Atractylenolide I Inhibits Nicotine-Induced Macrophage Pyroptosis and Alleviates Atherogenesis by Suppressing the TLR4/ROS/TXNIP/NLRP3 Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. RNA Isolation and RT-qPCR
2.3. Western Blot Analysis
2.4. ELISA Assay
2.5. Detection of Caspase-1 Activity
2.6. LDH Release Assay
2.7. Hoechst/PI Staining Assay
2.8. Detection of Intracellular ROS Levels
2.9. Animal Treatment
2.10. Assessment of Atherosclerotic Lesions
2.11. Detection of Serum Biochemical Indices
2.12. Statistical Analyses
3. Results
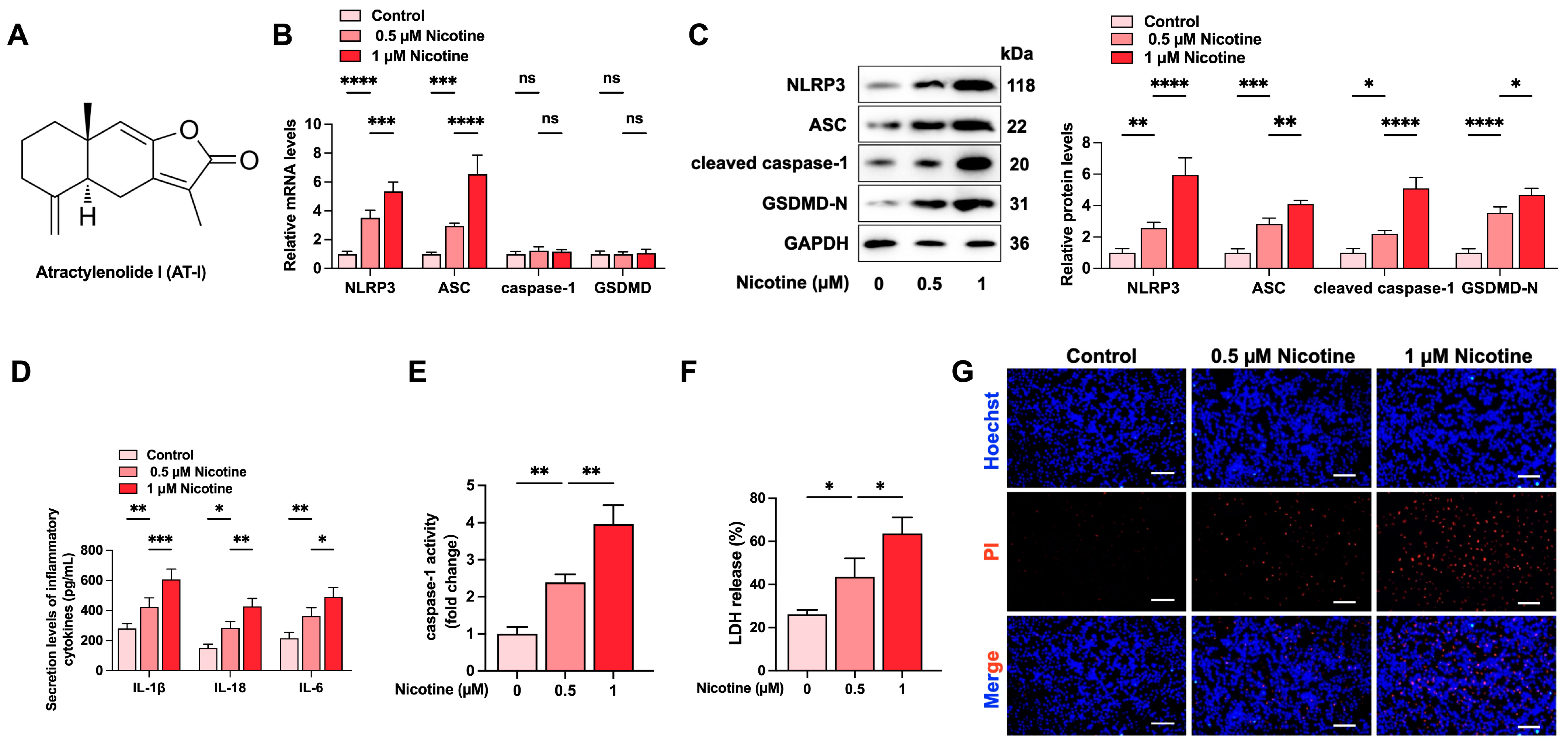
3.1. Nicotine Promotes NLRP3 Inflammasome Activation and Induces Pyroptosis in THP-1-Derived Macrophages
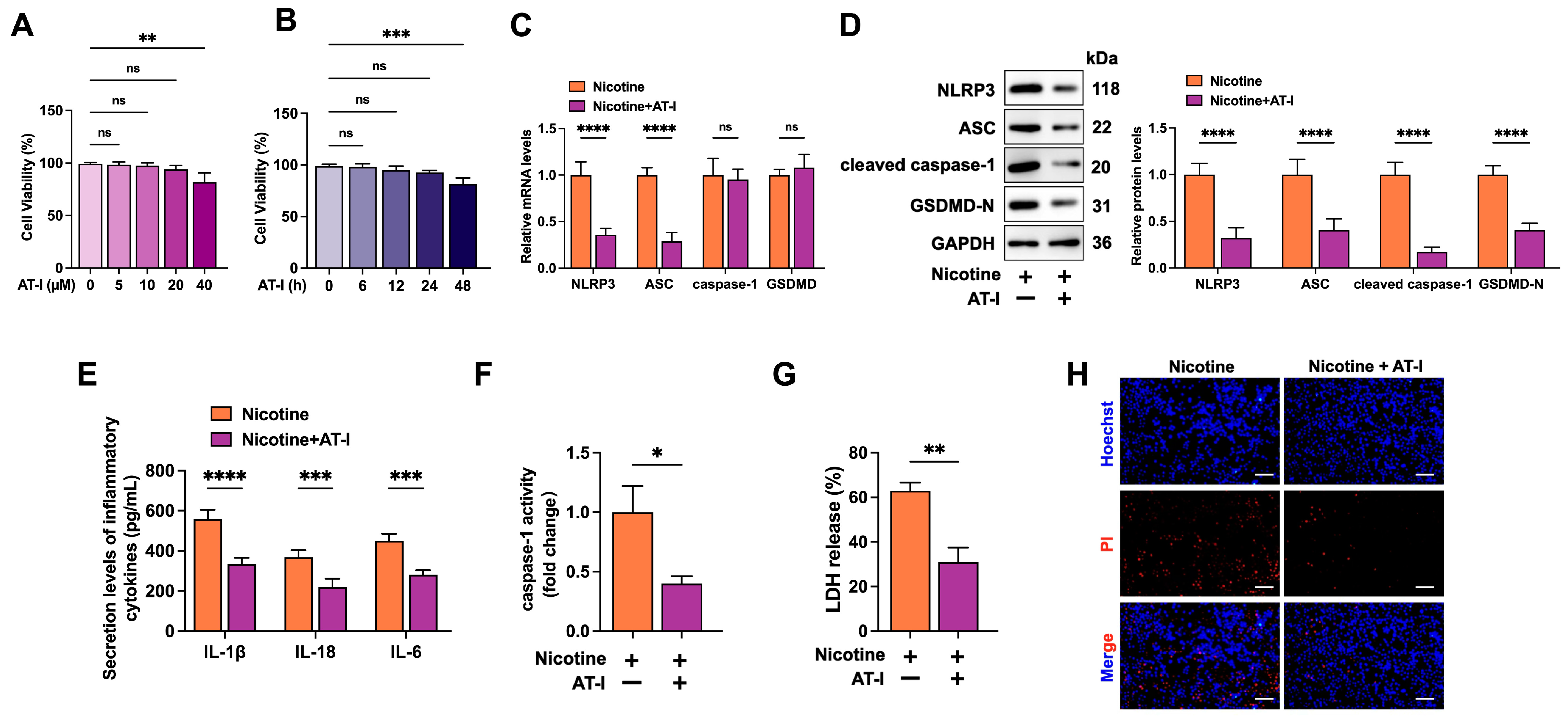
3.2. AT-I Inhibits Nicotine-Induced NLRP3 Inflammasome Activation and Pyroptosis in THP-1-Derived Macrophages
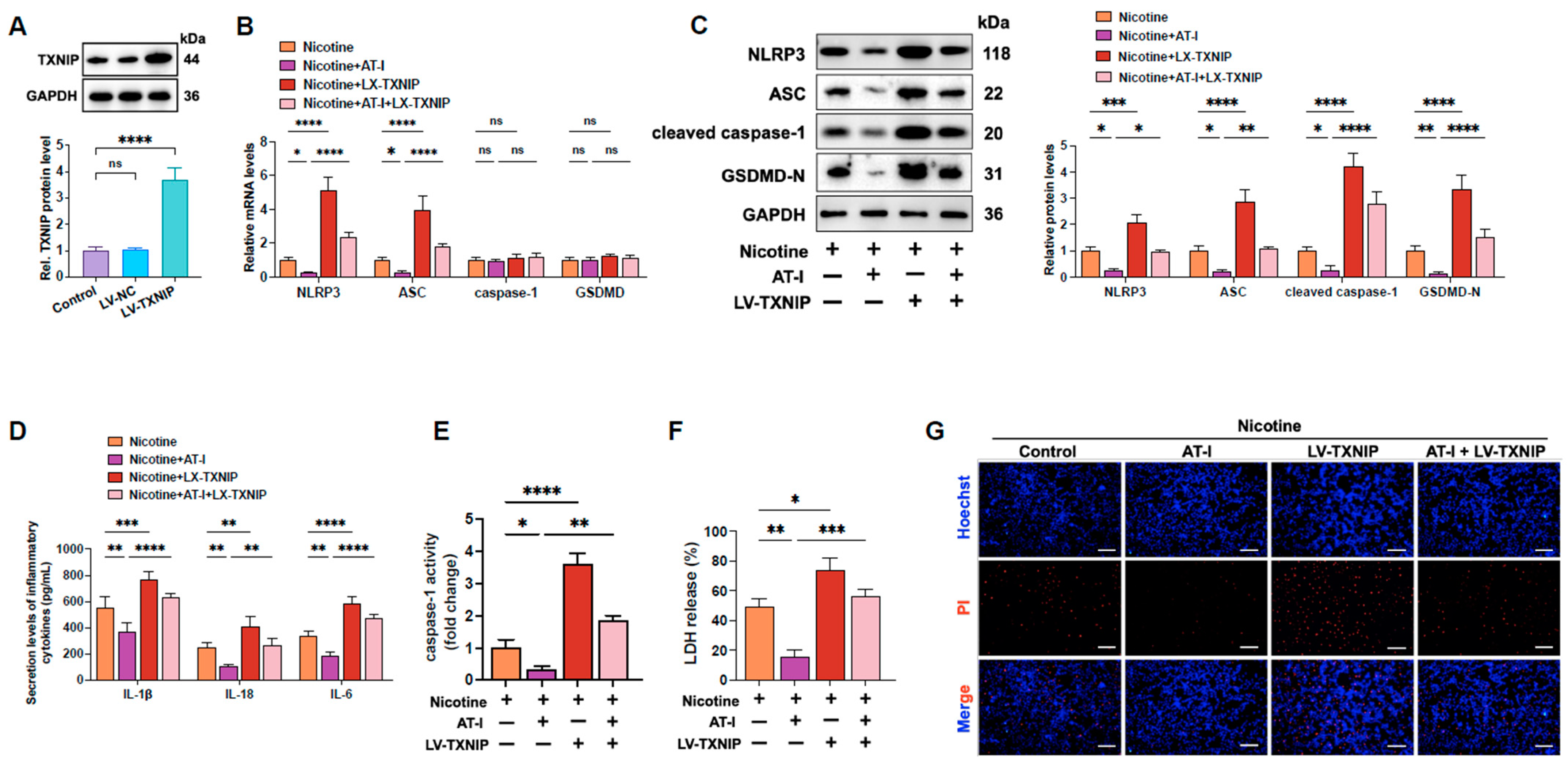
3.3. AT-I Inhibits Nicotine-Induced NLRP3 Inflammasome Activation and Pyroptosis in THP-1-Derived Macrophages by Decreasing TXNIP
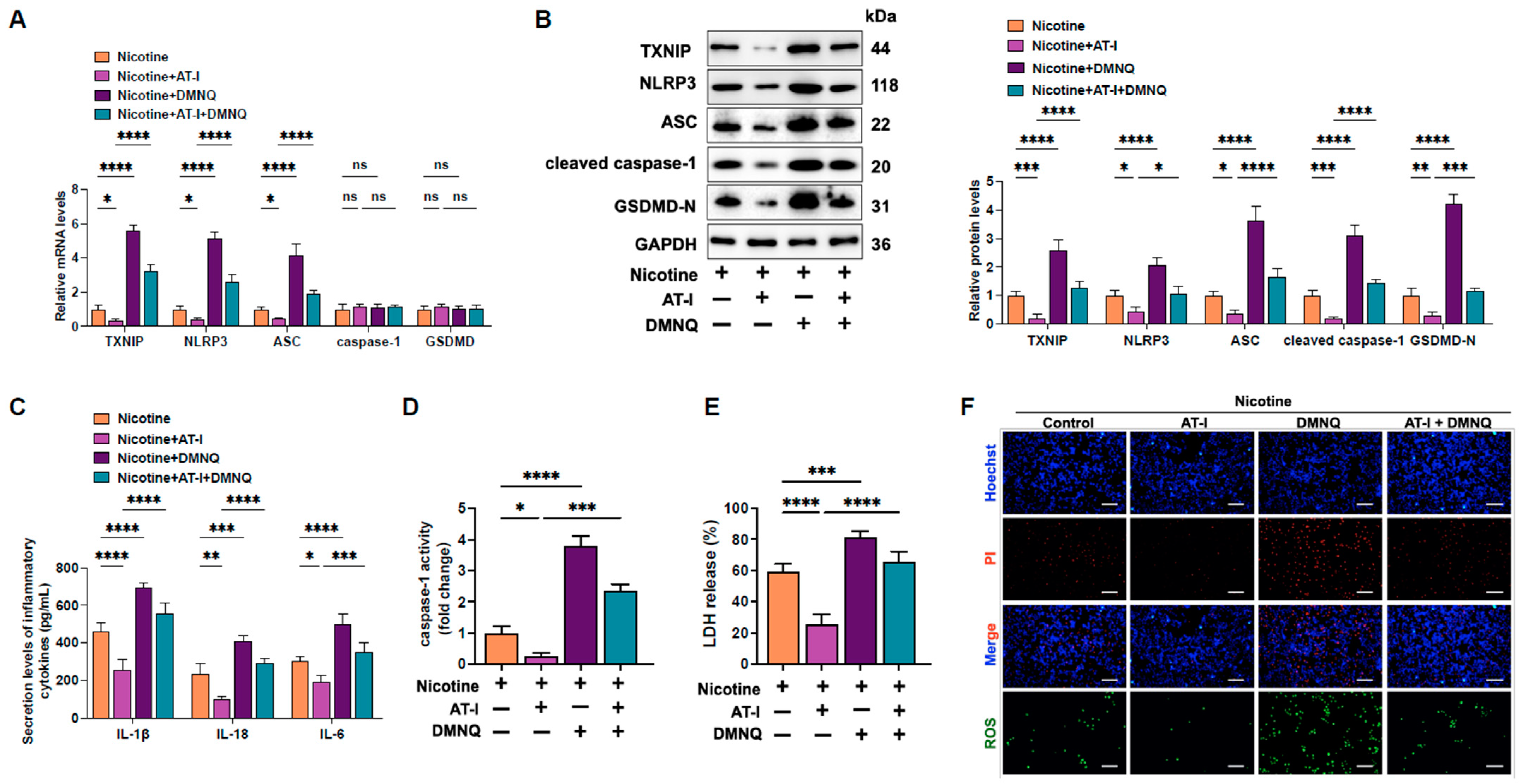
3.4. AT-I Represses Pyroptosis in Nicotine-Treated THP-1-Derived Macrophages by Inhibiting the ROS/TXNIP Pathway
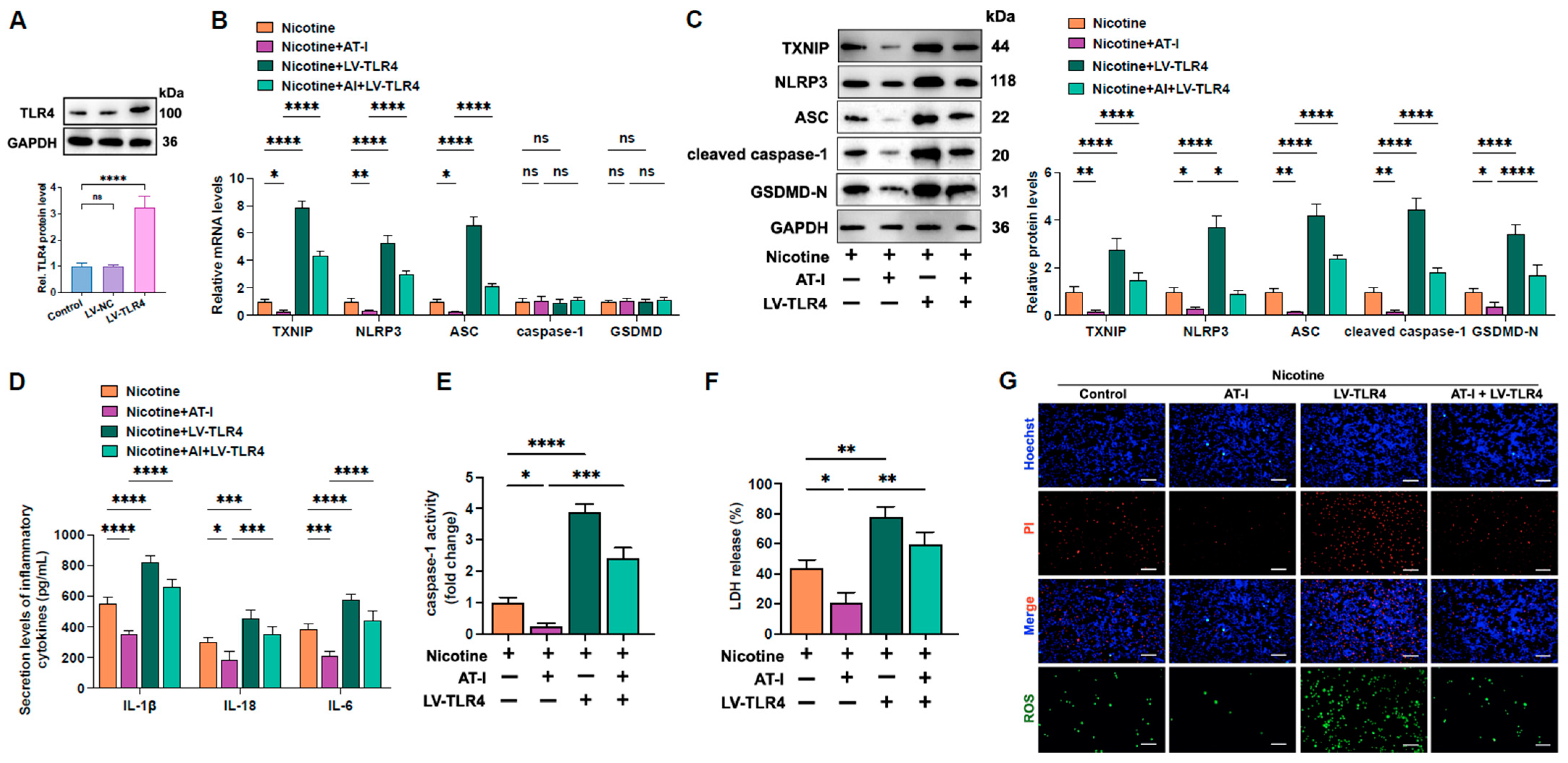
3.5. AT-I Represses Inflammation and Pyroptosis of Nicotine-Treated THP-1-Derived Macrophages Via Inhibition of the TLR4/ROS/TXNIP/NLRP3 Pathway
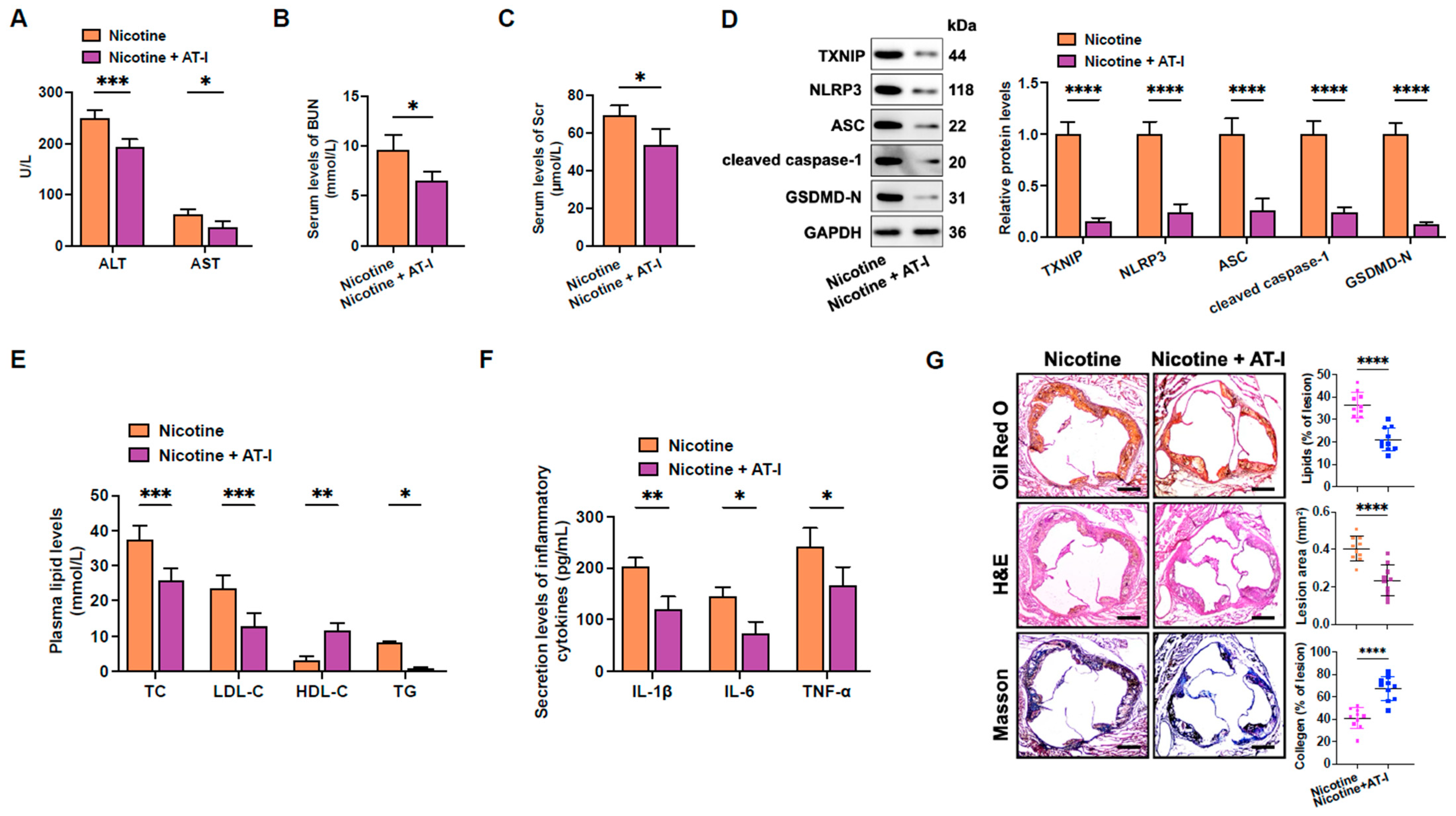
3.6. AT-I Mitigates Nicotine-Induced Atherosclerosis in HFD-Fed apoE−/− Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rosenblit, P.D. Extreme Atherosclerotic Cardiovascular Disease (ASCVD) Risk Recognition. Curr. Diab Rep. 2019, 19, 61. [Google Scholar] [CrossRef] [PubMed]
- Adkar, S.S.; Leeper, N.J. Efferocytosis in atherosclerosis. Nat. Rev. Cardiol. 2024, 21, 762–779. [Google Scholar] [CrossRef] [PubMed]
- Svensson, E.C.; Madar, A.; Campbell, C.D.; He, Y.; Sultan, M.; Healey, M.L.; Xu, H.; D’Aco, K.; Fernandez, A.; Wache-Mainier, C.; et al. TET2-Driven Clonal Hematopoiesis and Response to Canakinumab: An Exploratory Analysis of the CANTOS Randomized Clinical Trial. JAMA Cardiol. 2022, 7, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Paramel Varghese, G.; Folkersen, L.; Strawbridge, R.J.; Halvorsen, B.; Yndestad, A.; Ranheim, T.; Krohg-Sørensen, K.; Skjelland, M.; Espevik, T.; Aukrust, P.; et al. NLRP3 Inflammasome Expression and Activation in Human Atherosclerosis. J. Am. Heart Assoc. 2016, 5, e003031. [Google Scholar] [CrossRef]
