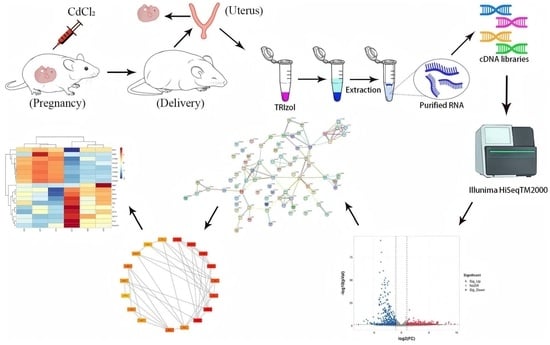
Cadmium Exposure Disrupts Uterine Energy Metabolism and Coagulation Homeostasis During Labor in Institute of Cancer Research Mice: Insights from Transcriptomic Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Animal Treatment
2.4. Transcriptomics Analysis
2.5. Gene Ontology and Pathway Enrichment
2.6. Protein–Protein Interaction Network and Identification of Hub Genes
3. Results
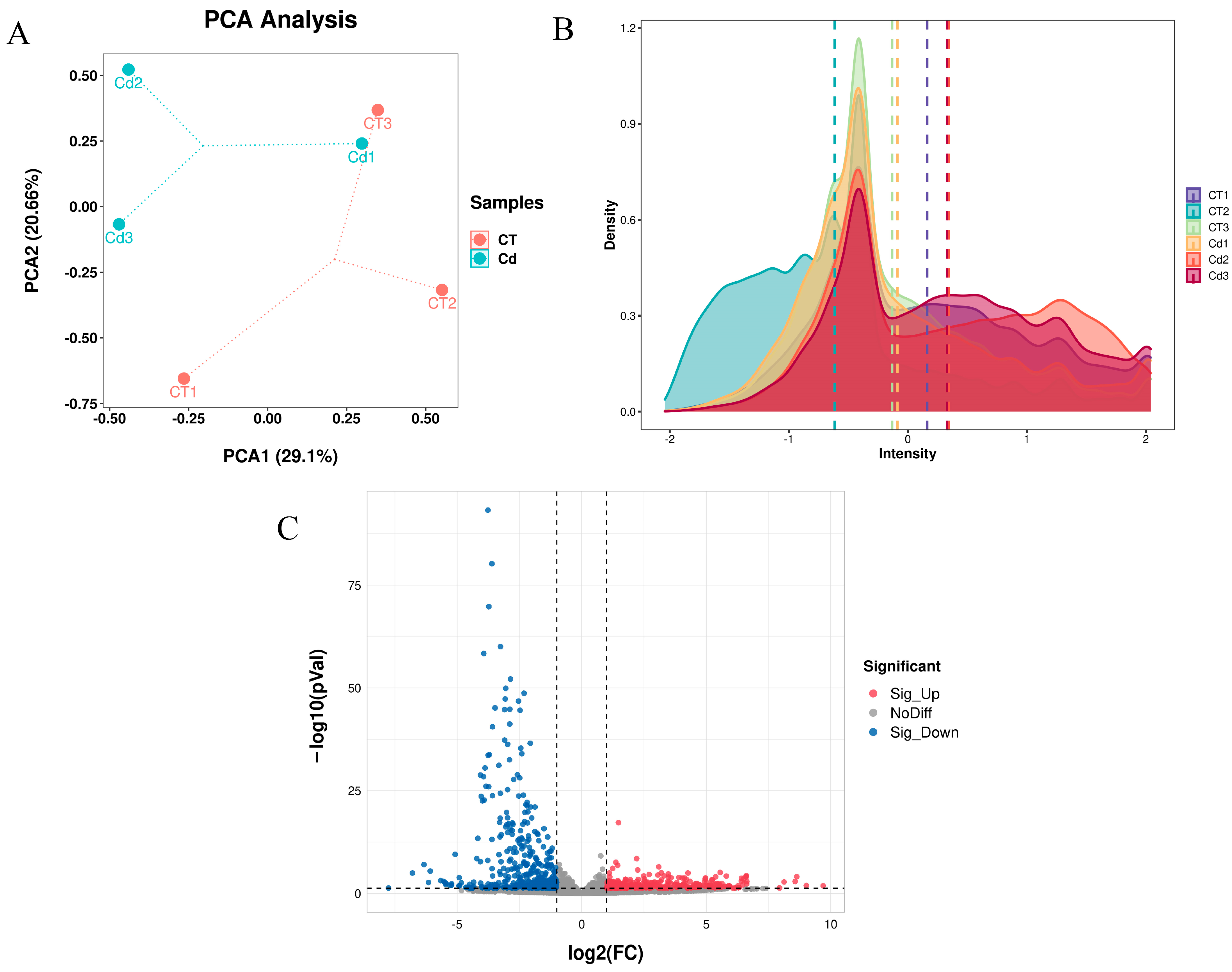
3.1. RNA-Seq and Differentially Expressed Genes
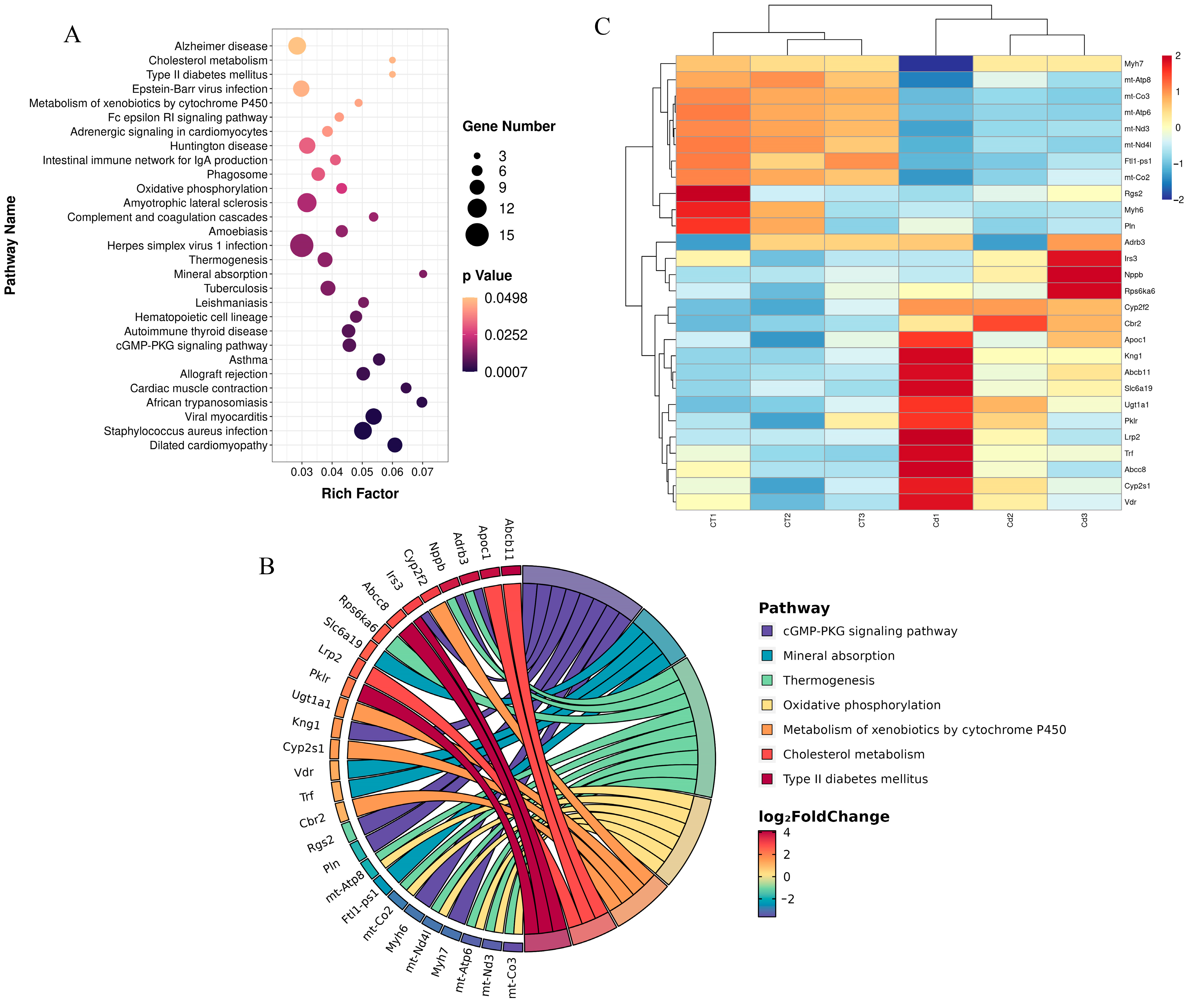
3.2. Kyoto Encyclopedia of Genes and Genomes Enrichment
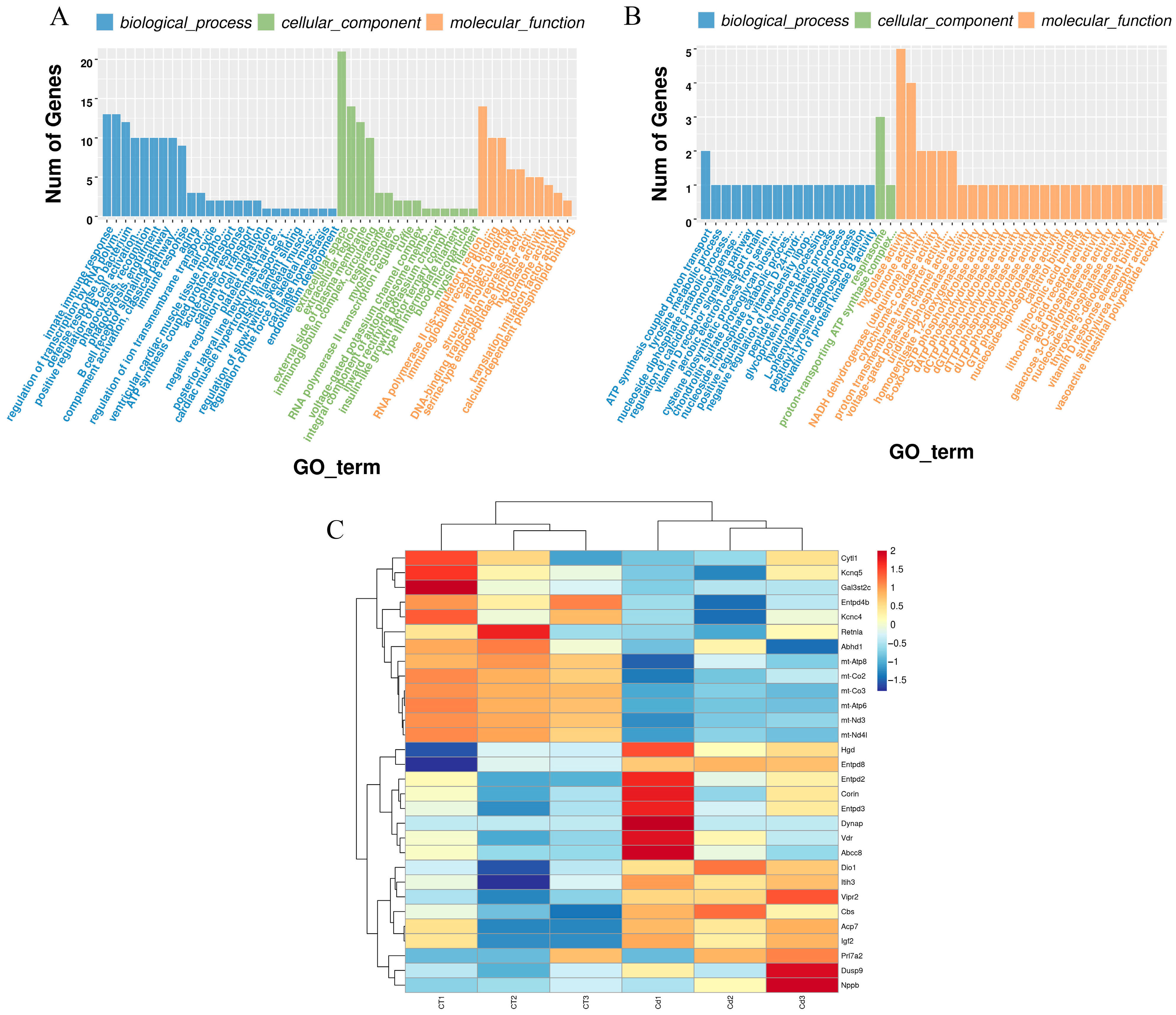
3.3. Gene Ontology Enrichment
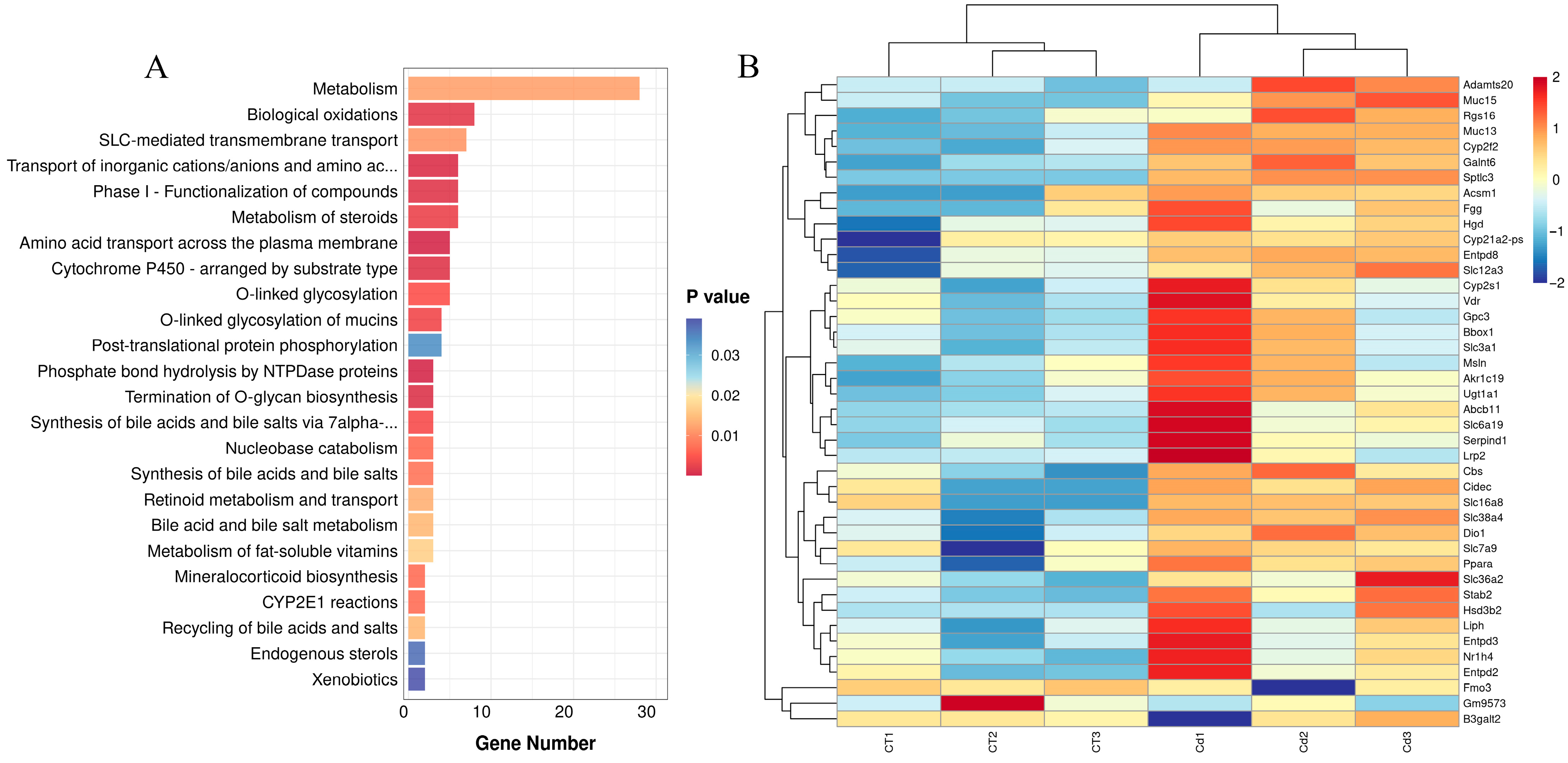
3.4. Reactome Enrichment
3.5. PANTHER Enrichmet
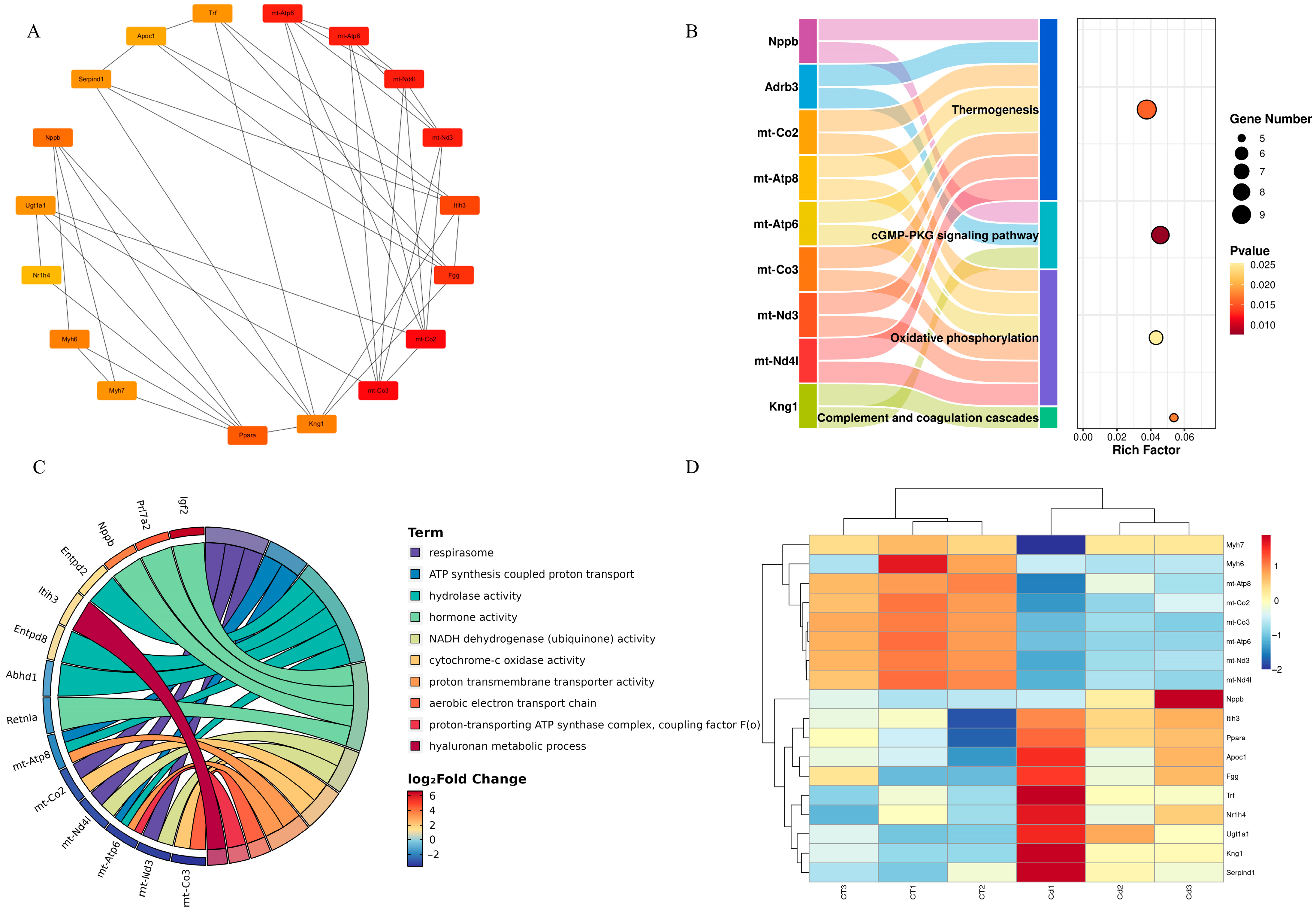
3.6. Integration of the Protein–Protein Interaction Network
3.7. Hub Genes and Their Functions
4. Discussion
4.1. Metabolism Was the Primary Mechanism of Cd Poisoning in the Uteri of ICR Mice in Labor
4.2. KEGG and GO Enrichment Analysis Further Confirmed That Mitochondrial Energy Metabolism Disorders and Coagulation Disorders Take Part in Metabolic Disorders Caused by Cd Poisoning in the Uteri of ICR Mice in Labor
4.3. PPI Network Analysis Further Confirmed That Mitochondrial Energy Metabolism Disorders and Coagulation Disorders Were Important Molecular Mechanisms of Cd-Caused Metabolic Disorders in the Uteri of ICR Mice in Labor
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friberg, L. Cadmium and the kidney. Environ. Health Perspect. 1984, 54, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liang, Y.; Hu, H.; Shaheen, S.M.; Zhong, H.; Tack, F.M.G.; Wu, M.; Li, Y.F.; Gao, Y.; Rinklebe, J.; et al. Speciation, transportation, and pathways of cadmium in soil-rice systems: A review on the environmental implications and remediation approaches for food safety. Environ. Int. 2021, 156, 106749. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiu, M.; Hao, Z.; Liu, Y.; Wang, S.; Chang, M.; Liu, X.; Sun, W.; Teng, X.; Wang, X. The mechanism of lycopene alleviating cadmium-inhibited glucose uptake ability of epithelioma papulosum cyprini cells: miR-375, oxidative stress, and actin cytoskeleton dysfunction. J. Environ. Manag. 2025, 380, 125143. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, E.; Kopff, A.; Fijałkowski, P.; Kopff, M.; Niedworok, J.; Błaszczyk, J.; Kedziora, J.; Tyślerowicz, P. Effect of anthocyanins on selected biochemical parameters in rats exposed to cadmium. Acta Biochim. Pol. 2003, 50, 543–548. [Google Scholar] [CrossRef]
- Verzelloni, P.; Urbano, T.; Wise, L.A.; Vinceti, M.; Filippini, T. Cadmium exposure and cardiovascular disease risk: A systematic review and dose-response meta-analysis. Environ. Pollut. 2024, 345, 123462. [Google Scholar] [CrossRef]
