DART–Triple Quadrupole Mass Spectrometry Method for Multi-Target and Fast Detection of Adulterants in Saffron
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Preparation of Calibration Curves in Saffron Extracts Adulterated with Safflower Extracts
2.3. Preparation of Calibration Curves in Saffron Powder Adulterated with Safflower and Turmeric Powder
2.4. Construction of Calibration Curves with Internal Standard
2.5. DART-MS Analysis
3. Results and Discussion
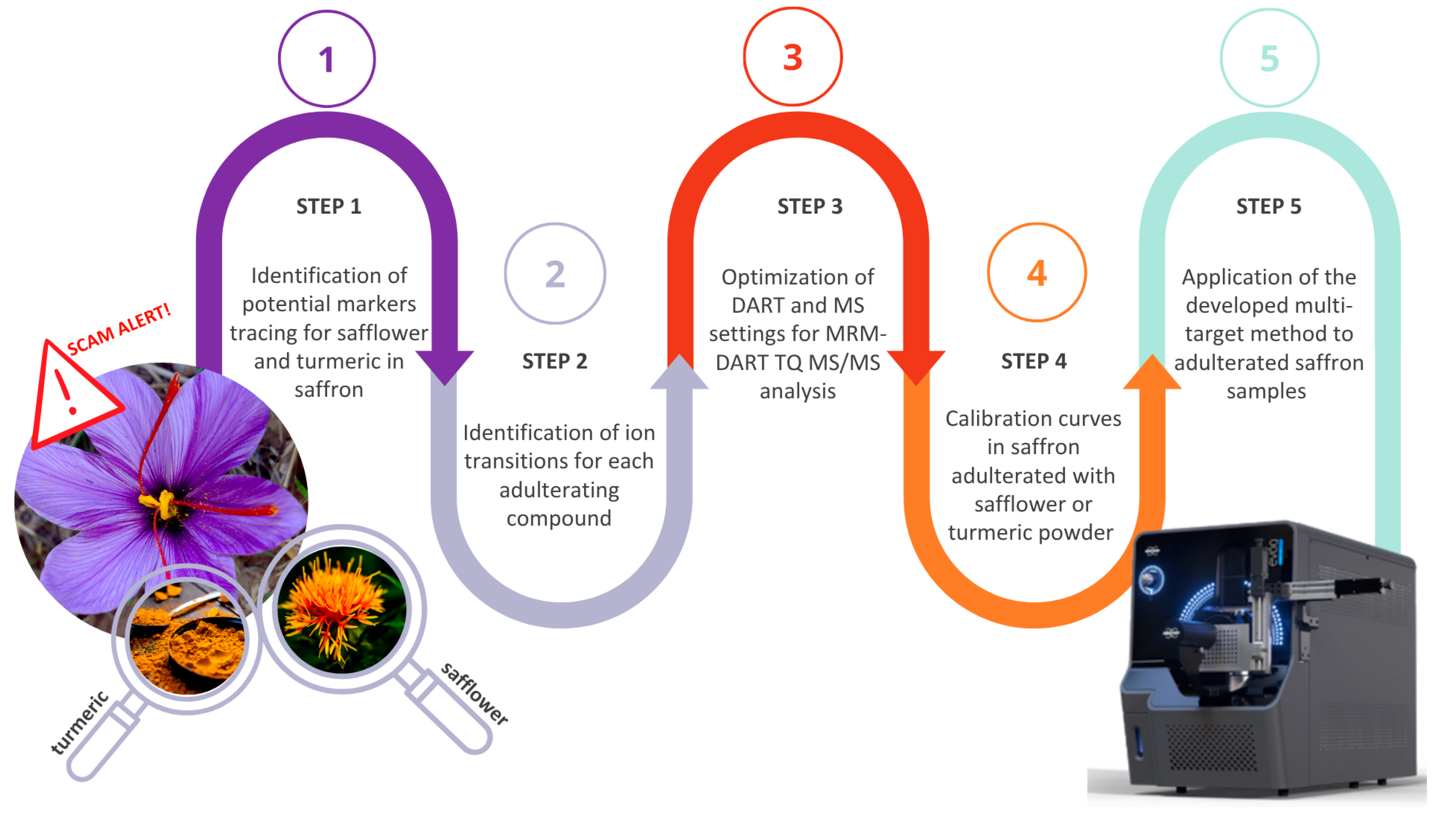
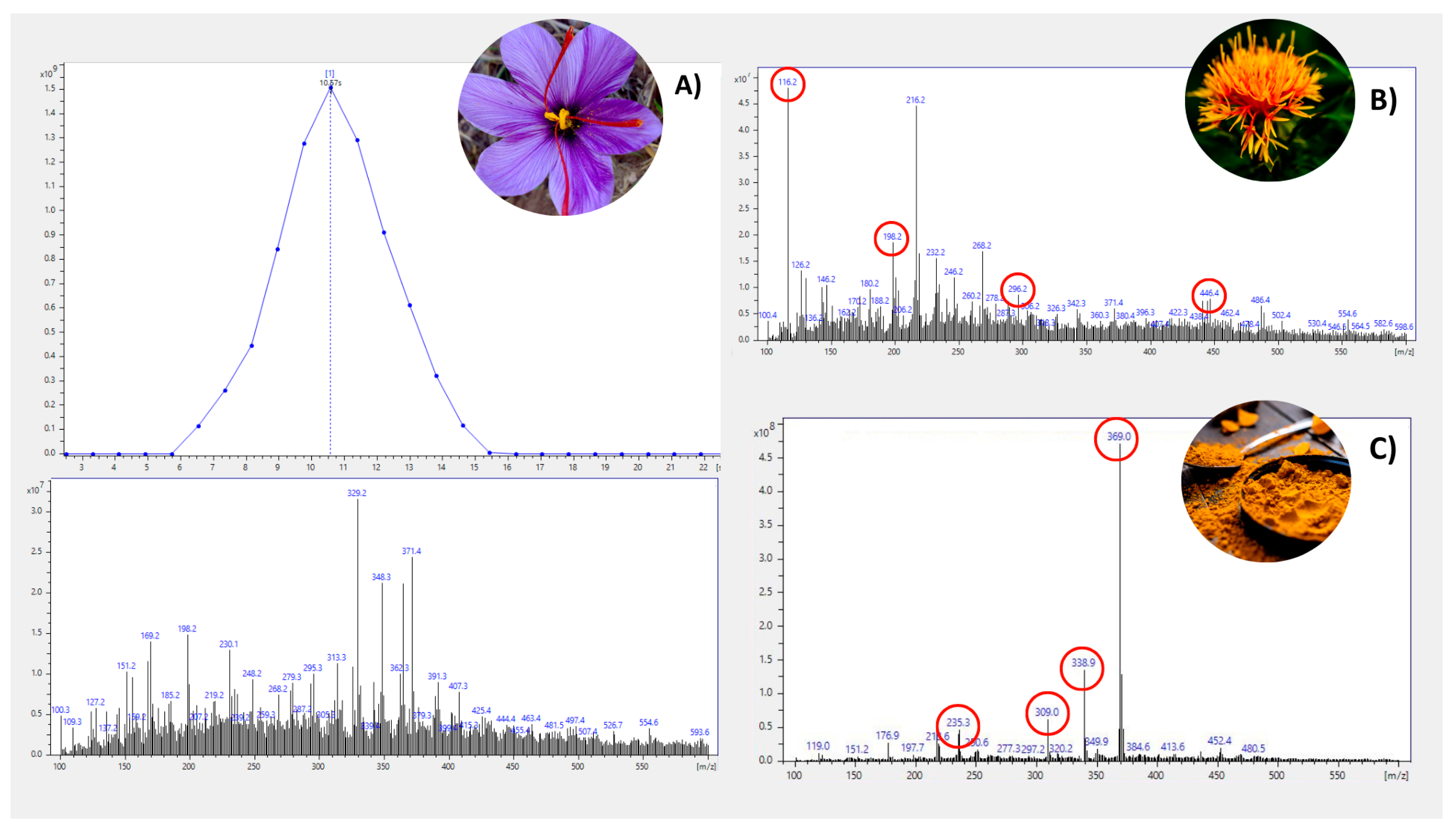
3.1. Selection of Safflower and Turmeric Markers and Method Optimization
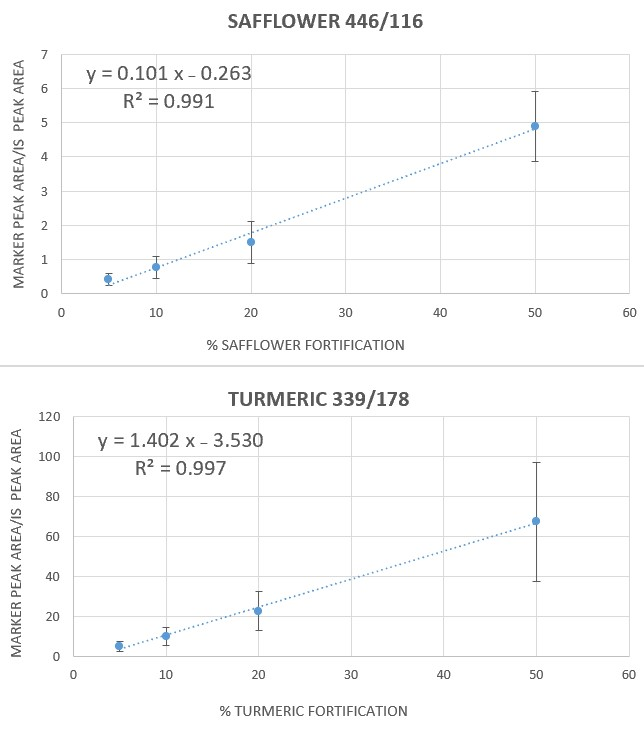
3.2. Calibration Curves in Artificially Fortified Saffron Extracts and Repeatability Study
