Combining Metabolomics and Proteomics to Reveal Key Serum Compounds Related to Canine Intervertebral Disc Herniation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Serum Sample Collection
2.3. Untargeted Metabolomic Analysis
2.4. Serum Zinc Determination
2.5. High-Resolution Mass-Spectrometry-Based Proteomic Analysis
2.6. Analytical Validation of Proteomic Results
2.7. Statistical and Bioinformatic Analyses
3. Results
3.1. Untargeted Serum Metabolomics of Canine IVDH Patients
3.2. Serum Zinc Levels
3.3. Proteomic Analysis
3.4. Metabolomics and Proteomics Data Integration
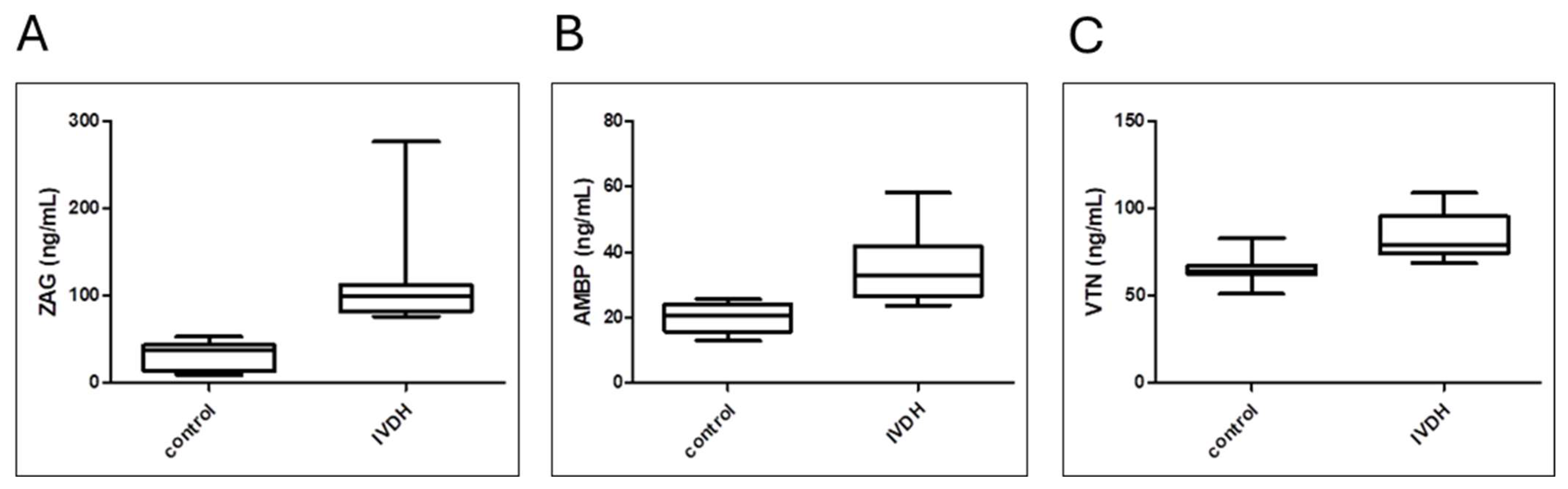
3.5. Analytical Validation of Proteomic Results by ELISA
4. Discussion
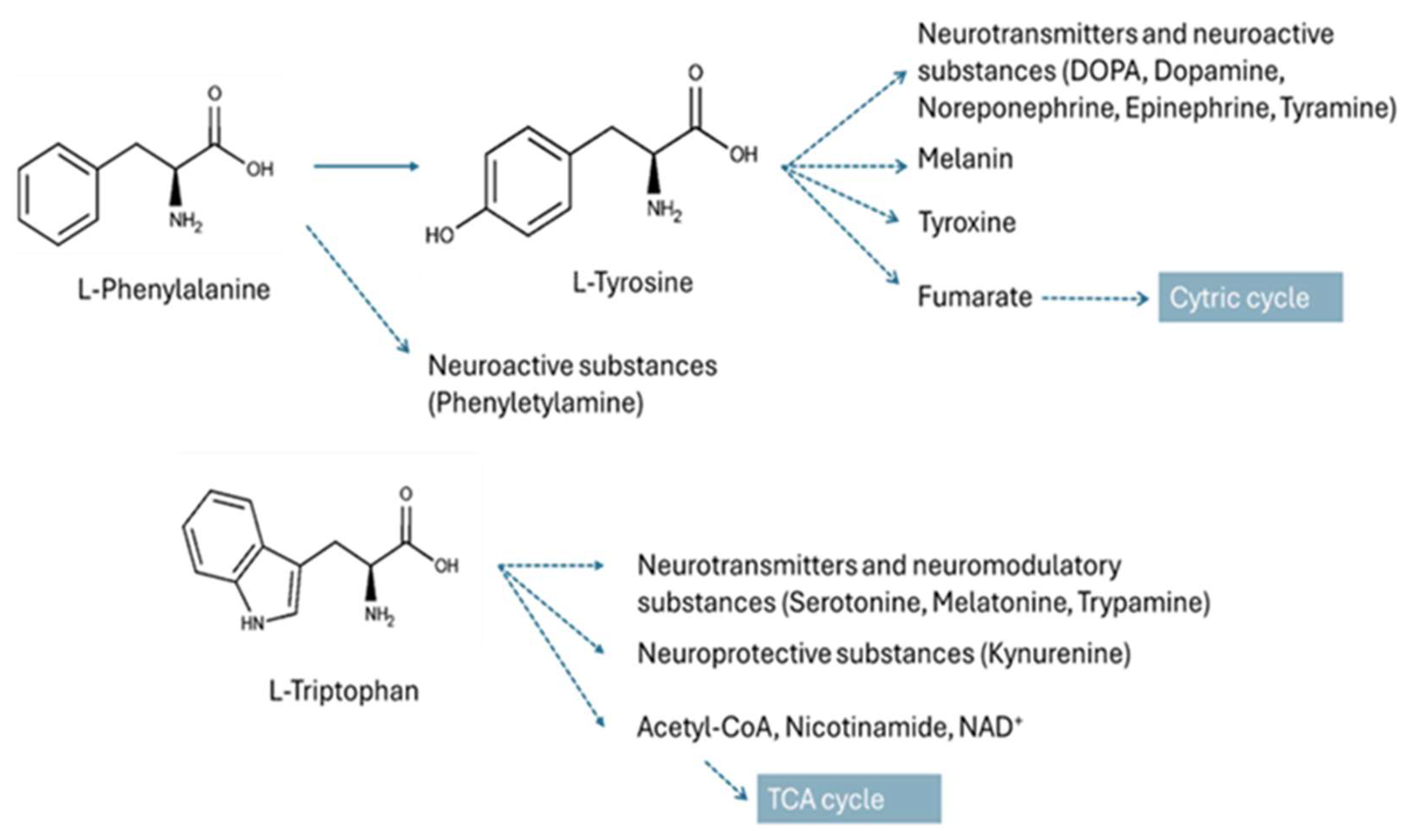
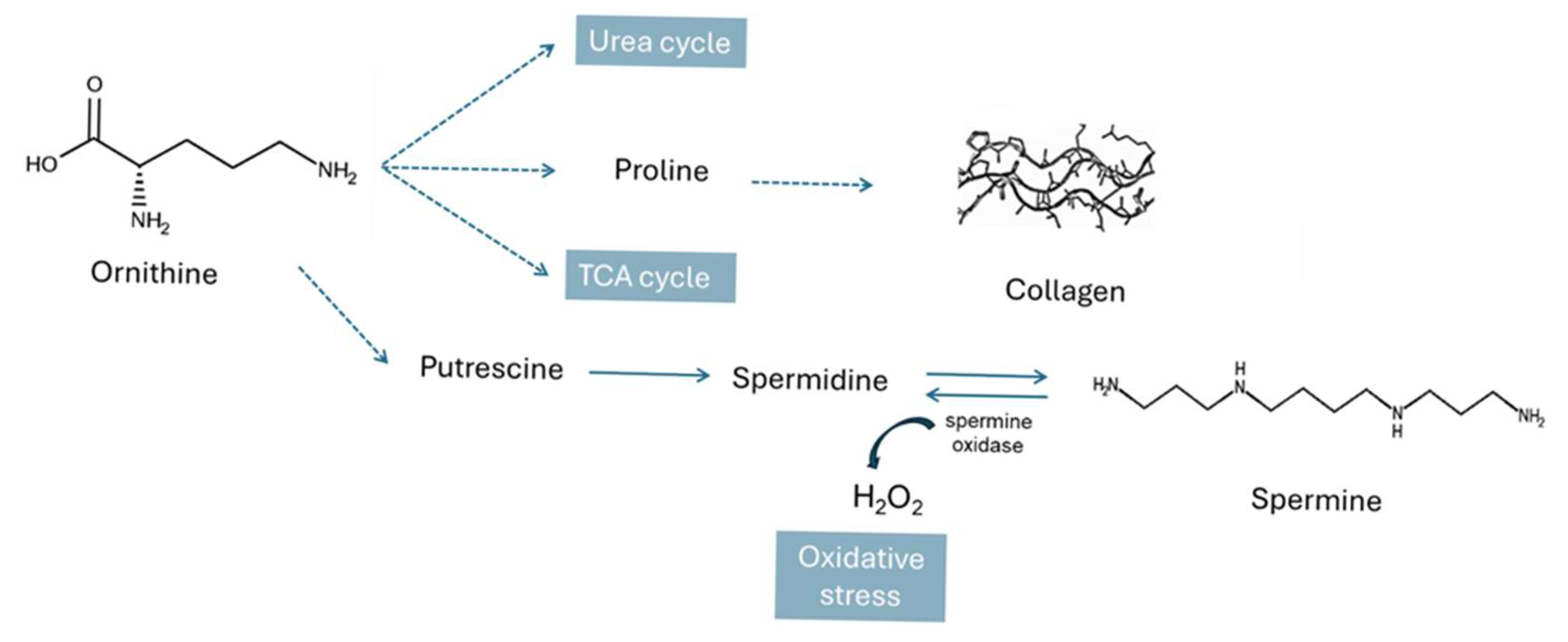
4.1. Metabolic Pathways Altered in IVDH
4.2. Serum Proteome Changes in Canine IVDH
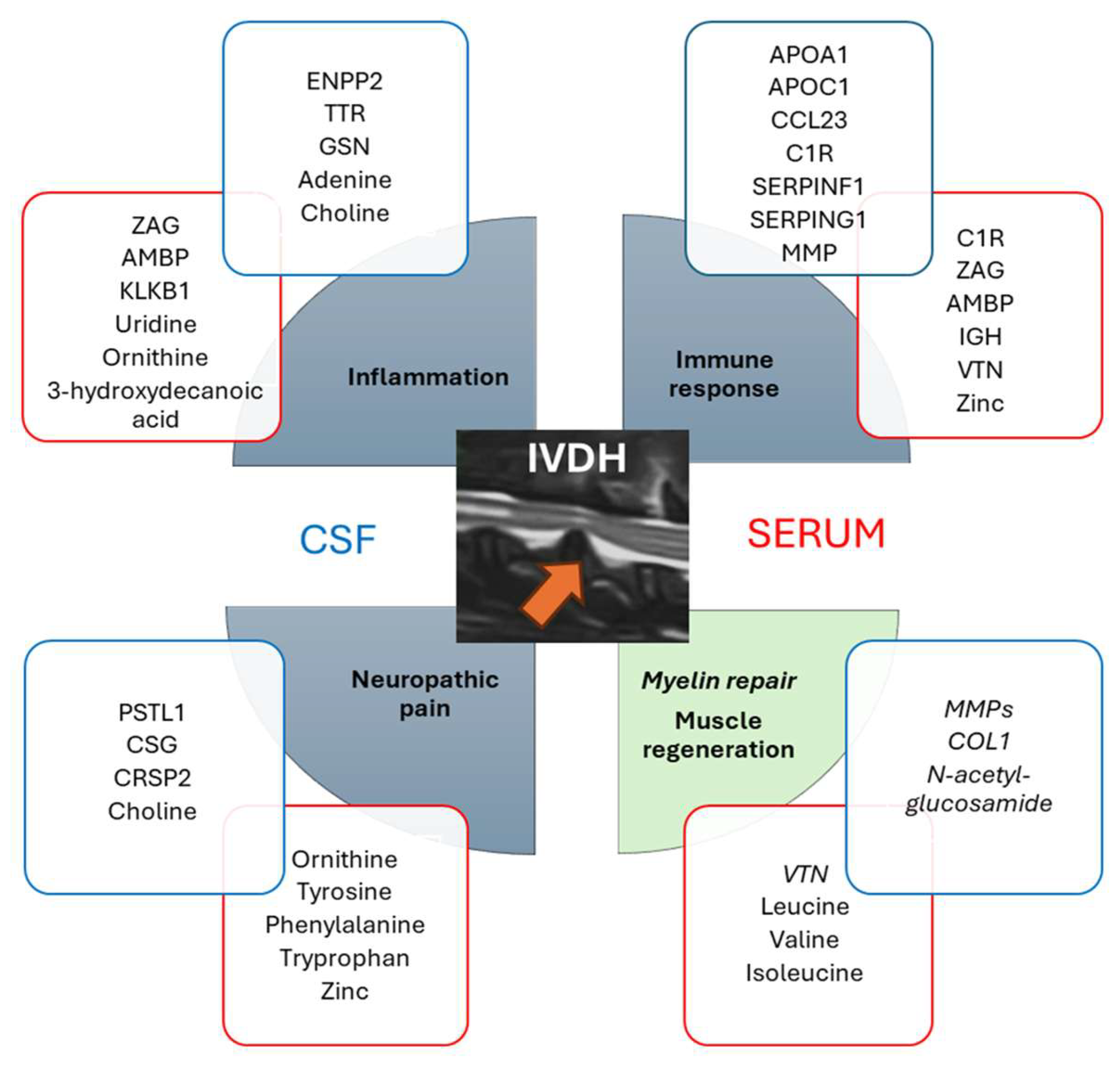
4.3. Compilation of Serum and CSF Omics Results Reveals a Cascade of Events Related to Canine IVDH
4.4. Strengths and Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGC | Automatic gain control |
| AMBP | Alpha-1-microglobulin/bikunin precursor |
| AOC3 | Amine oxidase copper containing 3 |
| BCAA | Branched-chain amino acid |
| CSF | Cerebrospinal fluid |
| CNS | Central nervous system |
| CT | Computed tomography |
| DDA | Data-dependent acquisition |
| FDR | False discovery rate |
| GO | Gene Ontology |
| HCD | Higher-energy collision dissociation |
| IVD | Intervertebral disc |
| IVDH | Intervertebral disc herniation |
| KLKB1 | Prekallikrein |
| MRI | Magnetic resonance imaging |
| NCE | Normalized collision energy |
| ROS | Reactive oxygen species |
| SERPINA5 | Protein C inhibitor |
| TMT | Tandem mass tag |
| VTN | Vitronectin |
| ZAG | Zinc-α2-glycoprotein |
References
- Adams, M.A.; Roughley, P.J. What Is Intervertebral Disc Degeneration, and What Causes It? Spine 2006, 31, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.N.; Kramer, J.S.; Stoker, A.M.; Bozynski, C.C.; Cook, C.R.; Stannard, J.T.; Choma, T.J.; Cook, J.L. Canine Models of Spine Disorders. JOR Spine 2020, 3, e1109. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Kim, H.; Jeon, W.J.; Yeo, C.; Kim, H.; Lee, J.; Lee, Y.J.; Ha, I.H. Animal Models of Intervertebral Disc Diseases: Advantages, Limitations, and Future Directions. Neurol. Int. 2024, 16, 1788–1818. [Google Scholar] [CrossRef] [PubMed]
- da Costa, R.C.; De Decker, S.; Lewis, M.J.; Volk, H.; Moore, S.A.; Olby, N.J.; Levine, J.M.; Lewis, M.J.; Jeffery, N.D.; Mullins, M.E.; et al. Diagnostic Imaging in Intervertebral Disc Disease. Front. Vet. Sci. 2020, 7, 588338. [Google Scholar] [CrossRef]
- Ravichandran, D.; Pillai, J.; Krishnamurthy, K. Genetics of Intervertebral Disc Disease: A Review. Clin. Anat. 2022, 35, 116–120. [Google Scholar] [CrossRef]
