Gut Microbiota Dysbiosis and Its Impact on Type 2 Diabetes: From Pathogenesis to Therapeutic Strategies
Abstract
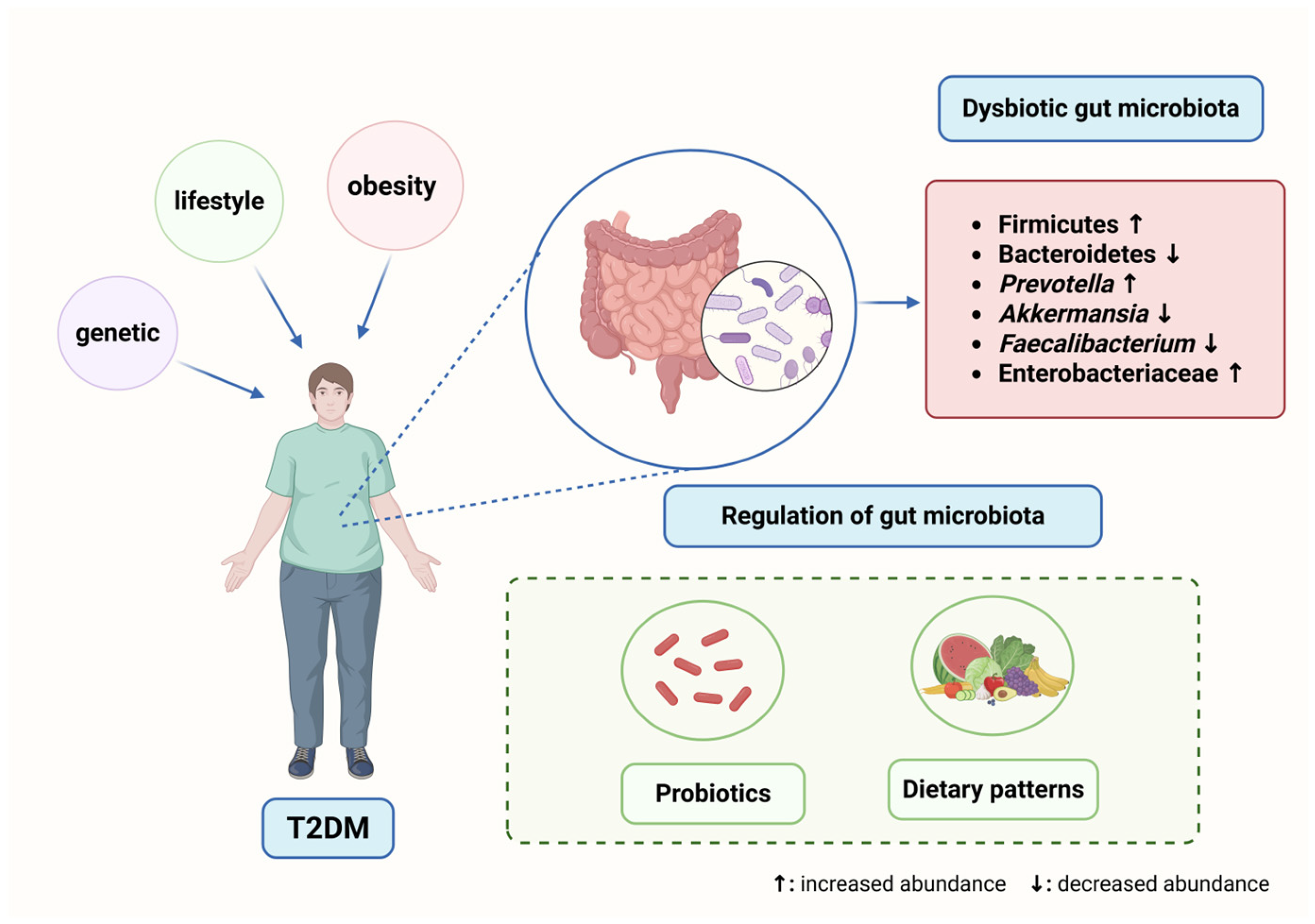
1. Introduction
2. Gut Microbiota in Healthy Individuals and T2DM
2.1. “Healthy” Gut Microbiota
2.2. Gut Microbiota Dysbiosis in T2DM
2.2.1. Compositional Shifts in the Gut Microbiota of T2DM Patients
2.2.2. Functional Shifts in the Gut Microbiota of T2DM Patients
| Reference | Year of Study | Population | Subjects Sample | Gut Microbiota | Abundance Compared to Control Subjects |
|---|---|---|---|---|---|
| Nadja Larsen et al. [19] | 2010 | Denmark | T2DM (n = 18), control group (n = 18) | Bacteroidetes, Proteobacteria, Bacilli, Lactobacillus, Prevotella spp. | increase |
| Firmicutes, Clostridia spp. | decrease | ||||
| Zhendong Mei et al. [35] | 2024 | America, Europe, Israel, and China | T2DM (n = 1851), prediabetes (n = 2770), normoglycemic (n = 2277) | Clostridium citroniae, Clostridium bolteae, Escherichia coli, Streptococcus parasanguinis, Streptococcus salivarius, Bacteroides fragilis, | increase |
| Coprococcus eutactus, Turicibacter sanguinis, Ruminococcus lactaris, Bacteroides plebeius, Butyrivibrio crossotus | decrease | ||||
| Xuangao Wu et al. [36] | 2022 | Asian (China, India, Japan, Thailand) | T2DM individuals (n = 551), healthy controls (n = 3378) | ET-L: Escherichia fergusonii, Collinsella aerofaciens, Streptococcus vestibularis, Bifidobacterium longum ET-P: Escherichia fergusonii, Megasphaera elsdenii, Oscillibacter valericigenes | increase |
| ET-L: Phocaeicola vulgatus, Bacteroides uniformis, Faecalibacterium prausnitzii ET-P: Bacteroides koreensis, Faecalibacterium prausnitzii | decrease | ||||
| Sunmin Park et al. [20] | 2023 | American | T2DM individuals (n = 1911), healthy controls (n = 872) | Enterocloster bolteae, Facalicatena fissicatena, Clostridium symbiosum, Faecalibacterium prausnitzii | increase |
| Bacteroides koreensis, Oscillibacter ruminantium, Bacteroides uniformis, Blautia wexlerae | decrease | ||||
| Matti O Ruuskanen et al. [10] | 2022 | Finnish | Incident T2DM (n = 432), cohort (n = 5572, 15.8 follow-up years) | Clostridium citroniae, Clostridium bolteae, Tyzzerella nexilis, Ruminococcus gnavus | increase |
| two Alistipes spp. | decrease | ||||
| Gertraud Maskarinec et al. [9] | 2021 | White, African American, Native Hawaiian, Japanese American, and Latino | T2DM (n = 307), normoglycemic participant (n = 735), prediabetes (n = 506), undiagnosed T2DM (n = 154) | Escherichia-Shigella, Lachnospiraceae | increase |
| Actinobacteria, Firmicutes, Clostridium sensu stricto 1, Lachnospira, Peptostreptococcaceae | decrease | ||||
| Hao Wu et al. [23] | 2020 | Swedish | NGT (n = 523), T2DM (at low risk, n = 226; at high risk, n = 297) | Clostridium bolteae, Clostridium clostridioforme | increase |
| Faecalibacterium spp., Clostridium spp., Alistipes spp., Pseudoflavonifractor spp., Oscillibacter spp. | decrease | ||||
| Camila Alvarez-Silva et al. [40] | 2021 | Denmark and India | T2DM (279 Danish and 294 Indian participants) | Danish: Bacteroidaceae, Christensenellaceae, Verrucomicrobiaceae, Desulfovibrionaceae, Rikenellaceae, Akkermansia, Alistipes, Bacteroides | increase |
| India: Lactobacillaceae, Leuconostocaceae, Burkholderiaceae, Prevotellaceae, Prevotella group 9, Megasphaera, Lactobacillus, Achromobacter | increase | ||||
| Fredrik H Karlsson et al. [41] | 2013 | European women | T2DM (n = 53), impaired glucose tolerance (IGT; n = 49), normal glucose tolerance (NGT; n = 43) | Clostridiales, Clostridium clostridioforme, Lactobacillus gasseri, Streptococcus mutans | increase |
| Roseburia, unidentified Clostridium species, multiple Clostridiales, Eubacterium eligens, Coriobacteriaceae, Bacteroides intestinalis | decrease | ||||
| Afshan Saleem et al. [42] | 2022 | Pakistanis | T2DM (n = 94) | Lactobacillaceae, Coriobacteriaceae, Libanicoccus, Lactobacillus, Collinsella, Senegalimassilia, Bifidobacterium, Slackia, Collinsella bouchesdurhonensis, Collinsella aerofaciens | increase |
| Ruminococcaceae, Prevotellaceae, Faecalibacterium, Oribacterium, Faecalibacterium prausnitzii | decrease |
3. Mechanism of Gut Dysbiosis in T2DM
3.1. SCFAs
3.1.1. Relationship Between SCFAs and Gut Barrier Function
3.1.2. Regulation of Insulin Sensitivity by SCFAs
3.1.3. Role of SCFAs in Fat Metabolism and Low-Grade Inflammation
3.2. BAs
BAs Regulate Metabolic Pathways via Receptor Activation
3.3. Amino Acids
3.4. Endotoxin
4. Potential Therapeutic Strategies Targeting Gut Microbiota
4.1. Probiotics and Prebiotics
4.2. Impact of Dietary Interventions on Gut Microbiota
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2025|Diabetes Care|American Diabetes Association. Available online: https://diabetesjournals.org/care/article/48/Supplement_1/S27/157566/2-Diagnosis-and-Classification-of-Diabetes (accessed on 18 April 2025).