3.3. Production of Matrix-Matched Calibration Curves in Saffron Powder Recovery Assessment and Analysis of Real Samples
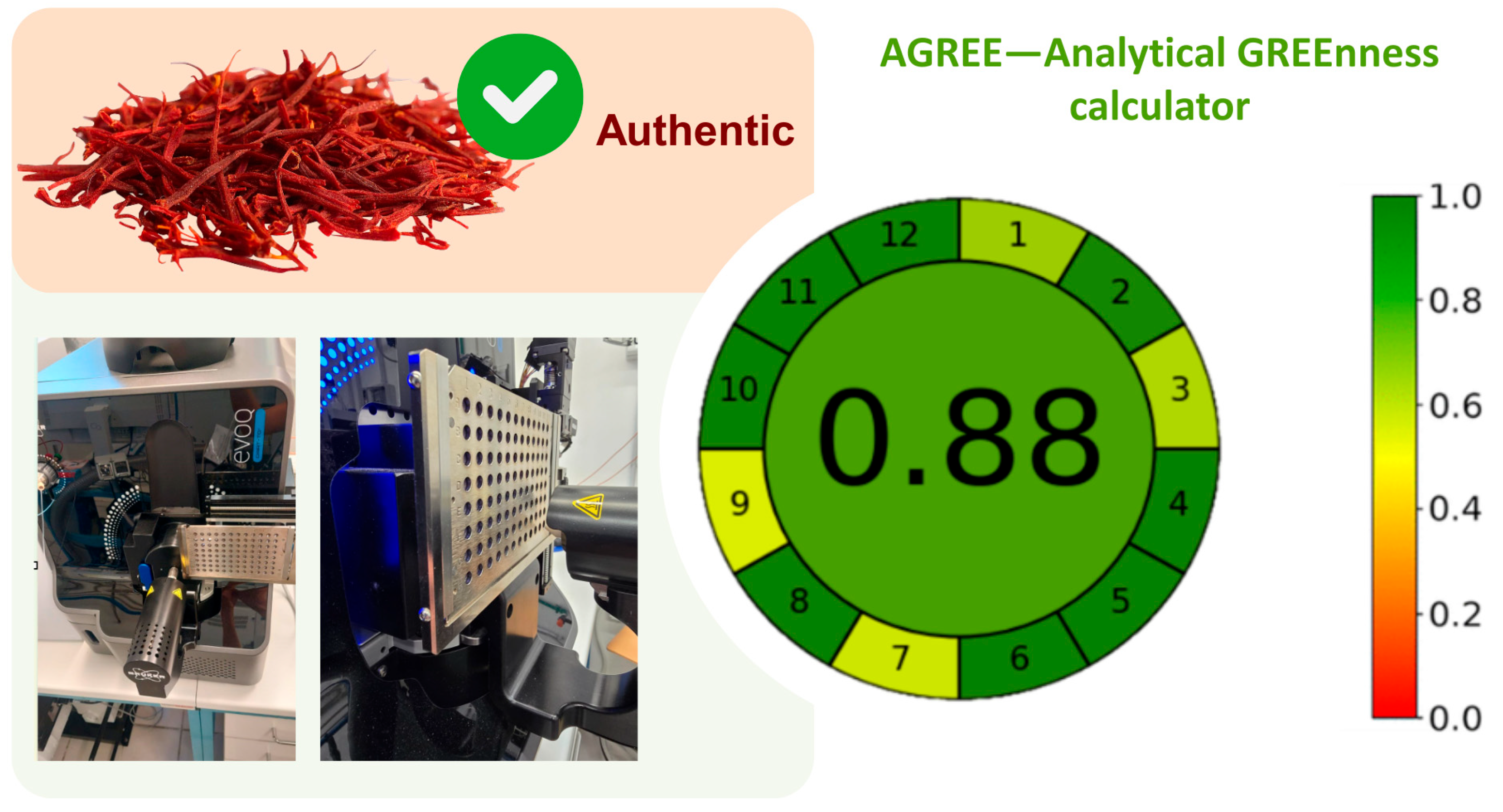
3.4. Greenness Assessment
3.5. Limitations and Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frusciante, L.; Geminiani, M.; Shabab, B.; Olmastroni, T.; Scavello, G.; Rossi, M.; Mastroeni, P.; Nyong’a, C.N.; Salvini, L.; Lamponi, S.; et al. Exploring the Antioxidant and Anti-Inflammatory Potential of Saffron (Crocus sativus) Tepals Extract within the Circular Bioeconomy. Antioxidants 2024, 13, 1082. [Google Scholar] [CrossRef]
- Mohammed, E.U.; Mostafa, M.H.; Hashem, S.N. Evaluation of the Antimicrobial Effect of Aqueous Extract of Saffron on Streptococcus Mutans in Oral Cavity. Al-Azhar J. Dent. 2024, 11, 13. [Google Scholar] [CrossRef]