- Levine, J.M.; Levine, G.J.; Porter, B.F.; Topp, K.; Noble-Haeusslein, L.J. Naturally Occurring Disk Herniation in Dogs: An Opportunity for Pre-Clinical Spinal Cord Injury Research. J. Neurotrauma 2011, 28, 675–688. [Google Scholar] [CrossRef]
- Fenn, J.; Olby, N.J. Classification of Intervertebral Disc Disease. Front. Vet. Sci. 2020, 7, 579025. [Google Scholar] [CrossRef]
- Slatter, D.H. Textbook of Small Animal Surgery, 3rd ed.; Saunders: Philadelphia, PA, USA, 2003. [Google Scholar]
- Liawrungrueang, W.; Kim, P.; Kotheeranurak, V.; Jitpakdee, K.; Sarasombath, P. Automatic Detection, Classification, and Grading of Lumbar Intervertebral Disc Degeneration Using an Artificial Neural Network Model. Diagnostics 2023, 13, 663. [Google Scholar] [CrossRef]
- Moore, S.A.; Tipold, A.; Olby, N.J.; Stein, V.; Granger, N. Current Approaches to the Management of Acute Thoracolumbar Disc Extrusion in Dogs. Front. Vet. Sci. 2020, 7, 610. [Google Scholar] [CrossRef]
- Binch, A.L.A.; Fitzgerald, J.C.; Growney, E.A.; Barry, F. Cell-Based Strategies for IVD Repair: Clinical Progress and Translational Obstacles. Nat. Rev. Rheumatol. 2021, 17, 158–175. [Google Scholar] [CrossRef]
- Tuck, M.K.; Chan, D.W.; Chia, D.; Godwin, A.K.; Grizzle, W.E.; Krueger, K.E.; Rom, W.; Sanda, M.; Sorbara, L.; Stass, S.; et al. Standard Operating Procedures for Serum and Plasma Collection: Early Detection Research Network Consensus Statement Standard Operating Procedure Integration Working Group. J. Proteome Res. 2009, 8, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Jesuthasan, A.; Ali, A.; Lee, J.K.W.; Rutherfurd-Markwick, K. Assessment of Changes in Physiological Markers in Different Body Fluids at Rest and after Exercise. Nutrients 2022, 14, 4685. [Google Scholar] [CrossRef] [PubMed]
- Horvatic, A.; Gelemanovic, A.; Pirkic, B.; Smolec, O.; Ljubic, B.B.; Rubic, I.; Eckersall, P.D.; Mrljak, V.; McLaughlin, M.; Samardzija, M.; et al. Multi-Omics Approach to Elucidate Cerebrospinal Fluid Changes in Dogs with Intervertebral Disc Herniation. Int. J. Mol. Sci. 2021, 22, 11678. [Google Scholar] [CrossRef]
- Guillemin, N.; Horvatić, A.; Kuleš, J.; Galan, A.; Mrljak, V.; Bhide, M. Omics Approaches to Probe Markers of Disease Resistance in Animal Sciences. Mol. Biosyst. 2016, 12, 2036–2046. [Google Scholar] [CrossRef]
- Schumacher-Schuh, A.; Bieger, A.; Borelli, W.V.; Portley, M.K.; Awad, P.S.; Bandres-Ciga, S. Advances in Proteomic and Metabolomic Profiling of Neurodegenerative Diseases. Front. Neurol. 2022, 12, 792227. [Google Scholar] [CrossRef]
- Yee, A.; Lam, M.P.Y.; Tam, V.; Chan, W.C.W.; Chu, I.K.; Cheah, K.S.E.; Cheung, K.M.C.; Chan, D. Fibrotic-like Changes in Degenerate Human Intervertebral Discs Revealed by Quantitative Proteomic Analysis. Osteoarthr. Cartil. 2016, 24, 503–513. [Google Scholar] [CrossRef]