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global Estimates of Diabetes Prevalence for 2017 and Projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wang, S.; Lin, H.; Wan, Q.; Zheng, R.; Zhu, Y.; Li, M.; Xu, Y.; Xu, M.; Zheng, J.; et al. Body Size, Insulin Sensitivity, Metabolic Health and Risk of Cardiovascular Disease in Chinese Adults: Insights from the China Cardiometabolic Disease and Cancer Cohort (4C) Study. Diabetes Obes. Metab. 2024, 26, 2176–2187. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, M.; Zeng, T.; Hu, R.; Xu, Y.; Xu, M.; Zhao, Z.; Chen, Y.; Wang, S.; Lin, H.; et al. Association Between Insulin Resistance and Cardiovascular Disease Risk Varies According to Glucose Tolerance Status: A Nationwide Prospective Cohort Study. Diabetes Care 2022, 45, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Scherer, P.E. Obesity, Diabetes, and Increased Cancer Progression. Diabetes Metab. J. 2021, 45, 799–812. [Google Scholar] [CrossRef]
- Srikanth, V.; Sinclair, A.J.; Hill-Briggs, F.; Moran, C.; Biessels, G.J. Type 2 Diabetes and Cognitive Dysfunction-towards Effective Management of Both Comorbidities. Lancet Diabetes Endocrinol. 2020, 8, 535–545. [Google Scholar] [CrossRef]
- Wang, T.; Lu, J.; Shi, L.; Chen, G.; Xu, M.; Xu, Y.; Su, Q.; Mu, Y.; Chen, L.; Hu, R.; et al. Association of Insulin Resistance and β-Cell Dysfunction with Incident Diabetes among Adults in China: A Nationwide, Population-Based, Prospective Cohort Study. Lancet Diabetes Endocrinol. 2020, 8, 115–124. [Google Scholar] [CrossRef]
- Li, M.; Xu, Y.; Wan, Q.; Shen, F.; Xu, M.; Zhao, Z.; Lu, J.; Gao, Z.; Chen, G.; Wang, T.; et al. Individual and Combined Associations of Modifiable Lifestyle and Metabolic Health Status With New-Onset Diabetes and Major Cardiovascular Events: The China Cardiometabolic Disease and Cancer Cohort (4C) Study. Diabetes Care 2020, 43, 1929–1936. [Google Scholar] [CrossRef]
- Maskarinec, G.; Raquinio, P.; Kristal, B.S.; Setiawan, V.W.; Wilkens, L.R.; Franke, A.A.; Lim, U.; Le Marchand, L.; Randolph, T.W.; Lampe, J.W.; et al. The Gut Microbiome and Type 2 Diabetes Status in the Multiethnic Cohort. PLoS ONE 2021, 16, e0250855. [Google Scholar] [CrossRef]
- Ruuskanen, M.O.; Erawijantari, P.P.; Havulinna, A.S.; Liu, Y.; Méric, G.; Tuomilehto, J.; Inouye, M.; Jousilahti, P.; Salomaa, V.; Jain, M.; et al. Gut Microbiome Composition Is Predictive of Incident Type 2 Diabetes in a Population Cohort of 5572 Finnish Adults. Diabetes Care 2022, 45, 811–818. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Chen, Y.; Cao, Z.; Liu, C.; Bao, R.; Wang, Y.; Huang, S.; Pan, S.; Qin, L.; et al. Akkermansia muciniphila Supplementation in Patients with Overweight/Obese Type 2 Diabetes: Efficacy Depends on Its Baseline Levels in the Gut. Cell Metab. 2025, 37, 592–605.e6. [Google Scholar] [CrossRef]
- Tremaroli, V.; Bäckhed, F. Functional Interactions between the Gut Microbiota and Host Metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Tsolis, R.M.; Bäumler, A.J. The Microbiome and Gut Homeostasis. Science 2022, 377, eabp9960. [Google Scholar] [CrossRef]
- Hays, K.E.; Pfaffinger, J.M.; Ryznar, R. The Interplay between Gut Microbiota, Short-Chain Fatty Acids, and Implications for Host Health and Disease. Gut Microbes 2024, 16, 2393270. [Google Scholar] [CrossRef]
- Collins, S.L.; Stine, J.G.; Bisanz, J.E.; Okafor, C.D.; Patterson, A.D. Bile Acids and the Gut Microbiota: Metabolic Interactions and Impacts on Disease. Nat. Rev. Microbiol. 2023, 21, 236–247. [Google Scholar] [CrossRef]
- Li, T.-T.; Chen, X.; Huo, D.; Arifuzzaman, M.; Qiao, S.; Jin, W.-B.; Shi, H.; Li, X.V.; Iliev, I.D.; Artis, D.; et al. Microbiota Metabolism of Intestinal Amino Acids Impacts Host Nutrient Homeostasis and Physiology. Cell Host Microbe 2024, 32, 661–675.e10. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Park, S.; Zhang, T.; Kang, S. Fecal Microbiota Composition, Their Interactions, and Metagenome Function in US Adults with Type 2 Diabetes According to Enterotypes. Int. J. Mol. Sci. 2023, 24, 9533. [Google Scholar] [CrossRef]
- Thingholm, L.B.; Rühlemann, M.C.; Koch, M.; Fuqua, B.; Laucke, G.; Boehm, R.; Bang, C.; Franzosa, E.A.; Hübenthal, M.; Rahnavard, A.; et al. Obese Individuals with and without Type 2 Diabetes Show Different Gut Microbial Functional Capacity and Composition. Cell Host Microbe 2019, 26, 252–264. [Google Scholar] [CrossRef]
- Chen, Z.; Radjabzadeh, D.; Chen, L.; Kurilshikov, A.; Kavousi, M.; Ahmadizar, F.; Ikram, M.A.; Uitterlinden, A.G.; Zhernakova, A.; Fu, J.; et al. Association of Insulin Resistance and Type 2 Diabetes With Gut Microbial Diversity. JAMA Netw. Open 2021, 4, e2118811. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Tremaroli, V.; Schmidt, C.; Lundqvist, A.; Olsson, L.M.; Krämer, M.; Gummesson, A.; Perkins, R.; Bergström, G.; Bäckhed, F. The Gut Microbiota in Prediabetes and Diabetes: A Population-Based Cross-Sectional Study. Cell Metab. 2020, 32, 379–390.e3. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Kubota, T.; Nakanishi, Y.; Tsugawa, H.; Suda, W.; Kwon, A.T.-J.; Yazaki, J.; Ikeda, K.; Nemoto, S.; Mochizuki, Y.; et al. Gut Microbial Carbohydrate Metabolism Contributes to Insulin Resistance. Nature 2023, 621, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.K.; Azmal, M.; Haque, A.S.N.B.; Meem, M.; Talukder, O.F.; Ghosh, A. Unlocking the Secrets of the Human Gut Microbiota: Comprehensive Review on Its Role in Different Diseases. World J. Gastroenterol. 2025, 31, 99913. [Google Scholar] [CrossRef]
- James, M.M.; Pal, N.; Sharma, P.; Kumawat, M.; Shubham, S.; Verma, V.; Tiwari, R.R.; Singh, B.; Nagpal, R.; Sarma, D.K.; et al. Role of Butyrogenic Firmicutes in Type-2 Diabetes. J. Diabetes Metab. Disord. 2022, 21, 1873–1882. [Google Scholar] [CrossRef]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial Signature of Dysbiosis in Gut Microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Y.; Li, X.; Sun, B. Dynamic Balancing of Intestinal Short-Chain Fatty Acids: The Crucial Role of Bacterial Metabolism. Trends Food Sci. Technol. 2020, 100, 118–130. [Google Scholar] [CrossRef]
- Pan, X.; Raaijmakers, J.M.; Carrión, V.J. Importance of Bacteroidetes in Host–Microbe Interactions and Ecosystem Functioning. Trends Microbiol. 2023, 31, 959–971. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A Metagenome-Wide Association Study of Gut Microbiota in Type 2 Diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Zhao, L.; Lou, H.; Peng, Y.; Chen, S.; Zhang, Y.; Li, X. Comprehensive Relationships between Gut Microbiome and Faecal Metabolome in Individuals with Type 2 Diabetes and Its Complications. Endocrine 2019, 66, 526–537. [Google Scholar] [CrossRef]
- Nuli, R.; Cai, J.; Kadeer, A.; Zhang, Y.; Mohemaiti, P. Integrative Analysis Toward Different Glucose Tolerance-Related Gut Microbiota and Diet. Front. Endocrinol. 2019, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Gaike, A.H.; Paul, D.; Bhute, S.; Dhotre, D.P.; Pande, P.; Upadhyaya, S.; Reddy, Y.; Sampath, R.; Ghosh, D.; Chandraprabha, D.; et al. The Gut Microbial Diversity of Newly Diagnosed Diabetics but Not of Prediabetics Is Significantly Different from That of Healthy Nondiabetics. mSystems 2020, 5, e00578-19. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Carbajal, A.; Pizano-Zárate, M.L.; Hernández-Quiroz, F.; Ortiz-Luna, G.F.; Morales-Hernández, R.M.; De Sales-Millán, A.; Hernández-Trejo, M.; García-Vite, A.; Beltrán-Lagunes, L.; Hoyo-Vadillo, C.; et al. Characterization of the Gut Microbiota of Individuals at Different T2D Stages Reveals a Complex Relationship with the Host. Microorganisms 2020, 8, 94. [Google Scholar] [CrossRef]
- Mei, Z.; Wang, F.; Bhosle, A.; Dong, D.; Mehta, R.; Ghazi, A.; Zhang, Y.; Liu, Y.; Rinott, E.; Ma, S.; et al. Strain-Specific Gut Microbial Signatures in Type 2 Diabetes Revealed by a Cross-Cohort Analysis of 8117 Metagenomes. Nat. Med. 2024, 30, 2265–2276. [Google Scholar] [CrossRef]
