Gut–Brain Inflammatory Pathways in Attention-Deficit/Hyperactivity Disorder: The Role and Therapeutic Potential of Diet
Abstract
1. Introduction
2. Key Nutrient and Dietary Patterns in ADHD and Related Interventions
2.1. Omega-3 Poly-Unsaturated Fatty Acids
2.2. Micronutrients
| Study | Study Type | Sample Size/Population | Supplement and Dosage † | ADHD Medication | Duration | Primary Outcomes | Key Findings |
|---|---|---|---|---|---|---|---|
| Hawkey and Nigg (2014) [82] | Meta-analysis of RCTs | 16 studies (N = 1408, age range 6–18 years, M = 9.7 years, with or without ADHD) | Omega-3 (total: 120–2513 mg; EPA: 0–1373 mg; DHA: 0–1140 mg) | In two studies, supplementation was an adjunct to ADHD medication | 7–24 weeks (M = 14.5 weeks) | ADHD symptom severity (parent- and teacher-rated; pooled ratings) | Significant reduction in hyperactivity-impulsivity (parent, teacher, pooled ratings) and inattention (parent and pooled ratings), compared to placebo. |
| Chang et al. (2018) [83] | Meta-analyses of RCTs | First meta-analysis (1): seven studies (N = 534). Second meta-analysis (2): three studies (N = 214). Age range 4–17 years | 1: Omega-3 (total: 120–1290 mg; EPA: 80–650 mg; DHA: 0–640 mg) 2: Omega-3 (total: 122–345 mg; EPA: 0–153 mg; DHA: 29–345 mg) | Not reported | Not stated | 1: ADHD clinical symptom scores (parent- and teacher-rated) 2: Cognitive measures associated with attention | 1: Significant reduction in parent-rated total, inattention, and hyperactivity symptoms; no effect on teacher-rated symptoms compared to placebo. 2: Significant improvement in omission and commission errors; no improvement in memory or information processing compared to placebo. |
| Abdullah et al. (2019) [85] | Systematic review of RCTs | Six studies (N = 564, age range 3–18 years) | Omega-3 (total: 100–1400 mg; EPA: 100–1000 mg; DHA: 0–400 mg) | In two studies, supplementation was an adjunct to ADHD medication | 8 weeks–4 months | ADHD symptom severity (parent- and/or teacher-rated; CPRS, CTRS) | Small, non-significant reduction in ADHD symptoms compared to placebo in five of six studies. |
| Händel et al. (2021) [86] | Meta-analysis of RCTs | 24 studies (N = 1755, age range 6–18 years) | Omega-3 and/or Omega-6 (PUFA type and dosage not reported) | In three studies, supplementation was an adjunct to ADHD medication | 8 weeks–12 months | ADHD symptom severity (parent- and teacher-rated) | No significant difference in ADHD symptoms based on parent or teacher ratings compared to placebo/control. |
| Gillies et al. (2023) [87] | Systematic review of RCTs and quasi-randomised trials | 36 studies (N > 2374) children and adolescents under 18 years | Omega-3 (EPA, DHA, ALA; 19 trials), Omega-6 (AA, LA, GLA; 2 trials), Combined Omega-3/6 (6 trials), Omega-3 + co-interventions (8 trials), Omega-3/6 + medication (1 trial) (EPA [22 studies]: 33–1039 mg; DHA [22 studies]: 2.7–3600 mg); ALA [2 studies]: 60–1080 mg; AA [2 studies]: 40–60 mg; LA [5 studies]: 240–360 mg; GLA [8 studies]: 6–345 mg) | In five omega-3 and one omega-3/6 trials, supplementation was an adjunct to ADHD medication | 2 weeks–6 months | ADHD symptom severity (parent-, teacher-, and clinician-rated) | Low-certainty evidence for improvement in ADHD symptoms. High-certainty evidence for no effect on parent-rated total, inattention, and hyperactivity/impulsivity symptoms. |
| Bos et al. (2015) [88] | Double-blind RCT | 40 boys with ADHD aged 8–14 years (M = 10.3 years): 20 intervention/20 placebo; 39 matched typically developing controls (Mage = 10.9 years): 20 intervention/19 placebo | Omega-3 (total: 1300 mg; EPA: 650 mg; DHA: 650 mg) | 38 of 40 ADHD participants were taking ADHD medication | 16 weeks | ADHD symptom severity (parent-rated; CBCL, SWAN) | Reduced inattention symptoms on the CBCL in both ADHD and control groups compared to placebo; no effect observed on the SWAN scale. |
| Rodríguez et al. (2019) [89] | Double-blind RCT | 66 participants aged 6–18 years (M = 11.7 years): 32 intervention/34 placebo | Omega-3 (total: 1240 mg; EPA: 90 mg; DHA: 1000 mg; DPA: 150 mg) | 24 of 32 participants in the intervention group and 24 of 34 in the placebo group were taking ADHD medication | 6 months | ADHD symptom severity (parent-rated; EDAH, CPRS) | Reduced inattention, hyperactivity, and overall ADHD scores on the EDAH scale compared to placebo. No between-group differences on CPRS scores. |
| San Mauro Martin et al. (2022) [90] * | Controlled trial | 31 participants aged 6–16 years (M = 10.7 years): 13 intervention/18 placebo | Omega-3 (total: 775 mg; EPA: 550 mg; DHA: 225 mg) | Not reported | 8 weeks | Impulsivity (child-report; BIS-11c) | Significantly lower levels of impulsivity compared to controls. |
| Dashti et al. (2014) [92] | Double-blind RCT | 85 participants aged 6–12 years (M = 8.2 years): 29 Ritalin/28 omega-3/28 placebo | Omega-3 (total: 1000 mg). A second intervention group received Ritalin | ADHD-medication-naïve | Duration not clearly reported | ADHD symptom severity (parent- and teacher-rated; CPRS, CTRS) | Significant reduction in ADHD symptom severity in both the omega-3 and Ritalin groups compared to placebo. No significant difference in symptom reduction between treatment groups. |
| Chang et al. (2019) [93] | Double-blind RCT | 92 participants aged 6–18 years: 48 intervention/44 placebo | Omega-3 (EPA only: 1200 mg) | ADHD-medication-naïve or medication-free for the past 6 months | 12 weeks | Blood PUFA levels; ADHD symptoms (CPT) | Greater improvement in focused attention (variability) in EPA group compared to placebo. Greater improvement in vigilance and focused attention (hit reaction time) in EPA group with lowest baseline EPA compared to placebo. Less improvement in impulsivity (commission errors) in EPA group than placebo. Less improvement in ADHD and emotional symptoms in EPA group with highest baseline EPA compared to placebo. |
| Lundbergh et al. (2022) [91] * | Double-blind randomised cross-over trial | 26 participants aged 18–40 years (M = 28 years) with ASD (14 with comorbid ADHD) | Omega-3 (total: 4000 mg; EPA: 2400 mg; DHA: 1600 mg) | Some participants were taking ADHD medication | Two 1-month supplement periods (omega-3 and placebo) | Sustained attention (d2-test); spatial working memory (Corsi); ADHD symptoms (adult-rated; CAARS) | Improvement in working memory and sustained attention compared to placebo. Participants with comorbid ADHD showed improvements in ADHD symptom scores. |
| Cornu et al. (2018) [94] | Double-blind RCT | 162 participants aged 6–15 years (M = 9.9 years): 80 intervention/82 placebo | Omega-3 (ages 6–8: total: 420 mg; EPA: 336 mg; DHA: 84 mg; ages 9–11: total: 630 mg; EPA: 504 mg; DHA: 126 mg; ages 12–15: total: 840 mg; EPA: 672 mg; DHA: 168 mg) | No ADHD medication for 1 month before and during the trial | 3 months | ADHD symptom severity (parent-rated; ADHD-RS-IV) | Greater reduction in ADHD symptoms observed in the placebo group compared to the intervention group. |
| Crippa et al. (2019) [95] | Double-blind RCT | 50 participants aged 7–14 years (M = 11.0 years): 25 intervention/25 placebo | Omega-3 (DHA only: 500 mg) | ADHD-medication-naïve | 6 months | ADHD symptom severity (parent-rated; ADHD-RS-IV) | Improvement in hyperactivity/impulsivity and total ADHD symptoms observed in both groups, with no significant difference between groups. |
| Widenhorn-Müller et al. (2014) [96] | Double-blind RCT | 95 participants aged 6–12 years (M = 8.9 years): 46 intervention/49 placebo | Omega-3 (total: 720 mg; EPA: 600 mg; DHA: 120 mg) | No ADHD medication for 6 months before and during the trial | 16 weeks | ADHD symptom severity (teacher- and parent-rated; DISYPS-II) | No significant changes in teacher- or parent-rated ADHD symptom scores compared to placebo. |
| Elliott et al. (2024) [104] | Meta-analysis of RCTs | Three studies (N = 124) for hyperactivity and two trials (N = 75) for inattention (participants under 25 years with low serum iron or iron deficiency) | Iron (ferrous sulphate: 80 mg or 300 mg; or ferrous fumarate: 200 mg if <30 kg; 400 mg if >30 kg) | In one study, supplementation was an adjunct to ADHD medication | 12 weeks | ADHD symptom severity (parent- and teacher-rated) | Non-significant improvements observed in hyperactivity and inattention scores compared to placebo. |
| Kumar et al. (2024) [105] * | Single-arm intervention | 32 children aged 4–12 years (M = 8.2 years; 23 were ferritin sufficient) | Iron (3 mg/kg) | ADHD-medication-naïve | 6 weeks | ADHD symptom severity (parent- and teacher-rated; CPRS, CTRS). Attention performance (CCTT) | Significant improvement in parent-rated hyperactivity and inattention; no significant change in teacher-rated symptoms. Significant improvement in inattention on CCTT. |
| Talebi et al. (2022) [106] | Meta-analysis of RCTs | Six studies (N = 489, age range 7–10 years) | Zinc (10–40 mg) | In four studies, supplementation was an adjunct to ADHD medication | 6–12 weeks | ADHD symptom severity (parent- and teacher-rated) | Significant reduction in total ADHD scores compared to control, but no significant changes on subscales. |
| Starobrat-Hermelin et al. (1997) [107] | RCT | 75 participants aged 7–12 years with magnesium deficiency: 50 intervention/25 control | Magnesium (approximately 200 mg) | Not reported | 6 months | ADHD symptom severity (parent- and teacher-rated; CPRS, CTRS) | Increased hair magnesium levels and decreased hyperactivity symptoms compared to control. |
| El Baza et al. (2016) [108] | RCT | 18 participants aged 6–16 years (M = 7.7 years) with magnesium deficiency: 9 intervention/9 control | Magnesium (200 mg) | All participants were taking ADHD medication | 8 weeks | ADHD symptom severity (parent-rated; CPRS) | Improvement in hyperactivity, impulsivity, and inattention compared to control. |
| Gan et al. (2019) [114] | Meta-analysis of RCTs | Four studies (N = 256, age range 2–18 years) | Vitamin D (1000 IU/day–50,000 IU/week) | In all studies, supplementation was an adjunct to ADHD medication | 6 weeks–3 months | ADHD symptom severity (clinician-, teacher-, and parent-rated) | Small improvements in ADHD total scores, inattention, hyperactivity, and behaviour scores compared to control. No improvement in oppositional scores. |
| Mirhosseini et al. (2024) [115] * | Double-blind RCT | 35 participants aged 7–13 years (M = 9.2 years) receiving neurofeedback therapy: 20 intervention/15 placebo | Vitamin D (50,000 IU/week) | No ADHD medication for one year before and during the trial | 2 months | Brain wave patterns (EEG); ADHD symptom severity (CPRS, ADHD-RS-IV) | Improvement in electrophysiological (EEG) measures; no improvement in ADHD symptom scores compared to placebo. |
| Hemamy et al. (2021) [116] | Double-blind RCT | 66 participants aged 6–12 years (M = 9.1 years) with vitamin D and magnesium deficiency: 33 intervention/33 placebo | Vitamin D (50,000 IU/week) and magnesium (6 mg/kg) | No difference in ADHD medication dose between groups | 8 weeks | Mental health status (parent-rated; SDQ) | Reduction in emotional, peer, internalizing problems, and total difficulties compared to placebo. No differences in conduct, hyperactivity, or prosocial behaviour. |
| Hemamy et al. (2020) [117] | Same study as above | Behaviour problems (parent-rated; CPRS-48) | Reduction in conduct, social, and anxiety/shyness scores, but not psychosomatic problems, compared to placebo. | ||||
