Analysis of Processing Impact on Raspberries Based on Broad-Spectrum Metabolomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Chromatographic Conditions
2.4. Mass Spectrometry Conditions
2.5. Metabolite Analysis
2.6. Network Pharmacology
2.6.1. Collection of Bioactive Components and Diabetic Nephropathy-Related Targets
2.6.2. Predicting Potential Targets and Constructing the Protein Interaction (PPI) Network
2.6.3. GO Function and KEGG Pathway Enrichment Analysis
2.7. Cell Experiments
2.7.1. Cell Culture and Subculture
2.7.2. Establishment of Glucose-Induced Cell Model
2.7.3. CCK-8 Method Used to Detect the Effects of Different Concentrations of Raspberries on Cell Activity
2.7.4. Western Blot Analysis
3. Results
3.1. Metabolomic Analysis of Raw and Salted Raspberry Products
3.2. Network Analysis
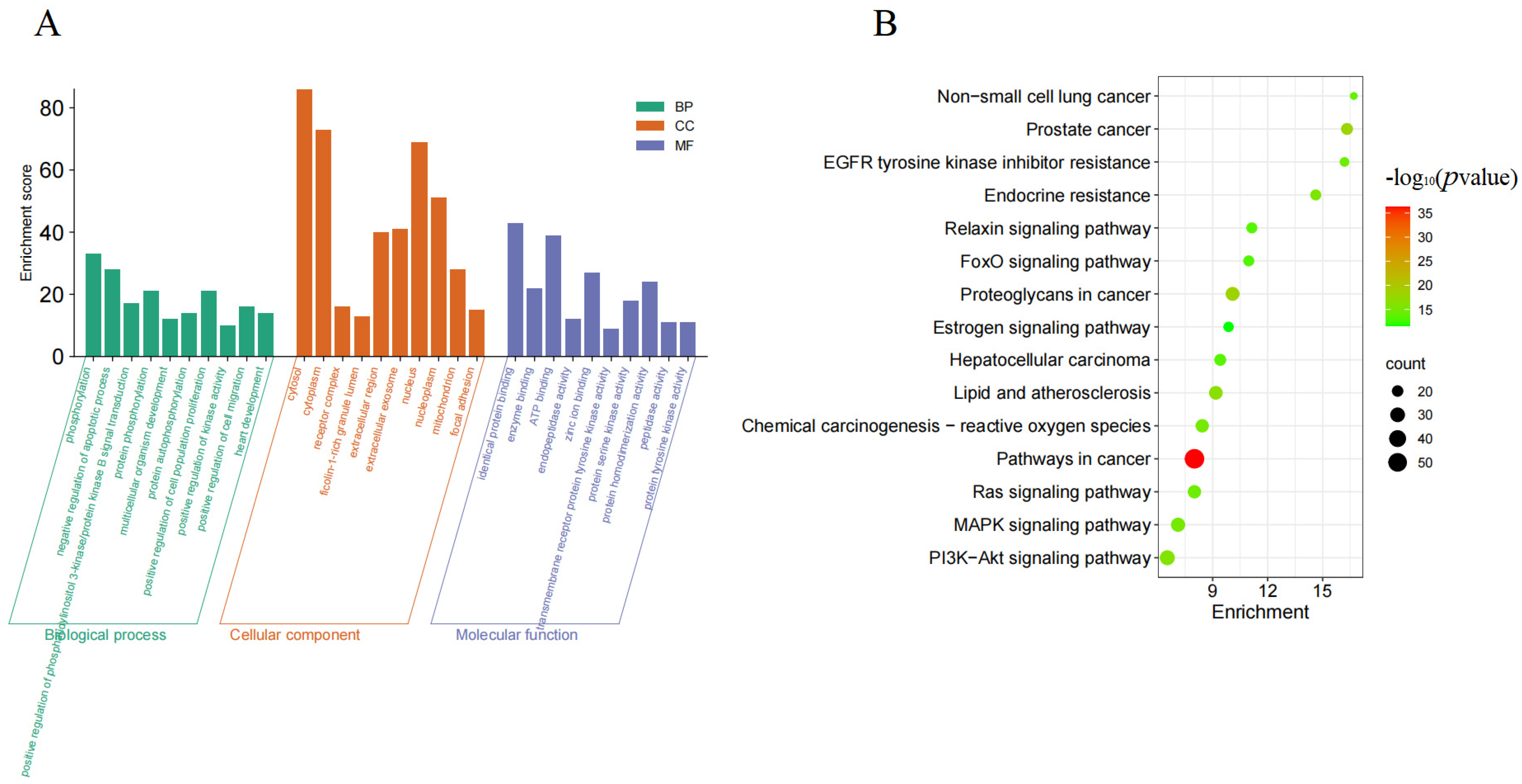
3.3. Analysis of GO and KEGG Results
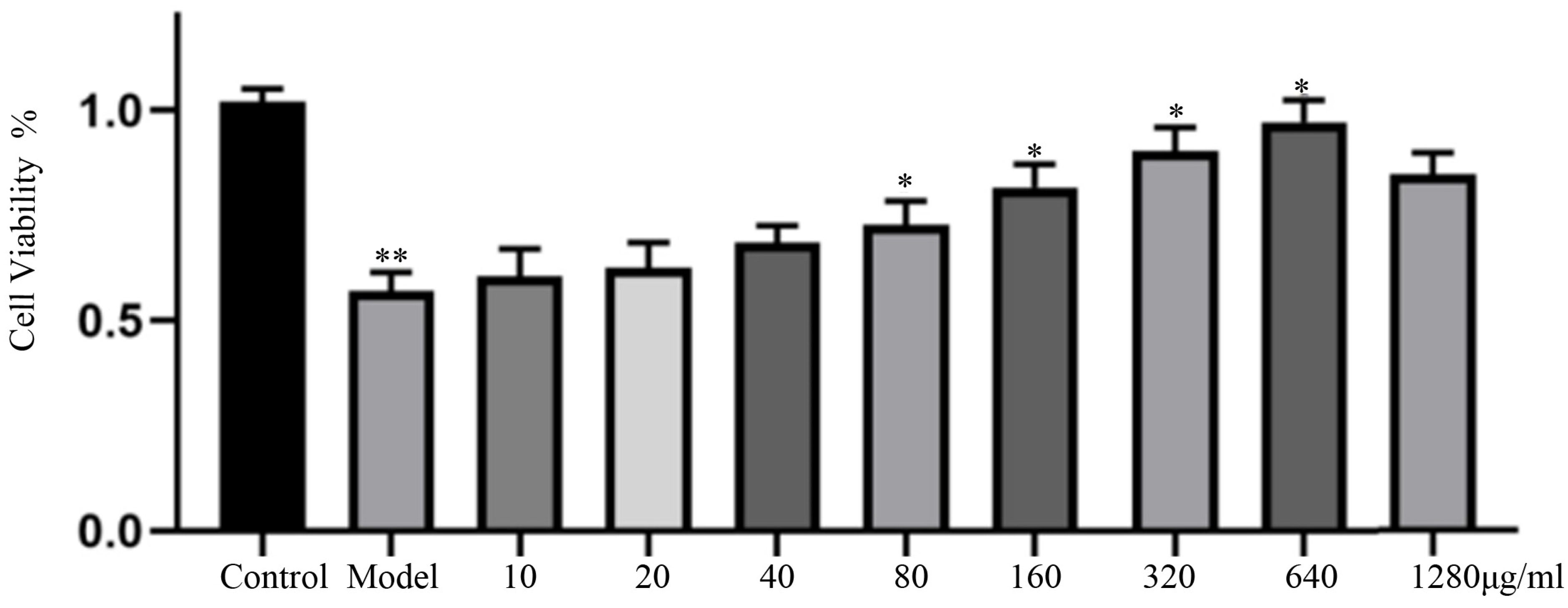
3.4. Effect of Salt-Processed Fupenzi on Cell Proliferation
3.5. Effect of Salt-Processed Fupenzi on the MAPK Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, K.; Sheng, L.-N.; Yang, B.; Chai, Z.; Xu, X.-W.; Zhang, X.-H.; Li, W.-Y.; Shen, G.-D.; Yuan, Y.-J.; Yang, J.-C.; et al. Utilization strategies of beam dumps for High energy Fragment Separator (HFRS) at HIAF. J. Instrum. 2024, 19, T12004. [Google Scholar] [CrossRef]
- Cabrera, L.E.; Buckner, C.; Then, V.; Mäki, S.; Vapalahti, O.; Vaheri, A.; Hepojoki, J.; Tietäväinen, J.; Mäkelä, S.; Mustonen, J.; et al. Circulating mucosal-like IgA responses increase with severity of Pamala Ortho hantavirus-caused hemorrhagic fever with renal syndrome. Front. Immunol. 2024, 15, 1480041. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, L.; Liu, Q.; Xiao, X.; Huang, W.; Wang, Y. Screening and Identification of HTNVpv Entry Inhibitors with High-throughput Pseudo virus-based Chemiluminescence. Virol. Sin. 2022, 37, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yang, X.; Shi, P.; Niu, G.; Zhang, S.; Gu, Z.; Guo, Q. Tissue-specific chemical expression and quantitative analysis of bioactive components of Moutan Cortex by laser-microdissection combined with UPLC-Q-Orbitrap-MS technique. J. Pharm. Biomed. Anal. 2025, 253, 116537. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, X.; Xia, M.; Yang, L.; Cheng, W.; Song, Q. Combining network pharmacology with chromatographic fingerprinting and multicomponent quantitative analysis for the quality evaluation of Moutan Cortex. Biomed. Chromatogr. BMC 2022, 36, e5434. