Neurotrophins and Proneurotrophins as Biomarkers for Overactive Bladder Syndrome in Aging Females
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Profiles
2.2. Demographic and Clinical Differences
2.3. Collection and Analysis of Urine Samples
2.4. Statistics
3. Results
3.1. Subject Characteristics
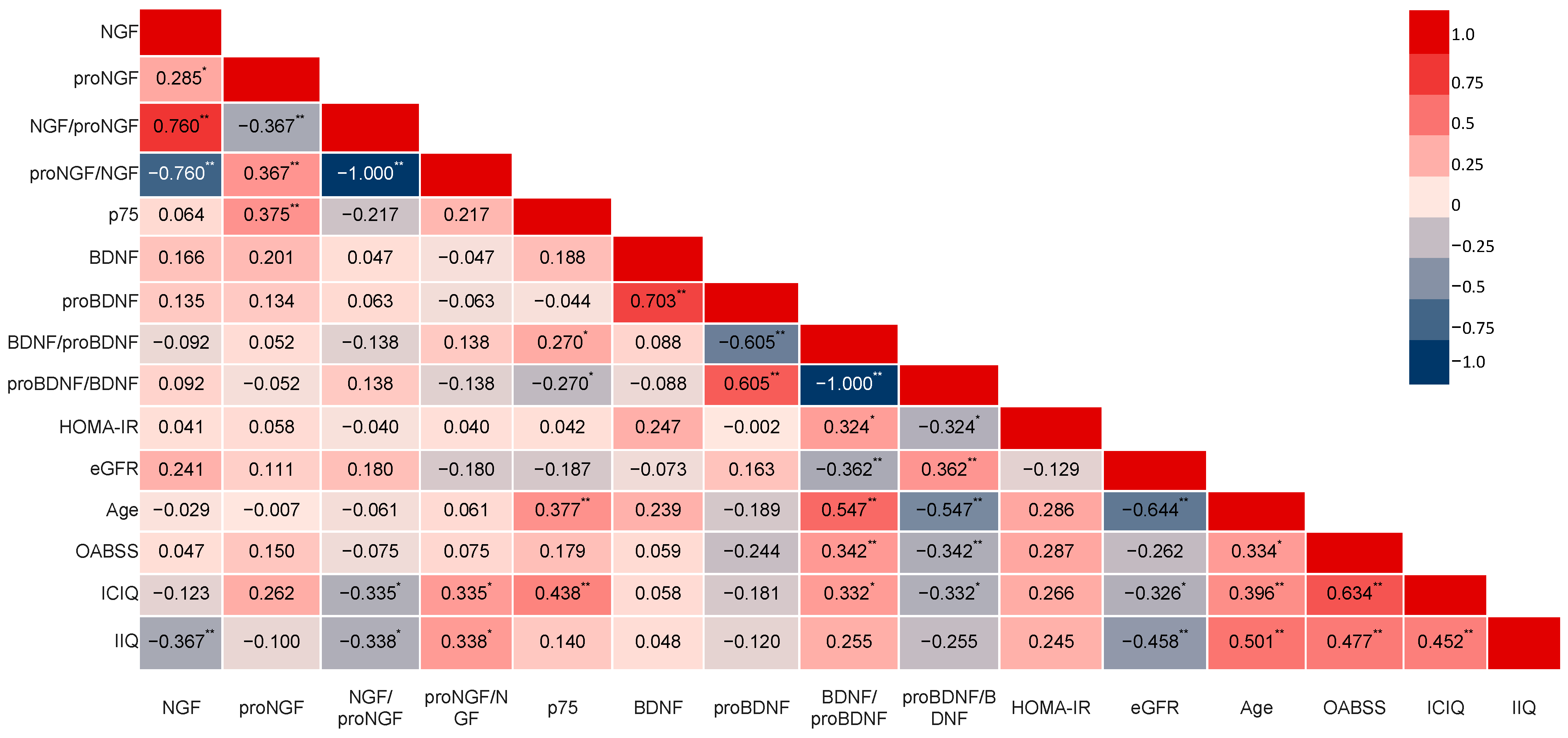
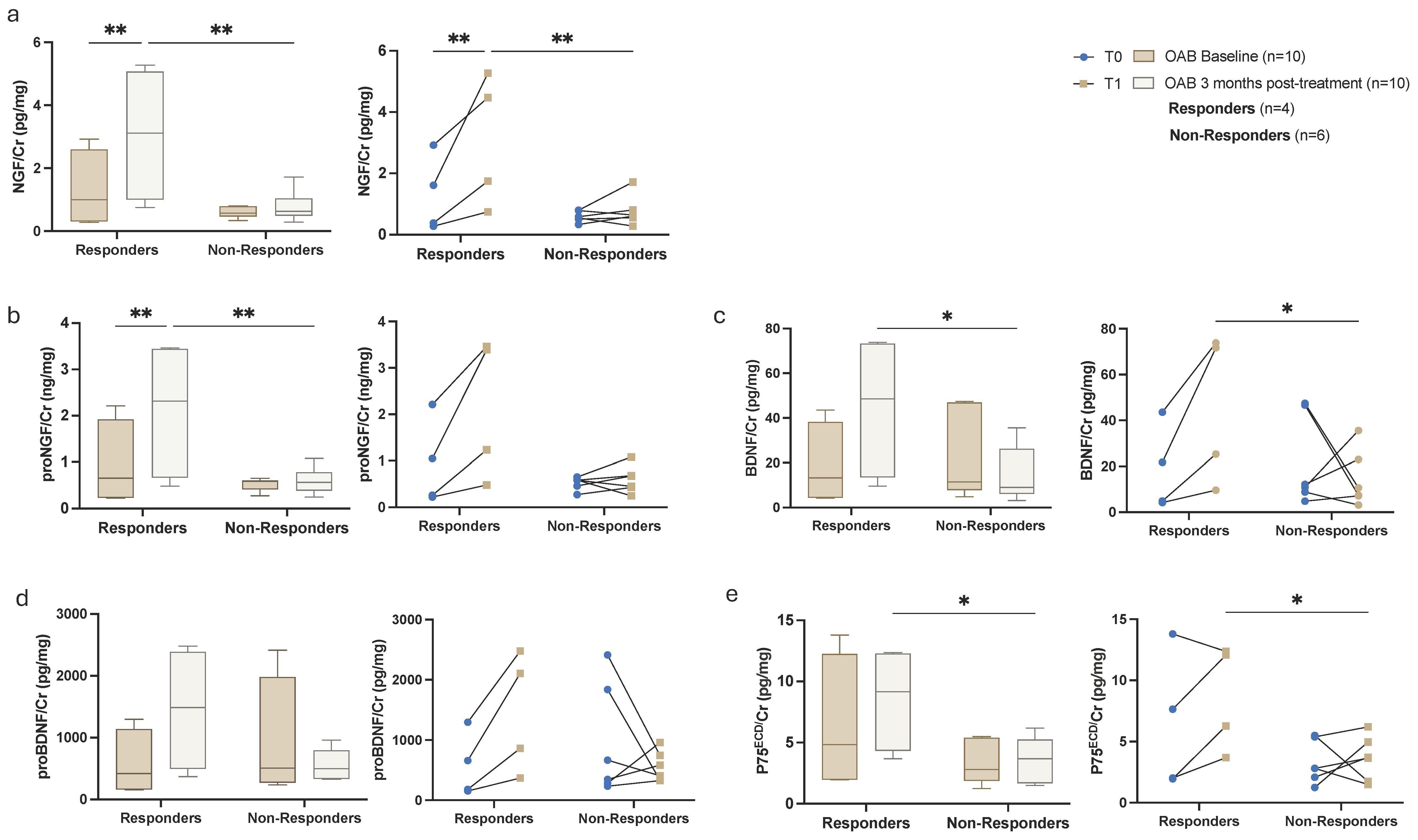
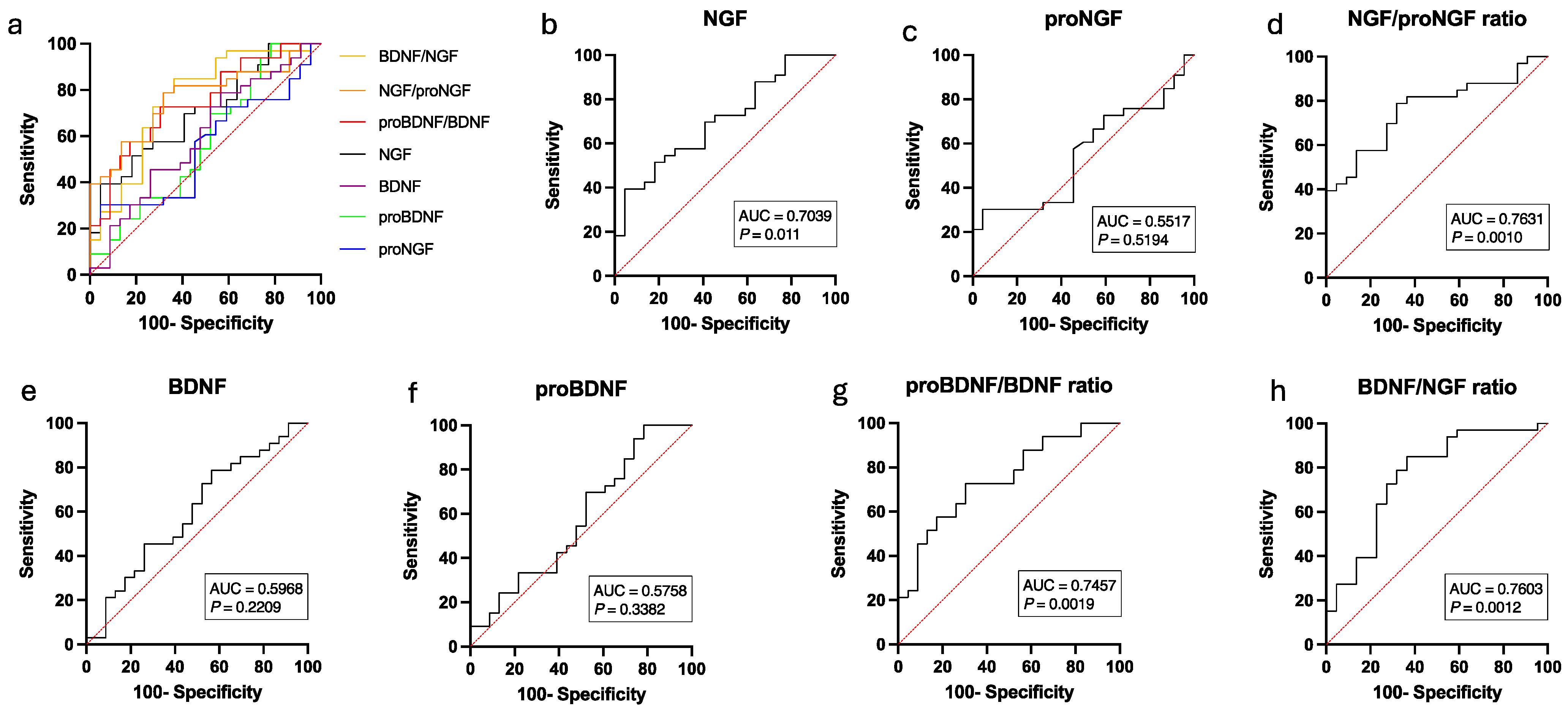
3.2. Biochemical Urinalysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BDNF | Brain-Derived Neurotrophic Factor |
| eGFR | Estimated Glomerular Filtration Rate |
| HOMA-IR | Homeostatic Model Assessment of Insulin Resistance |
| ICIQ-SF | International Consultation on Incontinence Questionnaire-Short Form |
| IIQ | Incontinence Impact Questionnaire |
| NGF | Nerve Growth Factor |
| OAB | Overactive Bladder Syndrome |
| OABSS | Overactive Bladder Symptom Score |
| p75ECD | p75 Extracellular Domain |
| proBDNF | Proform of Brain-Derived Neurotrophic Factor |
| proNGF | Proform of the Nerve Growth Factor |
Appendix A
| Confounders | CTR Group (n = 23) | OAB Group (n = 33) | p Value | |
|---|---|---|---|---|
| proNGF/Cr (ng/mg) | Age | 0.562 (0.372–0.752) | 0.689 (0.540–0.837) | 0.341 |
| HOMA-IR | 0.533 (0.339–0.728) | 0.675 (0.518–0.832) | 0.270 | |
| eGFR | 0.553 (0.373–0.733) | 0.695 (0.552–0.838) | 0.252 | |
| NGF/Cr (pg/mg) | Age | 2.201 (1.688–2.716) | 1.107 (0.703–1.510) | 0.003 * |
| HOMA-IR | 1.989 (1.455–2.523) | 1.043 (0.612–1.474) | 0.010 * | |
| eGFR | 1.882 (1.383–2.380) | 1.319 (0.924–1.715) | 0.102 | |
| proNGF/NGF ratio (mol/mol) | Age | 252.69 (19.346–486.03) | 820.18 (637.57–1002.79) | <0.001 * |
| HOMA-IR | 332.67 (85.548–579.80) | 846.93 (647.31–1046.55) | 0.003 * | |
| eGFR | 382.60 (152.59–612.61) | 733.57 (551.022–916.12) | 0.029 * | |
