New Players in Metabolic Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.2.1. Inclusion Criteria
- •
- Age (35–74);
- •
- BMI ≥ 30 kg/m2;
- •
- Prediabetes (fasting glucose between 6.1–6.9 mmol/L, values after a 120 min oral glucose tolerance test (OGTT) between 7.8–11.0 mmol/L, and/or an HbA1c between 5.7 and 6.4%);
- •
- Newly diagnosed diabetes (fasting glucose ≥7.0 mmol/L, values after a 120 min OGTT ≥11.1 mmol/L, and/or an HbA1c ≥6.5%), without therapy.
2.2.2. Exclusion Criteria
- •
- Liver dysfunction (liver enzymes ≥3 times above the reference range);
- •
- Chronic kidney disease (CKD) stages III–IV;
- •
- Heart failure (HF), classes III–IV NYHA;
- •
- Neoplastic disease.
2.3. Anthropometric Parameters
- •
- Weight, height, and waist and hip circumference;
- •
- Weight (kg) divided by height squared (m2) was used to compute BMI;
- •
- VAI = [WC/(36.68 + (1.88 × BMI)] × (TG/1.03) × (1.31/HDL) in males; VAI = [WC/(36.58 + (1.89 × BMI)] × (TG/0.81) × (1.52/HDL) for females.
2.4. Investigation of Glycemic Homeostasis
- •
- Using 75 g of glucose, the oral glucose tolerance test (OGTT) was conducted by measuring the glucose and immunoreactive insulin (IRI) at 0, 60, and 120 min.
- •
- If a patient’s HOMA index was greater than 2.5, they were deemed insulin resistant.
2.5. Metabolic Syndrome Was Defined by the Presence of Central Obesity (Defined as a Waist Circumference ≥80 cm for Women and ≥94 cm for Men) + Any Two of the Following Risk Factors (IDF Criteria)
- -
- Fasting glucose ≥ 5.6 mmol/L;
- -
- Blood pressure ≥ 130/≥ 85 mmHg;
- -
- Triglycerides ≥ 1.7 mmol/L;
- -
- HDL ≤ 1.03 mmol/L for men and ≤1.29 mmol/L for women.
2.6. Laboratory Investigation
- •
- Serum levels of FGF-21, sortilin, Metrnl, and nesfatin-1 were measured using standard ELISA techniques. Prior to the assay, the blood samples were centrifuged and kept at −80 °C.
2.7. Instrumental Investigation
- •
- Measurement of intima-media thickness (IMT) using a Panasonic Cardio Health station (Panasonic, Yogohoma, Japan);
- •
- Cardio-ankle vascular index (VaSera system, FUKUDA DENSHI CO., Tokyo, Japan);
- •
- Ankle-brachial index (ABI) (Elite Natus, Natus Medical Incorporated, Middleton, WI, USA);
- •
- Assessment of the autonomic nervous system through an evaluation of sudomotor function with FDA-approved Sudoscan (Itamar Medical Ltd., Caesarea, Israel);
- •
- Assessment of the peripheral nervous system through the neuropathy disability score.
