Primary Metabolic Variations in Maize Plants Affected by Different Levels of Nitrogen Supply
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth and Total Nitrogen Content Analysis
2.2. Amino Acid, Organic Acid, and Carbohydrate Content Measurement
2.3. Data Analysis
3. Results and Discussion
3.1. Growth Parameters and Total Nitrogen Content of Maize Seedlings Under Different Nitrogen Stress Levels
3.2. GC-MS Metabolite Changes in Maize Seedlings Under Nitrogen Deficiencies
3.3. Effect of Nitrogen Deficiencies on Primary Metabolite Changes
4. Conclusions and Future Perspective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| N | Nitrogen |
| GC-MS | Gas chromatography–mass spectrometry |
| LL | Lower leaf (leaves) |
| MB | Mibaek#2 |
| TCA | Tricarboxylic acid |
| TOR | Target of rapamycin |
| TYC | Taeyangchal |
| UL | Upper leaf (leaves) |
References
- Bhatla, S.C.; Lal, M.A. Plant Physiology, Development and Metabolism; Springer Nature: Singapore, 2018; ISBN 978-981-13-2022-4. [Google Scholar]
- Asibi, A.E.; Chai, Q.; Coulter, J.A. Mechanisms of Nitrogen Use in Maize. Agronomy 2019, 9, 775. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of Macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Amsterdam, The Netherlands, 2012; pp. 135–189. [Google Scholar] [CrossRef]
- Kusano, M.; Fukushima, A.; Redestig, H.; Saito, K. Metabolomic Approaches toward Understanding Nitrogen Metabolism in Plants. J. Exp. Bot. 2011, 62, 1439–1453. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Vyn, T.J. Physiological Perspectives of Changes over Time in Maize Yield Dependency on Nitrogen Uptake and Associated Nitrogen Efficiencies: A Review. Field Crops Res. 2012, 133, 48–67. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen Uptake, Assimilation and Remobilization in Plants: Challenges for Sustainable and Productive Agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Lu, Y.; Wang, Y.; Nie, C.; Xu, M.; Wang, L.; Bai, Y. Analysis of Metabolic Differences in Maize in Different Growth Stages under Nitrogen Stress Based on UPLC-QTOF-MS. Front. Plant Sci. 2023, 14, 1141232. [Google Scholar] [CrossRef]