- Radek, M.; Pacholczyk-Sienicka, B.; Jankowski, S.; Albrecht, Ł.; Grodzka, M.; Depta, A.; Radek, A. Assessing the Correlation between the Degree of Disc Degeneration on the Pfirrmann Scale and the Metabolites Identified in HR-MAS NMR Spectroscopy. Magn. Reson. Imaging 2016, 34, 376–380. [Google Scholar] [CrossRef]
- Seo, H.-J.; Cho, Y.-E.; Kim, T.; Shin, H.-I.; Kwun, I.-S. Zinc May Increase Bone Formation through Stimulating Cell Proliferation, Alkaline Phosphatase Activity and Collagen Synthesis in Osteoblastic MC3T3-E1 Cells. Nutr. Res. Pract. 2010, 4, 356. [Google Scholar] [CrossRef]
- Kiouri, D.P.; Chasapis, C.T.; Mavromoustakos, T.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and Its Binding Proteins: Essential Roles and Therapeutic Potential. Arch. Toxicol. 2024, 99, 23–41. [Google Scholar] [CrossRef]
- Staszkiewicz, R.; Bryś, K.; Gładysz, D.; Gralewski, M.; Garczarek, M.; Gadzieliński, M.; Wieczorek, J.; Marcol, W.; Ostenda, A.; Grabarek, B.O. Changes in Elements and Relationships among Elements in Intervertebral Disc Degeneration. Int. J. Environ. Res. Public Health 2022, 19, 9042. [Google Scholar] [CrossRef]
- Pereira, A.M.; Maia, M.R.G.; Fonseca, A.J.M.; Cabrita, A.R.J. Zinc in Dog Nutrition, Health and Disease: A Review. Animals 2021, 11, 978. [Google Scholar] [CrossRef] [PubMed]
- Baka, R.; Eckersall, D.; Horvatic, A.; Gelemanovic, A.; Mrljak, V.; McLaughlin, M.; Athanasiou, L.V.; Papaioannou, N.; Stylianaki, I.; Hanh, H.Q.; et al. Quantitative Proteomics of Cerebrospinal Fluid Using Tandem Mass Tags in Dogs with Recurrent Epileptic Seizures. J. Proteom. 2021, 231, 103997. [Google Scholar] [CrossRef] [PubMed]
- Gloaguen, Y.; Morton, F.; Daly, R.; Gurden, R.; Rogers, S.; Wandy, J.; Wilson, D.; Barrett, M.; Burgess, K. PiMP My Metabolome: An Integrated, Web-Based Tool for LC-MS Metabolomics Data. Bioinformatics 2017, 33, 4007–4009. [Google Scholar] [CrossRef]
- Holman, J.D.; Tabb, D.L.; Mallick, P. Employing ProteoWizard to Convert Raw Mass Spectrometry Data. Curr. Protoc. Bioinform. 2014, 46, 13.24.1–13.24.9. [Google Scholar] [CrossRef]
- Horvatic, A.; Guillemin, N.; Kaab, H.; McKeegan, D.; O’Reilly, E.; Bain, M.; Kules, J.; Eckersall, P.D. Quantitative Proteomics Using Tandem Mass Tags in Relation to the Acute Protein Response in Chicken Challenged with Escherichia coli Lipopolysaccharide Endotoxin. J. Proteom. 2019, 192, 64–77. [Google Scholar] [CrossRef]
- Di Guida, R.; Engel, J.; Allwood, J.W.; Weber, R.J.M.; Jones, M.R.; Sommer, U.; Viant, M.R.; Dunn, W.B. Non-Targeted UHPLC-MS Metabolomic Data Processing Methods: A Comparative Investigation of Normalisation, Missing Value Imputation, Transformation and Scaling. Metabolomics 2016, 12, 93. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Komsta, L. Outliers: Tests for Outliers; R Package Version 0.14; 2011. Available online: https://CRAN.R-project.org/package=outliers (accessed on 4 May 2022).
