Hyperuricemia—Especially “Metabolic Hyperuricemia”—Is Independently Associated with a Higher Risk of Steatotic Liver Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Methods
2.3. Ethics
2.4. Patient and Public Involvement
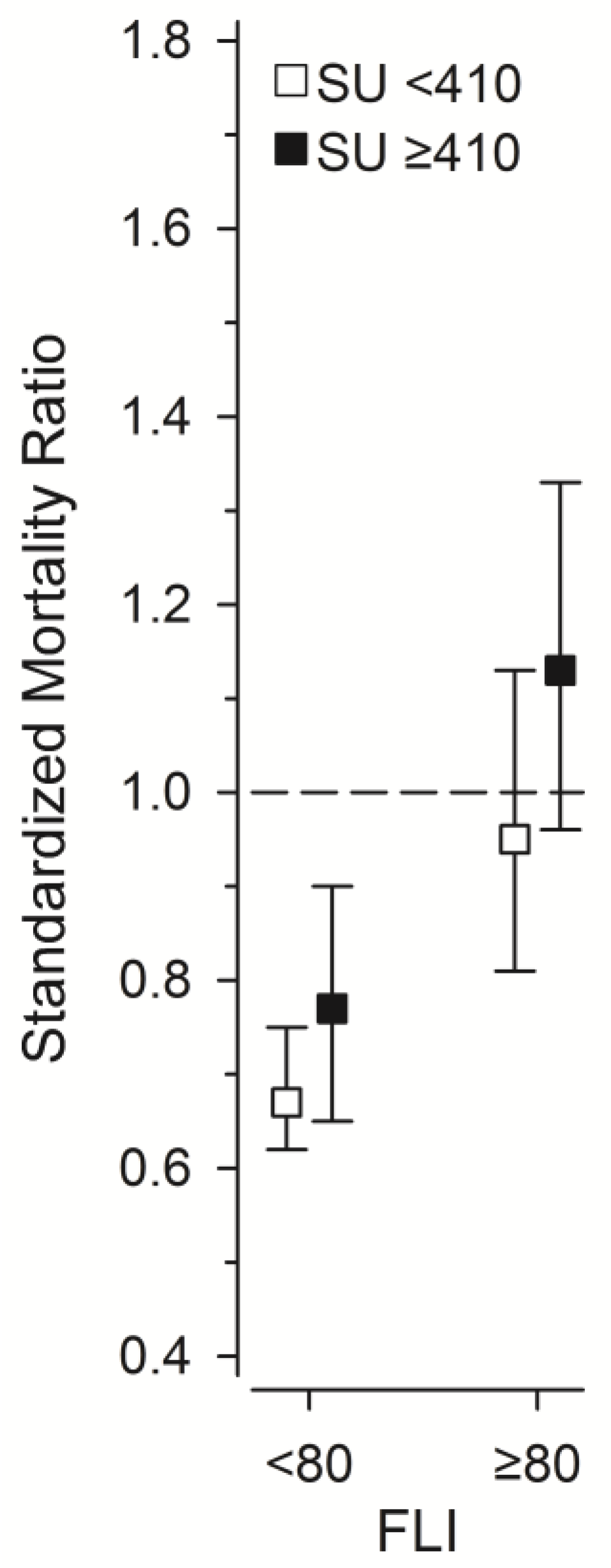
3. Results
- 4790 in the SU ≥ 410 μmol/L and FLI < 80 group,
- 4244 in the SU ≥ 410 μmol/L and FLI ≥ 80 group,
- 24,402 in the SU < 410 μmol/L and FLI < 80 group, and
- 4877 in the SU < 410 μmol/L and FLI ≥ 80 group.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SU | Serum urate |
| FLI | Fatty liver index |
| eGFR | Estimated glomerular filtration rate |
| NAFLD | Nonalcoholic fatty liver disease |
| MASLD | Metabolic dysfunction associated steatotic liver disease |
| XOR | Xanthine oxidoreductase |
| URAT1 | Urate transporter 1 |
| GLUT9 | Glucose transporter 9 |
| OAT4 | Organic anion transporter 4 |
| OAT1 | Organic anion transporter 1 |
| ABCG2 | ATP binding cassette subfamily G member 2 |
| NPT1 | Sodium-dependent phosphate transport protein 1 |
| NPT4 | Sodium-dependent phosphate transport protein 4 |
| ULT | Urate lowering therapy |
| URRAH | Uric Acid Right for Heart Health |
| ACR | American College of Rheumatology |
| GOAL | Good Aging in Lahti region |
| LDL-C | Low-density lipoprotein cholesterol |
| HDL-C | High-density lipoprotein cholesterol |
| CRP | C-reactive protein |
| BMI | Body mass index |
| GGT | Gamma-glutamyl transferase |
| CKD-EPI | Chronic Kidney Disease Epidemiology Collaboration |
| ICD-10 | International Statistical Classification of Diseases and Related Health Problems, 10th Revision |
| SD | Standard deviation |
| HR | Hazard ratio |
| CI | Confidence interval |
| SMR | Standardized mortality ratio |
| ROS | Reactive oxygen species |
| HMGB1 | High mobility group box 1 |
| RAGE | Receptor for advanced glycation endproducts |
| CAP | Controlled attenuation parameter |
References
- Timsans, J.; Palomäki, A.; Kauppi, M. Gout and Hyperuricemia: A Narrative Review of Their Comorbidities and Clinical Implications. J. Clin. Med. 2024, 13, 7616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Z. Why Does Hyperuricemia Not Necessarily Induce Gout? Biomolecules 2021, 11, 280. [Google Scholar] [CrossRef] [PubMed]
- Yip, K.; Cohen, R.E.; Pillinger, M.H. Asymptomatic hyperuricemia: Is it really asymptomatic? Curr. Opin. Rheumatol. 2020, 32, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Timsans, J.; Kauppi, J.E.; Kerola, A.M.; Lehto, T.M.; Kautiainen, H.; Kauppi, M.J. Hyperuricaemia: Prevalence and association with mortality in an elderly Finnish population. BMJ Open 2023, 13, e072110. [Google Scholar] [CrossRef]
- Juraschek, S.P.; Tunstall-Pedoe, H.; Woodward, M. Serum uric acid and the risk of mortality during 23 years follow-up in the Scottish Heart Health Extended Cohort Study. Atherosclerosis 2014, 233, 623–629. [Google Scholar] [CrossRef]
- Kikuchi, A.; Kawamoto, R.; Ninomiya, D.; Kumagi, T. Hyperuricemia is associated with all-cause mortality among males and females: Findings from a study on Japanese community-dwelling individuals. Metabol. Open 2022, 14, 100186. [Google Scholar] [CrossRef]
- Hu, L.; Hu, G.; Xu, B.P.; Zhu, L.; Zhou, W.; Wang, T.; Bao, H.; Cheng, X. U-shaped association of serum uric acid with all-cause and cause-specific mortality in US adults: A cohort study. J. Clin. Endocrinol. Metab. 2020, 105, dgz068. [Google Scholar] [CrossRef]
- Cho, S.K.; Chang, Y.; Kim, I.; Ryu, S. U-shaped association between serum uric acid level and risk of mortality: A cohort study. Arthritis Rheumatol. 2018, 70, 1122–1132. [Google Scholar] [CrossRef]
