Imprint Desorption Electrospray Ionization Mass Spectrometry Imaging (IDESI-MSI) Reveals Absorption of Triclopyr-Based Herbicide in Plants and Mouse Organs
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Sample Preparation and Treatment
2.3. Animal Feeding and Tissue Collection
2.4. Imprint Desorption Electrospray Ionization Mass Spectrometry Imaging (IDESI-MSI)
2.5. Data Analysis
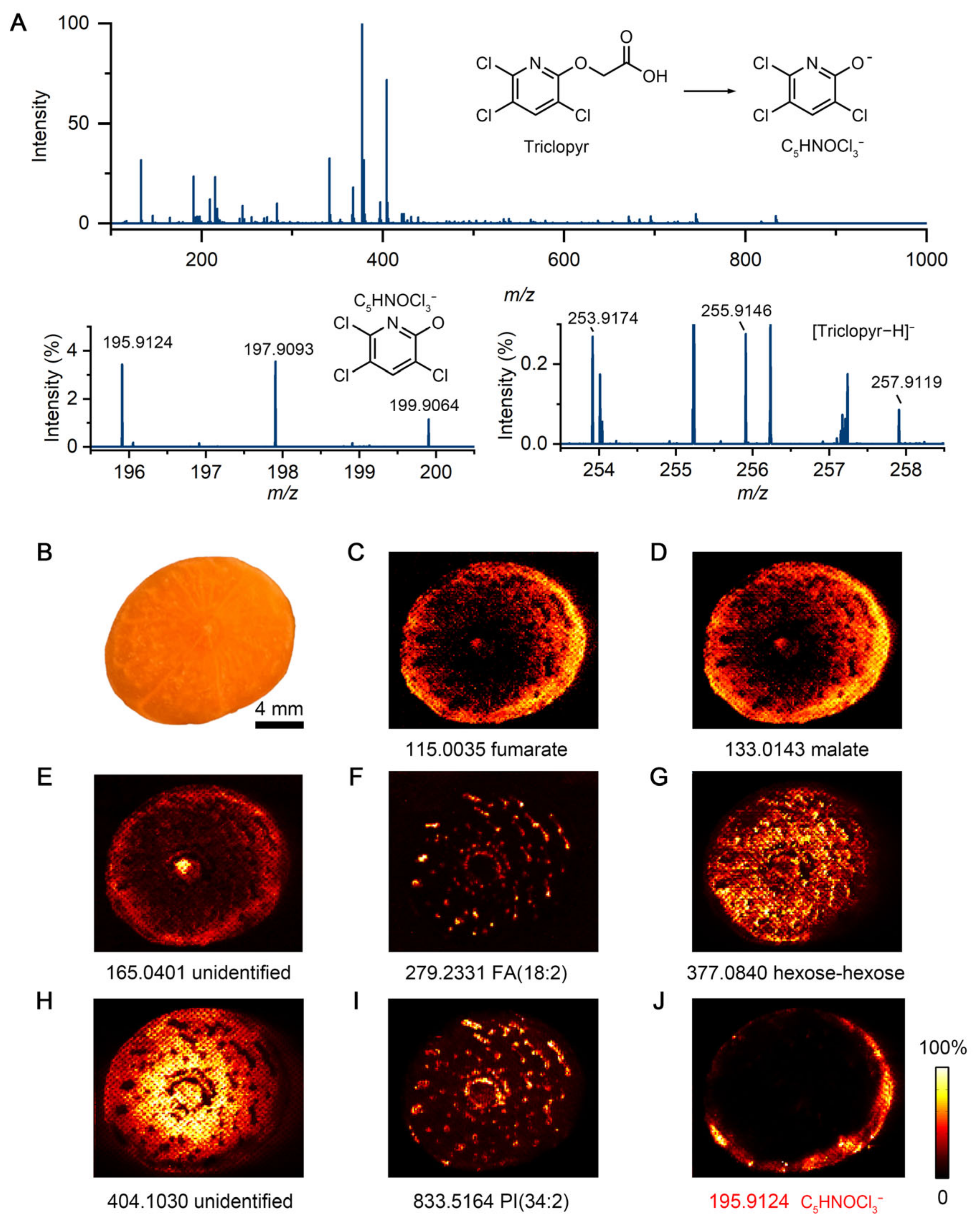
3. Results
3.1. IDESI-MSI of Carrot Exposed to Herbicide
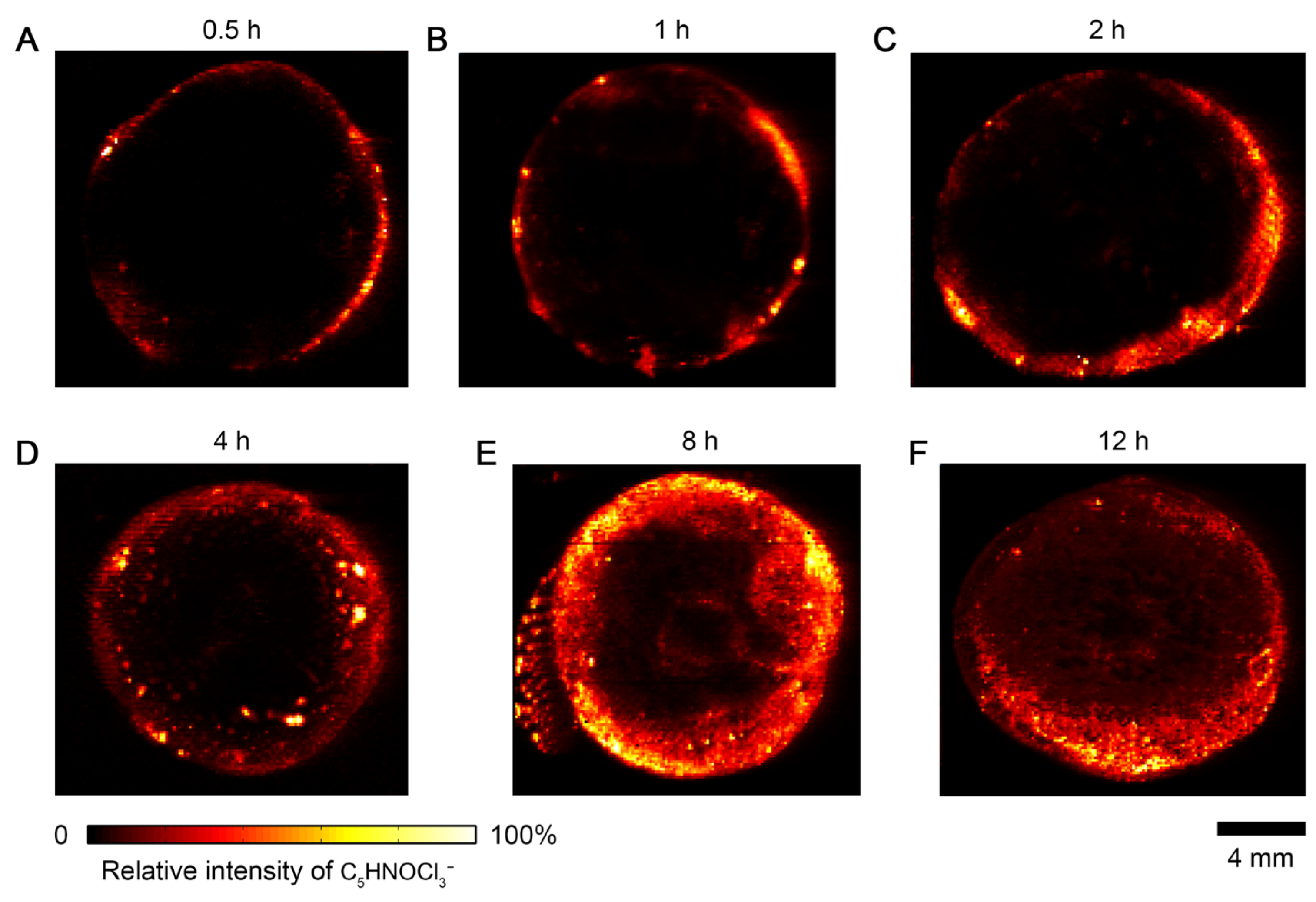
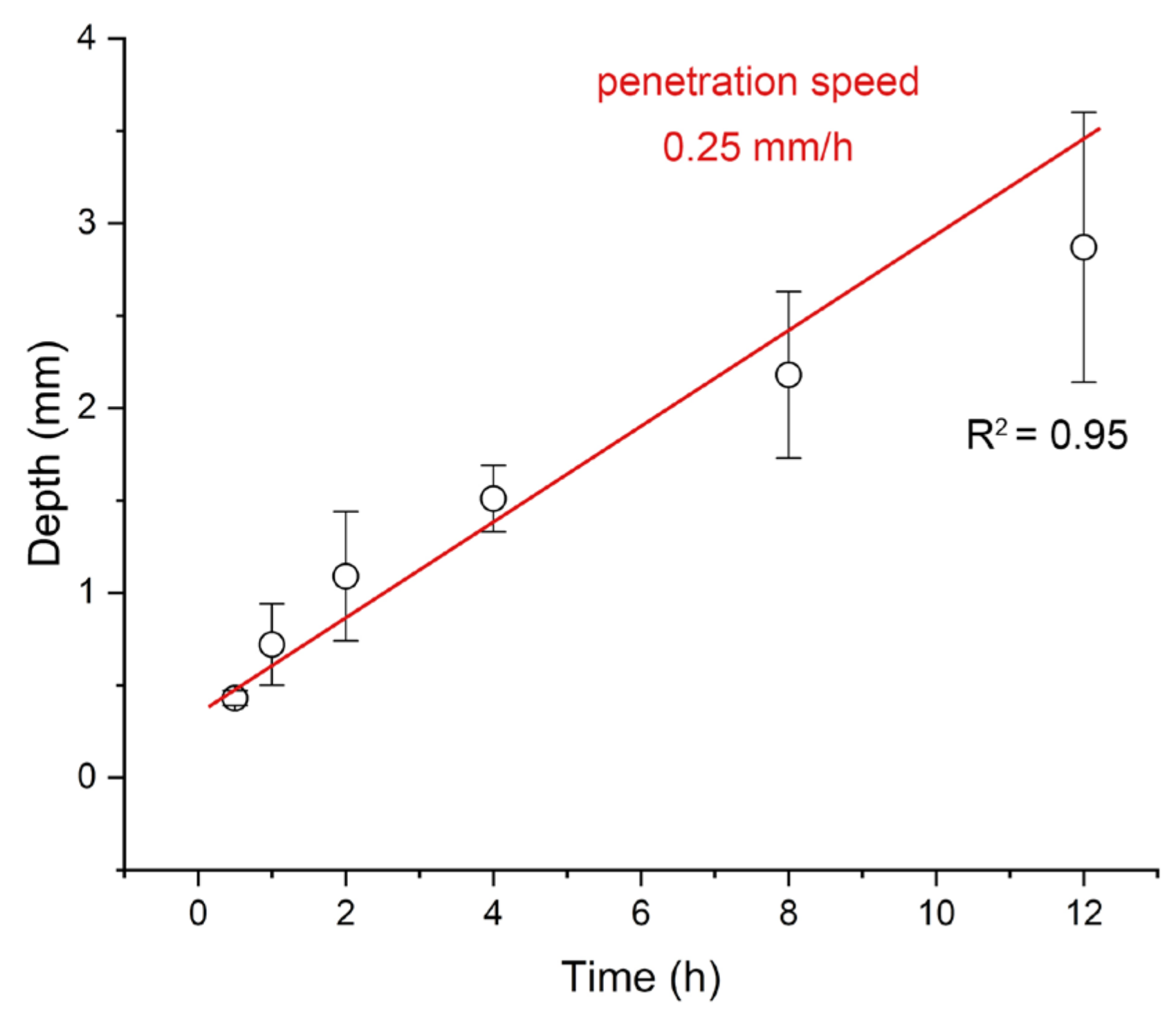
3.2. Time-Dependent Accumulation of Herbicide in Carrot
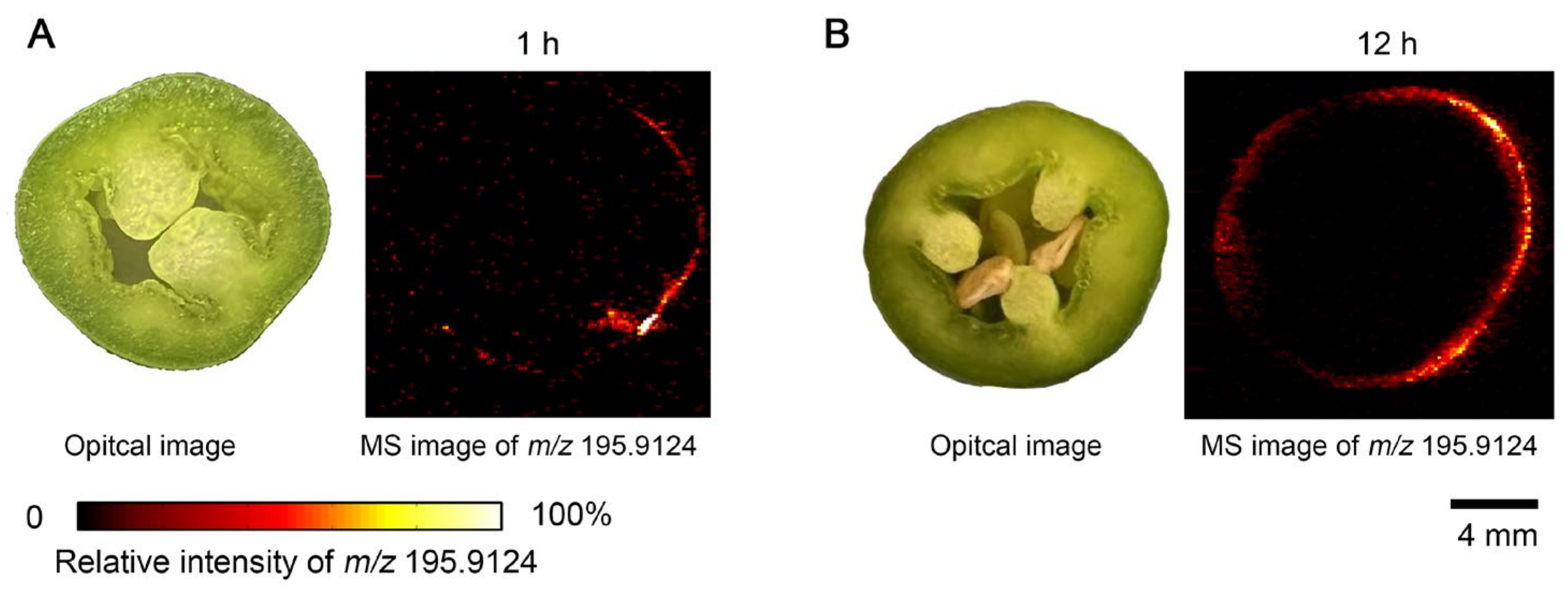
3.3. IDESI-MSI of Green Pepper Exposed to Herbicide
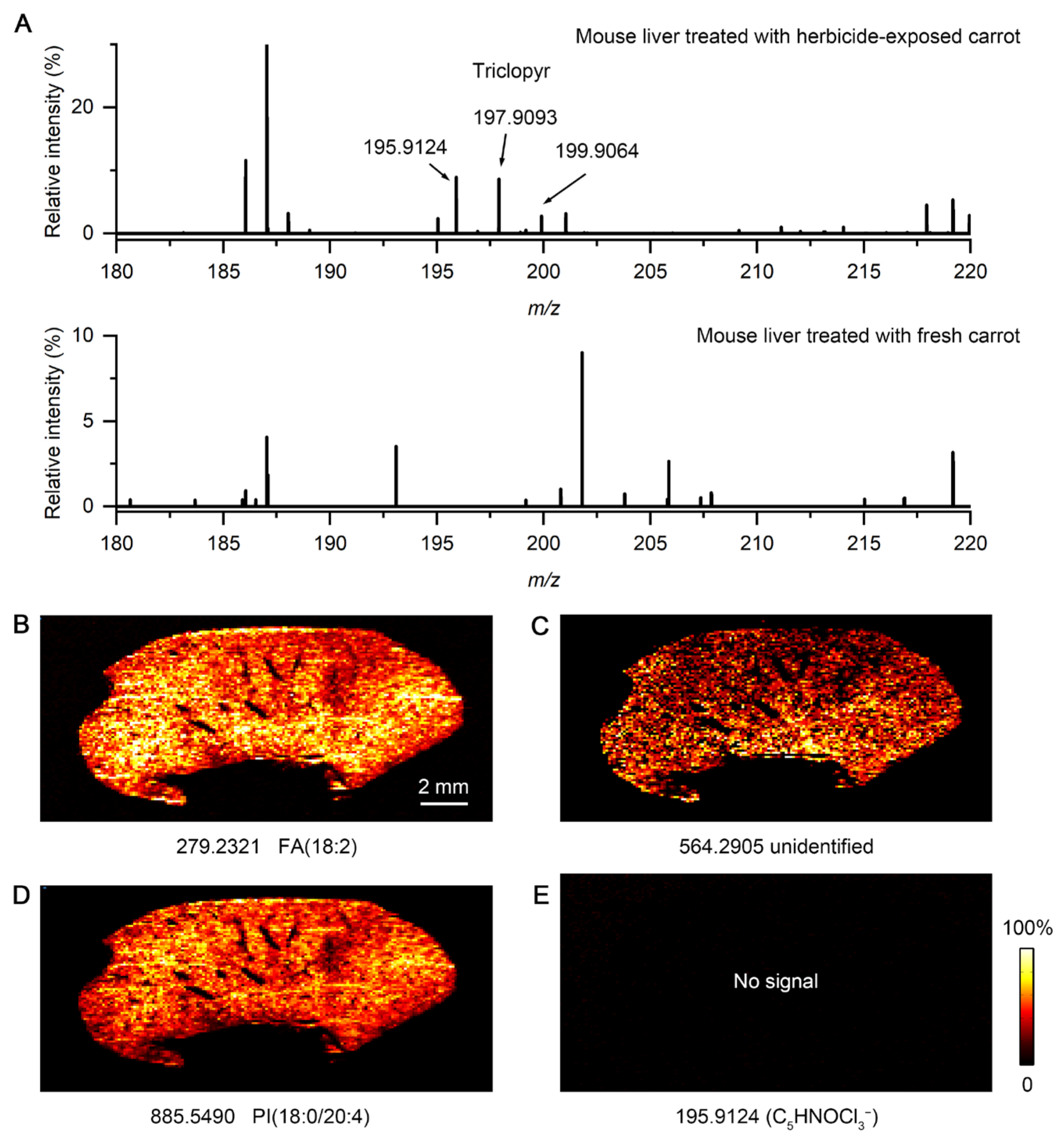
3.4. Accumulation of Herbicide in Mouse Organs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kniss, A.R. Long-term trends in the intensity and relative toxicity of herbicide use. Nat. Commun. 2017, 8, 14865. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, V.; Gill, J.P.; Datta, S.; Singh, S.; Dhaka, V.; Kapoor, D.; Wani, A.B.; Dhanjal, D.S.; Kumar, M.; et al. Herbicide Glyphosate: Toxicity and Microbial Degradation. Int. J. Environ. Res. Public Health 2020, 17, 7519. [Google Scholar] [CrossRef] [PubMed]