- Wickham, H. About the Ggplot2 Package. J. Appl. Comput. Math. 2016, 5, 321. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein–Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Shannon, P. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. ClusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, Y.; Anton, M.; Nielsen, J. GotEnzymes: An Extensive Database of Enzyme Parameter Predictions. Nucleic Acids Res. 2023, 51, D583–D586. [Google Scholar] [CrossRef] [PubMed]
- Alini, M.; Diwan, A.D.; Erwin, W.M.; Little, C.B.; Melrose, J. An Update on Animal Models of Intervertebral Disc Degeneration and Low Back Pain: Exploring the Potential of Artificial Intelligence to Improve Research Analysis and Development of Prospective Therapeutics. JOR Spine 2023, 6, e1230. [Google Scholar] [CrossRef] [PubMed]
- eBergknut, N.; Egenvall, A.; Hagman, R.; Gustås, P.; Hazewinkel, H.A.; Meij, B.P.; Lagerstedt, A.S. Incidence of intervertebral disk degeneration-related diseases and associated mortality rates in dogs. J. Am. Vet. Med. Assoc. 2012, 240, 1300–1309. [Google Scholar] [CrossRef]
- Spitzbarth, I.; Moore, S.A.; Stein, V.M.; Levine, J.M.; Olby, N.J.; Gjessing, K.M.; Davidson, R.M.; Lewis, M.J.; Jeffery, N.D.; da Costa, R.C.; et al. Current Insights Into the Pathology of Canine Intervertebral Disc Extrusion-Induced Spinal Cord Injury. Front. Vet. Sci. 2020, 7, 595796. [Google Scholar] [CrossRef]
- Foreman, M.; Vettorato, E.; Caine, A.; Monti, P.; Cherubini, G.B.; Eminaga, S. Serum C-Reactive Protein in Dogs with Paraplegia Secondary to Acute Intervertebral Disc Extrusion. J. Vet. Intern. Med. 2021, 35, 1857–1864. [Google Scholar] [CrossRef]
- Nishida, H.; Nakayama, M.; Tanaka, H.; Kamishina, H.; Izawa, T.; Hatoya, S.; Sugiura, K.; Suzuki, Y.; Ide, C.; Inaba, T. Evaluation of Serum Phosphorylated Neurofilament Subunit NF-H as a Prognostic Biomarker in Dogs with Thoracolumbar Intervertebral Disc Herniation. Vet. Surg. 2014, 43, 289–293. [Google Scholar] [CrossRef]
- Bilić, P.; Kuleš, J.; Galan, A.; Gomes de Pontes, L.; Guillemin, N.; Horvatić, A.; Festa Sabes, A.; Mrljak, V.; Eckersall, P.D. Proteomics in Veterinary Medicine and Animal Science: Neglected Scientific Opportunities with Immediate Impact. Proteomics 2018, 18, e1800047. [Google Scholar] [CrossRef]
- Ratan, K.C.; Sunil Kumar, B.V.; Mukhopadhyay, C.S.; Kashyap, N.; Sharma, V.; Singh, N.; Tazerji, S.S.; Kalantari, R.; Hajipour, P.; Malik, Y.S. Animal Wellness: The Power of Multiomics and Integrative Strategies. Vet. Med. Int. 2024, 2024, 4125118. [Google Scholar] [CrossRef]
- Francisco, V.; Ait Eldjoudi, D.; González-Rodríguez, M.; Ruiz-Fernández, C.; Cordero-Barreal, A.; Marques, P.; Sanz, M.J.; Real, J.T.; Lago, F.; Pino, J.; et al. Metabolomic Signature and Molecular Profile of Normal and Degenerated Human Intervertebral Disc Cells. Spine J. 2023, 23, 1549–1562. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, X.; Lv, J.; Mei, Y.; Luo, Y.; Li, F.; Liu, Z. Analysis of Key Differential Metabolites in Intervertebral Disc Degeneration Based on Untargeted Metabolomics. JOR Spine 2025, 8, e70032. [Google Scholar] [CrossRef] [PubMed]
- Bo, T.; Fujii, J. Primary Roles of Branched Chain Amino Acids (BCAAs) and Their Metabolism in Physiology and Metabolic Disorders. Molecules 2025, 30, 56. [Google Scholar] [CrossRef] [PubMed]
- Solmonson, A.; DeBerardinis, R.J. Lipoic Acid Metabolism and Mitochondrial Redox Regulation. J. Biol. Chem. 2018, 293, 7522–7530. [Google Scholar] [CrossRef] [PubMed]
- Mann, G.; Mora, S.; Madu, G.; Adegoke, O.A.J. Branched-Chain Amino Acids: Catabolism in Skeletal Muscle and Implications for Muscle and Whole-Body Metabolism. Front Physiol. 2021, 12, 702826. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, S.; Xie, C.; Fang, J. Uridine Metabolism and Its Role in Glucose, Lipid, and Amino Acid Homeostasis. BioMed Res. Int. 2020, 2020, 7091718. [Google Scholar] [CrossRef]
- Du, C.; Liu, W.-J.; Yang, J.; Zhao, S.-S.; Liu, H.-X. The Role of Branched-Chain Amino Acids and Branched-Chain α-Keto Acid Dehydrogenase Kinase in Metabolic Disorders. Front. Nutr. 2022, 9, 932670. [Google Scholar] [CrossRef]
- Yoo, H.-S.; Shanmugalingam, U.; Smith, P.D. Potential Roles of Branched-Chain Amino Acids in Neurodegeneration. Nutrition 2022, 103–104, 111762. [Google Scholar] [CrossRef]