- Wang, M.; Yu, F.; Zhang, Y.; Li, P. Programmed cell death in tumor immunity: Mechanistic insights and clinical implications. Front. Immunol. 2023, 14, 1309635. [Google Scholar] [CrossRef]
- Lyu, T.; Yin, Q. Research Progress on Pyroptosis in Hematological Malignancies. Curr. Treat. Options Oncol. 2023, 24, 1439–1450. [Google Scholar] [CrossRef]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nuñez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef]
- Fan, X.; Han, J.; Zhong, L.; Zheng, W.; Shao, R.; Zhang, Y.; Shi, S.; Lin, S.; Huang, Z.; Huang, W.; et al. Macrophage-Derived GSDMD Plays an Essential Role in Atherosclerosis an d Cross Talk Between Macrophages via the Mitochondria-STING-IRF3/NF-κB Axis. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1365–1378. [Google Scholar] [CrossRef]
- Weng, X.; Luo, X.; Dai, X.; Lv, Y.; Zhang, S.; Bai, X.; Bao, X.; Wang, Y.; Zhao, C.; Zeng, M.; et al. Apigenin inhibits macrophage pyroptosis through regulation of oxidativ e stress and the NF-κB pathway and ameliorates atherosclerosis. Phytother. Res. 2023, 37, 5300–5314. [Google Scholar] [CrossRef]
- Shao, X.; Zeng, W.; Wang, Q.; Liu, S.; Guo, Q.; Luo, D.; Luo, Q.; Wang, D.; Wang, L.; Zhang, Y.; et al. Fufang Zhenzhu Tiaozhi (FTZ) suppression of macrophage pyroptosis: Key to stabilizing rupture-prone plaques. J. Ethnopharmacol. 2024, 324, 117705. [Google Scholar] [CrossRef]
- Pan, X.; Xu, H.; Ding, Z.; Luo, S.; Li, Z.; Wan, R.; Jiang, J.; Chen, X.; Liu, S.; Chen, Z.; et al. Guizhitongluo Tablet inhibits atherosclerosis and foam cell formation through regulating Piezo1/NLRP3 mediated macrophage pyroptosis. Phytomedicine 2024, 132, 155827. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Report on the Global Tobacco Epidemic 2017: Monitoring Tobacco Use And prevention Policies; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- World Health Organization. WHO Report on the Global Tobacco Epidemic, 2011: Warning About the Dangers of Tobacco; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Fu, X.; Zong, T.; Yang, P.; Li, L.; Wang, S.; Wang, Z.; Li, M.; Li, X.; Zou, Y.; Zhang, Y.; et al. Nicotine: Regulatory roles and mechanisms in atherosclerosis progressi on. Food Chem. Toxicol. 