2.8. Statistical Analysis
3. Results
4. Discussion
4.1. Clinical Significance
4.2. Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.; Shaw, J. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Nakatake, Y.; Konishi, M.; Itoh, N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim. Biophys. Acta 2000, 1492, 203–206. [Google Scholar] [CrossRef]
- Xie, X.; Song, Y.; Chen, W.; Zhao, H.; Chu, N.; Wang, F. Association between circulating inflammatory proteins and gout: A Mendelian randomization study. Medicine 2025, 104, e42379. [Google Scholar] [CrossRef]
- Dolegowska, K.; Marchelek-Mysliwiec, M.; Nowosiad-Magda, M.; Slawinski, M.; Dolegowska, B. FGF19 subfamily members: FGF19 and FGF21. J. Physiol. Biochem. 2019, 75, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.S.; Madsen, P.; Christensen, E.I.; Nykjaer, A.; Gliemann, J.; Kasper, D.; Pohlmann, R.; Petersen, C.M. The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein. EMBO J. 2001, 20, 2180–2190. [Google Scholar] [CrossRef]
- Gao, A.; Cayabyab, F.S.; Chen, X.; Yang, J.; Wang, L.; Peng, T.; Lv, Y. Implications of Sortilin in Lipid Metabolism and Lipid Disorder Diseases. DNA Cell Biol. 2017, 36, 1050–1061. [Google Scholar] [CrossRef]
- Biscetti, F.; Nardella, E.; Rando, M.M.; Cecchini, A.L.; Bonadia, N.; Bruno, P.; Angelini, F.; Di Stasi, C.; Contegiacomo, A.; Santoliquido, A.; et al. Sortilin levels correlate with major cardiovascular events of diabetic patients with peripheral artery disease following revascularization: A prospective study. Cardiovasc. Diabetol. 2020, 19, 147. [Google Scholar] [CrossRef]
- Li, Z.Y.; Luo, H.Y.; Xu, F.; Xu, Y.; Ma, C.H.; Zhang, S.L.; Xu, S.; Ma, Y.Y.; Li, N.; Miao, C.Y. Metrnl protects intestinal barrier function by regulating tight junctions via the IKKβ/IκBα/NFκB/MLCK/MLC signaling pathway. Cell Death Discov. 2025, 11, 155. [Google Scholar] [CrossRef]
- Ushach, I.; Burkhardt, A.M.; Martinez, C.; Hevezi, P.A.; Gerber, P.A.; Buhren, B.A.; Schrumpf, H.; Valle-Rios, R.; Vazquez, M.I.; Homey, B.; et al. METEORIN-LIKE is a cytokine associated with barrier tissues and alternatively activated macrophages. Clin. Immunol. 2015, 156, 119–127. [Google Scholar] [CrossRef]