- Dodd, K.M.; Tee, A.R. Leucine and MTORC1: A Complex Relationship. Am. J. Physiol. Endocrinol. Metab. 2012, 302, 1329–1342. [Google Scholar] [CrossRef]
- Kato, H.; Miura, K.; Nakano, S.; Suzuki, K.; Bannai, M.; Inoue, Y. Leucine-Enriched Essential Amino Acids Attenuate Inflammation in Rat Muscle and Enhance Muscle Repair after Eccentric Contraction. Amino Acids 2016, 48, 2145–2155. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Cross, P.J.; Dobson, R.C.J.; Adams, L.E.; Savka, M.A.; Hudson, A.O. A Three-Ring Circus: Metabolism of the Three Proteogenic Aromatic Amino Acids and Their Role in the Health of Plants and Animals. Front. Mol. Biosci. 2018, 5, 29. [Google Scholar] [CrossRef]
- Fernstrom, J.D. Effects of the Diet on Brain Neurotransmitters. Metabolism 1977, 26, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yu, S.; Long, Y.; Shi, A.; Deng, J.; Ma, Y.; Wen, J.; Li, X.; Liu, S.; Zhang, Y.; et al. Tryptophan Metabolism: Mechanism-Oriented Therapy for Neurological and Psychiatric Disorders. Front. Immunol. 2022, 13, 985378. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Zhao, X.; Zhang, Y.; Jiao, Y.; Liu, Y.; Gao, C.; Zhang, J.; Ma, Y.; Wang, Z.; Li, T. Using Integrated Network Pharmacology and Metabolomics to Reveal the Mechanisms of the Combined Intervention of Ligustrazine and Sinomenine in CCI-Induced Neuropathic Pain Rats. Int. J. Mol. Sci. 2025, 26, 2604. [Google Scholar] [CrossRef]
- Ha, H.C.; Sirisoma, N.S.; Kuppusamy, P.; Zweier, J.L.; Woster, P.M.; Casero, R.A. The Natural Polyamine Spermine Functions Directly as a Free Radical Scavenger. Proc. Natl. Acad. Sci. USA 1998, 95, 11140–11145. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Qi, C.; Shen, L.; Wang, J.; Liu, X.; Zhang, N.; Bing, T.; Shangguan, D. Oxidative Degradation of Polyamines by Serum Supplement Causes Cytotoxicity on Cultured Cells. Sci. Rep. 2018, 8, 10384. [Google Scholar] [CrossRef]
- Narayanan, S.P.; Shosha, E.; Palani, C.D. Spermine Oxidase: A Promising Therapeutic Target for Neurodegeneration in Diabetic Retinopathy. Pharmacol. Res. 2019, 147, 104299. [Google Scholar] [CrossRef]
- Maccarrone, M.; Bari, M.; Battista, N.; Di Rienzo, M.; Falciglia, K.; Finazzi Agro, A.; Skulachev, V. Oxidation Products of Polyamines Induce Mitochondrial Uncoupling and Cytochrome c Release. FEBS Lett. 2001, 507, 30–34. [Google Scholar] [CrossRef]
- Duan, B.; Wang, Y.Z.; Yang, T.; Chu, X.P.; Yu, Y.; Huang, Y.; Cao, H.; Hansen, J.; Simon, R.P.; Zhu, M.X.; et al. Extracellular Spermine Exacerbates Ischemic Neuronal Injury through Sensitization of ASIC1a Channels to Extracellular Acidosis. J. Neurosci. 2011, 31, 2101–2112. [Google Scholar] [CrossRef]
- Cervelli, M.; Amendola, R.; Polticelli, F.; Mariottini, P. Spermine Oxidase: Ten Years After. Amino Acids 2012, 42, 441–450. [Google Scholar] [CrossRef]
- Liu, F.; Saul, A.B.; Pichavaram, P.; Xu, Z.; Rudraraju, M.; Somanath, P.R.; Smith, S.B.; Caldwell, R.B.; Narayanan, S.P. Pharmacological Inhibition of Spermine Oxidase Reduces Neurodegeneration and Improves Retinal Function in Diabetic Mice. J. Clin. Med. 2020, 9, 340. [Google Scholar] [CrossRef]
- Sivashanmugam, M.; Jaidev, J.; Umashankar, V.; Sulochana, K.N. Ornithine and Its Role in Metabolic Diseases: An Appraisal. Biomed. Pharmacother. 2017, 86, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ning, N.; Li, B.; Luo, D.; Qin, E.; Yu, W.; Wang, J.; Yang, G.; Nan, N.; He, Z.; et al. Longitudinal Metabolomics Reveals Ornithine Cycle Dysregulation Correlates With Inflammation and Coagulation in COVID-19 Severe Patients. Front. Microbiol. 2021, 12, 723818. [Google Scholar] [CrossRef] [PubMed]
- Mäntyselkä, P.; Ali-Sisto, T.; Kautiainen, H.; Niskanen, L.; Viinamäki, H.; Velagapudi, V.; Lehto, S.M. The Association Between Musculoskeletal Pain and Circulating Ornithine: A Population-Based Study. Pain Med. 2017, 18, 1145–1151. [Google Scholar] [CrossRef]
- Karna, E.; Szoka, L.; Huynh, T.Y.L.; Palka, J.A. Proline-Dependent Regulation of Collagen Metabolism. Cell. Mol. Life Sci. 2020, 77, 1911–1918. [Google Scholar] [CrossRef]
- Varma, S.; Orgel, J.P.R.O.; Schieber, J.D. Contrasting Local and Macroscopic Effects of Collagen Hydroxylation. Int. J. Mol. Sci. 2021, 22, 9068. [Google Scholar] [CrossRef]
