The Effect of Frailty on Body Composition and Its Impact on the Use of SGLT-2 Inhibitors and GLP-1RA in Older Persons with Diabetes
Abstract
1. Introduction
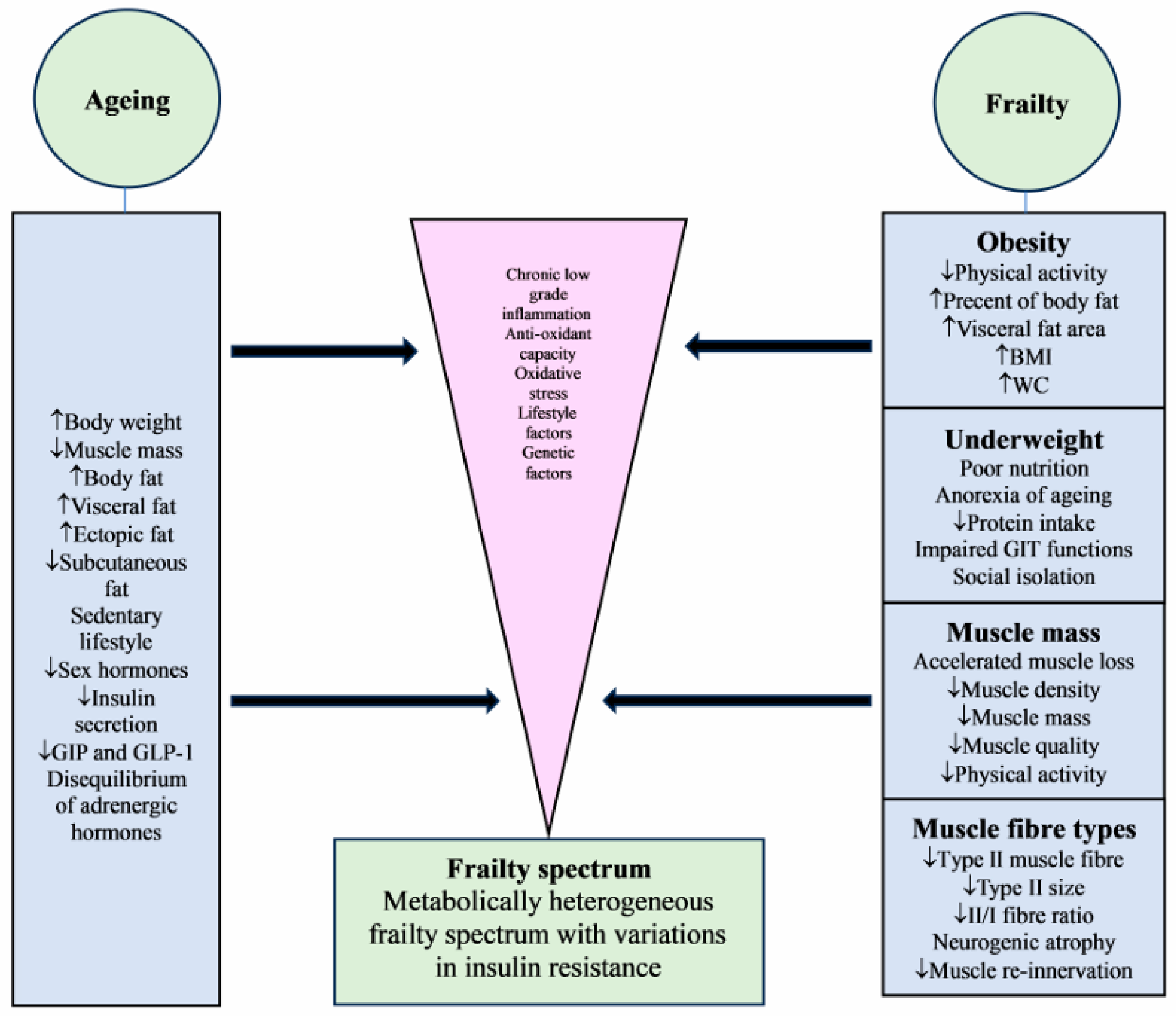
2. Body Composition—The Ageing Effect
3. Body Composition—The Frailty Effect
3.1. Frailty and Obesity
3.2. Frailty and Underweight
3.3. The U-Shaped Relationship
3.4. Frailty and Muscle Mass
3.5. Frailty and Muscle Fibres
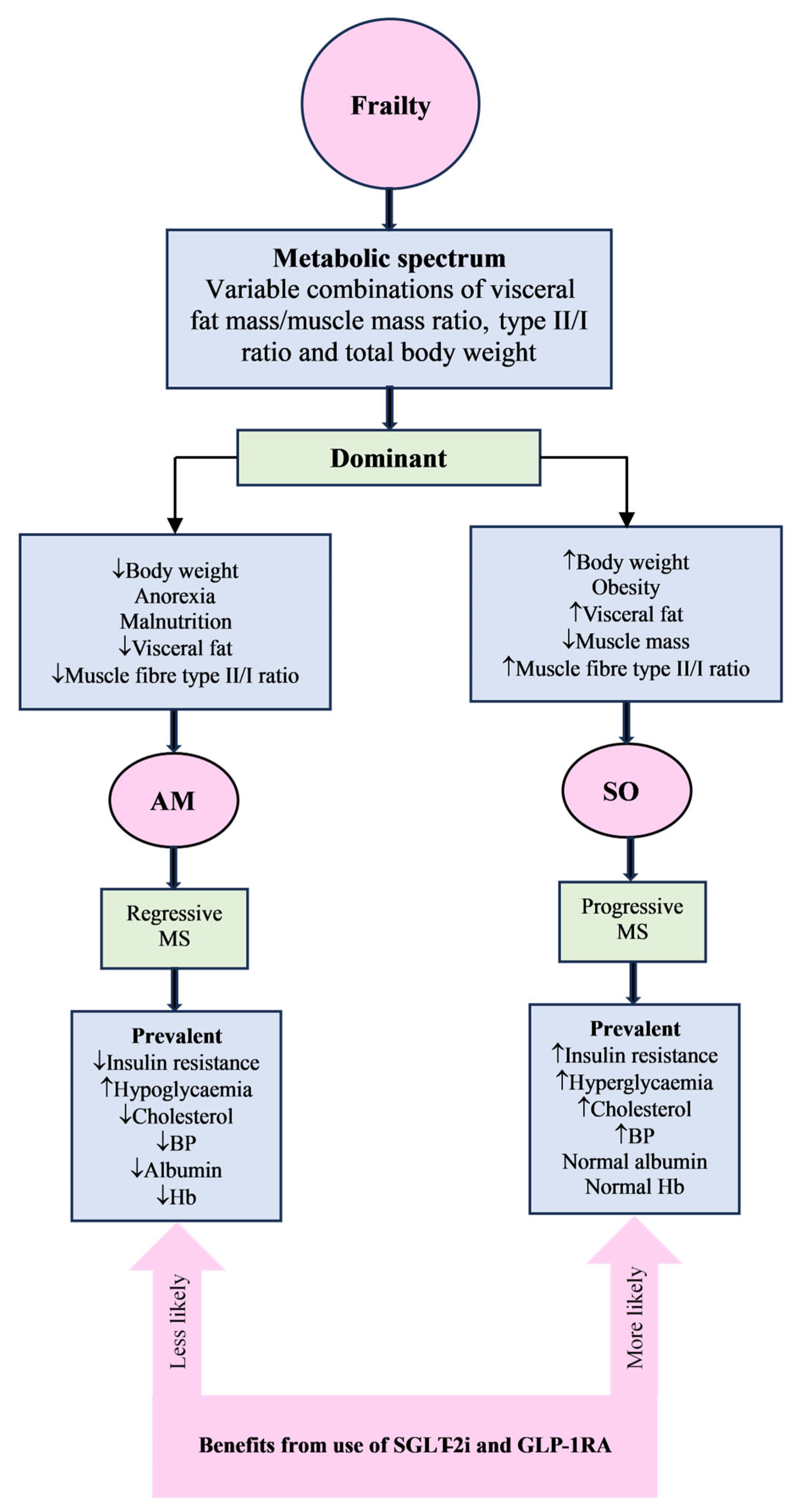
3.6. Frailty and Insulin Resistance
3.7. Frailty Metabolic Spectrum
4. SGLT-2 Inhibitors and GLP-1RA
4.1. Effect on Body Composition
4.2. Current Limitations of Clinical Guidelines
4.3. Use in Frailty
4.4. Clinical Implications
5. Conclusions
6. Future Perspectives
7. Key Points
- Frailty has a diverse and complex association with body composition.
- Frailty-induced body composition changes lead to the emergence of a metabolic spectrum of frail individuals with variable insulin resistance.
- The insulin resistance of frail patients will depend on the net effect of visceral fat and muscle mass ratio, dominant skeletal muscle fibre type, and total body weight.
- SGLT-2 inhibitors and GLP-1RA are metabolically active rather than hypoglycaemic agents; therefore, their effects will be different across the frailty spectrum.
- The obese frail are likely to benefit most from these agents due to the prevalence of high insulin resistance, while anorexic frail are likely to be intolerant to such therapy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.J.; Abdelhafiz, A.H.; Rodríguez-Mañas, L. Frailty and sarcopenia—Newly emerging and high impact complications of diabetes. J. Diabetes Complicat. 2017, 31, 1465–1473. [Google Scholar] [CrossRef]
- Chhetri, J.K.; Zheng, Z.; Xu, X.; Ma, C.; Chan, P. The prevalence and incidence of frailty in pre-diabetic and diabetic community dwelling older population: Results from Beijing longitudinal study of aging II (BLSA-II). BMC Geriatr. 2017, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Howrey, B.T.; Al Snih, S.; Markides, K.S. Frailty and diabetes among Mexican American older adults. Ann. Epidemiol. 2018, 28, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Castrejon-Perez, R.C.; Aguilar-Salinas, C.A.; Gutierrez-Robledo, L.M.; Cesari, M.; Pérez-Zepeda, M.U. Frailty, diabetes, and the convergence of chronic disease in an age-related condition: A population-based nationwide cross-sectional analysis of the Mexican nutrition and health survey. Aging Clin. Exp. Res. 2018, 30, 935–941. [Google Scholar] [CrossRef]
- Abdelhafiz, A.H.; Koay, L.; Sinclair, A.J. The Emergence of Frailty May Lead to a State of Burnt Out Type 2 Diabetes. J. Frailty Aging 2016, 5, 162–167. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 13. Older Adults: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48 (Suppl. S1), S266–S282. [Google Scholar] [CrossRef] [PubMed]
- LeRoith, D.; Biessels, G.J.; Braithwaite, S.S.; Casanueva, F.F.; Draznin, B.; Halter, J.B.; Hirsch, I.B.; McDonnell, M.E.; Molitch, M.E.; Murad, M.H.; et al. Treatment of Diabetes in Older Adults: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2019, 104, 1520–1574. [Google Scholar] [CrossRef]
- Distefano, G.; Goodpaster, B.H. Effects of exercise and aging on skeletal muscle. Cold Spring Harb. Perspect. Med. 2018, 8, a029785. [Google Scholar] [CrossRef]
- Choi, K.M. Sarcopenia and sarcopenic obesity. Korean J. Intern. Med. 2016, 31, 1054–1060. [Google Scholar] [CrossRef]
- Hughes, V.A.; Frontera, W.R.; Roubenoff, R.; Evans, W.J.; Singh, M.A. Longitudinal changes in body composition in older men and women: Role of body weight change and physical activity. Am. J. Clin. Nutr. 2002, 76, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, M.J.; Tchkonia, T.; Kirkland, J.L. Aging in adipocytes: Potential impact of inherent, depot-specific mechanisms. Exp. Gerontol. 2007, 42, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Grevendonk, L.; Connell, N.J.; McCrum, C.; Fealy, C.E.; Bilet, L.; Bruls, Y.M.H.; Mevenkamp, J.; Schrauwen-Hinderling, V.B.; Jörgensen, J.A.; Moonen-Kornips, E.; et al. Impact of aging and exercise on skeletal muscle mitochondrial capacity, energy metabolism, and physical function. Nat. Commun. 2021, 12, 4773. [Google Scholar] [CrossRef]
- Palmer, A.K.; Jensen, M.D. Metabolic changes in aging humans: Current evidence and therapeutic strategies. J. Clin. Investig. 2022, 132, e158451. [Google Scholar] [CrossRef] [PubMed]
- Aronoff, S.L.; Berkowitz, K.; Shreiner, B.; Want, L. Glucose Metabolism and Regulation: Beyond Insulin and Glucagon. Diabetes Spectr. 2004, 17, 183–190. [Google Scholar] [CrossRef]