- Chen, Z.T.; Weng, Z.X.; Lin, J.D.; Meng, Z.X. Myokines: Metabolic regulation in obesity and type 2 diabetes. Life Metab. 2024, 3, loae006. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.W.; Chen, J.; Chen, C.X.; Zheng, S.L.; Zhao, H.Y.; Miao, C.Y. Metrnl as a secreted protein: Discovery and cardiovascular research. Pharmacol. Ther. 2024, 263, 108730. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.Y. Aerobic Exercise Increases Meteorin-Like Protein in Muscle and Adipose Tissue of Chronic High-Fat Diet-Induced Obese Mice. BioMed Res. Int. 2018, 2018, 6283932. [Google Scholar] [CrossRef]
- Zhou, C.; Zeng, J.; Gao, X.; Chen, D.; Zhu, Q.; Feng, B.; Song, J. Association of serum Metrnl levels and high-density lipoprotein cholesterol in patients with type 2 diabetes mellitus: A cross-sectional study. PeerJ 2024, 12, e18264. [Google Scholar] [CrossRef]
- Saito, R.; Yamamoto, Y.; Fukano, R.; Mori, M.; Maruyama, T.; Ueta, Y. Acute effects of peripherally administered nicotine on food intake via the central anorectic peptide nesfatin-1/nucleobindin-2 in adult male rats. Peptides 2025, 189, 171409. [Google Scholar] [CrossRef] [PubMed]
- Khalili, S.; Khaniani, S.; Afkhami, F.; Derakhshan, M. NUCB2/=nesfatin-1: A potent meal regulatory hormone and its role in diabetes. Egypt. J. Med. Hum. Genet. 2017, 18, 105–109. [Google Scholar] [CrossRef]
- Gao, R.Y.; Hsu, B.G.; Wu, D.A.; Hou, J.S.; Chen, M.C. Serum Fibroblast Growth Factor 21 Levels Are Positively Associated with Metabolic Syndrome in Patients with Type 2 Diabetes. Int. J. Endocrinol. 2019, 2019, 5163245. [Google Scholar] [CrossRef]
- Novotny, D.; Vaverkova, H.; Karasek, D.; Lukes, J.; Slavik, L.; Malina, P.; Orsag, J. Evaluation of total adiponectin, adipocyte fatty acid binding protein and fibroblast growth factor 21 levels in individuals with metabolic syndrome. Physiol. Res. 2014, 63, 219–228. [Google Scholar] [CrossRef]
- Karamfilova, V.; Assyov, Y.; Nedeva, I.; Gateva, A.; Ivanova, I.; Cherkezov, N.; Mateva, L.; Kamenov, Z. Fibroblast Growth Factor 21 as a Marker of Prediabetes in Patients with Non-alcoholic Fatty Liver Disease. Turk. J. Gastroenterol. 2022, 33, 233–239. [Google Scholar] [CrossRef]