- Suzuki, M.; Takaishi, S.; Nagasaki, M.; Onozawa, Y.; Iino, I.; Maeda, H.; Komai, T.; Oda, T. Medium-Chain Fatty Acid-Sensing Receptor, GPR84, Is a Proinflammatory Receptor. J. Biol. Chem. 2013, 288, 10684–10691. [Google Scholar] [CrossRef]
- Tonin, A.M.; Amaral, A.U.; Busanello, E.N.B.; Grings, M.; Castilho, R.F.; Wajner, M. Long-Chain 3-Hydroxy Fatty Acids Accumulating in Long-Chain 3-Hydroxyacyl-CoA Dehydrogenase and Mitochondrial Trifunctional Protein Deficiencies Uncouple Oxidative Phosphorylation in Heart Mitochondria. J. Bioenerg. Biomembr. 2013, 45, 47–57. [Google Scholar] [CrossRef]
- Natarajan, S.K.; Ibdah, J.A. Role of 3-Hydroxy Fatty Acid-Induced Hepatic Lipotoxicity in Acute Fatty Liver of Pregnancy. Int. J. Mol. Sci. 2018, 19, 322. [Google Scholar] [CrossRef]
- Severyanova, L.A.; Lazarenko, V.A.; Plotnikov, D.V.; Dolgintsev, M.E.; Kriukov, A.A. L-Lysine as the Molecule Influencing Selective Brain Activity in Pain-Induced Behavior of Rats. Int. J. Mol. Sci. 2019, 20, 1899. [Google Scholar] [CrossRef]
- Huang, T.L.; Wu, C.C.; Yu, J.; Sumi, S.; Yang, K.C. L-Lysine Regulates Tumor Necrosis Factor-Alpha and Matrix Metalloproteinase-3 Expression in Human Osteoarthritic Chondrocytes. Process Biochem. 2016, 51, 904–911. [Google Scholar] [CrossRef]
- Rao, D.M.; Battu, S.; Dutt, K.R.; Ramesh, M. Medicinal Uses of L-Lysine: Past and Future. Int. J. Res. Pharm. Sci. 2011, 2, 637–642. [Google Scholar]
- Gospe, S.M. Pyridoxine-Dependent Seizures: Findings From Recent Studies Pose New Questions. Pediatr. Neurol. 2002, 26, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, T.; Liu, X.; Wen, Y.; Xu, T.; Yu, X.; Wei, X.; Ding, X.; Mo, L.; Yin, M.; et al. Overexpression of Zinc-A2-Glycoprotein Suppressed Seizures and Seizure-Related Neuroflammation in Pentylenetetrazol-Kindled Rats. J. Neuroinflammation 2018, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Navarro, I.; Vilchis-Gil, J.; Cossío-Torres, P.E.; Hernández-Mendoza, H.; Klünder-Klünder, M.; Layseca-Espinosa, E.; Galicia-Cruz, O.G.; Rios-Lugo, M.J. Serum Zinc-Alpha-2 Glycoprotein and Zinc Levels and Their Relationship with Insulin Resistance and Biochemical Parameters in Overweight and Obese Children. Biol. Trace Elem. Res. 2024. [Google Scholar] [CrossRef]
- Kitayama, T. Relationship between Neuropathic Pain and Zinc Ion. Glob. Drugs Ther. 2016, 1, 1–2. [Google Scholar] [CrossRef]
- Li, D.; Tian, H.; Li, X.; Mao, L.; Zhao, X.; Lin, J.; Lin, S.; Xu, C.; Liu, Y.; Guo, Y.; et al. Zinc Promotes Functional Recovery after Spinal Cord Injury by Activating Nrf2/HO-1 Defense Pathway and Inhibiting Inflammation of NLRP3 in Nerve Cells. Life Sci. 2020, 245, 117351. [Google Scholar] [CrossRef]
- Zentrichová, V.; Pechová, A.; Kovaříková, S. Zinc Concentration in Blood Serum of Healthy Dogs. Biol. Trace Elem. Res. 2023, 201, 3356–3366. [Google Scholar] [CrossRef]
- van den Berg, C.B.; Duvekot, J.J.; Güzel, C.; Hansson, S.R.; de Leeuw, T.G.; Steegers, E.A.P.; Versendaal, J.; Luider, T.M.; Stoop, M.P. Elevated Levels of Protein AMBP in Cerebrospinal Fluid of Women with Preeclampsia Compared to Normotensive Pregnant Women. Proteom. Clin. Appl. 2017, 11, 1600082. [Google Scholar] [CrossRef]
- Ruzha, Y.; Ni, J.; Quan, Z.; Li, H.; Qing, H. Role of Vitronectin and Its Receptors in Neuronal Function and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 12387. [Google Scholar] [CrossRef]
- Keasey, M.P.; Jia, C.; Pimentel, L.F.; Sante, R.R.; Lovins, C.; Hagg, T. Blood Vitronectin Is a Major Activator of LIF and IL-6 in the Brain through Integrin-FAK and UPAR Signaling. J. Cell Sci. 2018, 131, 202580. [Google Scholar] [CrossRef]
- Zhou, M.; Theologis, A.A.; O’Connell, G.D. Understanding the Etiopathogenesis of Lumbar Intervertebral Disc Herniation: From Clinical Evidence to Basic Scientific Research. JOR Spine 2024, 7, e1289. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.E.; Moore, H.B.; Kornblith, L.Z.; Neal, M.D.; Hoffman, M.; Mutch, N.J.; Schöchl, H.; Hunt, B.J.; Sauaia, A. Trauma-Induced Coagulopathy. Nat. Rev. Dis. Primers 2021, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. The Multi-Functional Serpin, Protein C Inhibitor: Beyond Thrombosis and Hemostasis. J. Thromb. Haemost. 2008, 6, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Vicente, V.; Estelles, A.; Moraleda, J.M.; Espafia, F.; Aznar, J. Fibrinolytic Changes During Acute Vascular Damage Induced by Mediterranean Spotted Fever. Fibrinolysis 1993, 7, 324–329. [Google Scholar] [CrossRef]
