1H NMR-Based Analysis to Determine the Metabolomics Profile of Solanum nigrum L. (Black Nightshade) Grown in Greenhouse Versus Open-Field Conditions
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sivakumar, D.; Phan, A.D.T.; Slabbert, R.M.; Sultanbawa, Y.; Remize, F. Phytochemical and nutritional quality changes during irrigation and postharvest processing of the underutilized vegetable African nightshade. Front. Nutr. 2020, 7, 576532. [Google Scholar] [CrossRef]
- Moussa, M.I.D.; Alashi, A.M.; Sossa-Vihotogbé, C.N.; Akponikpè, P.B.; Baco, M.N.; Djènontin, A.J.; Aluko, R.E.; Akissoé, N.H. Developments in research on the nutritional health-promoting properties of three traditional leafy vegetables commonly consumed in sub-Saharan Africa. J. Herb. Med. 2023, 40, 100668. [Google Scholar] [CrossRef]
- Kruger, M.; Sayed, N.; Langenhoven, M.; Holing, F. Composition of South African Foods: Vegetables and Fruit; Research Institute for Nutritional Diseases, South African Medical Research Council: Cape Town, South Africa, 1998; pp. 2–39. [Google Scholar]
- Steyn, N.P.; Olivier, J.; Winter, P.; Burger, S.; Nesamvuni, C. A survey of wild, green, leafy vegetables and their potential in combating micronutrient deficiencies in rural populations: Research in action. S. Afr. J. Sci. 2001, 97, 276–278. [Google Scholar]
- Weinberger, K.; Msuya, J.M. Indigenous Vegetables in Tanzania: Significance and Prospects; AVRDC-World Vegetable Center: Tainan City, Taiwan, 2004; Volume 600. [Google Scholar]
- Odhav, B.; Beekrum, S.; Akula, U.S.; Baijnath, H. Preliminary assessment of nutritional value of traditional leafy vegetables in KwaZulu-Natal, South Africa. J. Food Compost. Anal. 2007, 20, 430–435. [Google Scholar] [CrossRef]
- Managa, G.M.; Nemadodzi, L.E. Comparison of agronomic parameters and nutritional composition on red and green amaranth species grown in open field versus greenhouse environment. Agriculture 2023, 13, 685. [Google Scholar] [CrossRef]
- Managa, G.M.; Nemadodzi, L.E. Response of Black Nightshade to Different Cropping Systems and the Effect on Physiological Parameters and Mineral Composition. Adv. Agric. 2023, 2023, 3238867. [Google Scholar] [CrossRef]
- Van Averbeke, W.; Jansen van Rensburg, W.S.; Slabbert, M.M.; Chabalala, M.P.; Faber, M.; Van Jaarsveld, P.; Van Heerden, I.; Wenhold, F.; Oelofse, A. African leafy vegetables. In Nutritional Value and Water Use of African Leafy Vegetables for Improved Livelihoods: Report to the Water Research Commission & Department of Agriculture; Oelofse, A., Van Averbeke, W., Eds.; Forestry & Fisheries: Tokyo, Japan, 2012; pp. 39–59. [Google Scholar]
- Calumpang, C.L.F.; Saigo, T.; Watanabe, M.; Tohge, T. Cross-species comparison of fruit-metabolomics to elucidate metabolic regulation of fruit polyphenolics among solanaceous crops. Metabolites 2020, 10, 209. [Google Scholar] [CrossRef]
- Slimestad, R.; Verheul, M. Review of flavonoids and other phenolics from fruits of different tomato (Lycopersicon esculentum Mill.) cultivars. J. Sci. Food Agric. 2009, 89, 1255–1270. [Google Scholar] [CrossRef]
- Alseekh, S.; Tohge, T.; Wendenberg, R.; Scossa, F.; Omranian, N.l.; Li, J.; Kleessen, S.; Giavalisco, P.; Pleban, T.; Mueller-Roeber, B.; et al. Identification and Mode of Inheritance of Quantitative Trait Loci for Secondary Metabolite Abundance in Tomato. Plant Cell 2015, 27, 485–512. [Google Scholar] [CrossRef]
