Microbial Metabolites: A Sustainable Approach to Combat Plant Pests
Abstract
1. Introduction
2. Microbial Metabolites
3. Primary Metabolites from Microorganisms
3.1. Amino Acids in Plant Health and Defense
| Metabolites | Source Microorganisms | Mode of Action | Target Pests/Pathogens | References |
|---|---|---|---|---|
| Lipids | ||||
| Rhamnolipids (RLs) Fengycins (FGs) | Bacillus subtilis | Induce mycelial de-structuring and hyphal fusions | Botrytis cinerea and Sclerotinia sclerotiorum | [37] |
| Hormones | ||||
| Abscisic acid | Achromobacter xiloxidans, Bacillus pumilus | Enhances plant resistance | Broad-spectrum fungicide | [38] |
| Jasmonic acid (JA) | Pseudomonas, Bacillus, Azoospirillum | Induces systemic resistance (ISR) | Spodoptera exigua | [39] |
| Jasmonic acid (JA | Pochonia chlamydosporia | PR protein | Meloidogyne javanica | [40] |
| Salicylic acid (SA) | Pseudomonas, Bacillus, Azoospirillum | Induces systemic resistance (ISR) | Sclerotinia sclerotiorum | [41] |
| Ethylene (ET) | Paenibacillus lentimorbus | Induces systemic resistance (ISR) | Sclerotium rolfsii. | [42] |
| Indole acetic acid (IAA) | Dysoxylum gotadhora | Induces systemic resistance (ISR) | Verticillium dahliae and Fusarium oxysporum | [43] |
| Gibberelin | Rhizobium, Bacillus, and Penicillium | Induces systemic resistance (ISR) | All pathogens | [44] |
| Turanose sugar and hormones IAA, Gibberelic acid (GA) SA | B. amyloliquefaciens | Modulation of phytohormone signal | Rhizoctonia solani | [45] |
| Organic acids | ||||
| Lactic acid | Lactobacillus plantarum | Antibacterial and antifungal | Pseudomonas campestris, Ralstonia solanacearum, Xanthomonas campestris pv. vesicatoria, Pectobacterium carotovorum | [46] |
| Lactic acid | L. paracasei | Antibacterial and antifungal | R. solanacearum | [47] |
| Organic acid | Most of the microbes | Solubilizes cuticular proteins | Lepidopteran and dipteran pest | [8] |
| Organic acid | L. plantarum | Nematicidal effect | Meloidogyne incognita | [48] |
| Acetic, propionic, formic, benzoic acid | Lactobacillus sp. | Interferes with the membrane functions of the pathogen | Broad-spectrum | [49] |
| Others | ||||
| Meso-2,3-Butanediol | Klebsiella pneumoniae | Induction of systemic resistance | R. solanacearum | [50] |
| Proteinaceous and non-proteinaceous antifungal compounds | Lactobacillus plantarum | Antifungal compounds | Botrytis cinerea, Alternaria solani, Phytophthora drechsleri, Fusarium oxysporum and Glomerella cingulate | [51] |
3.2. Sugars and Their Role in Pest and Disease Control
3.3. Organic Acids as Biocontrol Agents
3.4. Lipids in Microbial Communication and Plant Interaction
3.5. Nucleotides and Their Regulatory Functions
4. Secondary Metabolites from Microorganisms
- Terpenes (e.g., volatiles, glycosides, sterols);
- Phenolics (e.g., flavonoids, coumarins);
- Nitrogen-containing compounds (e.g., alkaloids);
- Peptides (e.g., surfactin, iturin);
4.1. Terpenoids: Diverse Roles in Plant Protection
4.2. Polyketides
4.3. Phenolic Compounds
4.4. Alkaloids
4.5. Peptides
5. Mechanisms of Action of Microbial Metabolites Against Insects Pest and Diseases
5.1. Mechanisms of Action Against Insect Pests
5.1.1. Production of Toxins That Disrupt Insect Physiology
5.1.2. Interference with Insect Development and Reproduction
5.1.3. Repellent and Antifeedant Effects
5.1.4. Disruption of Insect Hormonal Balance
5.1.5. Induction of Plant Defense Mechanisms Against Diseases
5.2. Mechanisms of Action Against Plant Disease
5.2.1. Production of Toxins Against Fungi
5.2.2. Induction of Systemic Resistance in Plants
5.2.3. Enzyme Production to Degrade Pathogen Cell Walls
5.2.4. Production of Volatile Organic Compounds (VOCs)
5.2.5. Siderophore Production
5.2.6. Plant Growth Promotion
5.2.7. Cell Wall Reinforcement
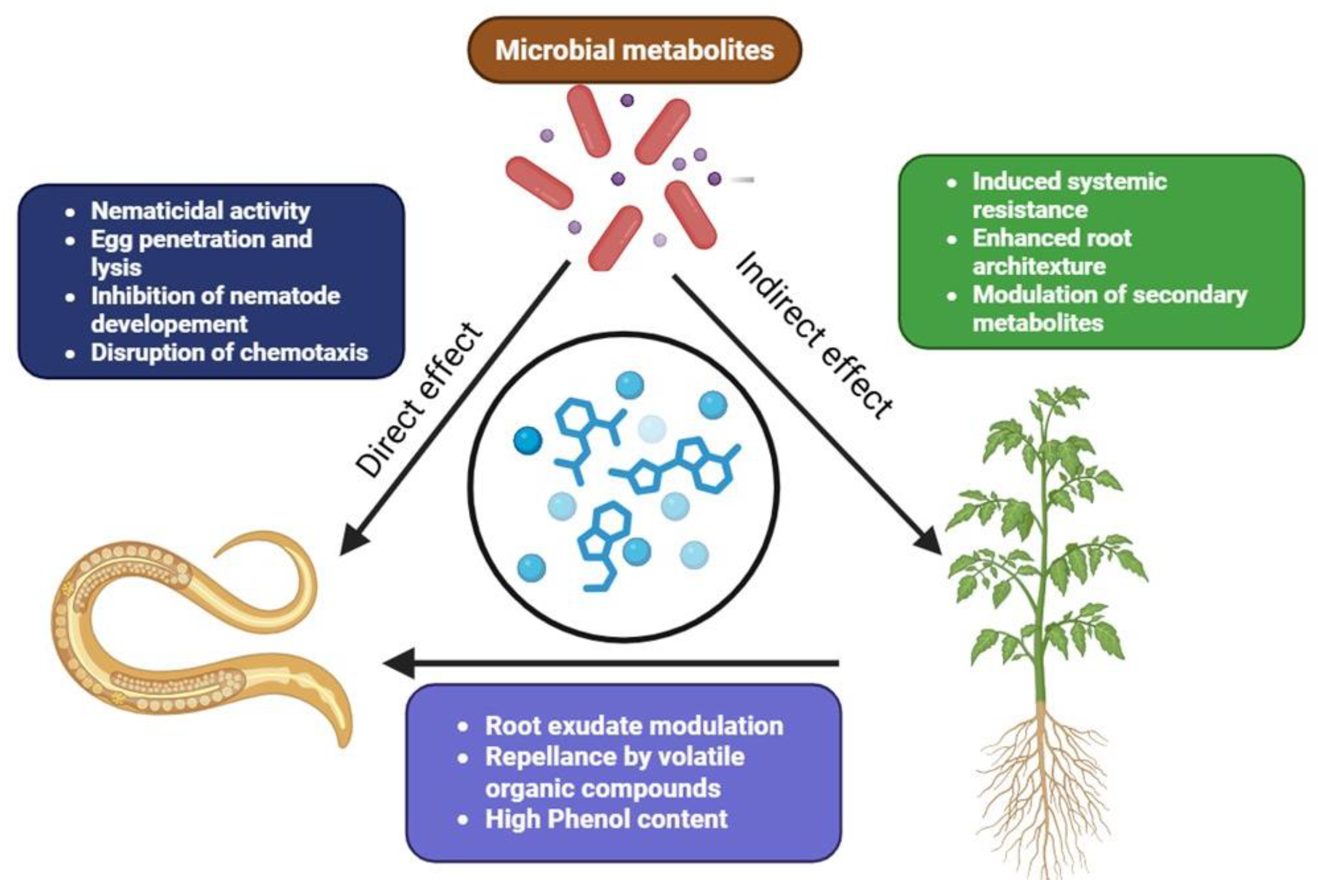
6. Mechanisms of Action Against Nematodes
6.1. Production of Nematicidal Toxins
6.2. Volatile Organic Compounds (VOCs) as Repellents
6.3. Induction of Plant Resistance to Nematode Infection
6.4. Production of Enzymes That Degrade Nematode Structures
| Metabolites | Source Microorganisms | Mode of Action | Target Pathogens | References |
|---|---|---|---|---|
| Terpenoids | ||||
| Abamectin | S. rochei | Nematicidal effect | M. incognita | [181] |
| Lactones | T. harnatum | Nematicidal effect | M. incognita | [182] |
| Lactones | Nigrospora sp. P. chalmydospora | Nematicidal effect | M. incognita | [183] |
| Milbemectin | S.bingchenggensis | Inhibits the reproduction of nematodes | M. javanica | [184] |
| Dimethyl disulfide (DMDS), methyl isovalerate (MIV) | Bacillus atrophaeus | Oxidative stress in nematodes lead to death | M. incognita | [185] |
| Dimethyl disulfide, S-methyl ester butanethioic acid | B. cereus | Fumigation and repellent activity | M. incognita | [186] |
| Benzeneacetaldehyde, 2-nonanone, decanal, 2-undecanone | B. megaterium | Nematicidal effect | M. incognita | [187] |
| 3-methoxy-2,5-dimethyl pyrazine,1-undecene, dimethyl disulfide | P. koreensis | Nematicidal effect | M. javanica | [188] |
| 1-octen-3-ol, 3-octanone | M. brunneum | Attraction and kill | M. hapla | [189] |
| 1,8-cineole | Annulohypoxylon sp. | Nematicidal effect | Bursaphelenchus xylophilus | [190] |
| 6-pentyl-2H-pyran-2-one | Trichoderma sp. | Nematicidal effect | B. xylophilus | [191] |
| Polyketides | ||||
| Bikaverin | F. oxysporum | Nematicidal effect | M. incognita Rotylenchulus reiniformis | [192] |
| Butyrolactone | Clonostachys rosea | Nematicidal effect | M. incognita | [193] |
| Alloaureothin and aureothin | Streptomyces sp. | Prevent egg hatching and juvenile mortality | B. xylophilus | [194] |
| 2,4-diacetylpholoroglucinol (DAPG) | P. fluroscens | Prevent egg hatching and juvenile mortality | M. incognita | [28] |
| 2,4-diacetylpholoroglucinol (DAPG) | P. fluroscens | Prevent egg hatching and juvenile mortality | M. javanica | [195] |
| Abamectin | S. avermitilis | Nematicidal effect | M. incognita | [196] |
| 4-heptanone | Daldinia concentrica | Nematicidal effect | M javanica | [197] |
| Phenols | ||||
| Trichostatin and dehydroxytrichostatin | S. nigrescens | Nematicidal effect | M. incognita | [198] |
| Trans cinnamic acid (t-CA) 5-phenylpent-4 enoic acid (PPA) and indole | Photorhabdus luminescens sonorensis | Nematicidal effect | M.incognita T. semipenetrans, | [199] |
| N-acetyltyramine. benzenepropanoic acid | Micromonospora sp. | Prevents egg hatching and juvenile mortality | M. incognita | [200] |
| Napthoquinone Fusarubin | Fusarium oxysporum | Affects the nervous system, leading to paralysis | M. incognita. | [193] |
| Nitrogen-containing compounds/Alkaloids | ||||
| Alkaloids | Penicillium bilaiae | Affects the nervous system, leading to paralysis and death | P. penetrans | [201] |
| Peptides | ||||
| Lucinostatin | Pacilomyces lilacinus | Prevents egg hatching and juvenile mortality | M. javanica | [202] |
| Rhabdopeptide | X. budapestensis | Nematicidal activity | M. incognita | [203] |
| Rhabdopeptides Fabclavines | Xenorhabdus sp. | Prevents egg hatching and juvenile mortality | M. javanica | [204] |
| Omphalotin | Omphalotis olearius | Disrupts nematode biology | M. incognita | [181] |
6.5. Modulation of Plant Metabolism and Immunity
6.6. Disruption of Nematode Feeding and Reproduction
7. Practical Applications of Secondary Metabolites Against Pests, Diseases, and Nematodes
7.1. Microbial Metabolites for Pest Management
7.2. Microbial Metabolites for Disease Management
7.3. Microbial Metabolites as Nematode Management
8. Formulation and Delivery Methods for Microbial Metabolites
9. Advances in Biotechnology and Synthetic Biology for Enhanced Metabolite Production
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reveglia, P.; Corso, G.; Evidente, A. Advances on Bioactive Metabolites with Potential for the Biocontrol of Plant Pathogenic Bacteria. Pathogens 2024, 13, 1000. [Google Scholar] [CrossRef] [PubMed]
- Sansinenea, E. Bacillus Spp.: As Plant Growth-Promoting Bacteria. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms: Discovery and Applications; Singh, H.B., Keswani, C., Reddy, M.S., Sansinenea, E., García-Estrada, C., Eds.; Springer: Singapore, 2019; pp. 225–237. [Google Scholar]
- Chen, Q.; Song, Y.; An, Y.; Lu, Y.; Zhong, G. Mechanisms and Impact of Rhizosphere Microbial Metabolites on Crop Health, Traits, Functional Components: A Comprehensive Review. Molecules 2024, 29, 5922. [Google Scholar] [CrossRef]
- Jayaprakashvel, M.; Mathivanan, N. Management of Plant Diseases by Microbial Metabolites. In Bacteria in Agrobiology: Plant Nutrient Management; Springer: Berlin/Heidelberg, Germany, 2011; pp. 237–265. [Google Scholar]