| NGF/proNGF ratio (mol/mol) | Age | 0.005 (0.004–0.006) | 0.002 (0.001–0.003) | 0.006 * |
| HOMA-IR | 0.005 (0.004–0.006) | 0.002 (0.001–0.003) | <0.001 * | |
| eGFR | 0.004 (0.003–0.005) | 0.003 (0.002–0.004) | 0.060 | |
| proBDNF/Cr (ng/mg) | Age | 1339.54 (776.55–1902.54) | 795.70 (343.89–1247.51) | 0.176 |
| HOMA-IR | 1602.51 (1014.97–2190.05) | 719.46 (231.27–1207.65) | 0.028 * | |
| eGFR | 1311.42 (775.95–1846.89) | 815.30 (380.44–1250.16) | 0.184 | |
| BDNF/Cr (pg/mg) | Age | 25.17 (12.856–37.492) | 26.48 (16.595–36.365) | 0.881 |
| HOMA-IR | 21.03 (9.421–32.646) | 25.46 (15.815–35.112) | 0.567 | |
| eGFR | 21.40 (9.520–33.281) | 29.11 (19.463–38.759) | 0.350 | |
| proBDNF/BDNF ratio (mol/mol) | Age | 25.55 (19.232–31.870) | 19.68 (14.613–24.755) | 0.193 |
| HOMA-IR | 31.47 (25.309–37.632) | 17.79 (12.674–22.913) | 0.002 * | |
| eGFR | 28.02 (21.733–34.306) | 17.96 (12.858–23.068) | 0.024 * | |
| BDNF/proBDNF ratio (mol/mol) | Age | 0.070 (0.035–0.105) | 0.090 (0.062–0.118) | 0.422 |
| HOMA-IR | 0.038 (0.006–0.071) | 0.093 (0.066–0.120) | 0.014 * | |
| eGFR | 0.052 -0.017–0.087) | 0.103 (0.074–0.131) | 0.041 * | |
| p75ECD/Cr (ng/mg) | Age | 3.168 (2.133–4.202) | 3.552 (2.722–4.383) | 0.600 |
| HOMA-IR | 2.747 (1.721–3.773) | 3.575 (2.723–4.428) | 0.229 | |
| eGFR | 2.896 (1.886–3.906) | 3.741 (2.921–4.562) | 0.229 |
References
- Cameron, A.P.; Chung, D.E.; Dielubanza, E.J.; Enemchukwu, E.; Ginsberg, D.A.; Helfand, B.T.; Linder, B.J.; Reynolds, W.S.; Rovner, E.S.; Souter, L.; et al. The AUA/SUFU Guideline on the Diagnosis and Treatment of Idiopathic Overactive Bladder. J. Urol. 2024, 212, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Gomelsky, A.; Dmochowski, R.R. Urinary Incontinence in the Aging Female: Etiology, Pathophysiology and Treatment Options. Aging Health 2011, 7, 79–88. [Google Scholar] [CrossRef]
