Application of Craniosacral Therapy Versus Blood Levels of Corticoliberin and Oxytocin in Male Firefighters Exposed to Occupational Stress—A Randomised Control Trial
Abstract
1. Introduction
- The application of craniosacral therapy changes the level of CRH;
- The application of craniosacral therapy changes the level OXT.
2. Materials and Methods
2.1. Participants
2.2. Randomisation
2.2.1. Corticotropin-Releasing Hormone and Oxytocin Assessment
2.2.2. Therapeutic Techniques of Craniosacral Therapy
2.2.3. Statistical Analysis
3. Results
3.1. Craniosacral Therapy Group
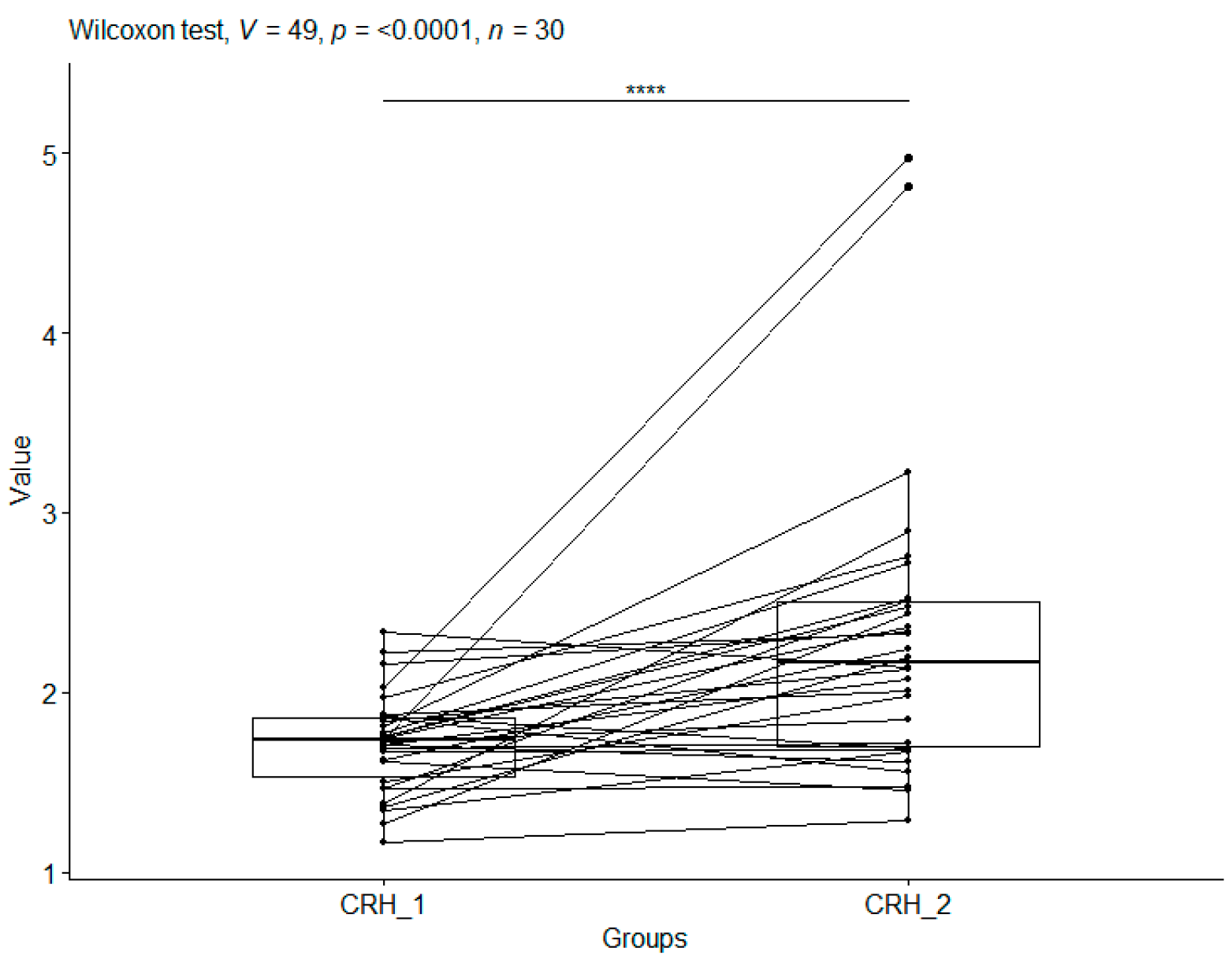
3.1.1. Corticotropin-Releasing Hormone
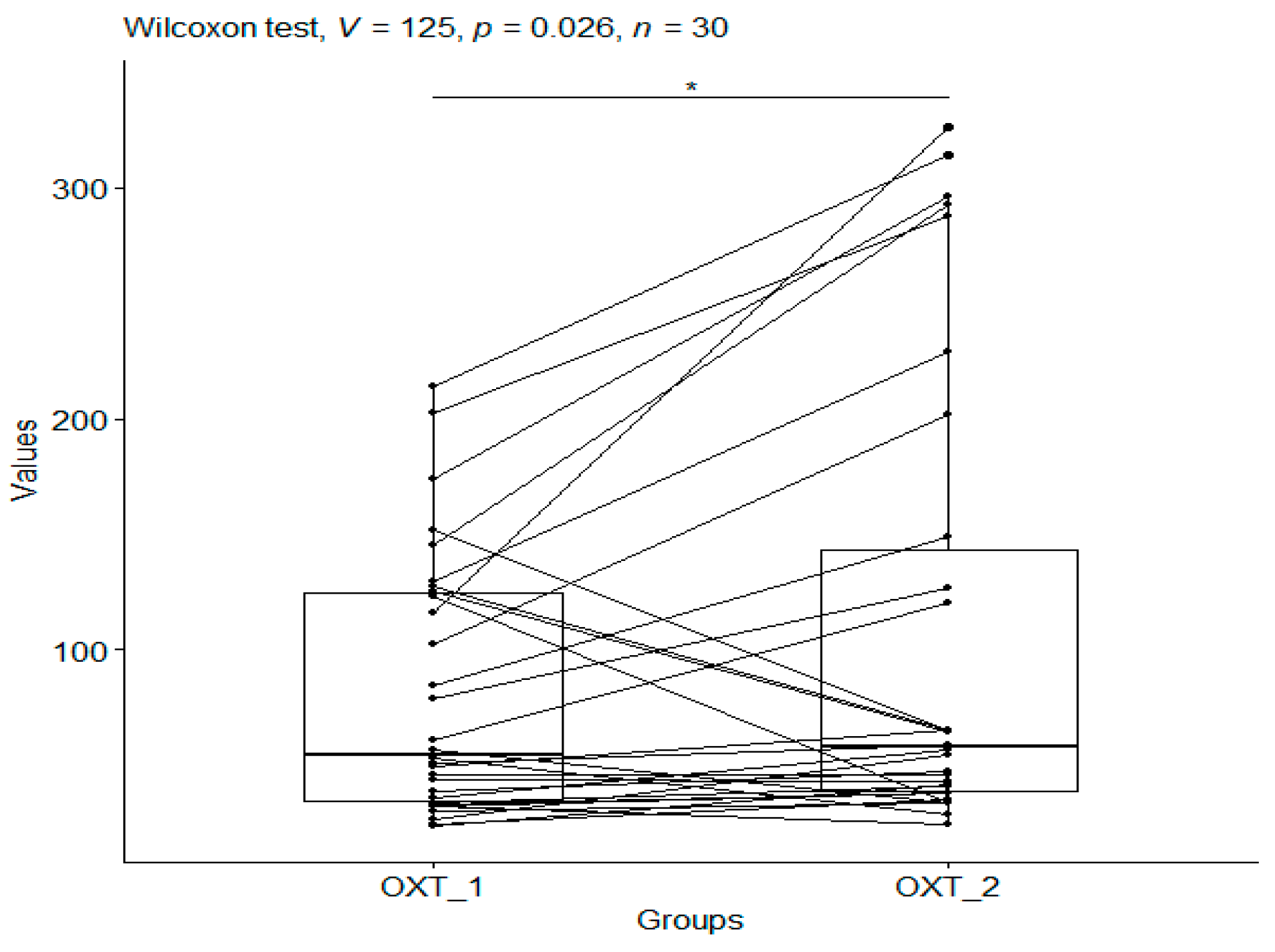
3.1.2. Oxytocin
3.2. Spearman’s Correlations
3.3. Control Group (Head Holding)
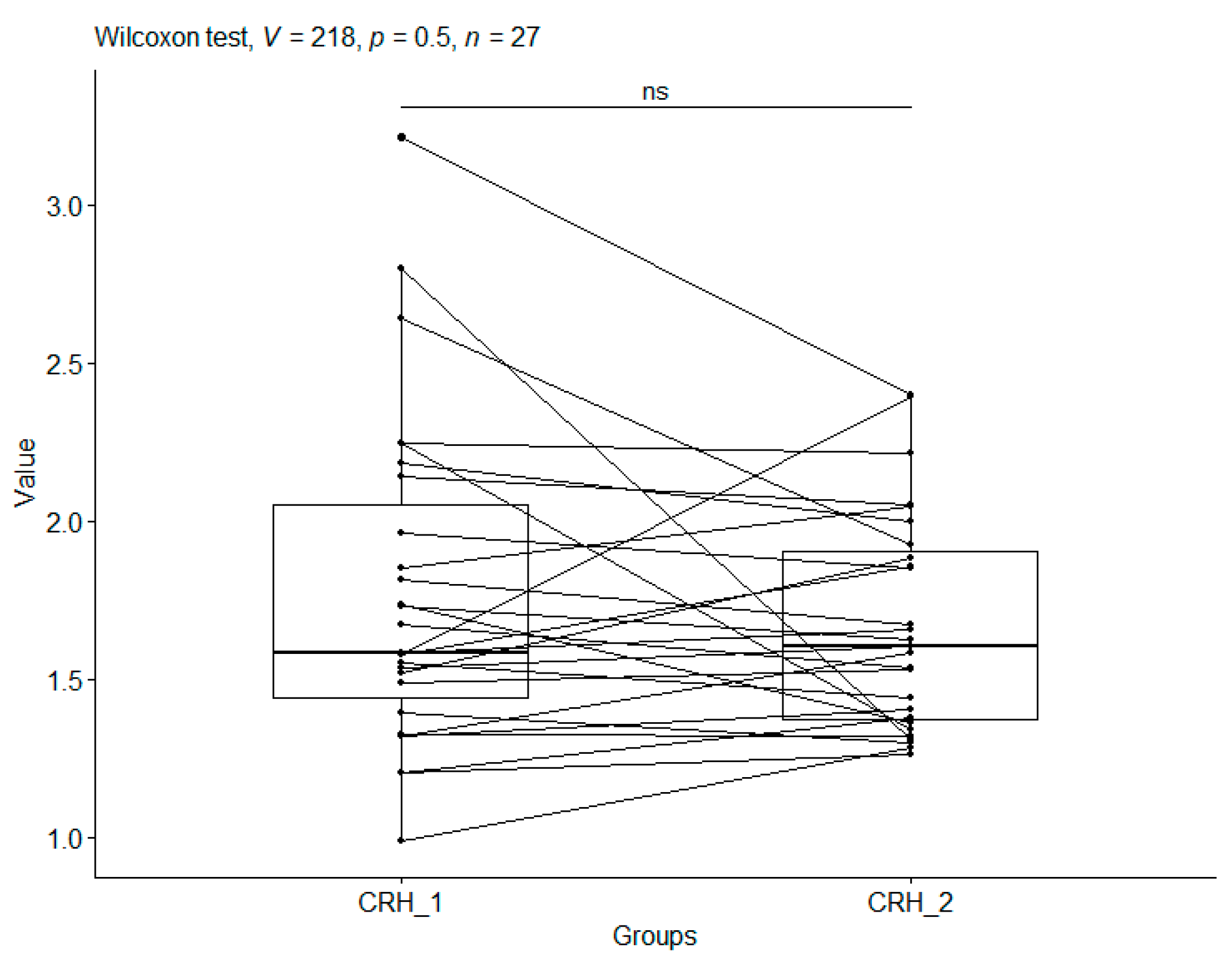
3.4. Corticotropin-Releasing Hormone
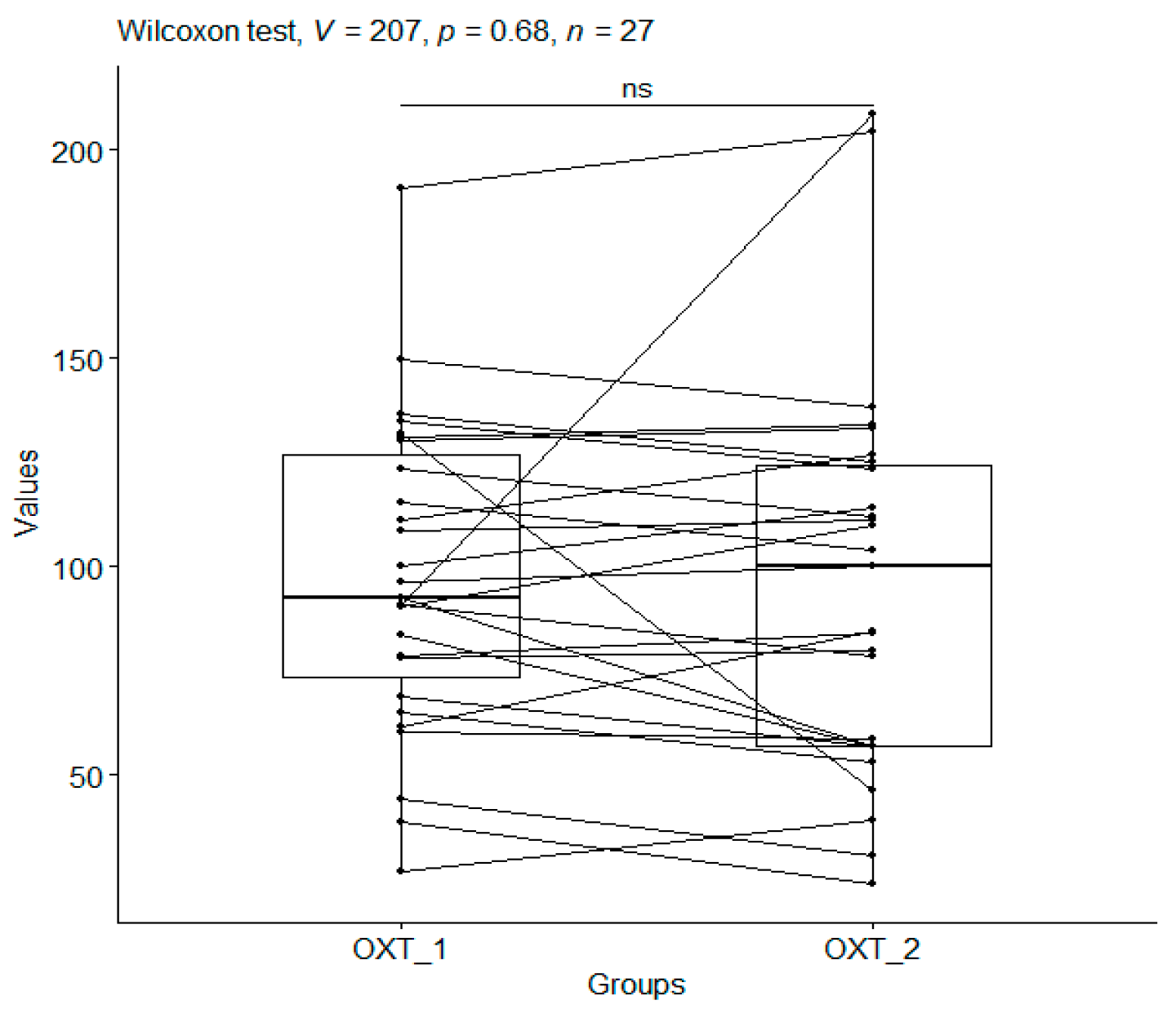
3.5. Oxytocin
3.6. Spearman’s Correlation
Treatment Group vs. Control Group
4. Discussion
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CS | Craniosacral therapy group |
| CO | Control group (head holding) |