| Rucklidge et al. (2018) [118] | Double-blind RCT | 93 participants aged 7–12 years (M = 9.8 years): 47 intervention/46 placebo | Broad-spectrum micronutrient formula (“Daily Essential Nutrients”: 13 vitamins, 17 minerals, four amino acids; titrated to 12 or 15 capsules) | No ADHD medication for 4 weeks before and during the trial | 10 weeks | ADHD symptom severity (clinician-rated ADHD-RS-IV; parent-rated CPRS-R:L); Clinical Global Impression-Improvement (CGI-I) | Improvement in overall functioning and inattention (CGI-I-ADHD scale), reduced impairment, and improved emotional regulation and aggression compared to placebo. In total, 32% on micronutrients showed clinical improvement in inattentive symptoms compared to 9% on placebo (CGI-I). No significant differences in clinician-, parent-, or teacher-rated ADHD symptoms from other measures between groups. |
| Rucklidge et al. (2014) [119] | Double-blind RCT | 80 participants aged 16 years or older (M = 35.2 years): 42 intervention/38 placebo | Broad-spectrum micronutrient formula (EMPowerPlus: 36 ingredients; titrated to 15 capsules) | No ADHD medication for 4 weeks before and during the trial | 8 weeks | ADHD symptom severity (self-, clinician-, and observer-rated; CAARS); Clinical Global Impression-Improvement (CGI-I) | Improvement in ADHD symptoms based on self- and observer-report, but not clinician-report. Improved clinician-rated global functioning and ADHD symptoms (from CGI-I) compared to placebo. |
| Gordon et al. (2015) [121] | Open-label reversal design intervention | 14 participants aged 8–12 years (M = 10.2 years) | Broad-spectrum micronutrient formula (EMPowerPlus: 36 ingredients; titrated to 15 capsules) | No ADHD medication for 4 weeks before and during the trial | Two 8-week treatment phases with 4-week washout periods | ADHD symptom severity (parent-rated CPRS-R:L); mental health status (parent-rated; SDQ); Clinical Global Impression-Improvement (CGI-I) | Improvement in ADHD symptoms, mood, and overall functioning during intervention phases; deterioration during washout periods. At the end of the second treatment phase, 79% rated as “much improved” or “very much improved” on overall clinical impression ratings; lower total difficulties, conduct problems, hyperactivity, and impact score and improved prosocial behaviour. |
| Johnstone et al. (2022) [122] | Double-blind RCT | 126 participants with irritability aged 6–12 years (M = 9.8 years): 71 intervention/55 placebo | Broad-spectrum micronutrient formula (“Daily Essential Nutrients”: 13 vitamins, 17 minerals, four amino acids; titrated to 9 or 12 capsules) | No ADHD medication for 4 weeks before and during the trial | 8 weeks | Composite of ADHD symptoms, oppositional defiant disorder, disruptive mood dysregulation, peer conflict, and impairment (parent-rated CASI-5); Clinical Global Impression-Improvement (CGI-I) | Both groups showed reduced CASI-5 scores; no significant difference between groups. 54% of the micronutrient group and 18% of the placebo group were responders based on CGI-I scores. |
2.3. Detrimental Dietary Patterns and Components and Elimination Diets
2.4. The Mediterranean Diet and Related Diets
3. Key Diet-Regulated Biological Pathways
3.1. Dietary Influence on Gut Dysbiosis and Inflammation in ADHD
3.2. MedDiet Modulation of Gut Microbiome and Inflammatory Pathways
3.2.1. The MedDiet
| Dietary Feature | Physiological Effect | Key References |
|---|---|---|
| High MedDiet adherence | Anti-Inflammatory and Antioxidant Effects | |
| Reduced inflammation and oxidative stress | [76,240,241,242] | |
| Gut Microbiota Composition and SCFA Production | ||
| Improved gut microbiota composition | [77,203,205,239,242,243,244] | |
| Reduced Escherichia coli and Ruminococcus gnavus [203,244] abundance | [203] | |
| Increased Bifidobacteria: E. coli ratio, Candida albicans, and total bacteria | ||
| Increased Faecalibacterium prausnitzii [205,244], Eubacterium eligens, and Bacteroides cellulosilyticus abundance | [205] | |
| Increased Prevotella abundance | [77,243] | |
| Increased levels of total SCFAs | [203] | |
| Enhanced Microbial Fibre Metabolism | ||
| Enhanced functions for fibre degradation | [205] | |
| Enhanced genes for microbial fibre degradation | [244] | |
| Fibre/prebiotics | Gut Barrier Integrity and Inflammation Regulation | |
| Reduced dysbiosis-induced gut permeability and chronic inflammation | [245] | |
| Gut Microbiota Composition and SCFA Production | ||
| Improved gut microbiota composition and increased levels of SCFAs | [246,247,248] | |
| Increased Bacteroidetes and Lactobacillus abundance Reduced Firmicutes and Fusobacterium abundance | [245] | |
| Polyphenols | Anti-Inflammatory and Antioxidant Effects | |
| Reduced oxidative stress and DNA damage Scavenging of harmful reactive species Inhibition of enzymes in inflammatory pathways Reduced plasma inflammatory markers | [249,250,251,252] | |
| Gut Microbiota Composition | ||
| Improved gut microbiota composition | [251,253,254,255] | |
| Increased Lactobacilli and Bifidobacterial abundance Reduced Clostridia abundance | [251] | |
| Plant-based foods | Gut Microbiota Composition | |
| Positive association with Eubacterium eligens Negative association with Flavonifractor and Ruminococcus torques | [202] | |
| Decreased F/B ratio and increased bacterial diversity | [256] | |
| EVOO | Inflammation, Oxidative Stress, and Apoptosis Regulation | |
| Reduced markers of inflammation and oxidative stress Increased anti-inflammatory factors | [257] | |
| Inhibition of inflammation, oxidative stress, and apoptosis | [258] | |
| Gut Microbiota Composition | ||
| Beneficial changes in gut microbiota composition | [259] | |
| Increased beneficial lactic acid-producing gut bacteria | [257] | |
| Omega-3 PUFAs | Inflammation and Oxidative Stress Regulation | |
| Reduced plasma inflammatory mediators and oxidative stress | [260] | |
| Improved inflammatory status | [261,262] | |
| Inhibition of pro-inflammatory cytokines | [263] | |
| Reduced metabolic endotoxemia and inflammation | [264] | |
| Gut Microbiota Composition and SCFA Production | ||
| Increased abundance of SCFA-producing bacterial genera | [265,266] | |
| Reduced fatty-liver associated genus Increased bacterial fermentation products | [266] | |
| Increased total and caecal SCFAs | [263,267] | |
| Increased bacterial alpha diversity | [267] | |
| Altered gut microbiota composition, with increased beneficial species and reduced pro-inflammatory strains | [264,268] | |
| Gut Barrier Integrity and Immune Regulation | ||
| Increased tight junction protein expression | [263] | |
| Improved intestinal barrier integrity | [264,267,268] | |
| Improved immune homeostasis and metabolic profile | [268] |
3.2.2. Fibre, Polyphenols, and Other Antioxidants
3.2.3. Extra-Virgin Olive Oil
3.2.4. Omega-3 PUFAs
4. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADHD | Attention-deficit/hyperactivity disorder |
| ASD | Autism spectrum disorder |
| US | United States |
| MedDiet | Mediterranean diet |
| PUFA | Poly-unsaturated fatty acid |
| EPA | Eicosapentaenoic acid |
| DHA | Docosahexaenoic acid |
| RCT | Randomised controlled trial |
| ARA | Arachidonic acid |
| SSB | Sugar-sweetened beverage |
| AFC | Artificial food colouring |
| CVD | Cardiovascular disease |
| EVOO | Extra-virgin olive oil |
| DASH | Dietary Approaches to Stop Hypertension |
| SCFA | Short chain fatty acid |
| F/B | Firmicutes to Bacteroidetes ratio |
| FODMAPS | Fermentable oligo-, di-, and monosaccharides and polyols |
References
- Biederman, J.; Petty, C.R.; Evans, M.; Small, J.; Faraone, S.V. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Res. 2010, 177, 299–304. [Google Scholar] [CrossRef]
- Thapar, A.; Cooper, M. Attention deficit hyperactivity disorder. Lancet 2016, 387, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Posner, J.; Polanczyk, G.V.; Sonuga-Barke, E. Attention-deficit hyperactivity disorder. Lancet 2020, 395, 450–462. [Google Scholar] [CrossRef]
- Faraone, S.V.; Rostain, A.L.; Blader, J.; Busch, B.; Childress, A.C.; Connor, D.F.; Newcorn, J.H. Practitioner Review: Emotional dysregulation in attention-deficit/hyperactivity disorder—Implications for clinical recognition and intervention. J. Child Psychol. Psychiatry 2019, 60, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, A.; Chavanon, M.-L.; Christiansen, H. Emotion dysregulation in adults with attention deficit hyperactivity disorder: A meta-analysis. BMC Psychiatry 2020, 20, 120. [Google Scholar] [CrossRef]
- Kooij, J.J.S.; Bijlenga, D.; Salerno, L.; Jaeschke, R.; Bitter, I.; Balázs, J.; Thome, J.; Dom, G.; Kasper, S.; Nunes Filipe, C.; et al. Updated European Consensus Statement on diagnosis and treatment of adult ADHD. Eur. Psychiatry 2019, 56, 14–34. [Google Scholar] [CrossRef] [PubMed]
- Hiscock, H.; Mulraney, M.; Heussler, H.; Rinehart, N.; Schuster, T.; Grobler, A.C.; Gold, L.; Mudiyanselage, S.B.; Hayes, N.; Sciberras, E. Impact of a behavioral intervention, delivered by pediatricians or psychologists, on sleep problems in children with ADHD: A cluster-randomized, translational trial. J. Child Psychol. Psychiatry 2019, 60, 1230–1241. [Google Scholar] [CrossRef]
- Becker, S.P.; Langberg, J.M.; Eadeh, H.-M.; Isaacson, P.A.; Bourchtein, E. Sleep and daytime sleepiness in adolescents with and without ADHD: Differences across ratings, daily diary, and actigraphy. J. Child Psychol. Psychiatry 2019, 60, 1021–1031. [Google Scholar] [CrossRef]
- McKeown, C.; Hisle-Gorman, E.; Eide, M.; Gorman, G.H.; Nylund, C.M. Association of constipation and fecal incontinence with attention-deficit/hyperactivity disorder. Pediatrics 2013, 132, e1210–e1215. [Google Scholar] [CrossRef]
- Curatolo, P.; D’Agati, E.; Moavero, R. The neurobiological basis of ADHD. Ital. J. Pediatr. 2010, 36, 79. [Google Scholar] [CrossRef]
- Willcutt, E.G.; Nigg, J.T.; Pennington, B.F.; Solanto, M.V.; Rohde, L.A.; Tannock, R.; Loo, S.K.; Carlson, C.L.; McBurnett, K.; Lahey, B.B. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. J. Abnorm. Psychol. 2012, 121, 991–1010. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.; Hodgkins, P.; Caci, H.; Young, S.; Kahle, J.; Woods, A.G.; Arnold, L.E. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: Effects of treatment and non-treatment. BMC Med. 2012, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Biederman, J.; Petty, C.R.; Monuteaux, M.C.; Fried, R.; Byrne, D.; Mirto, T.; Spencer, T.; Wilens, T.E.; Faraone, S.V. Adult Psychiatric Outcomes of Girls with Attention Deficit Hyperactivity Disorder: 11-Year Follow-Up in a Longitudinal Case-Control Study. Am. J. Psychiatry 2010, 167, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Hinshaw, S.P.; Owens, E.B.; Zalecki, C.; Huggins, S.P.; Montenegro-Nevado, A.J.; Schrodek, E.; Swanson, E.N. Prospective follow-up of girls with attention-deficit/hyperactivity disorder into early adulthood: Continuing impairment includes elevated risk for suicide attempts and self-injury. J. Consult. Clin. Psychol. 2012, 80, 1041–1051. [Google Scholar] [CrossRef]
- Choi, W.S.; Woo, Y.S.; Wang, S.M.; Lim, H.K.; Bahk, W.M. The prevalence of psychiatric comorbidities in adult ADHD compared with non-ADHD populations: A systematic literature review. PLoS ONE 2022, 17, e0277175. [Google Scholar] [CrossRef]
- Franke, B.; Michelini, G.; Asherson, P.; Banaschewski, T.; Bilbow, A.; Buitelaar, J.K.; Cormand, B.; Faraone, S.V.; Ginsberg, Y.; Haavik, J.; et al. Live fast, die young? A review on the developmental trajectories of ADHD across the lifespan. Eur. Neuropsychopharmacol. 2018, 28, 1059–1088. [Google Scholar] [CrossRef]