- Ribeiro, V.P.; Bajsa-Hirschel, J.; Tamang, P.; Harries, M.D.; Meepagala, K.M. Bioactive Secondary Metabolites from Curvularia Spp.: Natural Alternatives for Pest Management in Agriculture. J. Nat. Pestic. Res. 2025, 12, 100117. [Google Scholar] [CrossRef]
- Ortiz, A.; Sansinenea, E. Genetically Modified Plants Based on Bacillus Genes and Commercial Bacillus-Based Biopesticides for Sustainable Agriculture. Horticulturae 2023, 9, 963. [Google Scholar] [CrossRef]
- Wend, K.; Zorrilla, L.; Freimoser, F.; Gallet, A. Microbial Pesticides–Challenges and Future Perspectives for Testing and Safety Assessment with Respect to Human Health. Environ. Health 2024, 23, 49. [Google Scholar] [CrossRef]
- Subbanna, A.R.N.S.; Stanley, J.; Rajasekhara, H.; Mishra, K.K.; Pattanayak, A.; Bhowmick, R. Perspectives of Microbial Metabolites as Pesticides in Agricultural Pest Management. In Co-Evolution of Secondary Metabolites; Merillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–28. [Google Scholar]
- Rutledge, P.J.; Challis, G.L. Discovery of Microbial Natural Products by Activation of Silent Biosynthetic GENE clusters. Nat. Rev. Microbiol. 2015, 13, 509–523. [Google Scholar] [CrossRef]
- Keasling, J.D. Synthetic Biology and the Development of Tools for Metabolic Engineering. Metab. Eng. 2012, 14, 189–195. [Google Scholar] [CrossRef]
- Salazar, F.; Ortiz, A.; Sansinenea, E. Characterisation of Two Novel Bacteriocin-Like Substances Produced by Bacillus amyloliquefaciens ELI149 with Broad-Spectrum Antimicrobial Activity. J. Glob. Antimicrob. Resist. 2017, 11, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.R.; Villas-Boas, S.G. Extracellular Microbial Metabolomics: The State of the Art. Metabolites 2017, 7, 43. [Google Scholar] [CrossRef]
- Beale, D.J.; Kouremenos, K.A.; Palombo, E. Microbial Metabolomics: Applications in Clinical, Environmental, and Industrial Microbiology; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Macintyre, L.; Zhang, T.; Viegelmann, C.; Martinez, I.J.; Cheng, C.; Dowdells, C.; Abdelmohsen, U.R.; Gernert, C.; Hentschel, U.; Edrada-Ebel, R. Metabolomic Tools for Secondary Metabolite Discovery from Marine Microbial Symbionts. Mar. Drugs 2014, 12, 3416–3448. [Google Scholar] [CrossRef]
- Shahriar, S.A.; Islam, M.N.; Chun, C.N.W.; Kaur, P.; Rahim, M.A.; Islam, M.M.; Uddain, J.; Siddiquee, S. Microbial Metabolomics Interaction and Ecological Challenges of Trichoderma Species as Biocontrol Inoculant in Crop Rhizosphere. Agronomy 2022, 12, 900. [Google Scholar] [CrossRef]
- Shahid, I.; Han, J.; Hanooq, S.; Malik, K.A.; Borchers, C.H.; Mehnaz, S. Profiling of Metabolites of Bacillus Spp. and Their Application in Sustainable Plant Growth Promotion and Biocontrol. Front. Sustain. Food Syst. 2021, 5, 605195. [Google Scholar] [CrossRef]
- Villas-Bôas, S.G.; Roessner, U.; Hansen, M.A.E.; Smedsgaard, J.; Nielsen, J. Metabolome Analysis: An Introduction; Willey-Interscience: Hoboken, NJ, USA, 2007. [Google Scholar]
- Aldridge, B.B.; Rhee, K.Y. Microbial Metabolomics: Innovation, Application, Insight. Curr. Opin. Microbiol. 2014, 19, 90–96. [Google Scholar] [CrossRef]
- Vaidyanathan, S. Profiling Microbial Metabolomes: What Do We Stand to Gain? Metabolomics 2005, 1, 17–28. [Google Scholar] [CrossRef]
- Pinu, F.R.; Villas-Boas, S.G.; Aggio, R. Analysis of Intracellular Metabolites from Microorganisms: Quenching and Extraction Protocols. Metabolites 2017, 7, 53. [Google Scholar] [CrossRef]
- Pande, S.; Kost, C. Bacterial Unculturability and the Formation of Intercellular Metabolic Networks. Trends Microbiol. 2017, 25, 349–361. [Google Scholar] [CrossRef]
- Maharana, C.; Kumar, P.; Hubballi, A.; Raj, M.; Paschapur, A.; Bhat, C.; Singh, A.; Arns, S. Secondary Metabolites of Microbials as Potential Pesticides. In Sustainable Management of Potato Pests and Diseases; Springer: Berlin/Heidelberg, Germany, 2022; pp. 111–142. [Google Scholar]
- Harman, G.; Doni, F.; Khadka, R.B.; Uphoff, N. Endophytic Strains of Trichoderma Increase Plants’ Photosynthetic Capability. J. Appl. Microbiol. 2021, 130, 529–546. [Google Scholar] [CrossRef]
- Khan, S.; Srivastava, S.; Karnwal, A.; Malik, T. Streptomyces as a Promising Biological Control Agents for Plant Pathogens. Front. Microbiol. 2023, 14, 1285543. [Google Scholar] [CrossRef] [PubMed]
- Dara, S.K. Microbial Metabolites as Pesticides. In Microbes for Sustainable lnsect Pest Management: Hydrolytic Enzyme & Secondary Metabolite–Volume 2; Khan, M.A., Ahmad, W., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 75–88. [Google Scholar]
- Ruiz, B.; Chávez, A.; Forero, A.; García-Huante, Y.; Romero, A.; Sánchez, M.; Rocha, D.; Sánchez, B.; Rodríguez-Sanoja, R.; Sánchez, S.; et al. Production of Microbial Secondary Metabolites: Regulation by the Carbon Source. Crit. Rev. Microbiol. 2010, 36, 146–167. [Google Scholar] [CrossRef]
- Gan, L.; Zhang, Z.; Chen, J.; Shen, Z.; Chen, W.; Chen, S.; Li, J. Enhancement of Spinosad Production in Saccharopolyspora Spinosa by Overexpression of the Complete 74-kb Spinosyn Gene Cluster. Microb. Cell Factories 2025, 24, 1–10. [Google Scholar] [CrossRef]
- Radwan, W.H.; Abdelhafez, A.A.; Mahgoub, A.E.; Zayed, M.S. Streptomyces Avermitilis MICNEMA2022: A New Biorational Strain for Producing Abamectin as an Integrated Nematode Management Agent. BMC Microbiol. 2024, 24, 329. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Shen, X.; Jain, R.; Lin, Y.; Wang, J.; Sun, J.; Wang, J.; Yan, Y.; Yuan, Q. Synthesis of Chemicals by Metabolic Engineering of Microbes. Chem. Soc. Rev. 2015, 44, 3760–3785. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L.; Sanchez, S. Microbial Synthesis of Primary Metabolites: Current Trends and Future Prospects. In Fermentation Microbiology and Biotechnology, 4th ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 57–74. [Google Scholar]
- Wu, G. Amino Acids: Metabolism, Functions, and Nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-M.; Ye, D.-X.; Liu, Y.; Zhang, X.-Y.; Zhou, Y.-L.; Zhang, L.; Yang, X.-L. Peptides, New Tools for Plant Protection in Eco-Agriculture. Adv. Agrochem. 2023, 2, 58–78. [Google Scholar] [CrossRef]
- Moe, L.A. Amino Acids in the Rhizosphere: From Plants to Microbes. Am. J. Bot. 2013, 100, 1692–1705. [Google Scholar] [CrossRef]
- Rai, V.K. Role of Amino Acids in Plant Responses to Stresses. Biol. Plant. 2002, 45, 481–487. [Google Scholar] [CrossRef]
- Saeed, Q.; Xiukang, W.; Haider, F.U.; Kučerik, J.; Mumtaz, M.Z.; Holatko, J.; Naseem, M.; Kintl, A.; Ejaz, M.; Naveed, M.; et al. Rhizosphere Bacteria in Plant Growth Promotion, Biocontrol, and Bioremediation of Contaminated Sites: A Comprehensive Review of Effects and Mechanisms. Int. J. Mol. Sci. 2021, 22, 10529. [Google Scholar] [CrossRef]
- Moormann, J.; Heinemann, B.; Hildebrandt, T.M. News About Amino Acid Metabolism in Plant-Microbe Interactions. Trends Biochem. Sci. 2022, 47, 839–850. [Google Scholar] [CrossRef]
- Botcazon, C.; Bergia, T.; Lecouturier, D.; Dupuis, C.; Rochex, A.; Acket, S.; Nicot, P.; Leclère, V.; Sarazin, C.; Rippa, S. Rhamnolipids and Fengycins, very Promising Amphiphilic Antifungal Compounds from Bacteria Secretomes, Act on Sclerotiniaceae Fungi Through Different Mechanisms. Front. Microbiol. 2022, 13, 977633. [Google Scholar] [CrossRef]
- Kumar, A.; Radhakrishnan, E. Microbial Endophytes: Functional Biology and Applications; Woodhead Publishing: Cambridge, UK, 2020. [Google Scholar]
- Zebelo, S.; Song, Y.; Kloepper, J.W.; Fadamiro, H. Rhizobacteria Activates (+)-Δ-Cadinene Synthase Genes and Induces Systemic Resistance in Cotton Against Beet Armyworm (Spodoptera exigua). Plant Cell Environ. 2016, 39, 935–943. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhao, J.-L.; Bai, M.; Ping, X.-X.; Li, Y.-L.; Yang, Y.-H.; Mao, Z.-C.; Yang, G.-S.; Xie, B.-Y. Pochonia Chlamydosporia Isolate Pc-170-Induced Expression of Marker Genes for Defense Pathways in Tomatoes Challenged by Different Pathogens. Microorganisms 2021, 9, 1882. [Google Scholar] [CrossRef]
- He, S.; Huang, K.; Li, B.; Lu, G.; Wang, A. Functional Analysis of a Salicylate Hydroxylase in Sclerotinia sclerotiorum. J. Fungi 2023, 9, 1169. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.; Agrawal, L.; Singh, S.P.; Prateeksha; Singh, P.C.; Prasad, V.; Chauhan, P.S. Paenibacillus lentimorbus Induces Autophagy for Protecting Tomato from Sclerotium rolfsii Infection. Microbiol. Res. 2018, 215, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, N.; Ntemafack, A.; Chouhan, R.; Gandhi, S.G. Anti-Phytopathogenic and Plant Growth Promoting Potential of Endophytic Fungi Isolated from Dysoxylum gotadhora. Arch. Phytopathol. Plant Prot. 2022, 55, 454–473. [Google Scholar] [CrossRef]
- Wang, H.N.; Ke, X.; Jia, R.; Huang, L.G.; Liu, Z.Q.; Zheng, Y.G. Multivariate Modular Metabolic Engineering for Enhanced Gibberellic Acid Biosynthesis in Fusarium Fujikuroi. Bioresour. Technol. 2022, 364, 128033. [Google Scholar] [CrossRef]