- Wang, C.-Z.; Ma, Q.; Kim, S.; Wang, D.H.; Shoyama, Y.; Yuan, C. Effects of saffron and its active constituent crocin on cancer management: A narrative review. Longhua Chin. Med. 2022, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Abu-Izneid, T.; Rauf, A.; Khalil, A.A.; Olatunde, A.; Khalid, A.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Sahab Uddin, M.; Heydari, M.; Khayrullin, M.; et al. Nutritional and health beneficial properties of saffron (Crocus sativus L): A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2683–2706. [Google Scholar] [CrossRef] [PubMed]
- ISO 21983:2019; Guidelines for the Harvesting, Transportation, Separation of Stigma, Drying and Storage of Saffron Before Packing. ISO: Geneva, Switzerland, 2019.
- Semeniuc, C.A.; Mandrioli, M.; Urs, M.J.; Gallina Toschi, T. Quality and authenticity of saffron and sensory aspects. Int. J. Gastron. Food Sci. 2024, 38, 101067. [Google Scholar] [CrossRef]
- Salehi, A.; Shariatifar, N.; Pirhadi, M.; Zeinali, T. An overview on different detection methods of saffron (Crocus sativus L.) adulterants. J. Food Meas. Charact. 2022, 16, 4996–5006. [Google Scholar] [CrossRef]
- EUR 30877 EN; Results of an EU Wide Coordinated Control Plan to Establish the Prevalence of Fraudulent Practices in the Marketing of Herbs and Spices. Publications Office of the European Union: Gare, Luxembourg, 2021.
- ISO 3632-2:2010; Spices—Saffron (Crocus sativus L.)—Part 2: Test Methods. ISO: Geneva, Switzerland, 2010.
- ISO 3632-1:2011; Spices—Saffron (Crocus sativus L.)—Part 1: Specification. ISO: Geneva, Switzerland, 2011.