2021, 151, 112154. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Chen, H.; Long, J.; Song, J.; Xie, L.; Li, X. Atractylenolides (I, II, and III): A review of their pharmacology and pharmacokinetics. Arch. Pharm. Res. 2021, 44, 633–654. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, Q.L.; Hua, J.W.; Cheng, W.L.; Qin, L.P. The traditional uses, phytochemistry, and pharmacology of Atractylodes macrocephala Koidz.: A review. J. Ethnopharmacol. 2018, 226, 143–167. [Google Scholar] [CrossRef]
- Wang, C.C.; Lin, S.Y.; Cheng, H.C.; Hou, W.C. Pro-oxidant and cytotoxic activities of atractylenolide I in human promyeloleukemic HL-60 cells. Food Chem. Toxicol. 2006, 44, 1308–1315. [Google Scholar] [CrossRef]
- Fu, X.Q.; Chou, J.Y.; Li, T.; Zhu, P.L.; Li, J.K.; Yin, C.L.; Su, T.; Guo, H.; Lee, K.W.; Hossen, M.J.; et al. The JAK2/STAT3 pathway is involved in the anti-melanoma effects of atractylenolide I. Exp. Dermatol. 2018, 27, 201–204. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Liu, Z.; Guo, X.; Miao, Z.; Ma, S. Atractylenolide I Induces Apoptosis and Suppresses Glycolysis by Blocking the JAK2/STAT3 Signaling Pathway in Colorectal Cancer Cells. Front. Pharmacol. 2020, 11, 273. [Google Scholar] [CrossRef]
- Yu, R.; Yu, B.-x.; Chen, J.-f.; Lv, X.-y.; Yan, Z.-j.; Cheng, Y.; Ma, Q. Anti-tumor effects of Atractylenolide I on bladder cancer cells. J. Exp. Clin. Cancer Res. 2016, 35, 40. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, X.; Yu, F.; Liu, C.; Hu, F.; Liu, L.; Chen, J.; Wang, J. Atractylenolide I alleviates ischemia/reperfusion injury by preserving mitochondrial function and inhibiting caspase-3 activity. J. Int. Med. Res. 2021, 49, 300060521993315. [Google Scholar] [CrossRef]
- Chen, S.; Liu, X.; Zhou, X.; Lin, W.; Liu, M.; Ma, H.; Zhong, K.; Ma, Q.; Qin, C. Atractylenolide-I prevents abdominal aortic aneurysm formation through inhibiting inflammation. Front. Immunol. 2025, 16, 1486072. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Ma, J.; Zhao, W.R.; Shi, W.T.; Zhang, J.; Hu, Y.Y.; Yue, M.Y.; Zhou, W.L.; Yan, H.; Tang, J.Y.; et al. Qiangxinyin formula protects against isoproterenol-induced cardiac hypertrophy. Phytomedicine 2024, 130, 155717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-l.; Huang, W.-m.; Zeng, Q.-y. Atractylenolide I protects mice from lipopolysaccharide-induced acute lung injury. Eur. J. Pharmacol. 2015, 765, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhi, W.; Liu, F.; He, Z.; Wang, X.; Niu, X. Atractylenolide I restores HO-1 expression and inhibits Ox-LDL-induced VSMCs proliferation, migration and inflammatory responses in vitro. Exp. Cell Res. 2017, 353, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Duan, H.; He, L. Inhibitory effect of atractylenolide I on angiogenesis in chronic infl ammation in vivo and in vitro. Eur. J. Pharmacol. 2009, 612, 143–152. [Google Scholar] [CrossRef]
- Wang, D.; Lin, Z.; Zhou, Y.; Su, M.; Zhang, H.; Yu, L.; Li, M. Atractylenolide I ameliorates sepsis-induced cardiomyocyte injury by i nhibiting macrophage polarization through the modulation of the PARP1/ NLRP3 signaling pathway. Tissue Cell 2024, 89, 102424. [Google Scholar] [CrossRef]
- Li, C.-Q.; He, L.-C.; Jin, J.-Q. Atractylenolide I and atractylenolide III inhibit Lipopolysaccharide-i nduced TNF-alpha and NO production in macrophages. Phytother. Res. 2007, PTR 21, 347–353. [Google Scholar] [CrossRef]
- Mao, C.; Li, D.; Zhou, E.; Zhang, J.; Wang, C.; Xue, C. Nicotine exacerbates atherosclerosis through a macrophage-mediated end othelial injury pathway. Aging 2021, 13, 7627–7643. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, X.-B.; Liu, Y.; Wang, Q.; Tang, J.; Chen, M.-J. Piperlongumine inhibits esophageal squamous cell carcinoma in vitro and in vivo by triggering NRF2/ROS/TXNIP/NLRP3-dependent pyroptosis. Chem. Biol. Interact. 2024, 390, 110875. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, H.; Shen, D.; Liu, S.; Kong, W.; Zheng, K.; Yang, J.; Ge, L. Artemisinin attenuated ischemic stroke induced pyroptosis by inhibiting ROS/TXNIP/NLRP3/Caspase-1 signaling pathway. Biomed. Pharmacother. 2024, 177, 116894. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Chen, Y.; Li, X.; Jiang, Z. Decursinol angelate relieves inflammatory bowel disease by inhibiting the ROS/TXNIP/NLRP3 pathway and pyroptosis. Front. Pharmacol. 2025, 15, 1520040. [Google Scholar] [CrossRef]
