High-Calorie Diet Consumption Induces Lac-Phe Changes in the Brain in a Time-of-Day Manner Independent of Exercise
Abstract
1. Introduction
2. Materials and Methods
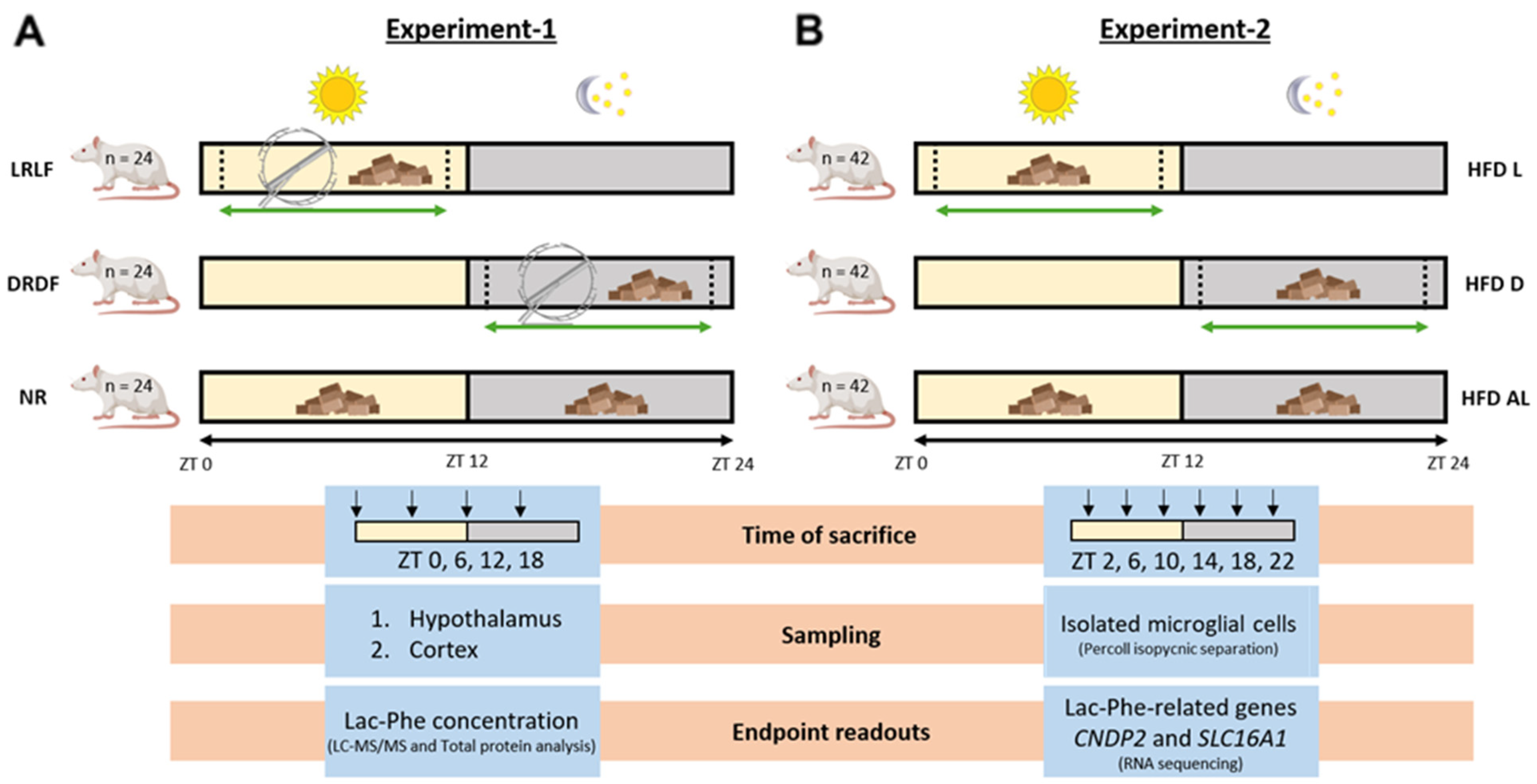
2.1. Study Design
2.2. Animal Experiments
2.2.1. Experiment-1: Animals for Brain Lac-Phe Measurements
2.2.2. Experiment-2: Time-Restricted Feeding Animals
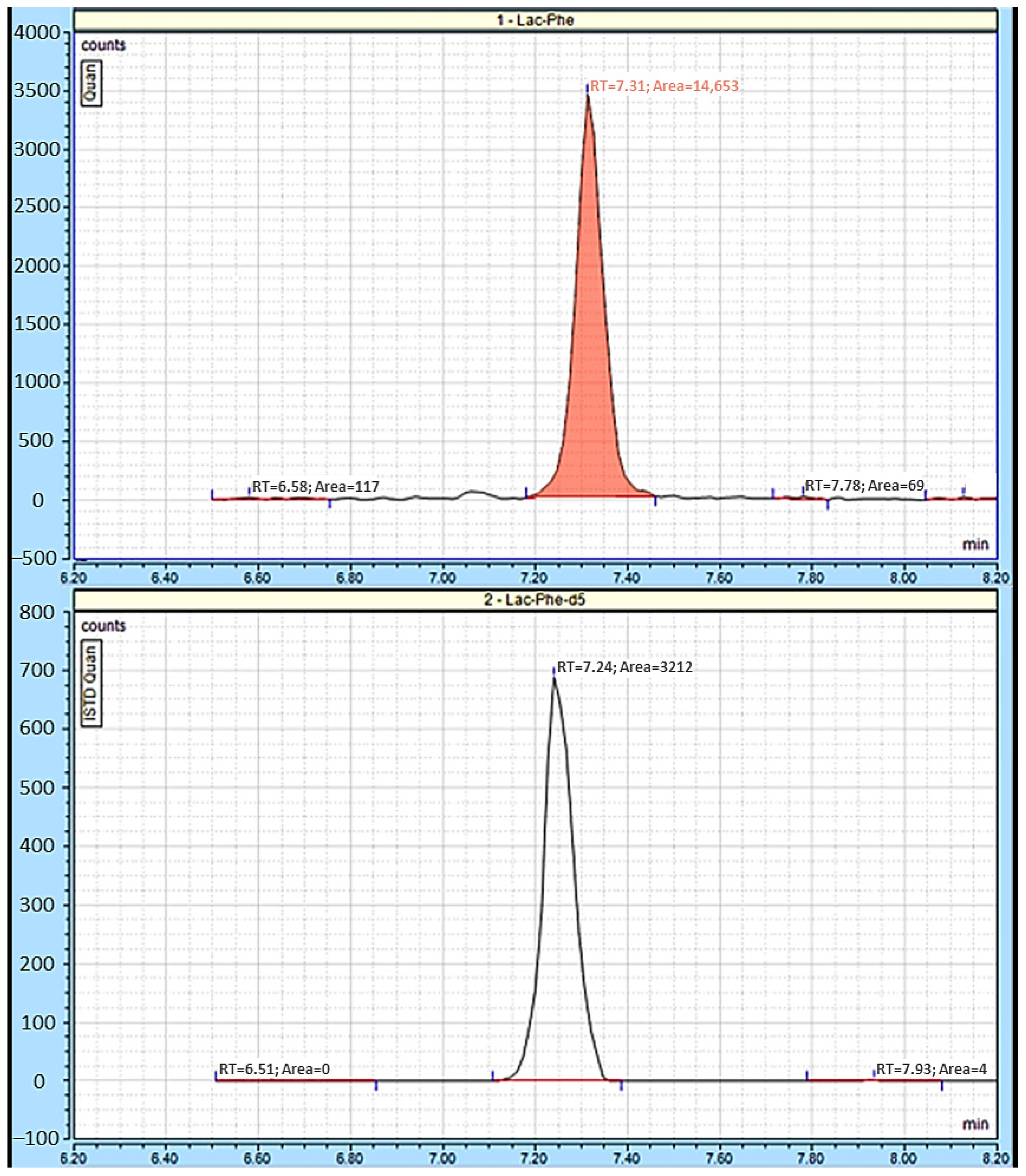
2.3. Quantitative Lac-Phe Measurement by LC-MS/MS in Hypothalamus and Cortex
2.4. Microglia Isolation
2.5. RNA Sequencing
2.6. Statistical Analyses
3. Results
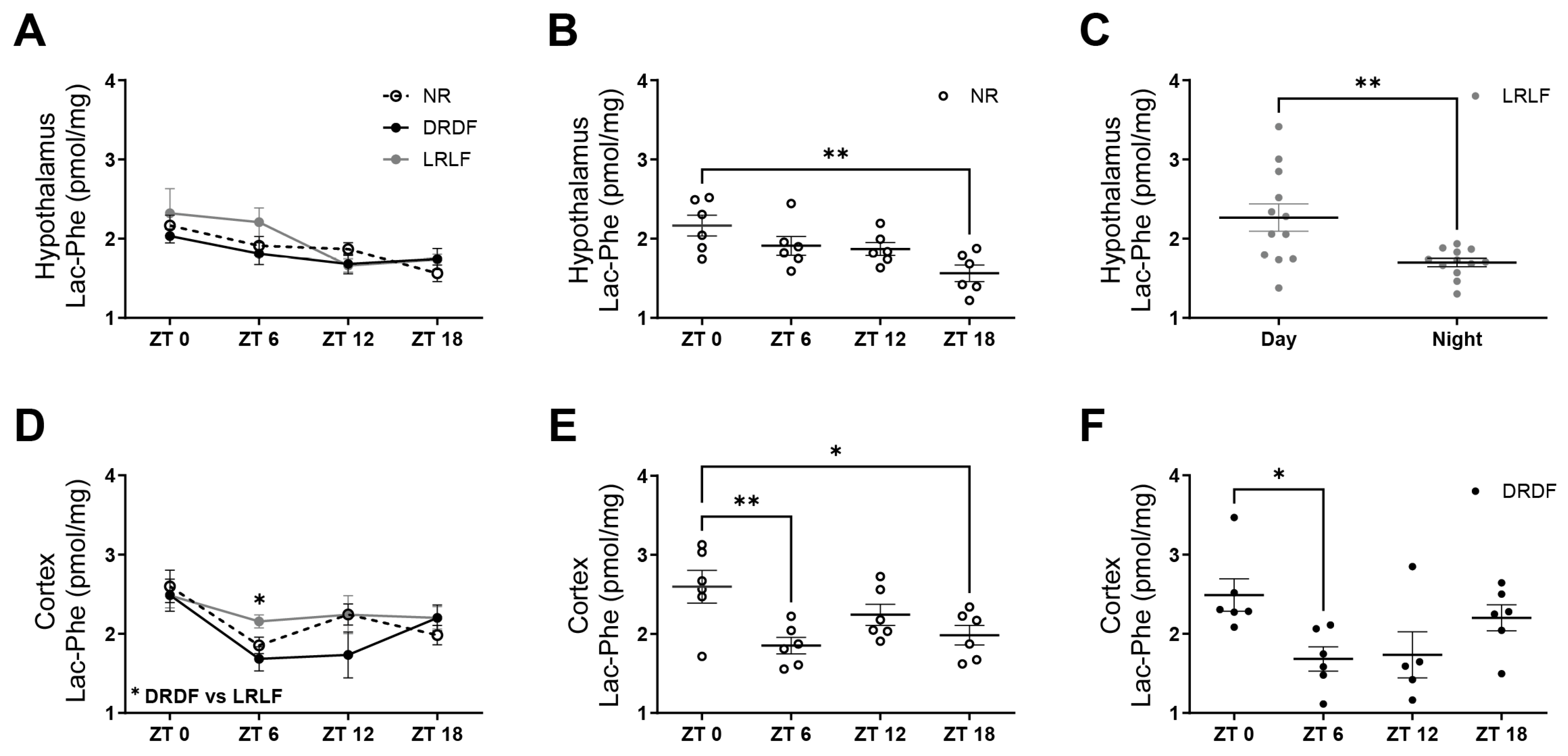
3.1. Experiment-1: Lac-Phe Levels Change in a Time-of-Day Dependent Manner in the Hypothalamus and Cortex Dependent on Exercise/Diet
3.2. Experiment-2: Circadian Expression of CNDP2 and SLC16A1 in Microglia After TRF HFD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abarca-Gómez, L.; Abdeen, Z.A.; Hamid, Z.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.A.; et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Ng, M.; Dai, X.; Cogen, R.M.; Abdelmasseh, M.; Abdollahi, A.; Abdullahi, A.; Aboagye, R.G.; Abukhadijah, H.J.; Adeyeoluwa, T.E.; Afolabi, A.A.; et al. National-level and state-level prevalence of overweight and obesity among children, adolescents, and adults in the USA, 1990–2021, and forecasts up to 2050. Lancet 2024, 404, 2278–2298. [Google Scholar] [CrossRef]
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Bray, G.A.; Frühbeck, G.; Ryan, D.H.; Wilding, J.P. Management of obesity. Lancet 2016, 387, 1947–1956. [Google Scholar] [PubMed]