- Ma, Y.; Su, Q.; Yue, C.; Zou, H.; Zhu, J.; Zhao, H.; Song, R.; Liu, Z. The Effect of Oxidative Stress-Induced Autophagy by Cadmium Exposure in Kidney, Liver, and Bone Damage, and Neurotoxicity. Int. J. Mol. Sci. 2022, 23, 13491. [Google Scholar] [CrossRef]
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, environmental exposure, and health outcomes. Environ. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, A. Cadmium toxicity: Effects on human reproduction and fertility. Rev. Environ. Health 2019, 34, 327–338. [Google Scholar] [CrossRef]
- Ali, W.; Ma, Y.; Zhu, J.; Zou, H.; Liu, Z. Mechanisms of Cadmium-Induced Testicular Injury: A Risk to Male Fertility. Cells 2022, 11, 3601. [Google Scholar] [CrossRef]
- Segal, T.R.; Giudice, L.C. Before the beginning: Environmental exposures and reproductive and obstetrical outcomes. Fertil. Steril. 2019, 112, 613–621. [Google Scholar] [CrossRef]
- Nishijo, M.; Nakagawa, H.; Honda, R.; Tanebe, K.; Saito, S.; Teranishi, H.; Tawara, K. Effects of maternal exposure to cadmium on pregnancy outcome and breast milk. Occup. Environ. Med. 2002, 59, 394–396, discussion 397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.K.; Li, X.; Han, X.X.; Sun, D.Y.; Wang, Y.Q.; Cao, Z.Z.; Liu, L.; Meng, Z.H.; Li, G.J.; Dong, Y.J.; et al. Cadmium induces spontaneous abortion by impairing endometrial stromal cell decidualization. Toxicology 2025, 511, 154069. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.; Bannigan, J. Cadmium: Toxic effects on the reproductive system and the embryo. Reprod. Toxicol. 2008, 25, 304–315. [Google Scholar] [CrossRef]
- Geng, H.X.; Wang, L. Cadmium: Toxic effects on placental and embryonic development. Environ. Toxicol. Pharmacol. 2019, 67, 102–107. [Google Scholar] [CrossRef]
- Hou, B.; Wang, F.; Liu, T.; Wang, Z. Reproductive toxicity of polystyrene microplastics: In vivo experimental study on testicular toxicity in mice. J. Hazard. Mater. 2021, 405, 124028. [Google Scholar] [CrossRef]
- Zhou, P.; Deng, F.; Yang, Z.; Cao, C.; Zhao, H.; Liu, F.; Zhong, K.; Fu, L.; Peng, T.; Sun, D.; et al. Ginsenoside Rb1 inhibits oxidative stress-induced ovarian granulosa cell injury through Akt-FoxO1 interaction. Sci. China Life Sci. 2022, 65, 2301–2315. [Google Scholar] [CrossRef]
- Young, R.C.; Marinescu, P.S.; Seligman, N.S. Monitoring uterine contractions during labor: Current challenges and future directions. Am. J. Obstet. Gynecol. 2023, 228, S1192–S1208. [Google Scholar] [CrossRef]