- Sabatino, L.; Scordino, M.; Gargano, M.; Belligno, A.; Traulo, P.; Gagliano, G. HPLC/PDA/ESI-MS Evaluation of Saffron (Crocus sativus L.) Adulteration. Nat. Prod. Commun. 2011, 6, 1873–1876. [Google Scholar] [CrossRef]
- Karoui, R.; Downey, G.; Blecker, C. Mid-Infrared Spectroscopy Coupled with Chemometrics: A Tool for the Analysis of Intact Food Systems and the Exploration of Their Molecular Structure−Quality Relationships—A Review. Chem. Rev. 2010, 110, 6144–6168. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Q.; Sun, S. Adulteration screening of botanical materials by a sensitive and model-free approach using infrared spectroscopic imaging and two-dimensional correlation infrared spectroscopy. J. Mol. Struct. 2016, 1124, 262–267. [Google Scholar] [CrossRef]
- Karimi, S.; Feizy, J.; Mehrjo, F.; Farrokhnia, M. Detection and quantification of food colorant adulteration in saffron sample using chemometric analysis of FT-IR spectra. RSC Adv. 2016, 6, 23085–23093. [Google Scholar] [CrossRef]
- Bilge, G.; Boyacı, İ.H.; Eseller, K.E.; Tamer, U.; Çakır, S. Analysis of bakery products by laser-induced breakdown spectroscopy. Food Chem. 2015, 181, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Agrawal, R.; Pathak, A.K.; Rai, A.K.; Rai, G.K. Laser-Induced Breakdown Spectroscopy: An Approach to Detect Adulteration in Turmeric. Spectrosc. Lett. Int. J. Rapid Commun 2013, 46, 155–159. [Google Scholar]
- Varliklioz Er, S.; Eksi-Kocak, H.; Yetim, H.; Boyaci, I.H. Novel Spectroscopic Method for Determination and Quantification of Saffron Adulteration. Food Anal. Methods 2017, 10, 1547–1555. [Google Scholar] [CrossRef]
- Yilmaz, A.; Nyberg, N.T.; Mølgaard, P.; Asili, J.; Jaroszewski, J.W. 1H NMR metabolic fingerprinting of saffron extracts. Metabolomics 2010, 6, 511–517. [Google Scholar] [CrossRef]
- Villa, C.; Costa, J.; Oliveira, M.B.P.P.; Mafra, I. Novel quantitative real-time PCR approach to determine safflower (Carthamus tinctorius) adulteration in saffron (Crocus sativus). Food Chem. 2017, 229, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Boudadi, I.; EL Merzougui, S.; Lachheb, M.; Lachguer, K.; Serghini, M.A. Design and optimisation of rapid quadruplex PCR technique to identify plant-derived adulterants in saffron. J. Food Sci. Technol. 2025, 1–11. [Google Scholar] [CrossRef]
- Ma, X.Q.; Zhu, D.Y.; Li, S.P.; Dong, T.T.X.; Tsim, K.W.K. Authentic Identification of Stigma Croci (Stigma of Crocus sativus) from its Adulterants by Molecular Genetic Analysis. Planta Med. 2001, 67, 183–186. [Google Scholar] [CrossRef]
- Bhooma, V.; Vassou, S.L.; Kaliappan, I.; Parani, M. Identification of adulteration in the market samples of saffron using morphology, HPLC, HPTLC, and DNA barcoding methods. Genome 2024, 67, 43–52. [Google Scholar] [CrossRef]
- Javanmardi, N.; Bagheri, A.; Moshtaghi, N.; Sharifi, A.; Kakhki, A.H. Identification of Safflower as a fraud in commercial Saffron using RAPD/SCAR marker. J. Mol. Cell Biol. 2011, 3, 31–37. [Google Scholar]
- Marieschi, M.; Torelli, A.; Bruni, R. Quality Control of Saffron (Crocus sativus L.): Development of SCARMarkers for the Detection of Plant Adulterants Used as BulkingAgents. J. Agric. Food Chem. 2012, 60, 10998–11004. [Google Scholar] [CrossRef]
- Soffritti, G.; Busconi, M.; Sánchez, R.A.; Thiercelin, J.-M.; Polissiou, M.; Roldán, M.; Fernández, J.A. Genetic and Epigenetic Approaches for the Possible Detection of Adulteration and Auto-Adulteration in Saffron (Crocus sativus L.) Spice. Molecules 2016, 21, 343. [Google Scholar] [CrossRef]
- Jiang, C.; Cao, L.; Yuan, Y.; Chen, M.; Jin, Y.; Huang, L. Barcoding Melting Curve Analysis for Rapid, Sensitive, and Discriminating Authentication of Saffron (Crocus sativus L.) from Its Adulterants. Biomed. Res. Int. 2014, 2014, 809037. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, G.M.; Losito, I.; De Angelis, E.; Arlorio, M.; Logrieco, A.F.; Monaci, L. Assessing fish authenticity by direct analysis in real time-high resolution mass spectrometry and multivariate analysis: Discrimination between wild- type and farmed salmon. Food Res. Int. 2019, 116, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Miano, B.; Righetti, L.; Piro, R.; Asta, C.D.; Folloni, S.; Galaverna, G.; Suman, M.; Miano, B.; Righetti, L.; Piro, R.; et al. Direct analysis real-time—High-resolution mass spectrometry for Triticum species authentication. Food Addit. Contam. Part A 2018, 35, 2291–2297. [Google Scholar] [CrossRef] [PubMed]