- Panahi, Y.; Bonakdaran, S.; Yaghoubi, M.A.; Keramati, M.R.; Haratian, M.; Sahebkar, A. Serum levels of fibroblast growth factor 21 in type 2 diabetic patients. Acta Endocrinol. 2016, 12, 257–261. [Google Scholar] [CrossRef]
- Jin, Q.R.; Bando, Y.; Miyawaki, K.; Shikama, Y.; Kosugi, C.; Aki, N.; Funaki, M.; Noji, S. Correlation of fibroblast growth factor 21 serum levels with metabolic parameters in Japanese subjects. J. Med. Investig. 2014, 61, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Mai, K.; Bobbert, T.; Groth, C.; Assmann, A.; Meinus, S.; Kraatz, J.; Andres, J.; Arafat, A.M.; Pfeiffer, A.F.; Möhlig, M.; et al. Physiological modulation of circulating FGF21: Relevance of free fatty acids and insulin. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E126–E130. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kang, Y.E.; Kim, J.M.; Choung, S.; Joung, K.H.; Kim, H.J.; Ku, B.J. Serum Meteorin-like protein levels decreased in patients newly diagnosed with type 2 diabetes. Diabetes Res. Clin. Pract. 2018, 135, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.L.; Li, Z.Y.; Zhang, Z.; Wang, D.S.; Xu, J.; Miao, C.Y. Evaluation of Two Commercial Enzyme-Linked Immunosorbent Assay Kits for the Detection of Human Circulating Metrnl. Chem. Pharm. Bull. 2018, 66, 391–398. [Google Scholar] [CrossRef]
- Chung, H.S.; Hwang, S.Y.; Choi, J.H.; Lee, H.J.; Kim, N.H.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; et al. Implications of circulating Meteorin-like (Metrnl) level in human subjects with type 2 diabetes. Diabetes Res. Clin. Pract. 2018, 136, 100–107. [Google Scholar] [CrossRef]
- AlKhairi, I.; Cherian, P.; Abu-Farha, M.; Madhoun, A.A.; Nizam, R.; Melhem, M.; Jamal, M.; Al-Sabah, S.; Ali, H.; Tuomilehto, J.; et al. Increased Expression of Meteorin-Like Hormone in Type 2 Diabetes and Obesity and Its Association with Irisin. Cells 2019, 8, 1283. [Google Scholar] [CrossRef]
- Li, Z.Y.; Song, J.; Zheng, S.L.; Fan, M.B.; Guan, Y.F.; Qu, Y.; Xu, J.; Wang, P.; Miao, C.Y. Adipocyte Metrnl Antagonizes Insulin Resistance Through PPARγ Signaling. Diabetes 2015, 64, 4011–4022. [Google Scholar] [CrossRef]