- Niño-Medina, G.; Urías-Orona, V.; Muy-Rangel, M.D.; Heredia, J. Structure and content of phenolics in eggplant (Solanum melongena)—A review. S Afr. J. Bot. 2017, 111, 161–169. [Google Scholar] [CrossRef]
- Wahyuni, Y.; Ballester, A.R.; Tikunov, Y.; De Vos, R.C.H.; Pelgrom, K.T.B.; Maharijaya, A.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Metabolomics and molecular marker analysis to explore pepper (Capsicum sp.) biodiversity. Metabolomics 2012, 9, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Lemos, C.; Reimer, J.J.; Wormit, A. Color for Life: Biosynthesis and Distribution of Phenolic Compounds in Pepper (Capsicum annuum). Agriculture 2019, 9, 81. [Google Scholar] [CrossRef]
- Mabuza, N.M. Agronomic, Genetic, and Nutritional Characterization of Nightshade (Solanum spp.) Accessions. Ph.D. Thesis, North-West University, Potchefstroom, South Africa, 2022. [Google Scholar]
- Azeez, J.O.; Van Averbeke, W.; Okorogbona, A.O.M. Differential responses in yield of pumpkin (Cucurbita maxima L.) and nightshade (Solanum retroflexum Dun.) to the application of three animal manures. Bioresour. Technol. 2010, 101, 2499–2505. [Google Scholar] [CrossRef]
- Sangija, F.; Kazosi, M.; Martin, M.; Matemu, A. Trends and constraints in the utilization of African nightshade (Solanum nigrum complex) in Tanzania: A case study of Kilimanjaro and Morogoro regions. Afr. J. Food Agric. Nutr. Dev. 2022, 22, 20623–20645. [Google Scholar] [CrossRef]
- Sangija, F.; Martin, H.; Matemu, A. African nightshades (Solanum nigrum complex): The potential contribution to human nutrition and livelihoods in sub-Saharan Africa. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3284–3318. [Google Scholar] [CrossRef]
- Yamauchi, N.; Watada, A.E. Regulated chlorophyll degradation in spinach leaves during storage. Water 1991, 116, 58–62. [Google Scholar] [CrossRef]
- Limantara, L.; Dettling, M.; Indrawati, R.; Brotosudarmo, T.H.P. Analysis on the chlorophyll content of commercial green leafy vegetables. Procedia Chem. 2015, 14, 225–231. [Google Scholar] [CrossRef]
- Kirigia, D.; Winkelmann, T.; Kasili, R.; Mibus, H. Nutritional composition in African nightshade (Solanum scabrum) influenced by harvesting methods, age and storage conditions. Postharvest Biol. Technol. 2019, 153, 142–151. [Google Scholar] [CrossRef]
- Kamau, E.H. Nutrient Composition, Phytochemical Content and Anti-Microbial Activity of African Nightshade (Solanum nigrum complex) Edible Berries. Ph.D. Thesis, Jomo Kenyatta University of Agriculture and Technology, Juja, Kenya, 2022. [Google Scholar]
- Schauer, N.; Fernie, A.R. Plant metabolomics: Towards biological function and mechanism. Trends Plant Sci. 2006, 11, 508–516. [Google Scholar] [CrossRef]
- Nemadodzi, L.E.; Vervoort, J.; Prinsloo, G. NMR-based metabolomic analysis and microbial composition of soil supporting Burkea africana growth. Metabolites 2020, 10, 402. [Google Scholar] [CrossRef]
- Hong, J.; Yang, L.; Zhang, D.; Shi, J. Plant metabolomics: An indispensable system biology tool for plant science. Int. J. Mol. Sci. 2016, 17, 767. [Google Scholar] [CrossRef]
- Moghe, G.; Kruse, L.H.; Petersen, M.; Scossa, F.; Fernie, A.R.; Gaquerel, E.; d’Auria, J.C. BAHD company: The ever-expanding roles of the BAHD acyltransferase gene family in plants. Annu. Rev. Plant Biol. 2023, 74, 165–194. [Google Scholar] [CrossRef]