- Szoke, E.; Shrayyef, M.Z.; Messing, S.; Woerle, H.J.; van Haeften, T.W.; Meyer, C.; Mitrakou, A.; Pimenta, W.; Gerich, J.E. Effect of aging on glucose homeostasis: Accelerated deterioration of β-cell function in individuals with impaired glucose tolerance. Diabetes Care 2008, 31, 539–543. [Google Scholar] [CrossRef]
- Kim, W.; Egan, J.M. The Role of Incretins in Glucose Homeostasis and Diabetes Treatment. Pharm. Rev. 2008, 60, 470–512. [Google Scholar] [CrossRef]
- Farilla, L.; Bulotta, A.; Hirshberg, B.; Li, C.S.; Khoury, N.; Noushmehr, H.; Bertolotto, C.; Di Mario, U.; Harlan, D.M.; Perfetti, R. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology 2003, 144, 5149–5158. [Google Scholar] [CrossRef]
- Basu, R.; Breda, E.; Oberg, A.L.; Powell, C.C.; Dalla Man, C.; Basu, A.; Vittone, J.L.; Klee, G.G.; Arora, P.; Jensen, M.D.; et al. Mechanisms of the age-associated deterioration in glucose tolerance: Contribution of alterations in insulin secretion, action, and clearance. Diabetes 2003, 52, 1738–1748. [Google Scholar] [CrossRef]
- Bergman, H.; Ferrucci, L.; Guralnik, J.; Hogan, D.B.; Hummel, S.; Karunananthan, S.; Wolfson, C. Frailty: An emerging research and clinical paradigm-issues and controversies. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 62A, 731–737. [Google Scholar] [CrossRef]
- Walston, J.; Hadley, E.C.; Ferrucci, L.; Guralnik, J.M.; Newman, A.B.; Studenski, S.A.; Ershler, W.B.; Harris, T.; Fried, L.P. Research agenda for frailty in older adults: Toward a better understanding of physiology and etiology: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J. Am. Geriatr. Soc. 2006, 54, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Castrejón-Pérez, R.C.; Gutiérrez-Robledo, L.M.; Cesari, M.; Pérez-Zepeda, M.U. Diabetes mellitus, hypertension and frailty: A population-based, cross-sectional study of Mexican older adults. Geriatr. Gerontol. Int. 2016, 17, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, S.; Harada, K.; Bae, S.; Makizako, H.; Doi, T.; Tsutsumimoto, K.; Hotta, R.; Nakakubo, S.; Park, H.; et al. Relationship between chronic kidney disease with diabetes or hypertension and frailty in community-dwelling Japanese older adults. Geriatr. Gerontol. Int. 2016, 17, 1527–1533. [Google Scholar] [CrossRef]
- Lee, C.G.; Boyko, E.J.; Strotmeyer, E.S.; Lewis, C.E.; Cawthon, P.M.; Hoffman, A.R.; Everson-Rose, S.A.; Barrett-Connor, E.; Orwoll, E.S. Association between insulin resistance and lean mass loss and fat mass gain in older men without diabetes mellitus. J. Am. Geriatr. Soc. 2011, 59, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Blaum, C.S.; Xue, Q.L.; Michelon, E.; Semba, R.D.; Fried, L.P. The association between obesity and the frailty syndrome in older women: The Women’s Health and Aging Studies. J. Am. Geriatr. Soc. 2005, 53, 927–934. [Google Scholar] [CrossRef]
- Jayanama, K.; Theou, O.; Godin, J.; Mayo, A.; Cahill, L.; Rockwood, K. Relationship of body mass index with frailty and all-cause mortality among middle-aged and older adults. BMC Med. 2022, 20, 404. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, S.; Han, P.; Zheng, K.; Chen, C.; Wu, Y.; Huang, C.; Guo, J.; Qi, Y.; Chen, X.; et al. The association between visceral fat obesity and prefrailty in Chinese older adults: A cross-sectional study. BMC Endocr. Disord. 2024, 24, 136. [Google Scholar] [CrossRef]
- Xu, W.; Tan, L.; Wang, H.F.; Jiang, T.; Tan, M.S.; Tan, L.; Zhao, Q.F.; Li, J.Q.; Wang, J.; Yu, J.T. Meta-analysis of modifiable risk factors for Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1299–1306. [Google Scholar] [CrossRef]
- Yuan, L.M.; Chang, M.; Wang, J. Abdominal obesity, body mass index and the risk of frailty in community-dwelling older adults: A systematic review and meta-analysis. Age Ageing 2021, 50, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, C.; Sousa-Santos, A.R.; Santos, A.; Borges, N.; Padrao, P.; Moreira, P.; Amaral, T.F. Frailty status is related to general and abdominal obesity in older adults. Nutr. Res. 2021, 85, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Uchai, S.; Andersen, L.F.; Hopstock, L.A.; Hjartåker, A. Body mass index, waist circumference and pre-frailty/frailty: The Tromsø study 1994–2016. BMJ Open 2023, 13, e065707. [Google Scholar] [CrossRef] [PubMed]
- Waters, D.L.; Hale, L.; Grant, A.M.; Herbison, P.; Goulding, A. Osteoporosis and gait and balance disturbances in older sarcopenic obese New Zealanders. Osteoporos. Int. 2010, 21, 351–357. [Google Scholar] [CrossRef]
- Liang, H.; Li, X.; Lin, X.; Ju, Y.; Leng, J. The correlation between nutrition and frailty and the receiver operating characteristic curve of different nutritional indexes for frailty. BMC Geriatr. 2021, 21, 619. [Google Scholar] [CrossRef]