| CRH | Corticotropin-releasing hormone |
| OXT | Oxytocin |
| 1 | First measurement before the start of this study in both groups |
| 2 | Second measurement after this study in both groups |
References
- Lebeaut, A.; Tran, J.K.; Vujanovic, A.A. Posttraumatic stress, alcohol use severity, and alcohol use motives among firefighters: The role of anxiety sensitivity. Addict. Behav. 2020, 106, 106353. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Horváth, K.; Juhász, B.; Kuti, D.; Ferenczi, S.; Kovács, K.J. Recruitment of Corticotropin-Releasing Hormone (CRH) Neurons in Categorically Distinct Stress Reactions in the Mouse Brain. Int. J. Mol. Sci. 2023, 24, 11736. [Google Scholar] [CrossRef] [PubMed]
- Cilz, N.I.; Cymerblit-Sabba, A.; Young, W.S. Oxytocin and vasopressin in the rodent hippocampus. Genes Brain Behav. 2019, 18, e12535. [Google Scholar] [CrossRef]
- Jiang, J.; Yang, M.; Tian, M.; Chen, Z.; Xiao, L.; Gong, Y. Intertwined associations between oxytocin, immune system and major depressive disorder. Biomed. Pharmacother. 2023, 163, 114852. [Google Scholar] [CrossRef]