- Torres-Rodríguez, J.V.; Salazar-Vidal, M.N.; Chávez Montes, R.A.; Massange-Sánchez, J.A.; Gillmor, C.S.; Sawers, R.J.H. Low Nitrogen Availability Inhibits the Phosphorus Starvation Response in Maize (Zea mays ssp. mays L.). BMC Plant Biol. 2021, 21, 259. [Google Scholar] [CrossRef]
- Schlüter, U.; Mascher, M.; Colmsee, C.; Scholz, U.; Bräutigam, A.; Fahnenstich, H.; Sonnewald, U. Maize Source Leaf Adaptation to Nitrogen Deficiency Affects Not Only Nitrogen and Carbon Metabolism but Also Control of Phosphate Homeostasis. Plant Physiol. 2012, 160, 1384–1406. [Google Scholar] [CrossRef]
- Plett, D.; Holtham, L.; Baumann, U.; Kalashyan, E.; Francis, K.; Enju, A.; Toubia, J.; Roessner, U.; Bacic, A.; Rafalski, A.; et al. Nitrogen Assimilation System in Maize Is Regulated by Developmental and Tissue-Specific Mechanisms. Plant Mol. Biol. 2016, 92, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Garnett, T.; Conn, V.; Plett, D.; Conn, S.; Zanghellini, J.; Mackenzie, N.; Enju, A.; Francis, K.; Holtham, L.; Roessner, U.; et al. The Response of the Maize Nitrate Transport System to Nitrogen Demand and Supply across the Lifecycle. New Phytol. 2013, 198, 82–94. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Vyn, T.J. Grain Nitrogen Source Changes over Time in Maize: A Review. Crop Sci. 2013, 53, 366–377. [Google Scholar] [CrossRef]
- Xue, Y.; Zhu, S.; Schultze-Kraft, R.; Liu, G.; Chen, Z. Dissection of Crop Metabolome Responses to Nitrogen, Phosphorus, Potassium, and Other Nutrient Deficiencies. Int. J. Mol. Sci. 2022, 23, 9079. [Google Scholar] [CrossRef]