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Li, C.; Qi, P.; Liang, T.; Feng, X.; Wang, M.; Liu, S.; Qiang, Z.; Jia, M.; Wei, X. Development and validation of a simple ultra-high-performance-tandem mass spectrometry method for the simultaneous determination of five bioactive components in rat plasma of Hedy sari Radix. Sep. Sci. Plus 2024, 7, 2300219. [Google Scholar] [CrossRef]
- Liu, L.Y.; He, S.J.; Luo, J.; Huang, J.K.; Yuan, J.X.; Yuan, C.J.; Zhang, J.L. Network pharmacology, molecular docking and experimental study on the mechanism of Curcumin’s anti-ferroptosis in melanocytes. Biochem. Biophys. Res. Commun. 2024, 736, 150871. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, X.; Wang, T.; Yu, J.; Wang, M.; Sun, J.; Yu, X.; Niu, N.; Chen, L. HPLC Fingerprint Combined with Chemometrics and Network Pharmacology for Q-Markers Prediction Analysis of Shaposhnikov divaricate. J. Anal. Test. 2024, 8, 83–94. [Google Scholar] [CrossRef]
- Li, J.; Wang, D.; Hao, X.; Li, Y.; Gao, H.; Fan, Y.; Fang, B.; Guo, Y. Exploring the high-quality ingredients and mechanisms of Da Chuanxiong Formula in the treatment of neuropathic pain based on network pharmacology, molecular docking, and molecular dynamics simulation. Biomed. Pharmacother. 2024, 178, 117195. [Google Scholar] [CrossRef]
- Wang, W.; Luan, F.; Shi, Y.; Zhang, X.; Guo, D.; Sun, J.; Zou, J.; Yuan, P. Combination of UHPLC-QE-MS and Network Pharmacology to Reveal the Mechanism of Fufang-Duzhong-Jiangu Granules for Treating Knee Osteoarthritis. Biomed. Chromatogr. BMC 2025, 39, e6051. [Google Scholar] [CrossRef]
- Singh, M.; Mohan, R.; Mishra, S.; Goyal, N.; Shanker, K.; Gupta, N.; Kumar, B. Ultra performance liquid chromatography coupled with principal component and cluster analysis of Swertia chirayita for adulteration check. J. Pharm. Biomed. Anal. 2019, 164, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Han, J.; Li, Q.; Nie, J.; Han, L.; Yu, Y.; Lv, C.; Lu, J. Dissection of the potential immunomodulatory activity materials and mechanism of Wu Shen Wan (a classical traditional Chinese medicine prescription) based on in vivo substances profiling and network pharmacology. Sep. Sci. Plus 2024, 7, 2300155. [Google Scholar] [CrossRef]
- Wang, R.; He, C.; Yang, Y.T.; Song, Q.; Zhou, H.; Peng, S.; Wang, F.; Fang, W. Ultra-performance liquid chromatography-quadrupole-time of flight tandem-mass spectrometry and liquid chromatograph-tandem mass spectrometer combined with chemometric analysis an approach for the quality evaluation of Mume Fructus. J. Sep. Sci. 2022, 45, 1884–1893. [Google Scholar] [CrossRef]