- Wu, X.; Park, S. Fecal Bacterial Community and Metagenome Function in Asians with Type 2 Diabetes, According to Enterotypes. Biomedicines 2022, 10, 2998. [Google Scholar] [CrossRef]
- Zhong, H.; Ren, H.; Lu, Y.; Fang, C.; Hou, G.; Yang, Z.; Chen, B.; Yang, F.; Zhao, Y.; Shi, Z.; et al. Distinct Gut Metagenomics and Metaproteomics Signatures in Prediabetics and Treatment-Naïve Type 2 Diabetics. EBioMedicine 2019, 47, 373–383. [Google Scholar] [CrossRef]
- Doumatey, A.P.; Adeyemo, A.; Zhou, J.; Lei, L.; Adebamowo, S.N.; Adebamowo, C.; Rotimi, C.N. Gut Microbiome Profiles Are Associated With Type 2 Diabetes in Urban Africans. Front. Cell Infect. Microbiol. 2020, 10, 63. [Google Scholar] [CrossRef]
- Yassour, M.; Lim, M.Y.; Yun, H.S.; Tickle, T.L.; Sung, J.; Song, Y.-M.; Lee, K.; Franzosa, E.A.; Morgan, X.C.; Gevers, D.; et al. Sub-Clinical Detection of Gut Microbial Biomarkers of Obesity and Type 2 Diabetes. Genome Med. 2016, 8, 17. [Google Scholar] [CrossRef]
- Alvarez-Silva, C.; Kashani, A.; Hansen, T.H.; Pinna, N.K.; Anjana, R.M.; Dutta, A.; Saxena, S.; Støy, J.; Kampmann, U.; Nielsen, T.; et al. Trans-Ethnic Gut Microbiota Signatures of Type 2 Diabetes in Denmark and India. Genome Med. 2021, 13, 37. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut Metagenome in European Women with Normal, Impaired and Diabetic Glucose Control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef]
- Saleem, A.; Ikram, A.; Dikareva, E.; Lahtinen, E.; Matharu, D.; Pajari, A.-M.; de Vos, W.M.; Hasan, F.; Salonen, A.; Jian, C. Unique Pakistani Gut Microbiota Highlights Population-Specific Microbiota Signatures of Type 2 Diabetes Mellitus. Gut Microbes 2022, 14, 2142009. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; Garelli, S.; Rampelli, S.; Agostini, A.; Matysik, S.; D’Amico, F.; Krautbauer, S.; Mazza, R.; Salituro, N.; Fanelli, F.; et al. Multi-Omics Gut Microbiome Signatures in Obese Women: Role of Diet and Uncontrolled Eating Behavior. BMC Med. 2022, 20, 500. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Zanini, J.; Pietzner, M.; Wheeler, E.; Kerrison, N.D.; Langenberg, C.; Wareham, N.J. Multi-Omic Prediction of Incident Type 2 Diabetes. Diabetologia 2024, 67, 102–112. [Google Scholar] [CrossRef]
- Agus, A.; Clément, K.; Sokol, H. Gut Microbiota-Derived Metabolites as Central Regulators in Metabolic Disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef]
- Xie, C.; Qi, C.; Zhang, J.; Wang, W.; Meng, X.; Aikepaer, A.; Lin, Y.; Su, C.; Liu, Y.; Feng, X.; et al. When Short-Chain Fatty Acids Meet Type 2 Diabetes Mellitus: Revealing Mechanisms, Envisioning Therapies. Biochem. Pharmacol. 2025, 233, 116791. [Google Scholar] [CrossRef]
- Huang, H.-H.; Lee, W.-J.; Chen, S.-C.; Chen, T.-F.; Lee, S.-D.; Chen, C.-Y. Bile Acid and Fibroblast Growth Factor 19 Regulation in Obese Diabetics, and Non-Alcoholic Fatty Liver Disease after Sleeve Gastrectomy. J. Clin. Med. 2019, 8, 815. [Google Scholar] [CrossRef]
- Yoshida, N.; Yamashita, T.; Osone, T.; Hosooka, T.; Shinohara, M.; Kitahama, S.; Sasaki, K.; Sasaki, D.; Yoneshiro, T.; Suzuki, T.; et al. Bacteroides Spp. Promotes Branched-Chain Amino Acid Catabolism in Brown Fat and Inhibits Obesity. iScience 2021, 24, 103342. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Abdi, S.; Sabico, S.; Alnaami, A.M.; Wani, K.A.; Ansari, M.G.A.; Khattak, M.N.K.; Khan, N.; Tripathi, G.; Chrousos, G.P.; et al. Gut-Derived Endotoxin and Telomere Length Attrition in Adults with and without Type 2 Diabetes. Biomolecules 2021, 11, 1693. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Cheng, Y.; Zhu, M.; Xiao, Z.; Ruan, G.; Wei, Y. Gut Microbiota: A New Target for T2DM Prevention and Treatment. Front. Endocrinol. 2022, 13, 958218. [Google Scholar] [CrossRef]
- Hamamah, S.; Iatcu, O.C.; Covasa, M. Nutrition at the Intersection between Gut Microbiota Eubiosis and Effective Management of Type 2 Diabetes. Nutrients 2024, 16, 269. [Google Scholar] [CrossRef]
- Fu, Y.; Li, S.; Xiao, Y.; Liu, G.; Fang, J. A Metabolite Perspective on the Involvement of the Gut Microbiota in Type 2 Diabetes. Int. J. Mol. Sci. 2023, 24, 14991. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef] [PubMed]
- van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-Chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Nishida, A.; Yamano, M.; Kimura, I. Short-Chain Fatty Acid Receptors and Gut Microbiota as Therapeutic Targets in Metabolic, Immune, and Neurological Diseases. Pharmacol. Ther. 2022, 239, 108273. [Google Scholar] [CrossRef]
- Clausen, M.R.; Mortensen, P.B. Kinetic Studies on Colonocyte Metabolism of Short Chain Fatty Acids and Glucose in Ulcerative Colitis. Gut 1995, 37, 684–689. [Google Scholar] [CrossRef]
- Nordgaard, I.; Hansen, B.S.; Mortensen, P.B. Importance of Colonic Support for Energy Absorption as Small-Bowel Failure Proceeds. Am. J. Clin. Nutr. 1996, 64, 222–231. [Google Scholar] [CrossRef]
- Hung, T.V.; Suzuki, T. Dietary Fermentable Fibers Attenuate Chronic Kidney Disease in Mice by Protecting the Intestinal Barrier. J. Nutr. 2018, 148, 552–561. [Google Scholar] [CrossRef]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glove, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef]
- Gaudier, E.; Jarry, A.; Blottière, H.M.; de Coppet, P.; Buisine, M.P.; Aubert, J.P.; Laboisse, C.; Cherbut, C.; Hoebler, C. Butyrate Specifically Modulates MUC Gene Expression in Intestinal Epithelial Goblet Cells Deprived of Glucose. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G1168–G1174. [Google Scholar] [CrossRef]
- Sugawara, Y.; Kanazawa, A.; Aida, M.; Yoshida, Y.; Yamashiro, Y.; Watada, H. Association of Gut Microbiota and Inflammatory Markers in Obese Patients with Type 2 Diabetes Mellitus: Post Hoc Analysis of a Synbiotic Interventional Study. Biosci. Microbiota Food Health 2022, 41, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Byndloss, M.; Devkota, S.; Duca, F.; Hendrik Niess, J.; Nieuwdorp, M.; Orho-Melander, M.; Sanz, Y.; Tremaroli, V.; Zhao, L. The Gut Microbiota and Diabetes: Research, Translation, and Clinical Applications-2023 Diabetes, Diabetes Care, and Diabetologia Expert Forum. Diabetes Care 2024, 47, 1491–1508. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G Protein-Coupled Receptors GPR41 and GPR43 Are Activated by Propionate and Other Short Chain Carboxylic Acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef] [PubMed]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.-Y.; Lannoy, V.; Decobecq, M.-E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional Characterization of Human Receptors for Short Chain Fatty Acids and Their Role in Polymorphonuclear Cell Activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef]
- Thangaraju, M.; Cresci, G.A.; Liu, K.; Ananth, S.; Gnanaprakasam, J.P.; Browning, D.D.; Mellinger, J.D.; Smith, S.B.; Digby, G.J.; Lambert, N.A.; et al. GPR109A Is a G-Protein-Coupled Receptor for the Bacterial Fermentation Product Butyrate and Functions as a Tumor Suppressor in Colon. Cancer Res. 2009, 69, 2826–2832. [Google Scholar] [CrossRef]
- Forbes, S.; Stafford, S.; Coope, G.; Heffron, H.; Real, K.; Newman, R.; Davenport, R.; Barnes, M.; Grosse, J.; Cox, H. Selective FFA2 Agonism Appears to Act via Intestinal PYY to Reduce Transit and Food Intake but Does Not Improve Glucose Tolerance in Mouse Models. Diabetes 2015, 64, 3763–3771. [Google Scholar] [CrossRef]
- Zeng, Y.; Wu, Y.; Zhang, Q.; Xiao, X. Crosstalk between Glucagon-like Peptide 1 and Gut Microbiota in Metabolic Diseases. mBio 2024, 15, e0203223. [Google Scholar] [CrossRef]
- den Besten, G.; Bleeker, A.; Gerding, A.; van Eunen, K.; Havinga, R.; van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.-J.; et al. Short-Chain Fatty Acids Protect Against High-Fat Diet-Induced Obesity via a PPARγ-Dependent Switch From Lipogenesis to Fat Oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, M.; Liu, Y.; Wei, F.; Li, X.; Feng, Y.; Jin, X.; Liu, D.; Guo, Y.; Hu, Y. Inulin-Enriched Megamonas Funiformis Ameliorates Metabolic Dysfunction-Associated Fatty Liver Disease by Producing Propionic Acid. NPJ Biofilms Microbiomes 2023, 9, 84. [Google Scholar] [CrossRef]