- Haylen, B.; De Ridder, D.; Freeman, R.; Swift, S.; Berghmans, B.; Lee, J.; Monga, A.; Petri, E.; Rizk, D.; Sand, P.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) Joint Report on the Terminology for Female Pelvic Floor Dysfunction. In Textbook of Female Urology and Urogynecology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 1090–1105. [Google Scholar] [CrossRef]
- Zillioux, J.; Slopnick, E.A.; Vasavada, S.P. Third-Line Therapy for Overactive Bladder in the Elderly: Nuances and Considerations. Neurourol. Urodyn. 2022, 41, 1967–1974. [Google Scholar] [CrossRef]
- Peyronnet, B.; Mironska, E.; Chapple, C.; Cardozo, L.; Oelke, M.; Dmochowski, R.; Amarenco, G.; Gamé, X.; Kirby, R.; Van Der Aa, F.; et al. A Comprehensive Review of Overactive Bladder Pathophysiology: On the Way to Tailored Treatment. Eur. Urol. 2019, 75, 988–1000. [Google Scholar] [CrossRef]
- Strimbu, K.; Tavel, J.A. What Are Biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466. [Google Scholar] [CrossRef]
- Sheng, W.; Zhang, H.; Ruth, K.-H. Could Urinary Nerve Growth Factor Be a Biomarker for Overactive Bladder? A Meta-Analysis. Neurourol. Urodyn. 2017, 36, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Tsiapakidou, S.; Apostolidis, A.; Pantazis, K.; Grimbizis, G.F.; Mikos, T. The Use of Urinary Biomarkers in the Diagnosis of Overactive Bladder in Female Patients. A Systematic Review and Meta-Analysis. Int. Urogynecol. J. 2021, 32, 3143–3155. [Google Scholar] [CrossRef] [PubMed]
- Suskind, A.M. The Aging Overactive Bladder: A Review of Aging-Related Changes from the Brain to the Bladder. Curr. Bladder Dysfunct. Rep. 2017, 12, 42–47. [Google Scholar] [CrossRef]