- Heinemann, B.; Hildebrandt, T.M. The Role of Amino Acid Metabolism in Signaling and Metabolic Adaptation to Stress-Induced Energy Deficiency in Plants. J. Exp. Bot. 2021, 72, 4634–4645. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, X.; Zhao, X.; Ding, W.; Wang, Y.; Xiong, Y. Diverse Nitrogen Signals Activate Convergent ROP2-TOR Signaling in Arabidopsis. Dev. Cell 2021, 56, 1283–1295.e5. [Google Scholar] [CrossRef]
- Ganie, A.H.; Ahmad, A.; Yousuf, P.Y.; Pandey, R.; Ahmad, S.; Aref, I.M.; Iqbal, M. Nitrogen-Regulated Changes in Total Amino Acid Profile of Maize Genotypes Having Contrasting Response to Nitrogen Deficit. Protoplasma 2017, 254, 2143–2153. [Google Scholar] [CrossRef] [PubMed]
- Amiour, N.; Imbaud, S.; Clément, G.; Agier, N.; Zivy, M.; Valot, B.; Balliau, T.; Armengaud, P.; Quilleré, I.; Cañas, R.; et al. The Use of Metabolomics Integrated with Transcriptomic and Proteomic Studies for Identifying Key Steps Involved in the Control of Nitrogen Metabolism in Crops Such as Maize. J. Exp. Bot. 2012, 63, 5017–5033. [Google Scholar] [CrossRef] [PubMed]
- Hirel, B.; Le Gouis, J.; Ney, B.; Gallais, A. The Challenge of Improving Nitrogen Use Efficiency in Crop Plants: Towards a More Central Role for Genetic Variability and Quantitative Genetics within Integrated Approaches. J. Exp. Bot. 2007, 58, 2369–2387. [Google Scholar] [CrossRef]
- Huang, W.-T.; Zheng, Z.-C.; Hua, D.; Chen, X.-F.; Zhang, J.; Chen, H.-H.; Ye, X.; Guo, J.-X.; Yang, L.-T.; Chen, L.-S. Adaptive Responses of Carbon and Nitrogen Metabolisms to Nitrogen-Deficiency in Citrus Sinensis Seedlings. BMC Plant Biol. 2022, 22, 370. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Chen, Y. The Physiological Response of Photosynthesis to Nitrogen Deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef]