- Löffler, D.; Landgraf, K.; Rockstroh, D.; Schwartze, J.T.; Dunzendorfer, H.; Kiess, W.; Körner, A. METRNL decreases during adipogenesis and inhibits adipocyte differentiation leading to adipocyte hypertrophy in humans. Int. J. Obes. 2017, 41, 112–119. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, J.; Tang, Y.; Bi, F.; Liu, J. The novel function of nesfatin-1: Antihyperglycemia. Biochem. Biophys. Res. Commun. 2010, 391, 1039–1104. [Google Scholar] [CrossRef]
- Zhai, T.; Li, S.Z.; Fan, X.T.; Tian, Z.; Lu, X.Q.; Dong, J. Circulating Nesfatin-1 Levels and Type 2 Diabetes: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2017, 2017, 7687098. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, L.; Yang, M.; Liu, H.; Boden, G.; Yang, G. Increased plasma levels of nesfatin-1 in patients with newly diagnosed type 2 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 2012, 120, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gao, L.; Tang, H.; Yin, Y.; Xiang, X.; Li, Y.; Zhao, J.; Mulholland, M.; Zhang, W. Peripheral effects of nesfatin-1 on glucose homeostasis. PLoS ONE 2013, 8, e71513. [Google Scholar] [CrossRef]
- Aydın, S. Multi-functional peptide hormone NUCB2/nesfatin-1. Endocrine 2013, 44, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Goebel-Stengel, M.; Wang, L. Central and peripheral expression and distribution of NUCB2/nesfatin-1. Curr. Pharm. Des. 2013, 19, 6935–6940. [Google Scholar] [CrossRef]
- Nykjaer, A.; Willnow, T.E. Sortilin: A receptor to regulate neuronal viability and function. Trends Neurosci. 2012, 35, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Demir, I.; Akan, O.Y.; Guler, A.; Bozkaya, G.; Aslanipour, B.; Calan, M. Relation of decreased circulating sortilin levels with unfavorable metabolic profiles in subjects with newly diagnosed type 2 diabetes mellitus. Am. J. Med. Sci. 2020, 359, 8–16. [Google Scholar] [CrossRef]
- Oh, T.J.; Ahn, C.H.; Kim, B.R.; Kim, K.M.; Moon, J.H.; Lim, S.; Park, K.S.; Lim, C.; Jang, H.; Choi, S.H. Circulating sortilin level as a potential biomarker for coronary atherosclerosis and diabetes mellitus. Cardiovasc. Diabetol. 2017, 16, 92. [Google Scholar] [CrossRef]

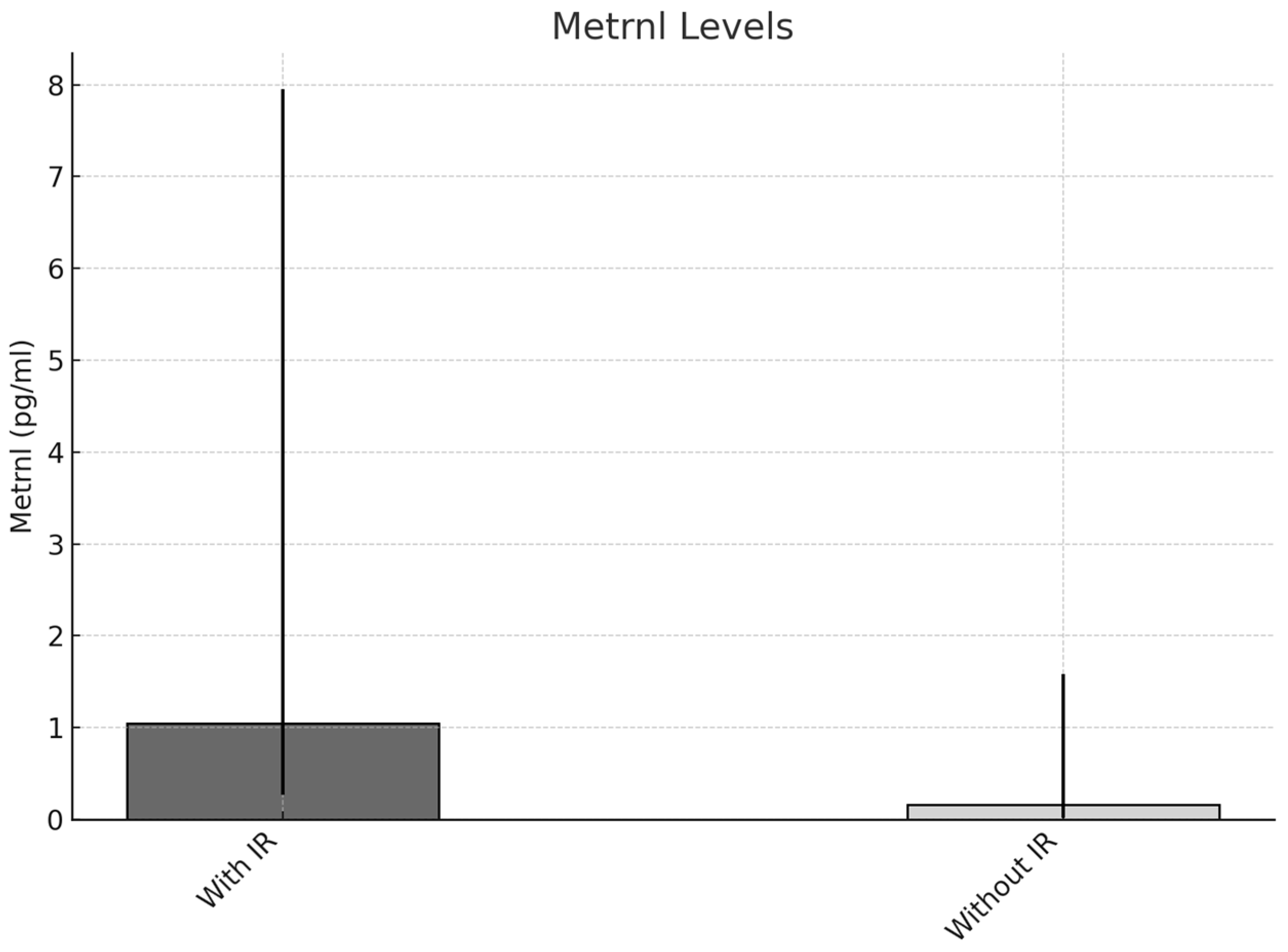
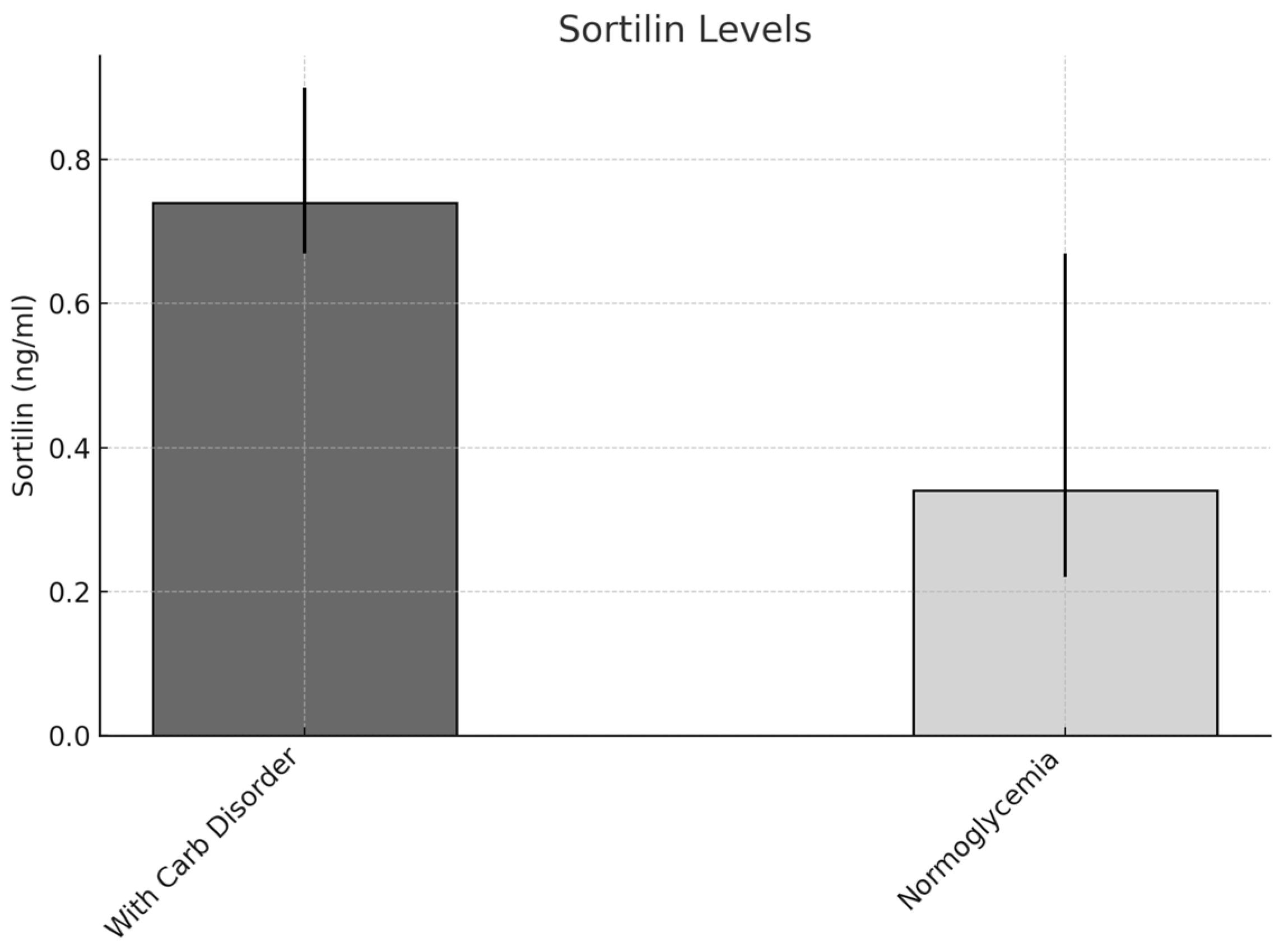