- Vonk, J.A.; Kraak, M.H.S. Herbicide Exposure and Toxicity to Aquatic Primary Producers. In Reviews of Environmental Contamination and Toxicology Volume 250; de Voogt, P., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 119–171. [Google Scholar]
- Giesy, J.P.; Dobson, S.; Solomon, K.R. Ecotoxicological Risk Assessment for Roundup® Herbicide. In Reviews of Environmental Contamination and Toxicology: Continuation of Residue Reviews; Ware, G.W., Ed.; Springer: New York, NY, USA, 2000; pp. 35–120. [Google Scholar]
- Williams, G.M.; Kroes, R.; Munro, I.C. Safety Evaluation and Risk Assessment of the Herbicide Roundup and Its Active Ingredient, Glyphosate, for Humans. Regul. Toxicol. Pharmacol. 2000, 31, 117–165. [Google Scholar] [CrossRef] [PubMed]
- Séralini, G.-E.; Clair, E.; Mesnage, R.; Gress, S.; Defarge, N.; Malatesta, M.; Hennequin, D.; de Vendômois, J.S. Republished study: Long-term toxicity of a Roundup herbicide and a Roundup-tolerantgenetically modified maize. Environ. Sci. Eur. 2014, 26, 14. [Google Scholar] [CrossRef]
- When Roundup Isn’t Roundup: Clearing up the Confusion Between Products. Available online: https://purduelandscapereport.org/article/when-roundup-isnt-roundup-clearing-up-the-confusion-between-products/ (accessed on 25 June 2025).