- Beta, A.; Giannouli, A.; Rizos, D.; Mantzou, A.; Deligeoroglou, E.; Bakas, P. Nerve Growth Factor and Brain-Derived Neurotrophic Factor as Potential Biomarkers of Mirabegron Efficacy in Patients with Overactive Bladder Syndrome. Int. Urogynecol. J. 2024, 35, 1317–1322. [Google Scholar] [CrossRef]
- Sağır, S.; Bayrak, Ö.; Şen, H.; Kul, S.; Erturhan, S.; Seçkiner, İ. Correlation between the NGF Levels and Questionnaire Forms in Patients Receiving Antimuscarinic Treatment and Those Receiving Onabotulinum Toxin-A Injection. Turk. J. Urol. 2021, 47, 223–228. [Google Scholar] [CrossRef]
- Ochodnicky, P.; Cruz, C.D.; Yoshimura, N.; Cruz, F. Neurotrophins as Regulators of Urinary Bladder Function. Nat. Rev. Urol. 2012, 9, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Mossa, A.H.; Cammisotto, P.G.; Shamout, S.; Campeau, L. Imbalance of Nerve Growth Factor Metabolism in Aging Women with Overactive Bladder Syndrome. World J. Urol. 2020, 39, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Mossa, A.H.; Shamout, S.; Cammisotto, P.; Campeau, L. Urinary Metabolomics Predict the Severity of Overactive Bladder Syndrome in an Aging Female Population. Int. Urogynecol. J. 2019, 31, 1023–1031. [Google Scholar] [CrossRef]
- Covarrubias, C.; Cammisotto, P.G.; Shamout, S.; Campeau, L. Decrease in the Ratio proBDNF/BDNF in the Urine of Aging Female Patients with OAB. Metabolites 2023, 13, 723. [Google Scholar] [CrossRef] [PubMed]
- Husdan, H.; Rapoport, A. Estimation of Creatinine by the Jaffe Reaction. Clin. Chem. 1968, 14, 222–238. [Google Scholar] [CrossRef]
- Bunn, F.; Kirby, M.; Pinkney, E.; Cardozo, L.; Chapple, C.; Chester, K.; Cruz, F.; Haab, F.; Kelleher, C.; Milsom, I.; et al. Is There a Link between Overactive Bladder and the Metabolic Syndrome in Women? A Systematic Review of Observational Studies. Int. J. Clin. Pract. 2015, 69, 199–217. [Google Scholar] [CrossRef]