- Liang, J.; Lin, X.; Jiang, C.; Liu, Y.; Hao, Z.; Qiu, M.; Liu, X.; Chen, D.; Teng, X.; Tang, Y. Molecular mechanism of apoptosis induced by 4-tBP in common carp (Cyprinus carpio L.) head kidneys was explored from various angles: Hippo pathway, miR-203a, oxidative stress, ER stress, and mitochondrial pathway. Aquaculture 2024, 589, 740981. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Zhang, M.; Qian, Y.; Li, E.; Teng, X.; Li, M. miR-199-5p mediates the regulation of autophagy by targeting mTOR signaling and involvement in ammonia detoxification under ammonia stress in yellow catfish (Pelteobagrus fulvidraco). Aquaculture 2024, 589, 740977. [Google Scholar] [CrossRef]
- Che, X.; Shang, X.; WeiXu; Xing, M.; Wei, H.; Li, W.; Li, Z.; Teng, X.; Geng, L. Selenium-enriched Lactiplantibacillus plantarum alleviates alkalinity stress-induced selective hepatic insulin resistance in common carp. Int. J. Biol. Macromol. 2025, 305, 141204. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Qiu, M.; Liu, Y.; Liu, Y.; Chang, M.; Liu, X.; Wang, Y.; Sun, W.; Teng, X.; Tang, Y. Co-exposure to ammonia and lipopolysaccharide-induced impaired energy metabolism via the miR-1599/HK2 axis and triggered autophagy, ER stress, and apoptosis in chicken cardiomyocytes. Poult. Sci. 2025, 104, 104965. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Hao, Z.; Qiu, M.; Liu, Y.; Chang, M.; Liu, X.; Wang, Y.; Tang, Y.; Sun, W.; Teng, X.; et al. Amino acid metabolism disorder and oxidative stress took part in EGCG alleviating Mn-caused ferroptosis via miR-9-5p/got1 axis. J. Hazard. Mater. 2025, 489, 137656. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, X.D.; Xu, X.; Cai, Y.B.; Zhu, Z.X.; Zhu, L.; Ren, K. Linc00657 promoted pyroptosis in THP-1-derived macrophages and exacerbated atherosclerosis via the miR-106b-5p/TXNIP/NLRP3 axis. Int. J. Biol. Macromol. 2023, 253, 126953. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, Y.; Ma, C.Y.; Xu, X.M.; Zhang, Y.Q.; Wang, C.G.; Jin, J.; Shen, X.; Gao, J.L.; Li, N.; et al. Dimethyl fumarate induces necroptosis in colon cancer cells through GSH depletion/ROS increase/MAPKs activation pathway. Br. J. Pharmacol. 2015, 172, 3929–3943. [Google Scholar] [CrossRef]