- Artaza-Artabe, I.; Sáez-López, P.; Sánchez-Hernández, N.; Fernández-Gutierrez, N.; Malafarina, V. The relationship between nutrition and frailty: Effects of protein intake, nutritional supplementation, vitamin D and exercise on muscle metabolism in the elderly. A systematic review. Maturitas 2016, 93, 89–99. [Google Scholar] [CrossRef]
- Landi, F.; Camprubi-Robles, M.; Bear, D.E.; Cederholm, T.; Malafarina, V.; Welch, A.A.; Cruz-Jentoft, A.J. Muscle loss: The new malnutrition challenge in clinical practice. Clin. Nutr. 2019, 38, 2113–2120. [Google Scholar] [CrossRef]
- Sanford, A.M. Anorexia of aging and its role for frailty. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 54–60. [Google Scholar] [CrossRef]
- Cox, N.J.; Ibrahim, K.; Sayer, A.A.; Robinson, S.M.; Roberts, H.C. Assessment and Treatment of the Anorexia of Aging: A Systematic Review. Nutrients 2019, 11, 144. [Google Scholar] [CrossRef]
- Morley, J.E. Pathophysiology of the anorexia of aging. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 27–32. [Google Scholar] [CrossRef]
- De Castro, J.M. Age-related changes in spontaneous food intake and hunger in humans. Appetite 1993, 21, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Calvani, R.; Martone, A.M.; Marzetti, E.; Onder, G.; Savera, G.; Lorenzi, M.; Serafini, E.; Bernabei, R.; Landi, F. Pre-hospital dietary intake correlates with muscle mass at the time of fracture in older hip-fractured patients. Front. Aging Neurosci. 2014, 6, 269. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, R.E.; Lang, I.A.; Llewellyn, D.J.; Rockwood, K. Frailty, body mass index, and abdominal obesity in older people. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 377–381. [Google Scholar] [CrossRef]
- Watanabe, D.; Yoshida, T.; Watanabe, Y.; Yamada, Y.; Kimura, M.; Kyoto-Kameoka Study Group. A U-Shaped Relationship between the Prevalence of Frailty and Body Mass Index in Community-Dwelling Japanese Older Adults: The Kyoto–Kameoka Study. J. Clin. Med. 2020, 9, 1367. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, M.; Downer, B.; Li, C.-Y.; Al Snih, S. Body mass index and physical frailty among older Mexican Americans: Findings from an 18-year follow up. PLoS ONE 2022, 17, e0274290. [Google Scholar] [CrossRef]
- Boutin, E.; Natella, P.A.; Schott, A.M.; Bastuji-Garin, S.; David, J.P.; Paillaud, E.; Rolland, Y.; Canouï-Poitrine, F. Interrelations between body mass index, frailty, and clinical adverse events in older community-dwelling women: The EPIDOS cohort study. Clin. Nutr. 2018, 37, 1638–1644. [Google Scholar] [CrossRef]
- Rietman, M.L.; Van Oostrom, S.H.; Picavet, H.S.J.; DollÃ, M.E.T.; Van Steeg, H.; Verschuren, W.M.M.; Spijkerman, A.M.W. The Association between BMI and Different Frailty Domains: A U-Shaped Curve? J. Nutr. Health Aging 2018, 22, 8–15. [Google Scholar] [CrossRef]
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L. Frailty: Implications for clinical practice and public health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef]
- Buckinx, F.; Landi, F.; Cesari, M.; Fielding, R.A.; Visser, M.; Engelke, K.; Maggi, S.; Dennison, E.; Al-Daghri, N.M.; Allepaerts, S.; et al. Pitfalls in the measurement of muscle mass: A need for a reference standard. J. Cachexia Sarcopenia Muscle 2018, 9, 269–278. [Google Scholar] [CrossRef]
- Kohara, K.; Okada, Y.; Ochi, M.; Ohara, M.; Nagai, T.; Tabara, Y.; Igase, M. Muscle mass decline, arterial stiffness, white matter hyperintensity, and cognitive impairment: Japan Shimanami Health Promoting Program study. J. Cachexia Sarcopenia Muscle 2017, 8, 557–566. [Google Scholar] [CrossRef]
- Cesari, M.; Leeuwenburgh, C.; Lauretani, F.; Onder, G.; Bandinelli, S.; Maraldi, C.; Guralnik, J.M.; Pahor, M.; Ferrucci, L. Frailty syndrome and skeletal muscle: Results from the Invecchiare in Chianti study. Am. J. Clin. Nutr. 2006, 83, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, J.; Shen, S.; Hong, X.; Zeng, X.; Yang, Y.; Liu, Z.; Chen, L.; Chen, X. Association Between Body Composition and Frailty in Elder Inpatients. Clin. Interv. Aging 2020, 15, 313–320. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Prell, T.; Grimm, A.; Axer, H. Uncovering sarcopenia and frailty in older adults by using muscle ultrasound-A narrative review. Front. Med. 2024, 11, 1333205. [Google Scholar] [CrossRef]
- Narici, M.V.; Maffulli, N. Sarcopenia: Characteristics, mechanisms and functional significance. Br. Med. Bull. 2010, 95, 139–159. [Google Scholar] [CrossRef]