- Chhibber, A.; Watanabe, A.H.; Chaisai, C.; Veettil, S.K.; Chaiyakunapruk, N. Global Economic Burden of Attention-Deficit/Hyperactivity Disorder: A Systematic Review. Pharmacoeconomics 2021, 39, 399–420. [Google Scholar] [CrossRef]
- Cenit, M.C.; Nuevo, I.C.; Codoner-Franch, P.; Dinan, T.G.; Sanz, Y. Gut microbiota and attention deficit hyperactivity disorder: New perspectives for a challenging condition. Eur. Child Adolesc. Psychiatry 2017, 26, 1081–1092. [Google Scholar] [CrossRef]
- Biederman, J.; Spencer, T. Attention-deficit/hyperactivity disorder (adhd) as a noradrenergic disorder. Biol. Psychiatry 1999, 46, 1234–1242. [Google Scholar] [CrossRef]
- Faraone, S.V.; Larsson, H. Genetics of attention deficit hyperactivity disorder. Mol. Psychiatry 2019, 24, 562–575. [Google Scholar] [CrossRef]
- Faraone, S.V.; Perlis, R.H.; Doyle, A.E.; Smoller, J.W.; Goralnick, J.J.; Holmgren, M.A.; Sklar, P. Molecular Genetics of Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 2005, 57, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Nikolas, M.A.; Burt, S.A. Genetic and environmental influences on ADHD symptom dimensions of inattention and hyperactivity: A meta-analysis. J. Abnorm. Psychol. 2010, 119, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Verlaet, A.; Briceno Noriega, D.; Hermans, N.; Savelkoul, H.F.J. Nutrition, immunological mechanisms and dietary immunomodulation in ADHD. Eur. Child Adolesc. Psychiatry 2014, 23, 519–529. [Google Scholar] [CrossRef]
- Braun, J.M.; Kahn, R.S.; Froehlich, T.; Auinger, P.; Lanphear, B.P. Exposures to Environmental Toxicants and Attention Deficit Hyperactivity Disorder in U.S. Children. Environ. Health Perspect. 2006, 114, 1904–1909. [Google Scholar] [CrossRef]
- Bull-Larsen, S.; Mohajeri, M.H. The Potential Influence of the Bacterial Microbiome on the Development and Progression of ADHD. Nutrients 2019, 11, 2805. [Google Scholar] [CrossRef]
- Grizenko, N.; Shayan, Y.R.; Polotskaia, A.; Ter-Stepanian, M.; Joober, R. Relation of maternal stress during pregnancy to symptom severity and response to treatment in children with ADHD. J. Psychiatry Neurosci. 2008, 33, 10–16. [Google Scholar]
- Johnson, S.P.; Kochhar, P.M.; Hennessy, E.M.; Marlow, N.D.; Wolke, D.P.; Hollis, C.P. Antecedents of Attention-Deficit/Hyperactivity Disorder Symptoms in Children Born Extremely Preterm. J. Dev. Behav. Pediatr. 2016, 37, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Olsen, J.; Vestergaard, M.; Obel, C. Attention-deficit/hyperactivity disorder in the offspring following prenatal maternal bereavement: A nationwide follow-up study in Denmark. Eur. Child Adolesc. Psychiatry 2010, 19, 747–753. [Google Scholar] [CrossRef]
- Linnet, K.M.; Dalsgaard, S.; Obel, C.; Wisborg, K.; Henriksen, T.B.; Rodriguez, A.; Kotimaa, A.; Moilanen, I.; Thomsen, P.H.; Olsen, J.; et al. Maternal Lifestyle Factors in Pregnancy Risk of Attention Deficit Hyperactivity Disorder and Associated Behaviors: Review of the Current Evidence. Am. J. Psychiatry 2003, 160, 1028–1040. [Google Scholar] [CrossRef]
- Mann, J.R.; McDermott, S. Are Maternal Genitourinary Infection and Pre-Eclampsia Associated With ADHD in School-Aged Children? J. Atten. Disord. 2011, 15, 667–673. [Google Scholar] [CrossRef]
- Park, S.; Kim, B.-N.; Kim, J.-W.; Shin, M.-S.; Yoo, H.J.; Cho, S.-C. Protective effect of breastfeeding with regard to children’s behavioral and cognitive problems. Nutr. J. 2014, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, S.K.; Epstein, J.N.; Bellinger, D.C.; Korrick, S.A. Pre- and postnatal risk factors for ADHD in a nonclinical pediatric population. J. Atten. Disord. 2013, 17, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Colvin, L.; Hagemann, E.; Bower, C. Environmental risk factors by gender associated with attention-deficit/hyperactivity disorder. Pediatrics 2014, 133, e14–e22. [Google Scholar] [CrossRef]
- Sucksdorff, M.; Lehtonen, L.; Chudal, R.; Suominen, A.; Joelsson, P.; Gissler, M.; Sourander, A. Preterm Birth and Poor Fetal Growth as Risk Factors of Attention-Deficit/ Hyperactivity Disorder. Pediatrics 2015, 136, e599–e608. [Google Scholar] [CrossRef]
- Thapar, A.; Cooper, M.; Eyre, O.; Langley, K. What have we learnt about the causes of ADHD? J. Child Psychol. Psychiatry 2013, 54, 3–16. [Google Scholar] [CrossRef]
- Jo, H.; Schieve, L.A.; Sharma, A.J.; Hinkle, S.N.; Li, R.; Lind, J.N. Maternal prepregnancy body mass index and child psychosocial development at 6 years of age. Pediatrics 2015, 135, e1198–e1209. [Google Scholar] [CrossRef]
- Rodriguez, A.; Miettunen, J.; Henriksen, T.B.; Olsen, J.; Obel, C.; Taanila, A.; Ebeling, H.; Linnet, K.M.; Moilanen, I.; Järvelin, M.R. Maternal adiposity prior to pregnancy is associated with ADHD symptoms in offspring: Evidence from three prospective pregnancy cohorts. Int. J. Obes. 2008, 32, 550–557. [Google Scholar] [CrossRef]
- Sanchez, C.E.; Barry, C.; Sabhlok, A.; Russell, K.; Majors, A.; Kollins, S.H.; Fuemmeler, B.F. Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: A meta-analysis. Obes. Rev. 2018, 19, 464–484. [Google Scholar] [CrossRef]
- Sciberras, E.; Mulraney, M.; Silva, D.; Coghill, D. Prenatal Risk Factors and the Etiology of ADHD-Review of Existing Evidence. Curr. Psychiatry Rep. 2017, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, P.B.; Clausen, T.D.; Petersen, A.H.; Hageman, I.; Pinborg, A.; Kessing, L.V.; Bergholt, T.; Rasmussen, S.C.; Keiding, N.; Løkkegaard, E.C.L. Investigating the effects of cesarean delivery and antibiotic use in early childhood on risk of later attention deficit hyperactivity disorder. Child Psychol. Psychiatry 2019, 60, 151–159. [Google Scholar] [CrossRef]
- Stadler, D.D.; Musser, E.D.; Holton, K.F.; Shannon, J.; Nigg, J.T. Recalled Initiation and Duration of Maternal Breastfeeding Among Children with and Without ADHD in a Well Characterized Case-Control Sample. J. Abnorm. Child Psychol. 2016, 44, 347–355. [Google Scholar] [CrossRef] [PubMed]
- del Campo, N.; Chamberlain, S.R.; Sahakian, B.J.; Robbins, T.W. The Roles of Dopamine and Noradrenaline in the Pathophysiology and Treatment of Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 2011, 69, e145–e157. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology. CNS Drugs 2009, 23, 33–41. [Google Scholar] [CrossRef]
- Banerjee, E.; Nandagopal, K. Does serotonin deficit mediate susceptibility to ADHD? Neurochem. Int. 2015, 82, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Quist, J.F.; Kennedy, J.L. Genetics of childhood disorders: XXIII. ADHD, Part 7: The serotonin system. J. Am. Acad. Child Adolesc. Psychiatry 2001, 40, 253–256. [Google Scholar] [CrossRef]
- Wang, L.-J.; Yu, Y.-H.; Fu, M.-L.; Yeh, W.-T.; Hsu, J.-L.; Yang, Y.-H.; Chen, W.J.; Chiang, B.-L.; Pan, W.-H. Attention deficit-hyperactivity disorder is associated with allergic symptoms and low levels of hemoglobin and serotonin. Sci. Rep. 2018, 8, 10229. [Google Scholar] [CrossRef]
- Lewis, N.; Villani, A.; Lagopoulos, J. Gut dysbiosis as a driver of neuroinflammation in attention-deficit/hyperactivity disorder: A review of current evidence. Neuroscience 2025, 569, 298–321. [Google Scholar] [CrossRef]
- Bailey, M.T.; Lubach, G.R.; Coe, C.L. Prenatal stress alters bacterial colonization of the gut in infant monkeys. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 414–421. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Lieber, A.D.; Wu, F.; Perez-Perez, G.I.; Chen, Y.; et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 2016, 8, 343ra82. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; De Jesus-Laboy, K.M.; Shen, N.; Cox, L.M.; Amir, A.; Gonzalez, A.; Bokulich, N.A.; Song, S.J.; Hoashi, M.; Rivera-Vinas, J.I.; et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat. Med. 2016, 22, 250–253. [Google Scholar] [CrossRef]
- Fouhy, F.; Watkins, C.; Hill, C.J.; O’Shea, C.-A.; Nagle, B.; Dempsey, E.M.; O’Toole, P.W.; Ross, R.P.; Ryan, C.A.; Stanton, C. Perinatal factors affect the gut microbiota up to four years after birth. Nat. Commun. 2019, 10, 1517. [Google Scholar] [CrossRef]
- Grölund, M.-M.; Lehtonen, O.-P.; Eerola, E.; Kero, P. Fecal Microflora in Healthy Infants Born by Different Methods of Delivery: Permanent Changes in Intestinal Flora After Cesarean Delivery. J. Pediatr. Gastroenterol. Nutr. 1999, 28, 19–25. [Google Scholar] [CrossRef]
- Levin, A.M.; Sitarik, A.R.; Havstad, S.L.; Fujimura, K.E.; Wegienka, G.; Cassidy-Bushrow, A.E.; Kim, H.; Zoratti, E.M.; Lukacs, N.W.; Boushey, H.A.; et al. Joint effects of pregnancy, sociocultural, and environmental factors on early life gut microbiome structure and diversity. Sci. Rep. 2016, 6, 31775. [Google Scholar] [CrossRef] [PubMed]
- Gilley, S.P.; Ruebel, M.L.; Sims, C.; Zhong, Y.; Turner, D.; Lan, R.S.; Pack, L.M.; Piccolo, B.D.; Chintapalli, S.V.; Abraham, A.; et al. Associations between maternal obesity and offspring gut microbiome in the first year of life. Pediatr. Obes. 2022, 17, e12921. [Google Scholar] [CrossRef] [PubMed]
- Groer, M.W.; Miller, E.M.; D’Agata, A.; Ho, T.T.B.; Dutra, S.V.; Yoo, J.Y.; Yee, A.L.; Gilbert, J.A.; Dishaw, L.J. Contributors to Dysbiosis in Very-Low-Birth-Weight Infants. J. Obstet. Gynecol. Neonatal Nurs. 2020, 49, 232–242. [Google Scholar] [CrossRef]
- Sandhu, K.V.; Sherwin, E.; Schellekens, H.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Feeding the microbiota-gut-brain axis: Diet, microbiome, and neuropsychiatry. Transl. Res. J. Lab. Clin. Med. 2017, 179, 223–244. [Google Scholar] [CrossRef]
- Papanastasiou, G.; Drigas, A.; Papanastasiou, P. The association of diet quality and lifestyle factors in children and adults with ADHD: A systematic review and meta-analysis. Sci. Electron. Arch. 2021, 14. [Google Scholar] [CrossRef]
- Altabella, L.; Zoratto, F.; Adriani, W.; Canese, R. MR imaging–detectable metabolic alterations in attention deficit/hyperactivity disorder: From preclinical to clinical studies. Am. J. Neuroradiol. 2014, 35 (Suppl. S6), S55–S63. [Google Scholar] [CrossRef]
- Cortese, S.; Adamo, N.; Del Giovane, C.; Mohr-Jensen, C.; Hayes, A.J.; Carucci, S.; Atkinson, L.Z.; Tessari, L.; Banaschewski, T.; Coghill, D.; et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: A systematic review and network meta-analysis. lancet. Psychiatry 2018, 5, 727–738. [Google Scholar] [CrossRef]
- De Crescenzo, F.; Cortese, S.; Adamo, N.; Janiri, L. Pharmacological and non-pharmacological treatment of adults with ADHD: A meta-review. Evid. Based Ment. Health 2017, 20, 4–11. [Google Scholar] [CrossRef]
- Gürkan, K.; Bilgiç, A.; Türkoğlu, S.; Kılıç, B.G.; Aysev, A.; Uslu, R. Depression, anxiety and obsessive-compulsive symptoms and quality of life in children with attention-deficit hyperactivity disorder (ADHD) during three-month methylphenidate treatment. J. Psychopharmacol. 2010, 24, 1810–1818. [Google Scholar] [CrossRef] [PubMed]
- Kuczenski, R.; Segal, D.S. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: Comparison with amphetamine. J. Neurochem. 1997, 68, 2032–2037. [Google Scholar] [CrossRef]
- Bonvicini, C.; Cortese, S.; Maj, C.; Baune, B.T.; Faraone, S.V.; Scassellati, C. DRD4 48 bp multiallelic variants as age-population-specific biomarkers in attention-deficit/hyperactivity disorder. Transl. Psychiatry 2020, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Childress, A.C.; Sallee, F.R. Attention-deficit/hyperactivity disorder with inadequate response to stimulants: Approaches to management. CNS Drugs 2014, 28, 121–129. [Google Scholar] [CrossRef]