- Boer, K.; den Hollander, I.A.; Meijers, J.C.; Levi, M. Tissue factor-dependent blood coagulation is enhanced following delivery irrespective of the mode of delivery. J. Thromb. Haemost. 2007, 5, 2415–2420. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Qiu, M.; Liu, Y.; Liu, Y.; Chang, M.; Liu, X.; Wang, Y.; Sun, W.; Teng, X.; Tang, Y. Co-exposure to ammonia and lipopolysaccharide-induced impaired energy metabolism via the miR-1599/HK2 axis and triggered autophagy, ER stress, and apoptosis in chicken cardiomyocytes. Poult. Sci. 2025, 104, 104965. [Google Scholar] [CrossRef]
- Rosen, H.; Yogev, Y. Assessment of uterine contractions in labor and delivery. Am. J. Obstet. Gynecol. 2023, 228, S1209–S1221. [Google Scholar] [CrossRef]
- Belyaeva, E.A.; Sokolova, T.V.; Emelyanova, L.V.; Zakharova, I.O. Mitochondrial electron transport chain in heavy metal-induced neurotoxicity: Effects of cadmium, mercury, and copper. Sci. World J. 2012, 2012, 136063. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, L.; Wen, B.; Wang, L.; Yang, F.; Bao, J.; Pan, X.; Zhang, G.; Ji, K.; Liu, H. Serpin family E member 1 enhances myometrium contractility by increasing ATP production during labor. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2024, 38, e23368. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Zong, S.; Wu, M.; Gu, J.; Yang, M. Architecture of Human Mitochondrial Respiratory Megacomplex I2III2IV2. Cell 2017, 170, 1247–1257.e12. [Google Scholar] [CrossRef]
- Miller, D.K.; Menezes, M.J.; Simons, C.; Riley, L.G.; Cooper, S.T.; Grimmond, S.M.; Thorburn, D.R.; Christodoulou, J.; Taft, R.J. Rapid identification of a novel complex I MT-ND3 m.10134C>A mutation in a Leigh syndrome patient. PLoS ONE 2014, 9, e104879. [Google Scholar] [CrossRef] [PubMed]
- Hirst, J. Mitochondrial complex I. Annu. Rev. Biochem. 2013, 82, 551–575. [Google Scholar] [CrossRef]
- Zhang, T.; Xi, Q.; Wang, D.; Li, J.; Wang, M.; Li, D.; Zhu, L.; Jin, L. Mitochondrial dysfunction and endoplasmic reticulum stress involved in oocyte aging: An analysis using single-cell RNA-sequencing of mouse oocytes. J. Ovarian Res. 2019, 12, 53. [Google Scholar] [CrossRef]
- Yi, Y.; Wang, G.; Zhang, W.; Yu, S.; Fei, J.; An, T.; Yi, J.; Li, F.; Huang, T.; Yang, J.; et al. Mitochondrial-cytochrome c oxidase II promotes glutaminolysis to sustain tumor cell survival upon glucose deprivation. Nat. Commun. 2025, 16, 212. [Google Scholar] [CrossRef]
- Bedi, M.; Das, S.; Das, J.; Mukherjee, S.; Basu, A.; Saha, S.; Ghosh, A. Mitochondrial proteome analysis reveals that an augmented cytochrome c oxidase assembly and activity potentiates respiratory capacity in sarcoma. Biochem. Biophys. Res. Commun. 2024, 736, 150501. [Google Scholar] [CrossRef]
- Wolters, J.E.J.; van Breda, S.G.J.; Grossmann, J.; Fortes, C.; Caiment, F.; Kleinjans, J.C.S. Integrated ‘omics analysis reveals new drug-induced mitochondrial perturbations in human hepatocytes. Toxicol. Lett. 2018, 289, 1–13. [Google Scholar] [CrossRef]
