UPLC-ESI-QTRAP-MS-Based Metabolomics Revealed Changes in Biostimulant-Related Metabolite Profiles in Zingiber mioga Flower Buds During Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Reagents
2.3. Extraction of Metabolites Related to Biostimulants
2.4. Analysis of Metabolites Related to Biostimulants
2.5. Statistical Analysis
3. Results and Discussion
3.1. Identification and Quantitative Descriptive Analysis of Biostimulant-Related Metabolites
3.2. Analysis Profiles of Biostimulant-Related Metabolites
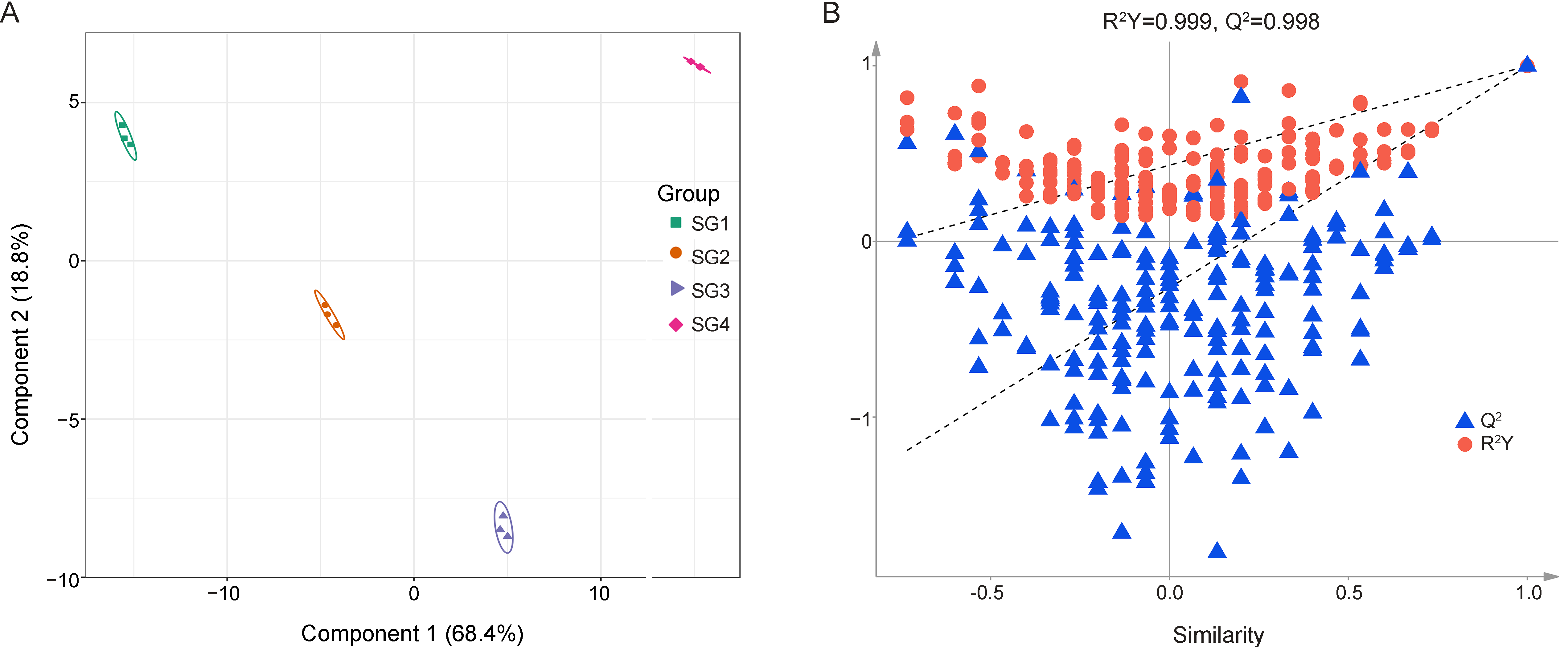
3.3. OPLS-DA Results
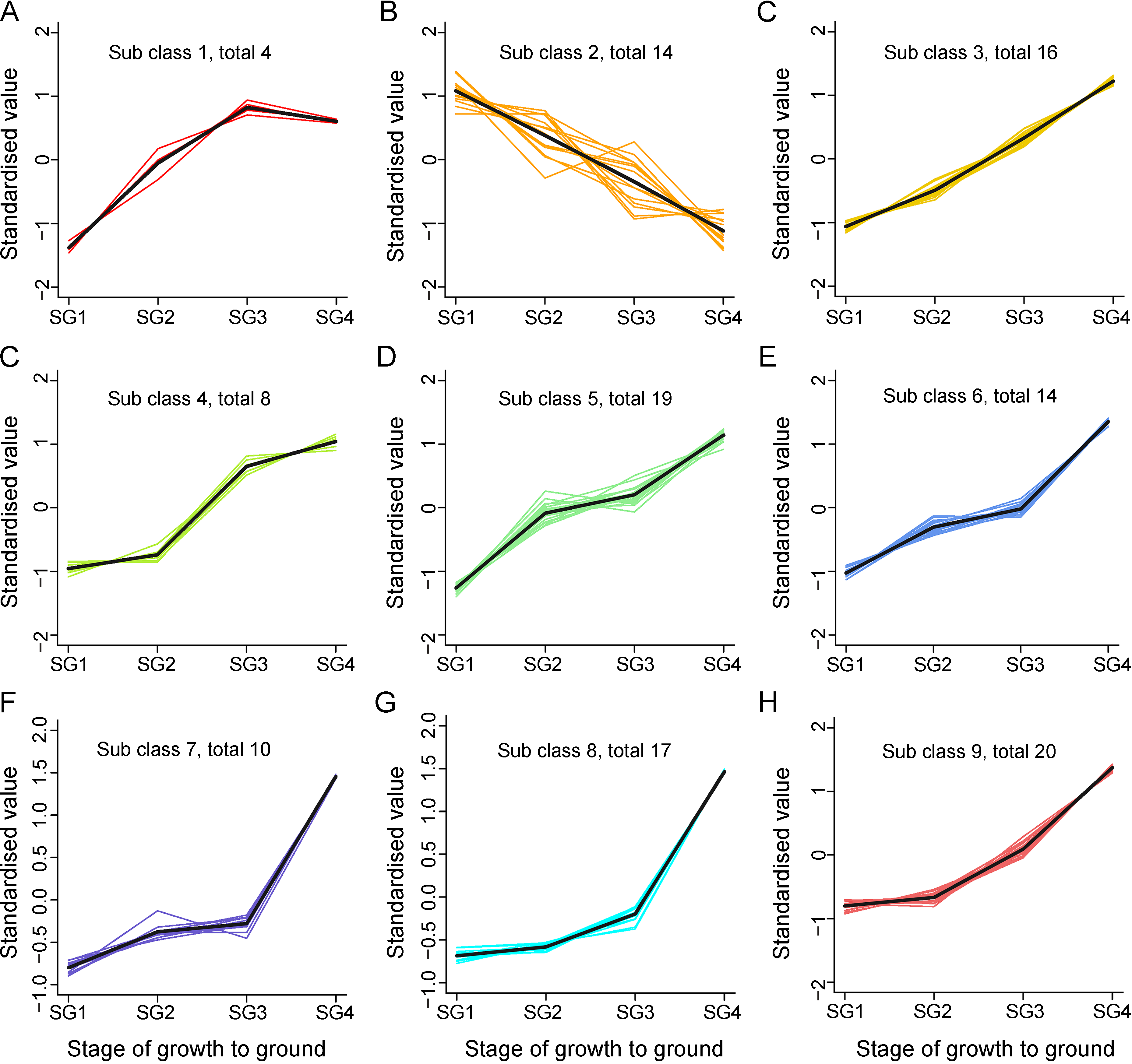
3.4. Identification and K-Means Cluster Analysis of Differential Metabolites
3.5. Systematic Analysis of Key Differential Metabolites
3.6. Correlation Analysis of Different Stages and Differential Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, D.; Ahn, J.; Jang, Y.; Ha, T.; Jung, C. Zingiber mioga reduces weight gain, insulin resistance and hepatic gluconeogenesis in diet-induced obese mice. Exp. Ther. Med. 2016, 12, 369–376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abe, M.; Ozawa, Y.; Uda, Y.; Morimitsu, Y.; Nakamura, Y.; Osawa, T. A novel labdane-type trialdehyde from myoga (Zingiber mioga Roscoe) that potently inhibits human platelet aggregation and human 5-lipoxygenase. Biosci. Biotech. Bioch. 2006, 70, 2494–2500. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, Z.; Xie, J. The dynamic changes in volatile metabolites provide a new understanding for the special flavor formation in Z. Mioga flower buds during the growth stages. Food Res. Int. 2024, 186, 114347. [Google Scholar] [CrossRef]
- Wei, S.; Liu, H.; Li, J.; Ren, T.; Xie, J. Metabolite variations of sugars, organic acids, fatty acids and amino acids in flower buds of Zingiber mioga Roscoe at different developmental stages. J. Food Compos. Anal. 2023, 116, 105050. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, Z.; Luo, P.; Xie, J. The dynamic variations of flavonoid metabolites in flower buds for Zingiber mioga at different developmental stages. J. Food Compos. Anal. 2023, 123, 105537. [Google Scholar] [CrossRef]
- Del Buono, D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lardon, R.; Mangelinckx, S.; Geelen, D. A practical guide to the discovery of biomolecules with biostimulant activity. J. Exp. Bot. 2024, 75, 3797–3817. [Google Scholar] [CrossRef]