- Verlaet, A.A.J.; Maasakkers, C.M.; Hermans, N.; Savelkoul, H.F.J. Rationale for Dietary Antioxidant Treatment of ADHD. Nutrients 2018, 10, 405. [Google Scholar] [CrossRef] [PubMed]
- Ching, C.; Eslick, G.D.; Poulton, A.S. Evaluation of Methylphenidate Safety and Maximum-Dose Titration Rationale in Attention-Deficit/Hyperactivity Disorder: A Meta-analysis. JAMA Pediatr 2019, 173, 630–639. [Google Scholar] [CrossRef]
- Dias, T.G.C.; Kieling, C.; Graeff-Martins, A.S.; Moriyama, T.S.; Rohde, L.A.; Polanczyk, G.V. Developments and challenges in the diagnosis and treatment of ADHD. Rev. Bras. Psiquiatr. 2013, 35 (Suppl. S1), S40–S50. [Google Scholar] [CrossRef]
- Ramstad, E.; Storebø, O.J.; Gerner, T.; Krogh, H.B.; Holmskov, M.; Magnusson, F.L.; Moreira-Maia, C.R.; Skoog, M.; Groth, C.; Gillies, D.; et al. Hallucinations and other psychotic symptoms in response to methylphenidate in children and adolescents with attention-deficit/hyperactivity disorder: A Cochrane systematic review with meta-analysis and trial sequential analysis#. Scand. J. Child Adolesc. Psychiatry Psychol. 2018, 6, 52–71. [Google Scholar] [CrossRef]
- Shyu, Y.-C.; Yuan, S.-S.; Lee, S.-Y.; Yang, C.-J.; Yang, K.-C.; Lee, T.-L.; Wang, L.-J. Attention-deficit/hyperactivity disorder, methylphenidate use and the risk of developing schizophrenia spectrum disorders: A nationwide population-based study in Taiwan. Schizophr. Res. 2015, 168, 161–167. [Google Scholar] [CrossRef]
- Antshel, K.M.; Faraone, S.V.; Gordon, M. Cognitive behavioral treatment outcomes in adolescent ADHD. J. Atten. Disord. 2014, 18, 483–495. [Google Scholar] [CrossRef]
- Cairncross, M.; Miller, C.J. The Effectiveness of Mindfulness-Based Therapies for ADHD: A Meta-Analytic Review. J. Atten. Disord. 2020, 24, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Cerrillo-Urbina, A.J.; García-Hermoso, A.; Sánchez-López, M.; Pardo-Guijarro, M.J.; Gómez, J.L.S.; Martínez-Vizcaíno, V. The effects of physical exercise in children with attention deficit hyperactivity disorder: A systematic review and meta-analysis of randomized control trials. Child Care Health Dev. 2015, 41, 779–788. [Google Scholar] [CrossRef]
- Nigg, J.T.; Lewis, K.; Edinger, T.; Falk, M. Meta-analysis of attention-deficit/hyperactivity disorder or attention-deficit/hyperactivity disorder symptoms, restriction diet, and synthetic food color additives. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 86–97.e8. [Google Scholar] [CrossRef]
- Del-Ponte, B.; Quinte, G.C.; Cruz, S.; Grellert, M.; Santos, I.S. Dietary patterns and attention deficit/hyperactivity disorder (ADHD): A systematic review and meta-analysis. J. Affect. Disord. 2019, 252, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018, 72, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R. Anti-inflammatory effects of the Mediterranean diet: The experience of the PREDIMED study. Proc. Nutr. Soc. 2010, 69, 333–340. [Google Scholar] [CrossRef]
- Buchman, M.; Kruse, C.; Broach, W.; Jensen, E.; Kales, S.; Vattem, D.; Prieto, M.S. Composition of Human Gut Microbiota After a Mediterranean Diet Intervention Among Fire Fighters (OR23-05-19). Curr. Dev. Nutr. 2019, 3 (Suppl. S1), nzz040.OR23-05-19. [Google Scholar] [CrossRef]
- Das, U.N. Essential fatty acids: Biochemistry, physiology and pathology. Biotechnol. J. 2006, 1, 420–439. [Google Scholar] [CrossRef]
- Richardson, A.J. The importance of omega-3 fatty acids for behaviour, cognition and mood. Scand. J. Nutr. 2003, 47, 92–98. [Google Scholar] [CrossRef][Green Version]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Fuentes-Albero, M.; Martínez-Martínez, M.I.; Cauli, O. Omega-3 Long-Chain Polyunsaturated Fatty Acids Intake in Children with Attention Deficit and Hyperactivity Disorder. Brain Sci. 2019, 9, 120. [Google Scholar] [CrossRef]
- Hawkey, E.; Nigg, J.T. Omega-3 fatty acid and ADHD: Blood level analysis and meta-analytic extension of supplementation trials. Clin. Psychol. Rev. 2014, 34, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.P.-C.; Su, K.-P.; Mondelli, V.; Pariante, C.M. Omega-3 Polyunsaturated Fatty Acids in Youths with Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-Analysis of Clinical Trials and Biological Studies. Neuropsychopharmacology 2018, 43, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Crippa, A.; Agostoni, C.; Mauri, M.; Molteni, M.; Nobile, M. Polyunsaturated Fatty Acids Are Associated With Behavior But Not With Cognition in Children With and Without ADHD: An Italian study. J. Atten. Disord. 2018, 22, 971–983. [Google Scholar] [CrossRef]
- Abdullah, M.; Jowett, B.; Whittaker, P.J.; Patterson, L. The effectiveness of omega-3 supplementation in reducing ADHD associated symptoms in children as measured by the Conners’ rating scales: A systematic review of randomized controlled trials. J. Psychiatr. Res. 2019, 110, 64–73. [Google Scholar] [CrossRef]
- Händel, M.N.; Rohde, J.F.; Rimestad, M.L.; Bandak, E.; Birkefoss, K.; Tendal, B.; Lemcke, S.; Callesen, H.E. Efficacy and Safety of Polyunsaturated Fatty Acids Supplementation in the Treatment of Attention Deficit Hyperactivity Disorder (ADHD) in Children and Adolescents: A Systematic Review and Meta-Analysis of Clinical Trials. Nutrients 2021, 13, 1226. [Google Scholar] [CrossRef]
- Gillies, D.; Leach, M.J.; Algorta, G.P. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst. Rev. 2023, 4, CD007986. [Google Scholar] [CrossRef] [PubMed]
- Bos, D.J.; Oranje, B.; Veerhoek, E.S.; Van Diepen, R.M.; Weusten, J.M.; Demmelmair, H.; Koletzko, B.; de Sain van der Velden, M.G.; Eilander, A.; Hoeksma, M.; et al. Reduced Symptoms of Inattention after Dietary Omega-3 Fatty Acid Supplementation in Boys with and without Attention Deficit/Hyperactivity Disorder. Neuropsychopharmacology 2015, 40, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.; García, T.; Areces, D.; Fernández, E.; García-Noriega, M.; Domingo, J.C. Supplementation with high-content docosahexaenoic acid triglyceride in attention-deficit hyperactivity disorder: A randomized double-blind placebo-controlled trial. Neuropsychiatr. Dis. Treat. 2019, 15, 1193–1209. [Google Scholar] [CrossRef]
- San Mauro Martin, I.; Sanz Rojo, S.; Gonzalez Cosano, L.; Conty de la Campa, R.; Garicano Vilar, E.; Blumenfeld Olivares, J.A. Impulsiveness in children with attention-deficit/hyperactivity disorder after an 8-week intervention with the Mediterranean diet and/or omega-3 fatty acids: A randomised clinical trial. Neurología 2022, 37, 513–523. [Google Scholar] [CrossRef]
- Lundbergh, B.; Enevoldsen, A.S.; Stark, K.D.; Ritz, C.; Lauritzen, L. Fish oil supplementation may improve attention, working memory and attention-deficit/hyperactivity disorder symptoms in adults with autism spectrum disorder: A randomised crossover trial. Br. J. Nutr. 2022, 128, 2398–2408. [Google Scholar] [CrossRef] [PubMed]
- Dashti, N.; Hekmat, H.; Soltani, H.R.; Rahimdel, A.; Javaherchian, M. Comparison of therapeutic effects of omega-3 and methylphenidate (ritalin(®)) in treating children with attention deficit hyperactivity disorder. Iran. J. Psychiatry Behav. Sci. 2014, 8, 7–11. [Google Scholar]
- Chang, J.P.-C.; Su, K.-P.; Mondelli, V.; Satyanarayanan, S.K.; Yang, H.-T.; Chiang, Y.-J.; Chen, H.-T.; Pariante, C.M. High-dose eicosapentaenoic acid (EPA) improves attention and vigilance in children and adolescents with attention deficit hyperactivity disorder (ADHD) and low endogenous EPA levels. Transl. Psychiatry 2019, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Cornu, C.; Mercier, C.; Ginhoux, T.; Masson, S.; Mouchet, J.; Nony, P.; Kassai, B.; Laudy, V.; Berquin, P.; Franc, N.; et al. A double-blind placebo-controlled randomised trial of omega-3 supplementation in children with moderate ADHD symptoms. Eur. Child Adolesc. Psychiatry 2018, 27, 377–384. [Google Scholar] [CrossRef]
- Crippa, A.; Tesei, A.; Sangiorgio, F.; Salandi, A.; Trabattoni, S.; Grazioli, S.; Agostoni, C.; Molteni, M.; Nobile, M. Behavioral and cognitive effects of docosahexaenoic acid in drug-naïve children with attention-deficit/hyperactivity disorder: A randomized, placebo-controlled clinical trial. Eur. Child Adolesc. Psychiatry 2019, 28, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Widenhorn-Müller, K.; Schwanda, S.; Scholz, E.; Spitzer, M.; Bode, H. Effect of supplementation with long-chain ω-3 polyunsaturated fatty acids on behavior and cognition in children with attention deficit/hyperactivity disorder (ADHD): A randomized placebo-controlled intervention trial. Prostaglandins Leukot. Essent. Fat. Acids 2014, 91, 49–60. [Google Scholar] [CrossRef]
- Donev, R.; Thome, J. Inflammation: Good or bad for ADHD? ADHD Atten. Deficit Hyperact. Disord. 2010, 2, 257–266. [Google Scholar] [CrossRef]
- Wang, L.-J.; Yu, Y.-H.; Fu, M.-L.; Yeh, W.-T.; Hsu, J.-L.; Yang, Y.-H.; Yang, H.-T.; Huang, S.-Y.; Wei, I.-L.; Chen, W.J.; et al. Dietary Profiles, Nutritional Biochemistry Status, and Attention-Deficit/Hyperactivity Disorder: Path Analysis for a Case-Control Study. J. Clin. Med. 2019, 8, 709. [Google Scholar] [CrossRef]
- Elbaz, F.; Zahra, S.; Hanafy, H. Magnesium, zinc and copper estimation in children with attention deficit hyperactivity disorder (ADHD). Egypt. J. Med. Hum. Genet. 2017, 18, 153–163. [Google Scholar] [CrossRef]
- Konofal, E.; Lecendreux, M.; Arnulf, I.; Mouren, M.-C. Iron deficiency in children with attention-deficit/hyperactivity disorder. Arch. Pediatr. Adolesc. Med. 2004, 158, 1113–1115. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, G.; Ran, Q.; Nie, L.; Liu, X.; Pan, Z.; He, L. Quantitative susceptibility mapping shows lower brain iron content in children with attention-deficit hyperactivity disorder. Hum. Brain Mapp. 2022, 43, 2495–2502. [Google Scholar] [CrossRef] [PubMed]
- Effatpanah, M.; Rezaei, M.; Effatpanah, H.; Effatpanah, Z.; Varkaneh, H.K.; Mousavi, S.M.; Fatahi, S.; Rinaldi, G.; Hashemi, R. Magnesium status and attention deficit hyperactivity disorder (ADHD): A meta-analysis. Psychiatry Res. 2019, 274, 228–234. [Google Scholar] [CrossRef]
- Smykiewicz, K.; Michalczewska, A.; Wierzejska, N.; Pach, M.; Nowak, A.; Fugas, A.; Chmielowiec, Z.; Partyka, A.; Dziedzic, M.; Dobrzańska, J. Magnesium as a potential complementary treatment for ADHD—A review of recent literature. J. Educ. Health Sport 2024, 68, 50663. [Google Scholar] [CrossRef]
- Elliott, S.D.; Vickers, M.L.; McKeon, G.; Eriksson, L.; Malacova, E.; Scott, J.G. Iron Supplementation in Management of Neurodevelopmental Disorders: Systematic Review, Meta-Analysis, and Qualitative Synthesis. J. Neuropsychiatry Clin. Neurosci. 2024, 36, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Juneja, M.; Kaushik, S.; Gupta, A. Iron Supplementation in Children with Attention Deficit Hyperactivity Disorder: A Single Centre Study. Indian Pediatr. 2024, 61, 745–749. [Google Scholar] [CrossRef]
- Talebi, S.; Miraghajani, M.; Ghavami, A.; Mohammadi, H. The effect of zinc supplementation in children with attention deficit hyperactivity disorder: A systematic review and dose-response meta-analysis of randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2022, 62, 9093–9102. [Google Scholar] [CrossRef]