- Del Dotto, V.; Musiani, F.; Baracca, A.; Solaini, G. Variants in Human ATP Synthase Mitochondrial Genes: Biochemical Dysfunctions, Associated Diseases, and Therapies. Int. J. Mol. Sci. 2024, 25, 2239. [Google Scholar] [CrossRef]
- Diodato, D.; Invernizzi, F.; Lamantea, E.; Fagiolari, G.; Parini, R.; Menni, F.; Parenti, G.; Bollani, L.; Pasquini, E.; Donati, M.A.; et al. Common and Novel TMEM70 Mutations in a Cohort of Italian Patients with Mitochondrial Encephalocardiomyopathy. JIMD Rep. 2015, 15, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Yang, X.; Tan, M.; Guo, J.; Ye, Y.; Deng, J.; Huang, Z.; Wang, H.; Su, W.; Cheng, J.; et al. NIR-enhanced Pt single atom/g-C(3)N(4) nanozymes as SOD/CAT mimics to rescue ATP energy crisis by regulating oxidative phosphorylation pathway for delaying osteoarthritis progression. Bioact. Mater. 2024, 36, 1–13. [Google Scholar] [CrossRef]
- Yan, G.; Li, X.; Cheng, X.; Peng, Y.; Long, B.; Fan, Q.; Wang, Z.; Zheng, Z.; Shi, M.; Yan, X. Proteomic profiling reveals oxidative phosphorylation pathway is suppressed in longissimus dorsi muscle of weaned piglets fed low-protein diet supplemented with limiting amino acids. Int. J. Biochem. Cell Biol. 2016, 79, 288–297. [Google Scholar] [CrossRef]
- Uvnäs-Moberg, K. The physiology and pharmacology of oxytocin in labor and in the peripartum period. Am. J. Obstet. Gynecol. 2024, 230, S740–S758. [Google Scholar] [CrossRef] [PubMed]
- Nasiadek, M.; Kilanowicz, A.; Darago, A.; Lazarenkow, A.; Michalska, M. The effect of cadmium on the coagulation and fibrinolytic system in women with uterine endometrial cancer and myoma. Int. J. Occup. Med. Environ. Health 2013, 26, 291–301. [Google Scholar] [CrossRef]
- Marongiu, F.; Marongiu, S.; Ruberto, M.F.; Faa, G.; Barcellona, D. Trace metals and the hemostatic system. Clin. Chim. Acta 2023, 547, 117458. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.S.; Wojdyla, D.; Say, L.; Gülmezoglu, A.M.; Van Look, P.F. WHO analysis of causes of maternal death: A systematic review. Lancet 2006, 367, 1066–1074. [Google Scholar] [CrossRef]
- Rein, C.M.; Desai, U.R.; Church, F.C. Serpin-glycosaminoglycan interactions. Methods Enzymol. 2011, 501, 105–137. [Google Scholar] [CrossRef]
- Tollefsen, D.M. Vascular dermatan sulfate and heparin cofactor II. Prog. Mol. Biol. Transl. Sci. 2010, 93, 351–372. [Google Scholar] [CrossRef]
- Lin, W.Y.; Zhu, R.; Zhang, Z.; Lu, X.; Wang, H.; He, W.; Hu, Y.; Tang, L. RNAi targeting heparin cofactor II promotes hemostasis in hemophilia A. Mol. Ther. Nucleic Acids 2021, 24, 658–668. [Google Scholar] [CrossRef]