- Hall, R.D.; Brouwer, I.D.; Fitzgerald, M.A. Plant metabolomics and its potential application for human nutrition. Plant Physiol. 2008, 132, 162–175. [Google Scholar] [CrossRef]
- Wolfender, J.L.; Rudaz, S.; Hae Choi, Y.; Kyong Kim, H. Plant metabolomics: From holistic data to relevant biomarkers. Curr. Med. Chem. 2013, 20, 1056–1090. [Google Scholar]
- Nkobole, N.; Prinsloo, G. 1H-NMR and LC-MS Based Metabolomics Analysis of Wild and Cultivated Amaranthus spp. Molecules 2021, 26, 795. [Google Scholar] [CrossRef]
- Maina, G.D.; Wanyika, H.; Gohole, L.; Evans, L.C. Influence of plant metabolites on flea beetle infestation in spider plant morphotypes. Univers. J. Plant Sci. 2015, 3, 49–57. [Google Scholar] [CrossRef]
- Guzzetti, L.; Davide, P.; Marynka, U.; Grazia, S.; Matilde, F.; Paola, F.; Nicola, T.; Andrea, F.; Luca, C.; Massimo, L. Assessment of dietary bioactive phenolic compounds and agricultural sustainability of an African leafy vegetable Corchorus olitorius L. Front. Nutr. 2021, 8, 667812. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Yang, X.; Qiu, Y.; Luo, J.; Wu, H.; Liu, X.; Zhao, G.; Hao, G.; Zheng, X.; Li, J. Metabolic and molecular mechanisms underlying the foliar Zn application induced increase of 2-acetyl-1-pyrroline conferring the ‘taro-like’aroma in pumpkin leaves. Front. Plant Sci. 2023, 14, 1127032. [Google Scholar] [CrossRef]
- Kirigia, D. Investigation of Physiological and Molecular Mechanisms for Quality Assurance in Post-Harvest Management of African Nightshade (Solanum scabrum Mill.) and Cowpea (Vigna unguiculata L. Walp). Ph.D. Thesis, Institutionelles Repositorium der Leibniz Universität Hannover, Hannover, Germany, 2018. [Google Scholar]
- Ngwene, B.; Neugart, S.; Baldermann, S.; Ravi, B.; Schreiner, M. Intercropping induces changes in specific secondary metabolite concentration in Ethiopian kale (Brassica carinata) and African nightshade (Solanum scabrum) under controlled conditions. Front. Plant Sci. 2017, 8, 1700. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Said, A.M.; Khalifa, S.A.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.-K.; Verpoorte, R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef]
- Mthimunye, L.M.; Managa, G.M.; Nemadodzi, L.E. The influence of Lablab purpureus growth on nitrogen availability and mineral composition concentration in nutrient-poor savanna soils. Agron 2023, 13, 622. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-based metabolomic analysis of plants. Nat. Protoc. 2010, 5, 536–549. [Google Scholar] [CrossRef]
- Maree, J.; Viljoen, A. Phytochemical distinction between Pelargonium sidoides and Pelargonium reniforme—A quality control perspective. S. Afri. J. Bot. 2012, 82, 83–91. [Google Scholar] [CrossRef]
- Fernie, A.R.; Aharoni, A.; Wilmitzer, L.; Stutt, M.; Tohge, T.; Kopka, J.; Carol, A.J.; Saito, K.; Fraser, P.D.; Deluca, V. Recommendations for reporting metabolite data. Plant Cell. 2011, 23, 2477–2482. [Google Scholar] [CrossRef]
- Mediani, A.; Abas, F.; Khatib, A.; Maulidiani, M.; Shaari, K.; Choi, Y.H.; Lajis, N. 1H-NMR-based metabolomics approach to understanding the drying effects on the phytochemicals in Cosmos caudatus. Food Res. Int. 2012, 49, 763–770. [Google Scholar] [CrossRef]
- Nemadodzi, L.E.; Managa, G.M. 1H NMR-Based Metabolomics Profile of Green and Red Amaranthus Grown in Open Field versus Greenhouse Cultivation System. Metabolites 2023, 14, 21. [Google Scholar] [CrossRef]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Cheng, D.; Shrivastava, S.; Tzur, D.; Gautam, B.; Hassanali, M. DrugBank: A knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008, 36, D901–D906. [Google Scholar] [CrossRef]