- Starobrat-Hermelin, B.; Kozielec, T. The effects of magnesium physiological supplementation on hyperactivity in children with attention deficit hyperactivity disorder (ADHD). Positive response to magnesium oral loading test. Magnes. Res. 1997, 10, 149–156. [Google Scholar]
- El Baza, F.; AlShahawi, H.A.; Zahra, S.; AbdelHakim, R.A. Magnesium supplementation in children with attention deficit hyperactivity disorder. Egypt. J. Med. Hum. Genet. 2016, 17, 63–70. [Google Scholar] [CrossRef]
- Altun, H.; Şahin, N.; Belge Kurutaş, E.; Güngör, O. Homocysteine, Pyridoxine, Folate and Vitamin B12 Levels in Children with Attention Deficit Hyperactivity Disorder. Psychiatr. Danub. 2018, 30, 310–316. [Google Scholar] [CrossRef]
- Yektaş, Ç.; Alpay, M.; Tufan, A.E. Comparison of serum B12, folate and homocysteine concentrations in children with autism spectrum disorder or attention deficit hyperactivity disorder and healthy controls. Neuropsychiatr. Dis. Treat. 2019, 15, 2213–2219. [Google Scholar] [CrossRef]
- Lukovac, T.; Hil, O.A.; Popović, M.; Jovanović, V.; Savić, T.; Pavlović, A.M.; Pavlović, D. Serum Biomarker Analysis in Pediatric ADHD: Implications of Homocysteine, Vitamin B12, Vitamin D, Ferritin, and Iron Levels. Children 2024, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- Khoshbakht, Y.; Bidaki, R.; Salehi-Abargouei, A. Vitamin D Status and Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-Analysis of Observational Studies. Adv. Nutr. 2018, 9, 9–20. [Google Scholar] [CrossRef]
- Elshahawi, H.; Amin, G.; Khalil, S.; Kasem, R.; Mostafa, A. Serum Vitamin D Level in Children with Attention Deficit Hyperactivity Disorder (ADHD). QJM Int. J. Med. 2024, 117 (Suppl. S2), hcae175.491. [Google Scholar] [CrossRef]
- Gan, J.; Galer, P.; Ma, D.; Chen, C.; Xiong, T. The Effect of Vitamin D Supplementation on Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Child Adolesc. Psychopharmacol. 2019, 29, 670–687. [Google Scholar] [CrossRef] [PubMed]
- Mirhosseini, H.; Maayeshi, N.; Hooshmandi, H.; Moradkhani, S.; Hosseinzadeh, M. The effect of vitamin D supplementation on the brain mapping and behavioral performance of children with ADHD: A double-blinded randomized controlled trials. Nutr. Neurosci. 2024, 27, 566–576. [Google Scholar] [CrossRef]
- Hemamy, M.; Pahlavani, N.; Amanollahi, A.; Islam, S.M.S.; McVicar, J.; Askari, G.; Malekahmadi, M. The effect of vitamin D and magnesium supplementation on the mental health status of attention-deficit hyperactive children: A randomized controlled trial. BMC Pediatr. 2021, 21, 178. [Google Scholar] [CrossRef]
- Hemamy, M.; Heidari-Beni, M.; Askari, G.; Karahmadi, M.; Maracy, M. Effect of Vitamin D and Magnesium Supplementation on Behavior Problems in Children with Attention-Deficit Hyperactivity Disorder. Int. J. Prev. Med. 2020, 11, 4. [Google Scholar] [CrossRef]
- Rucklidge, J.J.; Eggleston, M.J.F.; Johnstone, J.M.; Darling, K.; Frampton, C.M. Vitamin-mineral treatment improves aggression and emotional regulation in children with ADHD: A fully blinded, randomized, placebo-controlled trial. J. Child Psychol. Psychiatry 2018, 59, 232–246. [Google Scholar] [CrossRef]
- Rucklidge, J.J.; Frampton, C.M.; Gorman, B.; Boggis, A. Vitamin–mineral treatment of attention-deficit hyperactivity disorder in adults: Double-blind randomised placebo-controlled trial. Br. J. Psychiatry 2014, 204, 306–315. [Google Scholar] [CrossRef]
- Rucklidge, J.J.; Frampton, C.M.; Gorman, B.; Boggis, A. Vitamin-Mineral Treatment of ADHD in Adults. J. Atten. Disord. 2017, 21, 522–532. [Google Scholar] [CrossRef]
- Gordon, H.A.; Rucklidge, J.J.; Blampied, N.M.; Johnstone, J.M. Clinically Significant Symptom Reduction in Children with Attention-Deficit/Hyperactivity Disorder Treated with Micronutrients: An Open-Label Reversal Design Study. J. Child Adolesc. Psychopharmacol. 2015, 25, 783–798. [Google Scholar] [CrossRef]
- Johnstone, J.M.; Hatsu, I.; Tost, G.; Srikanth, P.; Eiterman, L.P.; Bruton, A.M.; Ast, H.K.; Robinette, L.M.; Stern, M.M.; Millington, E.G.; et al. Micronutrients for Attention-Deficit/Hyperactivity Disorder in Youths: A Placebo-Controlled Randomized Clinical Trial. J. Am. Acad. Child Adolesc. Psychiatry 2022, 61, 647–661. [Google Scholar] [CrossRef]
- Darling, K.A.; Eggleston, M.J.F.; Retallick-Brown, H.; Rucklidge, J.J. Mineral-Vitamin Treatment Associated with Remission in Attention-Deficit/Hyperactivity Disorder Symptoms and Related Problems: 1-Year Naturalistic Outcomes of a 10-Week Randomized Placebo-Controlled Trial. J. Child Adolesc. Psychopharmacol. 2019, 29, 688–704. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Bes-Rastrollo, M.; Román-Viñas, B.; Pfrimer, K.; Sánchez-Villegas, A.; Martínez-González, M.A. Dietary patterns and nutritional adequacy in a Mediterranean country. Br. J. Nutr. 2009, 101 (Suppl. S2), S21–S28. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.L.; Robinson, M.; Smith, G.J.; Ambrosini, G.L.; Piek, J.P.; Oddy, W.H. ADHD is associated with a “Western” dietary pattern in adolescents. J. Atten. Disord. 2011, 15, 403–411. [Google Scholar] [CrossRef] [PubMed]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota-Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Zhang, H.; Li, M.; Mo, L.; Luo, J.; Shen, Q.; Quan, W. Association between Western Dietary Patterns, Typical Food Groups, and Behavioral Health Disorders: An Updated Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2023, 16, 125. [Google Scholar] [CrossRef]
- Oddy, W.H.; Allen, K.L.; Trapp, G.S.A.; Ambrosini, G.L.; Black, L.J.; Huang, R.-C.; Rzehak, P.; Runions, K.C.; Pan, F.; Beilin, L.J.; et al. Dietary patterns, body mass index and inflammation: Pathways to depression and mental health problems in adolescents. Brain Behav. Immun. 2018, 69, 428–439. [Google Scholar] [CrossRef]
- Zielińska, M.; Łuszczki, E.; Michońska, I.; Dereń, K. The Mediterranean Diet and the Western Diet in Adolescent Depression-Current Reports. Nutrients 2022, 14, 4390. [Google Scholar] [CrossRef]
- Jacka, F.N.; Pasco, J.A.; Mykletun, A.; Williams, L.J.; Hodge, A.M.; O’Reilly, S.L.; Nicholson, G.C.; Kotowicz, M.A.; Berk, M. Association of Western and Traditional Diets with Depression and Anxiety in Women. Am. J. Psychiatry 2010, 167, 305–311. [Google Scholar] [CrossRef]
- Atak, S.; Boye, A.; Botoseneanu, A.; Pecina, S.; Liu, Z.-X. Diet and Cognition in Aging: Effects of High-Fat-Sugar Diets on Memory and Executive Functioning. Innov. Aging 2024, 8 (Suppl. S1), 943–944. [Google Scholar] [CrossRef]
- Jacka, F.N.; Cherbuin, N.; Anstey, K.J.; Sachdev, P.; Butterworth, P. Western diet is associated with a smaller hippocampus: A longitudinal investigation. BMC Med. 2015, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Attuquayefio, T.; Stevenson, R.J.; Oaten, M.J.; Francis, H.M. A four-day Western-style dietary intervention causes reductions in hippocampal-dependent learning and memory and interoceptive sensitivity. PLoS ONE 2017, 12, e0172645. [Google Scholar] [CrossRef]
- Abbasi, K.; Beigrezai, S.; Ghiasvand, R.; Pourmasoumi, M.; Mahaki, B. Dietary Patterns and Attention Deficit Hyperactivity Disorder Among Iranian Children: A Case-Control Study. J. Am. Coll. Nutr. 2019, 38, 76–83. [Google Scholar] [CrossRef]
- Rojo-Marticella, M.; Arija, V.; Alda, J.Á.; Morales-Hidalgo, P.; Esteban-Figuerola, P.; Canals, J. Do Children with Attention-Deficit/Hyperactivity Disorder Follow a Different Dietary Pattern than That of Their Control Peers? Nutrients 2022, 14, 1131. [Google Scholar] [CrossRef]
- Akin, S.; Gultekin, F.; Ekinci, O.; Kanik, A.; Ustundag, B.; Tunali, B.D.; Al-Bayati, M.B.A.; Yasoz, C. Processed meat products and snacks consumption in ADHD: A case-control study. North. Clin. Istanb. 2022, 9, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Darabi, Z.; Vasmehjani, A.A.; Darand, M.; Sangouni, A.A.; Hosseinzadeh, M. Adherence to Mediterranean diet and attention-deficit/hyperactivity disorder in children: A case control study. Clin. Nutr. ESPEN 2022, 47, 346–350. [Google Scholar] [CrossRef]
- Ríos-Hernández, A.; Alda, J.A.; Farran-Codina, A.; Ferreira-García, E.; Izquierdo-Pulido, M. The Mediterranean Diet and ADHD in Children and Adolescents. Pediatrics 2017, 139, e20162027. [Google Scholar] [CrossRef]
- San Mauro Martín, I.; Blumenfeld Olivares, J.A.; Garicano Vilar, E.; Echeverry López, M.; Garcia Bernat, M.; Quevedo Santos, Y.; Blanco López, M.; Elortegui Pascual, P.; Borregon Rivilla, E.; Rincón Barrado, M. Nutritional and environmental factors in attention-deficit hyperactivity disorder (ADHD): A cross-sectional study. Nutr. Neurosci. 2018, 21, 641–647. [Google Scholar] [CrossRef]
- Beşenek, M.; Yazıcı, M. Mediterranean diet habits and their effects on symptomatology among children and adolescents with attention deficit hyperac-tivity disorder. J. Clin. Psychiatry 2022, 25, 193–201. [Google Scholar] [CrossRef]
- Bayranj, Z.; Fotros, D.; Sohouli, M.H.; Rohani, P.; Eslahi, M.; Ferdosi, S.; Khodadadi, N.; Hosseinzadeh, M. The relation between MIND diet with odds of attention-deficit/hyperactivity disorder in Iranian children: A case-control study. Child Neuropsychol. 2024, 31, 331–345. [Google Scholar] [CrossRef]
- Jung, T.-H.; Hwang, H.-J.; Han, K.-S. Correlation of attention deficit hyperactivity disorder with gut microbiota according to the dietary intake of Korean elementary school students. PLoS ONE 2022, 17, e0275520. [Google Scholar] [CrossRef]
- Lee, K.-S.; Choi, Y.-J.; Lim, Y.-H.; Lee, J.Y.; Shin, M.-K.; Kim, B.-N.; Shin, C.H.; Lee, Y.A.; Kim, J.I.; Hong, Y.-C. Dietary patterns are associated with attention-deficit hyperactivity disorder (ADHD) symptoms among preschoolers in South Korea: A prospective cohort study. Nutr. Neurosci. 2022, 25, 603–611. [Google Scholar] [CrossRef]
- Robinette, L.M.; Hatsu, I.E.; Johnstone, J.M.; Tost, G.; Bruton, A.M.; Leung, B.M.Y.; Odei, J.B.; Orchard, T.; Gracious, B.L.; Arnold, L.E. Fruit and vegetable intake is inversely associated with severity of inattention in a pediatric population with ADHD symptoms: The MADDY Study. Nutr Neurosci 2023, 26, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Cho, S.-C.; Hong, Y.-C.; Oh, S.-Y.; Kim, J.-W.; Shin, M.-S.; Kim, B.-N.; Yoo, H.-J.; Cho, I.-H.; Bhang, S.-Y. Association between dietary behaviors and attention-deficit/hyperactivity disorder and learning disabilities in school-aged children. Psychiatry Res. 2012, 198, 468–476. [Google Scholar] [CrossRef] [PubMed]
- San Mauro Martin, I.; Sanz Rojo, S.; Garicano Vilar, E.; González Cosano, L.; Conty de la Campa, R.; Blumenfeld Olivares, J.A. Lifestyle factors, diet and attention-deficit/hyperactivity disorder in Spanish children—An observational study. Nutr. Neurosci. 2021, 24, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, P.; Cuenca-García, M.; Labayen, I.; Esteban-Cornejo, I.; Henriksson, H.; Kersting, M.; Vanhelst, J.; Widhalm, K.; Gottrand, F.; Moreno, L.A.; et al. Diet quality and attention capacity in European adolescents: The Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) study. Br. J. Nutr. 2017, 117, 1587–1595. [Google Scholar] [CrossRef]
- Yu, C.-J.; Du, J.-C.; Chiou, H.-C.; Feng, C.-C.; Chung, M.-Y.; Yang, W.; Chen, Y.-S.; Chien, L.-C.; Hwang, B.; Chen, M.-L. Sugar-Sweetened Beverage Consumption Is Adversely Associated with Childhood Attention Deficit/Hyperactivity Disorder. Int. J. Environ. Res. Public Health 2016, 13, 678. [Google Scholar] [CrossRef]
- Farsad-Naeimi, A.; Asjodi, F.; Omidian, M.; Askari, M.; Nouri, M.; Pizarro, A.B.; Daneshzad, E. Sugar consumption, sugar sweetened beverages and Attention Deficit Hyperactivity Disorder: A systematic review and meta-analysis. Complement. Ther. Med. 2020, 53, 102512. [Google Scholar] [CrossRef]