- Tseng, W.C.; Chen, Y.T.; Ou, S.M.; Shih, C.J.; Tarng, D.C.; Taiwan Geriatric Kidney Disease (TGKD) Research Group. U-shaped association between serum uric acid levels with cardiovascular and all-cause mortality in the elderly: The role of malnourishment. J. Am. Heart Assoc. 2018, 7, e007523. [Google Scholar] [CrossRef]
- Kim, K.; Go, S.; Son, H.E.; Ryu, J.Y.; Lee, H.; Heo, N.J.; Chin, H.J.; Park, J.H. Association between serum uric acid level and ESRD or death in a Korean population. J. Korean Med. Sci. 2020, 35, e254. [Google Scholar] [CrossRef]
- Cang, Y.; Xu, S.; Zhang, J.; Ju, J.; Chen, Z.; Wang, K.; Li, J.; Xu, Y. Serum uric acid revealed a U-shaped relationship with all-cause mortality and cardiovascular mortality in high atherosclerosis risk patients: The ASSURE study. Front. Cardiovasc. Med. 2021, 8, 641513. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, Z.; Wang, S.; Chang, R.; Liu, Y.; Wang, R.; Chen, H.; Liu, S.; Qian, C.; Cai, Y.; et al. Gender-specific and U-shaped relationship between serum uric acid and all-cause mortality among Chinese older adults: A national population-based longitudinal study. Int. J. Public Health 2023, 68, 1605934. [Google Scholar] [CrossRef] [PubMed]
- Maloberti, A.; Mengozzi, A.; Russo, E.; Cicero, A.F.G.; Angeli, F.; Agabiti Rosei, E.; Barbagallo, C.M.; Bernardino, B.; Bombelli, M.; Cappelli, F.; et al. The results of the URRAH (Uric Acid Right for Heart Health) project: A focus on hyperuricemia in relation to cardiovascular and kidney disease and its role in metabolic dysregulation. High Blood Press. Cardiovasc. Prev. 2023, 30, 411–425. [Google Scholar] [CrossRef]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef]
- Perez-Ruiz, F.; Calabozo, M.; Erauskin, G.G.; Ruibal, A.; Herrero-Beites, A.M. Renal underexcretion of uric acid is present in patients with apparent high urinary uric acid output. Arthritis Rheum. 2002, 47, 610–613. [Google Scholar] [CrossRef]
- Chung, S.; Kim, G.H. Urate transporters in the kidney: What clinicians need to know. Electrolyte Blood Press. 2021, 19, 1–9. [Google Scholar] [CrossRef]
- Russo, E.; Viazzi, F.; Pontremoli, R.; Barbagallo, C.M.; Bombelli, M.; Casiglia, E.; Cicero, A.F.; Cirillo, M.; Cirillo, P.; Desideri, G.; et al. Serum uric acid and kidney disease measures independently predict cardiovascular and total mortality: The Uric Acid Right for Heart Health (URRAH) Project. Front. Cardiovasc. Med. 2021, 8, 713652. [Google Scholar] [CrossRef]
- Timsans, J.; Kauppi, J.E.; Kerola, A.M.; Lehto, T.M.; Kautiainen, H.J.; Kauppi, M.J. Hyperuricaemia-associated all-cause mortality risk effect is increased by non-impaired kidney function—Is renal hyperuricaemia less dangerous? Eur. J. Intern. Med. 2024, 121, 56–62. [Google Scholar] [CrossRef]
- Timsans, J.; Kerola, A.M.; Rantalaiho, V.M.; Hakkarainen, K.N.; Kautiainen, H.J.; Kauppi, M.J. “Metabolic” type of hyperuricemia increases mortality mainly by leading to premature death from cardiovascular disease. Mayo Clin. Proc. 2024, 99, 1835–1837. [Google Scholar] [CrossRef]
- Casiglia, E.; Tikhonoff, V.; Virdis, A.; Grassi, G.; Angeli, F.; Barbagallo, C.M.; Bombelli, M.; Cicero, A.F.G.; Cirillo, M.; Cirillo, P.; et al. Serum uric acid / serum creatinine ratio as a predictor of cardiovascular events. Detection of prognostic cardiovascular cut-off values. J. Hypertens. 2023, 41, 180–186. [Google Scholar] [CrossRef]