- Morillas-España, A.; Ruiz-Nieto, Á.; Lafarga, T.; Acién, G.; Arbib, Z.; González-López, C.V. Biostimulant Capacity of Chlorella and Chlamydopodium species produced using wastewater and centrate. Biology 2022, 11, 1086. [Google Scholar] [CrossRef]
- Godlewska, K.; Pacyga, P.; Michalak, I.; Biesiada, A.; Szumny, A.; Pachura, N.; Piszcz, U. Systematic investigation of the effects of seven plant extracts on the physiological parameters, yield, and nutritional quality of Radish (Raphanus sativus var. sativus). Front. Plant Sci. 2021, 12, 651152. [Google Scholar] [CrossRef]
- Salvage, R.; Cannon, T.; Kingsmill, P.; Liu, F.; Fleming, C.C. A complex biostimulant based on plant flavonoids enhances potato growth and commercial yields. Front. Sustain. Food Syst. 2024, 8, 1368423. [Google Scholar] [CrossRef]
- Ahmad, S.; Lu, C.; Gao, J.; Ren, R.; Wei, Y.; Wu, J.; Jin, J.; Zheng, C.; Zhu, G.; Yang, F. Genetic insights into the regulatory pathways for continuous flowering in a unique orchid Arundina graminifolia. BMC Plant Biol. 2021, 21, 587. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Yang, K.; Chen, G.; Huang, J.; Hao, Y.; Tu, S.; Zhou, Y.; Zhao, K.; Chen, J.; Shi, X.; et al. Transcriptome mining of hormonal and floral integrators in the leafless flowers of three cymbidium orchids. Front. Plant Sci. 2022, 13, 1043099. [Google Scholar] [CrossRef] [PubMed]
- De La Peña, R.; Sattely, E.S. Rerouting plant terpene biosynthesis enables momilactone pathway elucidation. Nat. Chem. Biol. 2021, 17, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Susila, H.; Jurić, S.; Liu, L.; Gawarecka, K.; Chung, K.S.; Jin, S.; Kim, S.; Nasim, Z.; Youn, G.; Suh, M.C.; et al. Florigen sequestration in cellular membranes modulates temperature-responsive flowering. Science 2021, 373, 1137–1142. [Google Scholar] [CrossRef]
- Gillissen, B.; Bürkle, L.; André, B.; Kühn, C.; Rentsch, D.; Brandl, B.; Frommer, W.B. A new family of high-affinity transporters for adenine, cytosine, and purine derivatives in Arabidopsis. Plant Cell 2000, 12, 291–300. [Google Scholar] [CrossRef]