- Owagboriaye, F.O.; Dedeke, G.A.; Ademolu, K.O.; Olujimi, O.O.; Ashidi, J.S.; Adeyinka, A.A. Reproductive toxicity of Roundup herbicide exposure in male albino rat. Exp. Toxicol. Pathol. 2017, 69, 461–468. [Google Scholar] [CrossRef]
- Bartels, M.; Brown, C.; Chung, G.; Chan, M.; Terry, C.; Gehen, S.; Corvaro, M. Review of the pharmacokinetics and metabolism of triclopyr herbicide in mammals: Impact on safety assessments. Regul. Toxicol. Pharmacol. 2020, 116, 104714. [Google Scholar] [CrossRef]
- Shin, E.-H.; Choi, J.-H.; Abd El-Aty, A.M.; Khay, S.; Kim, S.-J.; Im, M.H.; Kwon, C.-H.; Shim, J.-H. Simultaneous determination of three acidic herbicide residues in food crops using HPLC and confirmation via LC-MS/MS. Biomed. Chromatogr. 2011, 25, 124–135. [Google Scholar] [CrossRef]
- Kaczyński, P. Clean-up and matrix effect in LC-MS/MS analysis of food of plant origin for high polar herbicides. Food Chem. 2017, 230, 524–531. [Google Scholar] [CrossRef]
- Pareja, L.; Cesio, V.; Heinzen, H.; Fernández-Alba, A.R. Evaluation of various QuEChERS based methods for the analysis of herbicides and other commonly used pesticides in polished rice by LC–MS/MS. Talanta 2011, 83, 1613–1622. [Google Scholar] [CrossRef]