- Jerez-Roig, J.; Booth, J.; Skelton, D.A.; Giné-Garriga, M.; Chastin, S.F.M.; Hagen, S. Is Urinary Incontinence Associated with Sedentary Behaviour in Older Women? Analysis of Data from the National Health and Nutrition Examination Survey. PLoS ONE 2020, 15, e0227195. [Google Scholar] [CrossRef]
- Nockher, W.A.; Renz, H. Neurotrophins in Clinical Diagnostics: Pathophysiology and Laboratory Investigation. Clin. Chim. Acta 2005, 352, 49–74. [Google Scholar] [CrossRef]
- Severini, C. Neurotrophic Factors in Health and Disease. Cells 2022, 12, 47. [Google Scholar] [CrossRef]
- D’Amico, F.; Lugarà, C.; Luppino, G.; Giuffrida, C.; Giorgianni, Y.; Patanè, E.M.; Manti, S.; Gambadauro, A.; La Rocca, M.; Abbate, T. The Influence of Neurotrophins on the Brain-Lung Axis: Conception, Pregnancy, and Neonatal Period. Curr. Issues Mol. Biol. 2024, 46, 2528–2543. [Google Scholar] [CrossRef]
- Brattico, E.; Bonetti, L.; Ferretti, G.; Vuust, P.; Matrone, C. Putting Cells in Motion: Advantages of Endogenous Boosting of BDNF Production. Cells 2021, 10, 183. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Y.; Tu, M.; Ye, Y.; Li, M.; Ran, R.; Zou, Z. Brain-Derived Neurotrophic Factor Levels across Psychiatric Disorders: A Systemic Review and Network Meta-Analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2024, 131, 110954. [Google Scholar] [CrossRef]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef]
- Thakkar, B.; Acevedo, E.O. BDNF as a Biomarker for Neuropathic Pain: Consideration of Mechanisms of Action and Associated Measurement Challenges. Brain Behav. 2023, 13, e2903. [Google Scholar] [CrossRef]