- Paungfoo-Lonhienne, C.; Lonhienne, T.G.A.; Mudge, S.R.; Schenk, P.M.; Christie, M.; Carroll, B.J.; Schmidt, S. DNA is taken up by root hairs and pollen, and stimulates root and pollen tube growth. Plant Physiol. 2010, 153, 799–805. [Google Scholar] [CrossRef]
- Pei, L.; Gao, Y.; Feng, L.; Zhang, Z.; Liu, N.; Yang, B.; Zhao, N. Phenolic acids and flavonoids play important roles in flower bud differentiation in Mikania micrantha: Transcriptomics and Metabolomics. Int. J. Mol. Sci. 2023, 24, 16550. [Google Scholar] [CrossRef]
- Wang, T.; Zou, Q.; Guo, Q.; Yang, F.; Wu, L.; Zhang, W. Widely targeted metabolomics analysis reveals the effect of flooding stress on the synthesis of flavonoids in Chrysanthemum morifolium. Molecules 2019, 24, 3695. [Google Scholar] [CrossRef]
- Xu, F. Study on Hormone Regulation of Alkaloid Metabolism and Auxin-Related Genes in Catharanthus roseus. Master’s Thesis, Shanghai Jiao Tong University, Shanghai, China, 2014. [Google Scholar]
- Chen, X.; Tian, Q.; Chen, Z.; Jiang, Q.; Hu, X.; Wang, D.; Wu, T.; Luo, X.; Yuan, L.; Yu, F. Integrative phenomics, metabolomics, and flavoromics reveal key quality indicators during the formation of flavor and bioactive compounds in Alpinia hainanensis (Zingiberaceae) fruit. Food Chem. 2025, 486, 144602. [Google Scholar] [CrossRef]
- Deng, M.; Yun, X.; Ren, S.; Qing, Z.; Luo, F. Plants of the genus Zingiber: A review of their ethnomedicine, phytochemistry and pharmacology. Molecules 2022, 27, 2826. [Google Scholar] [CrossRef]
- Tan, K.; Deng, K.; Zhang, Y.; Yang, G.; Yang, X. Biological characteristics of Zingiber striolatum germplasm resources in Zunyi. Guizhou Agric. Sci. 2016, 44, 9–14. [Google Scholar]
- Wang, C.; Zhang, Y.B.; Zhou, Q.; Chen, G.Y.; Zhao, D.W.; Xu, J. Analysis of nutritional components and metabolite differences in flower buds of different varieties (strains) of Zingiber striolatum Diels. Chi. J. Trop. Agric. 2024, 44, 50–56. [Google Scholar]
- Granato, D.; Santos, J.S.; Escher, G.B.; Ferreira, B.L.; Maggio, R.M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci. Technol. 2018, 72, 83–90. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Li, D.; Liu, Y.; Liu, B.; Meng, X. Characterization of metabolite profiles of white and green spears of Asparagus officinalis L. from Caoxian, East China. Food Res. Int. 2020, 128, 108869. [Google Scholar] [CrossRef]