- Srivastava, S.; Bist, V.; Srivastava, S.; Singh, P.C.; Trivedi, P.K.; Asif, M.H.; Chauhan, P.S.; Nautiyal, C.S. Unraveling Aspects of Bacillus amyloliquefaciens Mediated Enhanced Production of Rice under Biotic Stress of Rhizoctonia Solani. Front. Plant Sci. 2016, 7, 587. [Google Scholar] [CrossRef] [PubMed]
- Visser, R.; Holzapfel, W.H.; Bezuidenhout, J.J.; Kotzé, J.M. Antagonism of Lactic Acid Bacteria against Phytopathogenic Bacteria. Appl. Environ. Microbiol. 1986, 52, 552–555. [Google Scholar] [CrossRef]
- Murthy, K.N.M.M.; Savitha, J.; Srinivas, C. Lactic Acid Bacteria (LAB) as Plant Growth Promoting Bacteria (Pgpb) for the Control of Wilt of Tomato Caused by Ralstonia solanacearum. Pest Manag. Hortic. Ecosyst. 2012, 18, 60–65. [Google Scholar]
- Kim, S.; Kim, H.M.; Seo, H.J.; Yeon, J.; Park, A.R.; Yu, N.H.; Jeong, S.G.; Chang, J.Y.; Kim, J.C.; Park, H.W. Root-Knot Nematode (Meloidogyne incognita) Control Using a Combination of Lactiplantibacillus plantarum WiKim0090 and Copper Sulfate. J. Microbiol. Biotechnol. 2022, 32, 960–966. [Google Scholar] [CrossRef]
- Arena, M.P.; Silvain, A.; Normanno, G.; Grieco, F.; Drider, D.; Spano, G.; Fiocco, D. Use of Lactobacillus Plantarum Strains as a Bio-Control Strategy against Food-Borne Pathogenic Microorganisms. Front. Microbiol. 2016, 7, 464. [Google Scholar] [CrossRef]
- Kim, B.; Park, A.R.; Song, C.W.; Song, H.; Kim, J.-C. Biological Control Efficacy and Action Mechanism of Klebsiella pneumoniae Jck-2201 Producing Meso-2,3-Butanediol Against Tomato Bacterial Wilt. Front. Microbiol. 2022, 13, 914589. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Pan, H.X.; Tang, G.L. Production of Doramectin by Rational Engineering of the Avermectin Biosynthetic Pathway. Bioorg. Med. Chem. Lett. 2011, 21, 3320–3323. [Google Scholar] [CrossRef]
- Fincheira, P.; Quiroz, A. Microbial Volatiles as Plant Growth Inducers. Microbiol. Res. 2018, 208, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Meza, L.C.; Quintana-Obregón, E.A.; Vargas-Arispuro, I.; Falcón-Rodríguez, A.B.; Aispuro-Hernández, E.; Virgen-Ortiz, J.J.; Martínez-Téllez, M.Á. Oligosaccharins as Elicitors of Defense Responses in Wheat. Polymers 2021, 13, 3105. [Google Scholar] [CrossRef]
- Fürstenberg-Hägg, J.; Zagrobelny, M.; Bak, S. Plant Defense Against Insect Herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef] [PubMed]
- Tofalo, R.; Battistelli, N.; Perpetuini, G.; Valbonetti, L.; Rossetti, A.P.; Perla, C.; Zulli, C.; Arfelli, G. Oenococcus oeni Lifestyle Modulates Wine Volatilome and Malolactic Fermentation Outcome. Front. Microbiol. 2021, 12, 736789. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Lactic Acid Bacteria: Their Antimicrobial Compounds and Their Uses in Food Production. Ann. Biol. Res. 2010, 1, 218–228. [Google Scholar]
- Olaoye, O.A.; Onilude, A.A. Quantitative Estimation of Antimicrobials Produced by Lactic Acid Bacteria Isolated from Nigerian Beef. Int. Food Res. J. 2011, 18, 1104–1110. [Google Scholar]
- Jaffar, N.S.; Jawan, R.; Chong, K.P. The Potential of Lactic Acid Bacteria in Mediating the Control of Plant Diseases and Plant Growth Stimulation in Crop Production-A Mini Review. Front. Plant Sci. 2023, 13, 1047945. [Google Scholar] [CrossRef]
- Kim, H.M.; Park, J.H.; Choi, I.S.; Wi, S.G.; Ha, S.; Chun, H.H.; Hwang, I.M.; Chang, J.Y.; Choi, H.J.; Kim, J.C.; et al. Effective Approach to Organic Acid Production from Agricultural Kimchi Cabbage Waste and Its Potential Application. PLoS ONE 2018, 13, e0207801. [Google Scholar] [CrossRef]
- Alawamleh, A.; Ðurović, G.; Maddalena, G.; Guzzon, R.; Ganassi, S.; Hashmi, M.M.; Wäckers, F.; Anfora, G.; Cristofaro, A.D. Selection of Lactic Acid Bacteria Species and Strains for Efficient Trapping of Drosophila Suzukii. Insects 2021, 12, 153. [Google Scholar] [CrossRef] [PubMed]
- Macabuhay, A.; Arsova, B.; Walker, R.; Johnson, A.; Watt, M.; Roessner, U. Modulators or Facilitators? Roles of Lipids in Plant Root–Microbe Interactions. Trends Plant Sci. 2022, 27, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Abd-Allah, E.F.; Hashem, A.; Ezzat, S.M. Lipid Metabolism in Tomato and Bean as a Sensitive Monitor for Biocontrol of Wilt Diseases. Phytoparasitica 2006, 34, 516–522. [Google Scholar] [CrossRef]
- Siebers, M.; Brands, M.; Wewer, V.; Duan, Y.; Hölzl, G.; Dörmann, P. Lipids in Plant-Microbe Interactions. Biochim. Biophys. Acta 2016, 1861, 1379–1395. [Google Scholar] [CrossRef]
- Sansinenea, E.; Ortiz, A. Secondary Metabolites of Soil Bacillus Spp. Biotechnol. Lett. 2011, 33, 1523–1538. [Google Scholar] [CrossRef]
- Aamir, M.; Rai, K.; Zehra, A.; Dubey, M.; Kumar, S.; Shukla, V.; Upadhyay, R. Microbial Bioformulation-Based Plant Biostimulants: A Plausible Approach Toward Next Generation of Sustainable Agriculture. In Microbial Endophytes; Woodhead Publishing: Cambridge, UK, 2020; pp. 195–225. [Google Scholar]
- Abdel-Aziz, S.; Abo Elsoud, M.; Anise, A. Microbial Biosynthesis: A Repertory of Vital Natural Products. Food Biosynth. 2017, 1, 25–54. [Google Scholar]
- Firn, R.D.; Jones, C.G. The Evolution of Secondary Metabolism-a Unifying Model. Mol. Microbiol. 2000, 37, 989–994. [Google Scholar] [CrossRef]
- Junkins, E.N.; McWhirter, J.B.; McCall, L.-I.; Stevenson, B.S. Environmental Structure Impacts Microbial Composition and Secondary Metabolism. ISME Commun. 2022, 2, 15. [Google Scholar] [CrossRef]
- Chaabouni, I.; Guesmi, A.; Cherif, A. Secondary Metabolites of Bacillus: Potentials in Biotechnology. In Bacillus Thuringiensis Biotechnology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 347–366. [Google Scholar]
- Bills, G.F.; Gloer, J.B. Biologically Active Secondary Metabolites from the Fungi. Microbiol. Spectr. 2016, 4, 1087–1119. [Google Scholar] [CrossRef]
- Song, H.C.; Shen, W.Y.; Dong, J.Y. Nematicidal Metabolites from Gliocladium roseum Ymf1.00133. Appl. Biochem. Microbiol. 2016, 52, 324–330. [Google Scholar] [CrossRef]
- Dhanarasu, S. Chromatography and Its Applications. Chem. Biol. 2012, 1, 212. [Google Scholar] [CrossRef]
- Balthazar, C.; St-Onge, R.; Léger, G.; Lamarre, S.G.; Joly, D.L.; Filion, M. Pyoluteorin and 2,4-Diacetylphloroglucinol are Major Contributors to Pseudomonas protegens Pf-5 Biocontrol Against Botrytis cinerea in Cannabis. Front. Microbiol. 2022, 13, 945498. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Wang, P.; Zhao, W.; Su, Z.; Zhang, X.; Lu, X.; Li, S.; Ma, P.; Qinggang, G. Surfactin and Fengycin Contribute Differentially to the Biological Activity of Bacillus subtilis NCD-2 Against Cotton Verticillium Wilt. Biol. Control 2022, 174, 104999. [Google Scholar] [CrossRef]
- Tang, R.; Tan, H.; Dai, Y.; Li, L.a.; Huang, Y.; Yao, H.; Cai, Y.; Yu, G. Application of Antimicrobial Peptides in Plant Protection: Making Use of the Overlooked Merits. Front. Plant Sci. 2023, 14, 1139539. [Google Scholar] [CrossRef]
- Jayalakshmi, R.; Oviya, R.; Premalatha, K.; Mehetre, S.T.; Paramasivam, M.; Kannan, R.; Theradimani, M.; Pallavi, M.S.; Mukherjee, P.K.; Ramamoorthy, V. Production, Stability and Degradation of Trichoderma Gliotoxin in Growth Medium, Irrigation Water and Agricultural Soil. Sci. Rep. 2021, 11, 16536. [Google Scholar] [CrossRef]
- Tijerino, A.; Cardoza, R.E.; Moraga, J.; Malmierca, M.G.; Vicente, F.; Aleu, J.; Collado, I.G.; Gutiérrez, S.; Monte, E.; Hermosa, R. Overexpression of the Trichodiene Synthase Gene Tri5 Increases Trichodermin Production and Antimicrobial Activity in Trichoderma brevicompactum. Fungal. Genet. Biol. 2011, 48, 285–296. [Google Scholar] [CrossRef]
- Whalon, M.E.; Wingerd, B.A. Bt: Mode of Action and Use. Arch. Insect Biochem. Physiol. 2003, 54, 200–211. [Google Scholar] [CrossRef]
- Anand, U.; Pal, T.; Yadav, N.; Singh, V.K.; Tripathi, V.; Choudhary, K.K.; Shukla, A.K.; Sunita, K.; Kumar, A.; Bontempi, E.; et al. Current Scenario and Future Prospects of Endophytic Microbes: Promising Candidates for Abiotic and Biotic Stress Management for Agricultural and Environmental Sustainability. Microb. Ecol. 2023, 86, 1455–1486. [Google Scholar] [CrossRef]
- Kumar, V.; Nautiyal, C.S. Plant Abiotic and Biotic Stress Alleviation: From an Endophytic Microbial Perspective. Curr. Microbiol. 2022, 79, 311. [Google Scholar] [CrossRef]
- Toopaang, W.; Bunnak, W.; Srisuksam, C.; Wattananukit, W.; Tanticharoen, M.; Yang, Y.-L.; Amnuaykanjanasin, A. Microbial Polyketides and Their Roles in Insect Virulence: From Genomics to Biological Functions. Nat. Prod. Rep. 2022, 39, 2008–2029. [Google Scholar] [CrossRef]
- Waldron, C.; Madduri, K.; Crawford, K.; Merlo, D.J.; Treadway, P.; Broughton, M.C.; Baltz, R.H. A Cluster of Genes for the Biosynthesis of Spinosyns, Novel Macrolide Insect Control Agents Produced by Saccharopolyspora Spinosa. Antonie Van Leeuwenhoek 2000, 78, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Lasota, J.A.; Dybas, R.A. Avermectins, a Novel Class of Compounds: Implications for Use in Arthropod Pest Control. Annu. Rev. Entomol. 1991, 36, 91–117. [Google Scholar] [CrossRef] [PubMed]