| Obesity Group 1 | Prediabetes Group 2 | Diabetes Group 3 | 1/2 | 1/3 | 2/3 | |
|---|---|---|---|---|---|---|
| n | 80 | 60 | 60 | |||
| Age years | 53.54 ± 9.09 | 53.74 ± 11.65 | 55.92 ± 11.02 | ns | ns | p < 0.05 |
| BMI (kg/m2) | 34.65 ± 3.10 | 35.85 ± 4.97 | 34.69 ± 5.56 | ns | ns | ns |
| WHR | 0.90 ± 0.09 | 0.91 ± 0.10 | 0.96 ± 0.16 | p < 0.05 | ns | p < 0.05 |
| WSR | 0.64 ± 0.06 | 0.65 ± 0.07 | 0.65 ± 0.07 | ns | ns | ns |
| Waist cm | 104.58 ± 10.38 | 106.17 ± 11.68 | 108.36 ± 13.96 | ns | ns | ns |
| Obesity Group 1 | Prediabetes Group 2 | Diabetes Group 3 | 1/2 | 1/3 | 2/3 | |
|---|---|---|---|---|---|---|
| N | 80 | 60 | 60 | |||
| SBP (mmHg) | 130 [120–139.5] | 120 [112.5–130] | 130 [120–140] | ns | ns | ns |
| DBP (mmHg) | 80 [70–90] | 80 [72.3–80] | 80 [72.3–87.5] | ns | ns | ns |
| Tchol (mmol/L) | 5.33 (1.21) | 5.29 (1.08) | 5.55 (1.26) | ns | ns | ns |
| LDL (mmol/L) | 3.33 (1.05) | 3.20 (0.99) | 3.23 (1.10) | ns | ns | ns |
| HDL (mmol/L) | 1.35 (0.30) | 1.24(0.31) | 1.08 (0.30) | p < 0.05 | p < 0.05 | p < 0.05 |
| TG (mmol/L) | 1.29 [0.87–1.67] | 1.55 [1.28–2.07] | 1.94 [1.38–1.94] | p < 0.05 | p < 0.001 | p < 0.05 |
| Hypertesion | 76.3% | 85.5% | 84.7% | ns | ns | ns |
| Smoking % | 26.6% | 18.0% | 33.9% | ns | ns | ns |
| Dyslipidemia % | 46.3% | 62.9% | 79.3% | ns | p < 0.05 | ns |
| Variable | FGF21 | NEFA | METRNL | SORT |
|---|---|---|---|---|
| Age | 0.112 | −0.010 | −0.014 | −0.039 |
| BMI | 0.068 | 0.088 | 0.101 | 0.041 |
| WC (cm) | 0.176 | 0.175 | 0.288 * | 0.158 |
| HC (cm) | 0.029 | −0.112 | 0.010 | 0.091 |
| WHR | 0.093 | 0.297 * | 0.353 ** | 0.189 |
| WSR | 0.099 | 0.208 | 0.176 | 0.078 |
| VAI | 0.330 ** | 0.022 | 0.355 ** | 0.117 |
| FAT (%) | 0.064 | 0.048 | 0.145 | 0.031 |
| SBP (mmHg) | 0.003 | 0.011 | 0.068 | 0.047 |
| DBP (mmHg) | −0.007 | 0.075 | 0.125 | 0.013 |
| Tchol (mmol/L) | 0.042 | 0.048 | 0.021 | 0.063 |
| VLDL(mmol/L) | 0.296 ** | 0.021 | 0.127 | 0.189 |
| HDL (mmol/L) | −0.289 * | −0.002 | −0.032 | −0.157 |
| LDL (mmol/L) | 0.044 | 0.060 | 0.014 | 0.071 |
| TG (mmol/L) | 0.296 ** | 0.015 | 0.128 | 0.187 |
| Gluc 0 min (mmol/L) | 0.119 | 0.282 * | 0.190 | 0.393 ** |
| Gluc 60 min (mmol/L) | 0.047 | 0.227 | 0.081 | 0.126 |