- Dobson, D.A.; Fish, R.J.; de Vries, P.S.; Morrison, A.C.; Neerman-Arbez, M.; Wolberg, A.S. Regulation of fibrinogen synthesis. Thromb. Res. 2024, 242, 109134. [Google Scholar] [CrossRef] [PubMed]
- Sidelmann, J.J.; Gram, J.B.; Rasmussen, J.J.; Kistorp, C. Anabolic-Androgenic Steroid Abuse Impairs Fibrin Clot Lysis. Semin. Thromb. Hemost. 2021, 47, 11–17. [Google Scholar] [CrossRef]
- Sinnathamby, E.S.; Issa, P.P.; Roberts, L.; Norwood, H.; Malone, K.; Vemulapalli, H.; Ahmadzadeh, S.; Cornett, E.M.; Shekoohi, S.; Kaye, A.D. Hereditary Angioedema: Diagnosis, Clinical Implications, and Pathophysiology. Adv. Ther. 2023, 40, 814–827. [Google Scholar] [CrossRef] [PubMed]
- Merkulov, S.; Zhang, W.M.; Komar, A.A.; Schmaier, A.H.; Barnes, E.; Zhou, Y.; Lu, X.; Iwaki, T.; Castellino, F.J.; Luo, G.; et al. Deletion of murine kininogen gene 1 (mKng1) causes loss of plasma kininogen and delays thrombosis. Blood 2008, 111, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Langhauser, F.; Göb, E.; Kraft, P.; Geis, C.; Schmitt, J.; Brede, M.; Göbel, K.; Helluy, X.; Pham, M.; Bendszus, M.; et al. Kininogen deficiency protects from ischemic neurodegeneration in mice by reducing thrombosis, blood-brain barrier damage, and inflammation. Blood 2012, 120, 4082–4092. [Google Scholar] [CrossRef]
- Stahl, F.; Hitzmann, B.; Mutz, K.; Landgrebe, D.; Lübbecke, M.; Kasper, C.; Walter, J.; Scheper, T. Transcriptome analysis. Genom. Syst. Biol. Mamm. Cell Cult. 2012, 127, 1–25. [Google Scholar] [CrossRef]
- Hrdlickova, R.; Toloue, M.; Tian, B. RNA-Seq methods for transcriptome analysis. Wiley Interdiscip. Rev. RNA 2017, 8, e1364. [Google Scholar] [CrossRef] [PubMed]
- Che, X.; Shang, X.; Xu, W.; Xing, M.; Wei, H.; Li, W.; Li, Z.; Teng, X.; Geng, L. Selenium-enriched Lactiplantibacillus plantarum alleviates alkalinity stress-induced selective hepatic insulin resistance in common carp. Int. J. Biol. Macromol. 2025, 305, 141204. [Google Scholar] [CrossRef]
- Zhou, Q.; Hao, Z.; Qiu, M.; Liu, Y.; Chang, M.; Liu, X.; Wang, Y.; Tang, Y.; Sun, W.; Teng, X.; et al. Amino acid metabolism disorder and oxidative stress took part in EGCG alleviating Mn-caused ferroptosis via miR-9-5p/got1 axis. J. Hazard. Mater. 2025, 489, 137656. [Google Scholar] [CrossRef]
- Mao, F.; Wang, E.; Xu, J.; Lu, J.; Yan, G.; Fu, L.; Jiao, Y.; Wu, L.; Liu, T.; Li, Y. Transcriptome Analysis of Multiple Metabolic Tissues in High-Salt Diet-Fed Mice. Front. Endocrinol. 2022, 13, 887843. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8 (Suppl. 4), S11. [Google Scholar] [CrossRef]