- Guidi, L.; Lorefice, G.; Pardossi, A.; Malorgio, F.; Tognoni, F.; Soldatini, G.F. Growth and Photosynthesis of Lycopersicon esculentum (L.) Plants as Affected by Nitrogen Deficiency. Biol. Plant 1997, 39, 235–244. [Google Scholar] [CrossRef]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. SUGAR SENSING AND SIGNALING IN PLANTS: Conserved and Novel Mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef]
- Ning, P.; Peng, Y.; Fritschi, F.B. Carbohydrate Dynamics in Maize Leaves and Developing Ears in Response to Nitrogen Application. Agronomy 2018, 8, 302. [Google Scholar] [CrossRef]
- Ning, P.; Yang, L.; Li, C.; Fritschi, F.B. Post-Silking Carbon Partitioning under Nitrogen Deficiency Revealed Sink Limitation of Grain Yield in Maize. J. Exp. Bot. 2018, 69, 1707–1719. [Google Scholar] [CrossRef]
- Lu, Y.; Song, G.; Wang, Y.; Wang, L.; Xu, M.; Zhou, L.; Wang, L. Combining Nitrogen Effects and Metabolomics to Reveal the Response Mechanisms to Nitrogen Stress and the Potential for Nitrogen Reduction in Maize. J. Integr. Agric. 2023, 22, 2660–2672. [Google Scholar] [CrossRef]
- Sabermanesh, K.; Holtham, L.R.; George, J.; Roessner, U.; Boughton, B.A.; Heuer, S.; Tester, M.; Plett, D.C.; Garnett, T.P. Transition from a Maternal to External Nitrogen Source in Maize Seedlings. J. Integr. Plant Biol. 2017, 59, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Kim, M.; Geem, K.R.; Sung, J. Improving Nutrient Use Efficiency of Rice Under Alternative Wetting and Drying Irrigation Combined with Slow-Release Nitrogen Fertilization. Plants 2025, 14, 1530. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.T.; Yang, S.-O.; Choi, W.-S. Changes in Sucrose and the VcADR Gene Induced Sugar Accumulation in Blueberries during Ripening and after Chitosan Treatment. J. Berry Res. 2022, 12, 401–414. [Google Scholar] [CrossRef]
- Jiang, L.; Han, L.; Han, X.; Zhan, X.; Zuo, R.; Wu, Z.; Yuan, C. Effects of nitrogen on growth, root morphological traits, nitrogen uptake and utilization efficiency of maize seedlings. J. Plant Nutr. Fertil. 2011, 17, 247–253. [Google Scholar] [CrossRef]