- Ivanov, I.; Verhamme, I.M.; Sun, M.-F.; Mohammed, B.; Cheng, Q.; Matafonov, A.; Dickeson, S.K.; Joseph, K.; Kaplan, A.P.; Gailani, D. Protease Activity in Single-Chain Prekallikrein. Blood 2020, 135, 558–567. [Google Scholar] [CrossRef]
- Revenko, A.S.; Gao, D.; Crosby, J.R.; Bhattacharjee, G.; Zhao, C.; May, C.; Gailani, D.; Monia, B.P.; MacLeod, A.R. Selective Depletion of Plasma Prekallikrein or Coagulation Factor XII Inhibits Thrombosis in Mice without Increased Risk of Bleeding. Blood 2011, 118, 5302–5311. [Google Scholar] [CrossRef]
- Jaffa, A.A.; Jaffa, M.A.; Moussa, M.; Ahmed, I.A.; Karam, M.; Aldeen, K.S.; Al Sayegh, R.; El-Achkar, G.A.; Nasrallah, L.; Yehya, Y.; et al. Modulation of Neuro-Inflammatory Signals in Microglia by Plasma Prekallikrein and Neuronal Cell Debris. Front. Pharmacol. 2021, 12, 743059. [Google Scholar] [CrossRef]
- Agostinelli, E.; Tempera, G.; Viceconte, N.; Saccoccio, S.; Battaglia, V.; Grancara, S.; Toninello, A.; Stevanato, R. Potential Anticancer Application of Polyamine Oxidation Products Formed by Amine Oxidase: A New Therapeutic Approach. Amino Acids 2010, 38, 353–368. [Google Scholar] [CrossRef]
- Marttila-Ichihara, F.; Elima, K.; Auvinen, K.; Veres, T.Z.; Rantakari, P.; Weston, C.; Miyasaka, M.; Adams, D.; Jalkanen, S.; Salmi, M. Amine Oxidase Activity Regulates the Development of Pulmonary Fibrosis. FASEB J. 2017, 31, 2477–2491. [Google Scholar] [CrossRef]
- Yao, X. The Role of GABA in Spinal Cord Injury. Neurospine 2022, 19, 669–670. [Google Scholar] [CrossRef]
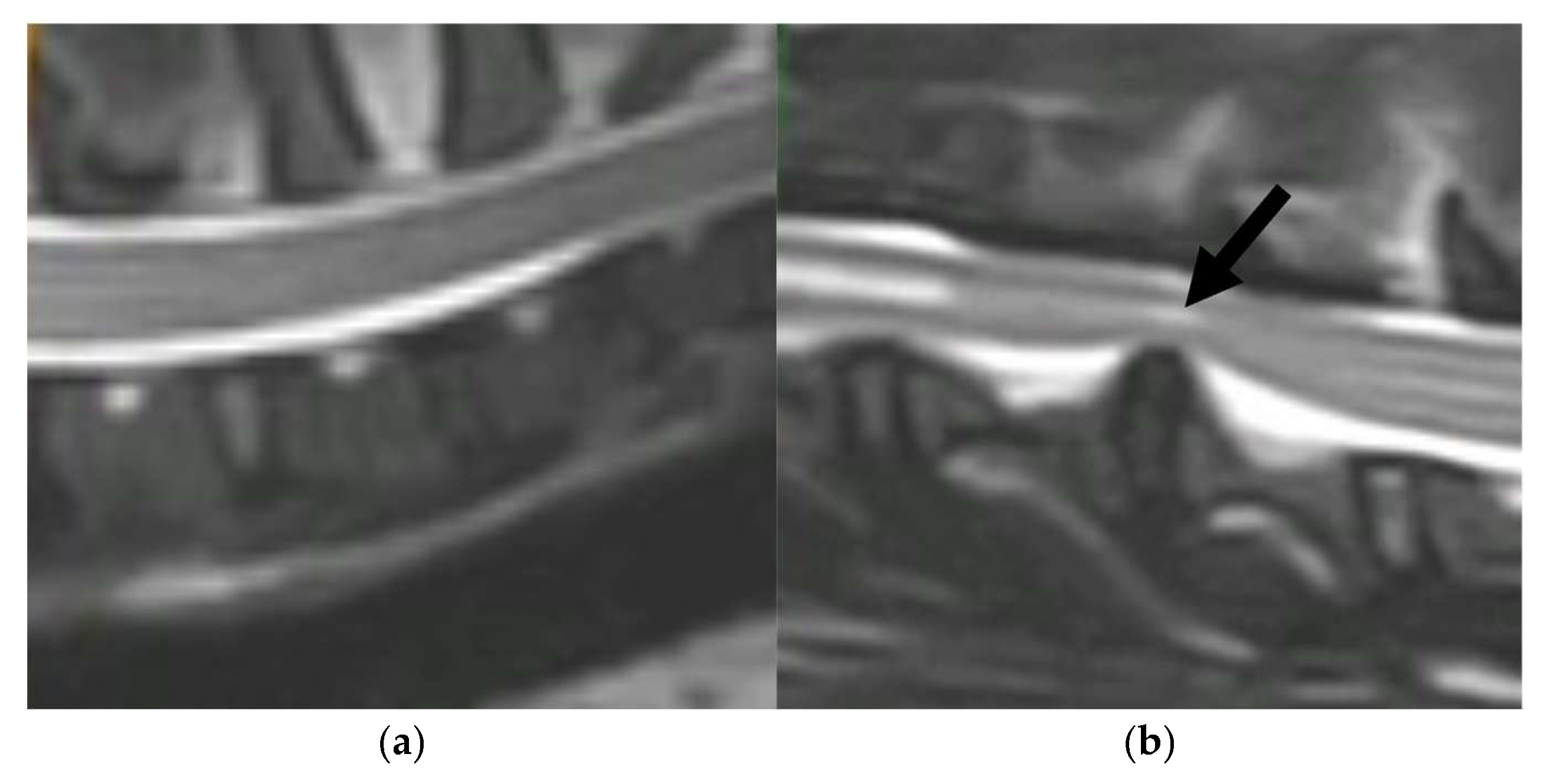
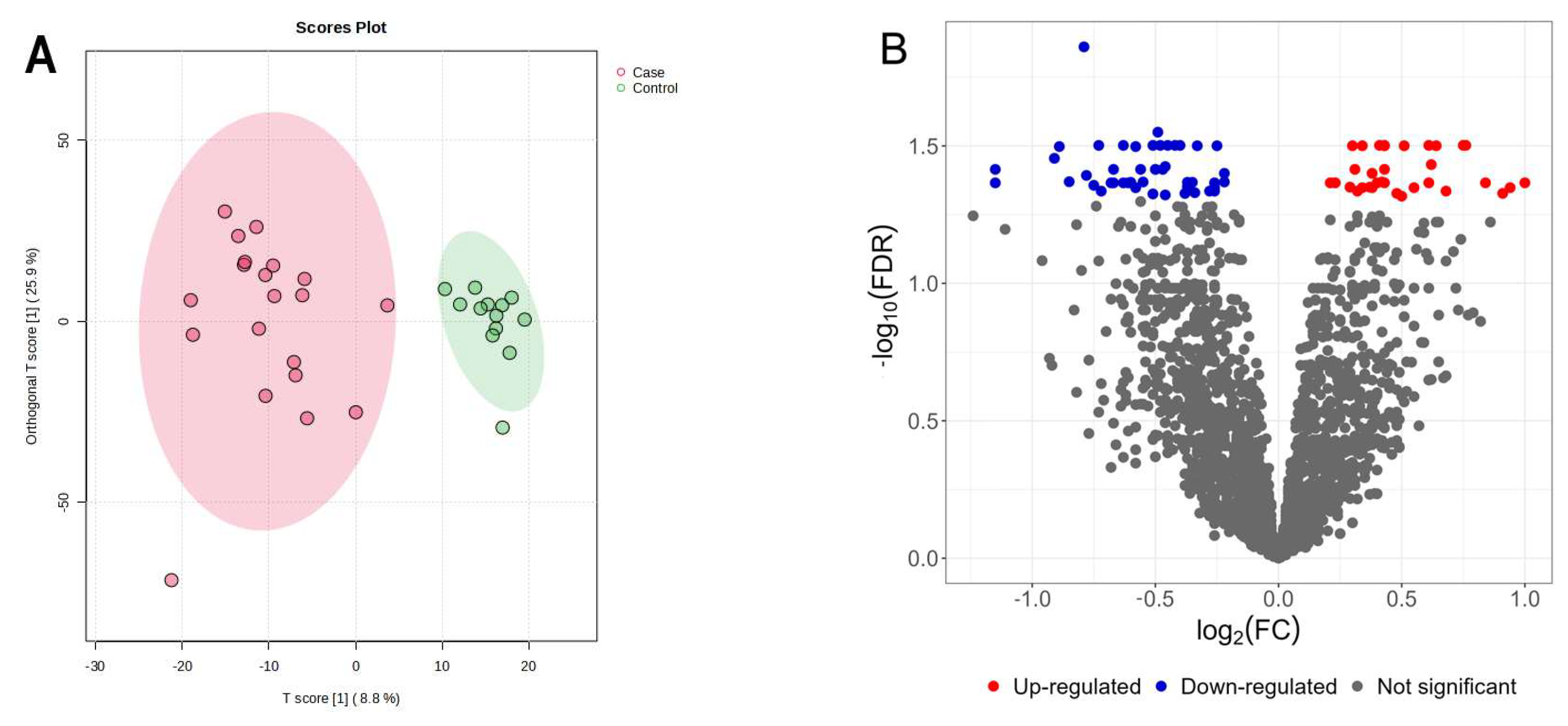
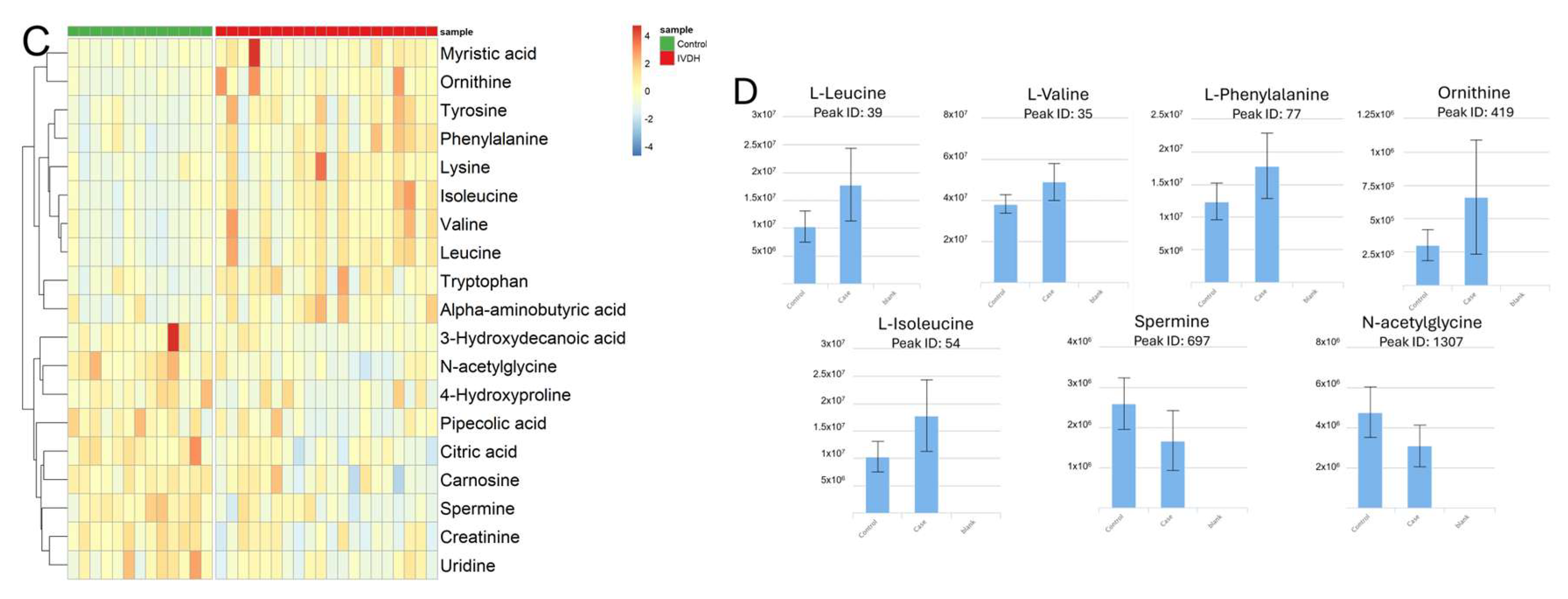



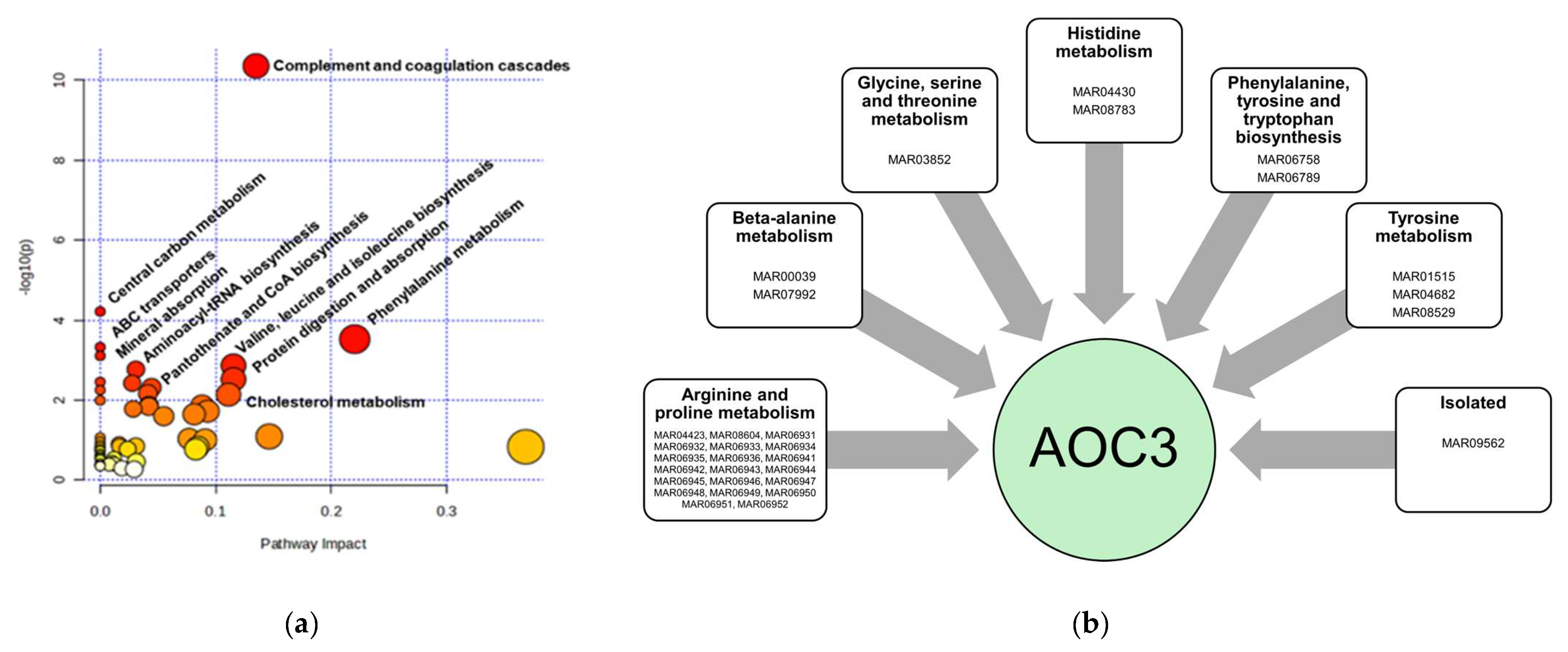
| Peak ID | Metabolite | HMDB ID | Log2 FC | p-Value | Adj. p-Value |
|---|---|---|---|---|---|
| 419 | Ornithine * | HMDB0000214 | 1.00 | 0.0008 | 0.043 |
| 130 | Lysine | HMDB0000182 | 0.82 | 0.0196 | 0.138 |
| 54 | Isoleucine * | HMDB0000172 | 0.75 | 0.0002 | 0.032 |
| 39 | Leucine * | HMDB0000687 | 0.61 | 0.0002 | 0.032 |
| 136 | Tyrosine | HMDB0000158 | 0.56 | 0.0045 | 0.075 |
| 1341 | Tryptophan | HMDB0000929 | 0.55 | 0.0099 | 0.104 |
| 147 | Alpha-aminobutyric acid | HMDB0000452 | 0.51 | 0.0136 | 0.115 |
| 77 | Phenylalanine * | HMDB0000159 | 0.50 | 0.0016 | 0.043 |
| 1213 | Myristic acid | HMDB0000806 | 0.46 | 0.0398 | 0.199 |
| 35 | Valine * | HMDB0000883 | 0.34 | 0.0003 | 0.032 |
| 1212 | Citric acid | HMDB0000094 | −0.21 | 0.0174 | 0.131 |
| 1551 | Uridine | HMDB0000296 | −0.26 | 0.0357 | 0.191 |
| 1326 | Creatinine | HMDB0000562 | −0.36 | 0.0034 | 0.064 |
| 1631 | 3-Hydroxydecanoic acid | HMDB0010725 | −0.50 | 0.0438 | 0.211 |
| 126 | Carnosine | HMDB0000033 | −0.55 | 0.0219 | 0.144 |
| 486 | Pipecolic acid | HMDB0000070 | −0.60 | 0.0240 | 0.151 |
| 1307 | N-acetyl glycine * | HMDB0000532 | −0.67 | 0.0005 | 0.039 |
| 697 | Spermine * | HMDB0001256 | −0.75 | 0.0013 | 0.044 |
| 1636 | 4-Hydroxyproline | HMDB0000725 | −0.96 | 0.0062 | 0.083 |
| Pathway | Total | Hits | p-Value | Regulation |
|---|---|---|---|---|
| Lipoic acid metabolism | 48 | 7 | 1.47 × 10−9 | ↑ |
| Nitrogen metabolism | 8 | 3 | 9.43 × 10−6 | ↑ |
| Steroid hormone biosynthesis | 4 | 2 | 0.000206 | ↑ |
| Steroid biosynthesis | 8 | 2 | 0.000948 | ↑ |
| Oxidative phosphorylation | 40 | 3 | 0.00150 | ↑ |
| Fatty acid biosynthesis | 36 | 2 | 0.00463 | ↓ |
| Carbon metabolism | 20 | 1 | 0.0405 | ↓ |
| NCBI Accession ID | p-Value | log2FC | Gene Symbol | Protein Name | Number of Unique Peptides | MW/kDa | Calc pI |
|---|---|---|---|---|---|---|---|
| 555289929 | 0.036 | −3.64 | KLKB1 | prekallikrein | 2 | 24 | 8.29 |
| 545508405 | 0.024 | −1.64 | SERPINA5 | plasma serine protease inhibitor | 7 | 45.8 | 8.44 |
| 124390007 | 0.048 | −1.36 | IGH | immunoglobulin heavy chain constant region CH1 | 3 | 11.5 | 4.83 |
| 545528321 | 0.036 | −0.66 | APOB | apolipoprotein B-100 | 42 | 518 | 6.95 |
| 296089 | 0.036 | 0.23 | APOH | apolipoprotein H | 13 | 38.4 | 8.13 |
| 70909945 | 0.036 | 0.67 | AZGP1 | zinc alpha-2-glycoprotein 1, partial | 4 | 20.2 | 4.88 |
| 560879429 | 0.036 | 0.67 | AZGP1 | zinc-alpha-2-glycoprotein precursor | 5 | 35.8 | 4.92 |