- Sack, C.; Vonderbrink, J.; Smoker, M.; Smith, R.E. Determination of Acid Herbicides Using Modified QuEChERS with Fast Switching ESI+/ESI– LC-MS/MS. J. Agric. Food Chem. 2015, 63, 9657–9665. [Google Scholar] [CrossRef]
- Xiao, X.; Guan, X.; Xu, Z.; Lu, Q. In-Situ Metabolic Profiling of Different Kinds of Rheum palmatum L. by Laser Desorption–Dielectric Barrier Discharge Ionization Mass Spectrometry Imaging. Metabolites 2024, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Dill, A.L.; Eberlin, L.S.; Cooks, R.G.; Ifa, D.R. Mass spectrometry imaging under ambient conditions. Mass Spectrom. Rev. 2013, 32, 218–243. [Google Scholar] [CrossRef] [PubMed]
- Chughtai, K.; Heeren, R.M.A. Mass Spectrometric Imaging for Biomedical Tissue Analysis. Chem. Rev. 2010, 110, 3237–3277. [Google Scholar] [CrossRef] [PubMed]
- Takáts, Z.; Wiseman, J.M.; Gologan, B.; Cooks, R.G. Mass Spectrometry Sampling Under Ambient Conditions with Desorption Electrospray Ionization. Science 2004, 306, 471–473. [Google Scholar] [CrossRef]
- Morato, N.M.; Cooks, R.G. Desorption Electrospray Ionization Mass Spectrometry: 20 Years. Acc. Chem. Res. 2023, 56, 2526–2536. [Google Scholar] [CrossRef]