- Goetz, L.; Jarvers, I.; Schleicher, D.; Mikan, K.; Brunner, R.; Kandsperger, S. The role of the endogenous oxytocin system under psychosocial stress conditions in adolescents sufering from anxiety disorder: Study protocol for a parallel group con-trolled trial. BMC Psychol. 2021, 9, 61. [Google Scholar] [CrossRef]
- Neumann, I.D.; Slattery, D.A. Oxytocin in general anxiety and social fear: A translational approach. Biol. Psychiatry 2016, 79, 213–221. [Google Scholar] [CrossRef]
- Gutkowska, J.; Jankowski, M.; Antunes-Rodrigues, J. The role of oxytocin in cardiovascular regulation. Braz. J. Med. Biol. Res. 2014, 47, 206–214. [Google Scholar] [CrossRef]
- Denkova, E.; Zanesco, A.P.; Rogers, S.L.; Jha, A.P. Is resilience trainable? An initial study comparing mindfulness and relaxa-tion training in firefighters. Psychiatry Res. 2020, 285, 112794. [Google Scholar] [CrossRef]
- Sawhney, G.; Jennings, K.S.; Britt, T.W.; Sliter, M.T. Occupational stress and mental health symptoms: Examining the moderating effect of work recovery strategies in firefighters. J. Occup. Health Psychol. 2018, 23, 443–456. [Google Scholar] [CrossRef]