- Yan, B.; Liao, P.; Han, Z.; Zhao, J.; Gao, H.; Liu, Y.; Chen, F.; Lei, P. Association of aging related genes and immune microenvironment with major depressive disorder. J. Affect. Disord. 2025, 369, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yi, X.; You, M.; Yang, H.; Zhang, S.; Huang, S.; Xie, L. Bulk-RNA and single-nuclei RNA seq analyses reveal the role of lactate metabolism-related genes in Alzheimer’s disease. Metab. Brain Dis. 2024, 39, 1469–1480. [Google Scholar] [CrossRef]
- Andersen, O.; Nielsen, J.B.; Svendsen, P. Oral cadmium chloride intoxication in mice: Effects of dose on tissue damage, intestinal absorption and relative organ distribution. Toxicology 1988, 48, 225–236. [Google Scholar] [CrossRef]
- Oliveira, H.; Spanò, M.; Santos, C.; Pereira Mde, L. Adverse effects of cadmium exposure on mouse sperm. Reprod. Toxicol. 2009, 28, 550–555. [Google Scholar] [CrossRef]
- Zargar, S.; Siddiqi, N.J.; Al Daihan, S.K.; Wani, T.A. Protective effects of quercetin on cadmium fluoride induced oxidative stress at different intervals of time in mouse liver. Acta Biochim. Pol. 2015, 62, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Y.; Bo, Q.L.; Ji, Y.L.; Liu, L.; Hu, Y.F.; Chen, Y.H.; Zhang, J.; Zhao, L.L.; Xu, D.X. Maternal cadmium exposure reduces placental zinc transport and induces fetal growth restriction in mice. Reprod. Toxicol. 2016, 63, 174–182. [Google Scholar] [CrossRef]
- Şensoy, E. Investigation of the effect of Cadmium chloride applied during pregnancy on the morphological parameters of mouse offspring and the protective role of melatonin. J. Hazard. Mater. Adv. 2023, 9, 100222. [Google Scholar] [CrossRef]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef]
- Yang, B.; Wang, Z.; Wang, S.; Li, X. Unveiling the Hub Genes Involved in Cadmium-Induced Hepatotoxicity. Biol. Trace Elem. Res. 2024, 203, 2186–2205. [Google Scholar] [CrossRef] [PubMed]
- Aghajanova, L.; Hamilton, A.E.; Giudice, L.C. Uterine receptivity to human embryonic implantation: Histology, biomarkers, and transcriptomics. Semin. Cell Dev. Biol. 2008, 19, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.O.D.; Maybin, J.A.; Armstrong, G.M.; Williams, A.R.W. Physiology of the Endometrium and Regulation of Menstruation. Physiol. Rev. 2020, 100, 1149–1179. [Google Scholar] [CrossRef]
- Teh, W.T.; McBain, J.; Rogers, P. What is the contribution of embryo-endometrial asynchrony to implantation failure? J. Assist. Reprod. Genet. 2016, 33, 1419–1430. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Wang, J.; Li, W.; Chen, Z.J.; Du, Y. Androgen-induced gut dysbiosis disrupts glucolipid metabolism and endocrinal functions in polycystic ovary syndrome. Microbiome 2021, 9, 101. [Google Scholar] [CrossRef]
- Sadowska, J.; Dudzińska, W.; Dziaduch, I. Effects of different models of sucrose intake on the oxidative status of the uterus and ovary of rats. PLoS ONE 2021, 16, e0251789. [Google Scholar] [CrossRef]