- Diaz, C.E.; Andres, M.F.; Lacret, R.; Cabrera, R.; Gimenez, C.; Kaushik, N.; Gonzalez-Coloma, A. Antifeedant, Antifungal and Nematicidal Compounds from the Endophyte Stemphylium Solani Isolated from Wormwood. Sci. Rep. 2024, 14, 13500. [Google Scholar] [CrossRef]
- Díaz-González, S.; Andrés, M.F.; González-Sanz, C.; Sacristán, S.; González-Coloma, A. Nematicidal and Antifeedant Activity of Ethyl Acetate Extracts from Culture Filtrates of Arabidopsis Thaliana Fungal Endophytes. Sci. Rep. 2025, 15, 11332. [Google Scholar] [CrossRef]
- Horikoshi, R.; Goto, K.; Mitomi, M.; Oyama, K.; Hirose, T.; Sunazuka, T.; Ōmura, S. Afidopyropen, a Novel Insecticide Originating from Microbial Secondary Extracts. Sci. Rep. 2022, 12, 2827. [Google Scholar] [CrossRef]
- Liu, F.; Wang, N.; Wang, Y.; Yu, Z. The Insecticidal Activity of Secondary Metabolites Produced by Streptomyces Sp. SA61 Against Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Microorganisms 2024, 12, 2031. [Google Scholar] [CrossRef]
- Horikoshi, R.; Goto, K.; Mitomi, M.; Oyama, K.; Sunazuka, T.; Ōmura, S. Insecticidal Properties of Pyripyropene A, a Microbial Secondary Metabolite, Against Agricultural Pests. J. Pestic. Sci. 2018, 43, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ruan, L.; Peng, D.; Li, L.; Sun, M.; Yu, Z. Thuringiensin: A Thermostable Secondary Metabolite from Bacillus thuringiensis with Insecticidal Activity Against a Wide Range of Insects. Toxins 2014, 6, 2229–2238. [Google Scholar] [CrossRef]
- Schrank, A.; Vainstein, M.H. Metarhizium anisopliae Enzymes and Toxins. Toxicon 2010, 56, 1267–1274. [Google Scholar] [CrossRef]
- Krasnoff, S.B.; Gupta, S. Identification and Directed Biosynthesis of Efrapeptins in the Fungus Tolypocladium geodes Gams (Deuteromycotina: Hyphomycetes). J. Chem. Ecol. 1991, 17, 1953–1962. [Google Scholar] [CrossRef]
- Olombrada, M.; Martínez-del-Pozo, A.; Medina, P.; Budia, F.; Gavilanes, J.G.; García-Ortega, L. Fungal Ribotoxins: Natural Protein-Based Weapons Against Insects. Toxicon 2014, 83, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Binnington, K.C.; Baule, V.J. Naturally Occurring Insecticidal Molecules as Candidates for Genetic Engineering. In Molecular Approaches to Fundamental and Applied Entomology; Oakeshott, J., Whitten, M.J., Eds.; Springer: New York, NY, USA, 1993; pp. 38–89. [Google Scholar]
- Islam, S.M.N.; Chowdhury, M.Z.H.; Mim, M.F.; Momtaz, M.B.; Islam, T. Biocontrol Potential of Native Isolates of Beauveria bassiana Against Cotton Leafworm Spodoptera Litura (Fabricius). Sci. Rep. 2023, 13, 8331. [Google Scholar] [CrossRef] [PubMed]
- Stilwell, M.D.; Cao, M.; Goodrich-Blair, H.; Weibel, D.B. Studying the Symbiotic Bacterium Xenorhabdus Nematophila in Individual, Living Steinernema Carpocapsae Nematodes Using Microfluidic Systems. mSphere 2018, 3, e00530-17. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, D.; Prabhu, S.; Rani, W.B.; Anandham, R. Isolation and Characterization of Toxins from Xenorhabdus nematophilus Against Ferrisia Virgata (Ckll.) on Tuberose, Polianthes Tuberosa. Toxicon 2018, 146, 42–49. [Google Scholar] [CrossRef]
- Wright, D.; Zydenbos, S.; Wessman, P.; O’Callaghan, M.; Townsend, R.; Jackson, T.; van Koten, C.; Mansfield, S. Surface Coating aids Survival of Serratia entomophila (Enterobacteriaceae) in Granules for Surface Application. Biocontrol. Sci. Technol. 2017, 27, 1–17. [Google Scholar] [CrossRef]
- Koivunen, M.; Chanbusarakum, L.; Fernández, L.; Asolkar, R.; Tan, E.; Wallner, D.; Marrone, P. Development of a New Microbial Insecticide Based on Chromobacterium Subtsugae; Crickmore, N., Enkerli, J., Glazer, I., Lopez-Ferber, M., Tkaczukeditors, C., Ehlers, R.U., Eds.; International Organization for Biological and Integrated Control of Noxious Animals and Plants (OIBC/OILB), West Palaearctic Regional Section (WPRS/SROP): Dijon, France, 2009; pp. 183–186. [Google Scholar]
- Tu, Y. The Discovery and Development of Artemisinins and Antimalarial agentsindex. In From Artemisia Annua L. to Artemisinins; Tu, Y., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 413–426. [Google Scholar]
- Chandrasekaran, M.; Paramasivan, M.; Sahayarayan, J.J. Microbial Volatile Organic Compounds: An Alternative for Chemical Fertilizers in Sustainable Agriculture Development. Microorganisms 2023, 11, 42. [Google Scholar] [CrossRef]
- Thomas, G.; Withall, D.; Birkett, M. Harnessing Microbial Volatiles to Replace Pesticides and Fertilizers. Microb. Biotechnol. 2020, 13, 1366–1376. [Google Scholar] [CrossRef]
- Kanchiswamy, C.N.; Malnoy, M.; Maffei, M.E. Bioprospecting Bacterial and Fungal Volatiles for Sustainable Agriculture. Trends Plant Sci. 2015, 20, 206–211. [Google Scholar] [CrossRef]
- Der, C.; Courty, P.E.; Recorbet, G.; Wipf, D.; Simon-Plas, F.; Gerbeau-Pissot, P. Sterols, Pleiotropic Players in Plant-Microbe Interactions. Trends Plant Sci. 2024, 29, 524–534. [Google Scholar] [CrossRef]
- Risdian, C.; Mozef, T.; Wink, J. Biosynthesis of Polyketides in Streptomyces. Microorganisms 2019, 7, 124. [Google Scholar] [CrossRef]
- Yoon, Y.J.; Kim, E.S.; Hwang, Y.S.; Choi, C.Y. Avermectin: Biochemical and Molecular Basis of its Biosynthesis and Regulation. Appl. Microbiol. Biotechnol. 2004, 63, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Jansson, R.K.; Dybas, R.A. Avermectins: Biochemical Mode of Action, Biological Activity and Agricultural Importance. In Insecticides with Novel Modes of Action: Mechanisms and Application; Ishaaya, I., Degheele, D., Eds.; Springer: Berlin, Heidelberg, 1998; pp. 152–170. [Google Scholar]
- Li, S.; Yang, B.; Tan, G.-Y.; Ouyang, L.-M.; Qiu, S.; Wang, W.; Xiang, W.; Zhang, L. Polyketide Pesticides from Actinomycetes. Curr. Opin. Biotechnol. 2021, 69, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Kankariya, R.A.; Chaudhari, A.B.; Gavit, P.M.; Dandi, N.D. 2,4-Diacetylphloroglucinol: A Novel Biotech Bioactive Compound for Agriculture. In Microbial Interventions in Agriculture and Environment: Volume 1: Research Trends, Priorities and Prospects; Singh, D.P., Gupta, V.K., Prabha, R., Eds.; Springer: Singapore, 2019; pp. 419–452. [Google Scholar]
- Almario, J.; Bruto, M.; Vacheron, J.; Prigent-Combaret, C.; Moënne-Loccoz, Y.; Muller, D. Distribution of 2,4-Diacetylphloroglucinol Biosynthetic Genes among the Pseudomonas spp. Reveals Unexpected Polyphyletism. Front. Microbiol. 2017, 8, 1218. [Google Scholar] [CrossRef]
- Cesco, S.; Mimmo, T.; Tonon, G.; Tomasi, N.; Pinton, R.; Terzano, R.; Neumann, G.; Weisskopf, L.; Renella, G.; Landi, L.; et al. Plant-Borne Flavonoids Released into the Rhizosphere: Impact on Soil Bio-Activities Related to Plant Nutrition. A Review. Biol. Fertil. Soils 2012, 48, 123–149. [Google Scholar] [CrossRef]
- Gören Sağlam, N.; Albayrak, F.Ö.; Ortaş, İ. Endophytic Fungal Alkaloids: Production and Applications. In Fungal Endophytes Volume I: Biodiversity and Bioactive Materials; Springer: Berlin/Heidelberg, Germany, 2025; pp. 341–363. [Google Scholar]
- Bacetty, A.A.; Snook, M.E.; Glenn, A.E.; Noe, J.P.; Hill, N.; Culbreath, A.; Timper, P.; Nagabhyru, P.; Bacon, C.W. Toxicity of Endophyte-Infected Tall Fescue Alkaloids and Grass Metabolites on Pratylenchus scribneri. Phytopathology 2009, 99, 1336–1345. [Google Scholar] [CrossRef]
- Leadmon, C.E.; Sampson, J.K.; Maust, M.D.; Macias, A.M.; Rehner, S.A.; Kasson, M.T.; Panaccione, D.G. Several Metarhizium Species Produce Ergot Alkaloids in a Condition-Specific Manner. Appl. Environ. Microbiol. 2020, 86, e00373-20. [Google Scholar] [CrossRef]
- Shi, H.; Jiang, J.; Zhang, H.; Jiang, H.; Su, Z.; Liu, D.; Jie, L.; He, F. Antibacterial Spirooxindole Alkaloids from Penicillium brefeldianum Inhibit Dimorphism of Pathogenic Smut Fungi. Front. Microbiol. 2022, 13, 1046099. [Google Scholar] [CrossRef]
- Tang, Z.; Cao, X.; Zhang, H. Production of iturin A by Bacillus velezensis ND and Its Biological Control Characteristics. J. Basic Microbiol. 2023, 63, 179–189. [Google Scholar] [CrossRef]
- Benfield, A.H.; Henriques, S.T. Mode-of-Action of Antimicrobial Peptides: Membrane Disruption Vs. Intracellular Mechanisms. Front. Med. Technol. 2020, 2, 610997. [Google Scholar] [CrossRef]
- Sarkar, T.; Chetia, M.; Chatterjee, S. Antimicrobial Peptides and Proteins: From Nature’s Reservoir to the Laboratory and Beyond. Front. Chem. 2021, 9, 691532. [Google Scholar] [CrossRef]