- Cuenca-Lozano, M.F.; Ramírez-García, C.O. Occupational Hazards in Firefighting: Systematic Literature Review. Saf. Health Work 2023, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pineles, S.L.; Rasmusson, A.M.; Yehuda, R.; Lasko, N.B.; Macklin, M.L.; Pitman, R.K.; Orr, S.P. Predicting emotional responses to potentially traumatic events from pre-exposure waking cortisol levels: A longitudinal study of police and firefighters. Anxiety Stress Coping 2013, 26, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Ge, Y.; Tang, B.; Liu, Y.; Kang, P.; Wang, M.; Zhang, L. A meta-analysis of risk factors for combat-related PTSD among military personnel and veterans. PLoS ONE 2015, 10, e0120270. [Google Scholar] [CrossRef] [PubMed]
- Makara-Studzińska, M.; Golonka, K.; Izydorczyk, B. Self-Efficacy as a Moderator between Stress and Professional Burnout in Firefighters. Int. J. Environ. Res. Public Health 2019, 16, 183. [Google Scholar] [CrossRef]
- Vaulerin, J.; Colson, S.S.; Emile, M.; Scoffier-Mériaux, S.; d’Arripe-Longueville, F. The Big Five Personality Traits and French Firefighter Burnout: The Mediating Role of Achievement Goals. J. Occup. Environ. Med. 2016, 58, e128–e132. [Google Scholar] [CrossRef]