- Del-Ponte, B.; Anselmi, L.; Assunção, M.C.F.; Tovo-Rodrigues, L.; Munhoz, T.N.; Matijasevich, A.; Rohde, L.A.; Santos, I.S. Sugar consumption and attention-deficit/hyperactivity disorder (ADHD): A birth cohort study. J. Affect. Disord. 2019, 243, 290–296. [Google Scholar] [CrossRef]
- Schab, D.W.; Trinh, N.-H. Do artificial food colors promote hyperactivity in children with hyperactive syndromes? A meta-analysis of double-blind placebo-controlled trials. J. Dev. Behav. Pediatr. JDBP 2004, 25, 423–434. [Google Scholar] [CrossRef]
- Torp, N.M.U.; Thomsen, P.H. The use of diet interventions to treat symptoms of ADHD in children and adolescents—A systematic review of randomized controlled trials. Nord. J. Psychiatry 2020, 74, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Huberts-Bosch, A.; Bierens, M.; Ly, V.; van der Velde, J.; de Boer, H.; van Beek, G.; Appelman, D.; Visser, S.; Bos, L.H.P.; Reijmers, L.; et al. Short-term effects of an elimination diet and healthy diet in children with attention-deficit/hyperactivity disorder: A randomized-controlled trial. Eur. Child Adolesc. Psychiatry 2024, 33, 1503–1516. [Google Scholar] [CrossRef]
- Abd El Baaki, O.M.; Abd El Hamid, E.R.; Zaki, S.T.; Alwakkad, A.S.E.D.; Sabry, R.N.; Elsheikh, E.M. Diet modification impact on ADHD outcome. Bull. Natl. Res. Cent. 2021, 45, 15. [Google Scholar] [CrossRef]
- Hontelez, S.; Stobernack, T.; Pelsser, L.M.; van Baarlen, P.; Frankena, K.; Groefsema, M.M.; Kleerebezem, M.; Pereira, R.R.; Postma, E.M.; Smeets, P.A.M.; et al. Correlation between brain function and ADHD symptom changes in children with ADHD following a few-foods diet: An open-label intervention trial. Sci. Rep. 2021, 11, 22205. [Google Scholar] [CrossRef] [PubMed]
- Yorgidis, E.; Beiner, L.; Blazynski, N.; Schneider-Momm, K.; Clement, H.-W.; Rauh, R.; Schulz, E.; Clement, C.; Fleischhaker, C. Individual Behavioral Reactions in the Context of Food Sensitivities in Children with Attention-Deficit/Hyperactivity Disorder before and after an Oligoantigenic Diet. Nutrients 2021, 13, 2598. [Google Scholar] [CrossRef]
- Walz, G.; Blazynski, N.; Frey, L.; Schneider-Momm, K.; Clement, H.W.; Rauh, R.; Schulz, E.; Biscaldi, M.; Clement, C.; Fleischhaker, C. Long-Term Effects of an Oligoantigenic Diet in Children with Attention-Deficit/Hyperactivity Disorder (ADHD) on Core Symptomatology. Nutrients 2022, 14, 5111. [Google Scholar] [CrossRef]
- Long, L.; Peng, H.; Chen, X.; Wang, F.; Long, W.; Cheng, M.; Ma, J. The Impact of Integrating a Low-Lectin Diet with Traditional ADHD Treatments on Gut Microbiota Composition and Symptom Improvement in Children—A Cohort Study. Neuropsychiatr. Dis. Treat. 2024, 20, 535–549. [Google Scholar] [CrossRef]
- Canal, L.A.; Sáez, I.C.; del Castillo, M.C.F.; Antón, J.A.; de Prada Vicente, I.; Zaragozá, C.I.; Gallego, J.T.; Gómez, M.J.M. Gluten-Free Diet for the Treatment of ADHD. Pilot Study 2019, 3. [Google Scholar] [CrossRef]
- Lykogeorgou, M.; Karkelis, S.; Papadaki-Papandreou, O.; Nikita, M. PS-253b Gluten Free Diet For Children With Attention Deficit And Hyperactivity Disorder. Arch. Dis. Child. 2014, 99 (Suppl. S2), A204–A205. [Google Scholar] [CrossRef]
- Jacka, F.N. Targeting the gut to achieve improved outcomes in mood disorders. Bipolar Disord. 2019, 21, 88–89. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Psaltopoulou, T.; Sergentanis, T.N.; Panagiotakos, D.B.; Sergentanis, I.N.; Kosti, R.; Scarmeas, N. Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Ann. Neurol. 2013, 74, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Hardman, R.J.; Kennedy, G.; Macpherson, H.; Scholey, A.B.; Pipingas, A. Adherence to a Mediterranean-Style Diet and Effects on Cognition in Adults: A Qualitative Evaluation and Systematic Review of Longitudinal and Prospective Trials. Front. Nutr. 2016, 3, 212467. [Google Scholar] [CrossRef]
- Muñoz, M.A.; Fíto, M.; Marrugat, J.; Covas, M.I.; Schröder, H. Adherence to the Mediterranean diet is associated with better mental and physical health. Br. J. Nutr. 2009, 101, 1821–1827. [Google Scholar] [CrossRef]
- Juton, C.; Berruezo, P.; Rajmil, L.; Lerin, C.; Fíto, M.; Homs, C.; Según, G.; Gómez, S.F.; Schröder, H. Prospective Association between Adherence to the Mediterranean Diet and Health-Related Quality of Life in Spanish Children. Nutrients 2022, 14, 5304. [Google Scholar] [CrossRef]
- Caamaño-Navarrete, F.; Latorre-Román, P.; Párraga-Montilla, J.; Jerez-Mayorga, D.; Delgado-Floody, P. Selective Attention and Concentration Are Related to Lifestyle in Chilean Schoolchildren. Children 2021, 8, 856. [Google Scholar] [CrossRef]
- Karstens, A.J.; Tussing-Humphreys, L.; Zhan, L.; Rajendran, N.; Cohen, J.; Dion, C.; Zhou, X.J.; Lamar, M. Associations of the Mediterranean diet with cognitive and neuroimaging phenotypes of dementia in healthy older adults. Am. J. Clin. Nutr. 2019, 109, 361–368. [Google Scholar] [CrossRef]
- Parletta, N.; Zarnowiecki, D.; Cho, J.; Wilson, A.; Bogomolova, S.; Villani, A.; Itsiopoulos, C.; Niyonsenga, T.; Blunden, S.; Meyer, B.; et al. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: A randomized controlled trial (HELFIMED). Nutr. Neurosci. 2019, 22, 474–487. [Google Scholar] [CrossRef]
- Sánchez-Villegas, A.; Martínez-González, M.A.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Covas, M.I.; Arós, F.; Romaguera, D.; Gómez-Gracia, E.; Lapetra, J.; et al. Mediterranean dietary pattern and depression: The PREDIMED randomized trial. BMC Med. 2013, 11, 208. [Google Scholar] [CrossRef] [PubMed]
- Khoshbakht, Y.; Moghtaderi, F.; Bidaki, R.; Hosseinzadeh, M.; Salehi-Abargouei, A. The effect of dietary approaches to stop hypertension (DASH) diet on attention-deficit hyperactivity disorder (ADHD) symptoms: A randomized controlled clinical trial. Eur. J. Nutr. 2021, 60, 3647–3658. [Google Scholar] [CrossRef]
- Daliry, A.; Pereira, E.N.G.d.S. Role of Maternal Microbiota and Nutrition in Early-Life Neurodevelopmental Disorders. Nutrients 2021, 13, 3533. [Google Scholar] [CrossRef]
- Perea, V.; Urquizu, X.; Valverde, M.; Macias, M.; Carmona, A.; Esteve, E.; Escribano, G.; Pons, N.; Giménez, O.; Gironés, T.; et al. Influence of Maternal Diabetes on the Risk of Neurodevelopmental Disorders in Offspring in the Prenatal and Postnatal Periods. Diabetes Metab. J. 2022, 46, 912–922. [Google Scholar] [CrossRef]
- Borge, T.C.; Biele, G.; Papadopoulou, E.; Andersen, L.F.; Jacka, F.; Eggesbø, M.; Caspersen, I.H.; Aase, H.; Meltzer, H.M.; Brantsæter, A.L. The associations between maternal and child diet quality and child ADHD—Findings from a large Norwegian pregnancy cohort study. BMC Psychiatry 2021, 21, 139. [Google Scholar] [CrossRef] [PubMed]
- López-Vicente, M.; Ribas Fitó, N.; Vilor-Tejedor, N.; Garcia-Esteban, R.; Fernández-Barrés, S.; Dadvand, P.; Murcia, M.; Rebagliato, M.; Ibarluzea, J.; Lertxundi, A.; et al. Prenatal Omega-6:Omega-3 Ratio and Attention Deficit and Hyperactivity Disorder Symptoms. J. Pediatr. 2019, 209, 204–211.e4. [Google Scholar] [CrossRef]
- Villagomez, A.; Ramtekkar, U. Iron, Magnesium, Vitamin D, and Zinc Deficiencies in Children Presenting with Symptoms of Attention-Deficit/Hyperactivity Disorder. Children 2014, 1, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Stanger, O.; Fowler, B.; Piertzik, K.; Huemer, M.; Haschke-Becher, E.; Semmler, A.; Lorenzl, S.; Linnebank, M. Homocysteine, folate and vitamin B12 in neuropsychiatric diseases: Review and treatment recommendations. Expert Rev. Neurother. 2009, 9, 1393–1412. [Google Scholar] [CrossRef]
- Johnson, R.J.; Gold, M.S.; Johnson, D.R.; Ishimoto, T.; Lanaspa, M.A.; Zahniser, N.R.; Avena, N.M. Attention-Deficit/Hyperactivity Disorder: Is it time to Reappraise the Role of Sugar Consumption? Postgrad. Med. 2011, 123, 39–49. [Google Scholar] [CrossRef]
- Stilling, R.M.; Dinan, T.G.; Cryan, J.F. Microbial genes, brain & behaviour—Epigenetic regulation of the gut-brain axis. Genes Brain Behav. 2014, 13, 69–86. [Google Scholar] [CrossRef]
- Becattini, S.; Taur, Y.; Pamer, E.G. Antibiotic-Induced Changes in the Intestinal Microbiota and Disease. Trends Mol. Med. 2016, 22, 458–478. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S.J.S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef]
- Yang, N.J.; Chiu, I.M. Bacterial Signaling to the Nervous System through Toxins and Metabolites. J. Mol. Biol. 2017, 429, 587–605. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.-M.; Quigley, E.M.M.; Cryan, J.F.; Dinan, T.G. Early life stress alters behavior, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef]
- Belizário, J.E.; Napolitano, M. Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Front. Microbiol. 2015, 6, 1050. [Google Scholar] [CrossRef] [PubMed]
- Aarts, E.; Ederveen, T.H.A.; Naaijen, J.; Zwiers, M.P.; Boekhorst, J.; Timmerman, H.M.; Smeekens, S.P.; Netea, M.G.; Buitelaar, J.K.; Franke, B.; et al. Gut microbiome in ADHD and its relation to neural reward anticipation. PLoS ONE 2017, 12, e0183509. [Google Scholar] [CrossRef]
- Wan, L.; Ge, W.-R.; Zhang, S.; Sun, Y.-L.; Wang, B.; Yang, G. Case-Control Study of the Effects of Gut Microbiota Composition on Neurotransmitter Metabolic Pathways in Children with Attention Deficit Hyperactivity Disorder. Front. Neurosci. 2020, 14, 127. [Google Scholar] [CrossRef]
- Szopinska-Tokov, J.; Dam, S.; Naaijen, J.; Konstanti, P.; Rommelse, N.; Belzer, C.; Buitelaar, J.; Franke, B.; Aarts, E.; Vasquez, A.A. Investigating the Gut Microbiota Composition of Individuals with Attention-Deficit/Hyperactivity Disorder and Association with Symptoms. Microorganisms 2020, 8, 406. [Google Scholar] [CrossRef]
- Prehn-Kristensen, A.; Zimmermann, A.; Tittmann, L.; Lieb, W.; Schreiber, S.; Baving, L.; Fischer, A. Reduced microbiome alpha diversity in young patients with ADHD. PLoS ONE 2018, 13, e0200728. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-Y.; Zhou, Y.-Y.; Zhou, G.-L.; Li, Y.-C.; Yuan, J.; Li, X.-H.; Ruan, B. Gut microbiota profiles in treatment-naïve children with attention deficit hyperactivity disorder. Behav. Brain Res. 2018, 347, 408–413. [Google Scholar] [CrossRef]
- Wang, L.-J.; Yang, C.-Y.; Chou, W.-J.; Lee, M.-J.; Chou, M.-C.; Kuo, H.-C.; Yeh, Y.-M.; Lee, S.-Y.; Huang, L.-H.; Li, S.-C. Gut microbiota and dietary patterns in children with attention-deficit/hyperactivity disorder. Eur. Child Adolesc. Psychiatry 2020, 29, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Richarte, V.; Sánchez-Mora, C.; Corrales, M.; Fadeuilhe, C.; Vilar-Ribó, L.; Arribas, L.; Garcia, E.; Rosales-Ortiz, S.K.; Arias-Vasquez, A.; Soler-Artigas, M.; et al. Gut microbiota signature in treatment-naïve attention-deficit/hyperactivity disorder. Transl. Psychiatry 2021, 11, 382. [Google Scholar] [CrossRef]
- Touch, S.; Godefroy, E.; Rolhion, N.; Danne, C.; Oeuvray, C.; Straube, M.; Galbert, C.; Brot, L.; Salgueiro, I.A.; Chadi, S.; et al. Human CD4+CD8α+ Tregs induced by Faecalibacterium prausnitzii protect against intestinal inflammation. JCI Insight 2022, 7, e154722. [Google Scholar] [CrossRef]
- Wang, L.-J.; Yang, C.-Y.; Kuo, H.-C.; Chou, W.-J.; Tsai, C.-S.; Lee, S.-Y. Effect of Bifidobacterium bifidum on Clinical Characteristics and Gut Microbiota in Attention-Deficit/Hyperactivity Disorder. J. Pers. Med. 2022, 12, 227. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-J.; Li, S.-C.; Li, S.-W.; Kuo, H.-C.; Lee, S.-Y.; Huang, L.-H.; Chin, C.-Y.; Yang, C.-Y. Gut microbiota and plasma cytokine levels in patients with attention-deficit/hyperactivity disorder. Transl. Psychiatry 2022, 12, 76. [Google Scholar] [CrossRef]