- Li, B.; Hansen, S.H.; Janfelt, C. Direct imaging of plant metabolites in leaves and petals by desorption electrospray ionization mass spectrometry. Int. J. Mass Spectrom. 2013, 348, 15–22. [Google Scholar] [CrossRef]
- Tata, A.; Perez, C.J.; Ore, M.O.; Lostun, D.; Passas, A.; Morin, S.; Ifa, D.R. Evaluation of imprint DESI-MS substrates for the analysis of fungal metabolites. RSC Adv. 2015, 5, 75458–75464. [Google Scholar] [CrossRef]
- Wu, L.; Qi, K.; Liu, C.; Hu, Y.; Xu, M.; Pan, Y. Enhanced Coverage and Sensitivity of Imprint DESI Mass Spectrometry Imaging for Plant Leaf Metabolites by Post-photoionization. Anal. Chem. 2022, 94, 15108–15116. [Google Scholar] [CrossRef]
- Hemalatha, R.G.; Ganayee, M.A.; Pradeep, T. Electrospun Nanofiber Mats as “Smart Surfaces” for Desorption Electrospray Ionization Mass Spectrometry (DESI MS)-Based Analysis and Imprint Imaging. Anal. Chem. 2016, 88, 5710–5717. [Google Scholar] [CrossRef]
- Tata, A.; Perez, C.J.; Hamid, T.S.; Bayfield, M.A.; Ifa, D.R. Analysis of Metabolic Changes in Plant Pathosystems by Imprint Imaging DESI-MS. J. Am. Soc. Mass Spectrom. 2015, 26, 641–648. [Google Scholar] [CrossRef]