- Meng, X.; Chen, C.; Qian, J.; Cui, L.; Wang, S. Energy metabolism and maternal-fetal tolerance working in decidualization. Front. Immunol. 2023, 14, 1203719. [Google Scholar] [CrossRef]
- Qiu, M.; Hao, Z.; Liu, Y.; Liu, Y.; Chang, M.; Lin, X.; Liu, X.; Dong, N.; Sun, W.; Teng, X. ROS acted as an initial role in selenium nanoparticles alleviating insecticide chlorpyrifos-induced oxidative stress, pyroptosis, and intestinal barrier dysfunction in porcine intestinal epithelial cells. Pestic. Biochem. Physiol. 2025, 211, 106418. [Google Scholar] [CrossRef]
- Wei, X.; Li, X.; Liu, P.; Li, L.; Chen, H.; Li, D.; Liu, J.; Xie, L. Integrated physiological, biochemical, and transcriptomic analysis of thallium toxicity in zebrafish (Danio rerio) larvae. Sci. Total Environ. 2023, 859, 160265. [Google Scholar] [CrossRef]
- Cannino, G.; Ferruggia, E.; Luparello, C.; Rinaldi, A.M. Cadmium and mitochondria. Mitochondrion 2009, 9, 377–384. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, C.; Sun, L.; Wang, A.; Lan, X.; Xu, W.; Liang, Y.; Ma, S.; Xia, Q. In-depth transcriptome unveils the cadmium toxicology and a novel metallothionein in silkworm. Chemosphere 2021, 273, 128522. [Google Scholar] [CrossRef]
- Bimonte, V.M.; Besharat, Z.M.; Antonioni, A.; Cella, V.; Lenzi, A.; Ferretti, E.; Migliaccio, S. The endocrine disruptor cadmium: A new player in the pathophysiology of metabolic diseases. J. Endocrinol. Investig. 2021, 44, 1363–1377. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, X.-D.; Xu, X.; Cai, Y.-B.; Zhu, Z.-X.; Zhu, L.; Ren, K. Linc00657 promoted pyroptosis in THP-1-derived macrophages and exacerbated atherosclerosis via the miR-106b-5p/TXNIP/NLRP3 axis. Int. J. Biol. Macromol. 2023, 253, 126953. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Sakuma, M.; Fujie, T.; Kaji, T.; Yamamoto, C. Cadmium induces plasminogen activator inhibitor-1 via Smad2/3 signaling pathway in human endothelial EA.hy926 cells. J. Toxicol. Sci. 2021, 46, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Middleton, P.; Shepherd, E.; Gomersall, J.C. Venous thromboembolism prophylaxis for women at risk during pregnancy and the early postnatal period. Cochrane Database Syst. Rev. 2021, 3, Cd001689. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Sokolov, E.P.; Ponnappa, K.M. Cadmium exposure affects mitochondrial bioenergetics and gene expression of key mitochondrial proteins in the eastern oyster Crassostrea virginica Gmelin (Bivalvia: Ostreidae). Aquat. Toxicol. 2005, 73, 242–255. [Google Scholar] [CrossRef]