- Binder, D.L.; Sander, D.H.; Walters, D.T. Maize Response to Time of Nitrogen Application as Affected by Level of Nitrogen Deficiency. Agron. J. 2000, 92, 1228–1236. [Google Scholar] [CrossRef]
- Zhao, H.; Ni, S.; Cai, S.; Zhang, G. Comprehensive Dissection of Primary Metabolites in Response to Diverse Abiotic Stress in Barley at Seedling Stage. Plant Physiol. Biochem. 2021, 161, 54–64. [Google Scholar] [CrossRef]
- Sung, J.; Lee, S.; Lee, Y.; Ha, S.; Song, B.; Kim, T.; Waters, B.M.; Krishnan, H.B. Metabolomic Profiling from Leaves and Roots of Tomato (Solanum lycopersicum L.) Plants Grown under Nitrogen, Phosphorus or Potassium-Deficient Condition. Plant Sci. 2015, 241, 55–64. [Google Scholar] [CrossRef]
- Sung, J.; Yun, H.; Back, S.; Fernie, A.R.; Kim, Y.X.; Lee, Y.; Lee, S.; Lee, D.; Kim, J. Changes in Mineral Nutrient Concentrations and C-N Metabolism in Cabbage Shoots and Roots Following Macronutrient Deficiency. J. Plant Nutr. Soil. Sci. 2018, 181, 777–786. [Google Scholar] [CrossRef]
- Miller, A.J.; Fan, X.; Shen, Q.; Smith, S.J. Amino Acids and Nitrate as Signals for the Regulation of Nitrogen Acquisition. J. Exp. Bot. 2007, 59, 111–119. [Google Scholar] [CrossRef]
- Walker, M.C.; van der Donk, W.A. The Many Roles of Glutamate in Metabolism. J. Ind. Microbiol. Biotechnol. 2016, 43, 419–430. [Google Scholar] [CrossRef]
- Signorelli, S.; Tarkowski, Ł.P.; O’Leary, B.; Tabares-da Rosa, S.; Borsani, O.; Monza, J. GABA and Proline Metabolism in Response to Stress. In Hormones and Plant Response; Springer: Berlin/Heidelberg, Germany, 2021; pp. 291–314. [Google Scholar]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Xin, W.; Zhang, L.; Zhang, W.; Gao, J.; Yi, J.; Zhen, X.; Li, Z.; Zhao, Y.; Peng, C.; Zhao, C. An Integrated Analysis of the Rice Transcriptome and Metabolome Reveals Differential Regulation of Carbon and Nitrogen Metabolism in Response to Nitrogen Availability. Int. J. Mol. Sci. 2019, 20, 2349. [Google Scholar] [CrossRef]