- Giannoulis, M.G.; Martin, F.C.; Nair, K.S.; Umpleby, A.M.; Sonksen, P. Hormone replacement therapy and physical function in healthy older men. Time to talk hormones? Endocr. Rev. 2012, 33, 314–377. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Jacot, E.; Jequier, E.; Maeder, E.; Wahren, J.; Felber, J.P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 1981, 30, 1000–1007. [Google Scholar] [CrossRef]
- Pette, D.; Peuker, H.; Staron, R.S. The impact of biochemical methods for single fiber analysis. Acta Physiol. Scand. 1999, 166, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, E.J.; Bourey, R.E.; Rodnick, K.J.; Koranyi, L.; Permutt, M.A.; Holloszy, J.O. Glucose transporter protein content and glucose transport capacity in rat skeletal muscles. Am. J. Physiol. Endocrinol. Metab. 1990, 259, E593–E598. [Google Scholar] [CrossRef]
- Verdijk, L.B.; Snijders, T.; Beelen, M.; Savelberg, H.H.; Meijer, K.; Kuipers, H.; Van Loon, L.J. Characteristics of muscle fiber type are predictive of skeletal muscle mass and strength in elderly men. J. Am. Geriatr. Soc. 2010, 58, 2069–2075. [Google Scholar] [CrossRef]
- Kramer, I.F.; Snijders, T.; Smeets, J.S.J.; Leenders, M.; van Kranenburg, J.; den Hoed, M.; Verdijk, L.B.; Poeze, M.; van Loon, L.J.C. Extensive Type II Muscle Fiber Atrophy in Elderly Female Hip Fracture Patients. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Sonjak, V.; Jacob, K.; Morais, J.A.; Rivera-Zengotita, M.; Spendiff, S.; Spake, C.; Taivassalo, T.; Chevalier, S.; Hepple, R.T. Fidelity of muscle fibre reinnervation modulates ageing muscle impact in elderly women. J. Physiol. 2019, 597, 5009–5023. [Google Scholar] [CrossRef] [PubMed]
- St-Jean-Pelletier, F.; Pion, C.H.; Leduc-Gaudet, J.P.; Sgarioto, N.; Zovilé, I.; Barbat-Artigas, S.; Reynaud, O.; Alkaterji, F.; Lemieux, F.C.; Grenon, A.; et al. The impact of ageing, physical activity, and pre-frailty on skeletal muscle phenotype, mitochondrial content, and intramyocellular lipids in men. J. Cachexia Sarcopenia Muscle 2017, 8, 213–228. [Google Scholar] [CrossRef]
- Pérez-Tasigchana, R.F.; León-Muñoz, L.M.; Lopez-Garcia, E.; Gutierrez-Fisac, J.L.; Laclaustra, M.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. Metabolic syndrome and insulin resistance are associated with frailty in older adults: A prospective cohort study. Age Ageing 2017, 46, 807–812. [Google Scholar] [CrossRef]
- Ke, Z.; Wen, H.; Huang, R.; Xu, X.; Yang, K.; Liu, W.; Wang, S.; Zhang, X.; Guo, Y.; Liao, X.; et al. Long-term insulin resistance is associated with frailty, frailty progression, and cardiovascular disease. J. Cachexia Sarcopenia Muscle 2024, 15, 1578–1586. [Google Scholar] [CrossRef]
- Peng, P.S.; Kao, T.W.; Chang, P.K.; Chen, W.L.; Peng, P.J.; Wu, L.W. Association between HOMA-IR and Frailty among U. S. Middle-aged and Elderly Population. Sci. Rep. 2019, 9, 4238. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Li, Y.M.; Wang, C.Q.; Chen, G.Q.; Lian, Y. Association between non-insulin-based insulin resistance indicators and frailty progression: A national cohort study and mendelian randomization analysis. Cardiovasc. Diabetol. 2025, 24, 31. [Google Scholar] [CrossRef]
- Li, H.; Meng, Y.; He, S.; Tan, X.; Zhang, Y.; Zhang, X.; Wang, L.; Zheng, W. Macrophages, chronic inflammation, and insulin resistance. Cells 2022, 11, 3001. [Google Scholar] [CrossRef]
- Goulet, E.D.; Hassaine, A.; Dionne, I.J.; Gaudreau, P.; Khalil, A.; Fulop, T.; Shatenstein, B.; Tessier, D.; Morais, J.A. Frailty in the elderly is associated with insulin resistance of glucose metabolism in the postabsorptive state only in the presence of increased abdominal fat. Exp. Gerontol. 2009, 44, 740–744. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Varadhan, R.; Weiss, C.O.; Fried, L.P.; Cappola, A.R. Frailty Status and Altered Glucose-Insulin Dynamics. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1300–1306. [Google Scholar] [CrossRef]
- Taguchi, A.; Wartschow, L.; White, M. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science 2007, 317, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Vischer, U.M.; Safar, M.E.; Safar, H.; Iaria, P.; LeDudal, K.; Henry, O.; Herrmann, F.R.; Ducimetiere, P.; Blacher, J. Cardiometabolic determinants of mortality in a geriatric population: Is there a “reverse metabolic syndrome”? Diabetes Metab. 2009, 35, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lim, W.S.; Jin, X.; Zin Nyunt, M.S.; Fulop, T.; Gao, Q.; Lim, S.C.; Larbi, A.; Ng, T.P. Lower insulin level is associated with sarcopenia in community-dwelling frail and non-frail older adults. Front. Med. 2022, 9, 971622. [Google Scholar] [CrossRef]