- Simon, T.G.; Roelstraete, B.; Khalili, H.; Hagström, H.; Ludvigsson, J.F. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: Results from a nationwide cohort. Gut 2021, 70, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Glass, L.M.; Hunt, C.M.; Fuchs, M.; Su, G.L. Comorbidities and nonalcoholic fatty liver disease: The chicken, the egg, or both? Fed. Pract. 2019, 36, 64–71. [Google Scholar] [PubMed]
- Le, M.H.; Yeo, Y.H.; Zou, B.; Barnet, S.; Henry, L.; Cheung, R.; Nguyen, M.H. Forecasted 2040 global prevalence of nonalcoholic fatty liver disease using hierarchical Bayesian approach. Clin. Mol. Hepatol. 2022, 28, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, C.; Yu, C.; Xu, L.; Miao, M. Association of serum uric acid level with non-alcoholic fatty liver disease: A cross-sectional study. J. Hepatol. 2009, 50, 1029–1034. [Google Scholar] [CrossRef]
- Yu, C.; Zhou, X.; Wang, T.; Zhu, L.; Zhou, W.; Bao, H.; Cheng, X. Positive correlation between fatty liver index and hyperuricemia in hypertensive Chinese adults: A H-type hypertension registry study. Front. Endocrinol. 2023, 14, 1183666. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, T.; Manji, L.; Liu, Y.; Chang, Q.; Zhao, Y.; Ding, Y.; Xia, Y. Association Between Serum Uric Acid and Non-Alcoholic Fatty Liver Disease: An Updated Systematic Review and Meta-Analysis. Clin. Epidemiol. 2023, 15, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Timsans, J.; Kauppi, J.; Kautiainen, H.; Kauppi, M. Hyperuricemia—Especially “Metabolic Hyperuricemia”—Is Independently Associated with Higher Risk of Fatty Liver. Arthritis Rheumatol. 2023, 75 (Suppl. S9). Available online: https://acrabstracts.org/abstract/hyperuricemia-especially-metabolic-hyperuricemia-is-independently-associated-with-higher-risk-of-fatty-liver/ (accessed on 9 April 2025).
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Reinshagen, M.; Kabisch, S.; Pfeiffer, A.F.H.; Spranger, J. Liver Fat Scores for Noninvasive Diagnosis and Monitoring of Nonalcoholic Fatty Liver Disease in Epidemiological and Clinical Studies. J. Clin. Transl. Hepatol. 2023, 11, 1212–1227. [Google Scholar] [CrossRef]
- Koehler, E.M.; Schouten, J.N.; Hansen, B.E.; Hofman, A.; Stricker, B.H.; Janssen, H.L. External validation of the fatty liver index for identifying nonalcoholic fatty liver disease in a population-based study. Clin. Gastroenterol. Hepatol. 2013, 11, 1201–1204. [Google Scholar] [CrossRef]
- Cuthbertson, D.J.; Weickert, M.O.; Lythgoe, D.; Sprung, V.S.; Dobson, R.; Shoajee-Moradie, F.; Umpleby, M.; Pfeiffer, A.F.; Thomas, E.L.; Bell, J.D.; et al. External validation of the fatty liver index and lipid accumulation product indices, using 1H-magnetic resonance spectroscopy, to identify hepatic steatosis in healthy controls and obese, insulin-resistant individuals. Eur. J. Endocrinol. 2014, 171, 561–569. [Google Scholar] [CrossRef]
- Huang, X.; Xu, M.; Chen, Y.; Peng, K.; Huang, Y.; Wang, P.; Ding, L.; Lin, L.; Xu, Y.; Chen, Y.; et al. Validation of the Fatty Liver Index for Nonalcoholic Fatty Liver Disease in Middle-Aged and Elderly Chinese. Medicine 2015, 94, e1682. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.L.; Wu, W.C.; Fang, K.C.; Wang, Y.C.; Huo, T.I.; Huang, Y.H.; Yang, H.I.; Su, C.W.; Lin, H.C.; Lee, F.Y.; et al. External validation of fatty liver index for identifying ultrasonographic fatty liver in a large-scale cross-sectional study in Taiwan. PLoS ONE 2015, 10, e0120443. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Gitto, S.; Fogacci, F.; Rosticci, M.; Giovannini, M.; D’Addato, S.; Andreone, P.; Borghi, C. Brisighella Heart Study Group Medical and Surgical Sciences Dept., University of Bologna. Fatty liver index is associated to pulse wave velocity in healthy subjects: Data from the Brisighella Heart Study. Eur. J. Intern. Med. 2018, 53, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Fan, J.; Zhang, X.; Lili, Y.; Lin, D.; Huang, C.; Li, F.; Sun, K. Fatty Liver Index and Its Association with 10-Year Atherosclerotic Cardiovascular Disease Risk: Insights from a Population-Based Cross-Sectional Study in China. Metabolites 2023, 13, 850. [Google Scholar] [CrossRef]