- Tao, Y.; Ma, Z.; Hou, W.; Zhu, Y.; Zhang, L.; Li, C.; Shi, C. Neuroticism Trait and Mental Health Among Chinese Firefighters: The Moderating Role of Perceived Organizational Support and the Mediating Role of Burnout—A Path Analysis. Front. Public Health 2022, 10, 870772. [Google Scholar] [CrossRef]
- Wójcik, M.; Placek, K.; Bordoni, B. Application of craniosacral therapy in practice. Fizjoterapia Pol. 2023, 23, 136–144. [Google Scholar] [CrossRef]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 are Essential Components of Distinct Mechanically Activated Cation Channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2019, 11, 487. [Google Scholar] [CrossRef]
- Schueler, M.; Messlinger, K.; Dux, M.; Neuhuber, W.L.; De, R. Extracranial Projections of Meningeal Afferents and Their Impact on Meningeal Nociception and Headache. Pain 2013, 154, 1622–1631. [Google Scholar] [CrossRef]
- Terrier, L.M.; Hadjikhani, N.; Destrieux, C. The Trigeminal Pathways. J. Neurol. 2022, 269, 3443–3460. [Google Scholar] [CrossRef] [PubMed]
- Panneton, W.M.; Gan, Q. The Mammalian Diving Response: Inroads to Its Neural Control. Front. Neurosci. 2020, 14, 524. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, M.; Siatkowski, I.; Żekanowska, E. A proposal for the use of craniosacral therapy in firefighter cadets to decrease cortisol levels and improve postural stability. J. Men’s Health 2022, 18, 140. [Google Scholar] [CrossRef]
- Wójcik, M.; Bordoni, B.; Siatkowski, I.; Żekanowska, E. The Effect of Craniosacral Therapy on Blood Levels of Stress Hormones in Male Firefighter Cadets: A Randomized Clinical Trial. Behav. Sci. 2023, 13, 914. [Google Scholar] [CrossRef]
- Wójcik, M.; Siatkowski, I. The efect of cranial techniques on the heart rate variability response to psychological stress test in frefghter cadets. Sci. Rep. 2023, 13, 7780. [Google Scholar] [CrossRef]
- Liem, T. Praktyka Osteopatii Czaszkowo-Krzyżowej. Wrocław; MedPharm: Guildford, UK, 2022; pp. 21–662. [Google Scholar]
- Development Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.r-project.org (accessed on 20 May 2023).