- Srikanthan, P.; Hevener, A.L.; Karlamangla, A.S. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: Findings from the National health and nutrition examination survey III. PLoS ONE 2010, 5, e10805. [Google Scholar] [CrossRef]
- Chung, J.-Y.; Kang, H.-T.; Lee, D.-C.; Lee, H.R.; Lee, Y.J. Body composition and its association with cardiometabolic risk factors in the elderly: A focus on sarcopenic obesity. Arch. Gerontol. Geriatr. 2013, 56, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafiz, A.H.; Keegan, G.L.; Sinclair, A.J. Metabolic Characteristics of Frail Older People with Diabetes Mellitus-A Systematic Search for Phenotypes. Metabolites 2023, 13, 705. [Google Scholar] [CrossRef]
- Abdelhafiz, A.H.; Chakravorty, P.; Gupta, S.; Haque, A.; Sinclair, A.J. Can hypoglycaemic medications be withdrawn in older people with type 2 diabetes? Int. J. Clin. Pract. 2014, 68, 790–792. [Google Scholar] [CrossRef]
- Sjöblom, P.; AndersTengblad; Löfgren, U.B.; Lannering, C.; Anderberg, N.; Rosenqvist, U.; Mölstad, S.; Ostgren, C.J. Can diabetes medication be reduced in elderly patients? An observational study of diabetes drug withdrawal in nursing home patients with tight glycaemic control. Diabetes Res. Clin. Pract. 2008, 82, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafiz, A.H.; Koay, L.; Sinclair, A.J. The emergence of frailty may burn out type 2 diabetes-a hypothesis. Int. J. Clin. Pract. 2016, 70, 358–359. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Vu, H.T.T.; Nguyen, T.N.; Dao, H.T.; Nguyen, T.X.; Nguyen, H.T.T.; Dang, A.K.; Nguyen, A.T.; Pham, T.; Vu, G.T.; et al. Assessment of nutritional status in older diabetic outpatients and related factors in Hanoi, Vietnam. J. Multidiscip. Heal. 2019, 12, 601–606. [Google Scholar] [CrossRef]
- Daradkeh, G.; Essa, M.M.; Al-Adawi, S.S.; Koshy, R.P.; Al-Asmi, A.; Waly, M.I. Nutritional status and cognitive impairment in elderly. Pak. J. Biol. Sci. 2014, 17, 1098–1105. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vellas, B.; Lauque, S.; Gillette-Guyonnet, S.; Andrieu, S.; Cortes, F.; Nourhashémi, F.; Cantet, C.; Ousset, P.J.; Grandjean, H.; REAL.FR Group. Impact of nutritional status on the evolution of Alzheimer’s disease and on response to acetylcholinesterase inhibitor treatment. J. Nutr. Health Aging 2005, 9, 75–80. [Google Scholar] [PubMed]
- Adame Perez, S.I.; Senior, P.A.; Field, C.J.; Jindal, K.; Mager, D.R. Frailty, Health-Related Quality of Life, Cognition, Depression, Vitamin D and Health-Care Utilization in an Ambulatory Adult Population with Type 1 or Type 2 Diabetes Mellitus and Chronic Kidney Disease: A Cross-Sectional Analysis. Can. J. Diabetes 2019, 43, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, A.; Taniguchi, Y.; Seino, S.; Yokoyama, Y.; Amano, H.; Fujiwara, Y.; Shinkai, S. Combined effect of diabetes and frailty on mortality and incident disability in older Japanese adults. Geriatr. Gerontol. Int. 2019, 19, 423–428. [Google Scholar] [CrossRef]
- Bilgin, S.; Aktas, G.; Kurtkulagi, O.; Atak, B.M.; Duman, T.T. Edmonton frail score is associated with diabetic control in elderly type 2 diabetic subjects. J. Diabetes Metab. Disord. 2020, 19, 511–514. [Google Scholar] [CrossRef]
- Lin, C.L.; Yu, N.C.; Wu, H.C.; Liu, Y.C. Risk factors associated with frailty in older adults with type 2 diabetes: A cross-sectional study. J. Clin. Nurs. 2022, 31, 967–974. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Z.; Ma, B.; Fan, L.; Yi, N.; Lu, B.; Wang, Q.; Liu, R. GLP-1 Improves Adipocyte Insulin Sensitivity Following Induction of Endoplasmic Reticulum Stress. Front. Pharmacol. 2018, 9, 1168. [Google Scholar] [CrossRef]
- Guo, C.; Huang, T.; Chen, A.; Chen, X.; Wang, L.; Shen, F.; Gu, X. Glucagon-like peptide 1 improves insulin resistance in vitro through anti-inflammation of macrophages. Braz. J. Med. Biol. Res. 2016, 49, e5826. [Google Scholar] [CrossRef]
- Puddu, A.; Mach, F.; Nencioni, A.; Viviani, G.L.; Montecucco, F. An Emerging Role of Glucagon-Like Peptide-1 in Preventing Advanced-Glycation-End-Product-Mediated Damages in Diabetes. Mediat. Inflamm. 2013, 2013, 591056. [Google Scholar] [CrossRef]
- Hao, T.; Zhang, H.; Li, S.; Tian, H. Glucagon-Like Peptide 1 Receptor Agonist Ameliorates the Insulin Resistance Function of Islet Beta Cells Via the Activation of Pdx-1/Jak Signaling Transduction in C57/Bl6 Mice With High-Fat Diet-Induced Diabetes. Int. J. Mol. Med. 2017, 39, 1029–1036. [Google Scholar] [CrossRef]