- Chung, T.H.; Kim, J.K.; Kim, J.H.; Lee, Y.J. Fatty Liver Index as a Simple and Useful Predictor for 10-Year Cardiovascular Disease Risks Determined by Framingham Risk Score in the General Korean Population. J. Gastrointestin. Liver Dis. 2021, 30, 221–226. [Google Scholar] [CrossRef]
- Werner, K.; Pihlsgård, M.; Elmståhl, S.; Legrand, H.; Nyman, U.; Christensson, A. Combining Cystatin C and Creatinine Yields a Reliable Glomerular Filtration Rate Estimation in Older Adults in Contrast to β-Trace Protein and β2-Microglobulin. Nephron 2017, 137, 29–37. [Google Scholar] [CrossRef]
- Kumar, A.U.A.; Browne, L.D.; Li, X.; Adeeb, F.; Perez-Ruiz, F.; Fraser, A.D.; Stack, A.G. Temporal trends in hyperuricaemia in the Irish health system from 2006–2014: A cohort study. PLoS ONE 2018, 13, e0198197. [Google Scholar] [CrossRef]
- Winder, M.; Owczarek, A.J.; Mossakowska, M.; Broczek, K.; Grodzicki, T.; Wierucki, Ł.; Chudek, J. Prevalence of Hyperuricemia and the Use of Allopurinol in Older Poles—Results from a Population-Based PolSenior Study. Int. J. Environ. Res. Public Health 2021, 18, 387. [Google Scholar] [CrossRef]
- Burke, B.T.; Köttgen, A.; Law, A.; Windham, B.G.; Segev, D.; Baer, A.N.; Coresh, J.; McAdams-DeMarco, M.A. Physical Function, Hyperuricemia, and Gout in Older Adults. Arthritis Care Res. 2015, 67, 1730–1738. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Bulló, M.; Babio, N.; Martínez-González, M.A.; Estruch, R.; Covas, M.I.; Wärnberg, J.; Arós, F.; Lapetra, J.; Serra-Majem, L.; et al. Mediterranean Diet and Risk of Hyperuricemia in Elderly Participants at High Cardiovascular Risk. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1263–1270. [Google Scholar] [CrossRef]
- Alqahtani, S.A.; Schattenberg, J.M. NAFLD in the Elderly. Clin. Interv. Aging 2021, 16, 1633–1649. [Google Scholar] [CrossRef] [PubMed]
- Pitisuttithum, P.; Treeprasertsuk, S. Nonalcoholic Fatty Liver Disease (NAFLD) among Older Adults. Port. Hypertens. Cirrhos. 2022, 1, 184–191. [Google Scholar] [CrossRef]
- Lonardo, A.; Loria, P.; Leonardi, F.; Borsatti, A.; Neri, P.; Pulvirenti, M.; Verrone, A.M.; Bagni, A.; Bertolotti, M.; Ganazzi, D.; et al. Fasting insulin and uric acid levels but not indices of iron metabolism are independent predictors of non-alcoholic fatty liver disease: A case-control study. Dig. Liver Dis. 2002, 34, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, R.; Calabrese, F.; Palermo, F.; Milazzo, M.; Italia, A.; Sapienza, C.; Occhipinti, S.; Anzalone, M.G.; Castellino, P. The Relationship Between Serum Uric Acid and Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Retrospective Study. Dig. Liver Dis. 2014, 46 (Suppl. S1), S114. [Google Scholar] [CrossRef]
- Shih, M.H.; Lazo, M.; Liu, S.H.; Bonekamp, S.; Hernaez, R.; Clark, J.M. Association Between Serum Uric Acid and Nonalcoholic Fatty Liver Disease in the US Population. J. Formos. Med. Assoc. 2015, 114, 314–320. [Google Scholar] [CrossRef]