- Gołyszny, M. An “old” and “new” neuropeptides as modulators of the stress axis (hypothalamus–pituitary–adrenal). Varia Medica 2018, 2, 5. [Google Scholar]
- Wei, F.; Xian, D.; He, Y.; Yan, Z.; Deng, X.; Chen, Y.; Zhao, L.; Zhang, Y.; Li, W.; Ma, B.; et al. Effects of maternal deprivation and environmental enrichment on anxiety-like and depression-like behaviors correlate with oxytocin system and CRH level in the medial-lateral habenula. Peptides 2022, 158, 170882. [Google Scholar] [CrossRef]
- Hurlemann, R.; Marsh, N. Deciphering the modulatory role of oxytocin in human altruism. Rev. Neurosci. 2017, 28, 335–342. [Google Scholar] [CrossRef]
- Guo, L.; Qi, Y.J.; Tan, H.; Dai, D.; Balesar, R.; Sluiter, A.; van Heerikhuize, J.; Hu, S.H.; Swaab, D.F.; Bao, A.M. Different oxytocin and corticotropin-releasing hormone system changes in bipolar disorder and major depressive disorder patients. EBioMedicine 2022, 84, 104266. [Google Scholar] [CrossRef]
- Zhang, Y.; Cong, D.; Liu, P.; Zhi, X.Y.; Shi, C.; Zhao, J.; Zhang, H. Study on the mechanism of regulating the hypothalamic cortical hormone releasing hormone/corticotropin releasing hormone type I receptor pathway by vibro-annular abdominal massage under the brain-intestine interaction in the treatment of insomnia. Medicine 2021, 100, e25854. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, C.; Wang, Y.; Guo, C.; Lu, P.; Mou, F.; Shao, S. Electroacupuncture alleviates anxiety and modulates amygdala CRH/CRHR1 signaling in single prolonged stress mice. Acupunct. Med. 2022, 40, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, B.B.; Nair, B.B.; Iremonger, K.J. Regulation of hypothalamic corticotropin-releasing hormone neurone excitability by oxytocin. J. Neuroendocrinol. 2017, 29, e12532. [Google Scholar] [CrossRef] [PubMed]
- Pati, D.; Krause, E.G.; Frazier, C.J. Intrahypothalamic effects of oxytocin on PVN CRH neurons in response to acute stress. Curr. Opin. Endocr. Metab. Res. 2022, 26, 100382. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.; Hopf, D.; Aguilar-Raab, C.; Scheele, D.; Neubauer, A.B.; Sailer, U.; Hurlemann, R.; Eckstein, M.; Ditzen, B. Affectionate touch and diurnal oxytocin levels: An ecological momentary assessment study. Elife 2023, 12, e81241. [Google Scholar] [CrossRef]
- Su, T.; Pei, L. Acupuncture and oxytocinergic system: The promising treatment for autism. Transl. Neurosci. 2021, 12, 96–102. [Google Scholar] [CrossRef]
- Mehta, U.M.; Gangadhar, B.N. Yoga: Balancing the excitation-inhibition equilibrium in psychiatric disorders. Prog. Brain Res. 2019, 244, 387–413. [Google Scholar] [CrossRef]
- Quintana, D.S.; Rokicki, J.; van der Meer, D.; Alnæs, D.; Kaufmann, T.; Córdova-Palomera, A.; Dieset, I.; Andreassen, O.A.; Westlye, L.T. Oxytocin pathway gene networks in the human brain. Nat. Commun. 2019, 10, 668. [Google Scholar] [CrossRef]
- Danoff, J.S.; Whelan, E.A.; Connelly, J.J. Is oxytocin receptor signaling really dispensable for social attachment? Compr. Psychoneuroendocrinol. 2023, 14, 100178. [Google Scholar] [CrossRef]
- Chen, R.; Wu, X.; Jiang, L.; Zhang, Y. Single-cell RNA-seq reveals hypothalamic cell diversity. Cell Rep. 2017, 18, 3227–3241. [Google Scholar] [CrossRef]