| Gluc 120 min (mmol/L) | 0.072 | 0.391 ** | 0.082 | 0.351 ** |
| IRI 0 min (mU/L) | 0.295 * | 0.238 * | 0.272 * | 0.142 |
| IRI 60 min (mU/L) | 0.318 ** | 0.138 | 0.285 * | 0.134 |
| IRI 120 min (mU/L) | 0.276 * | 0.242 | 0.122 | 0.186 |
| ASAT (U/L) | 0.098 | −0.113 | 0.099 | 0.174 |
| ALAT (U/L) | 0.235 * | −0.110 | 0.119 | 0.189 |
| GGT (U/L) | 0.418 *** | −0.009 | 0.074 | 0.239 * |
| Creat (mkmol/L) | 0.124 | −0.142 | −0.156 | 0.048 |
| Uric acid (mkmol/L) | 0.411 *** | −0.004 | 0.088 | 0.028 |
| HOMA-IR | 0.270 * | 0.256 * | 0.268 * | 0.100 |
| CPK (U/L) | 0.049 | −0.110 | 0.272 * | −0.030 |
| HbA1c% | 0.166 | 0.136 | 0.181 | 0.497 ** |
| ACR (mg/mmol) | 0.155 | −0.024 | 0.389 * | 0.082 |
| eGFR | −0.098 | 0.175 | 0.024 | 0.018 |
| 95% CI | ||||||
|---|---|---|---|---|---|---|
| Pathology State | Biomarker | Value | ORs | Upper limit | Lower limit | p |
| Metabolic syndrome | FGF-21 | ≥285.6/<285.6 | 11.400 | 3.001 | 43.307 | <0.001 |
| Insulin resistance | FGF-21 | ≥269/<269 | 8.000 | 2.272 | 28.170 | 0.001 |
| Insulin resistance | METRNL | ≥0.285/<0.285 | 6.400 | 1.859 | 22.036 | 0.003 |
| Dyslipidemia | FGF-21 | ≥275.7/<275.7 | 4.024 | 1.566 | 10.336 | 0.004 |
| Carbohydrate disorders | Sortilin | ≥0.59/<0.59 | 5.784 | 1.515 | 10.182 | 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nedeva, I.; Assyov, Y.; Karamfilova, V.; Kamenov, Z.; Dobrev, P.; Velikova, T.; Vodenicharov, V. New Players in Metabolic Syndrome. Metabolites 2025, 15, 380. https://doi.org/10.3390/metabo15060380
Nedeva I, Assyov Y, Karamfilova V, Kamenov Z, Dobrev P, Velikova T, Vodenicharov V. New Players in Metabolic Syndrome. Metabolites. 2025; 15(6):380. https://doi.org/10.3390/metabo15060380
Chicago/Turabian StyleNedeva, Iveta, Yavor Assyov, Vera Karamfilova, Zdravko Kamenov, Pavel Dobrev, Tsvetelina Velikova, and Vlayko Vodenicharov. 2025. "New Players in Metabolic Syndrome" Metabolites 15, no. 6: 380. https://doi.org/10.3390/metabo15060380
APA StyleNedeva, I., Assyov, Y., Karamfilova, V., Kamenov, Z., Dobrev, P., Velikova, T., & Vodenicharov, V. (2025). New Players in Metabolic Syndrome. Metabolites, 15(6), 380. https://doi.org/10.3390/metabo15060380