- Wang, D.; Liu, C.-D.; Tian, M.-L.; Tan, C.-Q.; Shu, G.; Jiang, Q.-Y.; Zhang, L.; Yin, Y. Propionate Promotes Intestinal Lipolysis and Metabolic Benefits via AMPK/LSD1 Pathway in Mice. J. Endocrinol. 2019, 243, 187–197. [Google Scholar] [CrossRef]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.K.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of Targeted Delivery of Propionate to the Human Colon on Appetite Regulation, Body Weight Maintenance and Adiposity in Overweight Adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Li, A.; Wang, H.; Ma, J.; Hou, J. Positive Regulation of Acetate in Adipocyte Differentiation and Lipid Deposition in Obese Mice. Nutrients 2023, 15, 3736. [Google Scholar] [CrossRef]
- Masui, R.; Sasaki, M.; Funaki, Y.; Ogasawara, N.; Mizuno, M.; Iida, A.; Izawa, S.; Kondo, Y.; Ito, Y.; Tamura, Y.; et al. G Protein-Coupled Receptor 43 Moderates Gut Inflammation through Cytokine Regulation from Mononuclear Cells. Inflamm. Bowel Dis. 2013, 19, 2848–2856. [Google Scholar] [CrossRef]
- Duan, H.; Wang, L.; Huangfu, M.; Li, H. The Impact of Microbiota-Derived Short-Chain Fatty Acids on Macrophage Activities in Disease: Mechanisms and Therapeutic Potentials. Biomed. Pharmacother. 2023, 165, 115276. [Google Scholar] [CrossRef]
- Li, G.; Lin, J.; Zhang, C.; Gao, H.; Lu, H.; Gao, X.; Zhu, R.; Li, Z.; Li, M.; Liu, Z. Microbiota Metabolite Butyrate Constrains Neutrophil Functions and Ameliorates Mucosal Inflammation in Inflammatory Bowel Disease. Gut Microbes 2021, 13, 1968257. [Google Scholar] [CrossRef]
- Hosseinkhani, F.; Heinken, A.; Thiele, I.; Lindenburg, P.W.; Harms, A.C.; Hankemeier, T. The Contribution of Gut Bacterial Metabolites in the Human Immune Signaling Pathway of Non-Communicable Diseases. Gut Microbes 2021, 13, 1882927. [Google Scholar] [CrossRef]
- Kim, C.H. Complex Regulatory Effects of Gut Microbial Short-Chain Fatty Acids on Immune Tolerance and Autoimmunity. Cell. Mol. Immunol. 2023, 20, 341–350. [Google Scholar] [CrossRef]
- Stellaard, F.; Lütjohann, D. Dynamics of the Enterohepatic Circulation of Bile Acids in Healthy Humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G55–G66. [Google Scholar] [CrossRef]
- Devlin, A.S.; Fischbach, M.A. A Biosynthetic Pathway for a Prominent Class of Microbiota-Derived Bile Acids. Nat. Chem. Biol. 2015, 11, 685–690. [Google Scholar] [CrossRef]
- Funabashi, M.; Grove, T.L.; Wang, M.; Varma, Y.; McFadden, M.E.; Brown, L.C.; Guo, C.; Higginbottom, S.; Almo, S.C.; Fischbach, M.A. A Metabolic Pathway for Bile Acid Dehydroxylation by the Gut Microbiome. Nature 2020, 582, 566–570. [Google Scholar] [CrossRef]
- Jones, B.V.; Begley, M.; Hill, C.; Gahan, C.G.M.; Marchesi, J.R. Functional and Comparative Metagenomic Analysis of Bile Salt Hydrolase Activity in the Human Gut Microbiome. Proc. Natl. Acad. Sci. USA 2008, 105, 13580–13585. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Nakama, R.; Tamaki, M.; Masuda, N.; Oda, H. Isolation and Characterization of Thirteen Intestinal Microorganisms Capable of 7 Alpha-Dehydroxylating Bile Acids. Appl. Environ. Microbiol. 1981, 41, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, S.; Li, M.; Gao, Z.; Xu, Y.; Zhao, X.; Hu, C.; Zhang, Y.; Liu, R.; Hu, R.; et al. Association of Serum Bile Acids Profile and Pathway Dysregulation With the Risk of Developing Diabetes Among Normoglycemic Chinese Adults: Findings From the 4C Study. Diabetes Care 2021, 44, 499–510. [Google Scholar] [CrossRef]
- Lu, Q.; Chen, J.; Jiang, L.; Geng, T.; Tian, S.; Liao, Y.; Yang, K.; Zheng, Y.; He, M.; Tang, H.; et al. Gut Microbiota-Derived Secondary Bile Acids, Bile Acids Receptor Polymorphisms, and Risk of Cardiovascular Disease in Individuals with Newly Diagnosed Type 2 Diabetes: A Cohort Study. Am. J. Clin. Nutr. 2024, 119, 324–332. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, L.; Wang, J.; Lu, M.; Liu, D.; Zhou, C.; Liu, Z. Metabolomic Profiling Reveals the Step-Wise Alteration of Bile Acid Metabolism in Patients with Diabetic Kidney Disease. Nutr. Diabetes 2024, 14, 85. [Google Scholar] [CrossRef]
- Lin, S.; Wang, S.; Wang, P.; Tang, C.; Wang, Z.; Chen, L.; Luo, G.; Chen, H.; Liu, Y.; Feng, B.; et al. Bile Acids and Their Receptors in Regulation of Gut Health and Diseases. Prog. Lipid Res. 2023, 89, 101210. [Google Scholar] [CrossRef]
- Forman, B.M.; Goode, E.; Chen, J.; Oro, A.E.; Bradley, D.J.; Perlmann, T.; Noonan, D.J.; Burka, L.T.; McMorris, T.; Lamph, W.W.; et al. Identification of a Nuclear Receptor That Is Activated by Farnesol Metabolites. Cell 1995, 81, 687–693. [Google Scholar] [CrossRef]
- Duboc, H.; Taché, Y.; Hofmann, A.F. The Bile Acid TGR5 Membrane Receptor: From Basic Research to Clinical Application. Dig. Liver Dis. 2014, 46, 302–312. [Google Scholar] [CrossRef]
- Fujino, T.; Une, M.; Imanaka, T.; Inoue, K.; Nishimaki-Mogami, T. Structure-Activity Relationship of Bile Acids and Bile Acid Analogs in Regard to FXR Activation. J. Lipid Res. 2004, 45, 132–138. [Google Scholar] [CrossRef]
- Thomas, C.; Pellicciari, R.; Pruzanski, M.; Auwerx, J.; Schoonjans, K. Targeting Bile-Acid Signalling for Metabolic Diseases. Nat. Rev. Drug Discov. 2008, 7, 678–693. [Google Scholar] [CrossRef]
- Ticho, A.L.; Malhotra, P.; Dudeja, P.K.; Gill, R.K.; Alrefai, W.A. Intestinal Absorption of Bile Acids in Health and Disease. Compr. Physiol. 2019, 10, 21–56. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Jin, L.; Huang, W. Bile Acids, Intestinal Barrier Dysfunction, and Related Diseases. Cells 2023, 12, 1888. [Google Scholar] [CrossRef]
- Song, M.; Ye, J.; Zhang, F.; Su, H.; Yang, X.; He, H.; Liu, F.; Zhu, X.; Wang, L.; Gao, P.; et al. Chenodeoxycholic Acid (CDCA) Protects against the Lipopolysaccharide-Induced Impairment of the Intestinal Epithelial Barrier Function via the FXR-MLCK Pathway. J. Agric. Food Chem. 2019, 67, 8868–8874. [Google Scholar] [CrossRef] [PubMed]
- Katafuchi, T.; Makishima, M. Molecular Basis of Bile Acid-FXR-FGF15/19 Signaling Axis. Int. J. Mol. Sci. 2022, 23, 6046. [Google Scholar] [CrossRef]
- Katsuma, S.; Hirasawa, A.; Tsujimoto, G. Bile Acids Promote Glucagon-like Peptide-1 Secretion through TGR5 in a Murine Enteroendocrine Cell Line STC-1. Biochem. Biophys. Res. Commun. 2005, 329, 386–390. [Google Scholar] [CrossRef]
- Porez, G.; Prawitt, J.; Gross, B.; Staels, B. Bile Acid Receptors as Targets for the Treatment of Dyslipidemia and Cardiovascular Disease. J. Lipid Res. 2012, 53, 1723–1737. [Google Scholar] [CrossRef]
- Portincasa, P.; Di Ciaula, A.; Garruti, G.; Vacca, M.; De Angelis, M.; Wang, D.Q.-H. Bile Acids and GPBAR-1: Dynamic Interaction Involving Genes, Environment and Gut Microbiome. Nutrients 2020, 12, 3709. [Google Scholar] [CrossRef]
- Fiorucci, S.; Distrutti, E.; Carino, A.; Zampella, A.; Biagioli, M. Bile Acids and Their Receptors in Metabolic Disorders. Prog. Lipid Res. 2021, 82, 101094. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite Profiles and the Risk of Developing Diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Wang, S.; Li, M.; Lin, H.; Wang, G.; Xu, Y.; Zhao, X.; Hu, C.; Zhang, Y.; Zheng, R.; Hu, R.; et al. Amino Acids, Microbiota-Related Metabolites, and the Risk of Incident Diabetes among Normoglycemic Chinese Adults: Findings from the 4C Study. Cell Rep. Med. 2022, 3, 100727. [Google Scholar] [CrossRef]
- Mansoori, S.; Ho, M.Y.-M.; Ng, K.K.-W.; Cheng, K.K.-Y. Branched-Chain Amino Acid Metabolism: Pathophysiological Mechanism and Therapeutic Intervention in Metabolic Diseases. Obes. Rev. 2025, 26, e13856. [Google Scholar] [CrossRef] [PubMed]