- Sreekuar, A.; Poisson, L.M.; Rajendiran, T.M.; Khan, A.P.; Cao, Q.; Yu, J.; Laxman, B.; Mehra, R.; Lonigro, R.J.; Li, Y.; et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 2009, 457, 910–914. [Google Scholar] [CrossRef]
- Kandror, O.; DeLeon, A.; Goldberg, A.L. Trehalose synthesis is induced upon exposure of Escherichia coli to cold and is essential for viability at low temperatures. Proc. Natl. Acad. Sci. USA 2002, 99, 9727–9732. [Google Scholar] [CrossRef]
- Abukutsa-Onyango, M.; Kavagi, P.; Amoke, P.; Habwe, F.O. Iron and protein content of priority African indigenous vegetables in the Lake Victoria basin. J. Agric. Sci. Technol. 2010, 4, 67–69. [Google Scholar]
- Kamga, R.T.; Kouamé, C.; Atangana, A.R.; Chagomoka, T.; Ndango, R. Nutritional evaluation of five African indigenous vegetables. J. Hort. Res. 2013, 21, 99–106. [Google Scholar] [CrossRef]
- Grivetti, L.E.; Ogle, B.M. Value of traditional foods in meeting macro-and micronutrient needs: The wild plant connection. Nutr. Res. Rev. 2017, 13, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Leong, B.J.; Last, R.L. Tip of the trichome: Evolution of acylsugar metabolic diversity in Solanaceae. Curr. Opin. Plant Biol. 2019, 49, 8–16. [Google Scholar] [CrossRef]
- Lou, Y.R.; Anthony, T.M.; Fiesel, P.D.; Arking, R.E.; Christensen, E.M.; Jones, A.D.; Last, R.L. It happened again: Convergent evolution of acylglucose specialized metabolism in black nightshade and wild tomato. Sci. Adv. 2021, 7, eabj8726. [Google Scholar] [CrossRef]
- Fang, C.; Fernie, A.R.; Luo, J. Exploring the diversity of plant metabolism. Trends Plant Sci. 2019, 24, 83–98. [Google Scholar] [CrossRef]
- Yamaki, S. Metabolism and accumulation of sugars translocated to fruit and their regulation. Jpn. Soc. Hortic. Sci. 2010, 79, 1–15. [Google Scholar] [CrossRef]
- Jia, H.; Wang, Y.; Sun, M.; Li, B.; Han, Y.; Zhao, Y.; Li, X.; Ding, N.; Li, C.; Ji, W.; et al. Sucrose functions as a signal involved in the regulation of strawberry fruit development and ripening. New Phytol. 2013, 198, 453–465. [Google Scholar] [CrossRef]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef]
- Araya, T.; Noguchi, K.; Terashima, I. Effects of carbohydrate accumulation on photosynthesis differ between sink and source leaves of Phaseolus vulgaris L. Plant Cell Physiol. 2006, 47, 644–652. [Google Scholar] [CrossRef]
- Fan, X.W.; Sun, J.L.; Cai, Z.; Zhang, F.; Li, Y.Z.; Palta, J.A. MeSWEET15a/b genes play a role in the resistance of cassava (Manihot esculenta crantz) to water and salt stress by modulating sugar distribution. Plant Physiol Biochem. 2023, 194, 394–405. [Google Scholar] [CrossRef]
- Luo, Y.; Li, F.; Wang, G.P.; Yang, X.H.; Wang, W. Exogenously-supplied trehalose protects thylakoid membranes of winter wheat from heat-induced damage. Plant Biol. 2010, 54, 495–501. [Google Scholar] [CrossRef]
- Feng, H.; Acosta-Gamboa, L.; Kruse, L.H.; Tracy, J.D.; Chung, S.H.; Nava Fereira, A.R.; Shakir, S.; Xu, H.; Sunter, G.; Gore, M.A.; et al. Acylsugars protect Nicotiana benthamiana against insect herbivory and desiccation. Plant Mol. Biol. 2021, 109, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Halford, N.G.; Curtis, T.Y.; Muttucumaru, N.; Postles, J.; Mottram, D.S. Sugars in crop plants. Ann. Appl. Biol. 2011, 158, 1–25. [Google Scholar] [CrossRef]