- Al Kaddissi, S.; Legeay, A.; Elia, A.C.; Gonzalez, P.; Floriani, M.; Cavalie, I.; Massabuau, J.C.; Gilbin, R.; Simon, O. Mitochondrial gene expression, antioxidant responses, and histopathology after cadmium exposure. Environ. Toxicol. 2014, 29, 893–907. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Huang, P.H.; Chen, Y.H.; Sung, S.H.; Chiang, K.H.; Chen, J.W.; Lin, S.J. Plasma heparin cofactor II activity is an independent predictor of future cardiovascular events in patients after acute myocardial infarction. Coron. Artery Dis. 2008, 19, 597–602. [Google Scholar] [CrossRef]
- Mohammed, B.M.; Sun, M.F.; Cheng, Q.; Litvak, M.; McCrae, K.R.; Emsley, J.; McCarty, O.J.T.; Gailani, D. High molecular weight kininogen interactions with the homologs prekallikrein and factor XI: Importance to surface-induced coagulation. J. Thromb. Haemost. 2024, 22, 225–237. [Google Scholar] [CrossRef]

| Sample | Raw Reads | Clean Reads | Q20 Rate (%) | Q30 Rate (%) | GC Content (%) |
|---|---|---|---|---|---|
| CT1 | 80,294,094 | 78,488,568 | 98.75 | 96.43 | 48.45 |
| CT2 | 62,142,356 | 60,731,292 | 98.7 | 96.29 | 49.08 |
| CT3 | 74,052,894 | 72,426,350 | 98.76 | 96.44 | 49.36 |
| Cd1 | 74,803,466 | 74,798,244 | 99.08 | 96.95 | 49.89 |
| Cd2 | 89,375,270 | 89,369,498 | 99.15 | 97.16 | 48.78 |
| Cd3 | 87,522,738 | 87,516,872 | 98.91 | 96.48 | 49.53 |
| Pathway | ID | p-Value | Input |
|---|---|---|---|
| Cytoskeletal regulation by Rho GTPase | P00016 | 0.037541 | Myh6, Myh7, Tubb1 |
| Insulin/IGF pathway–mitogen-activated protein kinase kinase/MAP kinase cascade | P00032 | 0.041579 | Igf2, Irs3 |
| Nicotinic acetylcholine receptor signaling pathway | P00044 | 0.046343 | Myo16, Myh6, Myh7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Bai, Y.; Wang, Y.; Cai, Y. Cadmium Exposure Disrupts Uterine Energy Metabolism and Coagulation Homeostasis During Labor in Institute of Cancer Research Mice: Insights from Transcriptomic Analysis. Metabolites 2025, 15, 339. https://doi.org/10.3390/metabo15050339
Wang Y, Bai Y, Wang Y, Cai Y. Cadmium Exposure Disrupts Uterine Energy Metabolism and Coagulation Homeostasis During Labor in Institute of Cancer Research Mice: Insights from Transcriptomic Analysis. Metabolites. 2025; 15(5):339. https://doi.org/10.3390/metabo15050339
Chicago/Turabian StyleWang, Yueyang, Yichen Bai, Yi Wang, and Yan Cai. 2025. "Cadmium Exposure Disrupts Uterine Energy Metabolism and Coagulation Homeostasis During Labor in Institute of Cancer Research Mice: Insights from Transcriptomic Analysis" Metabolites 15, no. 5: 339. https://doi.org/10.3390/metabo15050339
APA StyleWang, Y., Bai, Y., Wang, Y., & Cai, Y. (2025). Cadmium Exposure Disrupts Uterine Energy Metabolism and Coagulation Homeostasis During Labor in Institute of Cancer Research Mice: Insights from Transcriptomic Analysis. Metabolites, 15(5), 339. https://doi.org/10.3390/metabo15050339