- Abdualkader, A.M.; Karwi, Q.G.; Lopaschuk, G.D.; Al Batran, R. The Role of Branched-Chain Amino Acids and Their Downstream Metabolites in Mediating Insulin Resistance. J. Pharm. Pharm. Sci. 2024, 27, 13040. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A Branched-Chain Amino Acid-Related Metabolic Signature That Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, I.; Ardavani, A.; Vanweert, F.; Mellett, A.; Atherton, P.J.; Idris, I. The Association between Circulating Branched Chain Amino Acids and the Temporal Risk of Developing Type 2 Diabetes Mellitus: A Systematic Review & Meta-Analysis. Nutrients 2022, 14, 4411. [Google Scholar] [CrossRef]
- Jewell, J.L.; Kim, Y.C.; Russell, R.C.; Yu, F.-X.; Park, H.W.; Plouffe, S.W.; Tagliabracci, V.S.; Guan, K.-L. Differential Regulation of mTORC1 by Leucine and Glutamine. Science 2015, 347, 194–198. [Google Scholar] [CrossRef]
- Tremblay, F.; Krebs, M.; Dombrowski, L.; Brehm, A.; Bernroider, E.; Roth, E.; Nowotny, P.; Waldhäusl, W.; Marette, A.; Roden, M. Overactivation of S6 Kinase 1 as a Cause of Human Insulin Resistance During Increased Amino Acid Availability. Diabetes 2005, 54, 2674–2684. [Google Scholar] [CrossRef]
- Alessi, D.R.; Kozlowski, M.T.; Weng, Q.P.; Morrice, N.; Avruch, J. 3-Phosphoinositide-Dependent Protein Kinase 1 (PDK1) Phosphorylates and Activates the P70 S6 Kinase In Vivo and In Vitro. Curr. Biol. 1998, 8, 69–81. [Google Scholar] [CrossRef]
- Krebs, M.; Brehm, A.; Krssak, M.; Anderwald, C.; Bernroider, E.; Nowotny, P.; Roth, E.; Chandramouli, V.; Landau, B.R.; Waldhäusl, W.; et al. Direct and Indirect Effects of Amino Acids on Hepatic Glucose Metabolism in Humans. Diabetologia 2003, 46, 917–925. [Google Scholar] [CrossRef]
- Saha, A.K.; Xu, X.J.; Lawson, E.; Deoliveira, R.; Brandon, A.E.; Kraegen, E.W.; Ruderman, N.B. Downregulation of AMPK Accompanies Leucine- and Glucose-Induced Increases in Protein Synthesis and Insulin Resistance in Rat Skeletal Muscle. Diabetes 2010, 59, 2426–2434. [Google Scholar] [CrossRef]
- Shi, C.-X.; Zhao, M.-X.; Shu, X.-D.; Xiong, X.-Q.; Wang, J.-J.; Gao, X.-Y.; Chen, Q.; Li, Y.-H.; Kang, Y.-M.; Zhu, G.-Q. β-Aminoisobutyric Acid Attenuates Hepatic Endoplasmic Reticulum Stress and Glucose/Lipid Metabolic Disturbance in Mice with Type 2 Diabetes. Sci. Rep. 2016, 6, 21924. [Google Scholar] [CrossRef]
- Xiao, F.; Huang, Z.; Li, H.; Yu, J.; Wang, C.; Chen, S.; Meng, Q.; Cheng, Y.; Gao, X.; Li, J.; et al. Leucine Deprivation Increases Hepatic Insulin Sensitivity via GCN2/mTOR/S6K1 and AMPK Pathways. Diabetes 2011, 60, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, X.; Li, C.; Wang, D.; Shen, Y.; Lu, J.; Zhao, L.; Li, X.; Gao, H. BCAA Mediated Microbiota-Liver-Heart Crosstalk Regulates Diabetic Cardiomyopathy via FGF21. Microbiome 2024, 12, 157. [Google Scholar] [CrossRef] [PubMed]
- Gojda, J.; Cahova, M. Gut Microbiota as the Link between Elevated BCAA Serum Levels and Insulin Resistance. Biomolecules 2021, 11, 1414. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.A.; Joshi, M.; Ho Jeoung, N. Mechanisms Responsible for Regulation of Branched-Chain Amino Acid Catabolism. Biochem. Biophys. Res. Commun. 2004, 313, 391–396. [Google Scholar] [CrossRef]
- Patrick, M.; Gu, Z.; Zhang, G.; Max Wynn, R.; Kaphle, P.; Cao, H.; Vu, H.; Cai, F.; Gao, X.; Zhang, Y.; et al. Metabolon Formation Regulates Branched-Chain Amino Acid Oxidation and Homeostasis. Nat. Metab. 2022, 4, 1775–1791. [Google Scholar] [CrossRef]
- Kagamiyama, H.; Hayashi, H. Branched-Chain Amino-Acid Aminotransferase of Escherichia Coli. Methods Enzym. 2000, 324, 103–113. [Google Scholar] [CrossRef]
- Williams, B.B.; Van Benschoten, A.H.; Cimermancic, P.; Donia, M.S.; Zimmermann, M.; Taketani, M.; Ishihara, A.; Kashyap, P.C.; Fraser, J.S.; Fischbach, M.A. Discovery and Characterization of Gut Microbiota Decarboxylases That Can Produce the Neurotransmitter Tryptamine. Cell Host Microbe 2014, 16, 495–503. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human Gut Microbes Impact Host Serum Metabolome and Insulin Sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Würtz, P.; Soininen, P.; Kangas, A.J.; Rönnemaa, T.; Lehtimäki, T.; Kähönen, M.; Viikari, J.S.; Raitakari, O.T.; Ala-Korpela, M. Branched-Chain and Aromatic Amino Acids Are Predictors of Insulin Resistance in Young Adults. Diabetes Care 2013, 36, 648–655. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial Tryptophan Catabolites in Health and Disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A Gut Bacterial Pathway Metabolizes Aromatic Amino Acids into Nine Circulating Metabolites. Nature 2017, 551, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Li, J.; Yu, B.; Moon, J.-Y.; Chai, J.C.; Merino, J.; Hu, J.; Ruiz-Canela, M.; Rebholz, C.; Wang, Z.; et al. Host and Gut Microbial Tryptophan Metabolism and Type 2 Diabetes: An Integrative Analysis of Host Genetics, Diet, Gut Microbiome and Circulating Metabolites in Cohort Studies. Gut 2022, 71, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Dehhaghi, M.; Panahi, H.K.S.; Heng, B.; Guillemin, G.J. The Gut Microbiota, Kynurenine Pathway, and Immune System Interaction in the Development of Brain Cancer. Front. Cell Dev. Biol. 2020, 8, 562812. [Google Scholar] [CrossRef]
- Yan, T.; Shi, L.; Liu, T.; Zhang, X.; Yang, M.; Peng, W.; Sun, X.; Yan, L.; Dai, X.; Yang, X. Diet-Rich in Wheat Bran Modulates Tryptophan Metabolism and AhR/IL-22 Signalling Mediated Metabolic Health and Gut Dysbacteriosis: A Novel Prebiotic-like Activity of Wheat Bran. Food Res. Int. 2023, 163, 112179. [Google Scholar] [CrossRef]
- Sehgal, R.; de Mello, V.D.; Männistö, V.; Lindström, J.; Tuomilehto, J.; Pihlajamäki, J.; Uusitupa, M. Indolepropionic Acid, a Gut Bacteria-Produced Tryptophan Metabolite and the Risk of Type 2 Diabetes and Non-Alcoholic Fatty Liver Disease. Nutrients 2022, 14, 4695. [Google Scholar] [CrossRef]
- de Mello, V.D.; Paananen, J.; Lindström, J.; Lankinen, M.A.; Shi, L.; Kuusisto, J.; Pihlajamäki, J.; Auriola, S.; Lehtonen, M.; Rolandsson, O.; et al. Indolepropionic Acid and Novel Lipid Metabolites Are Associated with a Lower Risk of Type 2 Diabetes in the Finnish Diabetes Prevention Study. Sci. Rep. 2017, 7, 46337. [Google Scholar] [CrossRef]
- Shimada, Y.; Kinoshita, M.; Harada, K.; Mizutani, M.; Masahata, K.; Kayama, H.; Takeda, K. Commensal Bacteria-Dependent Indole Production Enhances Epithelial Barrier Function in the Colon. PLoS ONE 2013, 8, e80604. [Google Scholar] [CrossRef]
- Mawe, G.M.; Hoffman, J.M. Serotonin Signaling in the Gastrointestinal Tract. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in Gut Microbiota Control Inflammation in Obese Mice through a Mechanism Involving GLP-2-Driven Improvement of Gut Permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Amar, J.; Chabo, C.; Waget, A.; Klopp, P.; Vachoux, C.; Bermúdez-Humarán, L.G.; Smirnova, N.; Bergé, M.; Sulpice, T.; Lahtinen, S.; et al. Intestinal Mucosal Adherence and Translocation of Commensal Bacteria at the Early Onset of Type 2 Diabetes: Molecular Mechanisms and Probiotic Treatment. EMBO Mol. Med. 2011, 3, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Dunzendorfer, S.; Lee, H.-K.; Soldau, K.; Tobias, P.S. TLR4 Is the Signaling but Not the Lipopolysaccharide Uptake Receptor. J. Immunol. 2004, 173, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Creely, S.J.; McTernan, P.G.; Kusminski, C.M.; Fisher, F.M.; Da Silva, N.F.; Khanolkar, M.; Evans, M.; Harte, A.L.; Kumar, S. Lipopolysaccharide Activates an Innate Immune System Response in Human Adipose Tissue in Obesity and Type 2 Diabetes. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E740–E747. [Google Scholar] [CrossRef]
- Singh, A.; Boden, G.; Rao, A.K. Tissue Factor and Toll-like Receptor (TLR)4 in Hyperglycaemia-Hyperinsulinaemia. Effects in Healthy Subjects, and Type 1 and Type 2 Diabetes Mellitus. Thromb. Haemost. 2015, 113, 750–758. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Liu, Y.; He, T.; Yang, T.-T.; Wu, J.; Cianflone, K.; Lu, H.-L. Anaphylatoxin C5a Induces Inflammation and Reduces Insulin Sensitivity by Activating TLR4/NF-kB/PI3K Signaling Pathway in 3T3-L1 Adipocytes. Biomed. Pharmacother. 2018, 103, 955–964. [Google Scholar] [CrossRef]