- Qiu, M.; Hao, Z.; Liu, Y.; Liu, Y.; Chang, M.; Lin, X.; Liu, X.; Dong, N.; Sun, W.; Teng, X. ROS acted as an initial role in selenium nanoparticles alleviating insecticide chlorpyrifos-induced oxidative stress, pyroptosis, and intestinal barrier dysfunction in porcine intestinal epithelial cells. Pestic. Biochem. Physiol. 2025, 211, 106418. [Google Scholar] [CrossRef]
- Cong, L.; Liu, X.; Bai, Y.; Qin, Q.; Zhao, L.; Shi, Y.; Bai, Y.; Guo, Z. Melatonin alleviates pyroptosis by regulating the SIRT3/FOXO3α/ROS axis and interacting with apoptosis in Atherosclerosis progression. Biol. Res. 2023, 56, 62. [Google Scholar] [CrossRef]
- Choi, E.-H.; Park, S.-J. TXNIP: A key protein in the cellular stress response pathway and a potential therapeutic target. Exp. Mol. Med. 2023, 55, 1348–1356. [Google Scholar] [CrossRef]
- Chen, M.; Wang, R.; Liao, L.; Li, Y.; Sun, X.; Wu, H.; Lan, Q.; Deng, Z.; Liu, P.; Xu, T.; et al. DanShen Decoction targets miR-93-5p to provide protection against MI/RI by regulating the TXNIP/NLRP3/Caspase-1 signaling pathway. Phytomedicine 2024, 135, 156225. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Draga, D.; Zhou, C.; Su, T.; Zou, C.; Gu, Q.; Lahm, T.; Zheng, Z.; Qiu, Q. miR-590-3p Inhibits Pyroptosis in Diabetic Retinopathy by Targeting NLRP1 and Inactivating the NOX4 Signaling Pathway. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4215–4223. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, Y.; Cardell, L.O. Nicotine exaggerates LPS-induced airway hyperreactivity via JNK-mediated up-regulation of Toll-like receptor 4. Am. J. Respir. Cell Mol. Biol. 2014, 51, 370–379. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, G.; Huang, J.; Ma, S.; Mi, K.; Cheng, J.; Zhu, Y.; Zha, X.; Huang, W. Atractylenolide I modulates ovarian cancer cell-mediated immunosuppression by blocking MD-2/TLR4 complex-mediated MyD88/NF-κB signaling in vitro. J. Transl. Med. 2016, 14, 104. [Google Scholar] [CrossRef]
- Long, F.; Lin, H.; Zhang, X.; Zhang, J.; Xiao, H.; Wang, T. Atractylenolide-I Suppresses Tumorigenesis of Breast Cancer by Inhibiting Toll-Like Receptor 4-Mediated Nuclear Factor-κB Signaling Pathway. Front. Pharmacol. 2020, 11, 598939. [Google Scholar] [CrossRef]