- Niu, X.; Thaochan, N.; Hu, Q. Diversity of Linear Non-Ribosomal Peptide in Biocontrol Fungi. J. Fungi 2020, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, D.; Finking, R.; Marahiel, M.A. Nonribosomal Peptides: From Genes to Products. Nat. Prod. Rep. 2003, 20, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Rajput, V.D.; Prazdnova, E.V.; Gurnani, M.; Bhardwaj, P.; Sharma, S.; Sushkova, S.; Mandzhieva, S.S.; Minkina, T.; Sudan, J.; et al. Nature’s Antimicrobial Arsenal: Non-Ribosomal Peptides from Pgpb for Plant Pathogen Biocontrol. Fermentation 2023, 9, 597. [Google Scholar] [CrossRef]
- Keswani, C.; Singh, H.B.; García-Estrada, C.; Caradus, J.; He, Y.W.; Mezaache-Aichour, S.; Glare, T.R.; Borriss, R.; Sansinenea, E. Antimicrobial Secondary Metabolites from Agriculturally Important Bacteria as Next-Generation Pesticides. Appl. Microbiol. Biotechnol. 2020, 104, 1013–1034. [Google Scholar] [CrossRef]
- Hussain, M.S.; Fareed, S.; Ansari, S.; Rahman, M.A.; Ahmad, I.Z.; Saeed, M. Current Approaches Toward Production of Secondary Plant Metabolites. J. Pharm. Bioallied. Sci. 2012, 4, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Geat, N.; Jadon, K.S.; Verma, A.; Sharma, R.; Rajput, L.S.; Mahla, H.R.; Kakani, R.K. Isolation and Characterization of Biocontrol Microbes for Development of Effective Microbial Consortia for Managing Rhizoctonia Bataticola Root Rot of Cluster Bean Under Hot Arid Climatic Conditions. Microorganisms 2024, 12, 2331. [Google Scholar] [CrossRef]
- Thangaraj, P.; Balamurali, A.S.; Muthusamy, N. Biological Control of Trichoderma spp.: Mechanisms of Action Against Phytopathogens, Insect Pests, and Its Multifaceted Roles in Agro-Ecosystems. Environ. Conserv. J. 2025, 26, 302–314. [Google Scholar] [CrossRef]
- Suganthi, M.; Arvinth, S.; Senthilkumar, P. Comparative Bioefficacy of Bacillus and Pseudomonas Chitinase Against Helopeltis Theivora in Tea (Camellia sinensis (L.) O.Kuntze. Physiol. Mol. Biol. Plants 2020, 26, 2053–2060. [Google Scholar] [CrossRef]
- Attia, Z.; Dalal, A.; Moshelion, M. Vascular Bundle Sheath and Mesophyll Cells Modulate Leaf Water Balance in Response to Chitin. Plant J. Cell Mol. Biol. 2020, 101, 1368–1377. [Google Scholar] [CrossRef]
- Shaana, O.M.; Nisha, M.S. Harnessing Bacteria for Sustainable Pest Management: A Biological Approach. Arch. Curr. Res. Int. 2025, 25, 262–274. [Google Scholar] [CrossRef]
- Bangaru, N. Bacillus thuringiensis Cry and Cyt Toxins: Mechanisms of Action, Resistance Management and Impact on Host Immune Responses. Res. J. Chem. Environ. 2025, 29, 101–110. [Google Scholar] [CrossRef]
- Shah, A.Z.; Ma, C.; Zhang, Y.; Zhang, Q.; Xu, G.; Yang, G. Decoyinine Induced Resistance in Rice against Small Brown Planthopper Laodelphax Striatellus. Insects 2022, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- McMillan, H.M. Plants Target Gut Microbes to Reduce Insect Herbivore Damage. Proc. Natl. Acad. Sci. USA 2023, 120, e2308568120. [Google Scholar] [CrossRef]
- Dai, H.; Liu, B.; Yang, L.; Yao, Y.; Liu, M.; Xiao, W.; Li, S.; Ji, R.; Sun, Y. Investigating the Regulatory Mechanism of the Sesquiterpenol Nerolidol from a Plant on Juvenile Hormone-Related Genes in the Insect Spodoptera exigua. Int. J. Mol. Sci. 2023, 24, 13330. [Google Scholar] [CrossRef]
- Gao, Q.; Song, L.; Sun, J.; Cao, H.Q.; Wang, L.; Lin, H.; Tang, F. Repellent Action and Contact Toxicity Mechanisms of the Essential oil Extracted from Chinese Chive Against Plutella Xylostella Larvae. Arch. Insect Biochem. Physiol. 2019, 100, e21509. [Google Scholar] [CrossRef]
- Kandasamy, D.; Gershenzon, J.; Andersson, M.N.; Hammerbacher, A. Volatile Organic Compounds Influence the Interaction of the Eurasian Spruce Bark Beetle (Ips typographus) with Its Fungal Symbionts. ISME J. 2019, 13, 1788–1800. [Google Scholar] [CrossRef]
- Mi, T.; Sheng, C.; Lee, C.K.; Nguyen, P.; Zhang, Y.V. Harnessing Insect Chemosensory and Mechanosensory Receptors Involved in Feeding for Precision Pest Management. Life 2025, 15, 110. [Google Scholar] [CrossRef]
- Durak, R.; Jedryczka, M.; Czajka, B.; Dampc, J.; Wielgusz, K.; Borowiak-Sobkowiak, B. Mild Abiotic Stress Affects Development and Stimulates Hormesis of Hemp Aphid Phorodon cannabis. Insects 2021, 12, 420. [Google Scholar] [CrossRef] [PubMed]
- Tyler, B.M. The Fog of War: How Network Buffering Protects Plants’ Defense Secrets from Pathogens. PLOS Genet. 2017, 13, e1006713. [Google Scholar] [CrossRef]
- Romera, F.J.; García, M.J.; Lucena, C.; Martínez-Medina, A.; Aparicio, M.A.; Ramos, J.; Alcántara, E.; Angulo, M.; Pérez-Vicente, R. Induced Systemic Resistance (Isr) and Fe Deficiency Responses in Dicot Plants. Front. Plant Sci. 2019, 10, 287. [Google Scholar] [CrossRef]
- Neha, T.; Kumar, A.; Sayrav, K.; Prasad, B. Isolation and Screening of Potent Antibiotic-Producing Actinomycete. J. Adv. Sci. Res. 2024, 15, 22–26. [Google Scholar] [CrossRef]
- Sukar, N.A.; Elazab, N.T. Molecular Characterization and Fungicidal Activity of Some Isolated Endophytic Fungi from Some Wild Plants in Egypt. J. Plant Prot. Pathol. 2020, 11, 627–634. [Google Scholar] [CrossRef]
- Sulaiman, M.; Nissapatorn, V.; Rahmatullah, M.; Paul, A.K.; Rajagopal, M.; Rusdi, N.A.; Seelan, J.S.S.; Suleiman, M.; Zakaria, Z.A.; Wiart, C. Antimicrobial Secondary Metabolites from the Mangrove Plants of Asia and the Pacific. Mar. Drugs 2022, 20, 643. [Google Scholar] [CrossRef]
- Agarwal, S.; Sharma, G.; Mathur, V. Plant Endophytes: A Treasure House of Antimicrobial Compounds. In Medicinal Plants and Antimicrobial Therapies; Kumar, V., Shriram, V., Dey, A., Eds.; Springer Nature: Singapore, 2024; pp. 107–123. [Google Scholar]
- Intra, B.; Mungsuntisuk, I.; Nihira, T.; Igarashi, Y.; Panbangred, W. Identification of Actinomycetes from Plant Rhizospheric Soils with Inhibitory Activity Against Colletotrichum spp., the Causative Agent of Anthracnose Disease. BMC Res. Notes 2011, 4, 98. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural Products in Crop Protection. Bioorganic Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef]
- Saxena, S.; Pandey, A.K. Microbial Metabolites as Eco-Friendly Agrochemicals for the Next Millennium. Appl. Microbiol. Biotechnol. 2001, 55, 395–403. [Google Scholar] [CrossRef]
- Ruiu, L. Insect Pathogenic Bacteria in Integrated Pest Management. Insects 2015, 6, 352–367. [Google Scholar] [CrossRef]
- Valan Arasu, M.; Esmail, G.A.; Al-Dhabi, N.A. Hypersaline Actinomycetes and Their Biological Applications. In Actinobacteria-Basics and Biotechnological Applications; Dhanasekaran, D., Jiang, Y., Eds.; IntechOpen: Rijeka, Croatia, 2016. [Google Scholar]
- Zaid, R.; Koren, R.; Kligun, E.; Gupta, R.; Leibman-Markus, M.; Mukherjee, P.K.; Kenerley, C.M.; Bar, M.; Horwitz, B.A. Gliotoxin, an Immunosuppressive Fungal Metabolite, Primes Plant Immunity: Evidence from Trichoderma virens-Tomato Interaction. mBio 2022, 13, e0038922. [Google Scholar] [CrossRef] [PubMed]
- Chengzeng, Z.; Yichao, G.; Bin, W. Harzianolides H-J: Three New Butenolides Isolated from the Fungus Trichoderma harzianum. Nat. Prod. J. 2025, 15, 1–5. [Google Scholar] [CrossRef]
- Montesinos, L.; Bundó, M.; Izquierdo, E.; Campo, S.; Badosa, E.; Rossignol, M.; Montesinos, E.; San Segundo, B.; Coca, M. Production of Biologically Active Cecropin a Peptide in Rice Seed Oil Bodies. PLoS ONE 2016, 11, e0146919. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Liang, J.; Wu, L.; Gao, W.; Jiang, J. Iturin a Extracted from Bacillus subtilis Wl-2 Affects Phytophthora Infestans via Cell Structure Disruption, Oxidative Stress, and Energy Supply Dysfunction. Front. Microbiol. 2020, 11, 536083. [Google Scholar] [CrossRef] [PubMed]
- Gimenez, D.; Phelan, A.; Murphy, C.D.; Cobb, S.L. Fengycin a Analogues with Enhanced Chemical Stability and Antifungal Properties. Org. Lett. 2021, 23, 4672–4676. [Google Scholar] [CrossRef]
- Liu, L.; Jin, X.; Lu, X.; Guo, L.; Lu, P.; Yu, H.; Lv, B. Mechanisms of Surfactin from Bacillus subtilis Sf1 against Fusarium foetens: A Novel Pathogen Inducing Potato Wilt. J. Fungi 2023, 9, 367. [Google Scholar] [CrossRef]
- Clough, S.E.; Jousset, A.; Elphinstone, J.G.; Friman, V.P. Combining in Vitro and in Vivo Screening to Identify Efficient Pseudomonas Biocontrol Strains Against the Phytopathogenic Bacterium Ralstonia solanacearum. Microbiologyopen 2022, 11, e1283. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dou, G.; Gao, M.; Ren, F.; Li, R.; Zhang, X.; Yan, D.-H. Annulohypoxylon sp. Fpyf3050 Produces Volatile Organic Compounds against the Pine Wood Nematode, Bursaphelenchus xylophilus. Nematology 2020, 22, 245–255. [Google Scholar] [CrossRef]
- Chen, D.M.; Yang, H.J.; Huang, J.G.; Yuan, L. Lysobacter enzymogenes Le16 Autolysates Have Potential as Biocontrol Agents-Lysobacter sp. Autolysates as Biofungicide. J. Appl. Microbiol. 2020, 129, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Cui, R.; Wang, D.; Yi, Y.; Feng, D. Antagonistic Effects and Action Mechanism of Bacillus velezensis HX-6 Against Ralstonia solanacearum. Chin. J. Pestic. Sci. 2025, 27, 322–331. [Google Scholar]