| 545504920 | 0.048 | 0.73 | F5 | coagulation factor V | 2 | 251.7 | 5.77 |
| 1418267888 | 0.048 | 0.73 | VTN | vitronectin | 6 | 53.7 | 5.15 |
| 1239899336 | 0.048 | 0.80 | C6 | complement component C6 | 6 | 105.5 | 6.43 |
| 73997275 | 0.024 | 0.81 | C1S | complement C1s subcomponent | 5 | 77.5 | 4.93 |
| 163954 | 0.024 | 0.86 | CLU | glycoprotein 80 | 13 | 51.8 | 5.91 |
| 57091057 | 0.048 | 0.88 | AOC3 | membrane primary amine oxidase isoform X1 | 4 | 84.2 | 6.37 |
| 1239918266 | 0.048 | 0.88 | AOC3 | membrane primary amine oxidase isoform X3 | 4 | 74.6 | 6.61 |
| 1418340066 | 0.048 | 0.88 | AOC3 | membrane primary amine oxidase isoform X2 | 4 | 84 | 6.25 |
| 1418515495 | 0.048 | 0.91 | AHSG | alpha-2-HS-glycoprotein | 7 | 39.2 | 5.31 |
| 1418256182 | 0.024 | 0.91 | C1R | complement C1r subcomponent | 9 | 80.1 | 6.24 |
| 345777712 | 0.048 | 1.05 | AMBP | protein AMBP | 2 | 38.8 | 6.35 |
| 1418324502 | 0.048 | 1.31 | MGAM2 | putative maltase-glucoamylase-like protein FLJ16351 | 2 | 282.1 | 5.38 |
| 1101972892 | 0.024 | 1.70 | HBA1 | globin A1 | 7 | 16.2 | 8.44 |
| 103484123 | 0.024 | 1.72 | HBD | globin, partial | 2 | 6.1 | 9.25 |
| 1418222276 | 0.024 | 1.75 | HBB | hemoglobin subunit beta-like | 6 | 12.8 | 6.68 |
| Pathway | Total | Hits | p-Value | Regulation |
|---|---|---|---|---|
| Platelet degranulation | 89 | 2 | 0.00104 | ↑ |
| Response to elevated platelet cytosolic Ca2+ | 94 | 2 | 0.00116 | ↑ |
| Platelet activation, signaling, and aggregation | 220 | 2 | 0.00621 | ↑ |
| Scavenging of heme from plasma | 15 | 1 | 0.00896 | ↑ |
| Binding and uptake of ligands by scavenger receptors | 15 | 1 | 0.00896 | ↑ |
| Formation of fibrin clot (clotting cascade) | 29 | 1 | 0.0173 | ↑ |
| Molecules associated with elastic fibers | 38 | 1 | 0.0226 | ↑ |
| Elastic fiber formation | 45 | 1 | 0.0267 | ↑ |
| Hemostasis | 511 | 2 | 0.0316 | ↑ |
| Integrin cell surface interactions | 85 | 1 | 0.0500 | ↑ |
| LDL-mediated lipid transport | 4 | 1 | 0.00060 | ↓ |
| Chylomicron-mediated lipid transport | 12 | 1 | 0.00180 | ↓ |
| Platelet sensitization by LDL | 19 | 1 | 0.00285 | ↓ |
| Lipoprotein metabolism | 22 | 1 | 0.00329 | ↓ |
| Lipid digestion, mobilization, and transport | 41 | 1 | 0.00614 | ↓ |
| Retinoid metabolism and transport | 41 | 1 | 0.00614 | ↓ |
| Platelet homeostasis | 88 | 1 | 0.0132 | ↓ |
| Cell surface interactions at the vascular wall | 99 | 1 | 0.0148 | ↓ |
| Protein | Control Group Protein Concentration (Median/Range) | IVDH Group Protein Concentration (Median/Range) | p-Value * |
|---|---|---|---|
| zinc alpha 2-glycoprotein (ZAG) | 37.97 ng/mL (13.55–43.41 ng/mL) | 98.92 ng/mL (81.45–112.5 ng/mL) | 0.0001 |
| alpha-1-microglobulin/bikunin precursor (AMBP) | 20.41 ng/mL (15.76–24.06 ng/mL) | 33.08 ng/mL (26.44–41.8 ng/mL) | 0.0004 |
| vitronectin (VTN) | 64.28 ng/mL (62.17–67.18 ng/mL) | 78.84 ng/mL (74.39–95.45 ng/mL) | 0.0031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horvatić, A.; Kuleš, J.; Gelemanović, A.; Smolec, O.; Pirkić, B.; Pećin, M.; Rubić, I.; Mrljak, V.; Samardžija, M.; Lipar, M. Combining Metabolomics and Proteomics to Reveal Key Serum Compounds Related to Canine Intervertebral Disc Herniation. Metabolites 2025, 15, 396. https://doi.org/10.3390/metabo15060396
Horvatić A, Kuleš J, Gelemanović A, Smolec O, Pirkić B, Pećin M, Rubić I, Mrljak V, Samardžija M, Lipar M. Combining Metabolomics and Proteomics to Reveal Key Serum Compounds Related to Canine Intervertebral Disc Herniation. Metabolites. 2025; 15(6):396. https://doi.org/10.3390/metabo15060396
Chicago/Turabian StyleHorvatić, Anita, Josipa Kuleš, Andrea Gelemanović, Ozren Smolec, Boris Pirkić, Marko Pećin, Ivana Rubić, Vladimir Mrljak, Marko Samardžija, and Marija Lipar. 2025. "Combining Metabolomics and Proteomics to Reveal Key Serum Compounds Related to Canine Intervertebral Disc Herniation" Metabolites 15, no. 6: 396. https://doi.org/10.3390/metabo15060396
APA StyleHorvatić, A., Kuleš, J., Gelemanović, A., Smolec, O., Pirkić, B., Pećin, M., Rubić, I., Mrljak, V., Samardžija, M., & Lipar, M. (2025). Combining Metabolomics and Proteomics to Reveal Key Serum Compounds Related to Canine Intervertebral Disc Herniation. Metabolites, 15(6), 396. https://doi.org/10.3390/metabo15060396









