19 | 749.6 ± 331.7 4,19 |
| Non-adherent | 15.8 ± 8.6 3,7,19 | 515.9 ± 264.5 4,19 | ||
| Schulpis et al. [20] | 2002 | Adherent 17 | 2.35 ± 1.3 2,12 | 98.5 ± 22.3 2,13 |
| Non-adherent 18 | 5.8 ± 2.1 2,12 | 240.8 ± 62 2,13 | ||
| Robinson et al. [38] | 2000 | Regular 20 | 476 ± 258 16,22 | 468.7 ± 199.7 9,23 |
| Irregular 21 | 399.8 ± 184.8 16,22 | 303.6 ± 115.2 9,23 |
| Certainty Assessment | No. of Patients | Effect | Certainty | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome and No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Adherence | Non-Adherence | Absolute (95% CI) | |
| Vitamin B12—5 | non-randomised studies | serious a | serious b | not serious | serious c | all plausible residual confounding would reduce the demonstrated effect | 189 | 139 | SMD 0.38 SD lower (1.625 lower to 0.866 higher) | ⨁◯◯◯ Very low a,b,c |
| Folate—5 | non-randomised studies | serious a | serious d | not serious | serious c | all plausible residual confounding would reduce the demonstrated effect | 189 | 139 | SMD 0.041 SD lower (1.03 lower to 0.949 higher)) | ⨁◯◯◯ Very low a,c,d |
| Certainty Assessment | No. of Patients | Effect | Certainty | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome and No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Adherence | Non-Adherence | Absolute (95% CI) | |
| Vitamin B12—4 | non-randomised studies | serious a | not serious b | not serious | not serious | all plausible residual confounding would reduce the demonstrated effect | 85 | 105 | SMD 1.08 SD higher (0.754 higher to 1.405 higher) | ⨁⨁◯◯ Low a,b,c |
| Folate—3 | non-randomised studies | serious c | serious d | not serious | serious e | all plausible residual confounding would reduce the demonstrated effect | 59 | 90 | SMD 0.63 SD higher (0.116 higher to 1.145 higher) | ⨁◯◯◯ Very low a,c,d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bokayeva, K.; Jamka, M.; Walkowiak, D.; Duś-Żuchowska, M.; Kałużny, Ł.; Wichłacz-Trojanowska, N.; Chrobot, A.; Mozrzymas, R.; Sultanova, G.; Herzig, K.-H.; et al. Vitamin B12 and Folate in Adherent and Non-Adherent Individuals with Phenylketonuria: A Cross-Sectional Study, Systematic Review, and Meta-Analysis. Metabolites 2025, 15, 438. https://doi.org/10.3390/metabo15070438
Bokayeva K, Jamka M, Walkowiak D, Duś-Żuchowska M, Kałużny Ł, Wichłacz-Trojanowska N, Chrobot A, Mozrzymas R, Sultanova G, Herzig K-H, et al. Vitamin B12 and Folate in Adherent and Non-Adherent Individuals with Phenylketonuria: A Cross-Sectional Study, Systematic Review, and Meta-Analysis. Metabolites. 2025; 15(7):438. https://doi.org/10.3390/metabo15070438
Chicago/Turabian StyleBokayeva, Kamila, Małgorzata Jamka, Dariusz Walkowiak, Monika Duś-Żuchowska, Łukasz Kałużny, Natalia Wichłacz-Trojanowska, Agnieszka Chrobot, Renata Mozrzymas, Gulnara Sultanova, Karl-Heinz Herzig, and et al. 2025. "Vitamin B12 and Folate in Adherent and Non-Adherent Individuals with Phenylketonuria: A Cross-Sectional Study, Systematic Review, and Meta-Analysis" Metabolites 15, no. 7: 438. https://doi.org/10.3390/metabo15070438
APA StyleBokayeva, K., Jamka, M., Walkowiak, D., Duś-Żuchowska, M., Kałużny, Ł., Wichłacz-Trojanowska, N., Chrobot, A., Mozrzymas, R., Sultanova, G., Herzig, K.-H., & Walkowiak, J. (2025). Vitamin B12 and Folate in Adherent and Non-Adherent Individuals with Phenylketonuria: A Cross-Sectional Study, Systematic Review, and Meta-Analysis. Metabolites, 15(7), 438. https://doi.org/10.3390/metabo15070438