- Kawamori, D.; Shirakawa, J.; Liew, C.W.; Hu, J.; Morioka, T.; Duttaroy, A.; Burkey, B.; Kulkarni, R.N. Glp-1 Signalling Compensates for Impaired Insulin Signalling in Regulating Beta Cell Proliferation in Betairko Mice. Diabetologia 2017, 60, 1442–1453. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, M.; Nian, H.; Mayfield, D.; Devin, J.K.; Gamboa, J.L.; Yu, C.; Silver, H.J.; Niswender, K.; Luther, J.M.; Brown, N.J. Weight Loss-Independent Effect of Liraglutide on Insulin Sensitivity in Individuals With Obesity and Prediabetes. Diabetes 2024, 73, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.K.; Nikooienejad, A.; Bray, R.; Cui, X.; Wilson, J.; Duffin, K.; Milicevic, Z.; Haupt, A.; Robins, D.A. Dual GIP and GLP-1 Receptor Agonist Tirzepatide Improves Beta-cell Function and Insulin Sensitivity in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2021, 106, 388–396. [Google Scholar] [CrossRef]
- Yan, H.; Huang, C.; Shen, X.; Li, J.; Zhou, S.; Li, W. GLP-1 RAs and SGLT-2 Inhibitors for Insulin Resistance in Nonalcoholic Fatty Liver Disease: Systematic Review and Network Meta-Analysis. Front. Endocrinol. 2022, 13, 923606. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Patoulias, D.; Fragakis, N.; Mantzoros, C.S. Effect of glucagon-like peptide-1 receptor agonists and co-agonists on body composition: Systematic review and network meta-analysis. Metabolism 2025, 164, 156113. [Google Scholar] [CrossRef]
- De La Flor, J.C.; Coto Morales, B.; Basabe, E.; Rey Hernandez, M.; Zamora González-Mariño, R.; Rodríguez Tudero, C.; Benites Flores, I.; Espinoza, C.; Cieza Terrones, M.; Cigarrán Guldris, S.; et al. Effects of Sodium-Glucose Cotransporter-2 Inhibitors on Body Composition and Fluid Status in Cardiovascular Rehabilitation Patients with Coronary Artery Disease and Heart Failure. Medicina 2024, 60, 2096. [Google Scholar] [CrossRef]
- Pan, R.; Zhang, Y.; Wang, R.; Xu, Y.; Ji, H.; Zhao, Y. Effect of SGLT-2 inhibitors on body composition in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. PLoS ONE 2022, 17, e0279889. [Google Scholar] [CrossRef]
- Al Jobori, H.; Daniele, G.; Adams, J.; Cersosimo, E.; Solis-Herrera, C.; Triplitt, C.; DeFronzo, R.A.; Abdul-Ghani, M. Empagliflozin Treatment Is Associated With Improved Beta-Cell Function in Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2018, 103, 1402–1407. [Google Scholar] [CrossRef]
- Waseda, N.; Satoh, H.; Yoshida, C.; Ikeda, F.; Kanazawa, A.; Watada, H. Effects of SGLT2 Inhibitors on Insulin Secretion and Insulin Resistance—Results from a Cross-Sectional Study. Diabetes 2018, 67 (Suppl. S1), 1187-P. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tobin, J.D.; Andres, R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am. J. Physiol. 1979, 237, E214–E223. [Google Scholar] [CrossRef]
- Ferrannini, E.; Muscelli, E.; Frascerra, S.; Baldi, S.; Mari, A.; Heise, T.; Broedl, U.C.; Woerle, H.J. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J. Clin. Investig. 2014, 124, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, P.; Bergenstal, R.M.; Toural, E.; Inzucchi, S.E.; Zinman, B.; Hantel, S.; Kiš, S.G.; Kaspers, S.; George, J.T.; Fitchett, D. Efficacy and safety of empagliflozin in older patients in the EMPA-REG OUTCOME trial. Age Ageing 2019, 48, 859–866. [Google Scholar] [CrossRef]
- Cahn, A.; Mosenzon, O.; Wiviott, S.D.; Rozenberg, A.; Yanuv, I.; Goodrich, E.L.; Murphy, S.A.; Bhatt, D.L.; Leiter, L.A.; McGuire, D.K.; et al. Efficacy and safety of dapagliflozin in the elderly: Analysis from the DECLARE-TIMI 58 Study. Diabetes Care 2020, 43, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Longo, M.; Maiorino, M.I.; Bellastella, G.; Chiodini, P.; Solerte, S.B.; Esposito, K. Efficacy of SGLT-2 inhibitors in older adults with diabetes: Systematic review with meta-analysis of cardiovascular outcome trials. Diabetes Res. Clin. Pract. 2020, 108114. [Google Scholar] [CrossRef]
- Gilbert, M.P.; Bain, S.C.; Franek, E.; Jodar-Gimeno, E.; Nauck, M.A.; Pratley, R.; Réa, R.R.; Kerr Saraiva, J.F.; Rasmussen, S.; Tornøe, K.; et al. Effect of liraglutide on cardiovascular outcomes in elderly patients: A post hoc analysis of a randomized controlled trial. Ann. Intern. Med. 2019, 170, 423–426. [Google Scholar] [CrossRef]
- Emmerton, D.; Abdelhafiz, A. Newer anti-diabetic therapies with low hypoglycemic risk-potential advantages for frail older people. Hosp. Pract. 2021, 49, 164–175. [Google Scholar] [CrossRef]
- International Diabetes Federation Global Guidelines for Managing Older People with Type 2 Diabetes. 2013. Available online: https://ifa.ngo/wp-content/uploads/2014/02/IDF-Guideline-for-Older-People.pdf (accessed on 30 April 2025).