- Koch, K.E. Carbohydrate-modulated gene expression in plants. Annu. Rev. Plant Biol. 1996, 47, 509–540. [Google Scholar] [CrossRef]
- Kaplan, F.; Guy, C.L. β-Amylase induction and the protective role of maltose during temperature shock. Plant Physiol. 2004, 135, 1674–1684. [Google Scholar] [CrossRef]
- Ritte, G.; Raschke, K. Metabolite export of isolated guardcell chloroplasts of Vicia faba. New Phytol. 2003, 159, 195–202. [Google Scholar] [CrossRef]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef]
- Stępniowska, A.; Cieplińska, P.; Fac, W.; Gorska, J. Selected Alkaloids Used in the Cosmetics Industry. J. Cosmet. Sci. 2021, 72, 229–245. [Google Scholar]
- Korekar, G.; Kumar, A.; Ugale, C. Occurrence, fate, persistence and remediation of caffeine: A review. Environ. Sci. Pollut. Res. 2020, 27, 34715–34733. [Google Scholar] [CrossRef]
- Nonthakaew, A.; Matan, N.; Aewsiri, T.; Matan, N. Caffeine in foods and its antimicrobial activity. Int. Food Res. J. 2015, 22, 9. [Google Scholar]
- Kamau, E.H.; Mathara, J.M.; Kenji, G.M. Sugar Content and Physical Characterization of Four Selected African Nightshade (Solanum nigrum) Edible Berries. Eur. J. Agric. Food Sci. 2020, 2. [Google Scholar] [CrossRef]
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. Braz. J. Plant Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef]
- Jiang, Y.; Kusama, K.; Satoh, K.; Takayama, F.; Watanabe, S.; Sakagami, H. Induction of cytotoxicity by chlorogenic acid in human oral tumor cell lines. Phytomedicine 2000, 7, 483–491. [Google Scholar] [CrossRef]
- Yagasaki, K.; Miura, Y.; Okauchi, R.; Furuse, T. Inhibitory effects of chlorogenic acid and its related compounds on the invasion of hepatoma cells in culture. Cytotechnology 2000, 33, 229–235. [Google Scholar] [CrossRef]
- Belkaid, A.; Currie, J.C.; Desgagnés, J.; Annabi, B. The chemopreventive properties of chlorogenic acid reveal a potential new role for the microsomal glucose-6-phosphate translocase in brain tumor progression. Cancer Cell Int. 2006, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Zhou, C.Y.; Qiu, C.H.; Lu, X.M.; Wang, Y.T. Chlorogenic acid induced apoptosis and inhibition of proliferation in human acute promyelocytic leukemia HL-60 cells. Mol. Med. Rep. 2013, 8, 1106–1110. [Google Scholar] [CrossRef]
- Noratto, G.; Porter, W.; Byrne, D.; Cisneros-Zevallos, L. Identifying peach and plum polyphenols with chemopreventive potential against estrogen-independent breast cancer cells. J. Agric. Food Chem. 2009, 57, 5219–5226. [Google Scholar] [CrossRef]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of chlorogenic acid on regulating glucose and lipids metabolism: A review. Evid.-Based Complement. Altern. Med. 2013, 2013, 801457. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Ballevre, O.; Luo, H.; Zhang, W. Antihypertensive effects and mechanisms of chlorogenic acids. Hypertens Res. 2012, 35, 370–374. [Google Scholar] [CrossRef]