- Suh, Y.S.; Ko, K.J.; Kim, T.H.; Lee, H.S.; Sung, H.H.; Cho, W.J.; Lee, K.-S. Urinary Nerve Growth Factor as a Potential Biomarker of Treatment Outcomes in Overactive Bladder Patients. Int. Neurourol. J. 2017, 21, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.P.; Wiseman, J.B.; Smith, A.R.; Merion, R.M.; Gillespie, B.W.; Bradley, C.S.; Amundsen, C.L.; Yang, C.C.; Lai, H.H.; DeLancey, J.O.L.; et al. Are Three-day Voiding Diaries Feasible and Reliable? Results from the Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN) Cohort. Neurourol. Urodyn. 2019, 38, 2185–2193. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Geng, B.; Xu, X.; Harmanli, O. Current State of Bladder Diary: A Survey and Review of the Literature. Int. Urogynecol. J. 2023, 34, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Lee, S.C.; Jeong, C.W.; Hong, S.K.; Byun, S.-S.; Lee, S.E. Clinical and Urodynamic Differences among Women with Overactive Bladder According to the Presence of Detrusor Overactivity. Int. Urogynecol. J. 2013, 24, 255–261. [Google Scholar] [CrossRef]
- Bodmer, N.S.; Wirth, C.; Birkhäuser, V.; Sartori, A.M.; Leitner, L.; Averbeck, M.A.; De Wachter, S.; Finazzi Agro, E.; Gammie, A.; Goldman, H.B.; et al. Randomised Controlled Trials Assessing the Clinical Value of Urodynamic Studies: A Systematic Review and Meta-Analysis. Eur. Urol. Open Sci. 2022, 44, 131–141. [Google Scholar] [CrossRef]
- Abdel-Fattah, M.; Chapple, C.; Cooper, D.; Breeman, S.; Bell-Gorrod, H.; Kuppanda, P.; Guerrero, K.; Dixon, S.; Cotterill, N.; Ward, K.; et al. Invasive Urodynamic Investigations in the Management of Women with Refractory Overactive Bladder Symptoms (FUTURE) in the UK: A Multicentre, Superiority, Parallel, Open-Label, Randomised Controlled Trial. Lancet 2025, 405, 1057–1068. [Google Scholar] [CrossRef]
- Krhut, J.; Martan, A.; Zachoval, R.; Hanuš, T.; Švabík, K.; Zvara, P. Impact of Body Mass Index on Treatment Efficacy of Mirabegron for Overactive Bladder in Females. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 196, 64–68. [Google Scholar] [CrossRef] [PubMed]