- Sirota, J.C.; McFann, K.; Targher, G.; Johnson, R.J.; Chonchol, M.; Jalal, D.I. Elevated Serum Uric Acid Levels Are Associated with Non-Alcoholic Fatty Liver Disease Independently of Metabolic Syndrome Features in the United States: Liver Ultrasound Data from the National Health and Nutrition Examination Survey. Metabolism 2013, 62, 392–399. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, Y.; Liu, P.; Liu, C.; Zhong, R.; Yu, X.; Xiao, L.; Du, M.; Yang, L.; Yuan, J.; et al. No Evidence for a Causal Link Between Serum Uric Acid and Non-Alcoholic Fatty Liver Disease from the Dongfeng-Tongji Cohort Study. Oxid. Med. Cell. Longev. 2022, 2022, 6687626. [Google Scholar] [CrossRef]
- Li, S.; Fu, Y.; Liu, Y.; Zhang, X.; Li, H.; Tian, L.; Zhuo, L.; Liu, M.; Cui, J. Serum Uric Acid Levels and Non-Alcoholic Fatty Liver Disease: A 2-Sample Bidirectional Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2022, 107, e3497–e3503. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M.; et al. Uric Acid Induces Hepatic Steatosis by Generation of Mitochondrial Oxidative Stress: Potential Role in Fructose-Dependent and -Independent Fatty Liver. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef]
- Xie, D.; Zhao, H.; Lu, J.; He, F.; Liu, W.; Yu, W.; Wang, Q.; Hisatome, I.; Yamamoto, T.; Koyama, H.; et al. High Uric Acid Induces Liver Fat Accumulation via ROS/JNK/AP-1 Signaling. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E1032–E1043. [Google Scholar] [CrossRef]
- Choi, J.; Joe, H.; Oh, J.E.; Cho, Y.J.; Shin, H.S.; Heo, N.H. The Correlation Between NAFLD and Serum Uric Acid to Serum Creatinine Ratio. PLoS ONE 2023, 18, e0288666. [Google Scholar]
- Sookoian, S.; Pirola, C.J. The Serum Uric Acid/Creatinine Ratio is Associated with Nonalcoholic Fatty Liver Disease in the General Population. J. Physiol. Biochem. 2022, 79, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Arroyave-Ospina, J.C.; Wu, Z.; Geng, Y.; Moshage, H. Role of Oxidative Stress in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Implications for Prevention and Therapy. Antioxidants 2021, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Nasiri-Ansari, N.; Androutsakos, T.; Flessa, C.M.; Kyrou, I.; Siasos, G.; Randeva, H.S.; Kassi, E.; Papavassiliou, A.G. Endothelial Cell Dysfunction and Nonalcoholic Fatty Liver Disease (NAFLD): A Concise Review. Cells 2022, 11, 2511. [Google Scholar] [CrossRef]
- Cai, W.; Duan, X.M.; Liu, Y.; Yu, J.; Tang, Y.L.; Liu, Z.L.; Jiang, S.; Zhang, C.P.; Liu, J.Y.; Xu, J.X. Uric Acid Induces Endothelial Dysfunction by Activating the HMGB1/RAGE Signaling Pathway. BioMed Res. Int. 2017, 2017, 4391920. [Google Scholar] [CrossRef]
- Yagi, C.; Kusunoki, Y.; Tsunoda, T.; Murase, T.; Nakamura, T.; Osugi, K.; Ohigashi, M.; Morimoto, A.; Miyoshi, A.; Kakutani-Hatayama, M.; et al. Xanthine oxidoreductase activity is correlated with hepatic steatosis. Sci. Rep. 2022, 12, 12282. [Google Scholar] [CrossRef]
- Sato, K.; Naganuma, A.; Nagashima, T.; Arai, Y.; Mikami, Y.; Nakajima, Y.; Kanayama, Y.; Murakami, T.; Uehara, S.; Uehara, D.; et al. A Newly Developed Method-Based Xanthine Oxidoreductase Activities in Various Human Liver Diseases. Biomedicines 2023, 11, 1445. [Google Scholar] [CrossRef]
- Kawachi, Y.; Fujishima, Y.; Nishizawa, H.; Nakamura, T.; Akari, S.; Murase, T.; Saito, T.; Miyazaki, Y.; Nagao, H.; Fukuda, S.; et al. Increased plasma XOR activity induced by NAFLD/NASH and its possible involvement in vascular neointimal proliferation. JCI Insight 2021, 6, e144762. [Google Scholar] [CrossRef]