- Wu, X.; Qin, R.; Wu, H.; Yao, G.; Zhang, Y.; Li, P.; Xu, Y.; Zhang, Z.; Yin, Z.; Xu, H. Nanoparticle-immersed paper imprinting mass spectrometry imaging reveals uptake and translocation mechanism of pesticides in plants. Nano Res. 2020, 13, 611–620. [Google Scholar] [CrossRef]
- Qin, R.; Li, P.; Du, M.; Ma, L.; Huang, Y.; Yin, Z.; Zhang, Y.; Chen, D.; Xu, H.; Wu, X. Spatiotemporal Visualization of Insecticides and Fungicides within Fruits and Vegetables Using Gold Nanoparticle-Immersed Paper Imprinting Mass Spectrometry Imaging. Nanomaterials 2021, 11, 1327. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xu, Y.; Huang, Y.; Chen, Y.; Zhao, Y.; Yan, B.; Zhu, X.; Chen, Z.; Xu, H.; Yin, Z.; et al. Mapping Molecular Signatures in Plant Leaves, Flowers, and Fruits by a TiO2 Nanotube-Based Plasmonic Chip for Imprint Mass Spectrometry Imaging. ACS Agric. Sci. Technol. 2023, 3, 119–130. [Google Scholar] [CrossRef]
- Li, J.; Wei, R.; Meng, Y.; Zare, R.N. Evaluation of Oil-Absorbing Film for Imprint Desorption Electrospray Ionization Mass Spectrometry Imaging (IDESI-MSI) on Biological Samples. Metabolites 2024, 14, 160. [Google Scholar] [CrossRef]
- Oh, B.J.; Kim, K.D.; Kim, Y.S. Effect of Cuticular Wax Layers of Green and Red Pepper Fruits on Infection by Colletotrichum gloeosporioides. J. Phytopathol. 1999, 147, 547–552. [Google Scholar] [CrossRef]