- Nukarinen, E.; Nägele, T.; Pedrotti, L.; Wurzinger, B.; Mair, A.; Landgraf, R.; Börnke, F.; Hanson, J.; Teige, M.; Baena-Gonzalez, E.; et al. Quantitative Phosphoproteomics Reveals the Role of the AMPK Plant Ortholog SnRK1 as a Metabolic Master Regulator under Energy Deprivation. Sci. Rep. 2016, 6, 31697. [Google Scholar] [CrossRef]
- Simons, M.; Saha, R.; Guillard, L.; Clément, G.; Armengaud, P.; Cañas, R.; Maranas, C.D.; Lea, P.J.; Hirel, B. Nitrogen-Use Efficiency in Maize (Zea mays L.): From ‘Omics’ Studies to Metabolic Modelling. J. Exp. Bot. 2014, 65, 5657–5671. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Han, G.; Meng, Z.; Lin, L.; Sui, N. Roles of Malic Enzymes in Plant Development and Stress Responses. Plant Signal Behav. 2019, 14, e1644596. [Google Scholar] [CrossRef]
- Lillo, C.; Lea, U.S.; Ruoff, P. Nutrient Depletion as a Key Factor for Manipulating Gene Expression and Product Formation in Different Branches of the Flavonoid Pathway. Plant Cell Environ. 2008, 31, 587–601. [Google Scholar] [CrossRef]
- Kanojia, A.; Shrestha, D.K.; Dijkwel, P.P. Primary Metabolic Processes as Drivers of Leaf Ageing. Cell Mol. Life Sci. 2021, 78, 6351. [Google Scholar] [CrossRef] [PubMed]
- Coneva, V.; Guevara, D.; Rothstein, S.J.; Colasanti, J. Transcript and Metabolite Signature of Maize Source Leaves Suggests a Link between Transitory Starch to Sucrose Balance and the Autonomous Floral Transition. J. Exp. Bot. 2012, 63, 5079. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-M.; Lo, S.-F.; Ho, T.-H.D. Source–Sink Communication: Regulated by Hormone, Nutrient, and Stress Cross-Signaling. Trends Plant Sci. 2015, 20, 844–857. [Google Scholar] [CrossRef] [PubMed]
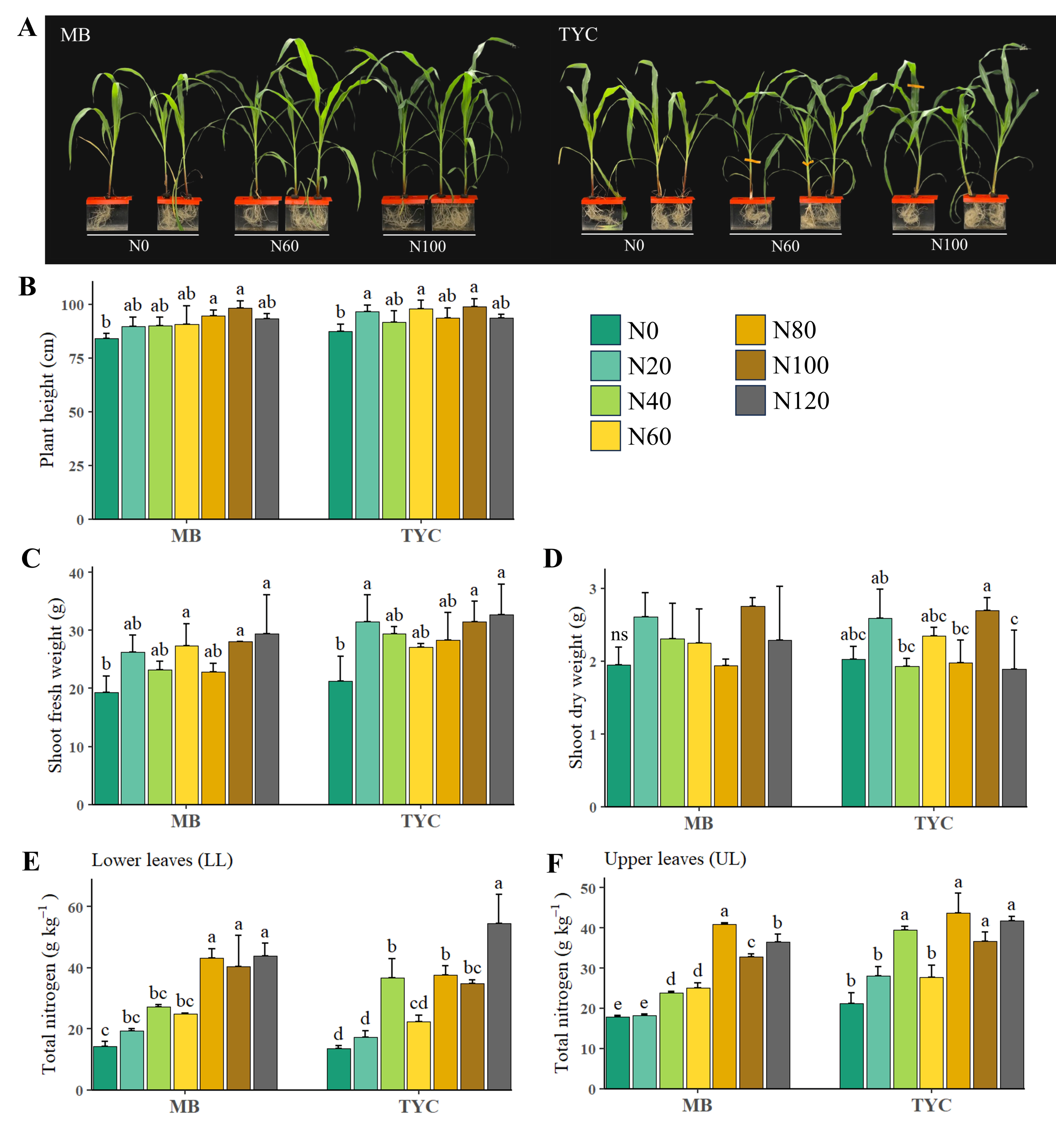
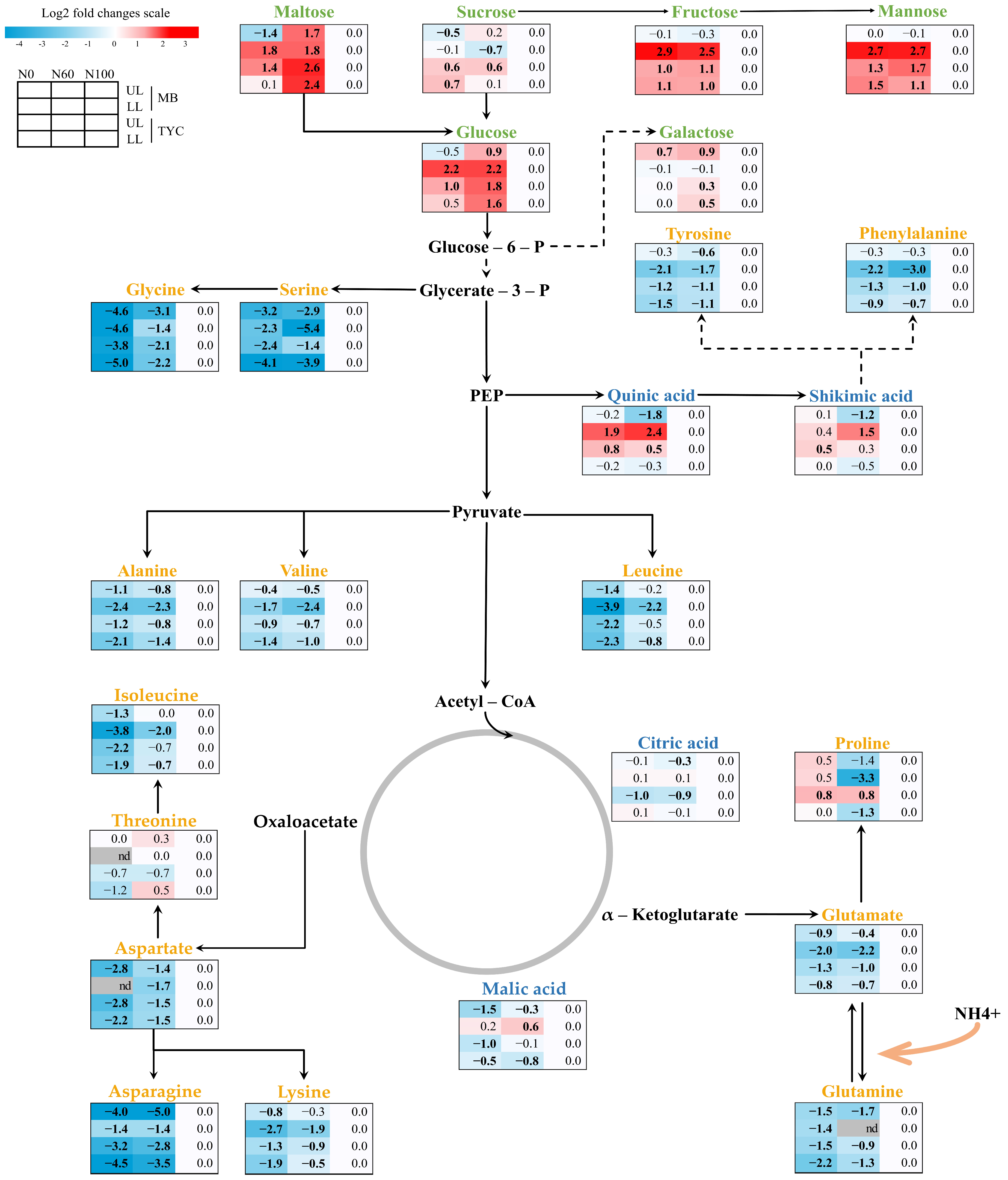