- Oppert, J.; Bellicha, A.; van Baak, M.A.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Carraça, E.V.; Encantado, J.; Ermolao, A.; Pramono, A.; et al. Exercise training in the management of overweight and obesity in adults: Synthesis of the evidence and recommendations from the European Association for the Study of Obesity Physical Activity Working Group. Obes. Rev. 2021, 22 (Suppl. S4), e13273. [Google Scholar] [CrossRef]
- McTiernan, A.; Sorensen, B.; Irwin, M.L.; Morgan, A.; Yasui, Y.; Rudolph, R.E.; Surawicz, C.; Lampe, J.W.; Lampe, P.D.; Ayub, K.; et al. Exercise effect on weight and body fat in men and women. Obesity 2007, 15, 1496–1512. [Google Scholar] [CrossRef]
- Li, V.L.; He, Y.; Contrepois, K.; Liu, H.; Kim, J.T.; Wiggenhorn, A.L.; Tanzo, J.T.; Tung, A.S.-H.; Lyu, X.; Zushin, P.-J.H.; et al. An exercise-inducible metabolite that suppresses feeding and obesity. Nature 2022, 606, 785–790. [Google Scholar]
- Xiao, S.; Li, V.L.; Long, J.Z. Lac-Phe (N-lactoyl-phenylalanine). Trends Endocrinol. Metab. 2024, 35, 758–759. [Google Scholar] [CrossRef]
- Jansen, R.S.; Addie, R.; Merkx, R.; Fish, A.; Mahakena, S.; Bleijerveld, O.B.; Altelaar, M.; Ijlst, L.; Wanders, R.J.; Borst, P.; et al. N-lactoyl-amino acids are ubiquitous metabolites that originate from CNDP2-mediated reverse proteolysis of lactate and amino acids. Proc. Natl. Acad. Sci. USA 2015, 112, 6601–6606. [Google Scholar] [CrossRef] [PubMed]