- Cucak, H.; Mayer, C.; Tonnesen, M.; Thomsen, L.H.; Grunnet, L.G.; Rosendahl, A. Macrophage Contact Dependent and Independent TLR4 Mechanisms Induce β-Cell Dysfunction and Apoptosis in a Mouse Model of Type 2 Diabetes. PLoS ONE 2014, 9, e90685. [Google Scholar] [CrossRef]
- Yu, Q.; Xu, T.; Ding, F.; Ding, Z.; Lin, R. Decreased Infiltration of Adipose Tissue Macrophages and Amplified Inflammation of Adipose Tissue in Obese Mice with Severe Acute Pancreatitis. Pancreatology 2021, 21, 1173–1182. [Google Scholar] [CrossRef]
- Xu, S.; Lu, F.; Gao, J.; Yuan, Y. Inflammation-Mediated Metabolic Regulation in Adipose Tissue. Obes. Rev. 2024, 25, e13724. [Google Scholar] [CrossRef]
- Saad, M.J.A.; Santos, A.; Prada, P.O. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef]
- Hersoug, L.-G.; Møller, P.; Loft, S. Role of Microbiota-Derived Lipopolysaccharide in Adipose Tissue Inflammation, Adipocyte Size and Pyroptosis during Obesity. Nutr. Res. Rev. 2018, 31, 153–163. [Google Scholar] [CrossRef]
- Amar, J.; Burcelin, R.; Ruidavets, J.B.; Cani, P.D.; Fauvel, J.; Alessi, M.C.; Chamontin, B.; Ferriéres, J. Energy Intake Is Associated with Endotoxemia in Apparently Healthy Men. Am. J. Clin. Nutr. 2008, 87, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, S.; Vanhoutte, P.M.; Woo, C.W.; Xu, A. Akkermansia Muciniphila Protects Against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe-/- Mice. Circulation 2016, 133, 2434–2446. [Google Scholar] [CrossRef] [PubMed]
- Hersoug, L.-G.; Møller, P.; Loft, S. Gut Microbiota-Derived Lipopolysaccharide Uptake and Trafficking to Adipose Tissue: Implications for Inflammation and Obesity. Obes. Rev. 2016, 17, 297–312. [Google Scholar] [CrossRef]
- Johnson-Henry, K.C.; Donato, K.A.; Shen-Tu, G.; Gordanpour, M.; Sherman, P.M. Lactobacillus Rhamnosus Strain GG Prevents Enterohemorrhagic Escherichia Coli O157:H7-Induced Changes in Epithelial Barrier Function. Infect. Immun. 2008, 76, 1340–1348. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, S.; Ye, Y.; Yin, S.; Fan, J.; Xia, M. Intermittent Fasting Improves Cardiometabolic Risk Factors and Alters Gut Microbiota in Metabolic Syndrome Patients. J. Clin. Endocrinol. Metab. 2021, 106, 64–79. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, W.; Wang, J.; Yu, J.; Yang, L. Intermittent Fasting Improves Lipid Metabolism Through Changes in Gut Microbiota in Diet-Induced Obese Mice. Med. Sci. Monit. 2020, 26, e926789-1–e926789-11. [Google Scholar] [CrossRef]
- Pinto, F.C.S.; Silva, A.A.M.; Souza, S.L. Repercussions of Intermittent Fasting on the Intestinal Microbiota Community and Body Composition: A Systematic Review. Nutr. Rev. 2022, 80, 613–628. [Google Scholar] [CrossRef]
- Ji, H.; Wan, Y.; Li, S.; Zhou, D.; Gu, F.; Sun, J.; Yan, X.; Le, Y.; Chen, T.; Nie, S.; et al. Remolding Probiotics for Effective Treatment of Type 2 Diabetes via Oral Administration. Biomaterials 2025, 315, 122970. [Google Scholar] [CrossRef]
- Bock, P.M.; Martins, A.F.; Schaan, B.D. Understanding How Pre- and Probiotics Affect the Gut Microbiome and Metabolic Health. Am. J. Physiol. Endocrinol. Metab. 2024, 327, E89–E102. [Google Scholar] [CrossRef]
- Kang, Y.; Kang, X.; Yang, H.; Liu, H.; Yang, X.; Liu, Q.; Tian, H.; Xue, Y.; Ren, P.; Kuang, X.; et al. Lactobacillus Acidophilus Ameliorates Obesity in Mice through Modulation of Gut Microbiota Dysbiosis and Intestinal Permeability. Pharmacol. Res. 2022, 175, 106020. [Google Scholar] [CrossRef]
- Kanazawa, A.; Aida, M.; Yoshida, Y.; Kaga, H.; Katahira, T.; Suzuki, L.; Tamaki, S.; Sato, J.; Goto, H.; Azuma, K.; et al. Effects of Synbiotic Supplementation on Chronic Inflammation and the Gut Microbiota in Obese Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Study. Nutrients 2021, 13, 558. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Li, J.; Zhang, L.; Chen, C.; Wei, Y.; Shen, Z.-A. Effects of Oral Supplementation of Probiotics on Body Weight and Visceral Fat in Obese Patients: A Meta-Analysis and Systematic Review. Sci. Rep. 2025, 15, 6355. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dilidaxi, D.; Wu, Y.; Sailike, J.; Sun, X.; Nabi, X.-H. Composite Probiotics Alleviate Type 2 Diabetes by Regulating Intestinal Microbiota and Inducing GLP-1 Secretion in Db/Db Mice. Biomed. Pharmacother. 2020, 125, 109914. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Sailike, J.; Sun, X.; Abuduwaili, N.; Tuoliuhan, H.; Yusufu, M.; Nabi, X.-H. Fourteen Composite Probiotics Alleviate Type 2 Diabetes through Modulating Gut Microbiota and Modifying M1/M2 Phenotype Macrophage in Db/Db Mice. Pharmacol. Res. 2020, 161, 105150. [Google Scholar] [CrossRef]
- Paul, P.; Kaul, R.; Harfouche, M.; Arabi, M.; Al-Najjar, Y.; Sarkar, A.; Saliba, R.; Chaari, A. The Effect of Microbiome-Modulating Probiotics, Prebiotics and Synbiotics on Glucose Homeostasis in Type 2 Diabetes: A Systematic Review, Meta-Analysis, and Meta-Regression of Clinical Trials. Pharmacol. Res. 2022, 185, 106520. [Google Scholar] [CrossRef]
- Naseri, K.; Saadati, S.; Ashtary-Larky, D.; Asbaghi, O.; Ghaemi, F.; Pashayee-Khamene, F.; Yari, Z.; de Courten, B. Probiotics and Synbiotics Supplementation Improve Glycemic Control Parameters in Subjects with Prediabetes and Type 2 Diabetes Mellitus: A GRADE-Assessed Systematic Review, Meta-Analysis, and Meta-Regression of Randomized Clinical Trials. Pharmacol. Res. 2022, 184, 106399. [Google Scholar] [CrossRef]
- Rittiphairoj, T.; Pongpirul, K.; Janchot, K.; Mueller, N.T.; Li, T. Probiotics Contribute to Glycemic Control in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Adv. Nutr. 2020, 12, 722–734. [Google Scholar] [CrossRef]
- Li, G.; Feng, H.; Mao, X.-L.; Deng, Y.-J.; Wang, X.-B.; Zhang, Q.; Guo, Y.; Xiao, S.-M. The Effects of Probiotics Supplementation on Glycaemic Control among Adults with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomised Clinical Trials. J. Transl. Med. 2023, 21, 442. [Google Scholar] [CrossRef]
- Tabrizi, R.; Moosazadeh, M.; Lankarani, K.B.; Akbari, M.; Heydari, S.T.; Kolahdooz, F.; Asemi, Z. The Effects of Synbiotic Supplementation on Glucose Metabolism and Lipid Profiles in Patients with Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Probiotics Antimicrob. Proteins 2018, 10, 329–342. [Google Scholar] [CrossRef]
- Baroni, I.; Fabrizi, D.; Luciani, M.; Magon, A.; Conte, G.; Angeli, G.D.; Paglione, G.; Ausili, D.; Caruso, R. Probiotics and Synbiotics for Glycemic Control in Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. 2024, 43, 1041–1061. [Google Scholar] [CrossRef]
- Jayedi, A.; Aletaha, A.; Zeraattalab-Motlagh, S.; Shahinfar, H.; Mohammadpour, S.; Mirrafiei, A.; Jibril, A.T.; Soltani, A.; Shab-Bidar, S. Comparative Efficacy and Safety of Probiotics, Prebiotics, and Synbiotics for Type 2 Diabetes Management: A Systematic Review and Network Meta-Analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2024, 18, 102923. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Wang, L.; Tian, P.; Jin, X.; Zhao, J.; Zhang, H.; Wang, G.; Zhu, M. The Effect of Probiotic Supplementation on Glucolipid Metabolism in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 3240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jiang, J.; Wang, C.; Li, S.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Meta-Analysis of Randomized Controlled Trials of the Effects of Probiotics on Type 2 Diabetes in Adults. Clin. Nutr. 2022, 41, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Bock, P.M.; Telo, G.H.; Ramalho, R.; Sbaraini, M.; Leivas, G.; Martins, A.F.; Schaan, B.D. The Effect of Probiotics, Prebiotics or Synbiotics on Metabolic Outcomes in Individuals with Diabetes: A Systematic Review and Meta-Analysis. Diabetologia 2021, 64, 26–41. [Google Scholar] [CrossRef]
- Dimba, N.R.; Mzimela, N.; Sosibo, A.M.; Khathi, A. Effectiveness of Prebiotics and Mediterranean and Plant-Based Diet on Gut Microbiota and Glycemic Control in Patients with Prediabetes or Type 2 Diabetes: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 3272. [Google Scholar] [CrossRef]