- Wang, R.; Wang, X.C.; Ou, J.M. Network pharmacology-based approach reveals that Fructus mume exerts therapeutic effects against ulcerative colitis via the AGE/RAGE signaling pathway. Arab. J. Chem. 2024, 17, 105534. [Google Scholar] [CrossRef]
- Zhang, X.; Liao, Y.; Cheng, M.; Yang, Z.; Li, B.; Hu, J. Study on the changes in chemical components and pharmacological effects of raspberries before and after salt treatment. J. Shizhen Tradit. Chin. Med. Pharm. 2020, 31, 82–84. [Google Scholar]
- Song, H.; Sun, H.; Fang, H.; Yang, L.; Zhao, Q.; Sun, Y.; Yan, G.; Han, Y.; Wang, X. Fingerprint profile analysis of hirudo polypeptide based on UHPLC–MS and its application. Sep. Sci. Plus 2024, 7, e202300218. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, Y.; Du, K.; Guo, J.; He, J.; Li, J.; Chang, Y.X. A ball mill-assisted vortex-enhanced matrix solid-phase dispersion method for the extraction and determi-nation of five phenolic compounds from Raspberry by high-performance liquid chromatography. Sep. Sci. Plus 2021, 4, 211–221. [Google Scholar] [CrossRef]
- Rasaily, S.; Haldipur, C.A.; Srividya, N. LC-Q-TOF-MS phenolic profiling, data mining for antiviral compounds, and in silico analysis against SARS-CoV-2 in heritage-pigmented rice metabolome. Food Biosci. 2025, 63, 105613. [Google Scholar] [CrossRef]
- Bayang, J.P.; Touwang, C.; Mamoudou, H.; Woudam, E.S.; Koubala, B.B. Variation of nutrients and bioactive compounds of five wild edible leafy vegetables from far north region of Cameroon. Food Chem. Adv. 2025, 6, 100849. [Google Scholar] [CrossRef]
- Chen, B.; Liu, Z.; Qu, D.; Wang, Y.; Huo, X.; Li, Z.; Sun, Y. Optimized extraction of secoiridoid glycosides from Gentiana radix by pressurized liquid extraction using a Box–Behnken Design. Sep. Sci. Plus 2018, 1, 556–563. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, M.H.; Yuan, S.W.; Lu, Y.; Wang, Q. A new monoterpene glycoside from and anticomplementary activity of its compounds. Nat. Prod. Res. 2021, 35, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Friesen, J.B.; Lankin, D.C.; McAlpine, J.B.; Nikolić, D.; Chen, S.N.; Pauli, G.F. Geraniol-Derived Monoterpenoid Glucosides from Rhodiola rosea: Resolving Structures by QM-HifSA Methodology. J. Nat. Prod. 2023, 86, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Morreale, G.; Possamai, T.; Panighel, A.; De Rosso, M.; Lovat, L.; Flamini, R.; Migliaro, D. First investigation on polyphenols and glycosidic aroma precursors in a spontaneous colour mu-tant of ‘Glera’, the principal grape variety of Prosecco sparkling wine. J. Sci. Food Agric. 