- Huang, K.; Zhang, B.; Shen, Z.-Y.; Cai, X.; Liu, Z.-Q.; Zheng, Y.-G. Enhanced Amphotericin B Production by Genetically Engineered Streptomyces nodosus. Microbiol. Res. 2021, 242, 126623. [Google Scholar] [CrossRef]
- Gu, Q.; Yang, Y.; Yuan, Q.; Shi, G.; Wu, L.; Lou, Z.; Huo, R.; Wu, H.; Borriss, R.; Gao, X. Bacillomycin D Produced by Bacillus amyloliquefaciens Is Involved in the Antagonistic Interaction with the Plant-Pathogenic Fungus Fusarium graminearum. Appl. Environ. Microbiol. 2017, 83, e01075-17. [Google Scholar] [CrossRef]
- Lin, X.; Kück, U. Cephalosporins as Key Lead Generation Beta-Lactam Antibiotics. Appl. Microbiol. Biotechnol. 2022, 106, 8007–8020. [Google Scholar] [CrossRef]
- Léger, G.; Novinscak, A.; Biessy, A.; Lamarre, S.; Filion, M. In Tuber Biocontrol of Potato Late Blight by a Collection of Phenazine-1-Carboxylic Acid-Producing Pseudomonas spp. Microorganisms 2021, 9, 2525. Microorganisms 2021, 9, 2525. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Chen, L.; Wang, X.W.; Zhang, T.; Zhao, P.B.; Song, X.Y.; Sun, C.Y.; Chen, X.L.; Zhou, B.C.; Zhang, Y.Z. Antimicrobial Peptaibols from Trichoderma pseudokoningii Induce Programmed Cell Death in Plant Fungal Pathogens. Microbiology 2012, 158, 166–175. [Google Scholar] [CrossRef]
- Durán, N.; Castro, G.R.; Portela, R.W.; Fávaro, W.J.; Durán, M.; Tasic, L.; Nakazato, G. Violacein and its Antifungal Activity: Comments and Potentialities. Lett. Appl. Microbiol. 2022, 75, 796–803. [Google Scholar] [CrossRef]
- Timmermann, T.; Poupin, M.J.; Vega, A.; Urrutia, C.; Ruz, G.A.; González, B. Gene Networks Underlying the Early Regulation of Paraburkholderia phytofirmans PsJN Induced Systemic Resistance in Arabidopsis. PLoS ONE 2019, 14, e0221358. [Google Scholar] [CrossRef]
- Nie, P.; Li, X.; Wang, S.; Guo, J.; Zhao, H.; Niu, D. Induced Systemic Resistance against Botrytis cinerea by Bacillus cereus Ar156 through a Ja/Et- and Npr1-Dependent Signaling Pathway and Activates Pamp-Triggered Immunity in Arabidopsis. Front. Plant Sci. 2017, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Panicker, S.; Sayyed, R. Hydrolytic Enzymes from Pgpr Against Plant Fungal Pathogens. In Antifungal Metabolites of Rhizobacteria for Sustainable Agriculture; Springer International Publishing: Cham, Switzerland, 2022; pp. 211–238. [Google Scholar]
- Agarwal, M.; Dheeman, S.; Dubey, R.C.; Kumar, P.; Maheshwari, D.K.; Bajpai, V.K. Differential Antagonistic Responses of Bacillus pumilus MSUA3 against Rhizoctonia solani and Fusarium oxysporum Causing Fungal Diseases in Fagopyrum esculentum Moench. Microbiol. Res. 2017, 205, 40–47. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Jung, H.; Koo, B.-K.; Han, J.A.; Lee, H.-S. Exploiting Bacterial Genera as Biocontrol Agents: Mechanisms, Interactions and Applications in Sustainable Agriculture. J. Plant Biol. 2023, 66, 485–498. [Google Scholar] [CrossRef]
- Ge, W.; Zhang, L.; Meng, F.; Tian, C. Study on Biocontrol Potential of Volatile Organic Compounds Produced by Pseudomonas atacamensis GZ-3 on Poplar Anthracnose. Ind. Crops Prod. 2025, 224, 120402. [Google Scholar] [CrossRef]
- Ghosh, P.; De, T.; Maiti, T. Role of Acc Deaminase as a Stress Ameliorating Enzyme of Plant Growth-Promoting Rhizobacteria Useful in Stress Agriculture: A Review. In Role of Rhizospheric Microbes in Soil: Volume 1: Stress Management and Agricultural Sustainability; Springer: Berlin/Heidelberg, Germany, 2018; pp. 57–106. [Google Scholar]
- Ehinmitan, E.; Losenge, T.; Mamati, E.; Ngumi, V.; Juma, P.; Siamalube, B. Biosolutions for Green Agriculture: Unveiling the Diverse Roles of Plant Growth-Promoting Rhizobacteria. Int. J. Microbiol. 2024, 2024, 6181491. [Google Scholar] [CrossRef]
- Toyota, K.; Watanabe, T. Recent Trends in Microbial Inoculants in Agriculture. Microbes Environ. JSME 2013, 28, 403–404. [Google Scholar] [CrossRef]
- Lorrai, R.; Ferrari, S. Host Cell Wall Damage During Pathogen Infection: Mechanisms of Perception and Role in Plant-Pathogen Interactions. Plants 2021, 10, 399. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zeng, Y.; Yan, X.; Wang, Z.; Guo, L.; Zhu, Y.; Wang, Y.; He, X. Volatile Organic Compounds of Bacillus velezensis Gj-7 against Meloidogyne hapla through Multiple Prevention and Control Modes. Molecules 2023, 28, 3182. [Google Scholar] [CrossRef] [PubMed]
- Settu, V.; Annaiyan, S.; Manu, J. Revealing the Genetic Arsenal of Bacillus firmus TNAU1: Unleashing Nematicidal and Plant Growth Promotion Traits. Physiol. Mol. Plant Pathol. 2023, 129, 102177. [Google Scholar] [CrossRef]
- Moreira, A.C.S.; Lopes, E.A.; Visôtto, L.E.; Soares, M.S.; Londe, M.L.A.; Ribeiro, L.B.; Terra, W.C.; Moreira, S.I.; Pedroso, M.P.; Pereira, L.F.; et al. Bacillus amyloliquefaciens Strain Banct02: An Antagonist with Multiple Mechanisms of Action Against Meloidogyne incognita. Plant Pathol. 2025, 74, 320–329. [Google Scholar] [CrossRef]
- Hu, L. Integration of Multiple Volatile Cues into Plant Defense Responses. New Phytol. 2022, 233, 618–623. [Google Scholar] [CrossRef]
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological Control of Plant-Parasitic Nematodes by Filamentous Fungi Inducers of Resistance: Trichoderma, Mycorrhizal and Endophytic Fungi. Front. Microbiol. 2020, 11, 992. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Kalia, N.; Bhardwaj, R.; Sidhu, G.P.S.; Sharma, A. Mechanisms of Plant Defense Under Pathogen Stress: A Review. Curr. Protein. Pept. Sci. 2021, 22, 376–395. [Google Scholar] [CrossRef]
- Fallahzadeh-Mamaghani, V.; Shahbazi-Ezmareh, R.; Shirzad, A.; Moslehi, S. Possible Mechanisms of Action of Bacillus wiedmannii AzBw1, a Biocontrol Agent of the Root-Knot Nematode, Meloidogyne arenaria. Egypt. J. Biol. Pest Control 2023, 33, 28. [Google Scholar] [CrossRef]
- Mani, J.; Kandasamy, D.; Vendan, R.T.; Sankarasubramanian, H.; Mannu, J.; Nagachandrabose, S. Unlocking the Potential of Streptomyces species as Promising Biological Control Agents Against Phytonematodes. Physiol. Mol. Plant Pathol. 2024, 134, 102465. [Google Scholar] [CrossRef]
- Baazeem, A.; Almanea, A.; Manikandan, P.; Alorabi, M.; Vijayaraghavan, P.; Abdel-Hadi, A. In Vitro Antibacterial, Antifungal, Nematocidal and Growth Promoting Activities of Trichoderma hamatum Fb10 and Its Secondary Metabolites. J. Fungi 2021, 7, 331. [Google Scholar] [CrossRef]
- Degenkolb, T.; Vilcinskas, A. Metabolites from Nematophagous Fungi and Nematicidal Natural Products from Fungi as an Alternative for Biological Control. Part I: Metabolites from Nematophagous Ascomycetes. Appl. Microbiol. Biotechnol. 2016, 100, 3799–3812. [Google Scholar] [CrossRef]
- Li, G.-H.; Zhang, K.-Q. Natural Nematicidal Metabolites and Advances in Their Biocontrol Capacity on Plant Parasitic Nematodes. Nat. Prod. Rep. 2023, 40, 646–675. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Ali, Q.; Farzand, A.; Khan, A.R.; Ling, H.; Gao, X. Nematicidal Volatiles from Bacillus atrophaeus Gbsc56 Promote Growth and Stimulate Induced Systemic Resistance in Tomato against Meloidogyne incognita. Int. J. Mol. Sci. 2021, 22, 5049. [Google Scholar] [CrossRef]
- Yin, N.; Liu, R.; Zhao, J.L.; Khan, R.A.A.; Li, Y.; Ling, J.; Liu, W.; Yang, Y.H.; Xie, B.Y.; Mao, Z.C. Volatile Organic Compounds of Bacillus cereus Strain Bc-Cm103 Exhibit Fumigation Activity against Meloidogyne incognita. Plant Dis. 2021, 105, 904–911. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, C.; Ma, L.; Zhang, K.; Duan, C.; Mo, M. Characterisation of Volatiles Produced from Bacillus megaterium YFM3.25 and Their Nematicidal Activity Against Meloidogyne incognita. Eur. J. Plant Pathol. 2009, 126, 417–422. [Google Scholar] [CrossRef]
- Wolfgang, A.; Taffner, J.; Guimarães, R.A.; Coyne, D.; Berg, G. Novel Strategies for Soil-Borne Diseases: Exploiting the Microbiome and Volatile-Based Mechanisms Toward Controlling Meloidogyne-Based Disease Complexes. Front. Microbiol. 2019, 10, 1296. [Google Scholar] [CrossRef]
- Khoja, S.; Eltayef, K.M.; Baxter, I.; Myrta, A.; Bull, J.C.; Butt, T. Volatiles of the Entomopathogenic Fungus, Metarhizium brunneum, Attract and Kill Plant Parasitic Nematodes. Biol. Control. 2021, 152, 104472. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; de Vries Reinder, H.; Chakraborty, P.; Song, C.; Zhao, X.; Scheffers, D.-J.; Roelfes, G.; Kuipers Oscar, P. Novel Modifications of Nonribosomal Peptides from Brevibacillus laterosporus Mg64 and Investigation of Their Mode of Action. Appl. Environ. Microbiol. 2020, 86, e01981-20. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, Z.; Lei, L.; Xia, Z.; Shao, L.; Zhang, K.; Li, G. Nematicidal Effect of Volatiles Produced by Trichoderma sp. J. Asia-Pac. Entomol. 2012, 15, 647–650. [Google Scholar] [CrossRef]