| CTR Group (n = 23) | OAB Group (n = 33) | p Value | |
|---|---|---|---|
| Demographic and Serum Analysis | |||
| Age (years), mean ± SD (range) | 56.62 ± 5.46 (50.0–72.0) | 69.49 ± 10.14 (50.0–86.0) | <0.0001 * |
| BMI (kg/m2) | 27.05 ± 5.30 | 27.36 ± 4.80 | 0.8247 |
| Systolic BP (mmHg) | 121.17 ± 12.80 | 127.76 ± 15.05 | 0.0930 |
| eGFR (mL/min/1.73 m2) | 96.39 ± 16.45 | 75.33 ± 18.08 | <0.0001 * |
| HbA1c (%) | 5.50 ± 0.44 | 5.70 ± 0.70 | 0.1581 |
| Fasting glucose (mmol/L) | 5.34 ± 0.74 | 5.97 ± 1.62 | 0.0885 |
| HOMA-IR | 2.03 ± 0.97 | 4.01 ± 3.71 | 0.0288 * |
| Total Chol/HDL | 3.39 ± 1.15 | 3.09 ± 0.72 | 0.2400 |
| Questionnaires’ scores | |||
| OABS (0–28) | 13.04 ± 4.89 | 21.03 ± 6.58 | <0.0001 * |
| ICIQ (0–22) | 4.13 ± 3.69 | 9.36 ± 4.49 | <0.0001 * |
| IIQ7 (0–100) | 2.08 ± 4.92 | 31.93 ± 24.04 | <0.0001 * |
| Voiding diary parameters | |||
| 24-h frequency | 8.74 ± 2.43 | 10.75 ± 3.07 | 0.0112 * |
| Daytime frequency | 8.13 ± 2.16 | 8.97 ± 2.34 | 0.1786 |
| Night frequency | 0.61 ± 0.78 | 1.78 ± 1.43 | 0.0007 * |
| 24-h voided volume (mL) | 2526.8 ± 2230.7 | 1779.2 ± 809.5 | 0.0821 |
| Nocturnal urine volume (mL) | 437.1 ± 291.02 | 429.7 ± 283.7 | 0.9229 |
| Mean voided volume (mL) | 310.2 ± 291.02 | 174.4 ± 78.24 | 0.0132 * |
| Maximum voided volume (mL) | 473.5 ± 190.13 | 329.6 ± 134.6 | 0.0016 * |
| CTR Group (n = 23) | p Value a | OAB Baseline (n = 10) | OAB 3-Months (n = 10) | p Value b | |
|---|---|---|---|---|---|
| proNGF/Cr (ng/mg) | 0.5261 (0.3519, 0.7497) | 0.5536 | 0.5838 (0.2724, 0.7499) | 0.6786 (0.4465, 1.774) | 0.0488 * |
| NGF/Cr (pg/mg) | 1.668 (1.173, 3.22) | 0.0034 * | 0.5671 (0.3697, 1.002) | 0.778 (0.595, 2.43) | 0.0273 * |