- Lund, J.; Clemmensen, C.; Schwartz, T.W. Outrunning obesity with Lac-Phe? Cell Metab. 2022, 34, 1085–1087. [Google Scholar] [CrossRef] [PubMed]
- Shiba, A.; de Goede, P.; Tandari, R.; Foppen, E.; Korpel, N.L.; Coopmans, T.V.; Hellings, T.P.; Jansen, M.W.; Ruitenberg, A.; Ritsema, W.I.; et al. Synergy between time-restricted feeding and time-restricted running is necessary to shift the muscle clock in male wistar rats. Neurobiol. Sleep Circadian Rhythm. 2024, 17, 100106. [Google Scholar] [CrossRef]
- Jia, M.; Meng, F.; Smerin, S.E.; Xing, G.; Zhang, L.; Su, D.M.; Benedek, D.; Ursano, R.; Su, Y.A.; Li, H. Biomarkers in an animal model for revealing neural, hematologic, and behavioral correlates of PTSD. J. Vis. Exp. 2012, 68, 3361. [Google Scholar]
- Nikodemova, M.; Watters, J.J. Efficient isolation of live microglia with preserved phenotypes from adult mouse brain. J. Neuroinflamm. 2012, 9, 147. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Kristiansen, K.; Wang, J. SOAP: Short oligonucleotide alignment program. Bioinformatics 2008, 24, 713–714. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Benelli, M.; Pescucci, C.; Marseglia, G.; Severgnini, M.; Torricelli, F.; Magi, A. Discovering chimeric transcripts in paired-end RNA-seq data by using EricScript. Bioinformatics 2012, 28, 3232–3239. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Park, J.W.; Lu, Z.-X.; Lin, L.; Henry, M.D.; Wu, Y.N.; Zhou, Q.; Xing, Y. rMATS: Robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc. Natl. Acad. Sci. USA 2014, 111, E5593–E5601. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Durinck, S.; Moreau, Y.; Kasprzyk, A.; Davis, S.; De Moor, B.; Brazma, A.; Huber, W. BioMart and Bioconductor: A powerful link between biological databases and microarray data analysis. Bioinformatics 2005, 21, 3439–3440. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef]
- Monsorno, K.; Buckinx, A.; Paolicelli, R.C. Microglial metabolic flexibility: Emerging roles for lactate. Trends Endocrinol. Metab. 2022, 33, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Moreira, T.J.; Pierre, K.; Maekawa, F.; Repond, C.; Cebere, A.; Liljequist, S.; Pellerin, L. Enhanced cerebral expression of MCT1 and MCT2 in a rat ischemia model occurs in activated microglial cells. J. Cereb. Blood Flow Metab. 2009, 29, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Wang, Z.; Liang, X.; Wang, Y.; Gao, L.; Ma, C. Monocarboxylate transporter 1 promotes classical microglial activation and pro-inflammatory effect via 6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase 3. J. Neuroinflammation 2019, 16, 240. [Google Scholar] [CrossRef]
- Scott, B.; Day, E.A.; O’brien, K.L.; Scanlan, J.; Cromwell, G.; Ni Scannail, A.; McDonnell, M.E.; Finlay, D.K.; Lynch, L. Metformin and feeding increase levels of the appetite-suppressing metabolite Lac-Phe in humans. Nat. Metab. 2024, 6, 651–658. [Google Scholar] [CrossRef]
- Xiao, S.; Li, V.L.; Lyu, X.; Chen, X.; Wei, W.; Abbasi, F.; Knowles, J.W.; Tung, A.S.-H.; Deng, S.; Tiwari, G.; et al. Lac-Phe mediates the effects of metformin on food intake and body weight. Nat. Metab. 2024, 6, 659–669. [Google Scholar] [CrossRef]