- Kundu, A.; Saha, S.; Walia, S.; Dutta, T.K. Anti-Nemic Secondary Metabolites Produced by Fusarium oxysporum f. sp. ciceris. J. Asia-Pac. Entomol. 2016, 19, 631–636. [Google Scholar] [CrossRef]
- Nagaraj, G.; Rengasamy, K.; Thiruvengadam, R.; Swarnakumari, N.; Saravanakumar, D. Nematicidal Action of Clonostachys rosea Against Meloidogyne incognita: In-Vitro and In-Silico Analyses. J. Taibah Univ. Sci. 2024, 18, 2288723. [Google Scholar] [CrossRef]
- Kang, M.-K.; Kim, J.-H.; Liu, M.-J.; Jin, C.-Z.; Park, D.-J.; Kim, J.; Sung, B.-H.; Kim, C.-J.; Son, K.-H. New Discovery on the Nematode Activity of Aureothin and Alloaureothin Isolated from Endophytic Bacteria Streptomyces sp. Ae170020. Sci. Rep. 2022, 12, 3947. [Google Scholar] [CrossRef]
- Meyer, S.L.; Halbrendt, J.M.; Carta, L.K.; Skantar, A.M.; Liu, T.; Abdelnabby, H.M.; Vinyard, B.T. Toxicity of 2,4-Diacetylphloroglucinol (Dapg) to Plant-Parasitic and Bacterial-Feeding Nematodes. J. Nematol. 2009, 41, 274–280. [Google Scholar] [PubMed]
- Siddiqui, I.; Shaukat, S. Suppression of Root-Knot Disease by Pseudomonas fluorescens Cha0 in Tomato: Importance of Bacterial Secondary Metabolite, 2,4-Diacetylpholoroglucinol. Soil Biol. Biochem. 2003, 35, 1615–1623. [Google Scholar] [CrossRef]
- Liarzi, O.; Bucki, P.; Braun Miyara, S.; Ezra, D. Bioactive Volatiles from an Endophytic Daldinia Cf. Concentrica Isolate Affect the Viability of the Plant Parasitic Nematode Meloidogyne javanica. PLoS ONE 2016, 11, e0168437. [Google Scholar] [CrossRef]
- Park, E.J.; Jang, H.J.; Park, J.Y.; Yang, Y.K.; Kim, M.J.; Park, C.S.; Lee, S.; Yun, B.S.; Lee, S.J.; Lee, S.W.; et al. Efficacy Evaluation of Streptomyces nigrescens Ka-1 Against the Root-Knot Nematode Meloidogyne incognita. Biol. Control 2023, 179, 105150. [Google Scholar] [CrossRef]
- Kusakabe, A.; Wang, C.; Xu, Y.M.; Molnár, I.; Stock, S.P. Selective Toxicity of Secondary Metabolites from the Entomopathogenic Bacterium Photorhabdus luminescens Sonorensis Against Selected Plant Parasitic Nematodes of the Tylenchina Suborder. Microbiol. Spectr. 2022, 10, e0257721. [Google Scholar] [CrossRef]
- Ran, Y.; Zhang, Y.; Wang, X.; Li, G. Nematicidal Metabolites from the Actinomycete Micromonospora sp. Wh06. Microorganisms 2022, 10, 2274. [Google Scholar] [CrossRef]
- Nakahara, S.; Kusano, M.; Fujioka, S.; Shimada, A.; Kimura, Y. Penipratynolene, a Novel Nematicide from Penicillium bilaiae Chalabuda. Biosci. Biotechnol. Biochem. 2004, 68, 257–259. [Google Scholar] [CrossRef]
- Park, J.O.; Hargreaves, J.R.; McConville, E.J.; Stirling, G.R.; Ghisalberti, E.L.; Sivasithamparam, K. Production of Leucinostatins and Nematicidal Activity of Australian Isolates of Paecilomyces lilacinus (Thom) Samson. Lett. Appl. Microbiol. 2004, 38, 271–276. [Google Scholar] [CrossRef]
- Bi, Y.; Gao, C.; Yu, Z. Rhabdopeptides from Xenorhabdus budapestensis Sn84 and Their Nematicidal Activities against Meloidogyne incognita. J. Agric. Food Chem. 2018, 66, 3833–3839. [Google Scholar] [CrossRef] [PubMed]
- Abebew, D.; Sayedain, F.S.; Bode, E.; Bode, H.B. Uncovering Nematicidal Natural Products from Xenorhabdus Bacteria. J. Agric. Food Chem. 2022, 70, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Anandakumar, S.; Rajadurai, G. Biological Control of Plant Parasitic-Nematodes by Plant Growth Promoting-Rhizobacteria. Plant Health Arch. 2023, 1, 5–7. [Google Scholar] [CrossRef]
- Aswathi, N.; Balakrishnan, N.; Srinivasan, T.; Kokiladevi, E.; Raghu, R. Diversity of Bt toxins and Their Utility in Pest Management. Egypt. J. Biol. Pest Control 2024, 34, 40. [Google Scholar] [CrossRef]
- Sparks, T.C.; Dripps, J.E.; Watson, G.B.; Paroonagian, D. Resistance and Cross-Resistance to the Spinosyns–A Review and Analysis. Pestic. Biochem. Physiol. 2012, 102, 1–10. [Google Scholar] [CrossRef]
- Martin, P.A.W.; Gundersen-Rindal, D.; Blackburn, M.; Buyer, J. Chromobacterium subtsugae sp. nov., a Betaproteobacterium Toxic to Colorado potato Beetle and Other Insect Pests. Int. J. Syst. Evol. Microbiol. 2007, 57, 993–999. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M.M. Xenorhabdus spp.: An Overview of the Useful Facets of Mutualistic Bacteria of Entomopathogenic Nematodes. Life 2022, 12, 1360. [Google Scholar] [CrossRef] [PubMed]
- Hariprasad, K.V. Recent Advancement in the Development of Biopesticides by Actinomycetes for the Control of Insect Pests. In Plant Growth Promoting Actinobacteria: A New Avenue for Enhancing the Productivity and Soil Fertility of Grain Legumes; Springer: Berlin/Heidelberg, Germany, 2016; pp. 47–62. [Google Scholar]
- He, H.; Ye, L.; Li, C.; Wang, H.; Guo, X.; Wang, X.; Zhang, Y.; Xiang, W. Sbbr/Sbba, an Important Arpa/Afsa-Like System, Regulates Milbemycin Production in Streptomyces bingchenggensis. Front. Microbiol. 2018, 9, 1064. [Google Scholar] [CrossRef]
- González-Jaramillo, L.M.; Aranda, F.J.; Teruel, J.A.; Villegas-Escobar, V.; Ortiz, A. Antimycotic Activity of Fengycin C Biosurfactant and its Interaction with Phosphatidylcholine Model Membranes. Colloids Surf. B Biointerfaces 2017, 156, 114–122. [Google Scholar] [CrossRef]
- Sharma, M.; Manhas, R.K. Purification and Characterization of Salvianolic acid B from Streptomyces sp. M4 Possessing Antifungal Activity Against Fungal Phytopathogens. Microbiol. Res. 2020, 237, 126478. [Google Scholar] [CrossRef]
- Shahid, M.; Singh, B.N.; Verma, S.; Choudhary, P.; Das, S.; Chakdar, H.; Murugan, K.; Goswami, S.K.; Saxena, A.K. Bioactive Antifungal Metabolites Produced by Streptomyces amritsarensis V31 Help to Control Diverse Phytopathogenic Fungi. Braz J. Microbiol. 2021, 52, 1687–1699. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ning, Q.; Yang, Y.; Liu, Y.; Niu, S.; Hu, X.; Pan, H.; Bu, Z.; Chen, N.; Guo, J.; et al. Endophytic Streptomyces hygroscopicus Osish-2-Mediated Balancing between Growth and Disease Resistance in Host Rice. mBio 2021, 12, e0156621. [Google Scholar] [CrossRef]
- Selim, M.S.M.; Abdelhamid, S.A.; Mohamed, S.S. Secondary Metabolites and Biodiversity of Actinomycetes. J. Genet. Eng. Biotechnol. 2021, 19, 72. [Google Scholar] [CrossRef]
- Silva, G.d.C.; Kitano, I.T.; Ribeiro, I.A.d.F.; Lacava, P.T. The Potential Use of Actinomycetes as Microbial Inoculants and Biopesticides in Agriculture. Front. Soil Sci. 2022, 2, 833181. [Google Scholar] [CrossRef]
- Rashad, F.M.; Fathy, H.M.; El-Zayat, A.S.; Elghonaimy, A.M. Isolation and Characterization of Multifunctional Streptomyces Species with Antimicrobial, Nematicidal and Phytohormone Activities from Marine Environments in Egypt. Microbiol. Res. 2015, 175, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Terra, W.C.; Campos, V.P.; Martins, S.J.; Costa, L.S.A.S.; da Silva, J.C.P.; Barros, A.F.; Lopez, L.E.; Santos, T.C.N.; Smant, G.; Oliveira, D.F. Volatile Organic Molecules from Fusarium oxysporum Strain 21 with Nematicidal Activity Against Meloidogyne incognita. Crop Prot. 2018, 106, 125–131. [Google Scholar] [CrossRef]
- Mishra, J.; Arora, N. Bioformulations for Plant Growth Promotion and Combating Phytopathogens: A Sustainable Approach. Bioformulations Sustain. Agric. 2016, 3–33. [Google Scholar]
- Bejarano, A.; Puopolo, G. Bioformulation of Microbial Biocontrol Agents for a Sustainable Agriculture. In How Research Can Stimulate the Development of Commercial Biological Control Against Plant Diseases; De Cal, A., Melgarejo, P., Magan, N., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 275–293. [Google Scholar]
- Khan, A.; Singh, A.V.; Gautam, S.S.; Agarwal, A.; Punetha, A.; Upadhayay, V.K.; Kukreti, B.; Bundela, V.; Jugran, A.K.; Goel, R. Microbial Bioformulation: A Microbial Assisted Biostimulating Fertilization Technique for Sustainable Agriculture. Front. Plant Sci. 2023, 14, 1270039. [Google Scholar] [CrossRef]
- Leggett, M.; Leland, J.; Kellar, K.; Epp, B. Formulation of Microbial Biocontrol agents–an Industrial Perspective. Can. J. Plant Pathol. 2011, 33, 101–107. [Google Scholar] [CrossRef]
- Temprano, F.J.; Albareda, M.; Camacho, M.; Daza, A.; Santamaría, C.; Rodríguez-Navarro, D.N. Survival of Several Rhizobium/Bradyrhizobium Strains on Different Inoculant Formulations and Inoculated Seeds. Int. Microbiol. 2002, 5, 81–86. [Google Scholar] [CrossRef]
- Fravel, D. ASDS Keynote Address Session 7. Hurdles and Bottlenecks on the Road to Biocontrol of Plant Pathogens. Australas. Plant Pathol. 1999, 28, 53–56. [Google Scholar] [CrossRef]
- Green, S.; Stewart-Wade, S.M.; Boland, G.J.; Teshler, M.P.; Liu, S.H. Formulating Microorganisms for Biological Control of Weeds. Plant-Microbe Interact. Biol. Control 1998, 249–280. [Google Scholar]
- Burges, H.D. Formulation of Mycoinsecticides. In Formulation of Microbial Biopesticides: Beneficial Microorganisms, Nematodes and Seed Treatments; Burges, H.D., Ed.; Springer: Dordrecht, The Netherlands, 1998; pp. 131–185. [Google Scholar]