- Lee, K.; Lee, J.-S.; Jang, H.-J.; Kim, S.-M.; Chang, M.S.; Park, S.H.; Kim, K.S.; Bae, J.; Park, J.-W.; Lee, B.; et al. Chlorogenic acid ameliorates brain damage and edema by inhibiting matrix metalloproteinase-2 and 9 in a rat model of focal cerebral ischemia. Eur. J. Pharmacol. 2012, 689, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Aseervatham, G.S.B.; Suryakala, U.D.; Sundaram, S.; Bose, P.C.; Sivasudha, T. Expression pattern of NMDA receptors reveals antiepileptic potential of apigenin 8-C-glucoside and chlorogenic acid in pilocarpine induced epileptic mice. Biomed Pharmacother. 2016, 82, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Gahalain, N. Reversal of reserpine-induced orofacial dyskinesia by chlorogenic acid in rats. Pharmacologia 2016, 7, 272–277. [Google Scholar] [CrossRef]
- Stewart, A.J.; Bozonnet, S.; Mullen, W.; Jenkins, G.I.; Lean, M.E.J.; Crozier, A. Occurrence of flavonols in tomatoes and tomato-based products. J. Agric. Food Chem. 2000, 48, 2663–2669. [Google Scholar] [CrossRef]
- Muir, S.R.; Collins, G.J.; Robinson, S.; Hughes, S.; Bovy, A.; De Vos, C.R.; Van Tunen, A.J.; Verhoeyen, M.E. Overexpression of petunia chalcone isomerase in tomato results in fruit containing increased levels of flavonols. Nat. Biotechnol. 2001, 19, 470–474. [Google Scholar] [CrossRef]
- Bovy, A.; De Vos, R.; Kemper, M.; Schijlen, E.; Pertejo, M.A.; Muir, S.; Collins, G.; Robinson, S.; Verhoeyen, M.; Hughes, S.; et al. High-flavonol tomatoes resulting from the heterologous expression of the maize transcription factor genes LC and C1. Plant Cell 2002, 14, 2509–2526. [Google Scholar] [CrossRef]
- Schijlen, E.; De Vos, C.R.; Martens, S.; Jonker, H.H.; Rosin, F.M.; Molthoff, J.W.; Tikunov, Y.M.; Angenent, G.C.; Van Tunen, A.J.; Bovy, A.G. RNA interference silencing of chalcone synthase, the first step in the flavonoid biosynthesis pathway, leads to parthenocarpic tomato fruits. Plant Physiol. 2007, 144, 1520–1530. [Google Scholar] [CrossRef]
- Mintz-Oron, S.; Mandel, T.; Rogachev, I.; Feldberg, L.; Lotan, O.; Yativ, M.; Wang, Z.; Jetter, R.; Venger, I.; Adato, A.; et al. Gene Expression and Metabolism in Tomato Fruit Surface Tissues. Plant Physiol. 2008, 147, 823–851. [Google Scholar] [CrossRef]
- Domigan, N.M.; Charlton, T.S.; Duncan, M.W.; Winterbourn, C.C.; Kettle, A.J. Chlorination of tyrosyl residues in peptides by myeloperoxidase and human neutrophils. J. Biol. Chem. 1995, 270, 16542–16548. [Google Scholar] [CrossRef]
- Kettle, A.J. Neutrophils convert tyrosyl residues in albumin to chlorotyrosine. FEBS Lett. 1996, 379, 103–106. [Google Scholar] [CrossRef]
- Bao Loan, H.N.B.; Kerkaert, B.; Cucu, T.; Mestdagh, F.; De Meulenaer, B. 3-chlorotyrosine formation versus other molecular changes induced by hypochlorous acid in proteins: A study using dairy proteins as a model. Lebenson. Wiss. Technol. 2016, 68, 145–152. [Google Scholar] [CrossRef]
- Stober, Q.J.; Dinnel, P.A.; Hurlburt, E.F.; DiJulio, D.H. Acute toxicity and behavioral responses of coho salmon (Oncorhynchus kisutch) and shiner perch (Cymatogaster aggregata) to chlorine in heated seawater. Water Res. 1980, 14, 347–354. [Google Scholar] [CrossRef]
- Vandekinderen, I.; Van Camp, J.; Devlieghere, F.; Veramme, K.; Denon, Q.; Ragaert, P.; De Meulenaer, B. Effect of decontamination agents on the microbial population, sensorial quality, and nutrient content of grated carrots (Daucus carota L.). J. Agric. Food Chem. 2008, 56, 5723–5731. [Google Scholar] [CrossRef]
- World Health Organization. Joint FAO/WHO Expert Meeting to Review Toxicological and Health Aspects of Bisphenol A: Final Report, Including Report of Stakeholder Meeting on Bisphenol A, 1–5 November 2010, Ottawa, Canada; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Bao Loan, H.N.B.; Devlieghere, F.; Van Hoeke, C.; De Meulenaer, B. 3-Chlorotyrosine formation in gilthead seabream (Sparus aurata) and European plaice (Pleuronectes platessa) fillets treated with sodium hypochlorite. Food Res. Int. 2015, 69, 164–169. [Google Scholar] [CrossRef]