- Milićević, N.; Brink, J.B.T.; Asbroek, A.L.T.; Bergen, A.A.; Felder-Schmittbuhl, M.-P. The circadian clock regulates RPE-mediated lactate transport via SLC16A1 (MCT1). Exp. Eye Res. 2020, 190, 107861. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jermei, J.; Jiao, H.; Shiba, A.; Goedhart, J.C.; Tandari, R.; Kalsbeek, A.; Struys, E.A.; Yi, C.-X. High-Calorie Diet Consumption Induces Lac-Phe Changes in the Brain in a Time-of-Day Manner Independent of Exercise. Metabolites 2025, 15, 375. https://doi.org/10.3390/metabo15060375
Jermei J, Jiao H, Shiba A, Goedhart JC, Tandari R, Kalsbeek A, Struys EA, Yi C-X. High-Calorie Diet Consumption Induces Lac-Phe Changes in the Brain in a Time-of-Day Manner Independent of Exercise. Metabolites. 2025; 15(6):375. https://doi.org/10.3390/metabo15060375
Chicago/Turabian StyleJermei, Jarne, Han Jiao, Ayano Shiba, Julia C. Goedhart, Roberta Tandari, Andries Kalsbeek, Eduard A. Struys, and Chun-Xia Yi. 2025. "High-Calorie Diet Consumption Induces Lac-Phe Changes in the Brain in a Time-of-Day Manner Independent of Exercise" Metabolites 15, no. 6: 375. https://doi.org/10.3390/metabo15060375
APA StyleJermei, J., Jiao, H., Shiba, A., Goedhart, J. C., Tandari, R., Kalsbeek, A., Struys, E. A., & Yi, C.-X. (2025). High-Calorie Diet Consumption Induces Lac-Phe Changes in the Brain in a Time-of-Day Manner Independent of Exercise. Metabolites, 15(6), 375. https://doi.org/10.3390/metabo15060375