- Sun, D.Q.; Wu, S.J.; Liu, W.Y.; Lu, Q.D.; Zhu, G.Q.; Shi, K.Q.; Braddock, M.; Song, D.; Zheng, M.H. Serum Uric Acid: A New Therapeutic Target for Nonalcoholic Fatty Liver Disease. Expert Opin. Ther. Targets 2016, 20, 375–387. [Google Scholar] [CrossRef]
- Nakatsu, Y.; Seno, Y.; Kushiyama, A.; Sakoda, H.; Fujishiro, M.; Katasako, A.; Mori, K.; Matsunaga, Y.; Fukushima, T.; Kanaoka, R.; et al. The Xanthine Oxidase Inhibitor Febuxostat Suppresses Development of Nonalcoholic Steatohepatitis in a Rodent Model. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G42–G51. [Google Scholar] [CrossRef]
- Kakimoto, M.; Fujii, M.; Sato, I.; Honma, K.; Nakayama, H.; Kirihara, S.; Fukuoka, T.; Ran, S.; Hirohata, S.; Kitamori, K.; et al. Antioxidant Action of Xanthine Oxidase Inhibitor Febuxostat Protects the Liver and Blood Vasculature in SHRSP5/Dmcr Rats. J. Appl. Biomed. 2023, 21, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.F.; Yu, C.H.; Xu, L.; Sa, X.Y.; Li, Y.M. Hypouricemic Therapy: A Novel Potential Therapeutic Option for Nonalcoholic Fatty Liver Disease. Hepatology 2010, 52, 1865–1866. [Google Scholar] [CrossRef] [PubMed]
- Atzori, S.; Pasha, Y.; Maurice, J.B.; Taylor-Robinson, S.D.; Campbell, L.; Lim, A.K.P. The Accuracy of Ultrasound Controlled Attenuation Parameter in Diagnosing Hepatic Fat Content. Hepat. Med. 2023, 15, 51–61. [Google Scholar] [CrossRef]
- Al-Shargi, A.; El Kholy, A.A.; Adel, A.; Hassany, M.; Shaheen, S.M. Allopurinol versus Febuxostat: A New Approach for the Management of Hepatic Steatosis in Metabolic Dysfunction-Associated Steatotic Liver Disease. Biomedicines 2023, 11, 3074. [Google Scholar] [CrossRef]
- Tykarski, A.; Filipiak, K.J.; Januszewicz, A.; Litwin, M.; Narkiewicz, K.; Prejbisz, A.; Ostalska-Nowicka, D.; Widecka, K.; Kostka-Jeziorny, K. Zasady Postępowania w Nadciśnieniu Tętniczym—2019 Rok. Nadciśnienie Tętnicze Prakt. 2019, 5, 1–86. [Google Scholar]
- Hisatome, I.; Ichida, K.; Mineo, I.; Ohtahara, A.; Ogino, K.; Kuwabara, M.; Ishizaka, N.; Uchida, S.; Kurajoh, M.; Kohagura, K.; et al. Japanese Society of Gout and Uric & Nucleic Acids 2019 guidelines for management of hyperuricemia and gout. Gout Uric Nucleic Acids 2020, 44, sp40. [Google Scholar]
- Lonardo, A.; Ballestri, S.; Bedogni, G.; Bellentani, S.; Tiribelli, C. The Fatty liver Index (FLI) 15 years later: A reappraisal. Metab. Target Organ Damage 2021, 1, 10. [Google Scholar] [CrossRef]
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology 2019, 70, 1457–1469. [Google Scholar] [CrossRef]
- Burra, P.; Bizzaro, D.; Gonta, A.; Shalaby, S.; Gambato, M.; Morelli, M.C.; Trapani, S.; Floreani, A.; Marra, F.; Brunetto, M.R.; et al. Special Interest Group Gender in Hepatology of the Italian Association for the Study of the Liver (AISF). Clinical impact of sexual dimorphism in non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). Liver Int. 2021, 41, 1713–1733. [Google Scholar] [CrossRef]
- Cherubini, A.; Della Torre, S.; Pelusi, S.; Valenti, L. Sexual dimorphism of metabolic dysfunction-associated steatotic liver disease. Trends Mol. Med. 2024, 30, 1126–1136. [Google Scholar] [CrossRef]
- McAdams DeMarco, M.A.; Maynard, J.W.; Baer, A.N.; Gelber, A.C.; Young, J.H.; Alonso, A.; Coresh, J. Diuretic Use, Increased Serum Urate Levels, and Risk of Incident Gout in a Population-Based Study of Adults with Hypertension: The Atherosclerosis Risk in Communities Cohort Study. Arthritis Rheum. 2012, 64, 121–129. [Google Scholar] [CrossRef] [PubMed]


| SU ≥ 410 μmol/L | SU < 410 μmol/L | p-Value | |||||
|---|---|---|---|---|---|---|---|
| FLI < 80 | FLI ≥ 80 | FLI < 80 | FLI ≥ 80 | SU | FLI | Interaction | |
| n = 346 | n = 310 | n = 1636 | n = 343 | ||||