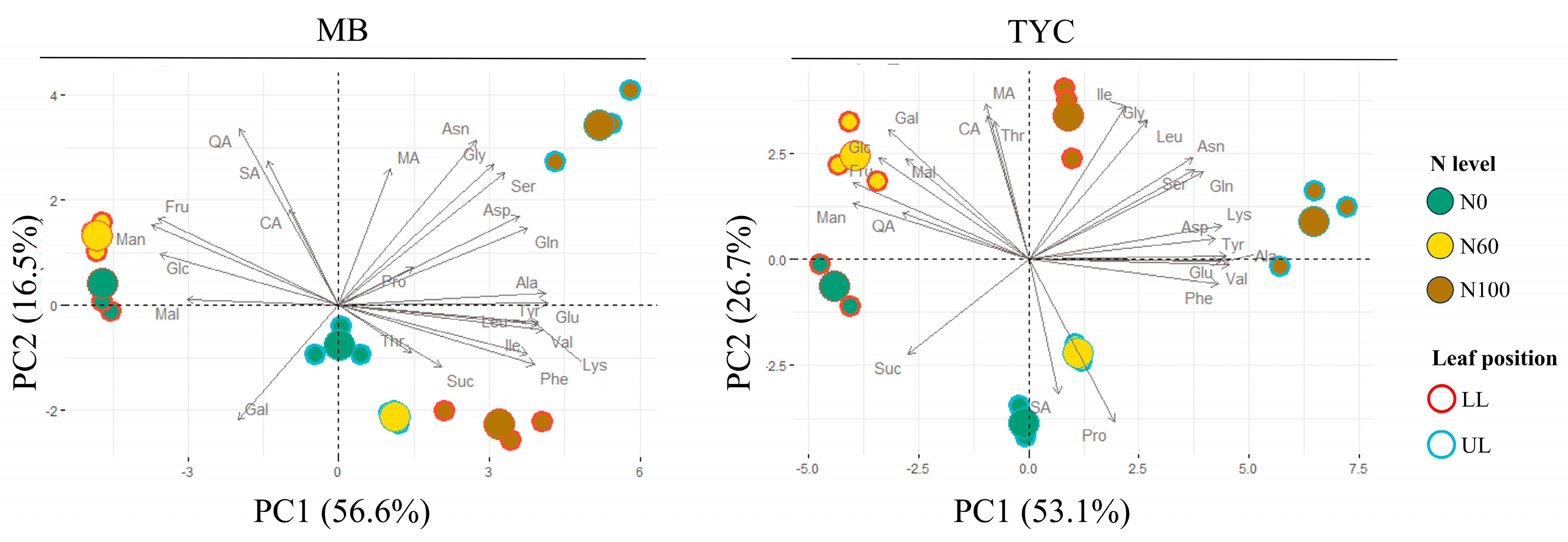
| Components | Unit | N0 | N20 | N40 | N60 | N80 | N100 | N120 |
|---|---|---|---|---|---|---|---|---|
| Ca(No3)2 | mM | 0 | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 |
| KNO3 | mM | 0 | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 |
| NH4NO3 | mM | 0 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 |
| CaCl2 | mM | 2.5 | 2 | 1.5 | 1 | 0.5 | 0 | 0 |
| KCl | mM | 2.5 | 2 | 1.5 | 1 | 0.5 | 0 | 0 |
| N concentration | mM | 0 | 1.7 | 3.4 | 5.1 | 6.8 | 8.5 | 10.2 |
| ANOVA | PH | SFW | SDW | TN |
|---|---|---|---|---|
| Cultivar | 0.138 (ns) | 0.005 (*) | 0.624 (ns) | 0.0000485 (*) |
| Treatment | 0.00021 (*) | 3.80 × 10−5 (*) | 0.000319 (*) | 6.07 × 10−29 (*) |
| Cultivar × Treatment | 0.537 (ns) | 0.975 (ns) | 0.687 (ns) | 2.00 × 10−5 (*) |
| Leaf group | 0.729 (ns) | |||
| Cultivar × Leaf group | 4.77 × 10−4 (*) | |||
| Treatment × Leaf group | 8.10 × 10−6 (*) | |||
| Cultivar × Treatment × Leaf group | 0.101 (ns) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.N.P.; Serwaa, R.N.; Sung, J. Primary Metabolic Variations in Maize Plants Affected by Different Levels of Nitrogen Supply. Metabolites 2025, 15, 519. https://doi.org/10.3390/metabo15080519
Nguyen TNP, Serwaa RN, Sung J. Primary Metabolic Variations in Maize Plants Affected by Different Levels of Nitrogen Supply. Metabolites. 2025; 15(8):519. https://doi.org/10.3390/metabo15080519
Chicago/Turabian StyleNguyen, The Ngoc Phuong, Rose Nimoh Serwaa, and Jwakyung Sung. 2025. "Primary Metabolic Variations in Maize Plants Affected by Different Levels of Nitrogen Supply" Metabolites 15, no. 8: 519. https://doi.org/10.3390/metabo15080519
APA StyleNguyen, T. N. P., Serwaa, R. N., & Sung, J. (2025). Primary Metabolic Variations in Maize Plants Affected by Different Levels of Nitrogen Supply. Metabolites, 15(8), 519. https://doi.org/10.3390/metabo15080519