- Bergt, C.; Pennathur, S.; Fu, X.; Byun, J.; O’Brien, K.; McDonald, T.O.; Singh, P.; Anantharamaiah, G.M.; Chait, A.; Brunzell, J.; et al. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc. Natl. Acad. Sci. USA 2004, 101, 13032–13037. [Google Scholar] [CrossRef]
- Buss, I.H.; Senthilmohan, R.; Darlow, B.A.; Mogridge, N.; Kettle, A.J.; Winterbourn, C.C. 3-Chlorotyrosine as a marker of protein damage by myeloperoxidase in tracheal aspirates from preterm infants: Association with adverse respiratory outcome. Pediatr. Res. 2003, 53, 455–462. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef]
- Davies, M.J.; Fu, S.; Wang, H.; Dean, R.T. Stable markers of oxidant damage to proteins and their application in the study of human disease. Free Radic. Biol. Med. 1999, 27, 1151–1163. [Google Scholar] [CrossRef]
- Robaszkiewicz, A.; Bartosz, G.; Soszynski, M. Detection of 3-chlorinated tyrosine residues in human cells by flow cytometry. J. Immunol. Methods 2011, 369, 141–145. [Google Scholar] [CrossRef]
- Tochtrop, G.P.; DeKoster, G.T.; Covey, D.F.; Cistola, D.P. A single hydroxyl group governs ligand site selectivity in human ileal bile acid binding protein. J. Am. Chem. Soc. 2004, 126, 1102–11029. [Google Scholar] [CrossRef]
- Mukidjam, E.; Barnes, S.; Elgavish, G.A. NMR studies of the binding of sodium and calcium ions to the bile salts glycocholate and taurocholate in dilute solution, as probed by the paramagnetic lanthanide dysprosium. J. Am. Chem. Soc. 1986, 108, 7082–7089. [Google Scholar] [CrossRef]
- Lücke, C.; Zhang, F.; Hamilton, J.A.; Sacchettini, J.C.; Rüterjans, H. Solution structure of ileal lipid binding protein in complex with glycocholate. Eur. J. Biochem. 2000, 267, 2929–2938. [Google Scholar] [CrossRef]
- Favretto, F.; Assfalg, M.; Gallo, M.; Cicero, D.O.; D’Onofrio, M.; Molinari, H. Ligand binding promiscuity of human liver fatty acid binding protein: Structural and dynamic insights from an interaction study with glycocholate and oleate. ChemBioChem 2013, 14, 1807–1819. [Google Scholar] [CrossRef]
- Hermens, W.A.J.J.; Hooymans, P.M.; Verhoef, J.C.; Merkus, F.W.H.M. Effects of absorption enhancers on human nasal tissue ciliary movement in vitro. Pharm. Res. 1990, 7, 144–146. [Google Scholar] [CrossRef]
- Merkus, F.W.H.M.; Schipper, N.G.M.; Hermens, W.A.J.J.; Romeijn, S.G.; Verhoef, J.C. Absorption enhancers in nasal drug delivery: Efficacy and safety. J. Control. Release 1993, 24, 201–208. [Google Scholar] [CrossRef]
- Morimoto, K.; Uehara, Y.; Iwanaga, K.; Kakemi, M.; Ohashi, Y.; Tanaka, A.; Nakai, Y. Influence of absorption enhancers (bile salts) and the preservative (Benzalkonium chloride) on mucociliary function and permeation barrier function in rabbit tracheas. Eur. J. Pharm. Sci. 1998, 6, 225–230. [Google Scholar] [CrossRef]
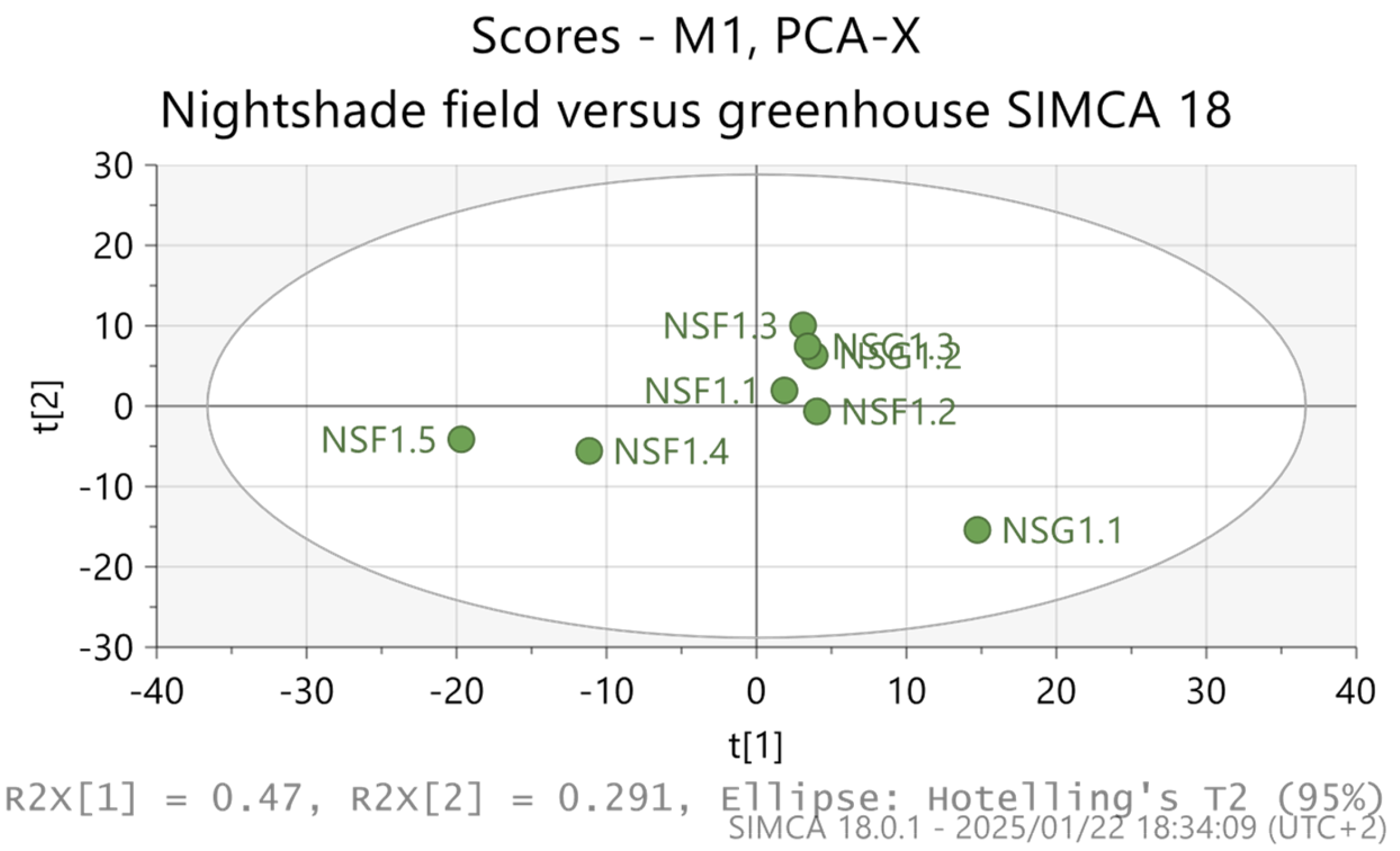



| Component | R2X (cum) | Q2 (cum) |
|---|---|---|
| 1 | 0.47 | 0.641 |
| 2 | 0.291 | 0.323 |
| Component | R2X (cum) | Q2 (cum) |
|---|---|---|
| 1 | 0.252 | 0.977 |
| 2 | 0.683 |
| Metabolites | Structural Fragment (Methyl Group) | NMR Region | Chenomx 9.0 | Human Metabolome Data Base |
|---|---|---|---|---|