- Pellegrini, S.; Sordi, V.; Bolla, A.M.; Saita, D.; Ferrarese, R.; Canducci, F.; Clementi, M.; Invernizzi, F.; Mariani, A.; Bonfanti, R.; et al. Duodenal Mucosa of Patients With Type 1 Diabetes Shows Distinctive Inflammatory Profile and Microbiota. J. Clin. Endocrinol. Metab. 2017, 102, 1468–1477. [Google Scholar] [CrossRef]
- Bastiaanssen, T.F.S.; Cowan, C.S.M.; Claesson, M.J.; Dinan, T.G.; Cryan, J.F. Making Sense of the Microbiome in Psychiatry. Int. J. Neuropsychopharmacol. 2019, 22, 37–52. [Google Scholar] [CrossRef]
- De Angelis, M.; Ferrocino, I.; Calabrese, F.M.; De Filippis, F.; Cavallo, N.; Siragusa, S.; Rampelli, S.; Di Cagno, R.; Rantsiou, K.; Vannini, L.; et al. Diet influences the functions of the human intestinal microbiome. Sci. Rep. 2020, 10, 4247. [Google Scholar] [CrossRef]
- Latorre-Pérez, A.; Hernández, M.; Iglesias, J.R.; Morán, J.; Pascual, J.; Porcar, M.; Vilanova, C.; Collado, L. The Spanish gut microbiome reveals links between microorganisms and Mediterranean diet. Sci. Rep. 2021, 11, 21602. [Google Scholar] [CrossRef] [PubMed]
- Mitsou, E.K.; Kakali, A.; Antonopoulou, S.; Mountzouris, K.C.; Yannakoulia, M.; Panagiotakos, D.B.; Kyriacou, A. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br. J. Nutr. 2017, 117, 1645–1655. [Google Scholar] [CrossRef]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Nguyen, L.H.; Li, Y.; Yan, Y.; Ma, W.; Rinott, E.; Ivey, K.L.; Shai, I.; Willett, W.C.; Hu, F.B.; et al. The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk. Nat. Med. 2021, 27, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front. Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef]
- Daniel, H.; Gholami, A.M.; Berry, D.; Desmarchelier, C.; Hahne, H.; Loh, G.; Mondot, S.; Lepage, P.; Rothballer, M.; Walker, A.; et al. High-fat diet alters gut microbiota physiology in mice. ISME J. 2014, 8, 295–308. [Google Scholar] [CrossRef]
- Ding, S.; Chi, M.M.; Scull, B.P.; Rigby, R.; Schwerbrock, N.M.; Magness, S.; Jobin, C.; Lund, P.K. High-fat diet: Bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS ONE 2010, 5, e12191. [Google Scholar] [CrossRef]
- Bailén, M.; Bressa, C.; Martínez-López, S.; González-Soltero, R.; Montalvo Lominchar, M.G.; San Juan, C.; Larrosa, M. Microbiota Features Associated With a High-Fat/Low-Fiber Diet in Healthy Adults. Front. Nutr. 2020, 7, 583608. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; Wilkinson, S.A.; Callaway, L.K.; McIntyre, H.D.; Morrison, M.; Dekker Nitert, M. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes 2018, 9, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Vela, G.; Stark, P.; Socha, M.; Sauer, A.K.; Hagmeyer, S.; Grabrucker, A.M. Zinc in Gut-Brain Interaction in Autism and Neurological Disorders. Neural Plast. 2015, 2015, 972791. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.K.; Kumar, S.; Rani, D.; Maurya, S.K.; Banerjee, P.; Verma, M.; Senapati, S. Implications of vitamin D deficiency in systemic inflammation and cardiovascular health. Crit. Rev. Food Sci. Nutr. 2024, 64, 10438–10455. [Google Scholar] [CrossRef] [PubMed]
- Giammanco, M.; Mazzola, M.; Giammanco, M.M.; Tomasello, G.; Carini, F. Omega-3 PUFAs and gut microbiota: A preventive approach for colorectal cancer. J. Biol. Res. 2021, 94, 94–99. [Google Scholar] [CrossRef]
- Talamonti, E.; Sasso, V.; To, H.; Haslam, R.P.; Napier, J.A.; Ulfhake, B.; Pernold, K.; Asadi, A.; Hessa, T.; Jacobsson, A.; et al. Impairment of DHA synthesis alters the expression of neuronal plasticity markers and the brain inflammatory status in mice. FASEB J. 2020, 34, 2024–2040. [Google Scholar] [CrossRef]
- Curone, G.; Biscarini, F.; Cotozzolo, E.; Menchetti, L.; Dal Bosco, A.; Riva, F.; Cremonesi, P.; Agradi, S.; Mattioli, S.; Castiglioni, B.; et al. Could Dietary Supplementation with Different Sources of N-3 Polyunsaturated Fatty Acids Modify the Rabbit Gut Microbiota? Antibiotics 2022, 11, 227. [Google Scholar] [CrossRef]
- Le Jan, D.; Siliman Misha, M.; Destrumelle, S.; Terceve, O.; Thorin, C.; Larcher, T.; Ledevin, M.; Desfontis, J.C.; Betti, E.; Mallem, Y. Omega-3 Fatty Acid and Vitamin D Supplementations Partially Reversed Metabolic Disorders and Restored Gut Microbiota in Obese Wistar Rats. Biology 2024, 13, 1070. [Google Scholar] [CrossRef]
- Kawano, Y.; Edwards, M.; Huang, Y.; Bilate, A.M.; Araujo, L.P.; Tanoue, T.; Atarashi, K.; Ladinsky, M.S.; Reiner, S.L.; Wang, H.H.; et al. Microbiota imbalance induced by dietary sugar disrupts immune-mediated protection from metabolic syndrome. Cell 2022, 185, 3501–3519.e20. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, X.; Zeng, M.; Qi, L.; Tang, X.; Wang, D.; Zhang, M.; Xie, Y.; Li, H.; Yang, X.; et al. A diet high in sugar and fat influences neurotransmitter metabolism and then affects brain function by altering the gut microbiota. Transl. Psychiatry 2021, 11, 328. [Google Scholar] [CrossRef]
- Gultekin, F.; Oner, M.E.; Savas, H.B.; Dogan, B. Food additives and microbiota. North. Clin. Istanb. 2020, 7, 192–200. [Google Scholar] [CrossRef]
- Yan, J.; Wang, D.; Li, K.; Chen, Q.; Lai, W.; Tian, L.; Lin, B.; Tan, Y.; Liu, X.; Xi, Z. Toxic effects of the food additives titanium dioxide and silica on the murine intestinal tract: Mechanisms related to intestinal barrier dysfunction involved by gut microbiota. Environ. Toxicol. Pharmacol. 2020, 80, 103485. [Google Scholar] [CrossRef] [PubMed]
- Hrncirova, L.; Hudcovic, T.; Sukova, E.; Machova, V.; Trckova, E.; Krejsek, J.; Hrncir, T. Human gut microbes are susceptible to antimicrobial food additives in vitro. Folia Microbiol. 2019, 64, 497–508. [Google Scholar] [CrossRef]
- Dieterich, W.; Schuppan, D.; Schink, M.; Schwappacher, R.; Wirtz, S.; Agaimy, A.; Neurath, M.F.; Zopf, Y. Influence of low FODMAP and gluten-free diets on disease activity and intestinal microbiota in patients with non-celiac gluten sensitivity. Clin. Nutr. 2019, 38, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.-Y.; Wang, J.-J.; Sun, J.-C.; You, Y.; Ying, J.-N.; Han, X.-M. Attention deficit hyperactivity disorder may be a highly inflammation and immune-associated disease. Mol. Med. Rep. 2017, 16, 5071–5077. [Google Scholar] [CrossRef] [PubMed]
- Quarantelli, M. MRI/MRS in neuroinflammation: Methodology and applications. Clin. Transl. Imaging 2015, 3, 475–489. [Google Scholar] [CrossRef]
- Corona, J.C. Role of Oxidative Stress and Neuroinflammation in Attention-Deficit/Hyperactivity Disorder. Antioxidants 2020, 9, 1039. [Google Scholar] [CrossRef]
- Kul, M.; Unal, F.; Kandemir, H.; Sarkarati, B.; Kilinc, K.; Kandemir, S.B. Evaluation of Oxidative Metabolism in Child and Adolescent Patients with Attention Deficit Hyperactivity Disorder. Psychiatry Investig. 2015, 12, 361–366. [Google Scholar] [CrossRef]
- Bisanz, J.E.; Enos, M.K.; PrayGod, G.; Seney, S.; Macklaim, J.M.; Chilton, S.; Willner, D.; Knight, R.; Fusch, C.; Fusch, G.; et al. Microbiota at Multiple Body Sites during Pregnancy in a Rural Tanzanian Population and Effects of Moringa-Supplemented Probiotic Yogurt. Appl. Environ. Microbiol. 2015, 81, 4965–4975. [Google Scholar] [CrossRef]
- Lundgren, S.N.; Madan, J.C.; Emond, J.A.; Morrison, H.G.; Christensen, B.C.; Karagas, M.R.; Hoen, A.G. Maternal diet during pregnancy is related with the infant stool microbiome in a delivery mode-dependent manner. Microbiome 2018, 6, 109. [Google Scholar] [CrossRef]
- Val-Laillet, D.; Besson, M.; Guérin, S.; Coquery, N.; Randuineau, G.; Kanzari, A.; Quesnel, H.; Bonhomme, N.; Bolhuis, J.E.; Kemp, B.; et al. A maternal Western diet during gestation and lactation modifies offspring’s microbiota activity, blood lipid levels, cognitive responses, and hippocampal neurogenesis in Yucatan pigs. FASEB J. 2017, 31, 2037–2049. [Google Scholar] [CrossRef]
- Zou, J.; Ngo, V.L.; Wang, Y.; Wang, Y.; Gewirtz, A.T. Maternal fiber deprivation alters microbiota in offspring, resulting in low-grade inflammation and predisposition to obesity. Cell Host Microbe 2023, 31, 45–57.e7. [Google Scholar] [CrossRef]
- House, J.S.; Mendez, M.; Maguire, R.L.; Gonzalez-Nahm, S.; Huang, Z.; Daniels, J.; Murphy, S.K.; Fuemmeler, B.F.; Wright, F.A.; Hoyo, C. Periconceptional Maternal Mediterranean Diet Is Associated With Favorable Offspring Behaviors and Altered CpG Methylation of Imprinted Genes. Front. Cell Dev. Biol. 2018, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Cendra-Duarte, E.; Canals, J.; Iglesias-Vázquez, L.; Jardí, C.; Martín-Luján, F.; Arija, V. Adherence to the Mediterranean diet during pregnancy and behavioural problems at 4 years of age. Matern. Child Nutr. 2024, 20, e13700. [Google Scholar] [CrossRef]
- Che, X.; Gross, S.M.; Wang, G.; Hong, X.; Pearson, C.; Bartell, T.; Wang, X. Impact of consuming a Mediterranean-style diet during pregnancy on neurodevelopmental disabilities in offspring: Results from the Boston Birth Cohort. Precis. Nutr. 2023, 2, e00047. [Google Scholar] [PubMed]
- Makrides, M.; Gould, J.F.; Gawlik, N.R.; Yelland, L.N.; Smithers, L.G.; Anderson, P.J.; Gibson, R.A. Four-Year Follow-up of Children Born to Women in a Randomized Trial of Prenatal DHA Supplementation. JAMA 2014, 311, 1802–1804. [Google Scholar] [CrossRef] [PubMed]
- Gould, J.F.; Treyvaud, K.; Yelland, L.N.; Anderson, P.J.; Smithers, L.G.; McPhee, A.J.; Makrides, M. Seven-Year Follow-up of Children Born to Women in a Randomized Trial of Prenatal DHA Supplementation. JAMA 2017, 317, 1173–1175. [Google Scholar] [CrossRef]
- Ramakrishnan, U.; Gonzalez-Casanova, I.; Schnaas, L.; DiGirolamo, A.; Quezada, A.D.; Pallo, B.C.; Hao, W.; Neufeld, L.M.; Rivera, J.A.; Stein, A.D.; et al. Prenatal supplementation with DHA improves attention at 5 y of age: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 104, 1075–1082. [Google Scholar] [CrossRef]
- Cani, P.D.; Matthias Van, H. Mediterranean diet, gut microbiota and health: When age and calories do not add up! Gut 2020, 69, 1167–1168. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Mediterranean dietary pattern, inflammation and endothelial function: A systematic review and meta-analysis of intervention trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 929–939. [Google Scholar] [CrossRef]
- Georgoulis, M.; Yiannakouris, N.; Tenta, R.; Fragopoulou, E.; Kechribari, I.; Lamprou, K.; Perraki, E.; Vagiakis, E.; Kontogianni, M.D. A weight-loss Mediterranean diet/lifestyle intervention ameliorates inflammation and oxidative stress in patients with obstructive sleep apnea: Results of the “MIMOSA” randomized clinical trial. Eur. J. Nutr. 2021, 60, 3799–3810. [Google Scholar] [CrossRef]
- Marlow, G.; Ellett, S.; Ferguson, I.R.; Zhu, S.; Karunasinghe, N.; Jesuthasan, A.C.; Han, D.Y.; Fraser, A.G.; Ferguson, L.R. Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn’s disease patients. Hum. Genom. 2013, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Rinott, E.; Meir, A.Y.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Scholz, M.U.; Koren, O.; Stampfer, M.J.; et al. The effects of the Green-Mediterranean diet on cardiometabolic health are linked to gut microbiome modifications: A randomized controlled trial. Genome Med. 2022, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Meslier, V.; Laiola, M.; Roager, H.M.; De Filippis, F.; Roume, H.; Quinquis, B.; Giacco, R.; Mennella, I.; Ferracane, R.; Pons, N.; et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 2020, 69, 1258–1268. [Google Scholar] [CrossRef]