- Gupta, D.; Chuang, J.C.; Mani, B.K.; Shankar, K.; Rodriguez, J.A.; Osborne-Lawrence, S.; Metzger, N.P.; Zigman, J.M. β1-adrenergic receptors mediate plasma acyl-ghrelin elevation and depressive-like behavior induced by chronic psychosocial stress. Neuropsychopharmacology 2019, 44, 1319–1327. [Google Scholar] [CrossRef]
- Svingen, S. PTSD and crime propensity: Stress systems, brain structures, and the nature of the relationship. Heliyon 2023, 9, e18381. [Google Scholar] [CrossRef] [PubMed]
- Kenkel, W.M.; Perkeybile, A.M.; Yee, J.R.; Pournajafi-Nazarloo, H.; Lillard, T.S.; Ferguson, E.F.; Wroblewski, K.L.; Ferris, C.F.; Carter, C.S.; Connelly, J.J. Behavioral and epigenetic consequences of oxytocin treatment at birth. Sci. Adv. 2019, 5, eaav2244. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.S.; Kenkel, W.M.; MacLean, E.L.; Wilson, S.R.; Perkeybile, A.M.; Yee, J.R.; Ferris, C.F.; Nazarloo, H.P.; Porges, S.W.; Davis, J.M.; et al. Is Oxytocin “Nature’s Medicine”? Pharmacol. Rev. 2020, 72, 829–861. [Google Scholar] [CrossRef] [PubMed]
| Comparison | r for CS | p-Value for CS | r for CO | p-Value for CO |
|---|---|---|---|---|
| CRH_1 vs. OXT_1 | 0.26 | 0.124 | 0.20 | 0.300 |
| CRH_2 vs. OXT_2 | −0.02 | 0.920 | 0.14 | 0.469 |
| CRH_1 vs. CRH_2 | 0.25 | 0.173 | 0.51 | 0.006 |
| OXT_1 vs. OXT_2 | 0.77 | <0.00001 | 0.73 | <0.00001 |
| Group | Mean | std | n | Min | Max | Q25 | Q50 | Q75 |
|---|---|---|---|---|---|---|---|---|
| CO_1 | 1.77 | 0.52 | 27 | 0.98 | 3.21 | 1.43 | 1.58 | 2.04 |
| CO_2 | 1.67 | 0.34 | 27 | 1.26 | 2.39 | 1.36 | 1.60 | 1.90 |
| CS_1 | 1.71 | 0.27 | 30 | 1.16 | 2.33 | 1.53 | 1.73 | 1.85 |
| CS_2 | 2.30 | 0.84 | 30 | 1.28 | 4.97 | 1.70 | 2.16 | 2.50 |
| Group | Mean | std | n | Min | Max | Q25 | Q50 | Q75 |
|---|---|---|---|---|---|---|---|---|
| CO_1 | 97.34 | 37.17 | 27 | 26.76 | 190.66 | 73.34 | 92.55 | 126.67 |
| CO_2 | 96.00 | 46.61 | 27 | 23.79 | 208.62 | 56.88 | 99.96 | 124.03 |
| CS_1 | 81.60 | 56.19 | 30 | 23.79 | 214.08 | 34.41 | 54.71 | 124.49 |
| CS_2 | 108.65 | 101.42 | 30 | 24.71 | 326.05 | 38.79 | 57.77 | 143.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik, M.; Siatkowski, I. Application of Craniosacral Therapy Versus Blood Levels of Corticoliberin and Oxytocin in Male Firefighters Exposed to Occupational Stress—A Randomised Control Trial. Metabolites 2025, 15, 374. https://doi.org/10.3390/metabo15060374
Wójcik M, Siatkowski I. Application of Craniosacral Therapy Versus Blood Levels of Corticoliberin and Oxytocin in Male Firefighters Exposed to Occupational Stress—A Randomised Control Trial. Metabolites. 2025; 15(6):374. https://doi.org/10.3390/metabo15060374
Chicago/Turabian StyleWójcik, Małgorzata, and Idzi Siatkowski. 2025. "Application of Craniosacral Therapy Versus Blood Levels of Corticoliberin and Oxytocin in Male Firefighters Exposed to Occupational Stress—A Randomised Control Trial" Metabolites 15, no. 6: 374. https://doi.org/10.3390/metabo15060374
APA StyleWójcik, M., & Siatkowski, I. (2025). Application of Craniosacral Therapy Versus Blood Levels of Corticoliberin and Oxytocin in Male Firefighters Exposed to Occupational Stress—A Randomised Control Trial. Metabolites, 15(6), 374. https://doi.org/10.3390/metabo15060374