| Alanine (1) | (CH3) | 1.45 1.46 | 1.5 3.8 | 1.47 3.77 |
| Glycocholate (2) | C (4); C (14) | 1.0 1.5 1.6 1.7 1.8 | 0.7 0.9 1.0 1.3 1.6 1.7 1.8 1.9 2.0 2.1 2.2 2.4 3.5 3.7 3.9 4.0 7.9 | N/A |
| Choline (3) | S-adenosyl-L-methionine | 3.2 | 3.2 3.5 4.1 | N/A |
| Betaine (4) | (CH3)3N+CH2COO | 3.3 3.9 | 3.3 3.9 | 3.25 3.89 [43] |
| Caffeine (5) | Methyl isocyanate CH3NCO | 3.3 3.5 3.9 | 3.3 3.5 3.9 7.9 | 3.22 3.34 4.04 8.49 [44] |
| Galactose (6) | 3-O-methylated or 4-O-methylated | 4.6 | 3.5 3.7 3.8 3.9 4.0 4.1 4.6 5.3 | N/A |
| Trehalose (7) | C16–19 C21–25 C24–28 | 5.19 | 3.4 3.6 3.8 3.9 5.2 | 3.42 3.49 3.63 3.75 3.81 3.84 3.85 4.12 [45] |
| Maltose (8) | C1 C4 | 5.38 | 3.3 3.4 3.6 3.7 3.8 3.9 4.0 4.6 5.2 5.4 | N/A |
| Sucrose (9) | 6-&6- | 5.4 | 3.4 3.6 3.7 3.8 3.9 4.0 4.2 5.4 | N/A |
| Chlorogenate (10) | [M-H] | 7.0 | 2.0 2.1 2.2 3.9 4.3 5.3 6.9 7.1 7.2 7.6 | N/A |
| 3-Chlorotyrosine (11) | 7.0 | 3.0 3.2 3.9 7.0 7.1 7.3 | 2.82 2.83 2.85 2.86 3.06 3.07 3.09 3.10 3.97 3.98 3.99 6.82 6.84 7.16 7.18 7.62 |
| Metabolites | Structural Fragment (Methyl Group) | NMR Region | Chenomx 9.0 | Human Metabolome Data Base |
|---|---|---|---|---|
| Alanine (1) | (CH3) | 1.45 1.46 | 1.5 3.8 | 1.47 3.77 |
| Glycocholate (2) | C (4); C (14) | 1.0 1.5 1.6 1.7 1.8 | 0.7 0.9 1.0 1.3 1.6 1.7 1.8 1.9 2.0 2.1 2.2 2.4 3.5 3.7 3.9 4.0 7.9 | N/A |
| Choline (3) | S-adenosyl-L-methionine | 3.2 | 3.2 3.5 4.1 | N/A |
| Betaine (4) | (CH3)3N+CH2COO | 3.3 3.9 | 3.3 3.9 | 3.25 3.89 [43] |
| Caffeine (5) | Methyl isocyanate CH3NCO | 3.3 3.5 3.9 | 3.3 3.5 3.9 7.9 | 3.22 3.34 4.04 8.49 [44] |
| Galactose (6) | 3-O-methylated or 4-O-methylated | 4.6 | 3.5 3.7 3.8 3.9 4.0 4.1 4.6 5.3 | N/A |
| Trehalose (7) | C16–19 C21–25 C24–28 | 5.19 | 3.4 3.6 3.8 3.9 5.2 | 3.42 3.49 3.63 3.75 3.81 3.84 3.85 4.12 [45] |
| Maltose (8) | C1 C4 | 5.38 | 3.3 3.4 3.6 3.7 3.8 3.9 4.0 4.6 5.2 5.4 | N/A |
| Sucrose (9) | 6-&6- | 5.4 | 3.4 3.6 3.7 3.8 3.9 4.0 4.2 5.4 | N/A |
| Chlorogenate (10) | [M-H] | 7.0 | 2.0 2.1 2.2 3.9 4.3 5.3 6.9 7.1 7.2 7.6 | N/A |
| 3- Chlorotyrosine (11) | 7.0 | 3.0 3.2 3.9 7.0 7.1 7.3 | 2.82 2.83 2.85 2.86 3.06 3.07 3.09 3.10 3.97 3.98 3.99 6.82 6.84 7.16 7.18 7.62 | |
| Glucose (12) | 3-O-methyl-D-glucose | 3.4 4.0 | 3.2 3.4 3.5 3.7 3.8 3.9 4.7 5.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemadodzi, L.E.; Managa, G.M.; Nemukondeni, N. 1H NMR-Based Analysis to Determine the Metabolomics Profile of Solanum nigrum L. (Black Nightshade) Grown in Greenhouse Versus Open-Field Conditions. Metabolites 2025, 15, 344. https://doi.org/10.3390/metabo15050344
Nemadodzi LE, Managa GM, Nemukondeni N. 1H NMR-Based Analysis to Determine the Metabolomics Profile of Solanum nigrum L. (Black Nightshade) Grown in Greenhouse Versus Open-Field Conditions. Metabolites. 2025; 15(5):344. https://doi.org/10.3390/metabo15050344
Chicago/Turabian StyleNemadodzi, Lufuno Ethel, Gudani Millicent Managa, and Ndivho Nemukondeni. 2025. "1H NMR-Based Analysis to Determine the Metabolomics Profile of Solanum nigrum L. (Black Nightshade) Grown in Greenhouse Versus Open-Field Conditions" Metabolites 15, no. 5: 344. https://doi.org/10.3390/metabo15050344
APA StyleNemadodzi, L. E., Managa, G. M., & Nemukondeni, N. (2025). 1H NMR-Based Analysis to Determine the Metabolomics Profile of Solanum nigrum L. (Black Nightshade) Grown in Greenhouse Versus Open-Field Conditions. Metabolites, 15(5), 344. https://doi.org/10.3390/metabo15050344