- Lei, L.; Yuan, X.; Fu, K.; Chen, Y.; Lu, Y.; Shou, N.; Wu, D.; Chen, X.; Shi, J.; Zhang, M.; et al. Pseudotargeted metabolomics revealed the adaptive mechanism of Draba oreades Schrenk at high altitude. Front. Plant Sci. 2022, 13, 1052640. [Google Scholar] [CrossRef]
- Kang, C.; Zhang, Y.; Zhang, M.; Qi, J.; Zhao, W.; Gu, J.; Guo, W.; Li, Y. Screening of specific quantitative peptides of beef by LC-MS/MS coupled with OPLS-DA. Food Chem. 2022, 387, 132932. [Google Scholar] [CrossRef]
- Leonardi, R.; Jackowski, S. Biosynthesis of pantothenic acid and coenzyme A. EcoSal Plus 2007, 2, 10.1128. [Google Scholar] [CrossRef]
- Cao, D.; Chabikwa, T.; Barbier, F.; Dun, E.A.; Fichtner, F.; Dong, L.; Kerr, S.C.; Beveridge, C.A. Auxin-independent effects of apical dominance induce changes in phytohormones correlated with bud outgrowth. Plant Physiol. 2023, 192, 1420–1434. [Google Scholar] [CrossRef]
- Qu, Y.; Chen, X.; Mao, X.; Huang, P.; Fu, X. Transcriptome analysis reveals the role of GA3 in regulating the asynchronism of floral bud differentiation and development in Heterodichogamous Cyclocarya paliurus (Batal.) Iljinskaja. Int. J. Mol. Sci. 2022, 23, 6763. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Zhou, Y.; Cheng, Z.; Liu, H. UPLC-ESI-QTRAP-MS-Based Metabolomics Revealed Changes in Biostimulant-Related Metabolite Profiles in Zingiber mioga Flower Buds During Development. Metabolites 2025, 15, 358. https://doi.org/10.3390/metabo15060358
Xie J, Zhou Y, Cheng Z, Liu H. UPLC-ESI-QTRAP-MS-Based Metabolomics Revealed Changes in Biostimulant-Related Metabolite Profiles in Zingiber mioga Flower Buds During Development. Metabolites. 2025; 15(6):358. https://doi.org/10.3390/metabo15060358
Chicago/Turabian StyleXie, Jiao, Yahan Zhou, Zhifei Cheng, and Huijuan Liu. 2025. "UPLC-ESI-QTRAP-MS-Based Metabolomics Revealed Changes in Biostimulant-Related Metabolite Profiles in Zingiber mioga Flower Buds During Development" Metabolites 15, no. 6: 358. https://doi.org/10.3390/metabo15060358
APA StyleXie, J., Zhou, Y., Cheng, Z., & Liu, H. (2025). UPLC-ESI-QTRAP-MS-Based Metabolomics Revealed Changes in Biostimulant-Related Metabolite Profiles in Zingiber mioga Flower Buds During Development. Metabolites, 15(6), 358. https://doi.org/10.3390/metabo15060358