| Female, n (%) | 110 (32) | 101 (33) | 996 (61) | 179 (52) | <0.001 | 0.12 | 0.057 |
| Age, mean (SD), years | 64 (8) | 62 (8) | 61 (8) | 62 (8) | 0.007 | 0.071 | <0.001 |
| BMI, mean (SD), kg/m2 | 26.9 (2.7) | 32.9 (4.7) | 26.0 (3.2) | 33.2 (4.4) | 0.095 | <0.001 | <0.001 |
| Waist circumference, mean (SD), cm | |||||||
| Women | 92 (7) | 110 (10) | 87 (9) | 110 (9) | <0.001 | <0.001 | <0.001 |
| Men | 97 (8) | 111 (9) | 94 (8) | 110 (9) | 0.001 | <0.001 | 0.14 |
| WHtR | 0.57 (0.05) | 0.66 (0.06) | 0.54 (0.06) | 0.66 (0.06) | <0.001 | <0.001 | <0.001 |
| LTPA, n (%) | 0.018 | <0.001 | 0.12 | ||||
| low | 99 (29) | 103 (34) | 339 (21) | 116 (35) | |||
| moderate | 143 (42) | 131 (43) | 696 (43) | 128 (38) | |||
| high | 101 (29) | 71 (23) | 589 (36) | 92 (27) | |||
| BP, mmHg, mean (SD) | |||||||
| systolic | 147 (19) | 152 (19) | 144 (19) | 151 (19) | 0.045 | <0.001 | 0.34 |
| diastolic | 86 (10) | 90 (10) | 85 (10) | 90 (10) | 0.23 | <0.001 | 0.29 |
| MAP | 106 (11) | 111 (11) | 104 (11) | 110 (11) | 0.068 | <0.001 | 0.25 |
| Fasting plasma glucose, mean (SD), mmol/L | 5.73 (1.06) | 6.32 (1.48) | 5.51 (1.13) | 6.32 (1.81) | 0.073 | <0.001 | 0.081 |
| Fasting plasma insulin, mean (SD) | 10.0 (8.9) | 17.7 (18.2) | 8.1 (8.0) | 14.2 (13.2) | <0.001 | <0.001 | 0.60 |
| Homa-IR, mean (SD) | 2.70 (3.16) | 5.19 (6.10) | 2.08 (2.98) | 4.29 (5.75) | <0.001 | <0.001 | 0.67 |
| Cholesterol, mean (SD), mmol/L | 5.62 (1.17) | 5.83 (1.14) | 5.76 (1.05) | 5.80 (1.16) | 0.27 | 0.015 | 0.11 |
| HDL, mean (SD), mmol/L | 1.43 (0.40) | 1.28 (0.35) | 1.60 (0.44) | 1.35 (0.39) | <0.001 | <0.001 | 0.018 |
| LDL, mean (SD), mmol/L | 3.50 (1.05) | 3.56 (0.99) | 3.57 (0.93) | 3.54 (0.99) | 0.57 | 0.73 | 0.33 |
| Triglycerides, mean (SD), mmol/L | 1.50 (0.71) | 2.33 (2.08) | 1.30 (0.60) | 2.01 (0.95) | <0.001 | <0.001 | 0.23 |
| eGFR, mean (SD), ml/min/1.73 m2 | 69.2 (16.0) | 72.1 (17.1) | 80.0 (14.2) | 79.3 (15.0) | <0.001 | 0.12 | 0.014 |
| Cystatin C, mean (SD), mg/L | 1.12 (0.25) | 1.10 (0.25) | 0.96 (0.17) | 1.01 (0.18) | <0.001 | 0.091 | <0.001 |
| GGT, mean (SD), U/L | 43.1 (35.5) | 80.5 (87.4) | 34.1 (31.5) | 73.6 (118.8) | 0.007 | <0.001 | 0.72 |
| ASAT, mean (SD), U/L | 32 (14) | 38 (18) | 29 (9) | 34 (22) | <0.001 | <0.001 | 0.70 |
| ALAT, mean (SD), U/L | 12 (9) | 16 (13) | 10 (6) | 15 (17) | 0.002 | <0.001 | 0.63 |
| hsCRP, mean (SD), mg/L | 3.1 (4.3) | 4.1 (6.7) | 2.3 (5.2) | 3.6 (4.5) | 0.013 | <0.001 | 0.71 |
| 25(OH)D, mean (SD), nmol/L | 36 (15) | 31 (17) | 36 (15) | 32 (14) | 0.71 | <0.001 | 0.91 |
| AUDIT score, mean (SD) | 3.5 (2.6) | 4.2 (2.9) | 2.9 (2.3) | 3.4 (2.8) | <0.001 | <0.001 | 0.32 |
| Smoking, n (%) | 49 (14) | 53 (17) | 263 (16) | 70 (20) | 0.16 | 0.050 | 0.79 |
| Education years, mean (SD) | 9.2 (3.3) | 9.4 (3.4) | 9.6 (3.3) | 9.0 (3.1) | 0.98 | 0.18 | 0.013 |
| Comorbidities, n (%) | |||||||
| DM | 36 (10) | 77 (25) | 120 (7) | 79 (23) | 0.075 | <0.001 | 0.30 |
| Hypertension | 138 (40) | 161 (52) | 451 (28) | 160 (47) | <0.001 | <0.001 | 0.084 |
| Musculoskeletal | 123 (36) | 119 (38) | 551 (34) | 138 (40) | 0.98 | 0.046 | 0.43 |
| CVD | 49 (14) | 42 (14) | 128 (8) | 38 (11) | 0.003 | 0.27 | 0.15 |
| Respiratory | 26 (8) | 36 (12) | 106 (6) | 29 (8) | 0.14 | 0.027 | 0.58 |
| MetS | 185 (53) | 283 (91) | 528 (32) | 300 (87) | <0.001 | <0.001 | 0.098 |
| Medication, n (%) | |||||||
| Antihypertensive * | 133 (38) | 144 (46) | 367 (22) | 145 (42) | <0.001 | <0.001 | 0.003 |
| Lipid lowering * | 77 (22) | 58 (19) | 224 (14) | 67 (20) | 0.031 | 0.40 | 0.009 |