- Sinclair, A.J.; Abdelhafiz, A.; Dunning, T.; Izquierdo, M.; Rodriguez Manas, L.; Bourdel-Marchasson, I.; Morley, J.E.; Munshi, M.; Woo, J.; Vellas, B. An international position statement on the management of frailty in diabetes mellitus: Summary of recommendations 2017. J. Frailty Aging. 2018, 7, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Bourdel-Marchasson, I.; Maggi, S.; Abdelhafiz, A.; Bellary, S.; Demurtas, J.; Forbes, A.; Ivory, P.; Rodríguez-Mañas, L.; Sieber, C.; Strandberg, T.; et al. Essential steps in primary care management of older people with Type 2 diabetes: An executive summary on behalf of the European geriatric medicine society (EuGMS) and the European diabetes working party for older people (EDWPOP) collaboration. Aging Clin. Exp. Res. 2023, 35, 2279–2291. [Google Scholar] [CrossRef]
- Ivers, N.M.; Jiang, M.; Alloo, J.; Singer, A.; Ngui, D.; Casey, C.G.; Yu, C.H. Diabetes Canada 2018 clinical practice guidelines: Key messages for family physicians caring for patients living with type 2 diabetes. Can. Fam. Physician 2019, 65, 14–24. [Google Scholar]
- Araki, E.; Goto, A.; Kondo, T.; Noda, M.; Noto, H.; Origasa, H.; Osawa, H.; Taguchi, A.; Tanizawa, Y.; Tobe, K.; et al. Japanese clinical practice guideline for diabetes 2019. Diabetol. Int. 2020, 11, 165–223. [Google Scholar] [CrossRef]
- Moon, J.S.; Kang, S.; Choi, J.H.; Lee, K.A.; Moon, J.H.; Chon, S.; Kim, D.J.; Kim, H.J.; Seo, J.A.; Kim, M.K.; et al. 2023 Clinical Practice Guidelines for Diabetes Management in Korea: Full Version Recommendation of the Korean Diabetes Association. Diabetes Metab. J. 2024, 48, 546–708. [Google Scholar] [CrossRef] [PubMed]
- Kutz, A.; Kim, D.H.; Wexler, D.J.; Liu, J.; Schneeweiss, S.; Glynn, R.J.; Patorno, E. Comparative Cardiovascular Effectiveness and Safety of SGLT-2 Inhibitors, GLP-1 Receptor Agonists, and DPP-4 Inhibitors According to Frailty in Type 2 Diabetes. Diabetes Care 2023, 46, 2004–2014. [Google Scholar] [CrossRef]
- Sinclair, A.J.; Abdelhafiz, A.H. The Use of SGLT-2 Inhibitors and GLP-1RA in Frail Older People with Diabetes: A Personalised Approach Is Required. Metabolites 2025, 15, 49. [Google Scholar] [CrossRef]
- Keller, H.H.; Carrier, N.; Slaughter, S.E.; Lengyel, C.; Steele, C.M.; Duizer, L.; Morrison, J.; Brown, K.S.; Chaudhury, H.; Yoon, M.N.; et al. Prevalence and Determinants of Poor Food Intake of Residents Living in Long-Term Care. J. Am. Med. Dir. Assoc. 2017, 18, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef]
- Khoonin, W.; Shantavasinkul, P.C.; Santivarangkna, C.; Praengam, K.; Trachootham, D. Eicosapentaenoic acid and branched-chain amino acids fortified complete nutrition drink improved muscle strength in older individuals with inadequate protein intake. Front. Nutr. 2023, 10, 1164469. [Google Scholar] [CrossRef] [PubMed]
- Lunati, M.E.; Cimino, V.; Gandolfi, A.; Trevisan, M.; Montefusco, L.; Pastore, I.; Pace, C.; Betella, N.; Favacchio, G.; Bulgheroni, M.; et al. SGLT2-inhibitors are effective and safe in the elderly: The SOLD study. Pharmacol. Res. 2022, 183, 106396. [Google Scholar] [CrossRef]
- Rolek, B.; Haber, M.; Gajewska, M.; Rogula, S.; Pietrasik, A.; Gąsecka, A. SGLT2 Inhibitors vs. GLP-1 Agonists to Treat the Heart, the Kidneys and the Brain. J. Cardiovasc. Dev. Dis. 2023, 10, 322. [Google Scholar] [CrossRef]
- Sohn, M.; Nam, S.; Nauck, M.A.; Lim, S. Long-term comparison of renal and metabolic outcomes after sodium–glucose co-transporter 2 inhibitor or glucagon-like peptide-1 receptor agonist therapy in type 2 diabetes. BMC Med. 2024, 22, 273. [Google Scholar] [CrossRef]
- Li, C.; Luo, J.; Jiang, M.; Wang, K. The Efficacy and Safety of the Combination Therapy With GLP-1 Receptor Agonists and SGLT-2 Inhibitors in Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis. Front. Pharmacol. 2022, 13, 838277. [Google Scholar] [CrossRef]
- Simms-Williams, N.; Treves, N.; Yin, H.; Lu, S.; Yu, O.; Pradhan, R.; Renoux, C.; Suissa, S.; Azoulay, L. Effect of combination treatment with glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors on incidence of cardiovascular and serious renal events: Population based cohort study. BMJ 2024, 385, e078242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lin, C.; Cai, X.; Jiao, R.; Bai, S.; Li, Z.; Lv, F.; Yang, W.; Liu, G.; Yang, X.; et al. One or two? Comparison of the cardiorenal effects between combination therapy and monotherapy with SGLT2i or GLP1RA. Diabetes Obes. Metab. 2025, 27, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafiz, A.; Bisht, S.; Kovacevic, I.; Pennells, D.; Sinclair, A. Insulin in Frail, Older People with Type 2 Diabetes—Low Threshold for Therapy. Diabetology 2022, 3, 369–383. [Google Scholar] [CrossRef]
- Hayes, K.N.; Berry, S.D.; Munshi, M.N.; Zullo, A.R. Adoption of sodium-glucose cotransporter-2 inhibitors among prescribers caring for nursing home residents. J. Am. Geriatr. Soc. 2023, 71, 2585–2592. [Google Scholar] [CrossRef] [PubMed]