2022, 102, 6623–6631. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ma, H.; Luo, J.; He, Y. Mechanism of Trigonella Foenum-graecum L. against Type 2 Diabetes Mellitus: Research on Network Pharmacology & Experimental Verification. Lett. Drug Des. Discov. 2025, 21, 4072–4084. [Google Scholar]




| Compounds | Formula | VIP | Log2FC | Type | Class |
|---|---|---|---|---|---|
| Ellagic acid | C14H6O8 | 1.60 | 2.64 | up | Phenolic acids |
| Kaempferol-3-O-(6″-galloyl)glucoside | C28H24O15 | 1.53 | 1.52 | up | Flavonols |
| Luteolin-7-O-glucoside (Cynaroside) | C21H20O11 | 1.52 | 1.55 | up | Flavones |
| 2,4,6-Trihydroxybenzoic acid | C7H6O5 | 1.48 | 1.36 | up | Phenolic acids |
| Dihydrokaempferol-3-O-glucoside | C21H22O11 | 1.47 | 1.13 | up | Flavanonols |
| Kaempferol-3-O-(6″-malonyl)glucoside | C24H22O14 | 1.36 | 1.16 | up | Flavonols |
| Yenhusomine | C21H23NO6 | 1.30 | 1.92 | up | Isoquinoline alkaloids |
| Raspberryide H | C36H56O10 | 1.17 | 1.11 | up | Triterpene saponins |
| Raspberryide F | C36H56O9 | 1.15 | 1.30 | up | Triterpene saponins |
| Palmatine | C21H22NO4+ | 1.12 | 3.50 | up | Alkaloids |
| Geraniinic acid B | C41H28O27 | 1.11 | 4.28 | up | Tannins |
| Sanguiin H1 | C34H26O22 | 1.06 | 2.79 | up | Tannins |
| Granatin A | C34H24O22 | 1.03 | 2.49 | up | Tannins |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liao, Q.; Wang, F.; Rui, X.; Liu, Y.; Wang, R. Analysis of Processing Impact on Raspberries Based on Broad-Spectrum Metabolomics. Metabolites 2025, 15, 435. https://doi.org/10.3390/metabo15070435
Wang X, Liao Q, Wang F, Rui X, Liu Y, Wang R. Analysis of Processing Impact on Raspberries Based on Broad-Spectrum Metabolomics. Metabolites. 2025; 15(7):435. https://doi.org/10.3390/metabo15070435
Chicago/Turabian StyleWang, Xiaoge, Qiyuan Liao, Fan Wang, Xuelin Rui, Yushan Liu, and Rui Wang. 2025. "Analysis of Processing Impact on Raspberries Based on Broad-Spectrum Metabolomics" Metabolites 15, no. 7: 435. https://doi.org/10.3390/metabo15070435
APA StyleWang, X., Liao, Q., Wang, F., Rui, X., Liu, Y., & Wang, R. (2025). Analysis of Processing Impact on Raspberries Based on Broad-Spectrum Metabolomics. Metabolites, 15(7), 435. https://doi.org/10.3390/metabo15070435