- Barrios-Gonzalez, J.; Fernández, F.; Tomasini, A. Microbial Secondary Metabolites Production and Strain Improvement. Indian J. Biotechnol. 2003, 2, 322–333. [Google Scholar]
- Elander, R.; Lowe, D. Fungal Biotechnology: An Overview. Handb. Appl. Mycol. 1994, 4, 1–34. [Google Scholar]
- Ke, J.; Wang, B.; Yoshikuni, Y. Microbiome Engineering: Synthetic Biology of Plant-Associated Microbiomes in Sustainable Agriculture. Trends Biotechnol. 2021, 39, 244–261. [Google Scholar] [CrossRef]
- Haskett, T.L.; Tkacz, A.; Poole, P.S. Engineering Rhizobacteria for Sustainable Agriculture. Isme J. 2021, 15, 949–964. [Google Scholar] [CrossRef]
- Patel, J.K.; Archana, G. Engineered Production of 2,4-Diacetylphloroglucinol in the Diazotrophic Endophytic Bacterium Pseudomonas sp. Ws5 and Its Beneficial Effect in Multiple Plant-Pathogen Systems. Appl. Soil Ecol. 2018, 124, 34–44. [Google Scholar] [CrossRef]
- Xu, L.; Wang, L.C.; Su, B.M.; Xu, X.Q.; Lin, J. Multi-Enzyme Cascade for Improving β-Hydroxy-α-Amino Acids Production by Engineering L-Threonine Transaldolase and Combining Acetaldehyde Elimination System. Bioresour. Technol. 2020, 310, 123439. [Google Scholar] [CrossRef]
- Han, S.-W.; Yoshikuni, Y. Microbiome Engineering for Sustainable Agriculture: Using Synthetic Biology to Enhance Nitrogen Metabolism in Plant-Associated Microbes. Curr. Opin. Microbiol. 2022, 68, 102172. [Google Scholar] [CrossRef]


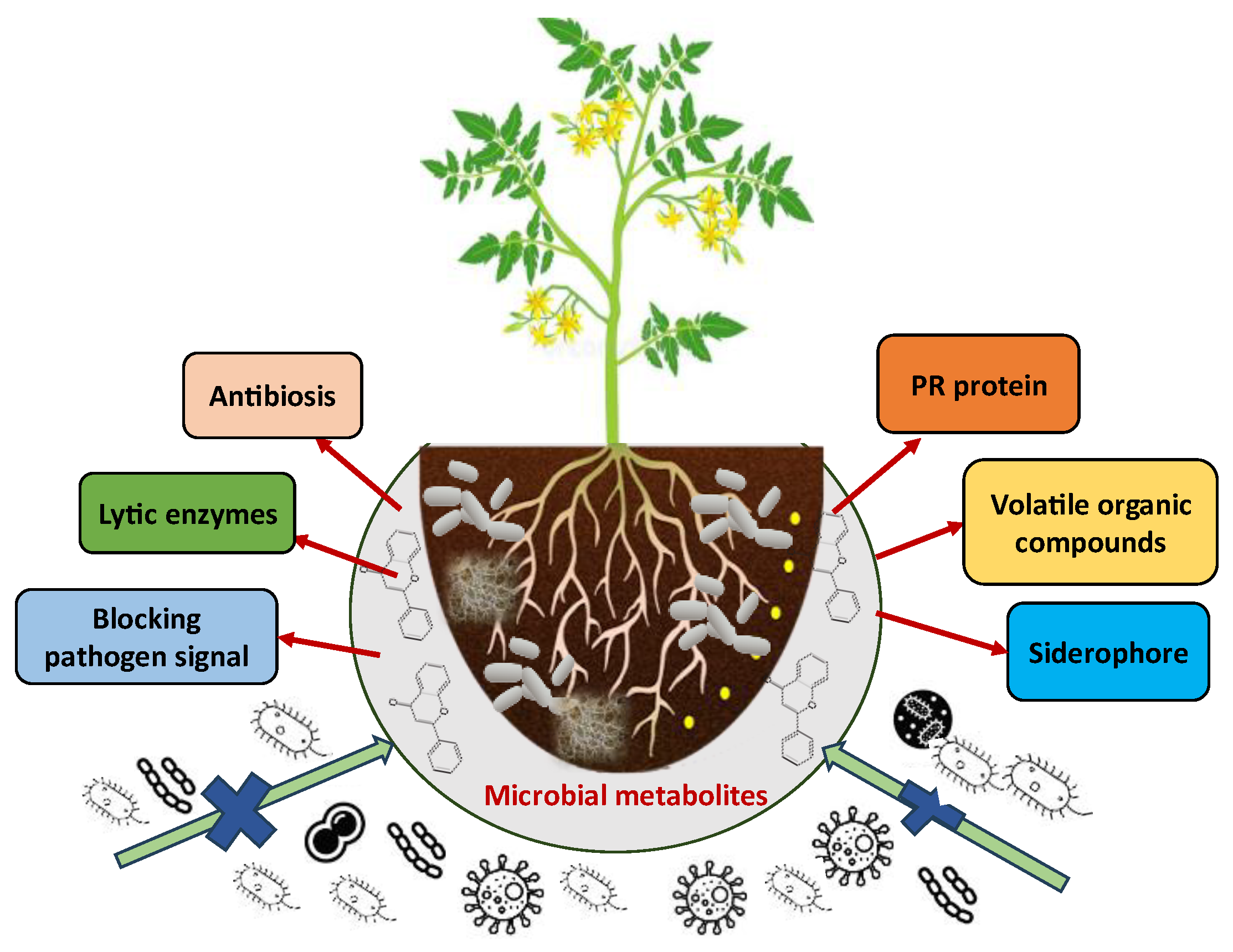


| Metabolites | Source Microorganisms | Mode of Action | Target Pest | References |
|---|---|---|---|---|
| Polyketide | ||||
| Dihydroxynaphthalene (DHN)-melanins | Cochliobolus heterostrophus, Alternaria, Colletotrichum, | Tolerant to UV-B and reactive oxygen species | Aphid, diamondback moth, beetle, and white fly | [81] |
| Rugulosin | Penicillium, Phialocephala scopiformis | Induction of programmed cell death | Budworm | [81] |
| Spinosyn | Saccharopolyspora spinosa | Damages nervous system, involuntary muscle contractions, tremors, and paralysis | Lepidoptera, Diptera, Thysanoptera | [82] |
| Avermectins | Streptomyces avermitilis | Disturbances in water balance, molting, metamorphosis, reproductive developments, dysfunction of nervous system | Broad-spectrum insecticide | [51,83] |
| Stempholone A, Stemphol | Stemphylium solani | Insect antifeedant | Spodoptera littoralis, Myzus persicae and Rhopalosiphum padi | [84] |
| Terpenoids | ||||
| 2,10-bisaboladien-1-ol | Alternaria, Dydimella, Penicillium, Fusarium | Insect antifeedant | Myzus persicae and S. littoralis. | [85] |
| Afidopyropen | Penicillium coprobium | Weakens the feeding inhibition | Sucking pests | [86] |
| Strekingmycin, phenalinolactone | Streptomyces sp. | Broad-spectrum Insecticide | Trialeurodes vaporariorum | [87] |
| Pyripyropene A | Aspergillus fumigatus | Weakens the feeding inhibition | Lepidopteran pests and aphids | [88] |
| Phenolics | ||||
| Stilbenes | Photorhabdus | Inhibits the growth of microbes on insect cadavers | Wax moth | [81] |
| Nucleoside analogs | ||||
| Thuringiensin | Bacillus thuringiensis (Bt) | Interferes with the RNA polymerase | Diptera, Lepidoptera, Coleoptera, Orthoptera, Hymenoptera, and Isoptera | [89] |
| Peptide-related compounds | ||||
| Fabclavines | Xenorhabdus sp. | Anti-symbiotic fungi and bacteria on the insect’s cuticle | Ant | [81] |
| Destruxins | Metarhizium anisopliae | Damages muscular and digestive system | Lepidopteran insects | [90] |
| Efrapeptins | Tolypocladium sp. | Inhibitors of intracellular protein transport | Insecticidal and miticidal effects | [91] |
| Hirsutellin | Hirsutella thompsonii | Inhibits protein synthesis | Aphids, mites, and fruit flies | [92] |
| Polyoxins and Nikkomycins | Streptomyces sp. | Inhibits chitin formation | Broad-spectrum insecticide | [93] |
| Others | ||||
| Crude extract | Beauveria bassiana | Insect antifeedant | S. litura | [94] |
| Crude extract | Xenorhabdus nematophila | Interferes with host AMPs, insecticidal toxins complex | Lepidoptera, Coleoptera, Diptera | [95,96] |
| Crude extract | Serratia entomophila | Colonization of foregut and cessation of feeding | New Zealand grass grub | [97] |
| Crude extract | Chromobacterium subtsugae | Insect antifeedant | Broad-spectrum insecticide | [98] |
| Metabolites | Source Microorganism | Target Pathogen | Mode of Action | References |
|---|---|---|---|---|
| Terpenoids | ||||
| Volatile–geosmin | Streptomyces spp. | Antibacterial, antifungal | May have allelopathic or inhibitory effects; soil microbes | [141] |
| Aminoglycosides | Streptomyces spp. | Broad spectrum | inhibit protein synthesis by binding to 30S ribosomal subunit | [142] |
| Viridin | Gliocladium virens | Antibacterial, antifungal | Antibacterial, antifungal; inhibits respiration | [143] |
| Trichodiene | Trichoderma and Fusarium spp. | Antibacterial, antifungal | Precursor of toxic trichothecenes; antifungal | [144] |
| Polyketides | ||||
| Koninginin A | Trichoderma koningii | Antibacterial, antifungal | Disrupts membrane integrity | [145] |
| Harzianum A | T. harzianum | Antibacterial, antifungal | Induces plant defense response | [8] |
| Lactone | T. harzianum | Antibacterial, antifungal | Disrupts membrane integrity, inhibits conidia germination | [146] |
| Lactone/butenolides | T. harzianum | Antifungal | Inhibits spore germination and hyphal growth | [8] |
| Lactone/butenolides | T. harzianum | Antifungal | Induces systemic resistance | [147] |
| Lactone/butenolides | T. harzianum | Antifungal | Affects cell wall synthesis | [148] |
| Macrolide | Streptomyces spp. | Broad-spectrum bacteria | Inhibits protein synthesis | [142] |
| Resistomycin | Streptomyces spp. | Broad spectrum | Inhibits cell proliferation | [141] |
| Nitrogen-containing compounds/Alkaloids | ||||
| Harzianopyridone | T.harzianum | Antifungal | Induces plant resistance | [8] |
| Gliotoxin | T. virens | Antifungal | Immunosuppressive; induces oxidative stress | [147] |
| Peptides | ||||
| Cecropin A | Hyalophora cecropia | F. oxysporum, Dickeya dadantii, F. verticillioides | Induces plant resistance | [149] |
| Iturin | B. subtilis | Broad-spectrum inhibitory effect | Interaction with cellular membranes, leading to disruption and subsequent cell death | [150] |
| Fengycin | B. subtilis | Fusarium, Alternaria and Botrytis | Disrupts the integrity of fungal cell membranes, leading to their lysis and subsequent death | [151] |
| Surfactin | B. amyloliquefaciens, B. subtilis, and B. pumilus | Fusarium, Lasiodiplodia, Colletotrichum, Botryosphaeria, Aspergillus, and Penicillium | Increases the permeability of cell membranes | [152] |
| Orfamide A | Pseudomonas strain | R. solanacearum | Direct contact inhibition | [153] |
| Brevibacillin | Brevibacillus laterosporus | X. campestris pv. campestris | Enhances plant resistance | [154] |
| Surfactin | Lysobacter enzymogenes | P. syringae pv. tabaci | Enhances plant resistance | [155] |
| Bacillomycin D (bmyA), fengycin (fenB) | B. velezensis | Ralstonia solanacearum | Antimicrobial compounds | [156] |
| Others | ||||
| Amphotericin B | Streptomyces nodosus | Exhibits antifungal activity | Combats fungal pathogen infections | [157] |
| Bacillomycin D | B. amyloliquefaciens | Antifungal and antibacterial property | Enhances the plant’s defense system | [158] |
| Cephalosporin | Acremonium chrysogenum | Broad spectrum | Inhibits bacterial cell wall synthesis | [159] |
| Phenazine-1- carboxylic acid (PCA) | Pseudomonas spp. | Phytophthora infestans | Enhances the plant’s defense system | [160] |
| Trichokonin VI | T. psedokoningii | Broad spectrum | Induces programmed cell death | [161] |
| Violocein | C. violaceum | Exhibits antifungal activity | Enhances the plant’s defense system | [162] |
| Tyrosol, phenethyl alcohol, 4-hydroxybenzaldehyde | Curvularia spp. | Colletotrichum fragariae | Enhances the plant’s defense system | [5] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prabhu, S.; Poorniammal, R.; Dufossé, L. Microbial Metabolites: A Sustainable Approach to Combat Plant Pests. Metabolites 2025, 15, 418. https://doi.org/10.3390/metabo15060418
Prabhu S, Poorniammal R, Dufossé L. Microbial Metabolites: A Sustainable Approach to Combat Plant Pests. Metabolites. 2025; 15(6):418. https://doi.org/10.3390/metabo15060418
Chicago/Turabian StylePrabhu, Somasundaram, Rajendran Poorniammal, and Laurent Dufossé. 2025. "Microbial Metabolites: A Sustainable Approach to Combat Plant Pests" Metabolites 15, no. 6: 418. https://doi.org/10.3390/metabo15060418
APA StylePrabhu, S., Poorniammal, R., & Dufossé, L. (2025). Microbial Metabolites: A Sustainable Approach to Combat Plant Pests. Metabolites, 15(6), 418. https://doi.org/10.3390/metabo15060418