| proNGF/NGF ratio mol/mol | 268.9 (164.3, 370.6) | 0.0002 * | 712.1 (658.0, 809.2) | 645.1 (560.6, 735.6) | 0.1309 |
| NGF/proNGF ratio mol/mol | 0.003 (0.002, 0.006) | 0.0002 * | 0.0015 (0.0012, 0.0017) | 0.0015 (0.0013, 0.0017) | 0.1055 |
| proBDNF/Cr (pg/mg) | 640.8 (364.5, 2028) | 0.3454 | 502.4 (220.8, 1433) | 662.9 (360.3, 1256) | 0.6953 |
| BDNF/Cr (pg/mg) | 10.32 (6.71, 28.54) | 0.3045 | 11.49 (4.869, 44.37) | 16.82 (7.384, 44.62) | 0.4922 |
| proBDNF/BDNF ratio mol/mol | 27,972 (15,710, 44,408) | 0.0095 * | 18,301 (14,718, 24,649) | 18,089 (14,402, 25,753) | 0.3223 |
| BDNF/proBDNF ratio mol/mol | 0.00004 (0.00002, 0.00006) | 0.0076 * | 0.00005 (0.00004, 0.00007) | 0.00005 (0.00003, 0.00007) | 0.2891 |
| p75ECD/Cr (ng/mg) | 2.614 (2.155, 3.012) | 0.1843 | 2.804 (2.005, 6.029) | 4.329 (3.167, 7.714) | 0.2324 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Covarrubias, C.; Cammisotto, P.G.; Campeau, L. Neurotrophins and Proneurotrophins as Biomarkers for Overactive Bladder Syndrome in Aging Females. Metabolites 2025, 15, 429. https://doi.org/10.3390/metabo15070429
Covarrubias C, Cammisotto PG, Campeau L. Neurotrophins and Proneurotrophins as Biomarkers for Overactive Bladder Syndrome in Aging Females. Metabolites. 2025; 15(7):429. https://doi.org/10.3390/metabo15070429
Chicago/Turabian StyleCovarrubias, Claudia, Philippe G. Cammisotto, and Lysanne Campeau. 2025. "Neurotrophins and Proneurotrophins as Biomarkers for Overactive Bladder Syndrome in Aging Females" Metabolites 15, no. 7: 429. https://doi.org/10.3390/metabo15070429
APA StyleCovarrubias, C., Cammisotto, P. G., & Campeau, L. (2025). Neurotrophins and Proneurotrophins as Biomarkers for Overactive Bladder Syndrome in Aging Females. Metabolites, 15(7), 429. https://doi.org/10.3390/metabo15070429