- Ojo, O.; Feng, Q.-Q.; Ojo, O.O.; Wang, X.-H. The Role of Dietary Fibre in Modulating Gut Microbiota Dysbiosis in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2020, 12, 3239. [Google Scholar] [CrossRef]
- Houghton, D.; Hardy, T.; Stewart, C.; Errington, L.; Day, C.P.; Trenell, M.I.; Avery, L. Systematic Review Assessing the Effectiveness of Dietary Intervention on Gut Microbiota in Adults with Type 2 Diabetes. Diabetologia 2018, 61, 1700–1711. [Google Scholar] [CrossRef]
- Ojo, O.; Wang, X.-H.; Ojo, O.O.; Adegboye, A.R.A. The Effects of Almonds on Gut Microbiota, Glycometabolism, and Inflammatory Markers in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2021, 13, 3377. [Google Scholar] [CrossRef]
- Mobini, R.; Tremaroli, V.; Ståhlman, M.; Karlsson, F.; Levin, M.; Ljungberg, M.; Sohlin, M.; Bertéus Forslund, H.; Perkins, R.; Bäckhed, F.; et al. Metabolic Effects of Lactobacillus Reuteri DSM 17938 in People with Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Obes. Metab. 2017, 19, 579–589. [Google Scholar] [CrossRef]
- Hsieh, M.-C.; Tsai, W.-H.; Jheng, Y.-P.; Su, S.-L.; Wang, S.-Y.; Lin, C.-C.; Chen, Y.-H.; Chang, W.-W. The Beneficial Effects of Lactobacillus Reuteri ADR-1 or ADR-3 Consumption on Type 2 Diabetes Mellitus: A Randomized, Double-Blinded, Placebo-Controlled Trial. Sci. Rep. 2018, 8, 16791. [Google Scholar] [CrossRef]
- Sanborn, V.E.; Azcarate-Peril, M.A.; Gunstad, J. Lactobacillus Rhamnosus GG and HbA1c in Middle Age and Older Adults without Type 2 Diabetes Mellitus: A Preliminary Randomized Study. Diabetes Metab. Syndr. 2020, 14, 907–909. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, A.S.; Larsen, N.; Pedersen-Skovsgaard, T.; Berg, R.M.G.; Møller, K.; Svendsen, K.D.; Jakobsen, M.; Pedersen, B.K. Effects of Lactobacillus Acidophilus NCFM on Insulin Sensitivity and the Systemic Inflammatory Response in Human Subjects. Br. J. Nutr. 2010, 104, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Hasanian-Langroudi, F.; Ghasemi, A.; Hedayati, M.; Siadat, S.D.; Tohidi, M. Novel Insight into the Effect of Probiotics in the Regulation of the Most Important Pathways Involved in the Pathogenesis of Type 2 Diabetes Mellitus. Probiotics Antimicrob. Proteins 2024, 16, 829–844. [Google Scholar] [CrossRef]
- Breton, J.; Tennoune, N.; Lucas, N.; Francois, M.; Legrand, R.; Jacquemot, J.; Goichon, A.; Guérin, C.; Peltier, J.; Pestel-Caron, M.; et al. Gut Commensal E. Coli Proteins Activate Host Satiety Pathways Following Nutrient-Induced Bacterial Growth. Cell Metab. 2016, 23, 324–334. [Google Scholar] [CrossRef]
- Memon, H.; Abdulla, F.; Reljic, T.; Alnuaimi, S.; Serdarevic, F.; Asimi, Z.V.; Kumar, A.; Semiz, S. Effects of Combined Treatment of Probiotics and Metformin in Management of Type 2 Diabetes: A Systematic Review and Meta-Analysis. Diabetes Res. Clin. Pract. 2023, 202, 110806. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Huang, H.; Zhang, C.; Zuo, H.-X.; Xu, P.; Niu, Y.-M.; Wu, S.-S. Inulin-Type Fructans Supplementation Improves Glycemic Control for the Prediabetes and Type 2 Diabetes Populations: Results from a GRADE-Assessed Systematic Review and Dose–Response Meta-Analysis of 33 Randomized Controlled Trials. J. Transl. Med. 2019, 17, 410. [Google Scholar] [CrossRef]
- Wang, Y.; Ames, N.P.; Tun, H.M.; Tosh, S.M.; Jones, P.J.; Khafipour, E. High Molecular Weight Barley β-Glucan Alters Gut Microbiota Toward Reduced Cardiovascular Disease Risk. Front. Microbiol. 2016, 7, 129. [Google Scholar] [CrossRef]
- Li, L.; Li, R.; Tian, Q.; Luo, Y.; Li, R.; Lin, X.; Ou, Y.; Guo, T.; Chen, X.; Pan, A.; et al. Effects of Healthy Low-Carbohydrate Diet and Time-Restricted Eating on Weight and Gut Microbiome in Adults with Overweight or Obesity: Feeding RCT. Cell Rep. Med. 2024, 5, 101801. [Google Scholar] [CrossRef]
- Pisanu, S.; Palmas, V.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Boi, F.; Loviselli, A.; et al. Impact of a Moderately Hypocaloric Mediterranean Diet on the Gut Microbiota Composition of Italian Obese Patients. Nutrients 2020, 12, 2707. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut Bacteria Selectively Promoted by Dietary Fibers Alleviate Type 2 Diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- Grootaert, C.; Van den Abbeele, P.; Marzorati, M.; Broekaert, W.F.; Courtin, C.M.; Delcour, J.A.; Verstraete, W.; Van de Wiele, T. Comparison of Prebiotic Effects of Arabinoxylan Oligosaccharides and Inulin in a Simulator of the Human Intestinal Microbial Ecosystem. FEMS Microbiol. Ecol. 2009, 69, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, S.; Wu, J.; Luo, L.; Qiao, S.; Li, R.; Xu, W.; Wang, N.; Zhao, B.; Wang, X.; et al. A Specific Gut Microbiota and Metabolomic Profiles Shifts Related to Antidiabetic Action: The Similar and Complementary Antidiabetic Properties of Type 3 Resistant Starch from Canna Edulis and Metformin. Pharmacol. Res. 2020, 159, 104985. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Chang, D.-M.; Wu, D.-J.; Peng, H.-Y.; Chuang, L.-M. Assessment of Blood Glucose Regulation and Safety of Resistant Starch Formula-Based Diet in Healthy Normal and Subjects With Type 2 Diabetes. Medicine 2015, 94, e1332. [Google Scholar] [CrossRef]
- Bodinham, C.L.; Smith, L.; Thomas, E.L.; Bell, J.D.; Swann, J.R.; Costabile, A.; Russell-Jones, D.; Umpleby, A.M.; Robertson, M.D. Efficacy of Increased Resistant Starch Consumption in Human Type 2 Diabetes. Endocr. Connect. 2014, 3, 75–84. [Google Scholar] [CrossRef]
- Flores-Hernández, M.N.; Martínez-Coria, H.; López-Valdés, H.E.; Arteaga-Silva, M.; Arrieta-Cruz, I.; Gutiérrez-Juárez, R. Efficacy of a High-Protein Diet to Lower Glycemic Levels in Type 2 Diabetes Mellitus: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 10959. [Google Scholar] [CrossRef]
- Markova, M.; Pivovarova, O.; Hornemann, S.; Sucher, S.; Frahnow, T.; Wegner, K.; Machann, J.; Petzke, K.J.; Hierholzer, J.; Lichtinghagen, R.; et al. Isocaloric Diets High in Animal or Plant Protein Reduce Liver Fat and Inflammation in Individuals With Type 2 Diabetes. Gastroenterology 2017, 152, 571–585.e8. [Google Scholar] [CrossRef]
- Malik, V.S.; Li, Y.; Tobias, D.K.; Pan, A.; Hu, F.B. Dietary Protein Intake and Risk of Type 2 Diabetes in US Men and Women. Am. J. Epidemiol. 2016, 183, 715–728. [Google Scholar] [CrossRef]
- Shang, X.; Scott, D.; Hodge, A.M.; English, D.R.; Giles, G.G.; Ebeling, P.R.; Sanders, K.M. Dietary Protein Intake and Risk of Type 2 Diabetes: Results from the Melbourne Collaborative Cohort Study and a Meta-Analysis of Prospective Studies. Am. J. Clin. Nutr. 2016, 104, 1352–1365. [Google Scholar] [CrossRef]
- Dowis, K.; Banga, S. The Potential Health Benefits of the Ketogenic Diet: A Narrative Review. Nutrients 2021, 13, 1654. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, H.; Qi, J.; Hu, A.; Jiang, Q.; Hou, Y.; Feng, Q.; Ojo, O.; Wang, X. An Almond-Based Low Carbohydrate Diet Improves Depression and Glycometabolism in Patients with Type 2 Diabetes through Modulating Gut Microbiota and GLP-1: A Randomized Controlled Trial. Nutrients 2020, 12, 3036. [Google Scholar] [CrossRef] [PubMed]
- Ağagündüz, D.; Icer, M.A.; Yesildemir, O.; Koçak, T.; Kocyigit, E.; Capasso, R. The Roles of Dietary Lipids and Lipidomics in Gut-Brain Axis in Type 2 Diabetes Mellitus. J. Transl. Med. 2023, 21, 240. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.A.; Mathew, T.C.; Dashti, A.A.; Asfar, S.; Al-Zaid, N.; Dashti, H.M. Effect of Low-Calorie versus Low-Carbohydrate Ketogenic Diet in Type 2 Diabetes. Nutrition 2012, 28, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Saslow, L.R.; Daubenmier, J.J.; Moskowitz, J.T.; Kim, S.; Murphy, E.J.; Phinney, S.D.; Ploutz-Snyder, R.; Goldman, V.; Cox, R.M.; Mason, A.E.; et al. Twelve-Month Outcomes of a Randomized Trial of a Moderate-Carbohydrate versus Very Low-Carbohydrate Diet in Overweight Adults with Type 2 Diabetes Mellitus or Prediabetes. Nutr. Diabetes 2017, 7, 304. [Google Scholar] [CrossRef]