- Hou, L.; He, Q.; Wang, Y.; Feng, X.; Mi, Y.; Li, S.; Deng, J.F.; Zhao, G. Nicotine induces macrophage pyroptosis via LINC01272/miR-515/KLF6 axis. Ecotoxicol. Environ. Saf. 2023, 263, 115265. [Google Scholar] [CrossRef]
- Xu, S.; Chen, H.; Ni, H.; Dai, Q. Targeting HDAC6 attenuates nicotine-induced macrophage pyroptosis via NF-κB/NLRP3 pathway. Atherosclerosis 2021, 317, 1–9. [Google Scholar] [CrossRef]
- Du, Z.; Ma, Z.; Lai, S.; Ding, Q.; Hu, Z.; Yang, W.; Qian, Q.; Zhu, L.; Dou, X.; Li, S. Atractylenolide I Ameliorates Acetaminophen-Induced Acute Liver Injury via the TLR4/MAPKs/NF-κB Signaling Pathways. Front. Pharmacol. 2022, 13, 797499. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Chen, R.; Zheng, J. Atractylenolide I inhibits lipopolysaccharide-induced inflammatory responses via mitogen-activated protein kinase pathways in RAW264.7 cells. Immunopharmacol. Immunotoxicol. 2014, 36, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, T.; Feng, Y.; Tong, Y.; Jia, Y.; Wang, C.; Cui, R.; Qu, K.; Liu, C.; Zhang, J. PPARγ Alleviates Sepsis-Induced Liver Injury by Inhibiting Hepatocyte Pyroptosis via Inhibition of the ROS/TXNIP/NLRP3 Signaling Pathway. Oxid. Med. Cell Longev. 2022, 2022, 1269747. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.J.; Yu, H.W.; Yang, Y.Z.; Wu, W.Y.; Chen, T.Y.; Jia, K.K.; Kang, L.L.; Jiao, R.Q.; Kong, L.D. Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biol. 2018, 18, 124–137. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, D.M.; Li, J.; Deng, S.H.; Liu, T.; Zhang, T.; He, M.; Zhao, Y.Y.; Xu, Y. Bixin Attenuates Experimental Autoimmune Encephalomyelitis by Suppressing TXNIP/NLRP3 Inflammasome Activity and Activating NRF2 Signaling. Front. Immunol. 2020, 11, 593368. [Google Scholar] [CrossRef]
- Gao, Y.; Li, S.; Zhang, S.; Zhang, Y.; Zhang, J.; Zhao, Y.; Chang, C.; Gao, X.; Chen, L.; Yang, G. Atractylenolide-I Attenuates MPTP/MPP(+)-Mediated Oxidative Stress in Parkinson’s Disease Through SIRT1/PGC-1α/Nrf2 Axis. Neurochem. Res. 2024, 50, 18. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, Y.; Xu, L.; Xu, J.; Xiong, Y.; Peng, Y.; Ding, K.; Zheng, S.; Yang, N.; Zhang, Z.; et al. Novel role for caspase 1 inhibitor VX765 in suppressing NLRP3 inflammasome assembly and atherosclerosis via promoting mitophagy and efferocytosis. Cell Death Dis. 2022, 13, 512. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, B.; Wang, Z.; Wang, D.; Ni, H.; Zhang, L.; Wang, Y. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics 2019, 9, 6901–6919. [Google Scholar] [CrossRef]
- Kabłak-Ziembicka, A.; Badacz, R.; Przewłocki, T. Clinical Application of Serum microRNAs in Atherosclerotic Coronary Artery Disease. J. Clin. Med. 2022, 11, 6849. [Google Scholar] [CrossRef]
- Liu, X.; Dong, Y.; Chen, S.; Zhang, G.; Zhang, M.; Gong, Y.; Li, X. Circulating MicroRNA-146a and MicroRNA-21 Predict Left Ventricular Remodeling after ST-Elevation Myocardial Infarction. Cardiology 2015, 132, 233–241. [Google Scholar] [CrossRef]
- Xue, Z.; Xi, Q.; Liu, H.; Guo, X.; Zhang, J.; Zhang, Z.; Li, Y.; Yang, G.; Zhou, D.; Yang, H.; et al. miR-21 promotes NLRP3 inflammasome activation to mediate pyroptosis and endotoxic shock. Cell Death Dis. 2019, 10, 461. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.-H.; Liu, X.; Wang, Y.-P.; Xu, X.; Zhu, L.; Zhang, W.; Ren, K. Atractylenolide I Inhibits Nicotine-Induced Macrophage Pyroptosis and Alleviates Atherogenesis by Suppressing the TLR4/ROS/TXNIP/NLRP3 Pathway. Metabolites 2025, 15, 329. https://doi.org/10.3390/metabo15050329
Li H-H, Liu X, Wang Y-P, Xu X, Zhu L, Zhang W, Ren K. Atractylenolide I Inhibits Nicotine-Induced Macrophage Pyroptosis and Alleviates Atherogenesis by Suppressing the TLR4/ROS/TXNIP/NLRP3 Pathway. Metabolites. 2025; 15(5):329. https://doi.org/10.3390/metabo15050329
Chicago/Turabian StyleLi, Huan-Huan, Xian Liu, Yu-Ping Wang, Xi Xu, Lin Zhu, Wei Zhang, and Kun Ren. 2025. "Atractylenolide I Inhibits Nicotine-Induced Macrophage Pyroptosis and Alleviates Atherogenesis by Suppressing the TLR4/ROS/TXNIP/NLRP3 Pathway" Metabolites 15, no. 5: 329. https://doi.org/10.3390/metabo15050329
APA StyleLi, H.-H., Liu, X., Wang, Y.-P., Xu, X., Zhu, L., Zhang, W., & Ren, K. (2025). Atractylenolide I Inhibits Nicotine-Induced Macrophage Pyroptosis and Alleviates Atherogenesis by Suppressing the TLR4/ROS/TXNIP/NLRP3 Pathway. Metabolites, 15(5), 329. https://doi.org/10.3390/metabo15050329