- Schmauder, F.; Creydt, M.; Fischer, M. Novel DART-MS approach for rapid and environmentally friendly determination of the geographical origin of hazelnuts (Corylus avellana L.). Food Chem. 2025, 467, 142265. [Google Scholar] [CrossRef]
- Zacometti, C.; Lefevre, S.; Massaro, A.; Gioia, T.; Lafeuille, A.F.J.; Suman, M.; Piro, R.; Candalino, I.F.; Tata, A. Thermal desorption direct analysis in real-time high-resolution mass spectrometry and machine learning allow the rapid authentication of ground black pepper and dried oregano: A proof-of-concept study. J. Mass. Spectrom. 2023, 58, e4953. [Google Scholar] [CrossRef]
- Fiorino, G.M.; Garino, C.; Arlorio, M.; Logrieco, A.F.; Losito, I.; Monaci, L. Overview on Untargeted Methods to Combat Food Frauds: A Focus on Fishery Products. J. Food Qual. 2018, 2018, 1581746. [Google Scholar] [CrossRef]
- Rubert, J.; Lacina, O.; Zachariasova, M.; Hajslova, J. Saffron authentication based on liquid chromatography high resolution tandem mass spectrometry and multivariate data analysis. Food Chem. 2016, 204, 201–209. [Google Scholar] [CrossRef]
- Guijarro-Díez, M.; Castro-Puyana, M.; Crego, A.L.; Marina, M.L. A novel method for the quality control of saffron through the simultaneous analysis of authenticity and adulteration markers by liquid chromatography-(quadrupole-time of flight)-mass spectrometry. Food Chem. 2017, 228, 403–410. [Google Scholar] [CrossRef]
- De Angelis, E.; Pilolli, R.; Bejjani, A.; Guagnano, R.; Garino, C.; Arlorio, M.; Monaci, L. Optimization of an Untargeted DART-HRMS Method Envisaging Identification of Potential Markers for Saffron Authenticity Assessment. Foods 2021, 10, 1238. [Google Scholar] [CrossRef]
- Pavlovich, M.J.; Musselman, B.; Hall, A.B. Direct analysis in real time—Mass spectrometry (DART-MS) in forensic and security applications. Mass. Spectrom. Rev. 2018, 37, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Armenta, S.; Garrigues, S.; De la Guardia, M. Green Analytical Chemistry. Trends Anal. Chem. 2008, 27, 497–511. [Google Scholar] [CrossRef]
- Sajid, M.; Płotka-Wasylka, J. Green analytical chemistry metrics: A review. Talanta 2022, 238, 123046. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef] [PubMed]
| Ion Transition | Calibration Curve Equation | R2 | LOD (% w/w) | LOQ (% w/w) |
|---|---|---|---|---|
| Safflower in saffron | ||||
| 116/70 | y = 0.0717 (±0.0063) x − 0.1786 (±0.174) | 0.985 | 7 | 24 |
| 446/116 | y = 0.1014 (±0.0067) x – 0.2633 (±0.185) | 0.991 | 5 | 18 |
| Turmeric in saffron | ||||
| 339/178 | y = 1.4027 (±0.052) x – 3.5298 (±1.445) | 0.997 | 3 | 10 |
| 368/285 | y = 4.2545 (±0.253) x − 5.9038 (±0.965) | 0.993 | 5 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monaci, L.; Luparelli, A.; Schirinzi, W.M.; Quintieri, L.; Verdu, A. DART–Triple Quadrupole Mass Spectrometry Method for Multi-Target and Fast Detection of Adulterants in Saffron. Metabolites 2025, 15, 357. https://doi.org/10.3390/metabo15060357
Monaci L, Luparelli A, Schirinzi WM, Quintieri L, Verdu A. DART–Triple Quadrupole Mass Spectrometry Method for Multi-Target and Fast Detection of Adulterants in Saffron. Metabolites. 2025; 15(6):357. https://doi.org/10.3390/metabo15060357
Chicago/Turabian StyleMonaci, Linda, Anna Luparelli, William Matteo Schirinzi, Laura Quintieri, and Alexandre Verdu. 2025. "DART–Triple Quadrupole Mass Spectrometry Method for Multi-Target and Fast Detection of Adulterants in Saffron" Metabolites 15, no. 6: 357. https://doi.org/10.3390/metabo15060357
APA StyleMonaci, L., Luparelli, A., Schirinzi, W. M., Quintieri, L., & Verdu, A. (2025). DART–Triple Quadrupole Mass Spectrometry Method for Multi-Target and Fast Detection of Adulterants in Saffron. Metabolites, 15(6), 357. https://doi.org/10.3390/metabo15060357