- Li, X.; Yang, J.; Zhou, X.; Dai, C.; Kong, M.; Xie, L.; Liu, C.; Liu, Y.; Li, D.; Ma, X.; et al. Ketogenic Diet-Induced Bile Acids Protect against Obesity through Reduced Calorie Absorption. Nat. Metab. 2024, 6, 1397–1414. [Google Scholar] [CrossRef]
- Lindefeldt, M.; Eng, A.; Darban, H.; Bjerkner, A.; Zetterström, C.K.; Allander, T.; Andersson, B.; Borenstein, E.; Dahlin, M.; Prast-Nielsen, S. The Ketogenic Diet Influences Taxonomic and Functional Composition of the Gut Microbiota in Children with Severe Epilepsy. npj Biofilms Microbiomes 2019, 5, 5. [Google Scholar] [CrossRef]
- Duncan, S.H.; Belenguer, A.; Holtrop, G.; Johnstone, A.M.; Flint, H.J.; Lobley, G.E. Reduced Dietary Intake of Carbohydrates by Obese Subjects Results in Decreased Concentrations of Butyrate and Butyrate-Producing Bacteria in Feces. Appl. Environ. Microbiol. 2007, 73, 1073–1078. [Google Scholar] [CrossRef]
- Godos, J.; Guglielmetti, M.; Ferraris, C.; Frias-Toral, E.; Domínguez Azpíroz, I.; Lipari, V.; Di Mauro, A.; Furnari, F.; Castellano, S.; Galvano, F.; et al. Mediterranean Diet and Quality of Life in Adults: A Systematic Review. Nutrients 2025, 17, 577. [Google Scholar] [CrossRef]
- Meslier, V.; Laiola, M.; Roager, H.M.; De Filippis, F.; Roume, H.; Quinquis, B.; Giacco, R.; Mennella, I.; Ferracane, R.; Pons, N.; et al. Mediterranean Diet Intervention in Overweight and Obese Subjects Lowers Plasma Cholesterol and Causes Changes in the Gut Microbiome and Metabolome Independently of Energy Intake. Gut 2020, 69, 1258–1268. [Google Scholar] [CrossRef]
- Vitale, M.; Giacco, R.; Laiola, M.; Della Pepa, G.; Luongo, D.; Mangione, A.; Salamone, D.; Vitaglione, P.; Ercolini, D.; Rivellese, A.A. Acute and Chronic Improvement in Postprandial Glucose Metabolism by a Diet Resembling the Traditional Mediterranean Dietary Pattern: Can SCFAs Play a Role? Clin. Nutr. 2021, 40, 428–437. [Google Scholar] [CrossRef]
- Ismael, S.; Silvestre, M.P.; Vasques, M.; Araújo, J.R.; Morais, J.; Duarte, M.I.; Pestana, D.; Faria, A.; Pereira-Leal, J.B.; Vaz, J.; et al. A Pilot Study on the Metabolic Impact of Mediterranean Diet in Type 2 Diabetes: Is Gut Microbiota the Key? Nutrients 2021, 13, 1228. [Google Scholar] [CrossRef] [PubMed]
- Forslund, K.; Hildebrand, F.; Nielsen, T.; Falony, G.; Le Chatelier, E.; Sunagawa, S.; Prifti, E.; Vieira-Silva, S.; Gudmundsdottir, V.; Krogh Pedersen, H.; et al. Disentangling Type 2 Diabetes and Metformin Treatment Signatures in the Human Gut Microbiota. Nature 2015, 528, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Takewaki, F.; Nakajima, H.; Takewaki, D.; Hashimoto, Y.; Majima, S.; Okada, H.; Senmaru, T.; Ushigome, E.; Hamaguchi, M.; Yamazaki, M.; et al. Habitual Dietary Intake Affects the Altered Pattern of Gut Microbiome by Acarbose in Patients with Type 2 Diabetes. Nutrients 2021, 13, 2107. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Hashimoto, Y.; Hamaguchi, M.; Kaji, A.; Sakai, R.; Inoue, R.; Kashiwagi, S.; Mizushima, K.; Uchiyama, K.; Takagi, T.; et al. Effects of Smoking on the Gut Microbiota in Individuals with Type 2 Diabetes Mellitus. Nutrients 2022, 14, 4800. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial Sweeteners Induce Glucose Intolerance by Altering the Gut Microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Plaza-Díaz, J.; Sáez-Lara, M.J.; Gil, A. Effects of Sweeteners on the Gut Microbiota: A Review of Experimental Studies and Clinical Trials. Adv. Nutr. 2019, 10, S31–S48. [Google Scholar] [CrossRef]

| References | Year | Intervention | Genus | Study Type | RCT | Patients | Sample Size | Main Results (Intervention Group vs. Control Group) |
|---|---|---|---|---|---|---|---|---|
| Paul et al. [156] | 2022 | Probiotics, prebiotics, and synbiotics | Probiotics: Lactobacillus, Bifidobacterium, Streptococcus, Lactococcus, Bacillus, Acetobacter, Propionibacterium, Akkermansia, Clostridium, amd Anaerobutyricum. Prebiotics: inulin/FOS, GOS, resistant dextrin, resistant starch, beta-glucan, and mixed/complex prebiotics. | Meta-analysis | 68 RCTs | T2DM | n = 3835 | fasting glucose ↓ HbA1c ↓ fasting insulin ↓ HOMA-IR ↓ QUICKI ↑ |
| Naseri et al. [157] | 2022 | Probiotics and synbiotics | / | Meta-analysis | 46 RCTs | T2DM | n = 3067 | FPG ↓ HbA1c ↓ fasting insulin ↓ HOMA-IR ↓ QUICKI ↑ |
| Rittiphairoj et al. [158] | 2021 | Probiotics | Probiotics: Lactobacillus, Bifidobacterium, Streptococcus, Lactococcus, Bacillus, Propionibacterium, Acetobacter, and Enterococcus. | Meta-analysis | 28 RCTs | Prediabetes/T2DM | n = 1947 | FPG ↓ |
| Li et al. [159] | 2023 | Probiotics | Probiotics: Lactobacillus, Bifidobacterium, Streptococcus, Lactococcus, Bacillus, Saccharomyces, Propionibacterium, and Acetobacter. | Meta-analysis | 30 RCTs | T2DM | n = 1827 | FPG ↓ HbA1c ↓ fasting insulin ↓ HOMA-IR ↓ |
| Tabrizi et al. [160] | 2017 | Synbiotic | Lactobacillus, Bifidobacterium. | Meta-analysis | 7 RCTs | T2DM/GDM | n = 482 | FPG ↓ insulin ↓ HOMA-β ↓ QUICKI ↑ |
| Baroni et al. [161] | 2024 | Probiotics/synbiotics | Probiotics: Lactobacillus, Bifidobacterium, Streptococcus, Lactococcus, Bacillus, Clostridium, Akkermansia, Anaerobutyricum, Propionibacterium, Acetobacter, and Saccharomyces. | Meta-analysis | 41 RCTs | T1DM/T2DM | n = 2991 | FPG ↓ HbA1c ↓ fasting insulin ↓ |
| Jayedi et al. [162] | 2024 | Probiotics, prebiotics, and synbiotics | Probiotics: Lactobacillus, Bifidobacterium, Streptococcus, Propionibacterium, and Bacillus. | Meta-analysis | 68 RCTs | T2DM | n = 4249 | FPG ↓ HbA1c ↓ |
| Xiao et al. [163] | 2023 | Probiotic | Probiotics: Lactobacillus, Bifidobacterium, Streptococcus, Propionibacterium, Acetobacter, and Saccharomyces. | Meta-analysis | 37 RCTs | T2DM | n = 2502 | FPG ↓ HbA1c ↓ fasting insulin ↓ HOMA-IR ↓ |
| Zhang et al. [164] | 2022 | Probiotics | / | Meta-analysis | 33 RCTs | T2DM | n = 1927 | FPG ↓ HbA1c ↓ fasting insulin(-) HOMA-IR ↓ |
| Bock et al. [165] | 2021 | Probiotics, prebiotics, or synbiotics | Probiotics: Lactobacillus, Bifidobacterium, Streptococcus, Lactococcus, Propionibacterium, Acetobacter, Bacillus, and Saccharomyces. | Meta-analysis | 38 RCTs | T1DM/T2DM | n = 2086 | FPG ↓ HbA1c(-) fasting insulin ↓ |
| Dimba et al. [166] | 2024 | Prebiotics/Mediterranean/plant-based diet | / | Meta-analysis | 8 RCTs | Prediabetes/T2DM | n = 488 | Prebiotics:FPG(-), HbA1c(-) MD:FPG ↓ HbA1c ↓ Plant-Based Diet:FPG(-),HbA1c(-) |
| Ojo et al. [167] | 2020 | Dietary fiber | Bifidobacterium ↑ | Meta-analysis | 9 RCTs | T2DM | n = 704 | HbA1c ↓ FPG(-) HOMA-IR(-) |
| Houghton et al. [168] | 2018 | Synbiotic supplementation, strict vegetarian diet, Ma-Pi diet, Type 2 diabetes diet with increased sardine intake, probiotic supplementation, prebiotic supplementation, digestive supplement | No specific genus change, but altered gut microbiota diversity and structure. | Meta-analysis | 8 RCTs | T2DM | n = 395 | HbA1c ↓ FPG(-), fasting insulin(-), HOMA-IR(-) |
| Ojo et al. [169] | 2021 | Almonds | Promoting short-chain fatty acid-producing bacteria | Meta-analysis | 8 RCTs | T2DM | n = 221 | HbA1c ↓ FBG(-), fasting insulin(-), HOMA-IR(-) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Ding, Y.; Wang, S.; Jiang, L. Gut Microbiota Dysbiosis and Its Impact on Type 2 Diabetes: From Pathogenesis to Therapeutic Strategies. Metabolites 2025, 15, 397. https://doi.org/10.3390/metabo15060397
Yu Y, Ding Y, Wang S, Jiang L. Gut Microbiota Dysbiosis and Its Impact on Type 2 Diabetes: From Pathogenesis to Therapeutic Strategies. Metabolites. 2025; 15(6):397. https://doi.org/10.3390/metabo15060397
Chicago/Turabian StyleYu, Yonghua, Yilan Ding, Shuangyuan Wang, and Lei Jiang. 2025. "Gut Microbiota Dysbiosis and Its Impact on Type 2 Diabetes: From Pathogenesis to Therapeutic Strategies" Metabolites 15, no. 6: 397. https://doi.org/10.3390/metabo15060397
APA StyleYu, Y., Ding, Y., Wang, S., & Jiang, L. (2025). Gut Microbiota Dysbiosis and Its Impact on Type 2 Diabetes: From Pathogenesis to Therapeutic Strategies. Metabolites, 15(6), 397. https://doi.org/10.3390/metabo15060397