- Wang, S.; Li, Q.; Zang, Y.; Zhao, Y.; Liu, N.; Wang, Y.; Xu, X.; Liu, L.; Mei, Q. Apple Polysaccharide inhibits microbial dysbiosis and chronic inflammation and modulates gut permeability in HFD-fed rats. Int. J. Biol. Macromol. 2017, 99, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Vanegas, S.M.; Meydani, M.; Barnett, J.B.; Goldin, B.; Kane, A.; Rasmussen, H.; Brown, C.; Vangay, P.; Knights, D.; Jonnalagadda, S.; et al. Substituting whole grains for refined grains in a 6-wk randomized trial has a modest effect on gut microbiota and immune and inflammatory markers of healthy adults. Am. J. Clin. Nutr. 2017, 105, 635–650. [Google Scholar] [CrossRef]
- Pihelgas, S.; Ehala-Aleksejev, K.; Kuldjärv, R.; Jõeleht, A.; Kazantseva, J.; Adamberg, K. Short-term pectin-enriched smoothie consumption has beneficial effects on the gut microbiota of low-fiber consumers. FEMS Microbes 2024, 5, xtae001. [Google Scholar] [CrossRef] [PubMed]
- Reimer, R.A.; Soto-Vaca, A.; Nicolucci, A.C.; Mayengbam, S.; Park, H.; Madsen, K.L.; Menon, R.; Vaughan, E.E. Effect of chicory inulin-type fructan–containing snack bars on the human gut microbiota in low dietary fiber consumers in a randomized crossover trial. Am. J. Clin. Nutr. 2020, 111, 1286–1296. [Google Scholar] [CrossRef]
- Larrosa, M.; Luceri, C.; Vivoli, E.; Pagliuca, C.; Lodovici, M.; Moneti, G.; Dolara, P. Polyphenol metabolites from colonic microbiota exert anti-inflammatory activity on different inflammation models. Mol. Nutr. Food Res. 2009, 53, 1044–1054. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef]
- Dvoráková, M.; Sivonová, M.; Trebatická, J.; Skodácek, I.; Waczuliková, I.; Muchová, J.; Duracková, Z. The effect of polyphenolic extract from pine bark, Pycnogenol on the level of glutathione in children suffering from attention deficit hyperactivity disorder (ADHD). Redox Rep. Communt Free Radic. Res. 2006, 11, 163–172. [Google Scholar] [CrossRef]
- Holscher, H.D.; Guetterman, H.M.; Swanson, K.S.; An, R.; Matthan, N.R.; Lichtenstein, A.H.; Novotny, J.A.; Baer, D.J. Walnut Consumption Alters the Gastrointestinal Microbiota, Microbially Derived Secondary Bile Acids, and Health Markers in Healthy Adults: A Randomized Controlled Trial. J. Nutr. 2018, 148, 861–867. [Google Scholar] [CrossRef]
- Guglielmetti, S.; Fracassetti, D.; Taverniti, V.; Del Bo, C.; Vendrame, S.; Klimis-Zacas, D.; Arioli, S.; Riso, P.; Porrini, M. Differential modulation of human intestinal bifidobacterium populations after consumption of a wild blueberry (Vaccinium angustifolium) drink. J. Agric. Food Chem. 2013, 61, 8134–8140. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, J.L.; Liu, X.; Charron, C.S.; Novotny, J.A.; Jeffery, E.H.; Seifried, H.E.; Ross, S.A.; Miller, M.J.; Swanson, K.S.; Holscher, H.D. Broccoli consumption affects the human gastrointestinal microbiota. J. Nutr. Biochem. 2019, 63, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Shahinozzaman, M.; Raychaudhuri, S.; Fan, S.; Obanda, D.N. Kale Attenuates Inflammation and Modulates Gut Microbial Composition and Function in C57BL/6J Mice with Diet-Induced Obesity. Microorganisms 2021, 9, 238. [Google Scholar] [CrossRef]
- Luisi, M.L.E.; Lucarini, L.; Biffi, B.; Rafanelli, E.; Pietramellara, G.; Durante, M.; Vidali, S.; Provensi, G.; Madiai, S.; Gheri, C.F.; et al. Effect of Mediterranean Diet Enriched in High Quality Extra Virgin Olive Oil on Oxidative Stress, Inflammation and Gut Microbiota in Obese and Normal Weight Adult Subjects. Front. Pharmacol. 2019, 10, 1366. [Google Scholar] [CrossRef]
- Tuşat, M.; Eroz, R.; Bölükbaş, F.; Özkan, E.; Erdal, H. Evaluation of the protective and therapeutic effects of extra virgin olive oil rich in phenol in experimental model of neonatal necrotizing enterocolitis by clinical disease score, ınflammation, apoptosis, and oxidative stress markers. Pediatr. Surg. Int. 2024, 40, 80. [Google Scholar] [CrossRef]
- Hidalgo, M.; Prieto, I.; Abriouel, H.; Cobo, A.; Benomar, N.; Gálvez, A.; Martínez-Cañamero, M. Effect of virgin and refined olive oil consumption on gut microbiota. Comparison to butter. Food Res. Int. 2014, 64, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Hariri, M.; Djazayery, A.; Djalali, M.; Saedisomeolia, A.; Rahimi, A.; Abdolahian, E. Effect of n-3 supplementation on hyperactivity, oxidative stress and inflammatory mediators in children with attention-deficit-hyperactivity disorder. Malays. J. Nutr. 2012, 18, 329–335. [Google Scholar]
- Oikonomou, E.; Vogiatzi, G.; Karlis, D.; Siasos, G.; Chrysohoou, C.; Zografos, T.; Lazaros, G.; Tsalamandris, S.; Mourouzis, K.; Georgiopoulos, G.; et al. Effects of omega-3 polyunsaturated fatty acids on fibrosis, endothelial function and myocardial performance, in ischemic heart failure patients. Clin. Nutr. 2019, 38, 1188–1197. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Ghayour-Mobarhan, M.; Rezaiean, S.; Hoseini, M.; Parizade, S.M.R.; Farhoudi, F.; Hosseininezhad, S.J.; Tavallaei, S.; Vejdani, A.; Azimi-Nezhad, M.; et al. Omega-3 fatty acid supplements improve the cardiovascular risk profile of subjects with metabolic syndrome, including markers of inflammation and auto-immunity. Acta Cardiol. 2009, 64, 321–327. [Google Scholar] [CrossRef]
- Xu, Z.; Tang, H.; Huang, F.; Qiao, Z.; Wang, X.; Yang, C.; Deng, Q. Algal Oil Rich in n-3 PUFA Alleviates DSS-Induced Colitis via Regulation of Gut Microbiota and Restoration of Intestinal Barrier. Front Microbiol 2020, 11, 615404. [Google Scholar] [CrossRef] [PubMed]
- Kaliannan, K.; Wang, B.; Li, X.-Y.; Kim, K.-J.; Kang, J.X. A host-microbiome interaction mediates the opposing effects of omega-6 and omega-3 fatty acids on metabolic endotoxemia. Sci. Rep. 2015, 5, 11276. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.; Mitra, S.; Croden, F.C.; Taylor, M.; Wood, H.M.; Perry, S.L.; Spencer, J.A.; Quirke, P.; Toogood, G.J.; Lawton, C.L.; et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 2018, 67, 1974. [Google Scholar] [CrossRef] [PubMed]
- Vijay, A.; Astbury, S.; Le Roy, C.; Spector, T.D.; Valdes, A.M. The prebiotic effects of omega-3 fatty acid supplementation: A six-week randomised intervention trial. Gut Microbes 2021, 13, 1863133. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Pham, V.; Seifert, N.; Richard, N.; Sybesma, W.; Steinert, R.E. The Polyunsaturated Fatty Acids Eicosapentaenoic Acid and Docosahexaenoic Acid, and Vitamin K1 Modulate the Gut Microbiome: A Study Using an In Vitro Shime Model. J. Diet. Suppl. 2024, 21, 135–153. [Google Scholar] [CrossRef]
- Lo Conte, M.; Antonini Cencicchio, M.; Ulaszewska, M.; Nobili, A.; Cosorich, I.; Ferrarese, R.; Massimino, L.; Andolfo, A.; Ungaro, F.; Mancini, N.; et al. A diet enriched in omega-3 PUFA and inulin prevents type 1 diabetes by restoring gut barrier integrity and immune homeostasis in NOD mice. Front. Immunol. 2022, 13, 1089987. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Dvořáková, M.; Ježová, D.; Blažíček, P.; Trebatická, J.; Škodáček, I.; Šuba, J.; Waczulíková, I.; Rohdewald, P.; Ďuračková, Z. Urinary catecholamines in children with attention deficit hyperactivity disorder (ADHD): Modulation by a polyphenolic extract from pine bark (Pycnogenol®). Nutr. Neurosci. 2007, 10, 151–157. [Google Scholar] [CrossRef]
- Kansal, S.; Catto-Smith, A.G.; Boniface, K.; Thomas, S.; Cameron, D.J.; Oliver, M.; Alex, G.; Kirkwood, C.D.; Wagner, J. Variation of Gut Mucosal Microbiome with Anti-Saccharomyces cerevisiae Antibody Status in Pediatric Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 696–703. [Google Scholar] [CrossRef]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol. Autism 2013, 4, 42. [Google Scholar] [CrossRef]
- Martínez, N.; Prieto, I.; Hidalgo, M.; Segarra, A.B.; Martínez-Rodríguez, A.M.; Cobo, A.; Ramírez, M.; Gálvez, A.; Martínez-Cañamero, M. Refined versus Extra Virgin Olive Oil High-Fat Diet Impact on Intestinal Microbiota of Mice and Its Relation to Different Physiological Variables. Microorganisms 2019, 7, 61. [Google Scholar] [CrossRef]
- De Santis, S.; Crupi, P.; Piacente, L.; Mestice, A.; Colabufo, N.A.; Amodio, L.; Pontrelli, P.; Gesualdo, L.; Moschetta, A.; Clodoveo, M.L.; et al. Extra virgin olive oil extract rich in secoiridoids induces an anti-inflammatory profile in peripheral blood mononuclear cells from obese children. Front. Nutr. 2022, 9, 1017090. [Google Scholar] [CrossRef] [PubMed]
- Kaisari, P.; Dourish, C.T.; Rotshtein, P.; Higgs, S. Associations Between Core Symptoms of Attention Deficit Hyperactivity Disorder and Both Binge and Restrictive Eating. Front. Psychiatry 2018, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Buja, A.; Manfredi, M.; Zampieri, C.; Minnicelli, A.; Bolda, R.; Brocadello, F.; Gatti, M.; Baldovin, T.; Baldo, V. Is emotional eating associated with behavioral traits and Mediterranean diet in children? A cross-sectional study. BMC Public Health 2022, 22, 1794. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsdottir, S.; Njardvik, U.; Bjarnason, R.; Olafsdottir, A.S. Changes in Eating Behaviors Following Taste Education Intervention: Focusing on Children with and without Neurodevelopmental Disorders and Their Families: A Randomized Controlled Trial. Nutrients 2022, 14, 4000. [Google Scholar] [CrossRef]
- Wise, R.A. Role of Brain Dopamine in Food Reward and Reinforcement. Philos. Trans. Biol. Sci. 2006, 361, 1149–1158. [Google Scholar] [CrossRef]
- Cerrillo-Urbina, A.J.; García-Hermoso, A.; María Jesús, P.-G.; Sánchez-López, M.; Santos-Gómez, J.L.; Martínez-Vizcaíno, V. The Effects of Long-Acting Stimulant and Nonstimulant Medications in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder: A Meta-Analysis of Randomized Controlled Trials. J. Child Adolesc. Psychopharmacol. 2018, 28, 494–507. [Google Scholar] [CrossRef]
- Zeisel, S.H. Precision (Personalized) Nutrition: Understanding Metabolic Heterogeneity. Annu. Rev. Food Sci. Technol. 2020, 11, 71–92. [Google Scholar] [CrossRef]
- Ordovas, J.M.; Ferguson, L.R.; Tai, E.S.; Mathers, J.C. Personalised nutrition and health. BMJ 2018, 361, bmj.k2173. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, J.; Noh, G.; Lee, S.S. The effects of elimination diet on nutritional status in subjects with atopic dermatitis. Nutr. Res. Pract. 2013, 7, 488–494. [Google Scholar] [CrossRef]
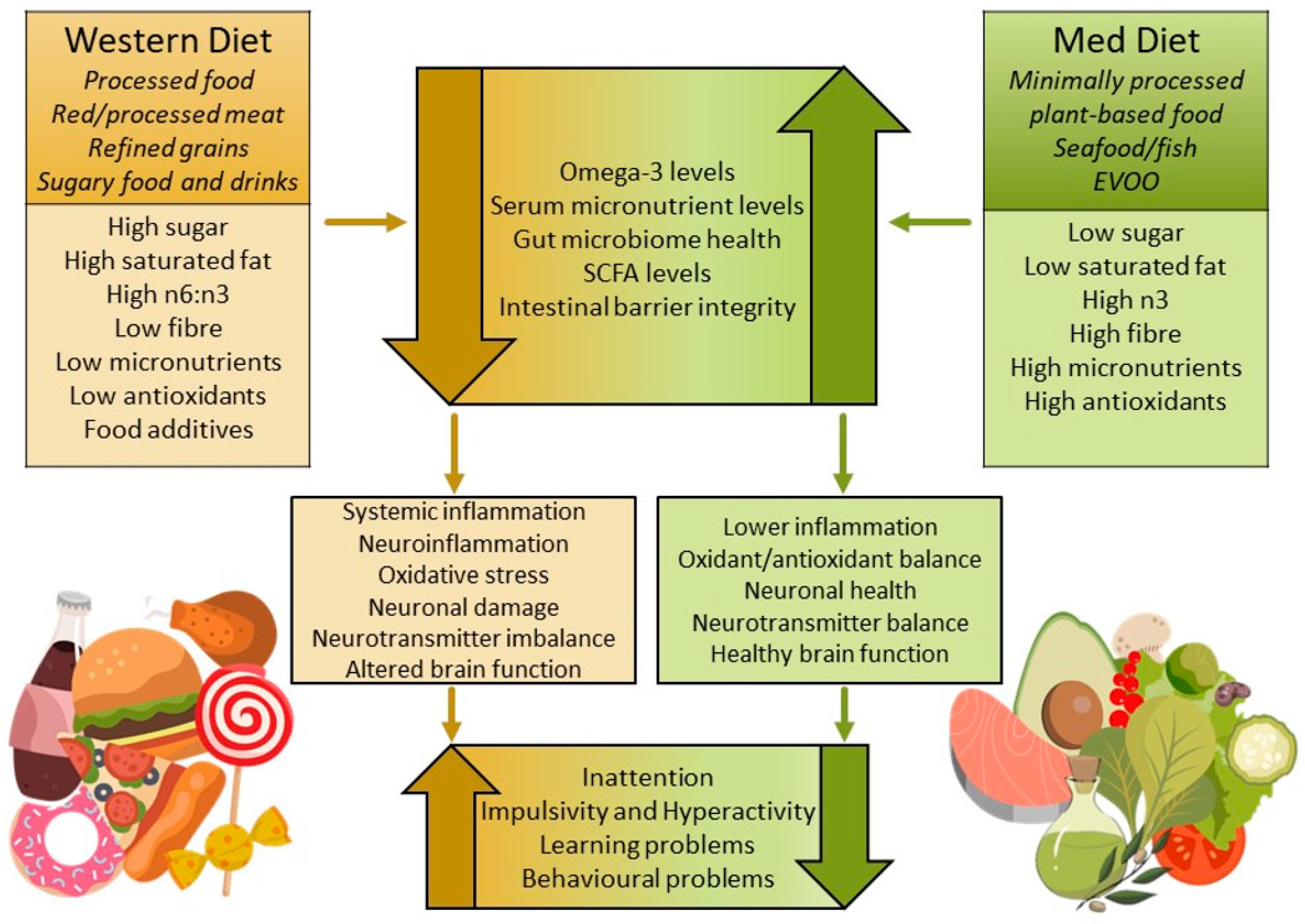
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewis, N.; Lagopoulos, J.; Villani, A. Gut–Brain Inflammatory Pathways in Attention-Deficit/Hyperactivity Disorder: The Role and Therapeutic Potential of Diet. Metabolites 2025, 15, 335. https://doi.org/10.3390/metabo15050335
Lewis N, Lagopoulos J, Villani A. Gut–Brain Inflammatory Pathways in Attention-Deficit/Hyperactivity Disorder: The Role and Therapeutic Potential of Diet. Metabolites. 2025; 15(5):335. https://doi.org/10.3390/metabo15050335
Chicago/Turabian StyleLewis, Naomi, Jim Lagopoulos, and Anthony Villani. 2025. "Gut–Brain Inflammatory Pathways in Attention-Deficit/Hyperactivity Disorder: The Role and Therapeutic Potential of Diet" Metabolites 15, no. 5: 335. https://doi.org/10.3390/metabo15050335
APA StyleLewis, N., Lagopoulos, J., & Villani, A. (2025). Gut–Brain Inflammatory Pathways in Attention-Deficit/Hyperactivity Disorder: The Role and Therapeutic Potential of Diet. Metabolites, 15(5), 335. https://doi.org/10.3390/metabo15050335