| Urate lowering ** | 35 (10) | 59 (19) | 27 (2) | 12 (3) | <0.001 | <0.001 | 0.94 |
| Cohabiting, n (%) | 255 (74) | 220 (71) | 1193 (73) | 219 (64) | 0.090 | 0.008 | 0.18 |
| eGFR > 67HR (95% CI) * | eGFR ≤ 67HR (95% CI) * | |
|---|---|---|
| FLI < 80 | 1.00 (Reference) | 1.89 (1.31 to 2.73) |
| FLI ≥ 80 | 2.30 (1.31 to 3.14) | 2.28 (1.49 to 3.50) |
| SU < 410 μmol/LHR (95% CI) * | SU ≥ 410 μmol/LHR (95% CI) * | |
|---|---|---|
| All-cause mortality | ||
| FLI < 80 | 1.00 (Reference) ** | 1.16 (0.95 to 1.40) |
| lFLI ≥ 80 | 1.34 (1.06 to 1.70) | 1.76 (1.39 to 2.23) |
| Cardiovascular mortality | ||
| FLI < 80 | 1.00 (Reference) ** | 1.16 (0.86 to 1.56) |
| FLI ≥ 80 | 1.26 (0.88 to 1.80) | 1.70 (1.20 to 2.41) |
| SU ≥ 410 μmol/L | SU < 410 μmol/L | ||||
|---|---|---|---|---|---|
| FLI < 80 (Group 1) | FLI ≥ 80 2 (Group 2) | FLI < 80 3 (Group 3) | FLI ≥ 80 4 (Group 4) | p-Value [*] | |
| Cancer and other neoplasms (ICD codes C00-D89) | [31] 5.5 (3.6 to 7.5) | [35] 8.4 (5.6 to 11.1) | [128] 6.1 (5.0 to 7.1) | [46] 10.1 (7.2 to 13.0) | 0.009 [1/4, 3/4] |
| Cardiovascular (ICD codes I00-I99) * | [61] 10.4 (7.8 to 13.1) | [70] 17.6 (13.5 to 21.8) | [176] 8.8 (7.5 to 10.1) | [63] 14.7 (11.1 to 18.4) | <0.001 [1/2, 2/3, 3/4] |
| Nervous system (ICD codes G00-G99) * | [11] 2.0 (0.8 to 3.2) | [13] 3.6 (1.6 to 5.5) | [74] 3.6 (2.8 to 4.4) | [10] 2.3 (0.9 to 3.7) | 0.20 |
| Respiratory system (ICD codes J00-J99) * | [12] 2.0 (0.9 to 3.2) | [7] 1.6 (0.4 to 2.8) | [15] 0.7 (0.4 to 1.1) | [2] 0.4 (0.0 to 1.1) | 0.030 [1/4] |
| Mental, behavioral and neurodevelopmental disorders (ICD codes F00-F99) * | [3] 0.5 (0.0 to 1.1) | [5] 1.4 (0.1 to 2.6) | [9] 0.5 (0.2 to 0.8) | [3] 0.7 (0.0 to1.6) | 0.26 |
| Gastrointestinal (ICD codes K00-K93) * | [6] 1.1 (0.2 to 2.0) | [4] 0.9 (0.0 to 1.8) | [13] 0.6 (0.3 to 0.9) | [3] 0.6 (0.0 to 1.3) | 0.59 |
| Endocrine (ICD codes E00-E99) * | [3] 0.5 (0.0 to 1.1) | [4] 0.8 (0.0 to 1.7) | [3] 0.1 (0.0 to 0.3) | [3] 0.6 (0.0 to 1.4) | 0.13 |
| External causes (ICD codes V01-Y98) | [3] 0.5 (0.1 to 1.1) | [1] 0.2 0.0 to 0.6) | [15] 0.7 (0.3 to 1.1) | [3] 0.7 (0.0 to 1.4) | 0.70 |
| Alcohol-related ** | [1] 0.4 (0.0 to 1.1) | [3] 0.6 (0.0 to 1.3) | [2] 0.1 (0.0 to 0.2) | [2] 0.4 (0.1 to 0.9) | 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timsans, J.; Kauppi, J.; Rantalaiho, V.; Kerola, A.; Hakkarainen, K.; Paldanius, M.; Kautiainen, H.; Kauppi, M. Hyperuricemia—Especially “Metabolic Hyperuricemia”—Is Independently Associated with a Higher Risk of Steatotic Liver Disease. Metabolites 2025, 15, 356. https://doi.org/10.3390/metabo15060356
Timsans J, Kauppi J, Rantalaiho V, Kerola A, Hakkarainen K, Paldanius M, Kautiainen H, Kauppi M. Hyperuricemia—Especially “Metabolic Hyperuricemia”—Is Independently Associated with a Higher Risk of Steatotic Liver Disease. Metabolites. 2025; 15(6):356. https://doi.org/10.3390/metabo15060356
Chicago/Turabian StyleTimsans, Janis, Jenni Kauppi, Vappu Rantalaiho, Anne Kerola, Kia Hakkarainen, Mika Paldanius, Hannu Kautiainen, and Markku Kauppi. 2025. "Hyperuricemia—Especially “Metabolic Hyperuricemia”—Is Independently Associated with a Higher Risk of Steatotic Liver Disease" Metabolites 15, no. 6: 356. https://doi.org/10.3390/metabo15060356
APA StyleTimsans, J., Kauppi, J., Rantalaiho, V., Kerola, A., Hakkarainen, K., Paldanius, M., Kautiainen, H., & Kauppi, M. (2025). Hyperuricemia—Especially “Metabolic Hyperuricemia”—Is Independently Associated with a Higher Risk of Steatotic Liver Disease. Metabolites, 15(6), 356. https://doi.org/10.3390/metabo15060356