| Type I | Type II |
|---|---|
| Red in colour, slow twitching and fatigues slowly. | Pale in colour, fast twitching and fatigues rapidly. |
| Has high aerobic oxidative capacity. | Has low anaerobic oxidative capacity. |
| Has high blood supply, high capillary and mitochondrial number. | Has low blood supply, low capillary and mitochondrial number. |
| Low lipid content. | High lipid content. |
| Low glycolytic capacity. | High glycolytic capacity. |
| Low insulin resistance. | High insulin resistance. |
| Declines less with increasing age and frailty. | Declines more with increasing age and frailty. |
| Guidelines | Frailty Inclusion | Recommendations |
|---|---|---|
| ADA [7] | Frailty is not defined, but screening for frailty, as a part of geriatric syndromes, is recommended. | SGLT-2i: Caution in individuals depending on caregivers for adequate fluid intake or have recurrent UTI. GLP-1RA: Injectables, which require visual, motor, and cognitive skills for administration, may not be preferred in people experiencing unexplained weight loss, suspected gastroparesis, or recurrent gastrointestinal problems. |
| ESE [8] | Frailty is defined, and screening using frailty screening tools is recommended. | SGLT-2i: Limit the dose in patients at risk of volume loss. GLP-1RA: Nausea is a common side effect and could be problematic in patients with compromised intake, especially those with progressing CKD. |
| IDF [107] | Defined and screening by frailty tool is recommended. | SGLT-2i: Associated with increased risk of genital and urinary tract infections, hypovolaemia, postural hypotension, and weight loss may limit their use in some older people. GLP-1RA: May not be appropriate for frail older people in whom weight loss can be detrimental. |
| IPS [108] | Frailty is defined, and screening using various frailty tools is recommended. | SGLT-2i: Watch for increased urinary frequency, incontinence, lower BP, genital infections, dehydration, and dose reduction required in the presence of renal impairment. GLP-1RA: Monitor for anorexia, weight loss, and dose reduction needed in moderate impairment. |
| EuGMS/EDWPOP [109] | Frailty is defined, and screening by easy-to-perform and validated tool, which does not necessarily require professional staff, is recommended as integral part of routine care. | SGLT2i: Not suitable for moderate–severe frailty or care home residents with weight loss. They increase risk of UTI, candidiasis, dehydration, hypotension, and diabetic ketoacidosis. GLP-1RA: Not suitable for subjects with CKD or care home residents with weight loss. |
| Diabetes Canada [110] | Defined and screening by frailty tool is recommended. | SGLT-2i: Could be considered for people < 75 years with evidence of CVD, relatively preserved renal function, and no other complex comorbidities. GLP-1RA: Older people may be more susceptible to dehydration and fractures than younger people treated with these agents, suggesting that they should be used cautiously. |
| JDS [111] | Not defined but recommend screening as part of geriatric syndromes. | General recommendation: SGLT-2i: Attention to dehydration and urogenital infections. GLP-1RA: Attention to gastrointestinal symptoms (nausea and vomiting) and weight loss. |
| KDS [112] | Defined and screening by frailty tools is recommended. | General recommendation: SGLT-2i: Caution due to risk of dehydration and weight loss. GLP-1RA: Their side effects significantly increase in people over 60 years without ASCVD. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinclair, A.; Siqueira, I.; Abdelhafiz, A. The Effect of Frailty on Body Composition and Its Impact on the Use of SGLT-2 Inhibitors and GLP-1RA in Older Persons with Diabetes. Metabolites 2025, 15, 381. https://doi.org/10.3390/metabo15060381
Sinclair A, Siqueira I, Abdelhafiz A. The Effect of Frailty on Body Composition and Its Impact on the Use of SGLT-2 Inhibitors and GLP-1RA in Older Persons with Diabetes. Metabolites. 2025; 15(6):381. https://doi.org/10.3390/metabo15060381
Chicago/Turabian StyleSinclair, Alan, Izel Siqueira, and Ahmed Abdelhafiz. 2025. "The Effect of Frailty on Body Composition and Its Impact on the Use of SGLT-2 Inhibitors and GLP-1RA in Older Persons with Diabetes" Metabolites 15, no. 6: 381. https://doi.org/10.3390/metabo15060381
APA StyleSinclair, A., Siqueira, I., & Abdelhafiz, A. (2025). The Effect of Frailty on Body Composition and Its Impact on the Use of SGLT-2 Inhibitors and GLP-1RA in Older Persons with Diabetes. Metabolites, 15(6), 381. https://doi.org/10.3390/metabo15060381