- Geerdink, R.B.; Kienhuis, P.G.M.; Brinkman, U.A.T. Optimization of instrumental parameters for flow injection analysis-thermospray tandem mass spectrometry. Chromatographia 1994, 39, 311–319. [Google Scholar] [CrossRef]
- Race, A.M.; Bunch, J. Optimisation of colour schemes to accurately display mass spectrometry imaging data based on human colour perception. Anal. Bioanal. Chem. 2015, 407, 2047–2054. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, S.; Franceschi, P.; Liu, Y. Profiling and visualization of organic acids in grape plants by desorption electrospray ionization imaging. Food Chem. 2025, 468, 142432. [Google Scholar] [CrossRef]
- Zhang, T.; Noll, S.E.; Peng, J.T.; Klair, A.; Tripka, A.; Stutzman, N.; Cheng, C.; Zare, R.N.; Dickinson, A.J. Chemical imaging reveals diverse functions of tricarboxylic acid metabolites in root growth and development. Nat. Commun. 2023, 14, 2567. [Google Scholar] [CrossRef]
- Baker, E.A.; Chamel, A.R. Herbicide penetration across isolated and intact leaf cuticles. Pestic. Sci. 1990, 29, 187–196. [Google Scholar] [CrossRef]
- Lin, R.; Xie, B.; Du, C.; Hang, W.; Huang, B. Pulsed micro-discharge ambient ionization mass spectrometry. Int. J. Mass Spectrom. 2018, 434, 123–129. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Tian, Y.; Wei, R.; Meng, Y.; Zare, R.N. Imprint Desorption Electrospray Ionization Mass Spectrometry Imaging (IDESI-MSI) Reveals Absorption of Triclopyr-Based Herbicide in Plants and Mouse Organs. Metabolites 2025, 15, 437. https://doi.org/10.3390/metabo15070437
Liu H, Tian Y, Wei R, Meng Y, Zare RN. Imprint Desorption Electrospray Ionization Mass Spectrometry Imaging (IDESI-MSI) Reveals Absorption of Triclopyr-Based Herbicide in Plants and Mouse Organs. Metabolites. 2025; 15(7):437. https://doi.org/10.3390/metabo15070437
Chicago/Turabian StyleLiu, Hanzhi, Yunshuo Tian, Ruolun Wei, Yifan Meng, and Richard N. Zare. 2025. "Imprint Desorption Electrospray Ionization Mass Spectrometry Imaging (IDESI-MSI) Reveals Absorption of Triclopyr-Based Herbicide in Plants and Mouse Organs" Metabolites 15, no. 7: 437. https://doi.org/10.3390/metabo15070437
APA StyleLiu, H., Tian, Y., Wei, R., Meng, Y., & Zare, R. N. (2025). Imprint Desorption Electrospray Ionization Mass Spectrometry Imaging (IDESI-MSI) Reveals Absorption of Triclopyr-Based Herbicide in Plants and Mouse Organs. Metabolites, 15(7), 437. https://doi.org/10.3390/metabo15070437






